Abstract
Simple Summary
Formaldehyde exposure is common due to inhalation and its presence in some food additives. Upon exposure to formaldehyde via any route, it is majorly metabolized by the liver. However, this metabolism impacts negatively on the liver, and in certain concentrations can result in liver damage referred to as hepatotoxicity. This toxicity is evident by a decrease in antioxidant markers as well as an increase in liver function enzymes, inflammatory markers as well as lipid profile in Wistar rats as shown by this study. To combat the deleterious effect of formaldehyde exposure, this study has shown that Ganoderma lucidum from red mushroom presents an excellent natural resource by ameliorating the aforementioned liver toxicity markers. This study should serve as a deterrent for those in the practice of using formaldehyde as food additives. Environment inspectors and governments should ensure that formaldehyde is kept below its toxicity threshold in work environments. However, in cases where hepatotoxicity has ensued or is suspected, Ganoderma lucidum could serve as a way to combat this toxicity but should be used under appropriate medical expert supervision.
Abstract
The majority of liver-related illnesses are caused by occupational and domestic exposure to toxic chemicals like formaldehyde (FA), which is widely common in Africa and the world at large. Hence, measures should be taken to protect humans from its hazardous effects. This study, therefore, examines the protective potential of Ganoderma lucidum (100 mg/kg body weight) on formaldehyde-induced (40%) liver oxido-inflammation in male rats. Male Wistar rats, 150–200 g, were allotted into four groups of 10 animals as follows: Group 1 was orally treated with 1 mg/mL distilled water, Group 2 was exposed to a 40% formaldehyde vapor environment for 30 min per day, Group 3 was orally treated with 100 mg/kg ethanol extract of Ganoderma lucidum, and Group 4 was co-administered formaldehyde and 100 mg/kg ethanol extract of Ganoderma lucidum. Rats were then sacrificed 24 h after administering the last dose of treatment, and the livers were excised. Ganoderma lucidum significantly reversed the formaldehyde-mediated reduction in body and organ weight. Ganoderma lucidum administration significantly prevented oxido-inflammation by reducing the levels of hydrogen peroxide and malondialdehyde and increasing the activity of antioxidant enzymes and glutathione contents, as well as the normal level of nitrite and myeloperoxidase production in FA-treated rats. Additionally, Ganoderma lucidum reversed a large decline in proinflammatory markers in formaldehyde. Furthermore, Ganoderma lucidum restores formaldehyde-induced histological alterations in the liver. Collectively, our results provide valuable information on the protective potential of Ganoderma lucidum in protecting formaldehyde-induced liver oxido-inflammation in male rats.
Keywords: Ganoderma lucidum, formaldehyde, liver, oxidative stress, inflammation
1. Introduction
Occupational and household formaldehyde is a common hydrophilic compound that is immediately retained through the lungs and, to a much lower extent, the skin. Health effects related to its exposure are pronounced when the body at sites like the eye, nose, skin, and throat has direct contact with the compound [1,2]. Researchers have deduced the relationship between the health effects and range of exposures, with some individuals becoming symptomatic at low levels of exposure. A few people may have gentle uneasiness while others have moderate or no inconvenience at comparative exposures. Mean level exposures are at their most elevated in the clinical dissection room or morgue [3]. Formaldehyde (FA) is a colorless, combustible and extremely reactive chemical at standard pressure and temperature [4]. It is broken down in the air and highly stable in liquid [5]. It rapidly diffuses in any tissues, e.g., the liver, through the oral or intraperitoneal route since it collaborates with various cell components [6]. Formalin was first used as a fixative and treating liquid; however, these days it is used in every field of daily life. The most appalling use of formalin is as food additive [7] and that’s why there is a drastic increase in human exposure to formalin intoxication. After intake, FA is readily absorbed from the gastrointestinal tract. In the liver, FA is largely metabolized to methanol and formate by aldehyde dehydrogenase 1 or mitochondrial aldehyde dehydrogenase 2, respectively. However in high concentrations of FA, toxicity arises in the hepatocytes [8].
Research conducted on FA exposure to animals shows hepatotoxicity and abnormal histological alterations in the gastrointestinal tract [9]. A low dose of FA has been shown to be mutagenic and carcinogenic and can manifest in a wide range of toxicities in different organs [10]. Gastrointestinal cancer can also be caused by drinking water containing a high concentration of FA [11]. In growing countries like India and Nigeria, the haphazard use of FA in lots of food items and drinking water has exposed a large percentage of citizens to a huge health hazards such as liver damage [12]. The liver is a large, composite organ that performs very important tasks in sugar, fat and protein digestion. It functions in the detoxification of metabolic wastes like ammonia. Together with the spleen, it is associated with the obliteration of the remnants of the erythrocyte and the re-use of its constituents. Bile synthesis and secretion are also present in the liver, lipoproteins and plasma proteins synthesis, as well as coagulating factors. It maintains a steady level of blood glucose via glycogenesis, glycogenolysis and gluconeogenesis. The liver also plays a significant role in the elimination and detoxification of drugs. Therefore, xenobiotics (for example, liquor and numerous drugs), malnutrition, infection, and anemia, can induce liver damage [13]. Hepatic damage is a common disease that mostly occurs as a result of oxidative stress and involves progressive growth from steatosis to hepatocellular carcinoma [14].
