Abstract
Investigations were carried out to study the effects of light-emitting diode (LED) lights on growth and development of isosteroidal alkaloids in embryogenic calli of Fritillaria cirrhosa D. Don, an important traditional Chinese medicine herb. Calli were cultured in glass bottles, each containing 100 mL of Murashige and Skoog’s basal medium supplemented with 2% sucrose and 0.4% gellan gum powder, a gelling agent. These bottles were incubated in a specially designed plant growth chamber equipped with eight different LED lights consisting of single or combinations of four different light spectra emitting blue (450 nm), green (525 nm), red (660 nm), and far-red (730 nm) light. After three months of incubation, morphological changes in embryogenic calli were recorded, and LC-MS/MS analysis of cultures was carried out for peimisine, sipeimine, peiminine, and peimine. The highest number of somatic embryos and the maximum fresh weight was recorded in calli incubated under red (9R), infrared (9IR), and a combination of red+blue+infrared (3R3B3IR), respectively, in decreasing order. The highest contents of peimisine, peiminine, and peimine were recorded under red (9R) and infrared (9IR) lights, respectively. Eight LED lights had significant effects on the morphogenesis of embryogenic calli of F. cirrhosa D. Don and contents of isosteroidal alkaloids.
Keywords: Fritillaria cirrhosa D. Don, alkaloid content, callus, in vitro culture, LED lights, light intensity
1. Introduction
Fritillaria, a bulbiferous and perennial monocot plant genus, belongs to the family Liliaceae. The genus consists of about 130 species distributed in the temperate regions of Central Asia and the Mediterranean region [1]. Though some Fritillaria species are grown as ornamental plants, several Fritillaria species possess valuable medicinal properties. Fritillaria bulbs composed of fleshy, farinaceous scales constitute essential plant parts and have been used to relieve cough for centuries [2].
In different Fritillaria species, a majority of bioactive compounds (86%) identified so far (~130) consist of isosteroidal alkaloid skeletons [3]. Alkaloids in Fritillaria bulbs are the main bioactive compounds responsible for relief from coughs [3,4]. However, the quantities and types of alkaloids vary depending on species [1,3,4,5].
In a recent study, it was found that peimine, an alkaloid from Fritillaria, blocked the Nav1.7 ion channel and inhibited the Kv1.3 ion channel in HEK 293 cell lines, indicating that the compound has a role in relieving pain and possesses anti-inflammatory properties [6]. More recently, Liu and co-workers investigated the potential effect and mechanism of six isosteroidal alkaloids on oxidative stress. The findings showed that F. cirrhosa D. Don bulbs might play a protective role in cellular oxidative stress by activating the Nrf2-mediated antioxidant pathway [7].
China is the center of diversity of the Fritillaria genus. F. cirrhosa D. Don (FC) is an important traditional Chinese medicine commonly known as “Chuanbeimu” (川貝母), and is one of the most exploited species. Due to scarcity, the price of wild F. cirrhosa D. Don bulbs escalated almost nine times from $60 to $560 USD between 2002–2017 [8]. Due to the excessive collection of FC bulbs from natural habitats, the species is now under protection [9]. Therefore, alternative propagation methods of FC bulbs and the production of critical isosteroidal alkaloids by tissue culture techniques must be optimized.
The culture of plant tissues and organs is an important bio-technique to produce secondary plant metabolites under controlled environmental conditions in a laboratory. Under culture conditions, callus (an undifferentiated mass of cells) can easily be induced, practically from any living plant part. Induced callus can be cultured and multiplied in vitro on a defined nutrient medium with or without plant growth regulators. There are several advantages of using callus cultures as a source of valuable secondary metabolites, including (i) ease of induction and multiplication; (ii) production of bioactive compounds throughout the year independent of season; (iii) whole plants do not need to be cultivated, especially rare and endangered species; (iv) amenability of scaling by bioreactors for mass production, etc. More recently, we have reported on the micropropagation of bulblets and the production of isosteroidal alkaloids in tissue culture-derived materials of F. cirrhosa D. Don [10]. Several recent studies have demonstrated that the light quality not only affects morphogenetic responses in plants but has significant effects on their physiological processes, including metabolic pathways and the production of secondary metabolites [11,12].
