Abstract
Campylobacter spp. is one of the most widespread infectious diseases of veterinary and public health significance. Globally, the incidence of campylobacteriosis has increased over the last decade in both developing and developed countries. Squamates (lizards, snakes and amphisbaenians) are a potential reservoir and source of transmission of campylobacteriosis to humans. This systematic review examined studies from the last 20 years that have reported squamate-associated human campylobacteriosis. It was found that C. fetus subsp. testudinum and C. fetus subsp. fetus were the most common species responsible for human campylobacteriosis from a squamate host. The common squamate hosts identified included bearded dragons (Pogona vitticeps), green iguana (Iguana iguana), western beaked gecko (Rhynchoedura ornate) and blotched blue-tongued skink (Tiliqua nigrolutea). People with underlying chronic illnesses, the immunocompromised and the elderly were identified as the most vulnerable population. Exposure to pet squamates, wild animals, consumption of reptilian cuisines and cross contamination with untreated water were risk factors associated with Campylobacter infections. Proper hand hygiene practices, responsible pet ownership, ‘One Health’ education and awareness on zoonotic diseases will help reduce the public health risks arising from Campylobacter exposure through squamates. Continued surveillance using molecular diagnostic methods will also enhance detection and response to squamate-linked campylobacteriosis.
Keywords: Campylobacter spp., campylobacteriosis, C. fetus subsp. testudinum, zoonosis, pet squamates, lizard, snake, reptile, One Health
1. Introduction
Globally, Campylobacter spp. is a common zoonotic pathogen of significant veterinary and public health concern [1,2]. It is the causative agent of campylobacteriosis, a gastrointestinal disease that has been increasing in incidence over the last decade [3,4,5,6]. The disease presents as gastroenteritis with fever, nausea, vomiting, abdominal pains and watery or bloody diarrhea [7]. While the disease may generally be a self-limiting enterocolitis, clearing on its own within a week, it may also manifest in serious long-term complications including extra-intestinal infections and autoimmune disorders such as Guillain-Barré syndrome, Miller-Fisher syndrome, cholecystitis, inflammatory bowel syndrome and reactive arthritis [7,8,9]. Over the last decade the incidence of campylobacteriosis has increased in both developed and developing countries [2]. In the USA, it is estimated that Campylobacter spp. causes over 1.3 million cases and approximately 130 deaths per year, with the Foodborne Diseases Active Surveillance Network (FoodNet) reporting an increase in annual incidence rate of human campylobacteriosis from 14.3 in 2012 to 19.5 cases per 100,000 population in 2019 [2,10,11,12,13]
Campylobacter spp. presents a threat to human and animal health because of its zoonotic potential, wide host range, ability to colonize diverse habitats, and emerging resistance to some of the commonly used antimicrobial drugs [14]. The virulence of different Campylobacter species and severity of the resulting enteritis is dependent on the pathogenesis mechanisms used, including adhesion to the intestinal wall, colonization of digestive tract, invasion of targeted cells and toxin production [15]. The infection process involves penetration of the gastrointestinal mucus by the bacteria using its high motility and spiral shape, adherence to the gut enterocytes and then inducing diarrhea through release of toxins mainly enterotoxins and cytotoxins [16]. While Campylobacter jejuni is a fastidious bacterial pathogen, its virulence is adversely affected by environmental stresses such as nutrient insufficiency, heat stress, absence of water, partial oxygen tension above 10%, low PH, UVB exposure and hydrostatic pressure [17]. However, it is able to develop survival mechanisms which include; persisting in the environment, especially in water, in a viable but non-culturable state [18], transition from rod to coccoid shape [19] and growth in biofilm [20]. By altering gene expression pathways, C. jejuni can also adapt to new growth temperatures when exposed to a sudden temperature upshift [21] and persist and grow intracellularly in non-phagocytic host cells through the use of gene encoding catalase (katA) enzyme [22]. While previous studies have provided useful information on virulence of Campylobacter spp., further research is needed to inform interpretation of different virulence associated markers or genes.
The Campylobacter genus displays wide taxonomic diversity currently comprising of 32 species and 9 subspecies [23]. Campylobacter spp. is responsible for 9% of all foodborne illnesses in the United States [10] and molecular typing techniques suggest that up to 80% of human infections are caused by Campylobacter strains associated with poultry hosts [24]. Campylobacter jejuni is the most common campylobacter species isolated from human cases with campylobacteriosis [2,25,26]. Additionally, C. jejuni causes over 80% of human campylobacteriosis cases, with 50–80% of the cases attributed to the chicken reservoir (both broilers and laying hens) [27,28].
The disease is not only a food-borne illness but is also transmitted through environmental reservoirs including animals [29,30]. Changes in land use, habitat loss, urbanization, encroachment of people into wildlife habitats and community composition are reported to influence wildlife health [31]. With human–wildlife interactions becoming more common, the likelihood of zoonotic spread of campylobacteriosis is increasing [28,32,33]. However, information about horizontal transmission of Campylobacter through non-foodborne routes is limited, and the zoonotic nature of the disease is often overlooked [32,34]. One potentially overlooked host is squamates [22]. Squamata is the largest order of reptiles comprising of three suborders: lizards (suborder: Lacertilia/Sauria), snakes (suborder: Serpentes/Ophidia) and worm lizards (suborder: Amphisbaenia) [35]. The suborder lizards includes skinks (family: Scincidae), dragons (family: Agamidae), monitor lizards/goannas (family: Varanidae), geckos (family: Gekkonidae) and flat-footed lizards (family: Pygopodidae) which are all adapted to diverse environments [35]. The squamates have been implicated in potentially aiding horizontal transmission of Campylobacter spp. either by cross-contamination through their feces, pet handling or generally as a result of close interaction with human habitats [34].
With the propensity to keep reptiles, including squamates, as pets increase globally [36,37,38], zoonotic disease transfer to humans continues to pose a serious challenge to the public and environmental health sector. This review examines the literature pertaining to squamate-linked campylobacteriosis in humans. Studies describing human campylobacteriosis cases linked to the handling of captive and wild squamates or cross-contamination through their feces are surveyed. Further, trends in emerging Campylobacter subspecies, the lizard and snake species involved in transmission and possible exposure routes were also explored. This information will inform more effective management strategies to reduce the risk of zoonotic transfer of Campylobacter from captive and wild squamates to humans.