Over 2 millennia, most Chinese medicines have made use of fungi for the management of a range of diseases [15]. Traditional oriental therapies have also benefited greatly from medicinal mushrooms, and fungal metabolites are widely used in the treatment of diseases. [16]. Additionally, mushrooms should not only be considered as food, as research has shown that they contain a lot of biologically active compounds [17]. Mushrooms have numerous compounds with some biological significance. The extensive list incorporates polysaccharides, phenolics, proteins, polysaccharide–protein complexes, lipid components, and terpenoids, alkaloids, little peptides and amino acids, nucleotides and nucleosides [18]. This extensive list refers to an extraordinary combination of organic properties, including cancer prevention agents [19], antitumor [20], antimicrobial [17], immunomodulatory [21], anti-inflammatory [22], antiatherogenic [23] and hypoglycemic activities [24]. Gandoerma lucidum (Lingzhi, Reishi), which has been used for quite a long time in Asian nations to improve wellbeing and advance life span, is widely perceived as a means of avoiding and treating many diseases, including malignant growth [25]. As far back as 1986, the lethal dose (LD50) has been reported to be 5000 mg/kg [26]. In 2006, reports investigated the beneficial roles of G. lucidum. Although the vast majority of persuasive information depends on laboratory and preclinical investigations, G. lucidum has gained consideration in non-Asian nations [27].
Investigations with refined G. lucidum triterpenes have indicated in vivo results, which can be used for drug development. However, the full use of G. lucidum preparation in corresponding and alternative medications is progressively beneficial because the specific component of G. lucidum could have synergistic or added substance impacts and could influence molecular signaling pathways and targets, finally prompting the destruction of malignant cells. This study is therefore performed to examine the protective potential of G. lucidum in formaldehyde-induced liver damage in experimental rats.
2. Materials and Methods
2.1. Fungi Material and Extraction
The whole basidiomata of G. lucidum was obtained from a village in Edo state. Identification was conducted at the Department of Agricultural Sciences, Joseph Ayo Babalola University. G. lucidum was air dried away from the direct sun rays and samples were milled to a total of 413 g of boorish powder. In total, 200 g of the quantity of coarse powder was soaked in 1 L ethanol for 72 h, decanted and concentrated, thus yielding a dark brown extract. The extract was weighed and stored in the refrigerator.
2.2. Chemicals
Thiobarbituric acid, 1-chloro-2,4-dinitrobenzene (CDNB), 5′,5′-dithiobis-2-nitrobenzoic acid (DTNB), xynelol orange, reduced glutathione (GSH), epinephrine and hydrogen peroxide (H2O2) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals and reagents were obtained from Cloud-Clone Inc., Wuhan, China.
2.3. Animals Care
Forty sexually matured Wistar strain male rats with the weight range of 150–200 g, were acquired in the animal colony, University of Ibadan, Nigeria. They were housed in a polycarbonate cage in an Assessment and Accreditation of Laboratory Animal care-certified animal facility and adherence to the protocol of the National Institute of Health on the Guide for the Care and Use of Laboratory Animals. Prior to acclimatization for 2 weeks, rats were kept under 12:12-h light:dark cycle and provided with NIH-07 diet and water ad libitum.
2.4. Experimental Design
Forty sexually matured Wistar strain male rats were divided into four groups of ten rats each and treated for thirty days (2 weeks of acclimatization inclusive) as described thus:
Group 1: were orally treated with 1 mg/mL distilled water.
Group 2: were exposed to 40% FA vapor environment for 30 min daily (the exposure was done by soaking 50 mL of FA in cotton wool and placed in a corner within the animal cage, thus exposing the animal to the vapor for a period of 2 weeks (40% FA at room temperature) [28].
Group 3: were orally treated with 100 mg/kg ethanol extract of G. lucidum.
Group 4: were co-administered FA and 100 mg/kg ethanol extract of G. lucidum (1/50 of LD50). The route of administration of G. lucidum was oral and that of FA was the same as in Group 2.
Rats were then sacrificed 24 h after the last administration via cervical dislocation. Liver samples were excised, weighed, homogenized, and then processed for further experiments.
2.5. Determination of Liver Function Parameters
Blood samples were collected after sacrifice and plasma samples were obtained using the standard method. Liver function biomarkers, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), triglycerides, total bilirubin, direct bilirubin, albumin and cholesterol were assayed according to the manufacturer’s procedure (Randox Laboratories, Crumlin, UK).