A recent review listed several studies on the effects of light quality on the production of secondary metabolites in different plant species [11]. In the present study, four isosteroidal alkaloids (peimisine, sipeimine, peiminine, and peimine) were analyzed considering their therapeutic effects. Peiminine (Pm) is one of the major isosteroidal alkaloids in Fritillaria that is reported to have extensive pharmacological activities, including anti-inflammatory [6], anti-cancer [13], and antioxidant [14] capabilities. In another report, sipeimine and peiminine from bulbs of F. wabuensis inhibited pro-inflammatory mediators in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophage cells [15]. More recently, it was demonstrated that peimine relieved inflammatory effects in IL-1β-induced chondrocytes, indicating that peimine might be a potential therapeutic agent for osteoarthritis [16]. Antitussive, expectorant, and anti-inflammatory effects of several alkaloids, including sipeimine, chuanbeinone, peiminine, and peimine isolated from Bulbus Fritillaria cirrhosa were demonstrated in mice by using a phytochemical method [17]. A most recent study confirmed the anti-cancer effects of sipeimine obtained from bulbs of F. cirrhosa against non-small cell lung cancer (NSCLC) both in vivo and in vitro [18]. This anti-cancer property of sipeimine is largely due to anti-inflammation action affected by NF-κB inhibition, making it a potential drug candidate for treating cancer at early stages [18]. Recently, Yin and co-workers have reported several therapeutic properties of peimine, including anti-cancer, anti-inflammatory, antitussive, expectorant, and sedative [19]. A more recent study has also demonstrated cough relief by peimine by affecting the systemic network of proteins and pathways [20].
The objective of the present study is to investigate the effects of different LED lights on growth and development in embryogenic calli and the contents of four isosteroidal alkaloids (peimisine, sipeimine, peiminine, and peimine) in in vitro cultures of F. cirrhosa D. Don. Findings in the study may be of help to produce certain alkaloids under laboratory conditions irrespective of the season and thus avoid having to collect F. cirrhosa D. Don bulbs from the wild.
2. Material and Methods
2.1. Callus Multiplication
Callus obtained in our previous experiments, as reported earlier [10], was further multiplied in the liquid medium. Callus was cultured in 125 mL Erlenmeyer flasks, each containing 20 mL basal salts and vitamins of Murashige and Skoog [21] medium (MSBM) supplemented with 2,4-D (0.5 mg/L) and 2% sucrose (Sigma). The culture flasks were placed on an orbital shaker (Model SK-302A, Sun Kaun Instruments Co., Taichung, Taiwan) set at 100 rpm and incubated at 25 ± 1 °C in the dark.
2.2. Influence of Different Light Spectra on Morphogenesis in Embryogenic Calli and Contents of Isosteroidal Alkaloids
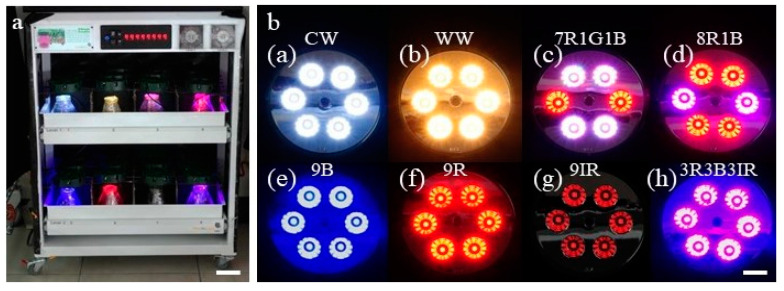
To investigate the effects of LED lights on the morphogenesis of embryogenic calli and the contents of isosteroidal alkaloids, embryogenic calli from liquid cultures were taken out and kept for 1 min on sterilized filter paper in a laminar flow before inoculation to glass bottles. Callus (3.0 g) was cultured in glass bottles (650 mL capacity), each containing 100 mL of MSBM medium with 2% sucrose and 0.4% gellan gum powder (GPP), a gelling agent (PhytoTechnology Laboratories®, USA). The pH of the medium was adjusted to 5.7 ± 0.1 before the addition of GPP and autoclaving at 1.05 kg/cm for 15 min. To facilitate LED light exposure to cultures, each bottle was closed with a piece of transparent, autoclavable plastic sheet. These bottles were incubated in a specially designed plant growth chamber equipped with eight different LED lights (Nano Bio Light Technology Co., Ltd., Taiwan). The chamber