Abstract
Recent studies have revealed synaptic dysfunction to be a hallmark of various psychiatric diseases, and that glial cells participate in synapse formation, development, and plasticity. Glial cells contribute to neuroinflammation and synaptic homeostasis, the latter being essential for maintaining the physiological function of the central nervous system (CNS). In particular, glial cells undergo gliotransmission and regulate neuronal activity in tripartite synapses via ion channels (gap junction hemichannel, volume regulated anion channel, and bestrophin-1), receptors (for neurotransmitters and cytokines), or transporters (GLT-1, GLAST, and GATs) that are expressed on glial cell membranes. In this review, we propose that dysfunction in neuron-glia interactions may contribute to the pathogenesis of neurodevelopmental disorders. Understanding the mechanisms of neuron-glia interaction for synapse formation and maturation will contribute to the development of novel therapeutic targets of neurodevelopmental disorders.
Keywords: neurodevelopmental disorder, neuron-glia interactions, ASD, ADHD, epilepsy
1. Introduction
Neurodevelopment occurs during the early stages of life. Maturation involves the consolidation of neural wiring, including synapse formation and synapse pruning [1]. Traditionally, synapse formation and pruning have been studied when investigating the generation and elimination of synapses. Synapses were thought of as the messenger for two adjusting neurons and that the elimination of synapses by pruning could establish synapse patterns that affect neuronal signaling [2,3]. However, recent studies have identified previously unknown features of glial cells that facilitate their interaction with neurons during brain development, such as the release of gliotransmitters and cytokines. In addition, synaptic changes induced by glial cells in the developmental stage, including synaptic patterning secondary to pruning, are now being investigated in relation to the neuron-glia interactions [4,5]. Glial cells release gliotransmitters such as gamma-aminobutyric acid (GABA), glutamate, and cytokines [6,7], which may affect neurons both directly and indirectly [8]. These substances are known to act at tripartite synapses [9].
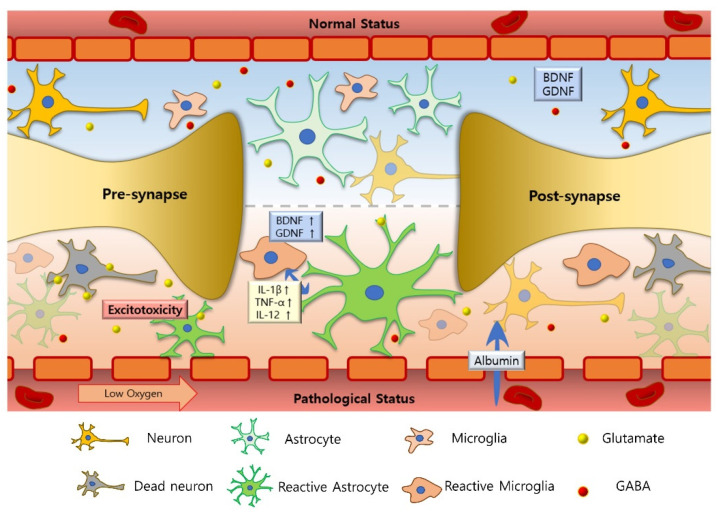
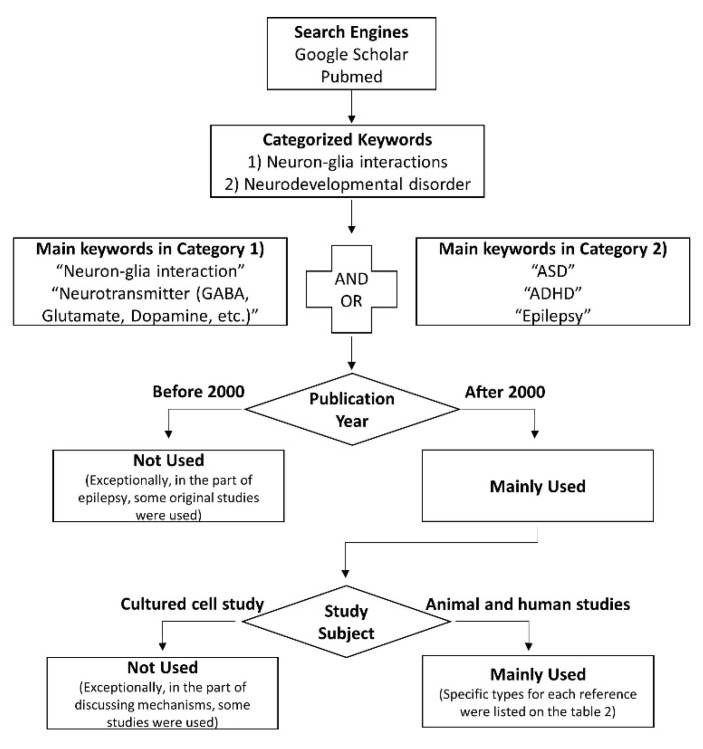
Another important concept in neuron-glia interactions is the tonic release of gliotransmitters, typically manifesting with extracellular glutamate and GABA. Tonic inhibition has been invaluable for studying neurodegenerative disorders such as Alzheimer’s disease [10] and Parkinson’s disease [11]. However, this constant stimulation of nerve cells has rarely been studied in neurodevelopmental disorders [12]. In this review article, we introduce the concept of neuron-glia interactions as applied to several representative neurodevelopmental disorders, including autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and epilepsy (Figure 1). The search strategy used for the references identified in this review is presented in Figure 2 as a flow chart, and the characteristics of the included studies are shown in Table 1.
Figure 1.
Changes associated with neuron-glia interactions in neurodevelopmental disorders. Inflammatory cytokines such as IL-12, IL-1β, and TNF-α are increased in neurodevelopmental disorders compared to normal individuals [13,14,15]. The increased cytokines activate astrocytes and microglia; these glial cells expand as a result of gliosis and release glutamate into the extracellular space, which kills the neurons. However, in patients with epilepsy, the blood-brain barrier opens, leading to the increased entry of albumin (↑) into the brain and astrocyte activation [16]. Unusually, in epilepsy, the released cytokines are known to facilitate functional neurogenesis and induce the release of neurotrophic factors such as neuronal growth factor (NGF), brain-derived neurotrophic factor (BDNF), and glial-cell derived neurotrophic factor (GDNF) (↑) [17,18,19,20].
Figure 2.
The search strategy used to identify references in this review.
Table 1.
Classification of the types and methodological components of papers contributing to the review.
| Types of Paper | |||||
|---|---|---|---|---|---|
| Review | Research | Others | |||
| Systematic | Animal | Human | Mathematical | ||
| [3,7,14,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] | Genetic | [1,2,4,5,8,9,10,11,12,17,18,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92] | Case | [13,15,16,93,94,95,96] | [97,98] |
| Meta-analysis | Pharmacological | [6,11,12,18,20,63,77,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122] | Cohort | [123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148] | |
| [149,150] | Environmental | [16,71,151,152,153,154,155,156] | Comparative | [157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178] | |
2. ASD (Autism Spectrum Disorder) and ADHD (Attention-Deficit/Hyperactivity Disorder)
ASD is a neurodevelopmental disorder that affects 1% of the population and there is currently no effective pharmacological treatment for ASD. There is a marked gender imbalance in ASD, with the rate being 4.3 times higher in the boys than in the girls [123]. For the majority of affected individuals, the cause of ASD remains unknown. ASD is characterized by a collection of neurobehavioral and neurological dysfunctions, including social and communication deficits, repetitive behaviors, and obsessive interests [21,179]. Previous studies have revealed that genetic and environmental factors contribute to autism. One of the environmental factors for ASD is maternal immune activation (MIA). Maternal viral infection, toxin exposure, and maternal obesity are linked to the dysfunction of inflammatory and immune pathways, which could increase the risk of behavioral deficits in offspring [124,125,151]. MIA is also known to be associated with ASD [99,100,126]. For example, mothers of children with ASD have been shown to have a higher frequency of allergies and autoimmune diseases compared to mothers with normally developing children [126]. In addition, the concentration of cytokines, including IFN-γ, IL-4, and IL-5, are reportedly elevated in the mid-gestation serum of females bearing a child with ASD [127,128]. Polyinosinic:polycytidylic acid (poly I:C) can mimic prenatal viral infection. MIA induced by the injection of poly I:C in a mother mouse model revealed that P2X7 purinergic receptors drive poly I:C-induced autism-like phenotypes such as social deficits and increased self-grooming [63]. This can induce an increase in inflammatory cytokines and impair neurogenesis, resulting in decreased Purkinje cell number and their density in the cerebellum as well as behavioral abnormalities in the fetal brain [101,157]. The decrease in GABAergic Purkinje cells in the cerebellum could cause a deficit in GABAergic transmission [101] and these neurodevelopmental dysfunctions may be related to ASD. In addition, MIA can alter the mammalian target of rapamycin (mTOR) pathway-associated genes, causing dysregulated inflammation [102]. MIA can be induced by poly I:C and prenatal virus infection [22].
On the other hand, it is known that environmental factors such as heavy metals can cause neurotoxicity. Lead (Pb) exposure disrupts Ca2+-dependent cell signaling and glutamatergic transmission though antagonization of the NMDA receptor [23]. Chronic methylmercury (MeHg) exposure directly inhibits Ca2+, glutamate, and GABA signaling [152]. Inhibition of the glutamine transporter in MeHg-exposed cultured astrocytes has been shown to result in a decrease in glutamate signaling [153]. In addition, GABA signaling decreased with modified GABAA receptor conformation upon MeHg exposure [152].
There are numerous autism-related genetic factors, including genes such as Shank3, neuroligin, neurexin, Pten, TSC1, MeCP2, and Scn1a [64,65,66,158]. In particular, Shank3, neuroligin, and neurexin are widely known genes associated with ASD [24,25]; the other genes described above have been revealed by large-scale exome sequencing analyses [159,160]. Neuroligin and neurexin connect pre- and post-synaptic neurons at the synapse and mediate trans-synaptic signaling. Mutations in neurexin or neuroligin genes have been implicated in autism and other cognitive disorders [161,162] and may induce perturbations in synaptic transmission [67].