2.6. Estimation of Antioxidant and Oxidative Stress Markers
The excised liver samples were homogenized accordingly using a 50 mM Tris–KCl buffer at pH 7.4 consisting of 1.15% KCl, then further centrifuged at 12,000× g for 15 min at 4 °C and afterward used for biochemical assays. Estimation of the protein concentration was done according to Bradford [29]. Claiborne [30] and Misra and Fridovich [31] methods were used to determine the activities of Catalase (CAT) and superoxide dismutase (SOD). Glutathione-S-transferase (GST) activity and the GSH level were determined according to Habig et al. [32] and Rotruck et al. [33]. Meanwhile, the level of lipid peroxidation (LPO) was determined according to Jollow et al. [34]. H2O2 generation was determined according to the standard method of Wolff [35]. All biochemical experiments were analyzed using a SpectraMax plate reader (Molecular Device, San Jose, CA, USA).
2.7. Assessment of Inflammatory Biomarkers
Myeloperoxidase (MPO) activity was determined according to the method described by Granell et al. [36], whereas the nitrite level concentration was assessed using an established protocol [37].
2.8. Determination of Proinflammatory Cytokines
Tumor Necrosis Factor (TNF-α), IL-1β and IL-6 concentrations in liver homogenates samples were assayed using rat TNF-α, IL-1β and IL-6 Elisa kits, respectively (Cloud-Clone Inc., Wuhan, China). A microplate antibody-coated plate was provided with the kit. All reagents, samples and working standards were prepared using standard procedures as provided by the kit manufacturers.
2.9. Histological Examination
Liver samples of rats that were removed were fixed with Bouin’s solution which was subsequently dehydrated in graded concentrations of alcohol. This was further cleared three times using xylene solution and was later embedded in paraffin wax. Microtome was then used to cut 4–5 mm of the paraffin waxed tissue on a slide and it was stained with haematoxylin (H) and eosin (E). The slides were then further viewed using a light microscope (Olympus CH; Olympus, Tokyo, Japan) and were snapped by pathologists.
2.10. Ethical Approval
All procedures involving animals performed in the study were performed in accordance with the ethical standards of our institution.
2.11. Statistical Analyses
Data were evaluated as mean ± SEM. Levels of statistical significance were analyzed with a one-way analysis of variance (ANOVA) which was further subjected to Bonferroni’s post hoc test using GraphPad Prism 6 software. p < 0.05 was considered significant.
3. Results
3.1. G. lucidum Suppressed FA-Induced Reduction in Body and Organ Weight
Table 1 represents the body weight gain and relative organ weight of control, G. lucidum and FA-treated rats. The result shows a significant reduction in the body and organ weight of the liver of rats administered formaldehyde as compared to the control. Additionally, there was a statistically significant increase in the body and organ weight gain of rats exposed to FA and G. lucidum when compared to the control.
Table 1.
Effect of Ganoderma lucidum and formaldehyde on average body weight and relative organ weight in rat.
| Control (g) | Formaldehyde (g) | Ganoderma lucidum | Ganoderma lucidum + Formaldehyde (g) | |
|---|---|---|---|---|
| Average Body weight | 42.35 ± 4.52 | 18.75 ± 5.45 a | 36.74 ± 6.32 | 25.45 ± 4.46 |
| Relative organ weight | 7.32 ± 0.47 | 4.76 ± 0.78 a | 8.76 ± 0.95 | 6.75 ± 0.52 a |
a—significantly different from control.
3.2. G. lucidum Inhibits FA-Induced Alteration in Hepatic Function Enzymes
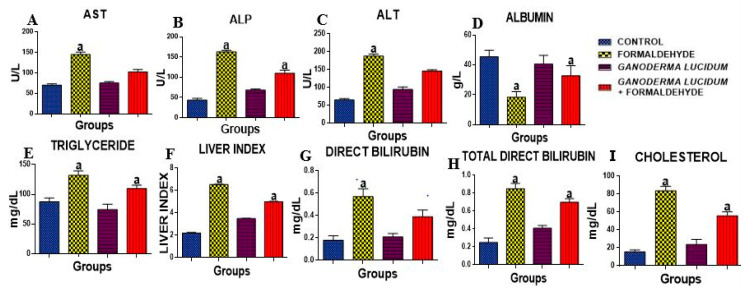
The activities of AST, ALT and ALP were significantly (p < 0.05) increased in the liver of rats administered FA as compared to the control. However, G. lucidum significantly reduced these levels when compared to the FA alone and control groups (Figure 1A–C). Additionally, the concentration of total cholesterol, direct bilirubin and total direct bilirubin was significantly increased in the liver of rats administered FA as compared to the control (Figure 1G–I). However, G. lucidum significantly reduced these levels when compared to the FA alone and control groups. The concentration of ALB was significantly (p < 0.05) decreased when compared to the control, but G. lucidum reversed this effect. The liver index shows an increase in rats administered FA as compared to the control. However, G. lucidum reversed this effect with an increase in the rats co-administered G. lucidum and FA (Figure 1F).
Figure 1.
