Abstract
Background
Although cardiac mitochondrial dysfunction is associated with heart failure (HF), this is a complex syndrome with two predominant etiologies, ischemic HF (iHF) and non-ischemic HF (niHF), and the exact impact of mitochondrial dysfunction in these two distinct forms of HF is unknown.
Methods and Results
To determine the impact of HF etiology on mitochondrial function, respiration was measured in permeabilized cardiac muscle fibers from patients with iHF (n=17), niHF (n=18), and healthy donor hearts (HdH). Oxidative phosphorylation capacity (OXPHOS), assessed as state 3 respiration, fell progressively from HdH to niHF, to iHF (Complex I+II: 54±1; 34±4; 27±3 pmol·s−1·mg−1) as did citrate synthase activity (CSA: 206±18; 129±6; 82±6 nmol·mg−1·min−1). Although still significantly lower than HdH, normalization of OXPHOS by CSA negated the difference in mass specific OXPHOS between iHF and niHF. Interestingly, Complex I state 2 respiration increased progressively from HdH, to niHF, to iHF, whether or not normalized for CSA (0.6±0.2; 1.1±0.3; 2.3±0.3; pmol·mg−1 ·CSA), such that the respiratory control ratio (RCR), fell in the same manner across groups. Finally, both the total free radical levels (60±6; 46±4 AU) and level of mitochondrial derived superoxide (1.0±0.2; 0.7±0.1 AU) were greater in iHF compared to niHF, respectively.
Conclusions
Thus, the HF-related attenuation in OXPHOS actually appears to be independent of etiology when the lower mitochondrial content of iHF is taken into account. However, these findings provide evidence of deleterious intrinsic mitochondrial changes in iHF, compared to niHF, including greater proton leak, attenuated OXPHOS efficiency, and augmented free radical levels.
Keywords: ischemic heart failure, non-ischemic heart failure, mitochondrial function, free radicals
INTRODUCTION
The heart is a vital organ with a high metabolic demand and is subsequently rich in mitochondria, with these energy producing organelles accounting for approximately 35% of cardiac tissue volume (1) and generating up to 90% of the heart’s requirement for ATP (2–4). Due to an impaired energetic state, as evidenced by a reduction in the phosphocreatine to ATP ratio, the heart in heart failure (HF) has been described as an ‘engine without fuel’ and mitochondrial dysfunction has been implicated as an important component of this disease (5,6). Such an attenuation in cardiac myocyte energy production may be the result of an alteration in the ratio of coupled, ATP producing, to uncoupled respiration, not ATP producing (7), potentially influencing the contractile capacity of the heart by creating a mismatch between energy supply and demand (1). However, previous studies focused upon HF and mitochondrial function have reported inconsistent results in terms of HF-related cellular adaptations including cell structure, mitochondrial substrate metabolism, and mitochondrial enzymes (8–10). These varied findings may, at least in part, be due to the differing impact of the multiple common comorbidities associated with HF such as insulin resistance, diabetes, and the subsequently elevated lipid accumulation in cardiac tissue(11,12)
It is apparent that the exact impact of cardiomyopathy on mitochondrial function is not well characterized and may actually be further complicated by the fact that HF is a complex syndrome with two predominant etiologies, iHF and niHF. The first and most common, iHF, is coronary artery disease (CAD)-dependent, while niHF is not related to CAD and includes dilated cardiomyopathy, hypertrophic cardiomyopathy, and restrictive cardiomyopathy. Interestingly, with such fundamentally different etiologies it is still unclear if iHF and niHF differ in terms of cardiomyopathy-related cellular and non-cellular metabolic adaptations (1,13) and mitochondrial free radical production (13,14). Indeed, although several studies have speculated that CAD-induced chronic ischemia may have quite a different effect on cardiac mitochondrial function than non-ischemic tissue in the same heart (1,12), the actual impact of HF etiology on mitochondrial function remains unknown. Interestingly, although the initial insult, the etiology, is different between iHF and niHF, the pathology is very similar, especially, amongst patients with end stage HF.
These unresolved issues regarding the impact of HF etiology are likely, at least in part, due to the limited opportunities to obtain human cardiac muscle, a failure to differentiate between HF etiologies, and may be further blurred by common co-morbidities. Therefore, using normal HdH as a reference and without the inclusion of patients with Diabetes, to minimize the effect of this co-morbidity, this study sought to examine the impact of HF etiology on mitochondrial function. Specifically, a comparison of mitochondrial function in cardiac muscle from patients with iHF and niHF was performed to test the following hypotheses: first, mitochondria in cardiac muscle from patients with HF would exhibit significantly attenuated OXPHOS than the HdH. Second, although, in mass specific terms, cardiac muscle from the iHF would exhibit lower OXPHOS than from patients with niHF, this difference would be negated by normalizing for mitochondrial content. However, finally, it was additionally hypothesized that accompanying this reduced mitochondrial content in iHF would be characterized by greater uncoupled respiration, leading to attenuated OXPHOS efficiency and greater free radicals in comparison to patients with niHF. Recognizing that large-scale clinical trials have reported that iHF and niHF respond differently to interventional drug therapies (11,15), the assessment of potential differences in mitochondrial function due to etiology is important in terms of accurately targeting specific patients with the appropriate treatment including pharmacological options14, 15
METHODS
Subjects
Cardiac tissue was collected from 35 patients with HF and 4 healthy donors. Data from these HdH have been previously published (16). HF patients were classified as iHF (n=17, evidence of CAD) and niHF (n=18, no evidence of CAD; exclusion criteria: hypertrophic cardiomyopathy, and acute heart failure). Cardiac tissue from patients with iHF and niHF was harvested from the apex of the left ventricle during either heart transplantation or left ventricular assist device (LVAD) implantation surgeries. Only intact cardiac tissue, free from scaring and fibrosis, was utilized in this study. All patients with HF were free from diabetes and there was no evidence of insulin resistance. The tissue from the HdH, not allocated for heart transplantation due to non-cardiac issues (e.g. heart size, incarceration, etc.) was also harvested from the apex of left ventricle during the procedures for the transplantation of other organs. Subjects or their legal representative (HdH) provided informed consent, and the study protocols were approved by the University of Utah and the Veteran’s Affairs Medical Center Institutional Review Boards. All protocols were carried out in accordance with the Declaration of Helsinki.