ADHD is one of the common neurodevelopmental disorders and contributes to children’s poor academic performance and deficits in social skills. Ten percent of school-aged children suffer from ADHD, and it is twice as common in males than in females [129]. The three traits that define ADHD are inattention, hyperactivity, and impulsivity [26,180]. Conventional treatment for children with ADHD, such as the neuroleptic drug methylphenidate, does not eliminate the root cause of the disorder and has some side effects, including sleep disorder [27]. Several animal models and human studies have attempted to elucidate the pathophysiologic mechanisms of ADHD. Recently, genome-wide association study (GWAS) and single nucleotide polymorphism (SNP) studies have identified ADHD-associated genes such as GIT1 [68,149]. In addition to genetics, some studies have suggested other possible causes and risk factors, including exposure to environmental materials during pregnancy or at a younger age and the use of alcohol and tobacco during pregnancy [163,164].
Glial cells comprise 70% of all the brain cells [28] and help maintain a healthy central nervous system (CNS) environment through its role in synaptic maturation and plasticity. Therefore, understanding how glia affects neuronal activity is essential.
2.1. Neuroinflammation in ASD and ADHD
Recent research efforts have focused on neuron-glia interactions in the CNS, including dysregulated immune activities in various types of glial cells as well as the changes between resting-state and reactive glial cells. Microglia are the resident immune cells of the CNS and act as primary mediators of neuroinflammation [29]. Astrocytes perform similar functions as microglia in terms of immunity [30]. Microglia in healthy CNS tissue have a ramified shape in the resting state. When microglia are activated, they become ameboid in association with the phagocytosis of cellular debris, antigens, and synapse pruning. In the brain tissue of patients with ASD, the activated form of microglia have been observed [181]. Glial cells are known to secrete neurotrophic factors such as anti-inflammatory cytokines (IL-4 and IL-10), neuronal growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-4/5 in the resting state [17,18]. In addition, microglia perform neuroprotective functions that contribute to neurogenesis and synaptic homeostasis [31]. Glial cells change their form when activated, responding to antigens associated with a variety of pathological states. Activated glial cells secrete proinflammatory cytokines such as IL-1β, IL-6, and TNF-α. However, cytokines derived from microglia could be cytotoxic to neurons and other glial cells, especially oligodendrocytes [13]. Excessive activation of glial cells drives systemic inflammation in the brain, which might cause synaptic elimination and dysfunctional synaptic plasticity [14]. Proinflammatory cytokines trigger the induction of excitotoxicity via an increase in glutamate release, alteration of ion channel expression, and immune activation of the blood-brain barrier. It has been suggested that an increased level of cytokines circulating in the blood due to immune activation could cause neurodevelopmental disorders in children during the neonatal period [130]. To confirm the effect of immune activation, more studies in children with ASD are required, especially considering that humans are subject to marked variations in the environmental and genetic factors. Cytokines secreted by glia affect various functions of the brain. For example, IL-1β is primarily a proinflammatory cytokine that increases in response to infection. This activates the innate immune system and induces the activation of immune cells such as T lymphocytes, B lymphocytes, and microglia. This dysregulation of IL-1β in the early stages of life should be considered when studying the mechanisms of neurodevelopmental problems, which include learning and memory deficits [69]. Highly elevated levels of IL-1β expression affect neural progenitor cell proliferation in the CNS and contribute to region-specific deviant brain growth in patients with ASD [131]. Neuropeptide neurotensin (NT) stimulates microglia to secrete IL-1β, which is increased in the cell cultures of human microglia obtained from children with ASD [103,132]. IL-1β increases the expression level of IL-6 and TNF-α, which are other proinflammatory cytokines contributing to the induction of apoptosis of neuronal cells [165]. IL-6 can modulate autism-like behaviors through impairments of synapse formation, dendritic spine development, and neuronal circuit balance [103,133]. However, IL-6 also has anti-inflammatory cytokine roles; this implies that IL-6 can participate in regulating metabolism and neuronal processes. Therefore, elevated IL-6 levels should be studied to evaluate its effect on the immune system.
TNF-α is secreted in a soluble/membrane-bound form and is known to induce apoptosis. In addition, microglia secrete chemokines, including CX3CR1 and CCL2. A function of chemokines is to recruit the immune cells. In the brain, macrophages secrete chemokines to attract microglia for phagocytosis. They release chemokines such as CX3CR1, CCL2, and CCL21, and they act as paracrine factors [166].
In addition, reactive microglia and astrocytes are known to produce reactive oxygen species (ROS). ROS are highly cytotoxic and lead to oxidative stress in neurons. Oxidative stress induces apoptosis; thus, chronic inflammation of the CNS may contribute to neuronal death by apoptosis triggered by ROS and inflammatory cytokines [32].
The abnormal cytokine profile of ASD patients suggests that immune system disturbance and abnormal neuroinflammation could be biomarkers of autism. These inflammatory changes in autism have been applied to rodent modes of ASD (Table 2).
Table 2.
ASD/ADHD models with observed neuron-glia interactions. ASD: autism spectrum disorder; ADHD: attention-deficit/hyperactivity disorder.
| ASD | Model |
|---|---|
| Genetic | Shank2 KO [24], Shank3 KO [70], NLGN3 R451C KI [67], NLXN1 KO [24], TSC1 HT [64], BTBR [70], MeCP2 mutant [65], Scn1a HT [64], PTEN mutant [158] |
| Pharmacological | Valproic acid (VPA) [71] |
| Environmental | Methyl mercury [127], maternal immune activation (MIA) [99,100,126], polyinosinic:polycytidylic acid (poly I:C) [63] |
| ADHD | Model |
| Genetic | Dopamine transporter (DAT) mutant [33], NK1R-KO [33], SNAP25 mutant [33], Git1 KO [160,161], Cdk5 KO [72], nicotinic acetylcholine receptor (nAChR) ß2-KO [33] |
| Pharmacological | Ethanol [162], methylazoxymethanol [104,105] |
| Environmental | Neonatal X-radiation, hypoxia, heavy metal exposure (lead, cadmium), oncogenic environmental exposure (polychlorinated biphenyl (PCB)) [33] |
NLGN: neuroligin; NLXN: neurexin; TSC: tuberous sclerosis; MeCP2, methyl CpG binding protein 2; Scn1: sodium channel protein type 1; PTEN: phosphatase and tensin homolog; NK1: neurokinin; SNAP: synaptosome associated protein.
Prenatal exposure to the antiepileptic drug valproic acid (VPA) has been reported to result in a high incidence of autism. Lucchina and Depino used a VPA-exposed ASD mouse model to study altered inflammation [71]. VPA-exposed mice showed reduced social interaction and increased depression-like behavior. In addition, long-lasting glial activation was observed in the hippocampus and cerebellum in association with lipopolysaccharide (LPS) stimulation. This observed activation of glial cells could help to explain the mechanism causing altered levels of cytokines in ASD [106].
Neuroinflammation in ADHD is a significant factor that can alter glial cell activity. This is supported by the finding that serum IL-6 levels in children with ADHD are increased compared to healthy children [134]. This implies that ADHD patients have dysregulated inflammatory responses [134]. Despite being an animal model of hypertension, the spontaneous hypertensive rat (SHR) has been used as a model of ADHD. The SHR model exhibits increased levels of inflammatory cytokines (IL-1b, IL-6, and TNF-α) in the serum, but decreased levels of TGF-β level. This model is limited by the presence of hypertension. However, the results of this rat model coincide with those of other studies on other older rodent models, used well earlier than the 1990s [73]. This highlights the need for a more suitable ADHD animal model and that research should be conducted in relation to the mechanism of ADHD.
An increase in oxidative stress was identified in a meta-analysis of patients with ADHD. The authors suggested that patients with ADHD had normal levels of antioxidant production, but the response to oxidative stress was insufficient [150].
On the other hand, it has been reported that methylphenidate hydrochloride (MP)-treated rats showed increased microglial activity in multiple regions of the brain (such as the insular cortex, hippocampus, and thalamus) [104]. MP is widely used in therapies for various psychiatric diseases, including ADHD and chronic sleep disorders. However, chronic MP treatment could mimic the pharmacokinetic profile of treated ADHD patients due to the blockage of the dopamine transporter (DAT) and norepinephrine transporter (NET) and the increased monoamine levels in the synapse. Chronic MP treatment affects the number of dopamine neurons in the substantia nigra (SN) as well as microgliosis. The mRNA expression of proinflammatory cytokine genes (IL-6 and TNF-α) is also increased in the acute MP-treated mouse brain [105]. Therefore, MP treatment is relevant to brain inflammation in ADHD.
2.2. Impaired E/I Balance in Patients with ASD and ADHD
One of the characteristic changes in ASD and ADHD is the E/I imbalance. Notably, increased glutamatergic excitation or reduced GABAergic inhibition occur in association with neuropsychiatric disorders including ASD, ADHD, and epilepsy. Previous studies have reported that astrocyte dysfunction associated with impaired glial glutamate uptake is a biomarker of numerous neuropsychiatric disorders [74]. Serum glutamate levels were elevated in children with ASD compared to controls. This serum glutamate change reflects altered glutamatergic neurotransmission [135]. Astrocytes are important in excitatory regulation to control extracellular glutamate levels via the glutamate transporters GLAST and GLT-1; in humans, these proteins are known as EAAT1 and EAAT2, respectively [34]. Various factors, including inflammatory cytokines, ROS, and genetic factors, affect the expression and function of glutamate transporters in astrocytes. GLT-1 knockouts have revealed astroglial dysfunction is associated with glutamate signaling. The study further showed an increase in repetitive behaviors and seizure severity in GLT-1 inducible knockout (iKO) mice. EPSC amplitude was also increased in GLT-1 iKO mice. GLAST affects locomotor hyperactivity and is exaggerated by the NMDAR antagonist, MK-801. This implies that the glial glutamate uptake is a deficit related to the pathophysiological risk factors for disease. This further suggests that GLAST KO contributes to hyperactivity related to psychiatric disorders [75].
Neuronal glutamatergic release and uptake are the principal signaling mechanisms in glutamatergic transmission. Astrocytes also participate in this signaling, and they uptake surplus glutamate that remains in the extracellular space to prevent the accumulation of glutamate and excitotoxicity. Generally, excitotoxicity is related to the activation of NMDARs by excessive glutamate, and brain cell death usually occurs after stimulation of glutamate receptors due to impaired reuptake of glutamate through GLT-1 transporters on glial cells [136]. It has been reported that the expression of these transporters is regulated by NF-κB [76]. One study in rats with upregulated GLT-1 expression, due to ceftriaxone, revealed that it severely impairs long-term depression (LTD) in mossy fibers of the rat hippocampal CA3 region. In addition, they revealed that chronic treatment with ceftriaxone altered long-term potentiation (LTP), which is associated with an increase in glutamate release [77].