Effect of formaldehyde and Ganoderma lucidum on hepatic enzyme markers. Data are presented as mean ± SD, n = 7; a: p < 0.05 vs. control; (A) AST (B) ALP (C) ALT (D) ALB (E) TRIG (F) LIVER INDEX (G) DIRECT BIL (H) TOTAL BIL (I) CHOL. AST: Aspartate aminotransferase, ALP: Alkaline Phosphatase, ALT: Alanine aminotransferase.
3.3. G. lucidum Attenuate FA-Induced Oxidative Damage in Rat Liver
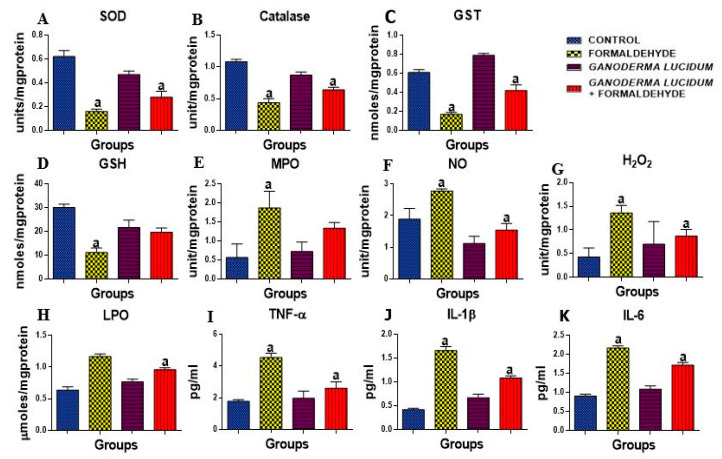
The antioxidant enzymes activities, SOD, CAT, GST and GSH level (Figure 2A–D), and also oxidative stress indices, H2O2 and MDA, were tested as presented in Figure 2G–H. There was a significant decrease in the liver activities of SOD, CAT, GST and the level of GSH in rats administered FA alone as compared to the control rats. Conversely, the co-administration of G. lucidum restored the level and activities of these enzymes. Furthermore, there was a significant increase in the levels of oxidative stress markers (H2O2 and MDA) in the liver of rats administered FA. However, the rats co-administered with G. lucidum revealed a significant decrease in the levels of H2O2 and MDA in the testes of treated rats as compared to FA alone as shown in Figure 2A,B.
Figure 2.
Effect of formaldehyde and Ganoderma lucidum on oxido-inflammatory markers. Data are presented as mean ± SD, n = 7; a: p < 0.05 vs. control, (A) SOD: superoxide dismutase, (B) CAT: catalase (C) GST: Glutathione-s-transferase (D) GSH: reduced glutathione, (E) MPO: myeloperoxidase, (F) NO: Nitric oxide (G) H2O2: Hydrogen peroxide (H) LPO: lipid peroxidation (I) TNFα: Tumor necrosis factor α. (J) IL-1β: Interleukin 1β, (K) IL-6: Interleukin 6.
3.4. G. lucidum Ameliorate FA-Induced Inflammation in Rat Liver
Nitrite level and the activity of MPO were determined in the liver of rats as shown in Figure 2E,F. An administration of FA alone resulted in a significant elevation of nitrite level and activity of MPO as compared to the control rats. On the contrary, rats co-administered with G. lucidum had a significantly decreased nitrite level and MPO activity compared to the livers of FA-administered rats. However, the administration of G. lucidum alone did not have an effect on nitrite level and the activity of MPO. Additionally, proinflammatory cytokines (TNF-α, MPO and IL-1β) were significantly (p < 0.05) increased in FA-administered rats when compared to the control as shown in Figure 2I–K; however, G. lucidum reversed this effect with a significant (p < 0.05) decrease in the proinflammatory cytokines as compared to the control.
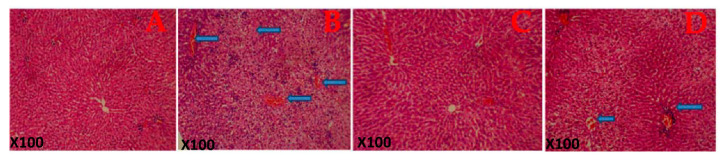
3.5. Histopathological Observations
Figure 3 shows the histological structure of the representative photomicrograph of the liver. The control rats and the G. lucidum-treated rats show a normal architecture. However, rats treated with FA alone show a diffuse periportal cellular infiltration with severe congestion indicating hepatic damage. Additionally, histological structures of the liver of rats co-administered with formaldehyde and G. lucidum at 100 mg/kg showed relatively normal features.
Figure 3.
Histological architecture of the liver of the rat in each group. (A) Control rat shows the normal architecture of the liver. (B) Rats administered Formaldehyde (FA) alone shows a diffuse periportal cellular infiltration with severe congestion indicating hepatic damage. (C) Rats administered Ganoderma lucidum show a normal architecture of the liver while (D) rats co-administered with formaldehyde and Ganoderma lucidum shows mild congestion. Magnification: X100.