2. Results
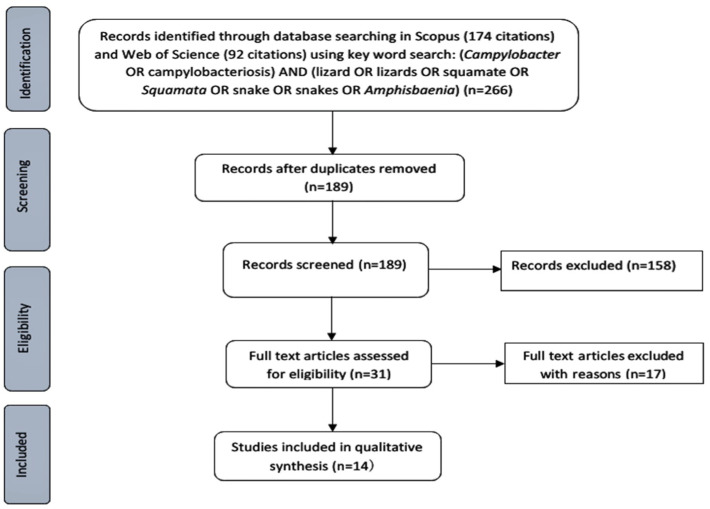
One hundred and eighty-nine papers were retrieved from SCOPUS and Web of Science using the search terms identified (Figure 1). After applying the inclusion and exclusion criteria described in Figure 1, a total of 14 papers were included for review; six case studies investigated the source of human campylobacteriosis cases linking them to a squamate source via testing, and eight environmental surveillance studies which screened different squamates for Campylobacter.
Figure 1.
Flow diagram of search methods and articles’ inclusion and exclusion criteria.
Table 1 provides a summarized list of squamate species identified from all studies included in this review and the associated Campylobacter species which they have been shown to transmit to humans. Campylobacter fetus subsp. fetus and C. fetus subsp. testudinum were identified as the most frequently isolated species in reptiles and the predominate causes of human campylobacteriosis linked to squamates. C. jejuni and C. iguaniorum have also been frequently isolated in squamates and reported to pose a potential health risk to humans. However, no human C. iguaniorum infections have been reported yet. [39]. The common lizard hosts identified included bearded dragons (Pogona vitticeps) [34,40], western beaked gecko (Rhynchoedura odura) [34], Hydrosaurus pustulatus [41], green iguana (Iguana iguana) [42], Pogona henrilawsonii, Sauromalus ater, Hemitheconyx caudicinctus [43] and blotched blue-tongued skink (Tiliqua nigrolutea)[44,45]. Five snake species were also identified namely; Heterodon nasicus, Orthriophis taeniurus, Boa constrictor, Python reticulatus [45] and Morelia amethistina [32].
Table 1.
Summary of squamate species and the respective Campylobacter spp. of human health significance that they have been shown to carry.
| Squamate Species | Campylobacter spp. | Sequence Data | Reference |
|---|---|---|---|
| Lizard (Pogona vitticeps) | C. iguaniorum | Whole Genome Sequencing (WGS) | [40] |
| Lizard (Iguana iguana) | C. iguaniorum subsp. nov | WGS | [42] |
| Snake (Heterodon nasicus) | C. fetus subsp. testudinum | WGS | [45] |
| Lizard (Tiliqua nigrolutea) | C. fetus | Multilocus sequence typing (MLST), PCR | [44] |
| Lizard (Pogona vitticeps) | C. jejuni | Quantitative PCR (qPCR) | [34] |
| Lizard (Rhynchoedura ornate) | C. jejuni | qPCR | [34] |
| Lizard (Hydrosaurus pustulatus) | C. fetus subsp. testudinum pet-3 | WGS | [41] |
| Lizard (Pogona henrilawsonii) | C. iguaniorum | MLST | [43] |
| Snake (Morelia amethistina) | C. fetus subsp. fetus | Multiplex PCR, MLST | [32] |
| Lizard (Hydrosaurus pustulatus) | C. fetus subsp. fetus | Multiplex PCR, MLST | [32] |
| Lizard (Sauromalus ater) | C. iguaniorum | MLST | [43] |
| Lizard (Hemitheconyx caudicinctus) | C. iguaniorum | MLST | [43] |
| Snake (Python reticulatus) | C. fetus subsp. testudinum | MLST | [45] |
| Lizard (Tiliqua rugosa) | C. fetus subsp. testudinum | MLST | [45] |
| Snake (Boa constrictor) | C. fetus subsp. testudinum | MLST | [45] |
| Snake (Orthriophis taeniurus) | C. fetus subsp. testudinum | MLST | [45] |
| Lizard (Tiliqua nigrolutea) | C. fetus subsp. testudinum | MLST | [45] |
The squamate surveillance studies (Table 2) and case reports (Table 3) identified in this review included reports of squamates contamination with Campylobacter from Australia [34], Korea [46], Taiwan [32,41], USA [47,48,49] China [50,51], United Kingdom [44] and Netherlands [39,40,42,43].
Table 2.
Surveillance studies investigating squamates as a potential risk for human campylobacteriosis.
| Country | Findings | Campylobacter spp. | Squamate | Comments | Reference |
|---|---|---|---|---|---|
| Taiwan | 179 reptile fecal samples obtained from chelonians, lizards and snakes. 12/179 (6.7%) were positive for Campylobacter spp.; 10/103 (9.7%) chelonians; 1/56 (1.7%) lizards and 1/20 (5%) of snakes were positive for C. fetus subsp. fetus. |
C. fetus subsp. fetus | Captive and wild lizards and snakes | Only the captive reptiles’ fecal samples tested positive for C. fetus. There were no positive isolates from the 23 reptiles collected from the wild fields. | [32] |
| Taiwan | Complete genome sequence of C. fetus subsp. testudinum strain pet-3 was isolated from a lizard | C. fetus subsp. testudinum strain pet-3 | Lizard (Hydrosaurus pustulatus) |
Isolated from humans, lizards, and turtles | [41] |
| USA | Polyphasic study to determine taxonomy of 13 C. fetus-like strains using MALDI-TOF MS yielded a novel Campylobacter fetus subsp. testudinum subsp. nov. | Five reptile C. fetus-like strains and eight C. fetus strains isolated from humans | Five reptiles | The 13 strains are closely related to C. fetus and they had multiple phenotypic biomarkers differentiating them from known C. fetus subspecies | [49] |
| Netherlands | C. iguaniorum is genetically related but distinct from C. fetus and C. hyointestinalis | C. iguaniorum | Bearded dragon (Pogona vitticeps) | C. iguaniorum isolated from a lizard. First whole genome sequence of C. iguaniorum was established. | [40] |
| Australia | 33% (17/51) of lizards’ feces collected from central Australia tested positive for C. jejuni by quantitative PCR | Campylobacter jejuni | 46 wild lizards (unknown); five captive lizards (Pogona vitticeps and Rhynchoedura ornate) | 3/5 (60%) of captive lizards; 14/46 (30%) wild lizard fecal samples were positive for C. jejuni. | [34] |
| Netherlands | Initial PCR and 16S rRNA showed the pathogens were most closely related to C. fetus and C. hyointestinalis. However, a polyphasic study involving characterization by 16S rRNA, atpA and MALDI-TOF MS showed divergence from all other known Campylobacter species. | C. iguaniorum subsp. nov | Five strains isolated from lizards and chelonians | Pathogen isolated from reptiles. Growth of the strains at ambient temperature may be an adaptation to their reptilian hosts which are identified as lizards and chelonians. | [42] |
| Netherlands | Campylobacter spp. through PCR as follows; 38% (62/163) in lizards, 32% (32/100) in snakes. Using culture; 3% (3/100) in snakes, and in 11% (18/163) lizards. |
C. iguaniorum, C. fetus subsp. testudinum and C. hyointestinalis |
Lizards (Pogona henrilawsonii, Sauromalus ater, Hemitheconyx caudicinctus) and snakes. | Lizards and snakes carry one or more of the intestinal epsilonproteobacteria. Presence of intestinal Campylobacter spp. was higher in lizards than in snakes. | [43] |
| Netherlands | Despite sharing the same host, no recent recombination was detected when genome comparison of C. iguaniorum and closely related C. fetus was done. Homology was higher between C. iguaniorum and C. fetus subsp. testudinum than between C. iguaniorum and mammalian C. fetus (C. fetus subsp. fetus & C. fetus subsp. venerealis). |
C. iguaniorum | Bearded dragon (Pogona vitticeps) and green iguana (Iguana iguana) | Primary reservoir reported to be reptiles, chelonians and lizards. C. iguaniorum strain 1485E and 2463D isolated from bearded dragon and green iguana respectively were genomically compared with reptilian C. fetus subsp. testudinum. | [39] |
Table 3.