Microglia also participate in glutamate signaling via the Xc- system. The Xc- transporter is a chloride-dependent antiporter that carries glutamate out of the cell and transports cysteine/cystine in at a ratio of 1:1 [35,78]. ROS are produced by the reactive form of microglia. ROS induce glutathione (GSH) shortages and initiate TLR4 signaling. TLR4 triggers elevated Xc- expression levels, resulting in cysteine/cystine influx and glutamate efflux [107]. ROS, TNF-α, and IL-1ß secreted from microglia impair EAAT function and increase extracellular glutamate levels. Taken together, reactive microglia interfere with neurotransmission by releasing excitotoxins such as glutamate, D-serin, adenosine triphosphate (ATP), and impaired glutamate uptake, and alter gliotransmitter release from astrocytes.
GABA is a representative inhibitory neurotransmitter that regulates the overall functions controlled by the brain, including learning and memory function [36,37]. Dysfunction in GABA transmission may be the pathological evidence of an E/I imbalance [38]. Astrocytes express GABA receptors (GABAR)—specifically, ionotropic GABAA and metabotropic GABAB receptors—and GABA transporters (GATs), including the GAT-1 and GAT-3 [79]. Through these receptors and transporters, glial cells actively interact with interneurons in a variety of brain regions [37]. In addition, astrocytes can regulate excitatory transmission by releasing ATP, which inhibits the activation of presynaptic adenosine receptors [80]. Furthermore, astrocytes synthesize GABA via monoamine oxidase B (MAOB) from putrescine in a different pathway to neurons [81]. The astrocytic bestrophin-1 (Best1) channel has been reported to mediate tonic inhibition by releasing GABA [12]. Therefore, astrocytes are crucial for regulating the E/I balance in the tripartite synapse. Additionally, reactive astrocytes can alter the expression level of membrane proteins and the activity of enzymes, which affect the tonic GABA levels. Previous studies reported a decrease in GABAergic interneurons and transmission in the ASD mouse model [82].
Presynaptic GAD1/2 is expressed in GABAergic neurons. Chao et al. studied an X-linked methyl-CpG-binding protein (MeCP2) mutation in a mouse model of ASD. They targeted the vesicular inhibitory amino acid transporter (Viaat, also known as Slc32al) to specifically delete the MeCP2 molecule in GABAergic neurons; therefore, GABAergic neuron-specific mutations in the Viaat-Mecp2-/y mice down-regulated MeCP2 levels in GABA-positive neurons. These mice showed increased autism-like behavior such as repetitive behaviors. In addition, reduced Gad1/2 levels have been linked with decreased miniature IPSC (mIPSC) amplitude and electroencephalography-measured hyperexcitability [65]. Another study used the BTBR T+tf/J mouse strain as an ASD model to analyze the impairment of inhibition. BTBR mice had decreased GAD65 expression in the insular cortex, which is related to communication and social behavior. BTBR mice also showed impaired mIPSC, which implies weak inhibition in the insular cortex. Besides, other ASD model mice (Shank3 KO, MeCP2 KO) showed decreased GAD65 and parvalbumin expression levels. Weakened inhibitory transmission may affect E/I balance deficits and result in autism-like behaviors in ASD [70]. It has been reported that the deletion of Nav1.1 voltage-dependent sodium channel in ASD mice (Scn1a+/−) results in impaired GABAergic transmission due to a deficit in the firing of GABAergic interneurons. The benzodiazepine clonazepam, which is a positive allosteric modulator for the GABAA receptor, recovered the behavior and GABAergic transmission [66].
Changes in glutamate and GABA levels in ADHD have also been investigated [39]. Changes in GABA levels have been reported in various regions, such as the cortex, striatum, and anterior cingulate cortex, and are an important trait of ADHD. Several studies have suggested that the decrease of GABA levels seen in ADHD patients might be related to the deficit of inhibitory actions in behaviors [137,138,167]. These decreased levels of GABA might be due to decreased glial GABA levels in the same region. The hippocampus and cerebellum have been reported to have reduced glial GABA levels, which might cause inattention and hyperactivity behaviors in animal models [83,84]. The GAT-1 KO mice exhibit hyperactivity, lack of attention, and learning deficiency [85].
However, other studies have reported no change or an increase in GABA levels. In children with ADHD, the subcortical GABA level was no different, while the GABA level in the subcortical and frontal regions in adults with ADHD was increased [182]. In addition, GABA release from striatal MSN neurons, by binding to the astrocytic GABAB receptors, activates neuronal spiking and contributes to the hyperactivity with disrupted attention [86]. In a study using cadherin 13 (Cdh13) KO mice, the GABA levels remained unaltered in the hippocampal CA1 region. However, the mIPSC increased in the KO mouse [72]. In addition, the glutamate level was altered in the ADHD patients, and the gene expression levels related to glutamate showed significant differences compared to the controls [168]. Regarding the increase and decrease in GABA or glutamate, it is suggested that relative changes and balance are more important than absolute concentrations. Animal models may have different trends depending on their molecular and genetic characteristics, and human studies differ depending on the stage of the person’s studies life when the studies were conducted. Further animal and human studies are required to investigate more specific approaches as different aspects of both types of study can occur depending on the region and circuit of the brain studied.
3. Epilepsy
Epilepsy has been studied for a long time and the neurophysiologic traits of epilepsy are well-known, namely the abnormal heterogeneous and dynamic neuronal spiking resulting in ictal events in the brain [93,97]. Despite extensive studies on epilepsy, there have been relatively few studies on the effect of glial cells. Recently, several studies have been conducted to determine the function of glial cells in the epileptic brain. In addition, a review article was published to help elucidate trends in epilepsy studies [40]. The newly understood glial cell function of releasing gliotransmitters, including glutamate and GABA, changing the synaptic environment, and inducing the release of cytokines have allowed epilepsy to focus more on the neuron and glial interactions [41,87,154].
Conventionally, the main reason for epilepsy has been thought to be an increase in neuron-oriented glutamate and a decrease in GABA levels [42,43,88]. However, some studies have reported that several functions of glia contribute to the pathogenesis of epilepsy [44,45]. Epileptogenic cues activate microglia and astrocytes. These activated glial cells can increase the synthesis and release of proinflammatory molecules. Thus, glial cells contribute to neuroinflammation [46].
3.1. Neuroinflammation in Epilepsy
Neuroinflammation is caused by the release of cytokines from a variety of sources in the brain. Epilepsy is a common symptom in patients with brain tumors, head injuries, and genetic diseases [89,94,139,140]. Epileptic patients have been reported to have high levels of IL-1ß, IL-1 receptors (IL-1Rs), IL-8, IL-12, MIP-1b, translocator protein (TSPO), toll-like receptor-4 (TLR4), high mobility group box 1 (HMGB1), TNF-α, and cytokine-related genes [15,47,89,139,140]. IL-1ß and TNF-α are the most widely studied, and the concentration of these cytokines is known to be important for protogenic status and inhibition of seizures [48]. Rodent knock-out of laforin, encoding for the gene Epm2a, and malin, encoding for the gene Epm2b, exhibit seizures that are genetically induced. In this Lafora disease (LD) rodent model, upregulated inflammatory-related genes have been reported through RNA-sequencing (Table 3). Approximately 60% of the upregulated proteins were found to be related to microglia [89]. LD is a genetically induced epilepsy. Laforin and malin levels are decreased when genetically mutated, and polyglucosan bodies (PGBs) accumulate in the brain. PGB has been studied in neurons for a long time, but the fact that astrocytes also have a considerable portion of PGBs is a recent discovery. Astrocytic PGBs are significantly increased in malin KO mice, indicating that astrocytic PGB could also cause LD [90,169]. Therefore, further studies on neuron-glia interactions and pathogenesis are required using this genetic model of epilepsy.
Table 3.
Epilepsy models with observed neuron-glia interactions.
| Epilepsy | Model |
|---|---|
| Genetic | AP (Activator Protein)-1 KO [108], SCN1A KO, DBA/2 KO [109] Lafora disease: Malin KO, Epm2a, Epm2b [89,90] |
| Pharmacological * | Pentylenetetrazol (PTZ), pilocarpine (PA), kainic acid (KA) |
| Environmental | Electrical stimulation, brain injury |
* To study epilepsy, pharmacological models are commonly and widely used.
Increased levels of inflammatory cytokines could act as a neuromodulator in the brain between glial cells and neurons [49,170]. Cell survival rates following cytokine release in epilepsy are highly dependent on concentrations [48]. Cytokines released from astrocytes reduce the survival rate of neurons by free nitric oxide (NO) and excitotoxicity to neurons, as described below [48,141]. Studies have also explored several treatments and recovery tests on glial cells in epilepsy. Treatment with vitamin E (α-tocopherol), miR-146a, and aucubin (AU) has been reported to reduce epileptic events [94,110,111]. Glial cell changes could reduce the inflammatory signals in the brain, and these reduced signals could stop stimulating the neurons around the glial cells. The α-tocopherol mentioned above could reduce IL-1ß and TNF-α levels by treatment in epileptic mice [112].
Compared to the control group, patients with epilepsies showed a several-fold increase in glial cells [111]. Activation of microglia and astrocytes is crucial to neurons and other types of cells that exist in proximity to glial cells [171]. Moreover, with the increase in inflammatory cytokines, chronically or overly activated glial cells are also frequently reported in epilepsy [111,142,143,155]. Chronic immune activation or the overexpression of TNF-α in glial cells can cause a synapse change that results in networks forming seizure-like activity patterns [50,91,98,113]. These reactive glial cells or gliosis imply that the recovery of glial cell function could reduce seizure events in epilepsy. In a post-traumatic epileptic rat model, increased glial reactivity was associated with the hyperexcitability of the neocortex region [155]. These activated glial cells have some loss-of-function changes. By recovering these glial cells, the frequency or amplitude of epileptic seizures could be rescued, which highlights the importance of glial cells in epileptic events. Through treatment with the antiserum of P2X7, an ionotropic receptor responding to extracellular ATP, seizure activity can be attenuated [114]. However, cytokines released from glial cells are not just neurotoxic. In the presence of IL-1β and TNF-α, astrocytes release NGF, BDNF, and glial-cell derived neurotrophic factor (GDNF) [51], which could explain why cytokines released in the brain cause neurodegeneration and neurogenesis in epilepsy [19,52].