Muscle fiber permeabilizing and mitochondrial respiration assessments
Following cardiac tissue collection, fat, fibrosis, scar and connective tissue was removed from the cardiac muscle in pre cooled buffer A (in mM: 2.77 CaK2EGTA, 7.23 K2EGTA, 6.56 MgCl2, 0.5 dithiothreitol (DTT), 50 K-MES, 20 imidazol, 20 taurine, 5.77 Na2ATP, 15 phosphocreatine, pH 7.1 at 4°C) and the sample remained in this solution until permeabilization (16).
All muscle samples were stored in precooled buffer A for no more than 30 min prior to the commencement of the permeabilizing procedures (17). Permeablizing procedures have been previously reported by our group (16). Briefly, muscle fibers were teased apart to increase permeability of the membrane and avoid limited diffusion of the substrates. After mild shaking for 30 min in buffer A with saponin (5 mg/ml), the muscle was rinsed twice in buffer B (in mM: 0.5 EGTA, 3 MgCl2.6H2O, 20 tautine,10 KH2PO4, 20 HEPES, 1 g BSA,60 potassium-lactobionate, 110 mannitol, 0.3 dithiothreitol, pH 7.1 ) for 10 min (16).
Mitochondrial respiratory oxygen (O2) flux (JO2) was assessed by a Clark type, high resolution, respirometer (Oxygraph, Hansatech, Kings Lynn, UK). The permeabilized muscle fibers (2–4 mg wet wt) were incubated in the respirometer with 2 ml of buffer B while being continuously stirred at 37°C. First, baseline muscle respiration was recorded, in the absence of respiratory substrates. To assess the function of each mitochondrial complex, O2 consumption was assessed using a series of respiratory substrates and inhibitors in the following order and final concentrations in the chamber: glutamate-malate (2:10 mM), ADP (5 mM), succinate (10 mM), cytochrome c (10 μM), rotenone (0.5 μM), antimycin-A (2.5 μM), oligomycin (2 μg/ml), N,N,N,N-tetramethyl- p-phenylenediamine (TMPD)-ascorbate (2:0.5 mM) (16). This allowed the determination of 1) Complex I state 2 respiration, the non-phosphorylating resting state that provides an index of proton leak, assessed in the presence of malate + glutamate, 2) Complex I, state 3 respiration, the ADP-activated state of oxidative phosphorylation, assessed in the presence of glutamate + malate + ADP, 3) Complex I+II, state 3 respiration, assessed in the presence of glutamate + malate + ADP + succinate 4) Complex II, state 3 respiration, assessed in the presence of glutamate + malate + ADP + succinate + rotenone 5) Complex IV respiration, assessed by the blocking of Complex 3 (antimycin A) and Complex V (oligomycin) followed by the addition of TMPD + ascorbate (16).
In each condition respiration rate was recorded for 3–4 min and the average of the last 30 seconds was used for data analysis. Mitochondrial membrane integrity was evaluated by cytochrome c induction. The rate of O2 consumption was measured in pmol of O2 per second and then expressed relative to muscle sample mass (pmol·sec·mg of wet weight). These respiration rates were further normalized by CS activity. The respiratory control ratio (RCR) was calculated as Complex I+II state 3/state 2 respiration. The substrate control ratio (SCR), an index of substrate utilization capacity at Complex II, was calculated as Complex I+II state 3/complex I state 3 (16).
Biochemical and Histochemical Analyses
Citrate synthase activity (CSA): CSA, an indicator of mitochondrial content (18), was measured as previously described (18,19). Briefly, the frozen cardiac muscle that had been used for mitochondrial respiration measurements, was homogenized in extraction buffer (50 mM triethanolamine and 1 mM EDTA) using a bead homogenizer (BioSystems, Hamburg, Germany). CSA was assessed by spectrophotometry at 412 nm of the absorbance of light while incubated with 200 μl of reaction buffer (2 μm acetyl-Coa, 200 μm 5,5’-dithiobis- (2-nitrobenzoic acid) (DTNB), 350 μM oxaloacetic acid, and 0.1% Triton-X) (16).
Mitochondrial DNA (mtDNA)
The frozen cardiac muscle was homogenized and the DNA dissolved in 100 μl of Tris-EDTA. Five μl of a 50 times DNA dilution was used for PCR amplification with QuantiTect SYBR Green PCR Master Mix (Qiagen, Hilden, Germany) containing 0.5 μM of each primer in a total volume of 25 μl. Levels of mtDNA and genomic DNA (gDNA), as determined by albumin content, was assessed by real-time PCR using a Biorad IQ real time PCR machine (Stratagene, La Jolla, CA, USA), as previously described (20). The copy number of mtDNA and gDNA was used as an estimate of mitochondrial concentration in cardiac muscle (21).
Free radical measurements in cardiac muscle from iHF and niHF
Total free radical levels in the cardiac muscle from both the iHF and niHF patients was assessed by 2’–7’-dichlorofluorescein-diacetate (DCFDA) fluorescence. Tissue was homogenized in 0.05% Trypsin-EDTA (Invitrogen, Carlsbad, CA) using a motor-driven tissue homogenizer (Qiagen Inc., Valencia, CA) and incubated for 30 min at 37 °C. Following centrifugation (5 min at 14,000 g), muscle lysates were incubated in DCF (invitrogen, Carlsbad, CA) dissolved in DMEM (final concentration of 20 μM, Invitrogen, Carlsbad, CA) for 30 min at 37 °C. Samples were centrifuged at 14,000 g for 5 min, pellets were re-suspended in 200 μl lysis buffer (50 mmol/L Hepes, 150 mmol/L NaCl, 10% Glycerol, 1% Triton X-100, 1.5 mmol/L MgCl2, 1 mmol/L, EGTA, 10 mmol/L Sodium Pyrophosphate, 100 mmol/L Sodium Fluoride and 100 μmol/L Sodium Vanadate, 1 mmol/L PMSF, 10 μg/ml Aprotinin, and 10 μg/ml Leupeptin) and incubated for 10 min at 4° C under constant agitation. Samples were then centrifuged (14,000 g for 5 min) and the supernatants transferred to a 96-well plate and fluorescence intensity was measured using a fluorescence plate reader (Biotek Instrument Inc, Winooski, USA). Data were normalized to protein content and the data were expressed as fold change (22).
