Abstract
Background:
EOC is one of the most lethal gynecological malignancy worldwide. Although the majority of EOC patients achieve clinical remission after induction therapy, over 80% relapse and succumb to the chemoresistant disease. Previous investigations have demonstrated the association of EGFR with resistance to cytotoxic chemotherapies, hormone therapy, and radiotherapy in the cancers. These studies have highlighted the role of EGFR as an attractive therapeutic target in cisplatin-resistant EOC cells.
Methods:
The human ovarian cell lines (SKOV3 and OVCAR3) were cultured according to ATCC recommendations. The MTT assay was used to determine the chemosensitivity of the cell lines in exposure to cisplatin and erlotinib. The qRT-PCR was applied to analyze the mRNA expression of the desired genes.
Results:
Erlotinib in combination with cisplatin reduced the cell proliferation in the chemoresistant EOC cells in comparison to monotherapy of the drugs (p < 0.05). Moreover, erlotinib/cisplatin combination synergistically decreased the expression of anti-apoptotic and also increased pro-apoptotic genes expression (p < 0.05). Cisplatin alone could increase the expression of MDR genes. The data suggested that EGFR and cisplatin drive chemoresistance in the EOC cells through MEKK signal transduction as well as through EGFR/MEKK pathways in the cells, respectively.
Conclusion:
Our findings propose that EGFR is an attractive therapeutic target in chemoresistant EOC to be exploited in translational oncology, and erlotinib/cisplatin combination treatment is a potential anti-cancer approach to overcome chemoresistance and inhibit the proliferation of the EOC cells.
Key Words: Cisplatin, Epidermal growth factor receptor, Ovarian cancer
INTRODUCTION
EPithelial ovarian cancer is one the most common cancer among gynecological malignancy and the fifth leading cause of cancer-related death among women globally[1]. It was estimated that nearly 14000 patients died from EOC, and 22000 new cases were diagnosed with EOC in the United States in 2018[2]. Advanced-stage diagnosis, peritoneal dissemination, and the establishment of therapy resistance are the major obstacles for the successful treatment of EOC and contribute to marginal overall survival rate[3].
Conventional treatment strategies for EOC includes radical debulking surgery, following by platinum-taxane-based regimens and adjuvant chemotherapies[3]. Despite advances in surgical debulking and chemotherapy regimens, over 80% of patients with late-stage disease relapse within a few months due to resistance to both chemotherapeutic agents and targeted therapies[4].
A large body of evidence has suggested that the ErbB family of receptor tyrosine kinases has a significant contribution to the initiation and progression of a variety of human malignancies[5]. The EGFR, a member of ErbB family of receptor tyrosine kinases, activates multiple downstream signaling pathways, including Ras/Raf/MAPK and PI3K/Akt, thereby promoting the proliferation, invasion, and metastasis of tumor cells[6]. Previous investigations have demonstrated that EGFR overexpression has been associated with resistance to cytotoxic chemotherapies, hormone therapy, and radiotherapy[7,8]. EGFR overexpression has been observed in 30–98% of EOC in all histologic subtypes[9]. Enhanced expression of EGFR and its ligands are correlated with advanced-stage disease, poor response to chemotherapies, dismal clinical outcome, and decreased recurrence-free survival[10]. Preclinical studies with cetuximab (an anti-EGFR mAb) as well as gefitinib and erlotinib (EGFR small molecule inhibitors) have displayed that EGFR-targeted therapies enhance the anti-tumor activity of the chemotherapeutic agents and radiotherapy in colorectal, pancreatic, non-small cell lung, and breast cancer cells[11-14]. These studies support the hypothesis that the inhibition of the EGFR pathway in combination with the chemotherapies might strengthen the antitumor activity of mentioned agents, leading to the increased apoptotic cell death.
Erlotinib is a reversible and highly specific EGFR tyrosine kinase inhibitor that is orally administrated in a variety of cancers[15]. Several randomized clinical trials have evaluated the efficacy and benefit of erlotinib in cancer, particularly in non-small cell lung cancer[16]. Cisplatin is also one of the most commonly used platinum-based chemotherapy agent administrated as the first-line standard treatment for EOC and in a broad range of cancers[17]. Cisplatin has been indicated to bind to the cellular components such as DNA and protein and inhibits molecular processes such as DNA replication and protein translation via forming DNA-cross link in the cells[18]. In the present study, we demonstrated the in vitro activity of erlotinib in the EOC cell lines and showed that EGFR blockade restores cisplatin sensitivity in these cells.
MATERIALS AND METHODS
Drugs
Erlotinib (anti-EGFR) and buparlisib (anti-PI3K) were purchased from Chemietek (Indianapolis, IN, USA) and Bay 11-7082 (anti- NFκB) and trametinib (anti-MEKK1/MEKK2) from Sigma (St. Louis, MO, USA). Cisplatin (DNA-damaging agent) was also acquired from the Pharmacy of Shariati Hospital (Tehran, Iran).
Human ovarian carcinoma cell lines
Human ovarian carcinoma cell lines, SKOV3 and OVCAR3, were obtained from the National Cell Bank of Iran, Pasteur Institute of Iran, Tehran. The cells were cultured, according to ATCC recommendations, in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin antibiotics (both from Gibco, Life Technologies, USA) in a humidified incubator in 5% CO2 at 37 °C.