Case studies investigating Campylobacter in squamates and links to human campylobacteriosis.
| Country | Findings | Campylobacter spp. | Squamate | Comments | Demographics | Reference |
|---|---|---|---|---|---|---|
| UK | Four isolates from ill patients were confirmed as reptile C. fetus strains using sap insertion PCR. Both strains (mammalian C. fetus and reptile C. fetus) were characterized by multilocus sequence typing to be sharing 92% nucleotide sequence identity. | Reptile C. fetus and classical mammalian C. fetus (C. fetus subsp. fetus and C. fetus subsp. venerealis) |
One snake (Heterodon nasicus) and one blotched blue-tongued skink (Tiliqua nigrolutea) | Reptile-like C. fetus strains have been isolated from cases of human disease. They showed capability of infecting humans despite having separate genomospecies. There was evidence of recombination. | Isolates from six clinically ill patients confirmed as reptile C. fetus strains using sap insertion PCR. | [44] |
| USA | Two Campylobacter spp. with markers of reptile origin were isolated from blood sample of a patient who was symptomatic due to recurrent bacteremia caused by C. fetus subsp. fetus. The second isolate was found 37 days after antibiotic therapy | Campylobacter fetus | Reptilian origin. Not reported how the patient acquired the pathogen. Chelonian cuisine or contact with pet reptile was suggested. | Pathogen was not able to be identified phenotypically at first. Molecular analysis (16S rRNA, then PCR, SapD sequencing) confirmed the pathogen was similar to C. fetus subsp. fetus and was of reptilian origin. | A febrile 27-year-old patient with precursor T-cell acute lymphoblastic leukemia. | [48] |
| China | Identification by multilocus sequence typing (MLST) 13 human cases of Campylobacter infection reported in Guangzhou in 2012 to 2013 |
Campylobacter fetus subsp. testudinum | Reptilian origin; Food or human–squamate contact was reported as most likely source as reptiles formed an integral part of Chinese cuisine. | Epidemiological data was unavailable for these nine cases. | 13 human cases of C. fetus reported. | [50] |
| Korea | Infectious spondylitis with bacteremia in a patient with chronic kidney disease was detected through 16S rRNA gene sequencing | C. fetus subsp. testudinum | Reptile | C. fetus spondylitis is a very rare disease. Confirmation of the identity of the squamate linked to the transmission was lacking. | 83-year-old male patient with end stage renal disease. | [46] |
| China | C. fetus subsp. testudinum strain 772 isolated from the ascites of a patient. Whole genome sequence of the C. fetus subsp. testudinum which is primarily isolated from reptile but can cause invasive infection in human was established. | C. fetus subsp. testudinum strain-772 | Reptilian food or human–squamate contact was reported as most likely source. | Complete genome sequence established. C. fetus subsp. testudinum from reptiles has zoonotic potential to cause infection in humans. | A patient with chronic kidney disease. | [51] |
| USA | Positive human infection with new subspecies of genetically distinct variant of C. fetus. | C. fetus subsp. testudinum subsp. nov | Reptile. Source reported to be related to traditional asian food or contact with reptile. | C. fetus association between reptiles and humans is well illustrated. Infection was related to exposure to foods of reptilian origin or due to human–reptile contact. | Positive cases in nine men of Asian origin, >60 years, with underlying illnesses | [47] |
In the studies analyzed for this review, molecular methods had been widely used to identify Campylobacter spp. isolates and analyze their epidemiology and population genetics. For example, in a reptile surveillance study by Wang et al. [32] involving 179 reptile fecal samples, 16S rRNA sequencing and biochemical methods were used to identify the positive Campylobacter fetus species. Using published subspecies-specific sequences and genomic data retrieved from GenBank and MLST database (http://pubmlst.org/), multiplex PCR was used to identify C. fetus subsp. fetus among the positive samples. Multilocus sequence typing (MLST) was then used to genotype the isolates and analyze for population genetics. Additionally, in a comparative genomics study by Gilbert et al. [39], C. fetus subsp. testudinum and C. iguaniorum isolates from reptiles’ (lizards, chelonians and snakes) fecal samples and human blood samples, were genomically compared with two strains of Campylobacter iguaniorum (Cig) isolated from lizards, Pogona vitticeps (Cig 1485E) and Iguana iguana (Cig 2463D). For all strains used in the study [39], comparison was done based on 16S rRNA, atpA gene sequences, MLST and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) to determine genotypic and phenotypic characters of the pathogens. The use of molecular methods such as whole genome sequencing (WGS) inform a better understanding of host adaptation, phylogeny and evolution of emerging Campylobacter strains differentiating them from recognized sub-species of C. fetus. The studies also confirmed zoonotic potential of Campylobacter spp. associated with squamate hosts.