To directly assess mitochondrial derived superoxide level, EPR spectroscopy (EMX X-band Bruker, Manning Park Billerica, MA) was performed on frozen cardiac tissue samples from the patients with iHF and niHF, utilizing mitoTempo–H probes (0.5 mM, Enzo Life Science, Inc.), as previously described (23,24).
Statistical Analyses
One way ANOVA was performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA) to detect differences in respiration, mitochondrial content, and free radical level. If a significant difference was detected, a Tukey’s post hoc test was used to identify this difference. Correlations between CSA, Complex IV respiration, and mtDNA copy number were assessed with a Pearson product moment correlation. For all analyses, a P value of <0.05 was considered significantly different. All data are expressed as mean ± SEM.
RESULTS
Subject Characteristics
The subject characteristics, disease conditions, and medications of the iHF and niHF patients are displayed in Table 1. The iHF and niHF patients were well-matched for age, physical, and blood chemistry characteristics. The 4 HdH came from 1 male and 3 females with an average age of 52±3 yrs. Thus, the HdH were of a similar age as the patients with iHF and niHF, however, other physical and blood chemistry characteristics were not available for these individuals.
Table 1.
Characteristic of the ischemic and non-ischemic heart failure (HF) patients
| Ischemic HF (n=17) | Non-ischemic HF (n=18) | |
|---|---|---|
| Age (years) | 57±3 | 53±4 |
| Gender (M/F) | 14/3 | 14/4 |
| BMI (kg/m2) | 27.1 | 27.2 |
| Systolic blood pressure (mm Hg) | 115±3 | 112±4 |
| Diastolic blood pressure (mm Hg) | 74±2 | 72±3 |
| NYHA classification: I-IV (n) | III(1)-IV(16) | III (2)-IV(16) |
| LVEF (%) | 21±2 % | 24±4 % |
| Type 2 diabetes as a co-morbidity | None | None |
| Hypertension | 6 | 7 |
| COPD | None | None |
| CKD | None | None |
| Anemia | 4 | 6 |
| Sleep disorder | 4 | 5 |
| Duration of HF symptoms, yrs | 6.4±5.3 | 5.9±4.2 |
| Medications | ||
| ACE inhibitor | 14/17 | 15/18 |
| angiotensin antagonist | 15/17 | 15/18 |
| Beta- blocker | 7/17 | 11/18 |
| Diuretics | 16/17 | 17/18 |
| Statins | 10/17 | 9/18 |
| Digoxin | 11/17 | 11/18 |
| Inotropic support | 7/17 | 3/18 |
| Plasma biochemistry | ||
| Total Cholesterol, mmol/l | 160±10 | 150±16 |
| HDL (mg/dL) | 40±1 | 42±2 |
| LDL (mg/dL) | 110±2 | 99±2 |
| Triglyceride (mg/dL) | 99±2 | 108±2 |
| Glucose (mg/dL) | 100±4 | 99±3 |
| Hematocrit (%) | 40±2 | 39±2 |
| RBC (M/uL) | 4.3±0.2 | 4.2±0.7 |
| WBC (K uL) | 8.9±0.2 | 7.7±0.4 |