Blood is another source that could introduce cytokines into the brain. Blood vessels are the main medium of all nutrients, oxygen, and cytokines. The change in permeability of the blood vessels changes the brain environment dynamically. Astrocytes are located close to blood vessels. The endfeet of astrocytes surround the vessels and contribute to neurovascular coupling (NVC) [20]. Astrocytic calcium levels are increased in the activated form. This astrocytic calcium signaling at the endfeet is related to ictal signals by the manipulation of vasoconstriction [20]. The endfeet of astrocytes are known to dilate the blood vessels at low oxygen levels [53]. In the biological model, low-oxygen levels resulted in increased epilepsy-like spiking patterns [41]. BBB dysfunction changes the permeability of molecules in the brain, and albumin flows into the hippocampus. Excessive albumin input changes the microenvironment and activates astrocytes [16,54]. The severity of BBB permeability dysfunction is positively correlated with seizure frequency [16].
Taken together, released cytokines following the activation of glial cells act on both neurodegeneration and neurogenesis in the epileptic brain. The dysfunction of the BBB could activate the release of these cytokines.
3.2. Impaired E/I Balance in Epilepsy
An increase in glutamate and a decrease in GABA levels was traditionally thought to be the main cause of epilepsy. In the AP-1 mouse model, the expression of GLAST is decreased [108]. In addition, the increase in glutamate levels could induce excitotoxicity and thus cause neuronal death [55]. Chronic epilepsy patients show significantly decreased numbers of neurons, even though the amount of neuronal loss is not known to be related to the type of seizure [144]. These excessive amounts of glutamate are buffered by glial cells in the brain [56,95]. In the sclerotic hippocampus of patients with mesial temporal lobe epilepsy (MTLE-HS), glial cells lose the glutamate metabolizing enzyme, glutamine synthetase [115]. The loss of astrocyte gap junction coupling is observed in human MTLE-HS [145].
Therefore, the main concept of epilepsy therapy relates to increasing the level of GABA or decreasing the level of glutamate. The tonic GABA could also reduce the severity of epilepsy in mice [12]. Changes in the GABAA receptor subunits have been observed in a pilocarpine-injected epileptic mouse model; this change in the GABAA receptor impaired both the phasic and tonic inhibitions [115]. GABA in astrocytes can be released via the Best1 channel and GAT [76]. Astrocytes can mediate approximately 75% of tonic inhibition by releasing GABA [116]. In epileptic mice induced using Best1 KO, the neural firing of the hippocampus was inhibited. Therefore, it has been suggested that astrocyte-released GABA could prevent seizure susceptibility [116]. This mechanism has the potential to be used as an anticonvulsant, as treatment with inhibitors of GABA uptake such as nipecotic acid relieve epilepsy in the audiogenic model DBA/2 [117]. Treatment with the glial GABA uptake selective inhibitor, THPO, delays the onset of epilepsy [109]. Thus, regulating glial GABA levels could be a useful target for epilepsy drug candidates and requires further studies [116]. Other treatments also alter GABA, releasing proteins in glial cells and thus suppressing neuronal activity. The expression level of GLAST is increased through treatment with saikosaponin (SSa) [108]. Through a high dose of AU, GLT-1 protein levels have been shown to be upregulated with GABA levels as well as the GABAA receptor subunit a1 (GABAARa1) [111].
Another reason for changes in neurotransmitter levels is the stimulation of cytokines in the brain [57]. They are mostly released from activated microglia in pathological conditions but could enable glial cells to release glutamate in a calcium-dependent manner [58,156]. In addition, proinflammatory cytokines, such as TNF-α and IL-1β, inhibit the ability of astrocytes to take up extracellular glutamate by reducing the mRNA level of glutamate transporters and also stimulating glia to release glutamate to the extracellular space [48,96]. The gap junction is one of the crucial proteins in astrocytes for communication and forming the astrocytic syncytium. In a kainic acid (KA)-induced epilepsy model, astrocytic connexins were shown to aggravate epileptic signals [118]. In addition, inflammatory cytokines inhibit astrocyte gap junction coupling in cell culture experiments. Following treatment with levetiracetam in LPS-induced gap junction coupling-inhibited mice, the uncoupling effects of LPS were entirely prevented [146]. Astrocytes and microglia are affected by cytokines and release glutamate from the microglia through the connexin 32 hemichannels as well as glutamate transporters [119]. Increased extracellular glutamate levels are thought to be the cause of epilepsy. This is shown by the conventionally used epileptic drugs. Carbamazepine decreases the astrocytic release of glutamate, which is increased in association with chronic cytokine release [120]. By increasing the expression of EAAT2, a glial cell glutamate transporter, pilocarpine-induced epilepsy can be relieved [121]. Blocking the astrocytic cannabinoid 1 (CB1) receptors have been shown to decrease the duration of epilepsy, even though the onset was unchanged [122]. In astrocytes, by increasing the astrocytic GLT-1, the extracellular glutamate level decreases, and these changes reduce the seizures in tuberous sclerosis (TSC)-conditioned KO mouse model [92]. In addition, the treatment of AU also attenuates the activation of glial cells [111]. It has long been known that excessive amounts of glutamate in the extracellular space can kill neurons [59]. The concentration of glutamate is maintained within the normal range through the uptake function of astrocytes and the adaptation range of glutamate release from the glial cells [60,61].
4. Conclusions
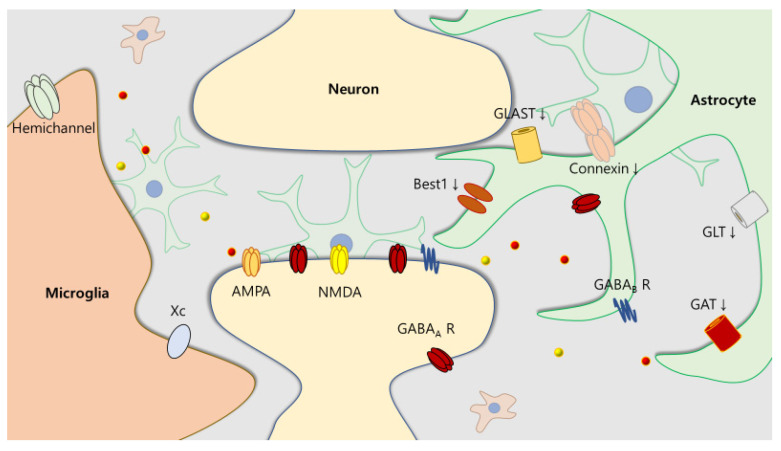
Our review highlights the presence of various glial interactions with neurons in several neurodevelopmental disorders. Some mechanisms, such as neuroinflammation, imbalance of excitation and inhibition, and neurotransmitters, are shared in ASD, ADHD, and epilepsy. Both patient- and animal model-based neurodevelopmental disorders demonstrate an increased level of cytokines in the brain. In addition, the alteration of neurotransmitters creates an imbalance relative to the normal status. This imbalance could be induced by changes in the expression levels of receptors and transporters, the modification of released gliotransmitters, and through dysfunction of uptake (Figure 3).
Figure 3.
Alteration of transporters and channels in neurodevelopmental disorders. Several transporters and channels on glial cells are responsible for the presence of a pathological state. In the epileptic brain, the expression of glutamate transporter (GLT), glutamate aspartate transporter (GLAST), GABA transporter (GAT) [79], and bestrophin-1 channel (Best1) [12] on astrocytes are decreased (↓). Changes in GABA receptors have been reported, and neurotransmitters such as glutamate and GABA are diffused by gap junction hemichannels composed of connexins [145] or microglial hemichannels [119].
Even with the high comorbidity of neurodevelopmental disorders, studies on the common mechanisms underlying ASD, ADHD, and epilepsy are rare. In addition, as the functions of neurons have been studied extensively previously, it is important to study the role of glial cells in connection with neurons. As therapeutics for neurodevelopmental disorders do not target the exact mechanisms of the disease, the drug resistance rate is relatively high. For example, there are no prominent therapies for the symptomatic relief of ASD. Methylphenidate, a drug commonly used for the treatment of ADHD, can cause hyperactivity, which is also a symptom of ADHD [104]. In the case of epilepsy, many types of anticonvulsants have been developed and are in clinical use. However, valproic acid is a commonly used anticonvulsant that can cause ASD in children [71]. These side effects caused by medications are due to the lack of understanding of the complex neuron-glia interactions. Concerning the development of new therapeutic targets, the common pathways in neurodevelopmental disorders give us hope that more feasible therapies with fewer side-effects can be developed for growing children.
In this review, we did not discuss the notable sex ratios of neurodevelopmental disorders. However, sex is one of the most important factors associated with this topic and is prominent in ASD and ADHD [123,129]. The different sex rates of children suffering from a disease are important to study the mechanisms and susceptibility of that disease. It is also important to recognize that neurodevelopmental disorders are accompanied by the behavioral and social effects of the medication. The response to the disease differs depending on the sex of the child, and doctors and parents need to tailor their recommendations accordingly [172]. Researchers suggest considering the sex effect not only from a sociocultural perspective but also in terms of biological effects [62,147].
The degree of sex imbalance is more prominent in ASD, but it also exists in ADHD [173]. Depending on the sex, the subtypes of ADHD are different, even if the severity of the disorder is similar [173,174]. ADHD is often reported to cause different problems that are sex-dependent, and the effect of ADHD on academic performance is also noted to differ by sex [175]. Some have reported that, even though the prevalence of ADHD varies by sex, specific alterations are not found according to sex, but instead that it should be considered during the diagnosis [176].
Finally, the effect of puberty should be considered in patients with neurodevelopmental disorders. Even though ADHD symptoms in adults are still controversial, the adult ADHD onset takes place during early childhood and is merely a continuation of those early onset symptoms [180]. Adults with ADHD show significant inattention as well as hyperactivity, and increased exposure to drug abuse [148,177]. The main theory behind neurodevelopmental disorders, including ADHD, is that they are not relieved over time, but instead that patients learn to manage the symptoms better [178]. Further studies in adults with ADHD are required to differentiate the characteristics of children with ADHD and adult ADHD, and determine the effect of time on neurodevelopmental disorders.