Anti-proliferative assays
The EOC cells were cultured in 96-well plates (2 × 103 cells/well). After incubation at 37 °C for 24 h, the cultures were treated with desired concentrations of the chemotherapy agents. Following 48 h of treatment, the proportion of viable cells was determined by MTT assay. After 48 and 72 hours, 20 µl of MTT solution (5 mg/ml; Sigma) was added to each well and incubated with 5% CO2 at 37 °C for 4 hours. The supernatant was removed, and 100 µl of DMSO was added to each well as a solvent. Cell viability percentage was assessed by spectrophotometry at 570 nm using Absorbance Microplate Reader (BioTek ELx800, USA). The cytotoxicity was reported as IC50 values calculated from full dose-response curves. Synergism was determined by drawing the normalized isobologram according to Chou-Talalay method (reviewed in[19]) using the Calcusyn software (Biosoft, Cambridge, UK). Untreated EOC cells were considered as the positive control, and blank wells were regarded as the negative control.
RNA extraction and cDNA synthesis
Trizol (Life Technologies, USA) was used to harvest the total RNA from the cultured cells. The quantity and quality of the extracted RNA were investigated by the 2% gel electrophoresis method. Then RNA concentration was measured by a Nanodrop (Thermo Scientific, USA). cDNA was prepared by the PrimeScript RT cDNA synthesis kit (Takara Bio, USA) according to the manual instruction. The total volume for this reaction was 20 μl that included 2 µg of total RNA, 4 μl of 5× buffer, 1 μl of dNTP, 1 μl of RNase Inhibitor, 2 μl of random hexamers, 1 μl of Moloney Murine Leukemia Virus reverse transcriptase, and DEPC water.
Analysis of gene expression by qRT-PCR
Changes in mRNA levels of the desired genes were measured by qRT-PCR on a LightCycler® 96 System (Roche Life Science, Germany) using SYBR green-based kit, RealQ Plus Master Mix Green (Ampliqon, Copenhagen, Denmark). The total volume was 20 μL, including 10 μL of SYBR Green, 1 μL of primer, 2 μL of cDNA, and DEPC water. Thermal cycling conditions were comprised of an activation step at 95 °C for 15 min, followed by 40 cycles, including a denaturation step at 95 °C for 10 seconds and at 60 °C for 1 min for annealing and extension, respectively. The primer sets are listed in Table 1. The target gene expression levels were normalized to HPRT1 levels. For calculation of relative expression, 2–ΔΔCT formula was used. HGPRT1 gene expression was considered as the positive control, and DEPC water was considered as the negative control.
Table 1.
Nucleotide sequences of the primers used for qRT-PCR
| Gene | Accession number | Forward primer | Reverse primer |
Amplicon
size (bp) |
|---|---|---|---|---|
| HPRT1 | NM_000194 | GGACAGTACGGGAGATCACAG | GCACTAATTTCCTTCAGGGATCG | 111 |
| FOXO1 | NM_002015 | TGATAACTGGAGTACATTTCGCC | CGGTCATAATGGGTGAGAGTCT | 80 |
| FOXO3 | NM_001455 | ACGGCTGACTGATATGGCAG | CGTGATGTTATCCAGCAGGTC | 85 |
| FOXO4 | NM_005938 | CACGTATGGATCCGGGGAAT | CCCCTCCGTGTGTACCTTTTC | 191 |
| P21 | NM_000389 | CCTGTCACTGTCTTGTACCCT | GCGTTTGGAGTGGTAGAAATCT | 130 |
| P27 | NM_004064 | AACGTGCGAGTGTCTAACGG | CCCTCTAGGGGTTTGTGATTCT | 209 |
| BAX | NM_001291428 | CGAGAGGTCTTTTTCCGAGTG | GTGGGCGTCCCAAAGTAGG | 242 |
| BCL2 | NM_000633 | CGGTGGGGTCATGTGTGTG | CGGTTCAGGTACTCAGTCATCC | 90 |