The non-food related risk factors associated with Campylobacter infections include handling of pet squamates and wild animals, cross-contamination with surface waters contaminated by wild animals or through contact with lizard feces [34,52]. Where squamates are reared for pet trade or as companion animals, transmission may occur through cross-contamination or contact with their feces when cleaning their vivaria. In communities where reptiles are reared for food, consumption of reptile cuisines predisposes humans to Campylobacter infections [50]. People with chronic underlying illnesses, the elderly and the immunocompromised [46,47,49,51], were identified as the most vulnerable population associated with Campylobacter infections linked to both consumption of reptilian cuisines and human–pet contact practices. The common clinical signs of human campylobacteriosis associated with a reptilian source included productive cough, fever, epigastric pain, diarrhea and general body weakness [46,47,48].
3. Discussion
Reptiles kept as pets offer aesthetic, economic and cultural value in many parts of the world both historically and currently in traditional and modern societies [53]. There are also reported mental and physical benefits which people derive from pet ownership and companionship [54]. However, the increasing popularity of exotic pets, including lizards, snakes and turtles [36,37,38,55], coupled with the emerging novel strains of Campylobacter in squamates warrants serious concern from public health practitioners as the pets may harbor diseases or aid in transmission of pathogens of zoonotic potential. An estimated 60% of all known infectious disease pathogens and up to 75% of emerging infectious diseases are zoonotic and able to infect other host species [56,57]. Direct and indirect contact of people with domesticated squamates coupled with failure to adhere to proper hand hygiene and pet care practices potentially presents a risk of transmitting Campylobacter spp., especially C. jejuni and variants of Campylobacter fetus to humans [41,47]. This review presents evidence that squamate-associated campylobacteriosis is a potential public health threat globally.
Campylobacter fetus is an opportunistic zoonotic species that poses public health risks to immunocompromised people, patients with underlying chronic illnesses, young children, pregnant women and the elderly [58]. One of its subspecies, C. fetus subsp. fetus has a wide host range in vertebrate hosts and has veterinary significance as it causes abortion in cattle and sheep. Additionally, C. fetus subsp. venerealis is host restricted and causes high economic losses in cattle through infertility, abortions and lowered pregnancy rates. The subspecies has also been isolated from blood samples in humans presenting with bacteremia, infective aneurysm and vaginosis [59]. Lastly, the zoonotic C. fetus subsp. testudinum strain that is primarily found in healthy reptiles, and also in ill snakes, is transmissible and pathogenic to humans [39,49,60,61]. However, despite high incidences of C. fetus infections from the three species, bovine genital campylobacteriosis is the only OIE notifiable disease from the Campylobacter genus requiring mandatory reporting to the World Organization for Animal Health (Office International des Epizooties—OIE) [62]. There is need for increased knowledge and education on the zoonotic and public health risks of campylobacteriosis at the human–animal-environment interface. Additionally, appropriate approaches need to be implemented to manage emerging zoonotic strains such as C. fetus subsp. testudinum which continue to remain underestimated in humans, as evidenced by studies analyzed in this review.
One example of a collaborative approach applicable to managing zoonotic infections is the One Health concept. One Health is a multidisciplinary and holistic concept that recognizes interconnections of different components of ecological communities and the inextricable link between human, animal and environmental health through interfaces with food, livestock, wildlife and pathogens in the environment [63]. Human campylobacteriosis associated with squamates’ exposure is thus a One Health issue due to its relevance to food safety, zoonoses and antimicrobial resistance; which are health threats addressed by World Health Organization (WHO), OIE and the Food and Agricultural Organization (FAO) [64,65,66]. In this regard, implementation of a coordinated One Health approach would foster interdisciplinary collaboration, communication and sharing of resources to develop effective surveillance techniques, molecular diagnostic and therapeutic interventions that enhance health outcomes at the human–wildlife–livestock-environment interface. A One Health Zoonotic Disease Prioritization tool bringing together experts from human, animal, wildlife and environment health sectors to prioritize endemic and emerging zoonoses of greatest national concern in a country/region was developed by the Centers for Disease Control and Prevention (CDC) and successfully utilized in prioritizing zoonoses in seven countries [67]. The One Health approach was also successfully applied in the UK through multi-agency coordination, improved biosecurity, surveillance and public health programs leading to decline of human Salmonella infections in the 1990s [68,69]. The approach may therefore also find relevance and application in campylobacteriosis prevention, detection and response. The OIE Wildlife working group continues to provide appropriate guidelines that address increasing risk of disease spill over from wildlife to humans and domestic animals through capture, handling, poorly regulated trade and consumption of wildlife.
Squamates can also play a role in cross contamination of other environmental sources of human campylobacteriosis [29,52]. This is particularly a concern with captive squamates which have increased interaction with the built environment. There is also a higher pathogen carriage rate and shedding in captive lizards compared with free-living wild lizards. For example, a Malaysian study by Cheng, Wong and Dykes [70] found 83.3% of captive pet lizards were positive for Salmonella while only 25% of free-living wild lizards tested positive. Stressed animals are also more likely to shed more pathogens [71]. Stress could be attributed to abiotic environmental challenges and confinement-specific stressors that contribute to reduced fitness of a captive animal [72], thus there is need for animal welfare concerns to be addressed so as to avoid stress levels that may lead to shedding of disease pathogens by household pet squamates and animals in petting zoos. Although research has focused more on transmission of zoonoses from farm animals to humans, household pets and animals in petting zoos have also been identified as potential sources of exposure to campylobacteriosis for people who may typically not live on or visit farms but have contact with these captive animals [73,74].
Pet care education and responsible pet ownership is crucial in addressing physiological stress on captive exotic pets through ensuring proper housing/enclosures, diet, cleanliness and hygiene, temperature, UV light, humidity control and veterinary health care [75,76]. A study by Vučinić et al. [77] on reptile ownership, demographics and reliance on veterinary care in Balkan countries noted that 40% of pet reptile owners had never contacted veterinarians about medical conditions of their pets. Reptiles pose a significant zoonotic risk to pet owners, zookeepers and veterinarians as well as to the immunocompromised, young children and the elderly [78]. Sensitization on pet-associated disease risks, adherence to proper hygiene and human–animal contact practices thus need to be upscaled.
Reptiles, particularly lizards and snakes, are the primary reservoirs of the emerging Campylobacter species. One of these subspecies is the novel Campylobacter iguaniorum strain which is closely related to C. fetus subsp. testudinum and both colonize the same reptilian hosts [39]. In a study by Gilbert et al. [39], C. iguaniorum isolated from reptilian hosts (Pogona vitticeps and Iguana iguana) was compared with genomes of closely related reptilian C. fetus clade (C. fetus, C. hyointestinalis and C. lanienae). Homology was highest between C. iguaniorum and reptilian C. hyointestinalis and C. fetus than between C. iguaniorum and mammalian C. fetus strains. This may explain the possibility of lateral gene transfer as a result of sharing same host. Other reptiles species such as turtles and tortoises that are phylogenetically distant from squamates [79], are also increasingly popular as pets in some European and Asian countries [80]. Freshwater turtles are farmed in China for human consumption, consequently posing reptilian-associated Campylobacter infection risks to humans [81] in situations where food safety and proper hygiene practices are not adhered to.