Funding
This study was supported by the National Research Foundation of Korea (grants NRF-2018R1D1A1B07045561 and NRF-2019M3C7A1031455 to B.E.Y) and by the Ministry of Environment of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Alyagor I., Berkun V., Keren-Shaul H., Marmor-Kollet N., David E., Mayseless O., Issman-Zecharya N., Amit I., Schuldiner O. Combining Developmental and Perturbation-Seq Uncovers Transcriptional Modules Orchestrating Neuronal Remodeling. Dev. Cell. 2018;47:38–52.e6. doi: 10.1016/j.devcel.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puro D.G., Nirenberg M. On the specificity of synapse formation. Proc. Natl. Acad. Sci. USA. 1976;73:3544–3548. doi: 10.1073/pnas.73.10.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki Y., Cai D.J. Creating Space for Synaptic Formation-A New Role for Microglia in Synaptic Plasticity. Cell. 2020;182:265–267. doi: 10.1016/j.cell.2020.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Simhal A.K., Zuo Y., Perez M.M., Madison D.V., Sapiro G., Micheva K.D. Multifaceted Changes in Synaptic Composition and Astrocytic Involvement in a Mouse Model of Fragile X Syndrome. Sci. Rep. 2019;9:13855. doi: 10.1038/s41598-019-50240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pannasch U., Freche D., Dallérac G., Ghézali G., Escartin C., Ezan P., Cohen-Salmon M., Benchenane K., Abudara V., Dufour A. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat. Neurosci. 2014;17:549–558. doi: 10.1038/nn.3662. [DOI] [PubMed] [Google Scholar]
- 6.Livne-Bar I., Lam S., Chan D., Guo X., Askar I., Nahirnyj A., Flanagan J.G., Sivak J.M. Pharmacologic inhibition of reactive gliosis blocks TNF-α-mediated neuronal apoptosis. Cell Death Dis. 2016;7:e2386. doi: 10.1038/cddis.2016.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deverman B.E., Patterson P.H. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Maubecin V., García-Hernández F., Williams J.T., Van Bockstaele E.J. Functional coupling between neurons and glia. J. Neurosci. 2000;20:4091–4098. doi: 10.1523/JNEUROSCI.20-11-04091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parpura V., Basarsky T.A., Liu F., Jeftinija K., Jeftinija S., Haydon P.G. Glutamate-mediated astrocyte–neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 10.Jo S., Yarishkin O., Hwang Y.J., Chun Y.E., Park M., Woo D.H., Bae J.Y., Kim T., Lee J., Chum H. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo J.Y., Nam M.H., Yoon H.H., Kim J., Hwang Y.J., Won W., Woo D.H., Lee J.A., Park H.J., Jo S. Aberrant Tonic Inhibition of Dopaminergic Neuronal Activity Causes Motor Symptoms in Animal Models of Parkinson’s Disease. Curr. Biol. 2020;30:276–291. doi: 10.1016/j.cub.2019.11.079. [DOI] [PubMed] [Google Scholar]
- 12.Pandit S., Neupane C., Woo J., Sharma R., Nam M.H., Lee G.S., Yi M.H., Shin N., Kim D.W., Cho H. Bestrophin1-mediated tonic GABA release from reactive astrocytes prevents the development of seizure-prone network in kainate-injected hippocampi. Glia. 2020;68:1065–1080. doi: 10.1002/glia.23762. [DOI] [PubMed] [Google Scholar]
- 13.Eftekharian M.M., Ghafouri-Fard S., Noroozi R., Omrani M.D., Arsang-Jang S., Ganji M., Gharzi V., Noroozi H., Komaki A., Mazdeh M. Cytokine profile in autistic patients. Cytokine. 2018;108:120–126. doi: 10.1016/j.cyto.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Patterson S.L. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology. 2015;96:11–18. doi: 10.1016/j.neuropharm.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liimatainen S., Fallah M., Kharazmi E., Peltola M., Peltola J. Interleukin-6 levels are increased in temporal lobe epilepsy but not in extra-temporal lobe epilepsy. J. Neurol. 2009;256:796–802. doi: 10.1007/s00415-009-5021-x. [DOI] [PubMed] [Google Scholar]
- 16.Van Vliet E.A., da Costa Araujo S., Redeker S., Van Schaik R., Aronica E., Gorter J.A. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 17.Elkabes S., DiCicco-Bloom E.M., Black I.B. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 1996;16:2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima K., Honda S., Tohyama Y., Imai Y., Kohsaka S., Kurihara T. Neurotrophin secretion from cultured microglia. J. Neurosci. Res. 2001;65:322–331. doi: 10.1002/jnr.1157. [DOI] [PubMed] [Google Scholar]
- 19.Jankowsky J.L., Patterson P.H. The role of cytokines and growth factors in seizures and their sequelae. Prog. Neurobiol. 2001;63:125–149. doi: 10.1016/S0301-0082(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C., Tabatabaei M., Bélanger S., Girouard H., Moeini M., Lu X., Lesage F. Astrocytic endfoot Ca2+ correlates with parenchymal vessel responses during 4-AP induced epilepsy: An in vivo two-photon lifetime microscopy study. J. Cereb. Blood Flow Metab. 2019;39:260–271. doi: 10.1177/0271678X17725417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delorme R., Ey E., Toro R., Leboyer M., Gillberg C., Bourgeron T. Progress toward treatments for synaptic defects in autism. Nat. Med. 2013;19:685–694. doi: 10.1038/nm.3193. [DOI] [PubMed] [Google Scholar]
- 22.Reisinger S., Khan D., Kong E., Berger A., Pollak A., Pollak D.D. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 2015;149:213–226. doi: 10.1016/j.pharmthera.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Neal A.P., Guilarte T.R. Molecular Neurobiology of Lead (Pb2+): Effects on Synaptic Function. Mol. Neurobiol. 2010;42:151–160. doi: 10.1007/s12035-010-8146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloan S.A., Barres B.A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 2014;27:75–81. doi: 10.1016/j.conb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Südhof T.C. Synaptic neurexin complexes: A molecular code for the logic of neural circuits. Cell. 2017;171:745–769. doi: 10.1016/j.cell.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loe I.M., Feldman H.M. Academic and educational outcomes of children with ADHD. J. Pediatr. Psychol. 2007;32:643–654. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- 27.Pringsheim T., Steeves T. Pharmacological treatment for Attention Deficit Hyperactivity Disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD007990.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Estes M.L., McAllister A.K. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci. 2015;16:469–486. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 30.Sofroniew M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greter M., Merad M. Regulation of microglia development and homeostasis. Glia. 2013;61:121–127. doi: 10.1002/glia.22408. [DOI] [PubMed] [Google Scholar]
- 32.Hanisch U.K., Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 33.Russell V.A. Overview of animal models of attention deficit hyperactivity disorder (ADHD) Curr. Protoc. Neurosci. 2011;54:9–35. doi: 10.1002/0471142301.ns0935s54. [DOI] [PubMed] [Google Scholar]
- 34.Pajarillo E., Rizor A., Lee J., Aschner M., Lee E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology. 2019;161:107559. doi: 10.1016/j.neuropharm.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McBean G.J. Cerebral cystine uptake: A tale of two transporters. Trends Pharmacol. Sci. 2002;23:299–302. doi: 10.1016/S0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Wilcke T., Fuchs E., Funke K., Vlachos A., Müller-Dahlhaus F., Puts N.A.J., Harris R.E., Edden R.A.E. GABA—From inhibition to cognition: Emerging concepts. Neuroscientist. 2018;24:501–515. doi: 10.1177/1073858417734530. [DOI] [PubMed] [Google Scholar]
- 37.Roux L., Buzsáki G. Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology. 2015;88:10–23. doi: 10.1016/j.neuropharm.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pizzarelli R., Cherubini E. Alterations of GABAergic signaling inautism spectrum disorders. Neural Plast. 2011:297153. doi: 10.1155/2011/297153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purkayastha P., Malapati A., Yogeeswari P., Sriram D. A review on GABA/glutamate pathway for therapeutic intervention of ASD and ADHD. Curr. Med. Chem. 2015;22:1850–1859. doi: 10.2174/0929867322666150209152712. [DOI] [PubMed] [Google Scholar]
- 40.Hubbard J.A., Hsu M.S., Fiacco T.A., Binder D.K. Glial cell changes in epilepsy: Overview of the clinical problem and therapeutic opportunities. Neurochem. Int. 2013;63:638–651. doi: 10.1016/j.neuint.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Anderson C.M., Swanson R.A. Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. doi: 10.1002/1098-1136(200010)32:1<1::AID-GLIA10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 42.Chapman A.G. Glutamate and epilepsy. J. Nutr. 2000;130:1043S–1045S. doi: 10.1093/jn/130.4.1043S. [DOI] [PubMed] [Google Scholar]
- 43.Treiman D.M. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42:8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- 44.Eroglu C., Barres B.A. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coulter D.A., Eid T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia. 2012;60:1215–1226. doi: 10.1002/glia.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vezzani A. Anti-inflammatory drugs in epilepsy: Does it impact epileptogenesis? Expert Opin. Drug Saf. 2015;14:583–592. doi: 10.1517/14740338.2015.1010508. [DOI] [PubMed] [Google Scholar]
- 47.Vezzani A., Moneta D., Richichi C., Perego C., De Simoni M.G. Functional role of proinflammatory and anti-inflammatory cytokines in seizures. Adv. Exp. Med. Biol. 2004;548:123–133. doi: 10.1007/978-1-4757-6376-8_10. [DOI] [PubMed] [Google Scholar]
- 48.Vezzani A., Ravizza T., Balosso S., Aronica E. Glia as a source of cytokines: Implications for neuronal excitability and survival. Epilepsia. 2008;49:24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 49.Hanisch U.K. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 50.Santello M., Volterra A. TNFα in synaptic function: Switching gears. Trends Neurosci. 2012;35:638–647. doi: 10.1016/j.tins.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Suzumura A., Takeuchi H., Zhang G., Kuno R., Mizuno T. Roles of glia-derived cytokines on neuronal degeneration and regeneration. Ann. N. Y. Acad. Sci. 2006;1088:219–229. doi: 10.1196/annals.1366.012. [DOI] [PubMed] [Google Scholar]
- 52.Bernardino L., Ferreira R., Cristovao A.J., Sales F., Malva J.O. Inflammation and neurogenesis in temporal lobe epilepsy. Curr. Drug Targets CNS Neurol. Disord. 2005;4:349–360. doi: 10.2174/1568007054546171. [DOI] [PubMed] [Google Scholar]