had two tiers, and each tier had four partitions (Figure 1a). Culture bottles were kept in these eight sections and exposed to different light spectra by eight specially designed LED lids (CW-5000K, WW-2700K, 8R1B, 7R1G1B, 3R3B3IR, 6R, 6B, and 6IR) (Figure 1b). LED lids CW-5000K and WW-2700K represented cool (C) and warm (W) white (W) light, while 5000K and 2700K represented color temperature, respectively. As reported in a previous study from our laboratory [22], six of these LED lids emitted single or combinations of four different light spectra with wavelengths such as blue (450 nm), green (525 nm), red (660 nm), and far-red (730 nm). The symbols in each LED lid code and the spectral distribution (quantum ratio) in eight LED lids were as follows: blue (B), green (G), red (R), infrared (IR), CW-5000K (28:43:29:0), WW-2700K (8:46:46:0), 8R1B (16:0:84:0), 7R1G1B (17:9:74:0), 3R3B3IR (57:0:43:37), 9R (0:0:100:0), 9B (100:0:0:0), and 9IR (0:0:0:100). In this work, the number (9, 7, 3, 1) in each LED lid code represents the number of LED chips in a particular lid. The light intensity of each LED lid was as follows: CW-5000K* (57 μmol m−2 s−1); WW-2700K (56 μmol m−2 s−1); 7R1G1B (56 μmol m−2 s−1); 8R1B (57 μmol m−2 s−1); 9B (57 μmol m−2 s−1); 9R (56); 9IR (10 μmol m−2 s−1); 3R3B3IR (56 μmol m−2 s−1).
Figure 1.
(a) Plant growth chamber with LED lights. Bar = 8.3 cm; (b) Eight different LED lights: (a) CW-5000 K, (b) WW-2700 K, (c) 7R1G1B, (d) 8R1B, (e) 9B, (f) 9R, (g) 9IR, (h) 3R3B3IR. Bar = 2 cm. Red (R): 660 nm; green (G): 525 nm; blue (B): 450 nm; infrared (IR): 730 nm.
The LED growth chamber was set on a 16 h light and 8 h dark cycle, kept in a culture room set at 25 ± 2 °C, and fully covered by a thick, dark cloth to cut off outside light. Morphological features of embryogenic callus under each light condition were recorded after three months of incubation. In addition, cultures under different LED lights were analyzed by LC-MS/MS for contents of peimisine, sipeimine, peiminine, and peimine.
2.3. Development of Bulblets from Somatic Embryos
For the development of bulblets, single somatic embryos (SEs), clusters with five somatic embryos each, and a single embryo with cotyledonary leaf obtained from cultures under CW5000K or WW2700K lights were further transferred to fresh MSBM medium in glass bottles as described in Section 2.2. Each bottle contained seven explants. These bottles were incubated in a culture room with a temperature set at 25 ± 2 °C, and under light (white fluorescent tubes, illumination intensity of 34 μmol m−2 s−1) with a 16 h light and 8 h dark cycle. Observations of bulblet development in all three types of SEs were recorded after three months of incubation.
Microphotographs of cultures were taken by a digital camera (Nikon D90, Tokyo, Japan). For SEM, samples of bulblets were frozen in liquid nitrogen and observed using a scanning electron microscope (JEOL JSM-6330F, Tokyo, Japan).
2.4. Chemicals and Other Materials
Peimisine, sipeimine, peiminine, and peimine standards were procured from SunHank Technology Co., Ltd., Taiwan. Ammonium formate, sodium acetate, and octadecyltrimethoxysilane were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ammonium hydroxide solution, ferric chloride hexahydrate, ethylene glycol, and acetonitrile were obtained from Fluka (Steinheim, Germany), Alfa Aesar (Heysham, UK), Acros Organics (Morris County, NJ, USA), and J. T. Baker (Phillipsburg, NJ, USA), respectively. Methanol and ethanol were purchased from Merck (Darmstadt, Germany). Ultrapure water (18.2 MΩ) was freshly obtained from a Millipore Simplicity system (MilliporeSigma, Bedford, MA, USA). The stock solutions (1 mg/mL) of each analyte were prepared in methanol separately and stored in the dark at −30 °C. The working solutions were prepared by diluting the stock solutions before use.