As shown in Table 1, C. iguaniorum, C. fetus subsp. fetus and C. fetus subsp. testudinum were the most common subspecies of Campylobacter associated with the ectothermic squamates. Although Campylobacter jejuni has also been isolated in lizards, it is typically found in mammals and birds, which are endotherms. This differential distribution could be explained by the optimal temperatures for growth in endotherms and ectotherms. The temperature range of ectothermic vertebrates is 5–46 °C while the optimal temperature for growth in C. iguaniorum and C. fetus subsp. testudinum is 20–37 °C [82]. Since the mean voluntary temperature for reptiles ranges between 20–35 °C, this temperature range may be an adaptation favoring growth of the pathogens in the reptilian host [40]. On the other hand, with mammals and birds having constant body temperature of 37 and 41–42 °C, respectively, these temperatures would favor the growth of thermophilic C. jejuni whose optimal growth temperature is 37–42 °C [17].
4. Materials and Methods
This systematic literature review is based on an adapted version of the PRISMA statement [83]. A systematic search of the databases SCOPUS and Web of Science was performed using the search strategy detailed in Figure 1. Briefly, articles written in English over the last 20 years, with the key words; “(Campylobacter OR campylobacteriosis) AND (lizard OR lizards OR snake OR Squamata OR squamate OR snakes OR Amphisbaenia)” were searched for in Scopus (Elsevier, Netherlands) (n = 174) and in Web of Science (Web of Science core collection, Clarivate analytics, United States) databases (n = 92). In Web of Science, the following Boolean search string was used; “TS=(Campylobacter OR campylobacteriosis) AND TS = (lizard* OR Lacertilia OR Serpentes OR snake* OR amphisbaenian* OR squamate OR Squamata OR Bipedidae OR Blanidae OR Cadeidae OR Rhineuridae OR Trogonophidae OR Dibamidae OR Gecko* OR Pygopodidae OR Agamidae OR Agamas OR dragon* OR Chamaeleonidae OR Chameleon* OR Corytophanidae OR basilisk OR Crotaphytidae OR Hoplocercidae OR clubtail* OR Iguanidae OR Iguana* OR Leiosauridae OR Liolaemidae OR swifts OR Opluridae OR Phrynosomatidae OR Polychrotidae OR Tropiduridae OR Alopoglossidae OR Gymnophthalmidae OR Lacertidae OR Teiidae OR Anguidae OR slowworm* OR Anniellidae OR Helodermatidae OR “gila monster*” OR Xenosauridae OR Lanthanotidae OR Shinisauridae OR Varanidae OR “monitor lizard*” OR Acrochordidae OR Aniliidae OR Anomochilidae OR Boidae OR boa* OR Bolyeriidae OR Colubridae OR colubrid* OR Cylindrophiidae OR Elapidae OR cobra* OR mamba* OR krait* OR elapid* OR asp* OR Homalopsidae OR Lamprophiidae OR Loxocemidae OR Pareatidae OR Tropidophiidae OR python* OR Uropeltidae OR viper* OR pitviper* OR rattlesnake* OR Xenodermatidae OR Xenopeltidae OR Gerrhopilidae OR Leptotyphlopidae OR Typhlopidae OR Xenotyphlopidae) AND TS = (((Public OR human) NEAR/2 (health OR disease OR contaminant*)))”.
Once duplicates were removed (n = 189), titles and abstracts of the results obtained were read and initially excluded if they were review articles, or did not refer to human campylobacteriosis or Campylobacter spp. infection in humans (n = 31). Articles were then read in full and excluded if they referred to non-squamate transmitted campylobacteriosis, or if they referred to a squamate-linked Campylobacter spp. infection that had not been confirmed through animal testing. Articles were included if they referred to squamate-transmitted campylobacteriosis confirmed through testing of the animal or comparing the animal and human Campylobacter spp. isolates. Environmental surveillance studies investigating squamates as a potential risk for human campylobacteriosis were also included.
5. Conclusions
There has been increasing popularity of pet squamates globally as well as rising incidence of campylobacteriosis over the last decade. This review provides evidence that squamates may harbor Campylobacter spp. and are able to transfer them to humans through contaminated food and water, pet handling or cross-contamination through their feces. Improved educational efforts especially in ‘One Health’ as an emerging approach recognizing the inextricable link between human, animal and environmental health, will help in ensuring the general public, farmers and pet reptile owners are aware of the potential risks and zoonotic implications of campylobacteriosis from pet lizards and snakes. Knowledge and awareness about zoonotic diseases should be enhanced through harmonized and collaborative approaches among human, veterinary, and public health personnel. Additionally, there is need for proper adherence to hand hygiene, pet care services and improved human–animal contact practices in homes and petting zoos. Lastly, continued surveillance of emerging Campylobacter species through use of laboratory diagnostic tools and modern molecular techniques will aid in detection that informs more effective management strategies, hence leading to improved public health outcomes.