| ABCG2 | NM_004827 | TGAGCCTACAACTGGCTTAGA | CCCTGCTTAGACATCCTTTTCAG | 75 |
| ABCB1 | NM_000927 | TTGCTGCTTACATTCAGGTTTCA | AGCCTATCTCCTGTCGCATTA | 105 |
| ABCC | NM_004996 | CTCTATCTCTCCCGACATGACC | AGCAGACGATCCACAGCAAAA | 94 |
| cIAP1 | NM_001166 | AGCACGATCTTGTCAGATTGG | GGCGGGGAAAGTTGAATATGTA | 102 |
| XIAP | NM_001167 | ATAGTGCCACGCAGTCTACAA | AGATGGCCTGTCTAAGGCAAA | 101 |
| MCL1 | NM_021960 | TGCTTCGGAAACTGGACATCA | TAGCCACAAAGGCACCAAAAG | 135 |
| CCND1 | NM_053056 | GCTGCGAAGTGGAAACCATC | CCTCCTTCTGCACACATTTGAA | 135 |
| MYC | NM_002467 | GTCAAGAGGCGAACACACAAC | TTGGACGGACAGGATGTATGC | 162 |
| Survivin | NM_001168 | CCAGATGACGACCCCATAGAG | TTGTTGGTTTCCTTTGCAATTTT | 152 |
| EGF | NM_001963 | TGTCCACGCAATGTGTCTGAA | CATTATCGGGTGAGGAACAACC | 133 |
| HB-EGF | NM_001945 | ATCGTGGGGCTTCTCATGTTT | TTAGTCATGCCCAACTTCACTTT | 86 |
| AREG | NM_001657 | GAGCCGACTATGACTACTCAGA | TCACTTTCCGTCTTGTTTTGGG | 121 |
| EREG | NM_001432 | GTGATTCCATCATGTATCCCAGG | GCCATTCATGTCAGAGCTACACT | 120 |
| BTC | NM_001729 | CCTGGGTCTAGTGATCCTTCA | CTTTCCGCTTTGATTGTGTGG | 131 |
| HER3 | NM_001982 | GGTGATGGGGAACCTTGAGAT | CTGTCACTTCTCGAATCCACTG | 80 |
| HER2 | NM_001005862 | CAGTGCAGCACAGAGACTCA | CCGGTGCACACTCACTTTTG | 103 |
| EGFR | NM_001346897 | AGACAGCTTCTTGCAGCGAT | TTCCAGACAAGCCACTCACC | 105 |
| HRG1A | NM_013964 | AAACCAAGAAAAGGCGGAGGAGCT | GAGGGCGATGCAGATGCCGG | 70 |
| HRG1B | NM_013956 | GCCAGCTTCTACAAGCATCTTGGGA | GGAGGGCGATGCAGATGCCG | 97 |
Statistical analysis
The data were graphed and analyzed using GraphPad Prism (version 6.01). Unpaired two-tailed Student’s t-test and One-way ANOVA were used for comparing the means between two and more than two independent groups, respectively. Heatmap was drawn via R software (3.5.1). All data were presented as mean ± SD, and all the experiments were conducted in triplicate for three independent times. A p value <0.05 was considered as statistically significant threshold.
RESULTS
Chemoresistance of the EOC cell lines
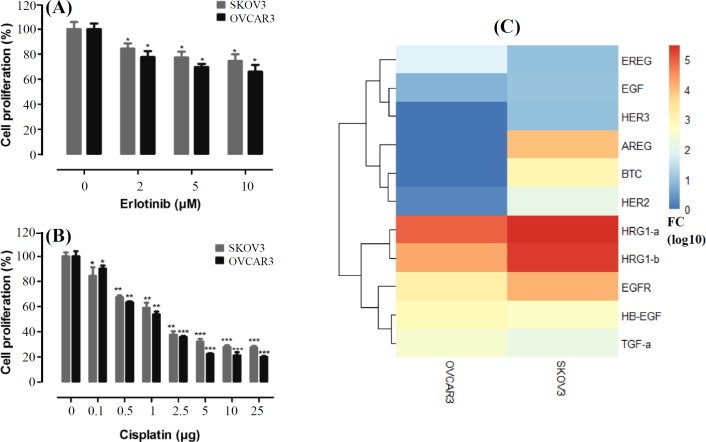
Since cisplatin is considered to be the first line conventional therapy in EOC, we tried to test the effects of cisplatin in combination with erlotinib on the cell viability of EOC cell lines. Chemoresponsiveness of the EOC cell lines, SKOV3 and OVCAR3, to cisplatin and erlotinib were measured by MTT assay and are presented in Figure 1A and 1B. The IC50 of cisplatin for SKOV3 and OVCAR3 was as 1.84 μg and 1.44 μg, respectively. These amounts were as 58.57 μM and 53.49 μM for erlotinib.
Fig. 1.
Erlotinib and cisplatin resistance profiles and the relative expression of EGF ligands and receptors. (A and B) SKOV3 and OVCAR3 cells exposed to erlotinib (2, 5, and 10 μM) and cisplatin (0.1, 0.5, 1, 2.5, 5, 10, and 25 µg). The data are presented as mean ± SD; (C) ErbB family expression profile in the cells. *p < 0.05, **p < 0.01, and ***p < 0.001
Expression of ErbB family in the EOC cells
To explore the expression of ErbB ligands and receptors, the relative expression of EREG, EGF, HER3, AREG, BTC, HER2, HRG-1A, HRG-1B, EGFR, HB-EGF, and TGF-α were investigated in SKOV3 and OVCAR3 cell lines by qRT-PCR. This screening experiment demonstrated that the expression of the ErbB family members is relatively expressed in those cells (Fig. 1C).