2.5. Preparation of Fe3O4@C18 Nanoparticle Composite
The adsorbent (magnetic nanoparticles, Fe3O4@C18) used for magnetic solid-phase extraction was synthesized according to the literature [23,24] with minor modification. Briefly, ferric chloride hexahydrate (2.7 g) and sodium acetate (7.2 g) were dissolved entirely in ethylene glycol (100 mL) before being poured into a Teflon-lined stainless steel autoclave heated at 200 °C for 8 h. The resultant Fe3O4 nanoparticles were washed with ethanol and dried. Afterward, the Fe3O4 nanoparticles (10 mg) were dispersed in a mixture of 970 µL ethanol, 10 µL water, and 20 µL octadecyltrimethoxysilane through sonication for 30 s, followed by shaking for 8 h at 45 °C. The derivatized Fe3O4@C18 was washed with ethanol, water, and acetonitrile three times, respectively. The final product was resuspended in 1 mL of acetonitrile (10 mg/mL).
2.6. Extraction Procedure
Ultra-pure water (2 mL) was added to each 0.1 g powdered sample of in vitro culture, 3-month-old in vitro derived bulblets, and 3-year-old wild type commercial bulbs before vortexing for 1 min and letting stand for 5 min. After centrifugation at 4000 rpm for 5 min, 0.8 mL of supernatant was collected. Then, 8 μL internal standard (50 μg/mL), 50 μL ammonia solution, and 140 μL Fe3O4@C18 were added into the supernatant with a vortex treatment for 30 s then left to stand still for 5 min. With the help of an external magnet, the analyte-adsorbed Fe3O4@C18 was rapidly separated from the supernatant and vortexed with 100 μL acetonitrile for 30 s to elute the analytes. After that, the external magnet was used to settle the magnetic adsorbent while the elution solution was filtered through a 0.22 μm PTFE (polytetrafluoroethylene) membrane for LC-MS/MS analysis.
2.7. LC-MS/MS Conditions
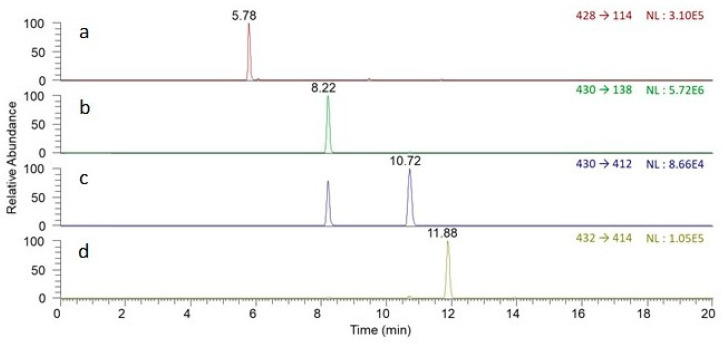
The analysis was performed with a Surveyor LC-MS/MS system (Thermo Scientific, Waltham, CA, USA). The chromatographic separation was achieved by using a Thermo Scientific Accucore C18 column (2.1 × 100 mm, 2.6 µm) at a constant column temperature of 30 °C with a flow rate of 0.2 mL/min. The mobile phase A was 10 mM ammonium formate aqueous solution containing 0.1% ammonia solution, and mobile phase B was methanol. The gradient elution program was started at 80% A for 1 min, dropped to 35% A in 1 min, decreased to 20% A in 2 min, held for 5 min, decreased to 10% A within 2 min, held for 3 min, resumed at 80% A within 1 min, and kept constant for 5 min. Figure 2 shows the typically extracted ion chromatograms of the mixed standard solution of peimisine, sipeimine, peiminine, and peimine at the concentration of 0.5 µg/mL.
Figure 2.
Extracted ion chromatograms of isosteroidal alkaloid standards (0.5 µg/mL): (a) Peimisine, (b) Sipeimine, (c) Peiminine, and (d) Peimine.