Author Contributions
Conceptualization: K.E.R. and H.W.; N.M.M. prepared the first draft, K.E.R., M.G.G. and H.W. edited first draft and provided academic input. All authors edited and contributed to the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hsieh Y.-H., Sulaiman I.M. Campylobacteriosis: An emerging infectious foodborne disease. Foodborne Dis. 2018:119–155. doi: 10.1016/B978-0-12-811444-5.00005-1. [DOI] [Google Scholar]
- 2.Kaakoush N.O., Castaño-Rodríguez N., Mitchell H.M., Man S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Igwaran A., Okoh A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon. 2019;5:e02814. doi: 10.1016/j.heliyon.2019.e02814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh Y.-H., Wang Y.F., Moura H., Miranda N., Simpson S., Gowrishankar R., Barr J., Kerdahi K., Sulaiman I.M. Application of MALDI-TOF MS systems in the rapid identification of Campylobacter spp. of public health importance. J. AOAC Int. 2018;101:761–768. doi: 10.5740/jaoacint.17-0266. [DOI] [PubMed] [Google Scholar]
- 5.Wilson D.J., Gabriel E., Leatherbarrow A.J., Cheesbrough J., Gee S., Bolton E., Fox A., Fearnhead P., Hart C.A., Diggle P.J. Tracing the source of campylobacteriosis. PLoS Genetics. 2008;4 doi: 10.1371/journal.pgen.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strachan N.J., Gormley F.J., Rotariu O., Ogden I.D., Miller G., Dunn G.M., Sheppard S.K., Dallas J.F., Reid T.M., Howie H. Attribution of Campylobacter infections in northeast Scotland to specific sources by use of multilocus sequence typing. J. Infect. Dis. 2009;199:1205–1208. doi: 10.1086/597417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du J., Luo J., Wang C., Li M., Wang B., Wang B., Chang H., Ji J., Sen K., He H. Emergence of genetic diversity and multi-drug resistant Campylobacter jejuni from wild birds in Beijing, China. Front. Microbiol. 2019;10:2433. doi: 10.3389/fmicb.2019.02433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pike B.L., Guerry P., Poly F. Global distribution of Campylobacter jejuni Penner serotypes: A systematic review. PloS ONE. 2013;8:e067375. doi: 10.1371/journal.pone.0067375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endtz H.P. Hunter’s Tropical Medicine and Emerging Infectious Diseases. 10th ed. Elsevier; Amsterdam, The Netherlands: 2020. Campylobacter Infections, Chapter 5. [Google Scholar]
- 10.Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.-A., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tack D.M., Ray L., Griffin P.M., Cieslak P.R., Dunn J., Rissman T., Jervis R., Lathrop S., Muse A., Duwell M. Preliminary incidence and trends of infections with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 US Sites, 2016–2019. Morb. Mortal. Wkly. Rep. 2020;69:509–514. doi: 10.15585/mmwr.mm6917a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Palacios G.M. The health burden of Campylobacter infection and the impact of antimicrobial resistance: Playing chicken. Clin. Infect. Dis. 2007;44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 13.Acheson D., Allos B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001;32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 14.Epps S.V., Harvey R.B., Hume M.E., Phillips T.D., Anderson R.C., Nisbet D.J. Foodborne Campylobacter: Infections, metabolism, pathogenesis and reservoirs. Int. J. Environ. Res. Public Health. 2013;10:6292–6304. doi: 10.3390/ijerph10126292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddad N., Marce C., Magras C., Cappelier J.-M. An overview of methods used to clarify pathogenesis mechanisms of Campylobacter jejuni. J. Food Prot. 2010;73:786–802. doi: 10.4315/0362-028X-73.4.786. [DOI] [PubMed] [Google Scholar]
- 16.Wallis M. The pathogenesis of Campylobacter jejuni. Br. J. Biomed. Sci. 1994;51:57–64. [PubMed] [Google Scholar]
- 17.Mihaljevic R.R., Sikic M., Klancnik A., Brumini G., Mozina S.S., Abram M. Environmental stress factors affecting survival and virulence of Campylobacter jejuni. Microb. Pathog. 2007;43:120–125. doi: 10.1016/j.micpath.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Baffone W., Casaroli A., Citterio B., Pierfelici L., Campana R., Vittoria E., Guaglianone E., Donelli G. Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model. Int. J. Food Microb. 2006;107:83–91. doi: 10.1016/j.ijfoodmicro.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Moran A., Upton M.E. Factors affecting production of coccoid forms by Campylobacter jejuni on solid media during incubation. J. Appl. Bacter. 1987;62:527–537. doi: 10.1111/j.1365-2672.1987.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 20.Joshua G.P., Guthrie-Irons C., Karlyshev A., Wren B. Biofilm formation in Campylobacter jejuni. Microbiology. 2006;152:387–396. doi: 10.1099/mic.0.28358-0. [DOI] [PubMed] [Google Scholar]
- 21.Stintzi A. Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J. Bacteriol. 2003;185:2009–2016. doi: 10.1128/JB.185.6.2009-2016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day W.A., Sajecki J.L., Pitts T.M., Joens L.A. Role of catalase in Campylobacter jejuni intracellular survival. Infect. Immun. 2000;68:6337–6345. doi: 10.1128/IAI.68.11.6337-6345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iraola G., Costa D. Pathogenomics of emerging Campylobacter Species. Clin. Microbiol. Rev. 2019;32:1–24. doi: 10.1128/CMR.00072-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newell D., Elvers K., Dopfer D., Hansson I., Jones P., James S., Gittins J., Stern N., Davies R., Connerton I. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl. Environ. Microbiol. 2011;77:8605–8614. doi: 10.1128/AEM.01090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taheri N., Fällman M., Wai S.N., Fahlgren A. Accumulation of virulence-associated proteins in Campylobacter jejuni outer membrane vesicles at human body temperature. J. Proteom. 2019;195:33–40. doi: 10.1016/j.jprot.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Kirkpatrick B.D., Tribble D.R. Update on human Campylobacter jejuni infections. Curr. Opin. Gastroenterol. 2011;27:1–7. doi: 10.1097/MOG.0b013e3283413763. [DOI] [PubMed] [Google Scholar]
- 27.European Food Safety Authority (EFSA) Panel on Biological Hazards (BIOHAZ) Scientific opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011;9:2105. doi: 10.2903/j.efsa.2011.2105. [DOI] [Google Scholar]
- 28.Navarro-Gonzalez N., Ugarte-Ruiz M., Domínguez L., Ruiz-Fons F. A European perspective on the transmission of foodborne pathogens at the wildlife–livestock–human interface. Food Saf. Risks Wildl. 2016:59–88. [Google Scholar]