BMI: body mass index; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; Type II DM: type II diabetes mellitus; COPD: chronic obstructive pulmonary disease; CKD: Chronic kidney disease; ACE inhibitor: angiotensin converting enzyme inhibitor; HDL: high-density lipoprotein; LDL: low-density lipoprotein; RBC: red blood cells; WBC: white blood cells. Data presented as mean ± SE.
Oxidative Phosphorylation capacity (OXPHOS) and mitochondrial content
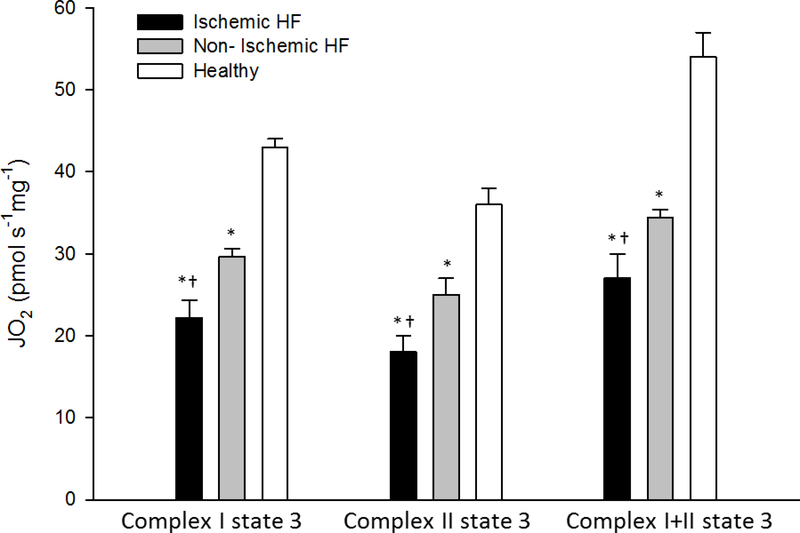
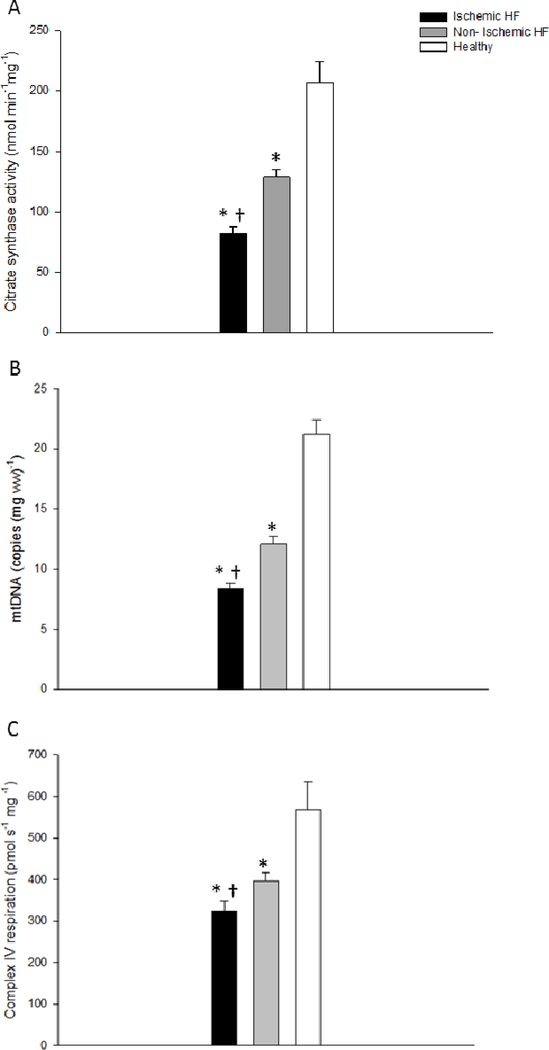
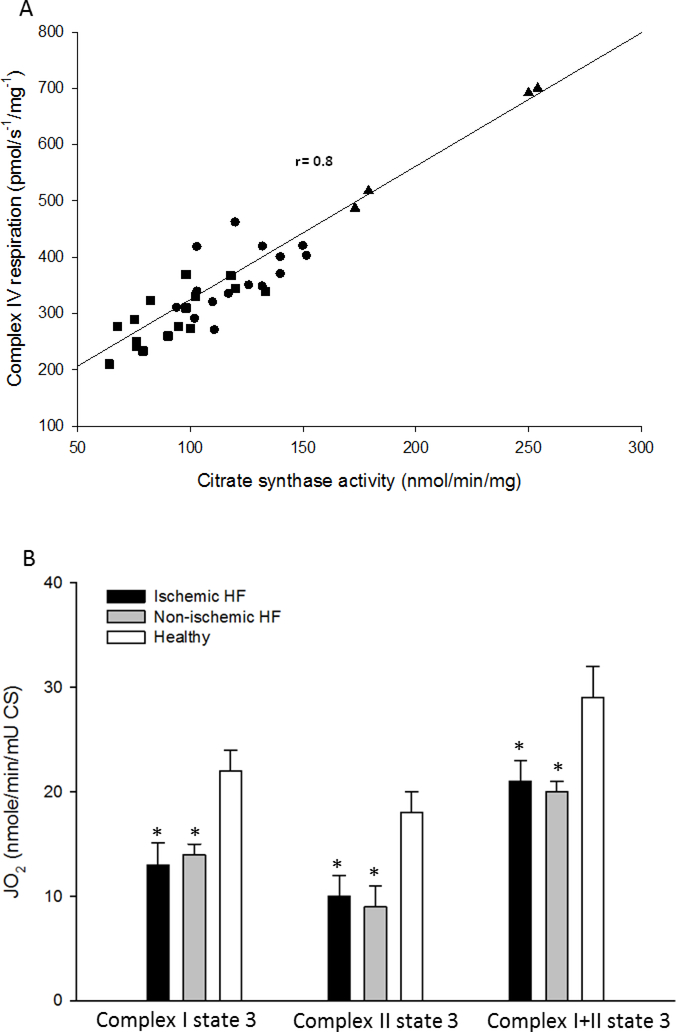
As illustrated in Figure 1, Complex I, Complex II, and Complex I+II state 3 respiration rates (OXPHOS), expressed per unit of wet weight, was consistently attenuated in both the iHF and niHF compared to the HdH (P<0.05). However, OXPHOS was also consistently attenuated in the iHF compared to niHF (P<0.05) (Figure 1). Markers of mitochondrial content, CSA, Complex IV respiration, and mtDNA all fell consistently from HdH to niHF, to iHF (Figure 2) and these measures were significantly correlated with each other to varying extents (CSA vs mtDNA: r = 0.36, P<0.05; Complex IV respiration vs mtDNA: r = 0.42 P<0.05; CSA vs Complex IV respiration: r =0.8, P<0.05, Figure 3A). When mitochondrial Complex I, Complex II, and Complex I+II state 3 respiration were normalized by CSA, OXPHOS for the HdH remained significantly greater than both iHF and niHF, but the difference between iHF and niHF was negated (Figure 3B). Also, SCR, an index of Complex II substrate utilization capacity, was not different across iHF, niHF, and HdH (1.3± 0.1, 1.3±0.1, 1.6±0.3, and P= 0.1, respectively).
Figure 1. Oxidative phosphorylation capacity assessed as Complex I, Complex II, and Complex I+II state 3 respiration in the cardiac muscle of healthy hearts and patients with ishchemic and non-ischemic heart failure (HF).
JO2, O2 flux. * Significantly different from healthy hearts, P<0.05; † Significantly different from non-ischemic HF, P<0.05. Data presented as mean ± SE.
Figure 2. Markers of mitochondrial content in cardiac muscle from healthy hearts, and patients with ischemic and non-ischemic heart failure (HF).
A: Citrate synthase activity, B: mitochondrial DNA copy number, and C: Complex IV respiration. JO2, O2 flux. * Significantly different from healthy hearts, P<0.05; † Significantly different from non-ischemic HF, P<0.05; mtDNA, mitochondrial deoxyribonucleic acid. Data presented as mean ± SE.
Figure 3. The significant relationship between citrate synthase activity (CSA) and Complex IV respiration (A) and oxidative phosphorylation capacity normalized by CSA (B) in cardiac muscle from healthy hearts and patients with ischemic and non-ischemic heart failure (HF).
* Significantly different from the healthy hearts, P<0.05. Healthy hearts, triangles; non-ischemic HF, circles; ischemic HF, squares. Data presented as mean ± SE in panel B.