Effect of erlotinib on the EOC cells
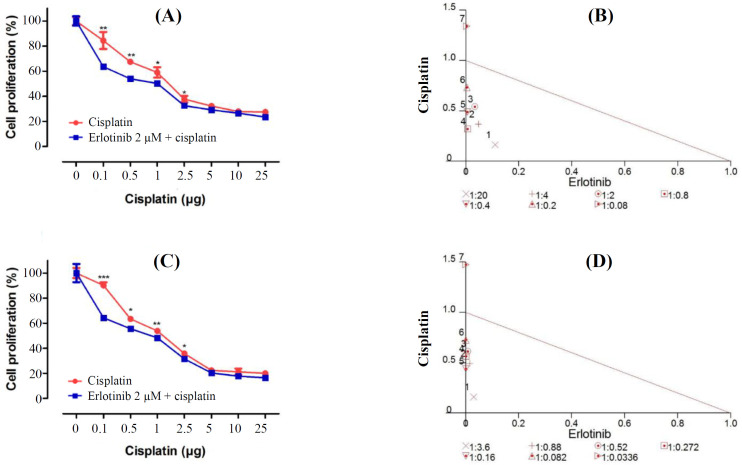
The effects of erlotinib in combination with cisplatin on the proliferative response of SKOV3 and OVCAR3 cells were evaluated. This combinatorial approach had synergistic effects on the growth inhibition. Cisplatin significantly reduced cell proliferation in combination with 2 μM of erlotinib, dose-dependently (p < 0.05). Moreover, 0.1 μg of cisplatin showed noticeable combinatorial effect with 2 μM of erlotinib in the EOC cells (p < 0.001). According to the normalized isobologram, 0.1 μg of cisplatin had the most powerful synergism with 2 μM of erlotinib in EOC cells. It has been suggested that erlotinib-mediated inhibition of EGFR increases the cisplatin responsiveness in SKOV3 and OVCAR3 cells (Fig. 2). For further investigation, 0.1 μg of cisplatin and 2 μM of erlotinib were chosen.
Fig. 2.
Synergistic effects of erlotinib and cisplatin on SKOV3 and OVCAR3 cell lines. (A) SKOV3 and (C) OVCAR3. (B and D) Normalized isobologram analysis represents the synergic effect of erlotinib (2 µM) and cisplatin (0.1, 0.5, 1, 2.5, 5, 10, and 25 µg) when using combination treatment in SKOV3 and OVCAR3 cell lines. The combination index was calculated with Calcusyn software. Points above, below, and over the isobologram effect line reflect antagonism, synergy, and additive effect, respectively. The numbers under the isobolograms indicate the doses of erlotinib and cisplatin in combination. Statistically significant values of *p < 0.05, **p < 0.01, and ***p < 0.001 were determined compared with the control
Effects of erlotinib/cisplatin combinatorial therapy
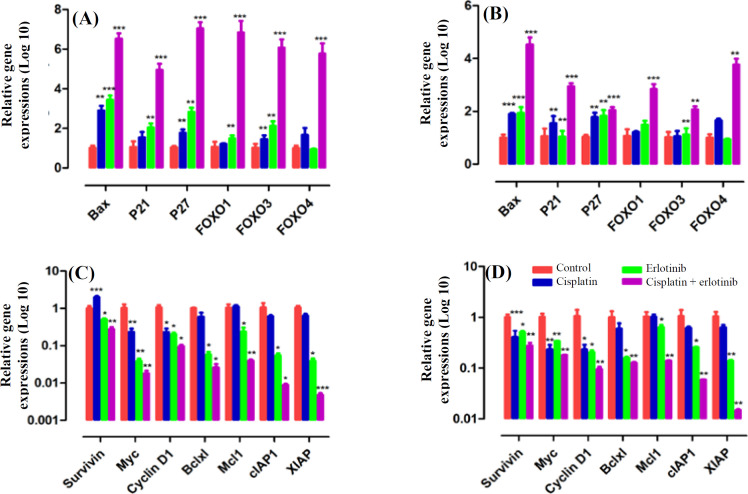
To demonstrate the anti-tumor effects of cisplatin in combination with erlotinib on the EOC cells, we investigated the effects of erlotinib, cisplatin, and combinatorial treatment on the expression of pro-apoptotic and anti-apoptotic genes. Accordingly, SKOV3 and OVCAR3 cells were exposed to cisplatin (0.1 μg) and erlotinib (2 μM) for 48 h. Erlotinib/ cisplatin combination treatment remarkably increased mRNA levels of pro-apoptotic genes such as BAX, p21, p27, FOXO1, FOXO3, and FOXO4, nearly five times in comparison to monotherapy of cisplatin or erlotinib (p < 0.001). Furthermore, we observed a significant reduction in the mRNA levels of anti-apoptotic genes, including survivin, MYC, Cyclin D1, BCL-xl, MCL1, cIAP1, and XIAP, approximately ten times in comparison with the controls and the chemotherapy alone (p < 0.01; Fig. 3).
Fig. 3.