- 53.MacVicar B.A., Newman E.A. Astrocyte regulation of blood flow in the brain. Cold Spring Harbor Perspect. Biol. 2015;7:1290–1300. doi: 10.1101/cshperspect.a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinemann U., Kaufer D., Friedman A. Blood-brain barrier dysfunction, TGFβ signaling, and astrocyte dysfunction in epilepsy. Glia. 2012;60:1251–1257. doi: 10.1002/glia.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y., Qin Z.H. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15:1382–1402. doi: 10.1007/s10495-010-0481-0. [DOI] [PubMed] [Google Scholar]
- 56.Barreto G.E., Gonzalez J., Torres Y., Morales L. Astrocytic-neuronal crosstalk: Implications for neuroprotection from brain injury. Neurosci. Res. 2011;71:107–113. doi: 10.1016/j.neures.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 57.De Simoni M.G., Imeri L. Cytokine-neurotransmitter interactions in the brain. Neurosignals Recept. 1998;7:33–44. doi: 10.1159/000014526. [DOI] [PubMed] [Google Scholar]
- 58.Vesce S., Rossi D., Brambilla L., Volterra A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int. Rev. Neurobiol. 2007;82:57–71. doi: 10.1016/S0074-7742(07)82003-4. [DOI] [PubMed] [Google Scholar]
- 59.Choi D.W., Rothman S.M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev. Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 60.Hertz L., Dringen R., Schousboe A., Robinson S.R. Astrocytes: Glutamate producers for neurons. J. Neurosci. Res. 1999;57:417–428. doi: 10.1002/(SICI)1097-4547(19990815)57:4<417::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 61.Tilleux S., Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- 62.Kreiser N.L., White S.W. ASD in females: Are we overstating the gender difference in diagnosis? Clin. Child Fam. Psychol. Rev. 2014;17:67–84. doi: 10.1007/s10567-013-0148-9. [DOI] [PubMed] [Google Scholar]
- 63.Horváth G., Otrokocsi L., Beko K., Baranyi M., Kittel Á., Fritz-Ruenes P.A., Sperlágh B. P2X7 Receptors Drive Poly(I:C) Induced Autism-like Behavior in Mice. J. Neurosci. 2019;39:2542–2561. doi: 10.1523/JNEUROSCI.1895-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai P.T., Hull C., Chu Y., Greene-Colozzi E., Sadowski A.R., Leech J.M., Steinberg J., Crawley J.N., Regehr W.G., Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chao H.T., Chen H., Samaco R.C., Xue M., Chahrour M., Yoo J., Neul J.L., Gong S., Lu H., Heintz N. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han S., Tai C., Westenbroek R.E., Yu F.H., Cheah C.S., Potter G.B., Rubenstein J.L., Scheuer T., De la Iglesia H.O., Catterall W.A. Autistic-like behaviour in Scn1a+/−mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hammer M., Krueger-Burg D., Tuffy L.P., Cooper B.H., Taschenberger H., Goswami S.P., Ehrenreich H., Jonas P., Variqueaux F., Rhee J.S. Perturbed hippocampal synaptic inhibition and γ-oscillations in a neuroligin-4 knockout mouse model of autism. Cell Rep. 2015;13:516–523. doi: 10.1016/j.celrep.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Won H., Mah W., Kim E., Kim J.W., Hahm E.K., Kim M.H., Cho S., Kim J., Jang H., Cho S.C. GIT1 is associated with ADHD in humans and ADHD-like behaviors in mice. Nat. Med. 2011;17:566–572. doi: 10.1038/nm.2330. [DOI] [PubMed] [Google Scholar]
- 69.Moore A.H., Wu M., Shaftel S.S., Graham K.A., O’Banion M.K. Sustained expression of interleukin-1β in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gogolla N., Takesian A.E., Feng G., Fagiolini M., Hensch T.K. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gumusoglu S.B., Fine R.S., Murray S.J., Bittle J.L., Stevens H.E. The role of IL-6 in neurodevelopment after prenatal stress. Brain Behav. Immun. 2017;65:274–283. doi: 10.1016/j.bbi.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rivero O., Selten M.M., Sich S., Popp S., Bacmeister L., Amendola E., Negwer M., Schuber D., Proft F., Kiser D. Cadherin-13, a risk gene for ADHD and comorbid disorders, impacts GABAergic function in hippocampus and cognition. Transl. Psychiatry. 2015;5:e655. doi: 10.1038/tp.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kozłowska A., Wojtacha P., Równiak M., Kolenkiewicz M., Huang A.C.W. ADHD pathogenesis in the immune, endocrine and nervous systems of juvenile and maturating SHR and WKY rats. Psychopharmacology. 2019;236:2937–2958. doi: 10.1007/s00213-019-5180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higashimori H., Schin C.S., Chiang M.S.R., Morel L., Shoneye T.A., Nelson D.L., Yang Y. Selective deletion of astroglial FMRP dysregulates glutamate transporter GLT1 and contributes to fragile X syndrome phenotypes in vivo. J. Neurosci. 2016;36:7079–7094. doi: 10.1523/JNEUROSCI.1069-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karlsson R.M., Tanaka K., Heilig M., Holmes A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: Rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol. Psychiatry. 2008;64:810–814. doi: 10.1016/j.biopsych.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghosh M., Yang Y., Rothstein J.D., Robinson M.B. Nuclear factor-κB contributes to neuron-dependent induction of glutamate transporter-1 expression in astrocytes. J. Neurosci. 2011;31:9159–9169. doi: 10.1523/JNEUROSCI.0302-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Omrani A., Melone M., Bellesi M., Safiulina V., Aida T., Tanaka K., Cherubin E., Conti F. Up-regulation of GLT-1 severely impairs LTD at mossy fibre—CA3 synapses. Pt 19J. Physiol. 2009;587:4575–4588. doi: 10.1113/jphysiol.2009.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin S., Colin C., Hinners I., Gervais A., Cheret C., Mallat M. System xc− and apolipoprotein E expressed by microglia have opposite effects on the neurotoxicity of amyloid-β peptide 1–40. J. Neurosci. 2006;26:3345–3356. doi: 10.1523/JNEUROSCI.5186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ribak C.E., Tong W.M., Brecha N.C. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J. Comp. Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 80.Boddum K., Jensen T.P., Magloire V., Kristiansen U., Rusakov D.A., Pavlov I., Walker M.C. Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat. Commun. 2016;7:1–10. doi: 10.1038/ncomms13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoon B.E., Woo J., Chun Y.E., Chun H., Jo S., Bae J.Y., Lee C.J. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J. Physiol. 2014;592:4951–4968. doi: 10.1113/jphysiol.2014.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silverman J.L., Pride M.C., Hayes J.E., Puhger K.R., Butler-Struben H.M., Baker S., Crawley J.N. GABA B receptor agonist R-baclofen reverses social deficits and reduces repetitive behavior in Two mouse models of autism. Neuropsychopharmacology. 2015;40:2228–2239. doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim Y.S., Woo J., Lee C.J., Yoon B.E. Decreased glial GABA and tonic inhibition in cerebellum of mouse model for attention-deficit/hyperactivity disorder (ADHD) Exp. Neurobiol. 2017;26:206–212. doi: 10.5607/en.2017.26.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sterley T.L., Howells F.M., Russell V.A. Evidence for reduced tonic levels of GABA in the hippocampus of an animal model of ADHD, the spontaneously hypertensive rat. Brain Res. 2013;1541:52–60. doi: 10.1016/j.brainres.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 85.Chen L., Yang X., Zhou X., Wang C., Gong X., Chen B., Chen Y. Hyperactivity and impaired attention in Gamma aminobutyric acid transporter subtype 1 gene knockout mice. Acta Neuropsychiatr. 2015;27:368–374. doi: 10.1017/neu.2015.37. [DOI] [PubMed] [Google Scholar]
- 86.Nagai J., Rajbhandari A.K., Gangwani M.R., Hachisuka A., Coppola G., Masmanidis S.C., Fanselow M.S., Khakh B.S. Hyperactivity with disrupted attention by activation of an astrocyte synaptogenic cue. Cell. 2019;177:1280–1292. doi: 10.1016/j.cell.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee S., Yoon B.E., Berglund K., Oh S.J., Park H., Shin H.S., Augustine G.J., Lee C.J. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- 88.Buckingham S.C., Campbell S.L., Haas B.R., Montana V., Robel S., Ogunrinu T., Sontheimer H. Glutamate release by primary brain tumors induces epileptic activity. Nat. Med. 2011;17:1269–1274. doi: 10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lahuerta M., Gonzalez D., Aguado C., Fathinajafabadi A., García-Giménez J.L., Moreno-Estellés M., Romá-Mateo C., Knecht E., Pallardó F.V., Sanz P. Reactive Glia-Derived Neuroinflammation: A Novel Hallmark in Lafora Progressive Myoclonus Epilepsy That Progresses with Age. Mol. Neurobiol. 2020;57:1607–1621. doi: 10.1007/s12035-019-01842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rubio-Villena C., Viana R., Bonet J., Garcia-Gimeno M.A., Casado M., Heredia M., Sanz P. Astrocytes: New players in progressive myoclonus epilepsy of Lafora type. Hum. Mol. Genet. 2018;27:1290–1300. doi: 10.1093/hmg/ddy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stellwagen D., Malenka R.C. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 92.Zeng L.H., Bero A.W., Zhang B., Holtzman D.M., Wong M. Modulation of astrocyte glutamate transporters decreases seizures in a mouse model of Tuberous Sclerosis Complex. Neurobiol. Dis. 2010;37:764–771. doi: 10.1016/j.nbd.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Truccolo W., Donoghue J.A., Hochberg L.R., Eskandar E.N., Madsen J.R., Anderson W.S., Brown E.N., Halgren E., Cash S.S. Single-neuron dynamics in human focal epilepsy. Nat. Neurosci. 2011;14:635–641. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iyer A., Zurolo E., Prabowo A., Fluiter K., Spliet W.G., van Rijen P.C., Gorter J.A., Aronica E. MicroRNA-146a: A key regulator of astrocyte-mediated inflammatory response. PLoS ONE. 2012;7:e44789. doi: 10.1371/journal.pone.0044789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Héja L., Barabás P., Nyitrai G., Kékesi K.A., Lasztóczi B., Toke O., Tárkányi G., Madsen K., Schousboe A., Dobolyi A., et al. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS ONE. 2009;4:e7153. doi: 10.1371/journal.pone.0007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu S., Sheng W.S., Ehrlich L.C., Peterson P.K., Chao C.C. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation. 2000;7:153–159. doi: 10.1159/000026433. [DOI] [PubMed] [Google Scholar]
- 97.Yao C., He Z., Nakano T., Shuai J. Spiking patterns of a neuron model to stimulus: Rich dynamics and oxygen’s role. Chaos. 2018;28:1–8. doi: 10.1063/1.5018707. [DOI] [PubMed] [Google Scholar]