Non-phosphorylating respiration and RCR
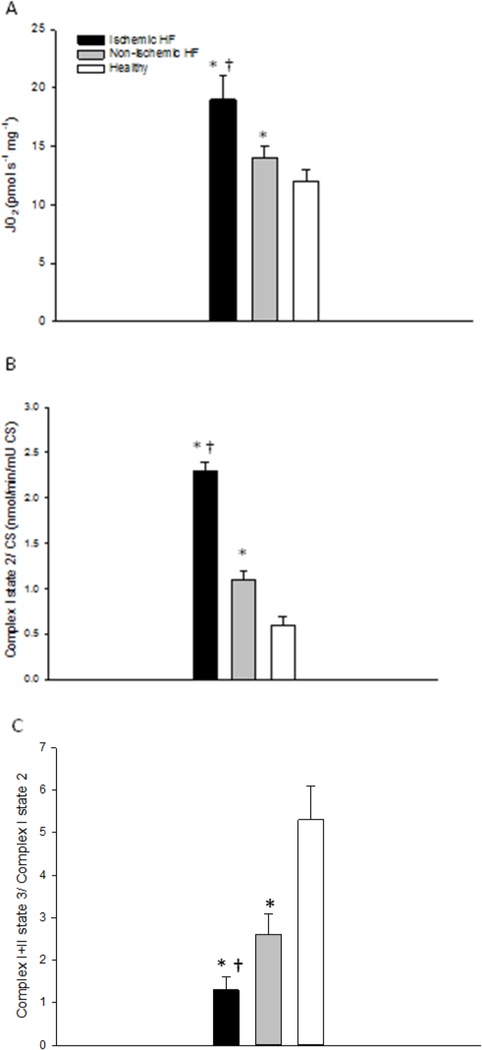
Complex I state 2 respiration, an index of non-phosphorylating proton leak, was significantly greater in iHF than niHF, but was still significantly greater in niHF than the HdH (P<0.05) (Figure 4A), and these differences were enhanced by normalizing for CSA (Figure 4B). As both complex I state 2 respiration normalized by tissue wet weight and CSA fell from iHF to niHF to HdH (P<0.05). RCR (Complex I+II, state 3 respiration/Complex I, state 2 respiration) exhibited a progressive and significant decline from HdH to niHF, to iHF (P<0.05) (Figure 4C).
Figure 4. Uncoupled mitochondrial respiration (complex I state 2) normalized by tissue wet weight (A) and then by citrate synthase activity (B), and respiratory control ratio (RCR, complex I+II state 3 / complex I state 2 respiration) (C) in cardiac muscle from healthy hearts and patients with ischemic and non-ischemic heart failure (HF).
* Significantly different from healthy hearts, P<0.05; † Significantly different from non-ischemic HF, P<0.05. Data presented as mean ± SE.
Cardiac tissue free radical levels in iHF and niHF
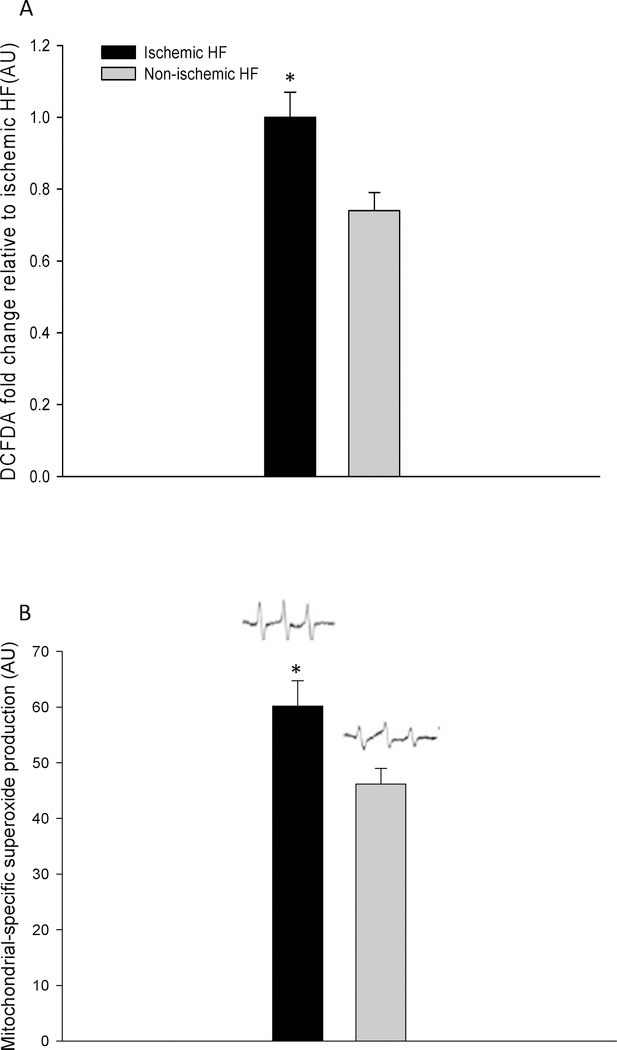
The total free radical level, assessed by DCFDA fluorescence, was greater in iHF compared to niHF (Figure 5A). Similarly, the level of mitochondrial specific superoxide, assessed by EPR spectroscopy, was significantly greater in iHF compared to niHF (Figure 5B).
Figure 5. Basal free radical levels in cardiac muscle from patients with ischemic heart failure (HF) and non-ischemic HF. A: Total Free radicals measured with, 2’–7’ dichlorofluorecein diacetae (DCFDA) fluorescence in whole-tissue extract. Data presented as fold change relative to Ischemic HF. B: Mitochondrial derived superoxide levels measured in frozen cardiac muscle utilizing the mitoTempo-H spin probe and electron paramagnetic resonance spectroscopy.
* Significantly different from non-ischemic HF, P < 0.05. Data presented as mean ± SE.
DISCUSSION
The study sought to examine the impact of HF etiology on mitochondrial respiratory function and, to our knowledge, this is the first investigation to comprehensively assess these processes in both iHF and niHF, compared to HdH. Consequently, there are several novel findings of this study. First, expressed per mg of tissue, OXPHOS, assessed as Complex I, II, and I+II state 3 respiration and mitochondrial content, assessed by CSA, fell progressively from HdH to niHF, to iHF. Second, although still lower than HdH, normalization of OXPHOS by CSA negated the difference in mass specific OXPHOS between iHF and niHF. Third, Complex I state 2 respiration increased progressively from HdH to niHF, to iHF, such that RCR, fell progressively from HdH to niHF, to iHF. Finally, measurements of both total free radical levels and mitochondrial derived superoxide levels were significantly greater in iHF compared to niHF. Thus, the HF-related attenuation in OXPHOS actually appears to be independent of etiology when the lower mitochondrial content of iHF is taken into account. However, this study also reveals deleterious intrinsic mitochondrial changes in iHF, compared to niHF, including greater proton leak, attenuated OXPHOS efficiency, and greater free radical levels. Identifying these etiology-specific mitochondrial function differences is important so as to accurately target specific patients with the appropriate treatments.