Erlotinib and cisplatin combination treatment inducing apoptosis and reducing anti-apoptotic gene expression. The EOC cells were treated with cisplatin (0.1 μg) and erlotinib (2 μM) for 48 h, then total RNA was harvested for qRT-PCR analysis. Gene expression levels were normalized to HPRT1. Erlotinib in combination with cisplatin dramatically diminished the gene expression of pro-apoptotic genes in SKOV3 (A) and OVCAR3 (B) cells. Anti-apoptotic genes expression levels were meaningfully depleted in erlotinib/cisplatin treatment in SKOV3 (C) and OVCAR3 (D) cell lines. *p < 0.05, **p < 0.01, and ***p < 0.001
Effect of chemoresistance through EGFR/MEKK pathway
Cisplatin underlying chemoresistance mechanisms in the EOC cell lines was investigated by treating SKOV3 and OVCAR3 cells with cisplatin (0.1 μg) and erlotinib (2 μM) for 48 h. Erlotinib/cisplatin combination treatment significantly reduced mRNA levels of MDR genes (ABCB1, ABCC, and ABCG2) compared to the control and the single-agent treatments (p < 0.05). Cisplatin alone marginally increased the MDR genes expression levels in the SKOV3 cells (p < 0.07, Fig. 4A and 4B). Furthermore, we explored how cisplatin enhanced chemoresistance in cancerous cell lines. The mRNA levels were analyzed by qRT-PCR. The data showed that cisplatin alone significantly increased HB-EGF (EGFR ligand) in the SKOV3 cells. HB-EGF expression just reduced in the cisplatin/erlotinib and cisplatin/trametinib combinatorial approaches significantly (p < 0.05), which showed that EGFR was driving chemoresistance in the EOC cells through MEKK1/MEKK2 signal transduction. The reduction of HB-EGF in the combinatorial approaches was so similar (two times) and significant. The data suggest that cisplatin drives chemoresistance through EGFR/MEKK pathways in the target cells (Fig. 4C).
Fig. 4.
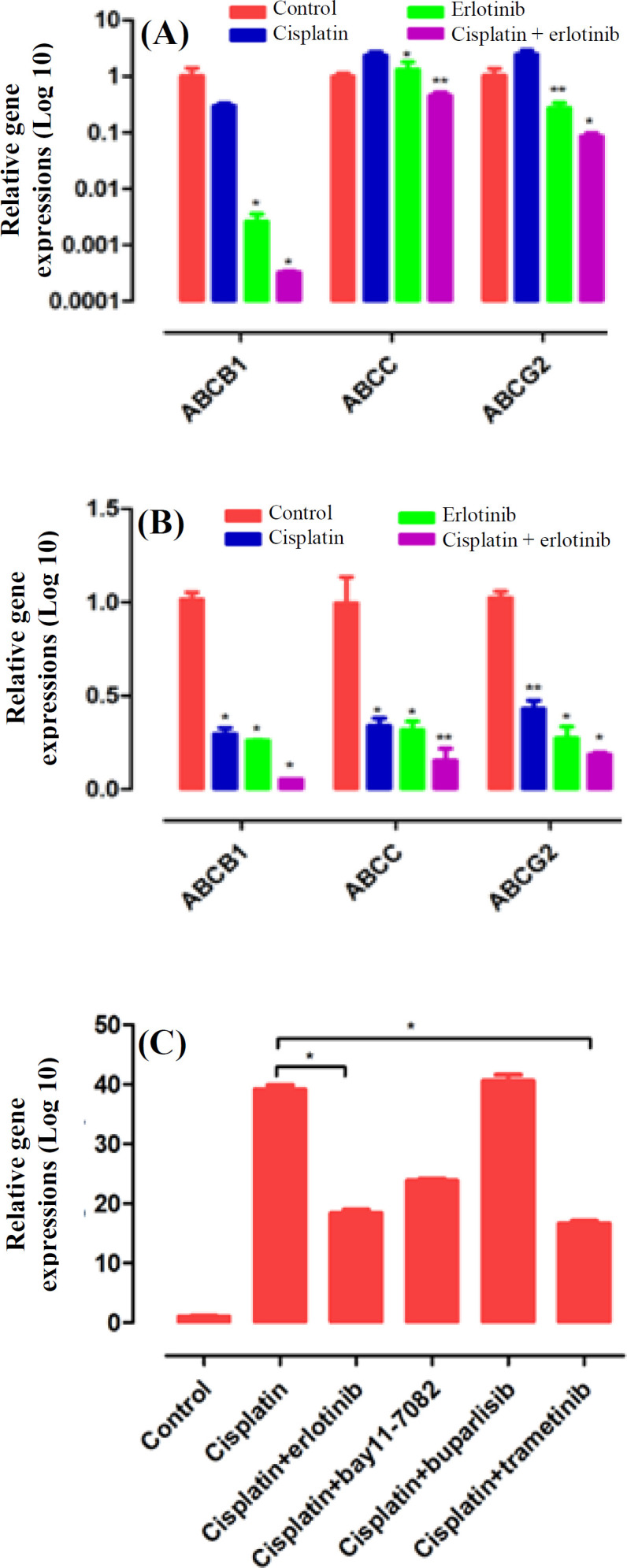
Cisplatin enhancement by chemoresistance through EGFR/MEKK pathway. The SKOV3 (A) and OVCAR3 (B) cell lines were treated with cisplatin (0.1 μg) and erlotinib (2 μM) for 48 h, then total RNA was harvested for qRT- PCR analysis. Erlotinib/cisplatin combination significantly reduced MDR genes in both cell lines with respect to the control. (C) SKOV3 cells were exposed to cisplatin (0.1 μg) as an igniter of chemoresistance, erlotinib (2 μM) as an anti-EGFR, bay11-7082 (5 μM) as an anti-NFκB, buparlisib (1 μM) as an anti-PI3K, and trametinib (5 μM) as an anti-MEKK1/MEKK2 for signaling dissection. After 48 h, RNA was harvested for qRT-PCR. HB-EGF (EGFR ligand) was evaluated in the treated cells
DISCUSSION
Despite advances in surgical debulking and chemotherapy regimens, EOC has exhibited marginal improvement in survival. Although most patients achieve a clinical remission after the induction therapy, resistance to chemotherapy will occur subsequently. Moreover, relapsed tumors have a poor response to other cytotoxic agents, as well. Hence, in order to improve the outcome of the EOC patients, it is of paramount importance to devise novel and more efficient therapies aimed at blocking pivotal signaling pathways responsible for therapy resistance[20]. Alternation in cellular signaling pathways after chemotherapy treatment may lead to the initiation of drug resistance[21].