4. Discussion
The use of natural products in the prevention and management of various illnesses has prominently increased in the last few years [38]. The present study established the promising chemopreventive potential of G. lucidum in the liver, preventing liver damage caused by FA exposure. The reestablishment of unhealthy liver functions was evident by the remarkable loss of body weight, and a significant reduction in the liver organ weight which can result from shrinkage in the liver as seen in the FA-administered group (Table 1), but this was prevented in the rats treated with G. lucidum. The hepatoprotective potential of G. lucidum against FA was investigated by determining ALT, AST and ALP. ALT is the important liver damage enzyme that catalyzes transamination reactions. The occurrence of conditions that can cause liver damage such as cancer, injury and hepatitis, will result in higher levels of this enzyme [14]. AST and ALP, the biomarkers of liver damage, are cytosolic and mitochondrial enzymes whose levels are usually increased in cases of chronic illness and necrosis due to loss of hepatocellular integrity. These enzymes are involved in the transfer of α-amino groups from alanine and aspartate to the α-keto group of ketoglutarate to form pyruvate and oxaloacetate, respectively [39]. As shown in figures, there is a significant increase (p < 0.05) in the levels of these enzymes in the group administered FA when compared to the control. However, treatment with 100 mg/kg G. lucidum significantly reduced the elevated levels, showing that G. lucidum exhibits a protective role against FA-induced liver damage in rats. This study is related to that of Lakshmi et al. [40] that showed the effects of Ganoderma lucidium on hepatic damage induced by benzo(a) pyrene. The elevated liver function enzymes were significantly reduced by Gandoerma lucidum administration.
Albumin is a measure of the synthetic function of the liver. A significant decrease in the albumin level in the FA-administered group could be traced to the reduction in protein synthesis that is an effect of FA. The carbonyl atom of FA reacts with the amino groups (nucleophilic sites) on the cell membranes forming hydroxymethyl amino acid derivatives [41]. However, treatment with 100 mg/kg G. Lucidum significantly increases the level of albumin. Cholesterol oxidation causes enzymatic increases in bile acids and contributes to hepatic cholesterol accumulation and hepatocellular injury. This is further explained by its significant increase in the FA-administered group as compared to the control. However, G. Lucidum treatment reduced the elevated cholesterol levels significantly when compared to the control. Total direct bilirubin is also an indicator of the destruction of erythrocytes and the proper functioning of the liver, gallbladder and bile ducts, and is a potential marker for liver damage. Triglycerides were also increased in the FA-administered group as compared to the control. However, G. Lucidum reduced the elevated cholesterol levels significantly when compared to the control. The liver index, the indicator of hepatic manifestation of metabolic disorders, was upregulated in the group administered FA, thus showing an impairment of the liver. However, upon administration of 100 mg/kg G. lucidum to the group induced with 40% FA, there was a significant downregulation in the increased liver index.
When the body metabolism is impaired, an increase in the production of toxic molecules such as free radicals and antioxidants, known as free radical scavengers, are needed to reduce or neutralize the free radical formation [42]. Our results show that FA has a direct effect on the hepatocytes and also an indirect effect through the circulatory and immune systems [43]. The hepatic destruction caused by FA causes oxidative stress and produces reactive oxygen species (ROS), as shown in the significant increase in H2O2 and LPO, which are known to be oxidative stress markers, and also a decrease in GSH, GST, catalase and SOD, which are antioxidant markers. These observations are accordance to Payani et al. [44] who reported that FA exposure significantly reduced the levels of enzymatic and non-enzymatic antioxidants. However, G. lucidum significantly increases the activities and levels of these antioxidant markers. These results indicated that animals treated with G. Lucidum cause a significant increase in the levels of antioxidant enzymes. These results are in line with the reports of other researchers that allude to the fact that Gandoderma lucidium has antioxidant activities both in vivo and in vitro [45,46,47]. These results indicated the hepatoprotective efficacy of G. Lucidum. Myeloperoxidase is one of the most important molecules released after the recruitment and activation of phagocytes and it is involved in the production of oxidative stress. Additionally, proinflammatory cytokines activate iNOS during liver injury to abnormally producing NO that contributes immensely to the pathogenesis and evolution of liver damage. The present study shows a distinct increase in the activity and level of MPO and NO in FA-administered rats’ livers as compared to the control. However, the reduced level of MPO and NO following G. lucidum treatment shows its potential to prevent inflammation in the liver of rats [48,49].