The impact of etiology on OXPHOS
In the human heart, 90% of ATP is produced by OXPHOS, therefore, an attenuation of this process in HF constitutes a major concern in terms of cardiac energy production (6,25,26). Indeed, the current study confirms and extends previous work that has typically observed attenuated Complex I state 3 respiration in both animal models of HF and patients with HF (13,27,28). Specifically, this study documents a HF-related attenuation in OXPHOS, per mg of tissue, assessed as Complex I, Complex II, and Complex I+II state 3 respiration, falling progressively from HdH to niHF, to iHF. Therefore, although previous studies have suggested that mitochondrial structure and function is not affected by HF etiology (29–31), the current findings clearly demonstrate that tissue mass specific OXPHOS is attenuated with HF in general, but that this limitation occurs to a greater extent in iHF compared to niHF. These conflicting results may be a consequence of the inclusion of co-morbidities such as diabetes and insulin resistance in prior studies, in contrast the current study excluded these confounding factors. Interestingly, when mass specific OXPHOS was normalized by CSA, a marker of mitochondrial content, considered valid in HF (16,18), the differences in respiration between iHF and niHF were negated. This suggests that OXPHOS per unit of mitochondrial content is similar between iHF and niHF and the observed differences in respiration were due to differences in the quantity rather than the quality of mitochondria between etiologies, with iHF exhibiting lower mitochondrial content and therefore lower mass specific OXPHOS.
Utilizing the innovative approach of examining mitochondrial respiration in cardiac tissue from ischemic and non-ischemic areas of the same heart, Stride et al. (13), inferred that iHF likely resulted in a mitochondrial defect that manifests predominantly at Complex II. However, the current findings suggest that the attenuated OXPHOS with HF, including complex II respiration, is attenuated as a consequence of lower mitochondrial content, and, therefore, this apparently limited function was no longer evident when respiration was normalized for CSA (Figure 3B). Additionally, in the current study, SCR, an index of substrate utilization capacity at Complex II, was similar between iHF and niHF, further supporting the conclusion that Complex II function is not specifically impacted by HF etiology.
Mitochondrial content in iHF and niHF
Interestingly, there is little accord as to the most appropriate and valid method to assess tissue mitochondrial content, however, suggested approaches include electron microscopy, CSA, mtDNA, and Complex IV respiration. Recently, by revealing a strong relationship between CSA, a reasonably well accepted method, and Complex IV respiration, in healthy cardiac, skeletal, and vascular smooth muscle, our group concluded that Complex IV respiration is an acceptable marker of mitochondrial content (16,18). The current data extend this previous conclusion to diseased tissue because across HdH, niHF, and iHG, complex IV was again highly correlated with CSA (Figure 3A). Additionally, mtDNA copy number revealed a similar pattern of reduction from HdH to niHF, to iHG as did CSA and Complex IV respiration (Figure 2). However, upon closer inspection it was apparent that by far the best agreement amongst these measures of mitochondrial content was between CSA and Complex IV respiration (Figure 3A), adding a degree of construct validity to both indices. In terms of HF, these findings of diminished mitochondrial content are well aligned with a previous study by Lemieux et al. (32), which reported a decrement in Complex IV enzyme activity in cardiac tissue from patients with HF compared to healthy controls. Therefore, in combination with prior work, the current findings suggest that both CSA and Complex IV respiration are viable markers of mitochondrial content in both healthy and diseased cardiac muscle.
Perhaps, mechanistically, as a result of diminished mitochondrial calcium handling capacity, the activation of caspase-3, and the triggering of apoptosis (33,34), several studies have reported a reduction in mitochondrial content in the cardiac tissue of patients with end-stage HF (Class IV). However, the specific impact of HF etiology on mitochondrial content has not been investigated (31,35). As already noted, recently, Stride et al., (13) investigated the impact of ischemia on mitochondrial content by examining ischemic and non-ischemic regions within the same heart. The authors determined that, in comparison to well perfused regions of the heart, mitochondrial content was reduced in the chronically ischemic areas, implicating a link between chronic ischemia and reduced mitochondrial content (13). The current study supports this contention by clearly revealing distinct etiology-dependent differences in mitochondrial content between iHF and niHF, with iHF resulting in reduced mitochondrial density (Figure. 2). The specific mechanism underlying the reduced mitochondrial content in iHF compared to niHF remains unclear, however, this finding is likely related to the impaired tissue oxygenation associated with chronic ischemia. This could result in chronic low–grade inflammation, the up-regulation of the pro-inflammatory cytokine tumor necrosis factor-α, and attenuated mitochondrial biogenesis, as evidenced by reduced PGC-1α (36,37). Additionally, impaired mitochondrial oxygenation attenuates respiratory complex function and adenine nucleotide translocase which may lead to membrane dysfunction and, eventually, cell death (38). Another, outcome of chronic ischemia is the accumulation of collagen and the development of fibrosis, reducing mitochondrial density per tissue mass, a phenomenon which has been documented to be more prevalent in iHF than niHF (39,40). Each of these factors deserve further attention to determine the mechanism responsible for the observed reduction in mitochondrial content in iHF compared to niHF
Non-phosphorylating respiration and RCR in iHF and niHF
The proton motive force, the proton gradient between the matrix and the intermembrane space of the mitochondria, facilitates ATP production (41) and in combination with non-phosphorylating proton conductance regulates the kinetics and efficiency of mitochondrial respiration (7,16). Interestingly, in the current study, despite greater mitochondrial content in niHF compared to iHF, tissue mass specific Complex I state 2 respiration, an indicator of proton leak, was greater in iHF compared to niHF (Figure 4A). Therefore, when normalized for CSA, this elevated Complex I state 2 respiration in the iHF compared niHF was not only maintained, but exaggerated, confirming that proton leak per mitochondrial content was greater in iHF compared to niHF. This general finding, of greater proton leak in HF, is well-aligned with previous studies suggesting greater mitochondrial uncoupling with mitochondrial membrane damage and subsequent reductions in ATP production in mice with HF (38,42). Furthermore, the current etiology specific findings suggest that chronic ischemia increases mitochondrial H+ ion membrane permeability that likely contributes to mitochondrial dysfunction. This difference in proton leak may be a fundamental difference which characterizes HF etiologies (43,44) (Figure 4A and B). Additionally, the RCR, the ratio of Complex I+II state 3 to Complex I state 2 respiration, provides an index of mitochondrial dysfunction, with a low RCR reflecting defects that lead to OXPHOS inefficiency (16,45). The current findings reveal a significantly lower RCR in iHF compared to niHF. This attenuated RCR in iHF compared to niHF provides additional novel evidence that HF etiology results in physiologically significant alterations in mitochondrial proton leak. Therefore, this study reveals that iHF exhibits an even greater attenuation in OXPHOS efficiency than niHF and this is predominantly due to distinct etiology-dependent uncoupled respiration.