EGFR pathway is a key regulator of chemoresponsiveness in human malignancies. It has been suggested that cisplatin can interact with the EGFR signaling pathway and could either promote or inhibit apoptosis[22]. A previous study has revealed that cisplatin-resistant human ovarian cancer cell lines have an enhanced rate of motility and invasion in vitro, which is associated with hyperactivation of EGFR[23]. To better clarify the events happening in the EGFR signaling pathway after cisplatin treatment and their roles in promoting chemoresistance in the EOC cells, we applied in vitro models of the EOC cells and found that cisplatin in combination with erlotinib synergistically reduced the cell growth ability of those cells. Expression data showed that erlotinib/cisplatin combination treatment dramatically decreased the mRNA levels of anti-apoptotic genes compared to the control. Studies have highlighted the role of BCL2 family and cyclin proteins in the development of chemoresistance in many types of cancers. It has also been displayed that BCL2 upregulation was associated with cisplatin-resistance in bladder, ovarian, lung, and breast cancer[24,25]. Downregulation of BCL2 and cyclin D1 enhanced cisplatin sensitivity in breast cancer cell lines[26]. A large body of evidence has demonstrated that the overexpressions of MYC, XIAP, and surviving are correlated with platinum and taxol resistance of the EOC cells[27,28]. In addition, our data showed that erlotinib in combination with cisplatin significantly up-regulated the expression of pro-apoptotic genes, including BAX, p21, p27, FOXO1, FOXO3, and FOXO4, compared to the control and the single-agent regiment. Vast evidence has revealed that the reduced expression of p27 and p21 is associated with chemotherapy resistance and poor prognosis in the patients[29,30]. BAX gene expression has been shown to associate with an increased progression-free and overall survival of the EOC patients[31]. A previous study has considered the forkhead transcription factor FOXO1 as a transcriptional factor, which can develop drug resistance in ovarian cancer[32]. EGFR drives proliferation, MDR genes expression, which efflux cisplatin out of the cells, cell survival, and apoptosis inhibition, ultimately resulting in chemoresistance in the cells. We showed that erlotinib induced apoptosis, reduced cell proliferation, and downregulated MDRs expression via blocking of EGFR.
Further to erlotinib-mediated potential anti-tumor activity, down-regulation of MDRs could lead to the accumulation of cisplatin in the cells and enhance cisplatin chemotherapeutic effects. One study showed that trametinib (MEKK inhibitor) remarkably reduced the cell growth and proliferation in combination with vincristine and doxorubicin through blocking the drug-efflux activity of ABCB1 transporter[33]. Cisplatin-resistant cells intrinsically up-regulate ATP-binding cassette transports (ABC) to efflux chemotherapy agents, consequently reducing the efficacy of the chemotherapies[34]. In addition, erlotinib can reduce drug efflux through the downregulation of ABC pump[35]. Phase 2 trial conducted by Nogueira‐ Rodrigues et al.[36] displayed that treatment with erlotinib in combination with cisplatin plus radiotherapy exerted significant effects on cervical cancer patients and remarkably improved overall and progression-free survival. Some in vitro studies considered EGFR family and related signaling transduction as critical players in chemoresistance via the induction of MDR pumps[37,38]. Also, EGFR inhibitor PD153035 can synergistically increase the anti-tumor effect of chemotherapy modalities via down-regulating ABCG2 in non-small cell lung cancer[37]. In fact, based on evidence, cisplatin treatment activates the EGFR and its downstream signaling pathway MEKK1/MEKK2, which has previously been shown to be implicated in chemoresistance in EOC[38].
Taken together, our results suggest that EGFR plays a key role in the development of chemoresistance in SKOV3 and OVCAR3 cells via the induction of MDR genes through the EGFR/MEKK1, MEKK2 pathway. Oure studies highlight the role of EGFR as an attractive therapeutic target and suggest that EGFR blocking-therapies seem to be promising strategies against chemoresistant EOC.
CONFLICT OF INTEREST.
None declared.