- 98.Savin C., Triesch J., Meyer-Hermann M. Epileptogenesis due to glia-mediated synaptic scaling. J. R. Soc. Interface. 2009;6:655–668. doi: 10.1098/rsif.2008.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi G.B., Yim Y.S., Wong H., Kim S., Kim H., Kim S.V., Hoeffer C.A., Littman D.R., Huh J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi L., Smith S.E., Malkova N., Tse D., Su Y., Patterson P.H. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav. Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lombardo M.V., Moon H.M., Su J., Palmer T.D., Courchesne E., Pramparo T. Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol. Psychiatry. 2018;23:1001–1013. doi: 10.1038/mp.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wei H., Chadman K.K., McCloskey D.P., Sheikh A.M., Malik M., Brown W.T., Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim. Biophys. Acta. 2012;1822:831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 104.Carias E., Hamilton J., Robison L.S., Delis F., Eiden R., Quattrin T., Hadjiargyrou M., Komatsu D., Thanos P.K. Chronic oral methylphenidate treatment increases microglial activation in rats. J. Neural Transm. 2018 doi: 10.1007/s00702-018-1931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sadasivan S., Pond B.B., Pani A.K., Qu C., Jiao Y., Smeyne R.J. Methylphenidate exposure induces dopamine neuron loss and activation of microglia in the basal ganglia of mice. PLoS ONE. 2012;7:e33693. doi: 10.1371/annotation/c76da2c1-ccb8-4797-94c1-359d3ceceeda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lucchina L., Depino A.M. Altered peripheral and central inflammatory responses in a mouse model of autism. Autism Res. 2014;7:273–289. doi: 10.1002/aur.1338. [DOI] [PubMed] [Google Scholar]
- 107.Taguchi K., Tamba M., Bannai S., Sato H. Induction of cystine/glutamate transporter in bacterial lipopolysaccharide induced endotoxemia in mice. J. Inflamm. 2007;4:20. doi: 10.1186/1476-9255-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao W., Bi Y., Ding L., Zhu W., Ye M. SSa ameliorates the Glu uptaking capacity of astrocytes in epilepsy via AP-1/miR-155/GLAST. Biochem. Biophys. Res. Commun. 2017;493:1329–1335. doi: 10.1016/j.bbrc.2017.09.139. [DOI] [PubMed] [Google Scholar]
- 109.Schousboe A., Larsson O.M., Wood J.D., Krogsgaard-Larsen P. Transport and Metabolism of 7-Aminobutyric Acid in Neurons and Glia: Implications for Epilepsy. Epilepsia. 1983;24:531–538. doi: 10.1111/j.1528-1157.1983.tb03417.x. [DOI] [PubMed] [Google Scholar]
- 110.Betti M., Minelli A., Ambrogini P., Ciuffoli S., Viola V., Galli F., Cuppini R. Dietary supplementation with α-tocopherol reduces neuroinflammation and neuronal degeneration in the rat brain after kainic acid-induced status epilepticus. Free Radic. Res. 2011;45:1136–1142. doi: 10.3109/10715762.2011.597750. [DOI] [PubMed] [Google Scholar]
- 111.Chen S., Zeng X., Zong W., Wang X., Chen L., Zhou L., Li C., Huang Q., Huang X., Zeng G. Aucubin alleviates seizures activity in Li-Pilocarpine-Induced epileptic mice: Involvement of inhibition of neuroinflammation and regulation of neurotransmission. Neurochem. Res. 2019;44:472–484. doi: 10.1007/s11064-018-2700-y. [DOI] [PubMed] [Google Scholar]
- 112.Ambrogini P., Minelli A., Galati C., Betti M., Lattanzi D., Ciffolilli S., Piroddi M., Galli F., Cuppini R. Post-seizure α-tocopherol treatment decreases neuroinflammation and neuronal degeneration induced by status epilepticus in rat hippocampus. Mol. Neurobiol. 2014;50:246–256. doi: 10.1007/s12035-014-8648-2. [DOI] [PubMed] [Google Scholar]
- 113.Steinmetz C.C., Turrigiano G.G. Tumor necrosis factor-α signaling maintains the ability of cortical synapses to express synaptic scaling. J. Neurosci. 2010;30:14685–14690. doi: 10.1523/JNEUROSCI.2210-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rappold P.M., Lynd-Balta E., Joseph S.A. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089:171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 115.Zhang N., Wei W., Mody I., Houser C.R. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J. Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Labarrera C., London M., Angelo K. Tonic inhibition sets the state of excitability in olfactory bulb granule cells. J. Physiol. 2013;591:1841–1850. doi: 10.1113/jphysiol.2012.241851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horton R.W., Collins J.F., Anlezark G.M., Meldrum B.S. Convulsant and anticonvulsant actions in DBA/2 mice of compounds blocking the reuptake of GABA. Eur. J. Pharmacol. 1979;59:75–83. doi: 10.1016/0014-2999(79)90026-8. [DOI] [PubMed] [Google Scholar]
- 118.Deshpande T., Li T., Henning L., Wu Z., Müller J., Seifert G., Steinhäuser C., Bedner P. Constitutive deletion of astrocytic connexins aggravates kainate-induced epilepsy. Glia. 2020;68:2136–2147. doi: 10.1002/glia.23832. [DOI] [PubMed] [Google Scholar]
- 119.Takeuchi H., Jin S., Wang J., Zhang G., Kawanokuchi J., Kuno R., Sonobe Y., Mizuno T., Suzumura A. Tumor necrosis factor-α induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 120.Okada M., Fukuyama K., Shiroyama T., Ueda Y. Carbamazepine attenuates astroglial l-glutamate release induced by pro-inflammatory cytokines via chronically activation of adenosine A2A receptor. Int. J. Mol. Sci. 2019;20:3727. doi: 10.3390/ijms20153727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kong Q., Takahashi K., Schulte D., Stouffer N., Lin Y., Lin C.L.G. Increased glial glutamate transporter EAAT2 expression reduces epileptogenic processes following pilocarpine-induced status epilepticus. Neurobiol. Dis. 2012;47:145–154. doi: 10.1016/j.nbd.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coiret G., Ster J., Grewe B., Wendling F., Helmchen F., Gerber U., Benquet P. Neuron to astrocyte communication via cannabinoid receptors is necessary for sustained epileptiform activity in rat hippocampus. PLoS ONE. 2012;7:e37320. doi: 10.1371/journal.pone.0037320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maenner M.J., Shaw K.A., Baio J., Washington A., Patrick M., DiRienzo M., Christensen D.L., Wiggins L.D., Pettygrove S., Andrews J.G. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill. Summ. 2020;69:1. doi: 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee B.K., Magnusson C., Gardner R.M., Blomström Å., Newschaffer C.J., Burstyn I., Karlsson H., Dalman C. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav. Immun. 2015;44:100–105. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim S.M., Han D.H., Lyoo H.S., Min K.J., Kim K.H., Renshaw P. Exposure to environmental toxins in mothers of children with autism spectrum disorder. Psychiatry Investig. 2010;7:122–127. doi: 10.4306/pi.2010.7.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Croen L.A., Grether J.K., Yoshida C.K., Odouli R., Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: A case-control study. Arch. Pediatr. Adolesc. Med. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- 127.Goines P.E., Croen L.A., Braunschweig D., Yoshida C.K., Grether J., Hansen R., Kharrazi M., Ashwood P., Van de Water J. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol. Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jones K.L., Croen L.A., Yoshida C.K., Heuer L., Hansen R., Zerbo O., DeLorenze G.N., Kharrazi M., Yolken R., Ashwood P. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol. Psychiatry. 2017;22:273–279. doi: 10.1038/mp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Danielson M.L., Bitsko R.H., Ghandour R.M., Holbrook J.R., Kogan M.D., Blumberg S.J. Prevalence of parent-reported ADHD diagnosis and associated treatment among US children and adolescents, 2016. J. Clin. Child Adolesc. Psychol. 2018;47:199–212. doi: 10.1080/15374416.2017.1417860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I., Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li X., Chauhan A., Sheikh A.M., Patil S., Chauhan V., Li X.M., Ji L., Brown T., Malik M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsilioni I., Patel A.B., Pantazopoulos H., Berretta S., Conti P., Leeman S.E., Theoharides T.C. IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin. Proc. Natl. Acad. Sci. USA. 2019;116:21659–21665. doi: 10.1073/pnas.1906817116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei H., Zou H., Sheikh A.M., Malik M., Dobkin C., Brown W.T., Li X. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J. Neuroinflamm. 2011;8:1–10. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Darwish A.H., Elgohary T.M., Nosair N.A. Serum Interleukin-6 Level in Children with Attention-Deficit Hyperactivity Disorder (ADHD) J. Child Neurol. 2019;34:61–67. doi: 10.1177/0883073818809831. [DOI] [PubMed] [Google Scholar]
- 135.Khalifa D., Shahin O., Salem D., Raafat O. Serum glutamate was elevated in children aged 3–10 years with autism spectrum disorders when they were compared with controls. Acta Paediatr. 2019;108:295–299. doi: 10.1111/apa.14477. [DOI] [PubMed] [Google Scholar]
- 136.Rothstein J.D., Martin L.J., Kuncl R.W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 137.Edden R.A., Crocetti D., Zhu H., Gilbert D.L., Mostofsky S.H. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2012;69:750–753. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Puts N.A., Ryan M., Oeltzschner G., Horska A., Edden R.A., Mahone E.M. Reduced striatal GABA in unmedicated children with ADHD at 7T. Pscyhiatry Res. Neuroimaging. 2020;301:111082. doi: 10.1016/j.pscychresns.2020.111082. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Z., Liu Q., Liu M., Wang H., Dong Y., Ji T., Liu X., Jiang Y., Cai L., Wu Y. Upregulation of HMGB1-TLR4 inflammatory pathway in focal cortical dysplasia type II. J. Neuroinflamm. 2018;15:1–11. doi: 10.1186/s12974-018-1078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ravizza T., Boer K., Redeker S., Spliet W.G.M., Van Rijen P.C., Troost D., Vezzani A., Aronica E. The IL-1β system in epilepsy-associated malformations of cortical development. Neurobiol. Dis. 2006;24:128–143. doi: 10.1016/j.nbd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 141.Chao C.C., Hu S., Sheng W.S., Bu D., Bukrinsky M.I., Peterson P.K. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia. 1996;16:276–284. doi: 10.1002/(SICI)1098-1136(199603)16:3<276::AID-GLIA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 142.Choi J., Nordli D.R., Alden T.D., DiPatri A., Laux L., Kelley K., Rosenow J., Schuele S.U., Raharam V., Koh S. Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J. Neuroinflamm. 2009;6:1–14. doi: 10.1186/1742-2094-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu J., Huang H., Zeng Q., Zhang X., Xu M., Cai Y., Wang Q., Huang Y., Peng Q., Deng L. Hippocampal neuron loss and astrogliosis in medial temporal lobe epileptic patients with mental disorders. J. Integr. Neurosci. 2019;18:127–132. doi: 10.31083/j.jin.2019.02.16. [DOI] [PubMed] [Google Scholar]