Etiology specific free radical levels in iHF and niHF
Previous studies have reported elevated free radicals in HF (1,46), however, the source of these free radicals, mitochondrial or non-mitochondrial, is not clear. Additionally, these previous investigations are somewhat confounded by the use of acute ischemia, in animal models, to induce free radical production (1,46). The current study utilized DCFDA to assess basal free radical levels in cardiac muscle from patients with iHF and niHF and revealed a greater total free radical level in the tissue from the patients with iHF (Figure 5A). Furthermore, the direct assessment of mitochondrial derived superoxide levels, utilizing the mitoTempo-H spin probe and EPR spectroscopy, revealed greater free radical levels in iHF compared to niHF (Figure 5B). Although speculative, it is tempting to imply that this may be a consequence of both a reduction in mitochondrial content and increased H+ membrane permeability in iHF compared to niHF (37,47).
Previously, Stride et al., (13) measured greater free radical production in ischemic compared to non-ischemic regions of the same heart in patients with HF. In agreement with this study, the current findings suggest that chronic ischemia may be an important factor contributing to increased mitochondrial derived free radical production in iHF. Potential mechanisms responsible for this augmented free radical production include attenuated Complex III function and diminished coenzyme Q binding protein (COQ10), both of which would result in a “bottleneck” for electron transport through the respiratory chain, allowing more time for free radical production (1,13). However, it is still somewhat controversial as to whether increased proton leak decreases free radical production due to decreased proton motive force and the subsequent attenuation of the coupled oxidative phosphorylation or if an increased proton leak reduces ATP/O2 ratio (P/O ratio), leading to an increase free radical production due to a decreased integrity of mitochondrial membranes (48,49). In actuality, the relationship between proton motive force and free radical formation is likely described by a U-shaped curve and thus it will not be possible to discern a single, simple, answer to this complex issue (50). Nonetheless, this study has identified greater total and mitochondrial derived free radical levels in the cardiac muscle of patients with iHF compared to niHF, revealing a clear etiology-dependent difference.
Experimental considerations
This study sought to examine the impact of HF etiology on mitochondrial function in cardiac muscle from patients with iHF and niHF and, as human cardiac tissue is not readily accessible, there are some experimental considerations germane to this study. First, as samples were facilitated by either heart transplantation or LVAD implantation, only tissue from end-stage iHF and niHF was available for analysis which limits insight into the progression to end-stage HF in these two etiologies and whether mitochondrial differences were present early on in the disease. Second, although efforts were made to minimize the variability in the causes of niHF (exclusion of hypertrophic cardiomyopathy and acute heart failure), this study did not have enough subjects to compare mitochondrial function across the different subtypes of non-ischemic cardiomyopathy. Third, although the cardiac tissue assessed in this study was minimally affected by scaring and fibrosis, because the tissue was harvested from the apex of the left ventricle in all patients, some of the tissue in iHF may have been close to previously infarcted and ischemic regions (i.e. anterior myocardial infarctions). These regions may have different characteristics, in terms of mitochondrial function, compared to the tissue more remote to the infarcted or ischemic region which, as there are no location-specific comparisons in this study, is a limitation of the current work. Finally, it should be acknowledged that this study only reported mitochondrial function in a small number of HdH to represent normal healthy function. However, of note, despite these experimental considerations, this study clearly documented HF related changes in mitochondrial function and identified potentially clinically significant etiology-specific differences in mitochondrial function between iHF and niHF.
Summary
The HF-related attenuation in OXPHOS appears to be independent of etiology when the lower mitochondrial content of iHF, compared to niHF, is taken into account. However, there are also deleterious intrinsic mitochondrial changes in iHF, compared to niHF, including greater proton leak, attenuated OXPHOS efficiency, and greater free radical levels. Identifying these etiology-specific mitochondrial function differences is important so as to accurately target specific patients with the appropriate treatments.
Acknowledgements
The authors thank the participants who donated heart tissue for this study.