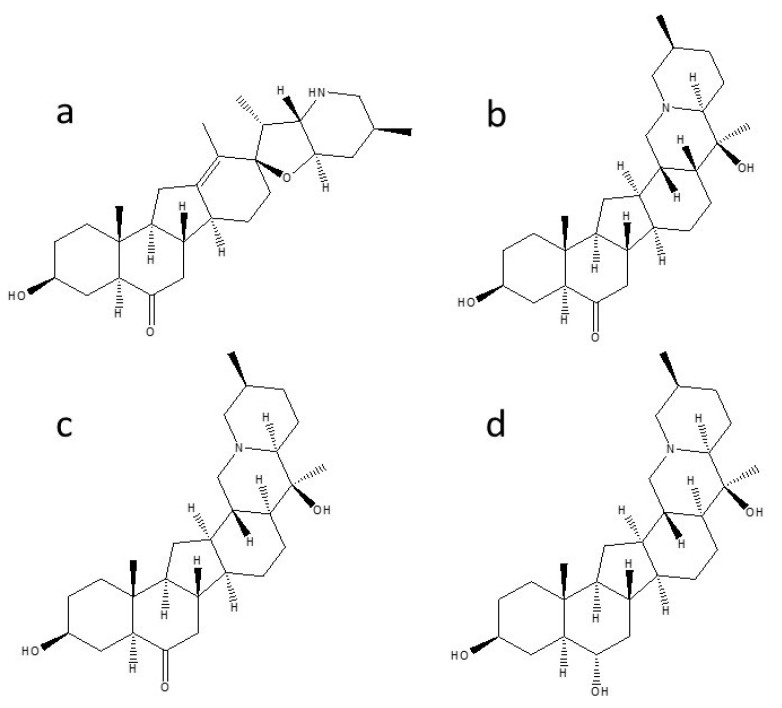
The mass spectrometer was equipped with an electrospray ionization (ESI) interface operating in positive ion mode. The selected reaction monitoring (SRM) mode was used to acquire the mass spectrometric data. The full width at half maximum (FWHM) of Q1 and Q3 was 0.7 for both. The optimal ESI source parameters were set as follows: the capillary temperature was 300 °C; spray voltage was 4600 V; the pressure of sheath gas, aux gas, and ion sweep gas was maintained at 45, 10, and 10 arb units, respectively. The ion transitions and optimal collision energy of selected reaction monitoring chosen for quantitative analysis were as follows: m/z 428→m/z 114 (44 eV) for peimisine; m/z 430→m/z 138 (48 eV) for sipeimine; m/z 430→m/z 412 (38 eV) for peiminine; m/z 432→m/z 414 (40 eV) for peimine. The retention time, protonated molecule ions (represented as [M+H]+), and the analytical parameters of the developed method for analysis of four alkaloids are listed in Table 1, and chemical structures are shown in Figure 3.
Table 1.
LC-MS/MS based method development and validation for four standards.
| Marker Compounds | tR a (min) | Mass/Charge (m/z) | Linearity and Range | Sensitivity c | |||
|---|---|---|---|---|---|---|---|
| Regression Equation b | Correlation Coefficient (r2) | Linear Range (μg/g) | LOD (ng/g) | LOQ (ng/g) | |||
| Peimisine | 5.78 | 428.316 | y = 0.0396x + 0.0630 | 0.9923 | 0.1–40 | 0.01 | 0.04 |
| Sipeimine | 8.22 | 430.332 | y = 0.5426x + 0.1531 | 0.9968 | 0.1–40 | 0.02 | 0.06 |
| Peiminine | 10.72 | 430.332 | y = 0.0047x + 0.0010 | 0.9967 | 0.1–40 | 0.97 | 3.23 |
| Peimine | 11.88 | 432.347 | y = 0.0067x + 0.0016 | 0.9977 | 0.1–40 | 0.56 | 1.88 |
a tR: Retention time. b The regression equations are presented as y = mx+c, and y and x are defined as peak area and concentration of the compound, respectively. c LOD: Limit of detection, S/N = 3; LOQ: limit of quantification, S/N = 10.
Figure 3.
Chemical structures of four isosteroidal alkaloids: (a) Peimisine, (b) Sipeimine, (c) Peimine, (d) Peiminine.
2.8. Statistical Analysis
Software SAS 9.1 was used for statistical analysis. Data were subjected to the least significant difference (LSD) test at a 5% probability level (p < 0.05) wherever possible. In Table 2 and Table 3, the number of replicates is 3 (three bottles under each LED light treatment). In Table 4, the number of replicates is 21 (each bottle had 7 explants, and each treatment had 3 bottles). The experiments were repeated three times, including LC-MS/MS analysis.