TNF, IL-1β and IL-6 play a major role in the pathogenesis of liver damage. TNFs are majorly a group of proinflammatory cytokines known to perform a crucial role in the instigation of liver damage with evidence that oxidative stress might act in conjunction with endotoxins to augment TNF production [50]. Interleukin 1β and 6 are potential biomarkers of acute or chronic liver toxicity. TNF, IL-1β and IL-6 are proinflammatory cytokines that are released into the bloodstream from the liver during hepatic toxic injury. Thus, biological agents suppressing these cytokines are known to have demonstrated huge therapeutic potential. As shown in our results, there was a significant upregulation in the levels of these cytokines in rat livers when administered FA. The significant downregulation of the levels of the cytokines was demonstrated in the group treated with 100 mg/kg G. Lucidum. This further indicates the hepatoprotective efficacy of G. Lucidum. The above result corroborates with the histopathological finding as shown in Figure 3 as rats administered FA show a diffuse periportal cellular infiltration with severe congestion indicating hepatic damage; however, G. lucidum was able to reverse this effect [51].
5. Conclusions
The results from this study demonstrated that exposure to FA led to a significant decline in antioxidant markers [52], with a concomitant increase in liver transaminases, lipid profile as well as inflammatory markers [53]. Additionally, G. lucidum possesses protective roles as it has the ability to restore antioxidant, lipid profile and anti-inflammatory statuses. Hence, G. lucidum may be a probable drug candidate to target liver damage [54,55].
Acknowledgments
The authors extend their appreciation to the researchers supporting project number (RSP-2020/201), King Saud University, Riyadh, Saudi Arabia.
Author Contributions
B.O.A., conceptualization, methodology, resources investigation writing original draft, review and editing; T.O.O., investigation, resources, writing original draft; O.A.A. (Oluwatosin Adefunke Adetuyi), methodology investigation, writing original draft; O.A.A. (Oluwaseun Abraham Adebisi), data curation, resources, methodology, writing original draft, review and editing; O.O.O., resources, conceptualization, supervision, writing original draft, review and editing; O.J.O., N.M., G.E.-S.B., A.M.B., and N.N.W., resources, writing original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Formalin Exposure: A Review of Known Health Hazards and the Role of Innovation in Improving Safety. Merit Medical Systems, Inc.; South Jordan, UT, USA: 2019. [Google Scholar]
- 2.Repetto R., Baliga S.S. Pesticides and the immune system: The public health risks. Executive summary. Cent. Eur. J. Public Health. 1996;4:263–265. doi: 10.1016/0261-2194(96)89826-7. [DOI] [PubMed] [Google Scholar]
- 3.Ellenhorn M., Schonwald G., Ordog J. Diagnosis and Treatment of Human Poisoning. Williams and Wikins; Los Angeles, CA, USA: 1997. [Google Scholar]
- 4.ATSDR . ATSDR’s Toxicological Profiles. Agency for Toxic Substances and Diseases; Atlanta, GA, USA: 2002. Toxicological Profile for Formaldehyde. [Google Scholar]
- 5.WHO . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 93 IARC; Lyon, France: 2010. [Google Scholar]
- 6.Cheng G., Shi Y., Sturla S.J., Jalas J.R., McIntee E.J., Villalta P.W., Wang M., Hecht S.S. Reactions of formaldehyde plus acetaldehyde with deoxyguanosine and DNA: Formation of cyclic deoxyguanosine adducts and formaldehyde cross-links. Chem. Res. Toxicol. 2003;16:145–152. doi: 10.1021/tx025614r. [DOI] [PubMed] [Google Scholar]
- 7.Restani P., Restelli A.R., Galli C.L. Formaldehyde and hexamethylenetetramine as food additives: Chemical interactions and toxicology. Food Addit. Contam. 1992;9:597–605. doi: 10.1080/02652039209374113. [DOI] [PubMed] [Google Scholar]
- 8.Teng S., Beard K., Pourahmad J., Moridani M., Easson E., Poon R., Brien P.J.O. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem. Biol. Interact. 2001;132:285–296. doi: 10.1016/S0009-2797(00)00272-6. [DOI] [PubMed] [Google Scholar]
- 9.Rumchev K.B., Spickett J.T., Bulsara M.K., Phillips M.R., Stick S.M. Domestic exposure to formaldehyde significantly increases the risk of asthma in young children. Eur. Respir. J. 2002;20:403–408. doi: 10.1183/09031936.02.00245002. [DOI] [PubMed] [Google Scholar]
- 10.Nouh W.G., Selim A.G. Toxopathological Studies on the Effect of Formalin and Copper Sulphate in Tilapia as A Commonly Used Disinfectant in Aquaculture. J. Appl. Environ. Biol. Sci. 2013;3:7–20. [Google Scholar]