- 29.Whiley H., Van den Akker B., Giglio S., Bentham R. The role of environmental reservoirs in human campylobacteriosis. Int. J. Environ. Res.Public Health. 2013;10:5886–5907. doi: 10.3390/ijerph10115886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rukambile E., Sintchenko V., Muscatello G., Kock R., Alders R. Infection, colonization and shedding of Campylobacter and Salmonella in animals and their contribution to human disease: A review. Zoonoses Public Health. 2019;66:562–578. doi: 10.1111/zph.12611. [DOI] [PubMed] [Google Scholar]
- 31.Murray M.H., Sánchez C.A., Becker D.J., Byers K.A., Worsley-Tonks K.E., Craft M.E. City sicker? A meta-analysis of wildlife health and urbanization. Front. Ecol. Environ. 2019;17:575–583. doi: 10.1002/fee.2126. [DOI] [Google Scholar]
- 32.Wang C.-M., Shia W.-Y., Jhou Y.-J., Shyu C.-L. Occurrence and molecular characterization of reptilian Campylobacter fetus strains isolated in Taiwan. Vet. Microbiol. 2013;164:67–76. doi: 10.1016/j.vetmic.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Bjelland A.M., Sandvik L.M., Skarstein M.M., Svendal L., Debenham J.J. Prevalence of Salmonella serovars isolated from reptiles in Norwegian zoos. Acta Vet. Scand. 2020;62:1–9. doi: 10.1186/s13028-020-0502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whiley H., McLean R., Ross K. Detection of Campylobacter jejuni in lizard faeces from central Australia using quantitative PCR. Pathogens. 2017;6:1. doi: 10.3390/pathogens6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cogger H. General description and definition of the order Squamata. Fauna Aust. 1993;2:157–171. [Google Scholar]
- 36.Alves R.R.N., de Araújo B.M.C., da Silva Policarpo I., Pereira H.M., Borges A.K.M., da Silva Vieira W.L., Vasconcellos A. Keeping reptiles as pets in Brazil: Ethnozoological and conservation aspects. J. Nat. Conserv. 2019;49:9–21. doi: 10.1016/j.jnc.2019.02.002. [DOI] [Google Scholar]
- 37.Benn A.L., McLelland D.J., Whittaker A.L. A review of welfare assessment methods in reptiles, and preliminary application of the welfare quality® protocol to the pygmy blue-tongue skink, Tiliqua adelaidensis, using animal-based measures. Animals. 2019;9:27. doi: 10.3390/ani9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuppli C.A., Fraser D., Bacon H.J. Welfare of non-traditional pets. Rev. Sci. Tech. 2014;33:221–231. doi: 10.20506/rst.33.1.2287. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert M.J., Miller W.G., Yee E., Kik M., Zomer A.L., Wagenaar J.A., Duim B. Comparative genomics of Campylobacter iguaniorum to unravel genetic regions associated with reptilian hosts. Genome Biol. Evol. 2016;8:3022–3029. doi: 10.1093/gbe/evw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert M.J., Miller W.G., Yee E., Kik M., Wagenaar J.A., Duim B. Complete genome sequence of Campylobacter iguaniorum strain 1485ET, isolated from a bearded dragon (Pogona vitticeps) Genome Announc. 2014;2:e00844-14. doi: 10.1128/genomeA.00844-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C.-M., Wu Z.-Y., Shia W.-Y., Jhou Y.-J., Tung K.-C., Shyu C.-L. Complete genome sequence of Campylobacter fetus subsp. testudinum strain Pet-3, isolated from a lizard (Hydrosaurus pustulatus) Genome Announc. 2015;3:e01420-14. doi: 10.1128/genomeA.01420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbert M.J., Kik M., Miller W.G., Duim B., Wagenaar J.A. Campylobacter iguaniorum sp. nov., isolated from reptiles. Int. J. Syst. Evol. Microbiol. 2015;65:975–982. doi: 10.1099/ijs.0.000048. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert M.J., Kik M., Timmerman A.J., Severs T.T., Kusters J.G., Duim B., Wagenaar J.A. Occurrence, diversity, and host association of intestinal Campylobacter, Arcobacter, and Helicobacter in reptiles. PLoS ONE. 2014;9:e101599. doi: 10.1371/journal.pone.0101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dingle K.E., Blaser M.J., Tu Z.-C., Pruckler J., Fitzgerald C., Van Bergen M.A., Lawson A.J., Owen R.J., Wagenaar J.A. Genetic relationships among reptilian and mammalian Campylobacter fetus strains determined by multilocus sequence typing. J. Clin. Microbiol. 2010;48:977–980. doi: 10.1128/JCM.01439-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilbert M.J., Miller W.G., Yee E., Zomer A.L., Van Der Graaf-Van Bloois L., Fitzgerald C., Forbes K.J., Méric G., Sheppard S.K., Wagenaar J.A. Comparative genomics of Campylobacter fetus from reptiles and mammals reveals divergent evolution in host-associated lineages. Genome Biol. Evol. 2016;8:2006–2019. doi: 10.1093/gbe/evw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi H.S., Shin S.U., Bae E.H., Ma S.K., Kim S.W. Infectious spondylitis in a patient with chronic kidney disease: Identification of Campylobacter fetus subsp. testudinum with 16S ribosomal RNA sequencing. Jpn. J. Infect. Dis. 2016;69:517–519. doi: 10.7883/yoken.JJID.2015.461. [DOI] [PubMed] [Google Scholar]
- 47.Patrick M.E., Gilbert M.J., Blaser M.J., Tauxe R.V., Wagenaar J.A., Fitzgerald C. Human infections with new subspecies of Campylobacter fetus. Emerg. Infect. Dis. 2013;19:1678–1680. doi: 10.3201/eid1910.130883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu Z.-C., Zeitlin G., Gagner J.-P., Keo T., Hanna B.A., Blaser M.J. Campylobacter fetus of reptile origin as a human pathogen. J. Clin. Microbiol. 2004;42:4405–4407. doi: 10.1128/JCM.42.9.4405-4407.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzgerald C., chao Tu Z., Patrick M., Stiles T., Lawson A.J., Santovenia M., Gilbert M.J., Van Bergen M., Joyce K., Pruckler J. Campylobacter fetus subsp. testudinum subsp. nov., isolated from humans and reptiles. Int. J. Syst. Evol. Microbiol. 2014;64:2944–2948. doi: 10.1099/ijs.0.057778-0. [DOI] [PubMed] [Google Scholar]
- 50.Hou S., Qu P., Wu Y., Zhang X., Hu Y., Deng Z., Wu X. The identification and multilocus sequence typing of nine Campylobacter fetus isolates from specimens of patients from 2012 to 2013. Chin. J. Prev. Med. 2015;49:744–746. [PubMed] [Google Scholar]
- 51.Hou S.-P., He P., Zhou Y., Wu X.-W. Complete genome sequence of Campylobacter fetus subsp. testudinum strain 772, isolated from ascites of a patient with chronic kidney disease. Genome Announc. 2018;6:e00432-18. doi: 10.1128/genomeA.00432-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levin R.E. Campylobacter jejuni: A review of its characteristics, pathogenicity, ecology, distribution, subspecies characterization and molecular methods of detection. Food Biotechnol. 2007;21:271–347. [Google Scholar]