Table 2.
Influence of different LED lights on the growth and development of embryogenic callus cultures *.
| LED Light Treatment | Av No. of Somatic Embryos/Bottle | Av No. Of Somatic Embryos with Cotyledonary Leaves/Bottle | Av Total Fresh Weight of Cultures/Bottle (g) | Morphological Features of Cultures (Somatic Embryos = SEs) |
|---|---|---|---|---|
| CW-5000K * | 63.7 ± 7.6 c ** | 12.0 ± 6.0 abc | 12.82 ± 1.09 c | SEs at all stages from globular to mature, SEs with long cotyledonary leaves. Color of cultures is light green. |
| WW-2700K | 122.0 ± 65.0 bc | 22.3 ± 13.9 a | 13.64 ± 2.82 c | SE stages are similar to CW-5000K. Color of cultures is light green. |
| 7R1G1B | 108.0 ± 38.2 bc | 8.5 ± 3.5 bc | 12.99 ± 2.02 c | A majority of SEs in early stages, including globular shapes. Cotyledonary leaves absent. Color of cultures is light green to light brown. |
| 8R1B | 157.3 ± 9.3 ab | 16.7 ± 8.3 ab | 15.23 ± 0.97 abc | Stages similar to CW-5000K. Color of cultures is light green. |
| 9B | 169.0 ± 66.1 ab | 3.7 ± 2.3 c | 14.61 ± 1.02 bc | SE stages similar to CW-5000K, but cotyledonary leaves shorter in length. Color of cultures is light green to light yellow. |
| 9R | 223.7 ± 57.5 a | 5.3 ± 4.9 c | 17.92 ± 0.77 a | A majority of SEs in the early stages, including globular shapes. Cotyledonary leaves absent. Color of cultures is white. |
| 9IR | 231.3 ± 62.3 a | 4.7 ± 0.6 c | 16.67 ± 1.85 ab | SE stages and color of cultures similar to 9R. Only a few cotyledonary leaves are seen. Color of cultures is white. |
| 3R3B3IR | 230.7 ± 23.4 a | 4.3 ± 2.5 c | 17.56 ± 2.35 a | SE at all stages from globular to mature but cotyledonary leaves shorter in length. Color of cultures is dark green. |
* Murashige and Skoog’s basal medium supplemented with 2% sucrose and 0.4% GPP. pH = 5.7 ± 0.1. Observations recorded after three months of incubation. ** Mean ± standard error. Means within each column followed by the same letter(s) are not significantly different at 5% level by Fisher’s protected LSD test.
Table 3.
LC-MS/MS analysis of four isosteroidal alkaloids in in vitro cultures exposed to eight different LED lights, in vitro derived bulblets (3 months old), and commercial Fritillaria cirrhosa D. Don bulbs (wild type, three years old).
| LED Light Treatment |
Plant Material | Isosteroidal Alkaloids (µg/g/dw) |
Total of Four Alkaloids (µg/g/dw) |
|||
|---|---|---|---|---|---|---|
| Peimisine | Sipeimine | Peimine | Peiminine | |||
| CW-5000K | In vitro cultures | ND * | ND | ND | 0.12 ± 0.20 b ** | 0.12 ± 0.20 c ** |
| WW-2700K | In vitro cultures | ND | ND | ND | 0.28 ± 0.27 b | 0.28 ± 0.27 c |
| 7R1G1B | In vitro cultures | ND | ND | ND | 0.19 ± 0.32 b | 0.19 ± 0.32 c |
| 8R1B | In vitro cultures | ND | ND | ND | ND | 0.00 ± 0.00 c |
| 9B | In vitro cultures | ND | ND | ND | 0.60 ± 0.43 b | 0.65 ± 0.45 c |
| 9R | In vitro cultures | 3.65 ± 1.68 b ** | ND | 0.38 ± 0.11 a | 2.40 ± 0.30 a | 6.42 ± 2.06 b |
| 9IR | In vitro cultures | 3.22 ± 3.28 b | ND | 0.05 ± 0.09 b | 2.21 ± 0.87 a | 5.48 ± 3.21 b |
| 3R3B3IR | In vitro cultures | ND | ND | ND | 0.26 ± 0.24 b | 0.26 ± 0.24 c |
| Fluorescent tube | In vitro bulblets (3 months old) |
0.91 ± 0.97 b | ND | ND | 2.98 ± 1.09 a | 3.90 ± 1.51 bc |
| Natural habitat | Commercial bulbs (wild type, 3 years old) | 68.4 ± 7.8 a | 0.6 ± 0.4 a | ND | ND | 69.0 ± 7.4 a |
* ND: Not detected. ** Mean ± standard error. Means within each column followed by the same letter(s) are not significantly different at 5% level by Fisher’s protected LSD test.