- 11.Takahashi M., Hasegawa R., Furukawa F., Toyoda K., Sato H., Hayashi Y. Effects of ethanol, potassium metabisulfite, formaldehyde and hydrogen peroxide on gastric carcinogenesis in rats after initiation with n-methyl-n′-nitro-n-nitrosoguanidine. Jpn. J. Cancer Res. GANN. 1986;77:118–124. doi: 10.20772/cancersci1985.77.2_118. [DOI] [PubMed] [Google Scholar]
- 12.Franklin P., Dingle P., Stick S. Raised exhaled nitric oxide in healthy children is associated with domestic formaldehyde levels. Am. J. Respir. Crit. Care Med. 2000;161:1757–1759. doi: 10.1164/ajrccm.161.5.9905061. [DOI] [PubMed] [Google Scholar]
- 13.Gowri Shankar N.L., Manavalan R., Venkappayya D., David Raj C. Hepatoprotective and antioxidant effects of Commiphora berryi (Arn) Engl bark extract against CCl4-induced oxidative damage in rats. Food Chem. Toxicol. 2008;46:3182–3185. doi: 10.1016/j.fct.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Kodavanti P.R.S., Joshi U.M., Young R.A., Meydrech E.F., Mehendale H.M. Protection of hepatotoxic and lethal effects of CCl4 by partial hepatectomy. Toxicol. Pathol. 1989;17:494–505. doi: 10.1177/019262338901700304. [DOI] [PubMed] [Google Scholar]
- 15.Ling-Sing Seow S., Naidu M., David P., Wong K.H., Sabaratnam V. Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells. BMC Complement. Altern. Med. 2013;13:157. doi: 10.1186/1472-6882-13-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindequist U., Niedermeyer T.H.J., Jülich W.D. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005;2:285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barros L., Baptista P., Estevinho L.M., Ferreira I.C.F.R. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. J. Agric. Food Chem. 2007;55:8766–8771. doi: 10.1021/jf071435+. [DOI] [PubMed] [Google Scholar]
- 18.Thu Z.M., Ko Myo K., Aung H.T., Clericuzio M., Armijos C., Vidari G. Bioactive phytochemical constituents of wild edible mushrooms from Southeast Asia. Molecules. 2020;25:1972. doi: 10.3390/molecules25081972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puttaraju N.G., Venkateshaiah S.U., Dharmesh S.M., Urs S.M.N., Somasundaram R. Antioxidant activity of indigenous edible mushrooms. J. Agric. Food Chem. 2006;54:9764–9772. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- 20.Moradali M.F., Mostafavi H., Ghods S., Hedjaroude G.A. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi) Int. Immunopharmacol. 2007;7:701–724. doi: 10.1016/j.intimp.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Borchers A.T., Keen C.L., Gershwini M.E. Mushrooms, Tumors, and Immunity: An Update. Exp. Biol. Med. 2004;229:393–406. doi: 10.1177/153537020422900507. [DOI] [PubMed] [Google Scholar]
- 22.Moro C., Palacios I., Lozano M., D’Arrigo M., Guillamón E., Villares A., Martínez J.A., García-Lafuente A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012;130:350–355. doi: 10.1016/j.foodchem.2011.07.049. [DOI] [Google Scholar]
- 23.Mori K., Kobayashi C., Tomita T., Inatomi S., Ikeda M. Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E-deficient mice. Nutr. Res. 2008;28:335–342. doi: 10.1016/j.nutres.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Hu S.H., Wang J.C., Lien J.L., Liaw E.T., Lee M.Y. Antihyperglycemic effect of polysaccharide from fermented broth of Pleurotus citrinopileatus. Appl. Microbiol. Biotechnol. 2006;70:107–113. doi: 10.1007/s00253-005-0043-5. [DOI] [PubMed] [Google Scholar]
- 25.Sliva D. Ganoderma lucidum (Reishi) in Cancer Treatment. Integr. Cancer Ther. 2003;2:358–364. doi: 10.1177/1534735403259066. [DOI] [PubMed] [Google Scholar]
- 26.Kim M.J., Kim H.W., Lee Y.S., Shim M.J., Choi E.C., Kim B. kak Studies on Safety of Ganoderma lucidum. Korean J. Mycol. 1986;14:49–59. [Google Scholar]
- 27.Paterson R.R.M. Ganoderma—A therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Njoya H.K., Ofusori D.A., Nwangwu S.C., Amegor O.F., Akinyeye A.J., Abayomi T.A. Histopathological effect of exposure of formaldehyde vapour on the trachea and lung of adult wistar rats. Int. J. Integr. Biol. 2009;7:160–165. [Google Scholar]
- 29.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Claiborne A. Catalase activity. In: Greenwald R.A., editor. Hand Book of Methods for Oxygen Radical Research. CRC Press; Boca Raton, FL, USA: 1985. [Google Scholar]
- 31.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 32.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 33.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: Biochemical role as a component of glatathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 34.Jollow D., Mitchell J.R., Zampaglione N., Gillette J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 35.Wolff S.P. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994 doi: 10.1016/S0076-6879(94)33021-2. [DOI] [Google Scholar]
- 36.Granell S., Gironella M., Bulbena O., Panés J., Mauri M., Sabater L., Aparisi L., Gelpi E., Closa D. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit. Care Med. 2003;31:525–530. doi: 10.1097/01.CCM.0000049948.64660.06. [DOI] [PubMed] [Google Scholar]