- 53.Mittermeier R.A., Carr J.L., Swingland I.R., Werner T.B., Mast R.B. Conservation of amphibians and reptiles. In: Adler K., editor. Herpetology: Current Research on the Biology of Amphibians and Reptiles. Society for the Study of Amphibians and Reptiles Publication; St. Louis, MO, USA: 1992. pp. 59–80. [Google Scholar]
- 54.Stull J.W., Peregrine A.S., Sargeant J.M., Weese J.S. Household knowledge, attitudes and practices related to pet contact and associated zoonoses in Ontario, Canada. BMC Public Health. 2012;12:553. doi: 10.1186/1471-2458-12-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alves R.R.N., Rocha L.A. Fauna at home: Animals as pets. Ethnozoology. 2018:303–321. doi: 10.1016/B978-0-12-809913-1.00016-8. [DOI] [Google Scholar]
- 56.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woolhouse M.E., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Culligan E.P., O’Connor J., Lynch C., O’Brien D., Vaughan C., Bolton D., Coffey A., Sleator R.D., Lucey B. Draft Genome Sequence of Campylobacter fetus subsp. fetus CITCf01, isolated from a patient with subacute bacterial endocarditis. Microbiol. Resour. Announc. 2019;8:e01556-18. doi: 10.1128/MRA.01556-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu F., Ma R., Wang Y., Zhang L. The clinical importance of Campylobacter concisus and other human hosted Campylobacter Species. Front. Cell. Infec. Microbiol. 2018;8:243. doi: 10.3389/fcimb.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calleros L., Betancor L., Iraola G., Méndez A., Morsella C., Paolicchi F., Silveyra S., Velilla A., Pérez R. Assessing the intra-species genetic variability in the clonal pathogen Campylobacter fetus: CRISPRs are highly polymorphic DNA markers. J. Microbiol. Methods. 2017;132:86–94. doi: 10.1016/j.mimet.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 61.Giacomelli M., Piccirillo A. Pet reptiles as potential reservoir of Campylobacter species with zoonotic potential. Vet. Record. 2014;174:479. doi: 10.1136/vr.102243. [DOI] [PubMed] [Google Scholar]
- 62.World Organization for Animal Health (OIE) OIE Terrestrial Manual. 7th ed. Paris, France: 2018. [(accessed on 30 August 2020)]. Chapter 3.4.4.—Bovine Genital Campylobacteriosis. Available online: https://www.oie.int/standard-setting/terrestrial-manual/access-online/ [Google Scholar]
- 63.Sato M.O., Sato M., Adsakwattana P., Fontanilla I.K. Preface to “Zoonotic Diseases and One Health”. In: Sato M.O., Sato M., Adsakwattana P., Fontanilla I.K., editors. Zoonotic Diseases and One Health, Special Edition. MDPI Pathogens; Basel, Switzerland: 2020. [Google Scholar]
- 64.Wielinga P.R., Schlundt J. Food Safety: At the center of a One Health approach for combating zoonoses. One Health: Hum.-Anim.-Environ. Interfaces Emerg. Infect. Dis. 2012:3–17. doi: 10.1007/82_2012_238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization (WHO) Taking A Multisectoral One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. FAO, OIE and WHO; Geneva, Switzerland: 2019. [Google Scholar]
- 66.Lubroth J. FAO and the One Health approach. In: Mackenzie J.S., Jeggo M., Daszak P., Richt J.A., editors. One Health: The Human-Animal-Environment Interfaces in Emerging Infectious Diseases. Volume 366 Springer; Berlin, Germany: 2012. [Google Scholar]
- 67.Salyer S.J., Silver R., Simone K., Behravesh C.B. Prioritizing zoonoses for global health capacity building—Themes from One Health zoonotic disease workshops in 7 countries, 2014–2016. Emerg. Infect. Dis. 2017;23:S55. doi: 10.3201/eid2313.170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cogan T., Humphrey T. The rise and fall of Salmonella enteritidis in the UK. J. Appl. Microbiol. 2003;94:114–119. doi: 10.1046/j.1365-2672.94.s1.13.x. [DOI] [PubMed] [Google Scholar]
- 69.Brown H.L., Passey J.L., Getino M., Pursley I., Basu P., Horton D.L., La Ragione R.M. The One Health European Joint Programme (OHEJP), 2018–2022: An exemplary One Health initiative. J. Med Microbiol. 2020;69:1037–1039. doi: 10.1099/jmm.0.001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng B.Y., Wong S.P., Dykes G.A. Salmonella associated with captive and wild lizards in Malaysia. Herpetol. Notes. 2014;7:145–147. [Google Scholar]
- 71.Rostagno M.H. Can stress in farm animals increase food safety risk? Foodborne Path. Dis. 2009;6:767–776. doi: 10.1089/fpd.2009.0315. [DOI] [PubMed] [Google Scholar]
- 72.Morgan K.N., Tromborg C.T. Sources of stress in captivity. App. Anim. Behav.Sci. 2007;102:262–302. doi: 10.1016/j.applanim.2006.05.032. [DOI] [Google Scholar]
- 73.Conrad C.C., Stanford K., Narvaez-Bravo C., Callaway T., McAllister T. Farm fairs and petting zoos: A review of animal contact as a source of zoonotic enteric disease. Foodborne Path. Dis. 2017;14:59–73. doi: 10.1089/fpd.2016.2185. [DOI] [PubMed] [Google Scholar]
- 74.Pintar K.D., Christidis T., Thomas M.K., Anderson M., Nesbitt A., Keithlin J., Marshall B., Pollari F. A systematic review and meta-analysis of the Campylobacter spp. prevalence and concentration in household pets and petting zoo animals for use in exposure assessments. PLoS ONE. 2015;10:e0144976. doi: 10.1371/journal.pone.0144976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Vosjoli P. The Lizard Keeper’s Handbook. i5 Publishing; Mount Joy, PA, USA: 2012. [Google Scholar]
- 76.Marshall C. Housing and husbandry of snakes and lizards. Vet. Nurs. J. 1993;8:38–45. doi: 10.1080/17415349.1993.11012510. [DOI] [Google Scholar]
- 77.Vučinić M., Hajzler I., Terzin J., Nenadović K., Janković L., Voslarova E., Vučićević M. Reptile ownership in Balkan countries: Demographics and reliance on veterinary advice. Anthrozoös. 2019;32:129–139. [Google Scholar]
- 78.Scheelings T.F., Lightfoot D., Holz P. Prevalence of Salmonella in Australian reptiles. J. Wildl. Dis. 2011;47:1–11. doi: 10.7589/0090-3558-47.1.1. [DOI] [PubMed] [Google Scholar]
- 79.Chiari Y., Cahais V., Galtier N., Delsuc F. Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria) BMC Biology. 2012;10:65. doi: 10.1186/1741-7007-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertrand S., Rimhanen-Finne R., Weill F., Rabsch W., Thornton L., Perevoščikovs J., Van Pelt W., Heck M., Eurosurveillance editorial team Salmonella infections associated with reptiles: The current situation in Europe. Eurosurveillance. 2008;13:18902. doi: 10.2807/ese.13.24.18902-en. [DOI] [PubMed] [Google Scholar]
- 81.Haitao S., Parham J.F., Zhiyong F., Meiling H., Feng Y. Evidence for the massive scale of turtle farming in China. Oryx. 2008;42:147–150. doi: 10.1017/S0030605308000562. [DOI] [Google Scholar]
- 82.Vitt L.J., Zug G.R., Caldwell J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles. 2nd ed. Academic press; Waltham, MA, USA: 2001. [Google Scholar]
- 83.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]