Table 4.
Development of bulblets in single embryo, a cluster of five embryos, and single embryo with cotyledonary leaf (3–6 cm long) in Fritillaria cirrhosa D. Don.
| Type of Somatic Embryo (SE) * | Percentage of Response (%) | Av No. of Bulblets/SE |
|---|---|---|
| Single embryo | 90.0 ± 10.0 a ** | 4.7 ± 1.3 a ** |
| Cluster of five embryos | 86.7 ± 12.0 a | 3.3 ± 1.5 ab |
| Embryo with the cotyledonary leaf | 43.6 ± 29.0 b | 1.1 ± 0.7 b |
* Culture medium MSBM supplemented with 2% sucrose, 0.4% GPP. Observations recorded after three months of incubation. ** Mean ± standard error. Means within each column followed by the same letter(s) are not significantly different at 5% level by Fisher’s protected LSD test.
3. Results and Discussion
3.1. Callus Proliferation
Callus of F. cirrhosa cultured in Murashige and Skoog’s liquid medium with 2,4-D, under agitated conditions in dark incubation for six weeks, proliferated readily. Callus not only grew in volume but also became embryogenic since early stages of embryos were observed (Figure 4). There are several reports from our laboratory where various secondary metabolites have been obtained from different culture systems [25], including callus cultures of several medicinally important plant species, e.g., Salvia miltiorrhiza Bunge [26] and Saussurea involucrata Kar. et Kir. [27]. These and numerous other reports demonstrate that callus cultures have tremendous potential for the sustainable and large-scale production of secondary metabolites used in pharmaceuticals. Biotechnological applications of plant callus cultures have been recently reviewed [28], and, according to the author, the full potential of callus plant culture technology has not yet been exploited. Callus cultures and suspension cell cultures offer a wide range of applications in agriculture and horticulture, including for Chinese medicinal plants. Genetically transformed callus cultures cannot only be used for the synthesis of bioactive compounds but also for the development of plants with traits [28].
Figure 4.
Embryogenic calli (EC) of F. cirrhosa D. Don growing in Murashige and Skoog’s liquid basal medium supplemented with 2,4-D (0.5 mg/mL) and 2% sucrose. The image is a photograph taken by a scanner of the bottom of the culture flask after six weeks of incubation (bar = 1.2 cm).
3.2. Influence of LED Lights on Morphogenesis of Embryogenic Calli (EC) of F. cirrhosa
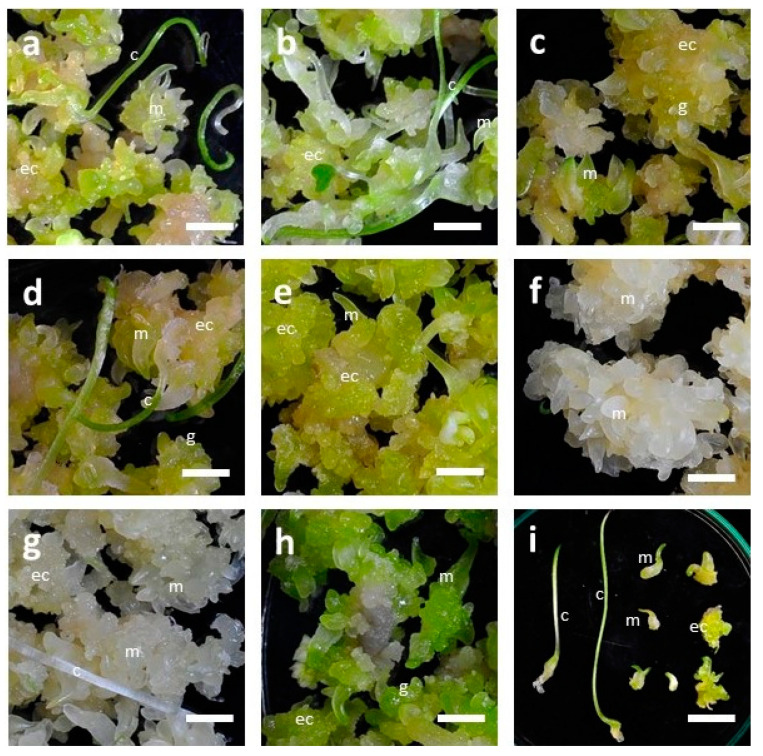
The eight LED lights had significant effects on the growth and development of embryogenic calli (EC) of F. cirrhosa (Table 2). Although the development of somatic embryos (SEs) in embryogenic calli were recorded under all the light treatments, the maximum number of SEs was recorded under red (9R, 223.7), infrared (9IR, 231.3), and a combination of red+blue+infrared light (3R3B3IR, 230.7), respectively. Among the red, infrared, and a combination of red+blue+infrared LED lights, there was a low significant difference concerning the number of SEs and the number of SEs with cotyledonary leaves (Table 2). LED lights also influenced the number of embryos with the development of cotyledonary