- 37.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 38.Oyebode O., Kandala N.B., Chilton P.J., Lilford R.J. Use of traditional medicine in middle-income countries: A WHO-SAGE study. Health Policy Plan. 2016;31:984–991. doi: 10.1093/heapol/czw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiff N.D., Giacino J.T., Kalmar K., Victor J.D., Baker K., Gerber M., Fritz B., Eisenberg B., O’Connor J., Kobylarz E.J., et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 40.Lakshmi B., Ajith T.A., Jose N., Janardhanan K.K. Antimutagenic activity of methanolic extract of Ganoderma lucidum and its effect on hepatic damage caused by benzo[a]pyrene. J. Ethnopharmacol. 2006;107:297–303. doi: 10.1016/j.jep.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Sabbioni G., Turesky R.J. Biomonitoring human albumin adducts: The past, the present, and the future. Chem. Res. Toxicol. 2017;30:332–366. doi: 10.1021/acs.chemrestox.6b00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z., Li Z., Ye Y., Xie L., Li W. Oxidative stress and liver cancer: Etiology and therapeutic targets. Oxid. Med. Cell. Longev. 2016;2016:7891574. doi: 10.1155/2016/7891574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beall C.M., Reichsman A.B. Hemoglobin levels in a Himalayan high altitude population. Am. J. Phys. Anthropol. 1984;63:301–306. doi: 10.1002/ajpa.1330630306. [DOI] [PubMed] [Google Scholar]
- 44.Payani S., Mamatha C., Chandraprakash C., Bhaskar M. Protective role of (Bronco-T) against formaldehyde induced antioxidant, oxidative and histopathological changes in lung of male Wistar rats. Toxicol. Rep. 2019;6:718–726. doi: 10.1016/j.toxrep.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao W., Jiang X., Deng W., Lai Y., Wu M., Zhang Z. Antioxidant activities of Ganoderma lucidum polysaccharides and their role on DNA damage in mice induced by cobalt-60 gamma-irradiation. Food Chem. Toxicol. 2012;50:303–309. doi: 10.1016/j.fct.2011.10.071. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh T.C., Wu J.M. Suppression of proliferation and oxidative stress by extracts of Ganoderma lucidum in the ovarian cancer cell line OVCAR-3. Int. J. Mol. Med. 2011;28:1065–1069. doi: 10.3892/ijmm.2011.788. [DOI] [PubMed] [Google Scholar]
- 47.Sohretoglu D., Huang S. Ganoderma lucidum Polysaccharides as An Anti-cancer Agent. Anticancer Agents Med. Chem. 2018;18:667–674. doi: 10.2174/1871520617666171113121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shalapour S., Karin M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joseph S., Sabulal B., George V., Antony K.R., Janardhanan K.K. Antitumor and anti-inflammatory activities of polysaccharides isolated from Ganoderma lucidum. Acta Pharm. 2011;61:335–342. doi: 10.2478/v10007-011-0030-6. [DOI] [PubMed] [Google Scholar]
- 50.Feagins A.R., Opriessnig T., Guenette D.K., Halbur P.G., Meng X.J. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Int. J. Food Microbiol. 2008;123:32–37. doi: 10.1016/j.ijfoodmicro.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Batiha G.-S., Alkazmi L.M., Wasef L.G., Beshbishy A.M., Nadwa E.H., Rashwan E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules. 2020;10:202. doi: 10.3390/biom10020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batiha G.-S., Beshbishy A.M., Wasef L., Elewa Y.H.A., El-Hack M.E.A., Taha A.E., Al-Sagheer A.A., Devkota H.P., Tufarelli V. Uncaria tomentosa (Willd. ex Schult.) DC.: A Review on Chemical Constituents and Biological Activities. Appl. Sci. 2020;10:2668. doi: 10.3390/app10082668. [DOI] [Google Scholar]
- 53.Ikram M., Beshbishy A.M., Kifayatullah M., Olukanni A., Zahoor M., Naeem M., Amin M., Shah M., Abdelaziz A.S., Ullah R., et al. Chemotherapeutic Potential of Carthamus oxycantha Root Extract as Antidiarrheal and In Vitro Antibacterial Activities. Antibiotics. 2020;9:226. doi: 10.3390/antibiotics9050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Rahman G.I.A., Behairy A., Elseddawy N.M., Batiha G.-S., Hozzein W.N., Khodeer D.M., Abd-Elhakim Y.M. Saussurea lappa Ethanolic Extract Attenuates Triamcinolone Acetonide-Induced Pulmonary and Splenic Tissue Damage in Rats via Modulation of Oxidative Stress, Inflammation, and Apoptosis. Antioxidants. 2020;9:396. doi: 10.3390/antiox9050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batiha G.-S., Olatunde A., El-Mleeh A., Hetta H.F., Al-Rejaie S., Alghamdi S., Zahoor M., Magdy Beshbishy A., Murata T., Zaragoza-Bastida A., et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium) Antibiotics. 2020;9:353. doi: 10.3390/antibiotics9060353. [DOI] [PMC free article] [PubMed] [Google Scholar]