- 144.Dam A.M. Epilepsy and neuron loss in the hippocampus. Epilepsia. 1980;21:617–629. doi: 10.1111/j.1528-1157.1980.tb04315.x. [DOI] [PubMed] [Google Scholar]
- 145.Eid T., Thomas M.J., Spencer D.D., Runden-Pran E., Lai J.C.K., Malthankar G.V., Kim J.H., Danbolt N.C., Ottersen O.P., De Lanerolle N.C. Loss of glutamine synthetase in the human epileptogenic hippocampus: Possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/S0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- 146.Bedner P., Dupper A., Hüttmann K., Müller J., Herde M.K., Dublin P., Deshpande T., Schramm J., Häussler U., Hass C.A. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain. 2015;138:1208–1222. doi: 10.1093/brain/awv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Conlon O., Volden J., Smith I.M., Duku E., Zwaigenbaum L., Waddell C., Szatmari P., Mirenda P., Vaillancourt T., Bennett T. Gender differences in pragmatic communication in school-aged children with autism spectrum disorder (ASD) J. Autism Dev. Disord. 2019;49:1937–1948. doi: 10.1007/s10803-018-03873-2. [DOI] [PubMed] [Google Scholar]
- 148.Lopez R., Dauvilliers Y., Jaussent I., Billieux J., Bayard S. A multidimensional approach of impulsivity in adult attention deficit hyperactivity disorder. Psychiatry Res. 2015;227:290–295. doi: 10.1016/j.psychres.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 149.Demontis D., Walters R.K., Martin J., Mattheisen M., Als T.D., Agerbo E., Baldursson G., Belliveau R., Bybjerg-Grauholm J., Bækvad-Hansen M. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 2019;51:63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Joseph N., Zhang-James Y., Perl A., Faraone S.V. Oxidative stress and ADHD: A meta-analysis. J. Atten. Disord. 2015;19:915–924. doi: 10.1177/1087054713510354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bilbo S.D., Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 152.Fonfría E., Rodríguez-Farré E., Suñol C. Mercury interaction with the GABA(A) receptor modulates the benzodiazepine binding site in primary cultures of mouse cerebellar granule cells. Neuropharmacology. 2001;41:819–833. doi: 10.1016/S0028-3908(01)00130-7. [DOI] [PubMed] [Google Scholar]
- 153.Allen J.W., Mutkus L.A., Aschner M. Mercuric chloride, but not methylmercury, inhibits glutamine synthetase activity in primary cultures of cortical astrocytes. Brain Res. 2001;891:148–157. doi: 10.1016/S0006-8993(00)03185-1. [DOI] [PubMed] [Google Scholar]
- 154.Lau L.T., Yu A.C.H. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J. Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- 155.D’Ambrosio R., Fairbanks J.P., Fender J.S., Born D.E., Doyle D.L., Miller J.W. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ida T., Hara M., Nakamura Y., Kozaki S., Tsunoda S., Ihara H. Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci. Lett. 2008;432:232–236. doi: 10.1016/j.neulet.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 157.Mandal M., Donnelly R., Elkabes S., Zhang P., Davini D., David B.T., Ponzio N.M. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav. Immun. 2013;33:33–45. doi: 10.1016/j.bbi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 158.Busch R.M., Srivastava S., Hogue O., Frazier T.W., Klass P., Hardan A., Martinez-Agosto J.A., Sahin M., Eng C., Developmental Synaptopathies Consortium et al. Neurobehavioral phenotype of autism spectrum disorder associated with germline heterozygous mutations in PTEN. Transl. Psychiatry. 2019;9:253. doi: 10.1038/s41398-019-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Satterstrom F.K., Walters R.K., Singh T., Wigdor E.M., Lescai F., Demontis D., Kosmicki J.A., Grove J., Stevens C., Bybjerg-Grauholm J. Autism spectrum disorder and attention deficit hyperactivity disorder have a similar burden of rare protein-truncating variants. Nat. Neurosci. 2019;22:1961–1965. doi: 10.1038/s41593-019-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.De Rubeis S., He X., Goldberg A.P., Poultney C.S., Samocha K., Cicek A.E., Kou Y., Liu L., Fromer M., Walker S. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Iossifov I., O’Roak B.J., Sanders S.J., Ronemus M., Krumm N., Levy D., Stessman H.A., Witherspoon K.T., Vives L., Patterson K.E. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sanders S.J., He X., Willsey A.J., Ercan-Sencicek A.G., Samocha K.E., Cicek A.E., Murtha M.T., Bal V.H., Bishop S.L., Dong S. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 2015;87:1215–1233. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ohlmeier M.D., Peters K., Wildt B.T.T., Zedler M., Ziegenbein M., Wiese B., Emrich H.M., Schneider U. Comorbidity of alcohol and substance dependence with attention-deficit/hyperactivity disorder (ADHD) Alcohol Alcohol. 2008;43:300–304. doi: 10.1093/alcalc/agn014. [DOI] [PubMed] [Google Scholar]
- 164.Ohlmeier M.D., Peters K., Kordon A., Seifert J., Wildt B.T., Wiese B., Ziegenbein M., Emrich H.M., Schneider U. Nicotine and alcohol dependence in patients with comorbid attention-deficit/hyperactivity disorder (ADHD) Alcohol Alcohol. 2007;42:539–543. doi: 10.1093/alcalc/agm069. [DOI] [PubMed] [Google Scholar]
- 165.Widera D., Mikenberg I., Elvers M., Kaltschmidt C., Kaltschmidt B. Tumor necrosis factor α triggers proliferation of adult neural stem cells via IKK/NF-κB signaling. BMC Neurosci. 2006;7:1–18. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Flynn G., Maru S., Loughlin J., Romero I.A., Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J. Neuroimmunol. 2003;136:84–93. doi: 10.1016/S0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 167.Ende G., Cackowski S., Van Eijk J., Sack M., Demirakca T., Kleindienst N., Bohus M., Sobanski E., Krause-Tuz A., Schmahl C. Impulsivity and aggression in female BPD and ADHD patients: Association with ACC glutamate and GABA concentrations. Neuropsychopharmacology. 2016;41:410–418. doi: 10.1038/npp.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Naaijen J., Bralten J., Poelmans G., Glennon J.C., Franke B., Buitelaar J.K. Glutamatergic and GABAergic gene sets in attention-deficit/hyperactivity disorder: Association to overlapping traits in ADHD and autism. Transl. Psychiatry. 2017;7:e999. doi: 10.1038/tp.2016.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Augé E., Pelegrí C., Manich G., Cabezón I., Guinovart J.J., Duran J., Vilaplana J. Astrocytes and neurons produce distinct types of polyglucosan bodies in Lafora disease. Glia. 2018;66:2094–2107. doi: 10.1002/glia.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guo W., Wang H., Watanabe M., Shimizu K., Zou S., LaGraize S.C., Wei F., Dubner R., Ren K. Glial–cytokine–neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Banks W.A., Plotkin S.R., Kastin A.J. Permeability of the blood-brain barrier to soluble cytokine receptors. Neuroimmunomodulation. 1995;2:161–165. doi: 10.1159/000096887. [DOI] [PubMed] [Google Scholar]
- 172.Dean M., Kasari C., Shih W., Frankel F., Whitney R., Landa R., Lord C., Orlich F., King B., Harwood R. The peer relationships of girls with ASD at school: Comparison to boys and girls with and without ASD. J. Child Psychol. Psychiatry. 2014;55:1218–1225. doi: 10.1111/jcpp.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mowlem F.D., Rosenqvist M.A., Martin J., Lichtenstein P., Asherson P., Larsson H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur. Child Adolesc. Psychiatry. 2019;28:481–489. doi: 10.1007/s00787-018-1211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bauermeister J.J., Shrout P.E., Chávez L., Rubio-Stipec M., Ramírez R., Padilla L., Anderson A., García P., Canino G. ADHD and gender: Are risks and sequela of ADHD the same for boys and girls? J. Child Psychol. Psychiatry. 2007;48:831–839. doi: 10.1111/j.1469-7610.2007.01750.x. [DOI] [PubMed] [Google Scholar]
- 175.Elkins I.J., Saunders G.R., Malone S.M., Keyes M.A., McGue M., Iacono W.G. Associations between childhood ADHD, gender, and adolescent alcohol and marijuana involvement: A causally informative design. Drug Alcohol Depend. 2018;184:33–41. doi: 10.1016/j.drugalcdep.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bauermeister J.J., Bird H.R., Shrout P.E., Chavez L., Ramírez R., Canino G. Short-term persistence of DSM-IV ADHD diagnoses: Influence of context, age, and gender. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:554–562. doi: 10.1016/j.jaac.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Greenfield B., Hechtman L., Stehli A., Wigal T. Sexual maturation among youth with ADHD and the impact of stimulant medication. Eur. Child Adolesc. Psychiatry. 2014;23:835–839. doi: 10.1007/s00787-014-0521-3. [DOI] [PubMed] [Google Scholar]
- 178.Semeijn E.J., Comijs H.C., De Vet H.C.W., Kooij J.J.S., Michielsen M., Beekman A.T.F., Deeg D.J.H. Lifetime stability of ADHD symptoms in older adults. ADHD Atten. Deficit Hyperact. Disord. 2016;8:13–20. doi: 10.1007/s12402-015-0178-x. [DOI] [PubMed] [Google Scholar]
- 179.Signs and Symptoms of Autism Spectrum Disorder. [(accessed on 6 September 2020)]; Available online: https://www.cdc.gov/ncbddd/autism/signs.html.
- 180.Symptoms and Diagnosis of ADHD. [(accessed on 6 September 2020)]; Available online: https://www.cdc.gov/ncbddd/adhd/diagnosis.html.
- 181.Lee A.S., Azmitia E.C., Whitaker-Azmitia P.M. Developmental microglial priming in postmortem autism spectrum disorder temporal cortex. Brain Behav. Immun. 2017;62:193–202. doi: 10.1016/j.bbi.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 182.Bollmann S., Ghisleni C., Poil S.S., Martin E., Ball J., Eich-Höchli D., Edden R.A.E., Klaver P., Michels L., Brandeis D. Developmental changes in gamma-aminobutyric acid levels in attention-deficit/hyperactivity disorder. Transl. Psychiatry. 2015;5:e589. doi: 10.1038/tp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]