References
- 1.Yousefi H, Momeny M, Ghaffari SH, Parsanejad N, Poursheikhani A, Javadikooshesh S, Zarrinrad G, Esmaeili F, Alishahi Z, Sabourinejad Z, Sankanian G, Shamsaiegahkani S, Bashash D, Shahsavani N, Tavakkoly-Bazzaz J, Alimoghaddam K, Ghavamzadeh A. IL-6/IL-6R pathway is a therapeutic target in chemoresistant ovarian cancer. Tumori journal. 2018;105(1):84–91. doi: 10.1177/0300891618784790. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Mia M, Ahmedin J, Rebecca L. Ovarian cancer statistics. CA: a cancer journal for clinicians. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottschalk N, Kimmig R, Lang S, Singh M, Brandau S. Anti-epidermal growth factor receptor (EGFR) antibodies overcome resistance of ovarian cancer cells to targeted therapy and natural cytotoxicity. International journal of molecular sciences. 2012;13(9):12000–12016. doi: 10.3390/ijms130912000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korkmaz T, Seber S, Basaran G. Review of the current role of targeted therapies as maintenance therapies in first and second line treatment of epithelial ovarian cancer; In the light of completed trials. Critical reviews in oncology/hematology. 2016;98:180–188. doi: 10.1016/j.critrevonc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Zhang P, Zhou M, Jiang H, Zhang H, Shi B. Exon 4 deletion variant of epidermal growth factor receptor enhances invasiveness and cisplatin resistance in epithelial ovarian cancer. Carcinogenesis. 2013;34(11):2639–2646. doi: 10.1093/carcin/bgt216. [DOI] [PubMed] [Google Scholar]
- 6.Granados ML, Hudson LG, Samudio-Ruiz SL. Contributions of the epidermal growth factor receptor to acquisition of platinum resistance in ovarian cancer cells. PLoS one . 2015;10(9):e0136893. doi: 10.1371/journal.pone.0136893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin cancer research. 2001;7(10):2958–2970. [PubMed] [Google Scholar]
- 8.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. Journal of clinical oncology. 2003;21(14):2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 9.Garces ÁHI, Dias MSF, Paulino E, Ferreira CGM, de Melo AC. Treatment of ovarian cancer beyond chemotherapy: Are we hitting the target? Cancer chemotherapy and pharmacology . 2015;75(2):221–234. doi: 10.1007/s00280-014-2581-y. [DOI] [PubMed] [Google Scholar]
- 10.Sheng Q, Liu J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. British journal of cancer. 2011;104(8):1241–1245. doi: 10.1038/bjc.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clinical cancer research . 2002;8(5):994–1003. [PubMed] [Google Scholar]
- 12.Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clinical cancer research. 2000;6(5):1936–1948. [PubMed] [Google Scholar]
- 13.Kishida O, Miyazaki Y, Murayama Y, Ogasa M, Miyazaki T, Yamamoto T, Watabe K, Tsustsuj S, Kiyohara T, Shimomura L, Shinomura Y. Gefitinib (Iressa, ZD1839) inhibits SN38-triggered EGF signals and IL-8 production in gastric cancer cells. Cancer chemotherapy and pharmacology. 2005;55(6):584–594. doi: 10.1007/s00280-004-0959-y. [DOI] [PubMed] [Google Scholar]
- 14.Li T, Ling YH, Goldman ID, Perez-Soler R. Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non–small cell lung cancer cells. Clinical cancer research . 2007;13(11):3413–3422. doi: 10.1158/1078-0432.CCR-06-2923. [DOI] [PubMed] [Google Scholar]
- 15.Madamsetty VS, Pal K, Dutta SK, Wang E, Thompson JR, Banerjee RK, Caulfield TR, Mody K, Yen Y, Mukhopadhyay D, Huanq HS. Design and evaluation of PEGylated liposomal formulation of a novel multikinase inhibitor for enhanced chemosensitivity and inhibition of metastatic pancreatic ductal adenocarcinoma. Bioconjugate chemistry. 2019;30(10):2703–2713. doi: 10.1021/acs.bioconjchem.9b00632. [DOI] [PubMed] [Google Scholar]
- 16.Bareschino M, Schettino C, Troiani T, Martinelli E, Morgillo F, Ciardiello F. Erlotinib in cancer treatment. Annals of oncology. 2007;18(6):35–41. doi: 10.1093/annonc/mdm222. [DOI] [PubMed] [Google Scholar]
- 17.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nature reviews clinical oncology . 2013;10(4):211–224. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasari SR, Velma V, Yedjou CG, Tchounwou PB. Preclinical assessment of low doses of cisplatin in the management of acute promyelocytic leukemia. International journal of cancer research and molecular mechanisms. 2015;1(3) doi: 10.16966/2381-3318.113. oi: 10.16966/2381-3318.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 20.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nature reviews cancer. 2005;5(5):355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 21.Macleod K, Mullen P, Sewell J, Rabiasz G, Lawrie S, Miller E, Smyth JF, Langdon SP. Altered ErbB receptor signaling and gene expression in cisplatin-resistant ovarian cancer. Cancer research. 2005;65(15):6789–6800. doi: 10.1158/0008-5472.CAN-04-2684. [DOI] [PubMed] [Google Scholar]
- 22.Benhar M, Engelberg D, Levitzki A. Cisplatin-induced activation of the EGF receptor. Oncogene. 2002;21(57):8723–8731. doi: 10.1038/sj.onc.1205980. [DOI] [PubMed] [Google Scholar]