Grants
R.S.R was supported, in part, by the National Institutes of Health (PO1HL-091830), and the United States Department of Veterans Affairs Rehabilitation Research and Development Service (E6910-R, E1697-R, E1433-P, and E9275-L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERECES
- 1.Stride N, Larsen S, Hey-Mogensen M et al. Decreased mitochondrial oxidative phosphorylation capacity in the human heart with left ventricular systolic dysfunction. Eur J Heart Fail 2013;15:150–7. [DOI] [PubMed] [Google Scholar]
- 2.Knaapen P, Germans T, Knuuti J et al. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation 2007;115:918–27. [DOI] [PubMed] [Google Scholar]
- 3.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol 1974;36:413–59. [DOI] [PubMed] [Google Scholar]
- 4.Opie LH. Metabolism of the heart in health and disease. I. Am Heart J 1968;76:685–98. [DOI] [PubMed] [Google Scholar]
- 5.Schwarzer M, Osterholt M, Lunkenbein A, Schrepper A, Amorim P, Doenst T. Mitochondrial ROS production and respiratory complex activity in rats with pressure overload-induced heart failure. J Physiol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray AJ, Cole MA, Lygate CA et al. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol 2008;44:694–700. [DOI] [PubMed] [Google Scholar]
- 7.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 2005;112:2686–95. [DOI] [PubMed] [Google Scholar]
- 8.Davila-Roman VG, Vedala G, Herrero P et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2002;40:271–7. [DOI] [PubMed] [Google Scholar]
- 9.Neglia D, De Caterina A, Marraccini P et al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2007;293:H3270–8. [DOI] [PubMed] [Google Scholar]
- 10.Follath F Ischemic versus non-ischemic heart failure: should the etiology be determined? Heart Fail Monit 2001;1:122–5. [PubMed] [Google Scholar]
- 11.Follath F, Cleland JG, Klein W, Murphy R. Etiology and response to drug treatment in heart failure. J Am Coll Cardiol 1998;32:1167–72. [DOI] [PubMed] [Google Scholar]
- 12.Chokshi A, Drosatos K, Cheema FH et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 2012;125:2844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stride N, Larsen S, Hey-Mogensen M et al. Impaired mitochondrial function in chronically ischemic human heart. Am J Physiol Heart Circ Physiol 2013;304:H1407–14. [DOI] [PubMed] [Google Scholar]
- 14.Dai DF, Santana LF, Vermulst M et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 2009;119:2789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleland JG, Bristow MR, Erdmann E, Remme WJ, Swedberg K, Waagstein F. Beta-blocking agents in heart failure. Should they be used and how? Eur Heart J 1996;17:1629–39. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Gifford JR, Andtbacka RH et al. Cardiac, skeletal, and smooth muscle mitochondrial respiration: are all mitochondria created equal? Am J Physiol Heart Circ Physiol 2014;307:H346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 2008;3:965–76. [DOI] [PubMed] [Google Scholar]
- 18.Larsen S, Nielsen J, Hansen CN et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 2012;590:3349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard M, Ritchie D, Wright KJ et al. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell 2010;9:1032–46. [DOI] [PubMed] [Google Scholar]
- 20.Rabol R, Svendsen PF, Skovbro M et al. Reduced skeletal muscle mitochondrial respiration and improved glucose metabolism in nondiabetic obese women during a very low calorie dietary intervention leading to rapid weight loss. Metabolism: clinical and experimental 2009;58:1145–52. [DOI] [PubMed] [Google Scholar]
- 21.Kraunsoe R, Boushel R, Hansen CN et al. Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. J Physiol 2010;588:2023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Kim KW, Yu BP, Chung HY. The effect of age on cyclooxygenase-2 gene expression: NF-kappaB activation and IkappaBalpha degradation. Free Radic Biol Med 2000;28:683–92. [DOI] [PubMed] [Google Scholar]
- 23.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 2014;592:2549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dikalov SI, Kirilyuk IA, Voinov M, Grigor’ev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res 2011;45:417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari R, Cargnoni A, Ceconi C. Anti-ischaemic effect of ivabradine. Pharmacol Res 2006;53:435–9. [DOI] [PubMed] [Google Scholar]
- 26.Neubauer S The failing heart--an engine out of fuel. N Engl J Med 2007;356:1140–51. [DOI] [PubMed] [Google Scholar]
- 27.Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol 2000;32:2361–7. [DOI] [PubMed] [Google Scholar]
- 28.Sanbe A, Tanonaka K, Hanaoka Y, Katoh T, Takeo S. Regional energy metabolism of failing hearts following myocardial infarction. J Mol Cell Cardiol 1993;25:995–1013. [DOI] [PubMed] [Google Scholar]
- 29.Sharov VG, Goussev A, Lesch M, Goldstein S, Sabbah HN. Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol 1998;30:1757–62. [DOI] [PubMed] [Google Scholar]
- 30.Jarreta D, Orus J, Barrientos A et al. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc Res 2000;45:860–5. [DOI] [PubMed] [Google Scholar]
- 31.Kalsi KK, Smolenski RT, Pritchard RD, Khaghani A, Seymour AM, Yacoub MH. Energetics and function of the failing human heart with dilated or hypertrophic cardiomyopathy. Eur J Clin Invest 1999;29:469–77. [DOI] [PubMed] [Google Scholar]
- 32.Lemieux H, Semsroth S, Antretter H, Hofer D, Gnaiger E. Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol 2011;43:1729–38. [DOI] [PubMed] [Google Scholar]
- 33.Petit PX, Lecoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon ML. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J Cell Biol 1995;130:157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolvetang EJ, Johnson KL, Krauer K, Ralph SJ, Linnane AW. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett 1994;339:40–4. [DOI] [PubMed] [Google Scholar]
- 35.Nascimben L, Ingwall JS, Pauletto P et al. Creatine kinase system in failing and nonfailing human myocardium. Circulation 1996;94:1894–901. [DOI] [PubMed] [Google Scholar]
- 36.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 2003;551:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci 2006;119:2855–62. [DOI] [PubMed] [Google Scholar]
- 38.Walters AM, Porter GA, Jr., Brookes PS. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res 2012;111:1222–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Querejeta R, Lopez B, Gonzalez A et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation 2004;110:1263–8. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee D, Sen S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest 1991;88:1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stuart JA, Brindle KM, Harper JA, Brand MD. Mitochondrial proton leak and the uncoupling proteins. J Bioenerg Biomembr 1999;31:517–25. [DOI] [PubMed] [Google Scholar]
- 42.Borutaite V, Mildaziene V, Brown GC, Brand MD. Control and kinetic analysis of ischemiadamaged heart mitochondria: which parts of the oxidative phosphorylation system are affected by ischemia? Biochim Biophys Acta 1995;1272:154–8. [DOI] [PubMed] [Google Scholar]
- 43.Brookes PS, Hulbert AJ, Brand MD. The proton permeability of liposomes made from mitochondrial inner membrane phospholipids: no effect of fatty acid composition. Biochim Biophys Acta 1997;1330:157–64. [DOI] [PubMed] [Google Scholar]
- 44.Brookes PS, Rolfe DF, Brand MD. The proton permeability of liposomes made from mitochondrial inner membrane phospholipids: comparison with isolated mitochondria. J Membr Biol 1997;155:167–74. [DOI] [PubMed] [Google Scholar]
- 45.Brand MD, Brindle KM, Buckingham JA, Harper JA, Rolfe DF, Stuart JA. The significance and mechanism of mitochondrial proton conductance. Int J Obes Relat Metab Disord 1999;23 Suppl 6:S4–11. [DOI] [PubMed] [Google Scholar]
- 46.Brown GC, Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion 2012;12:1–4. [DOI] [PubMed] [Google Scholar]
- 47.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 2008;294:H2121–8. [DOI] [PubMed] [Google Scholar]
- 48.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep 1997;17:3–8. [DOI] [PubMed] [Google Scholar]
- 49.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 2003;86:1101–7. [DOI] [PubMed] [Google Scholar]
- 50.Daiber A Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 2010;1797:897–906. [DOI] [PubMed] [Google Scholar]