- 23.Yue P, Zhang X, Paladino D, Sengupta B, Ahmad S, Holloway RW, Ingertsoll SB, Turkson J. Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells. Oncogene . 2012;31(18):2309–2322. doi: 10.1038/onc.2011.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D, Wu T, Wu J, Cheng YW, Chen YC, Lee M. Phosphorylation of paxillin confers cisplatin resistance in non-small cell lung cancer via activating ERK-mediated Bcl-2 expression. Oncogene . 2014;33(35):4385–4395. doi: 10.1038/onc.2013.389. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Ho JN, Jin H, Lee SC, Lee SE, Hong SK, Lee JW, Lee ES, Byun SS. Upregulated expression of BCL2, MCM7, and CCNE1 indicate cisplatin-resistance in the set of two human bladder cancer cell lines: T24 cisplatin sensitive and T24R2 cisplatin resistant bladder cancer cell lines. Investigative and clinical urology. 2016;57(1):63–72. doi: 10.4111/icu.2016.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yde CW, Issinger OG. Enhancing cisplatin sensitivity in MCF-7 human breast cancer cells by downregulation of Bcl-2 and cyclin D1. International journal of oncology. 2006;29:1397–1404. [PubMed] [Google Scholar]
- 27.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, Pilotti S, Zunino F, Daidone MG. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cellular and molecular life sciences CMLS . 2002;59(8):1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes-González JM, Armaiz-Peña GN, Mangala LS, Valiyeva F, Ivan C, Pradeep S, Echevarría-Vargas IM, Rivera-Reyes A, Sood AK, Vivas-Mejía PE. Targeting c-MYC in platinum-resistant ovarian cancer. Molecular cancer therapeutics. 2015;14(10):2260–2269. doi: 10.1158/1535-7163.MCT-14-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown I, Shalli K, McDonald SL, Moir SE, Hutcheon AW, Heys S. Reduced expression of p27 is a novel mechanism of docetaxel resistance in breast cancer cells. Breast cancer research . 2004;6(5):R601–R607. doi: 10.1186/bcr918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmider-Ross A, Pirsig O, Gottschalk E, Denkert C, Lichtenegger W, Reles A. Cyclin-dependant kinase inhibitors CIP1 (p21) and KIP1 (p27) in ovarian cancer. Journal of cancer research and clinical oncology . 2006;132:163–170. doi: 10.1007/s00432-005-0057-5. [DOI] [PubMed] [Google Scholar]
- 31.Schuyer M, van der Burg MEL, Henzen-Logmans SC, Fieret JH, Klijn JGM, Look MP. Reduced expression of BAX is associated with poor prognosis in patients with epithelial ovarian cancer: a multifactorial analysis of TP53, p21, BAX and BCL-2. British journal of cancer . 2001;85(9):1359–1367. doi: 10.1054/bjoc.2001.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J, San Ko Y, Yoon J, Kim MA, Park JW, Kim WH. The forkhead transcription factor FOXO1 mediates cisplatin resistance in gastric cancer cells by activating phosphoinositide 3-kinase/Akt pathway. Gastric cancer . 2014;17(3):423–430. doi: 10.1007/s10120-013-0314-2. [DOI] [PubMed] [Google Scholar]
- 33.Qiu JG, Zhang YJ, Li Y, Zhao JM, Zhang WJ, Jiang QW, Mei XL, Xue YQ, Qin WM, Yang Y, Zheng DW, Chen Y, Wei MN, Shi Z. Trametinib modulates cancer multidrug resistance by targeting ABCB1 transporter. Oncotarget . 2015;6(17):15494–15509. doi: 10.18632/oncotarget.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tonigold M, Rossmann A, Meinold M, Bette M, Märken M, Henkenius K, Giel G, Cai C, Rodepeter FR, Beneš V, Grénman R, Carey TE, Lage H, Stiewe T, Neubauer A, Werner JA, Brendel C, Mandic R, A cisplatin-resistant head, neck cancer cell line with cytoplasmic p53mut exhibits ATP-binding cassette transporter upregulation, high glutathione levels. Journal of cancer research and clinical oncology 2014; 140(10): 1689-1704. doi: 10.1007/s00432-014-1727-y. [DOI] [PubMed] [Google Scholar]
- 35.Lainey E, Sébert M, Thépot S, Scoazec M, Bouteloup C, Leroy C, De Botton S, Galluzzi L, Fenaux P, Kroemer G. Erlotinib antagonizes ABC transporters in acute myeloid leukemia. Cell cycle. 2012;11(21):4079–4092. doi: 10.4161/cc.22382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nogueira Rodrigues A, Moralez G, Grazziotin R, Carmo CC, Small IA, Alves FV, Mamede M, Erlich F, Viegas C. Triginelli SA, Ferreira CG, Phase 2 trial of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced cervical cancer. Cancer . 2014;120(8):1187–1193. doi: 10.1002/cncr.28471. [DOI] [PubMed] [Google Scholar]
- 37.Zhang GN, Zhang YK, Wang YJ, Gupta P, Ashby Jr CR, Alqahtani S, Deng T, Bates SE, Kaddoumi A, Wurpel JND, Lei YX, Chen ZS. Epidermal growth factor receptor (EGFR) inhibitor PD153035 reverses ABCG2-mediated multidrug resistance in non-small cell lung cancer: in vitro and in vivo. Cancer letters . 2018;424:19–29. doi: 10.1016/j.canlet.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Zhou JY, Wu GS. ERK-dependent MKP-1–mediated cisplatin resistance in human ovarian cancer cells. Cancer research . 2007;67(24):11933–11941. doi: 10.1158/0008-5472.CAN-07-5185. [DOI] [PubMed] [Google Scholar]