leaves. Calli exposed to white light (WW-2700K) developed a maximum number of SEs with cotyledonary leaves (22.3), followed by 8R1B (16.7) and CW-5000K (12.0), respectively. SEs under LED lights 9B, 9R, 9IR, and 3R3B3IR developed a reduced number of cotyledonary leaves (in the range of 3.7 to 5.3). There was a low significant difference in the total fresh weight of EC among different LED light treatments. The maximum total fresh weights (FW) of EC biomass, i.e., 17.92 g and 16.67 g, were recorded with treatments of red (9R) and a combination of red+blue+infrared light (3R3B3IR), respectively. Eight LED lights also influenced the growth and development, and morphological features of somatic embryos. Embryogenic calli exposed to different LED lights developed somatic embryos in different developmental stages from early globular to mature SEs with cotyledonary leaves (Figure 5a–i). However, the average number of SEs varied depending upon the LED light. LED light WW-2700K developed the maximum number of SEs with cotyledonary leaves (22.3), followed by 8R1B (16.7) (Table 2). Different LED lights also affected the overall color of the embryogenic calli mass (Figure 5a–h). Under red (9R) and infrared (9IR) treatments, cultures turned white (Figure 5f,g), while under red+blue+infrared (3R3B3IR), these were dark green (Figure 5h).
Figure 5.
Influence of different LED lights on the growth and development of embryogenic calli of F. cirrhosa D. Don: (a) CW-5000K; (b) WW-2700K; (c) 7R1G1B; (d) 8R1B; (e) 9B; (f) 9R; (g) 9IR; (h) 3R3B3IR; (i) Different stages of somatic embryos. For microphotographs, cultures are taken out of the bottles and transferred to sterilized Petri dishes. g: globular embryos; m: mature embryos; c: cotyledonary leaf; ec: embryogenic callus. (a–f, h, bar = 0.57 cm; g, bar = 1.4 cm). Culture medium is Murashige and Skoog’s basal medium supplemented with 2% sucrose and 0.4% GPP. Culture vessels are 650 mL glass bottles, each containing 100 mL medium. Observations recorded after three months of incubation in a specially designed LED light growth chamber.
Light is one of the essential components required by plants for photosynthesis. However, its quantity and duration (photoperiod) drastically affect plant growth and development [29]. Fluorescent tubes are the most common lighting source in a culture room in a typical tissue culture set up. However, in the recent past, due to several advantages, more advanced light-emitting diodes (LEDs) have been used as a source of light. LEDs are relatively cool, emit light of specific wavelengths (spectra), are much smaller in size, and are more durable compared to conventional ones [30]. Due to their efficiency in growth and development, LED lights have been used for micropropagation of many horticultural and agricultural crops [31,32,33]. Depending on requirements, different types of growth chambers equipped with LED lights can be designed. Since the supply of specific light spectra can be controlled in plant tissue culture systems via LED lights, the effects of individual or combinations of light spectra on plant growth and development can be investigated appropriately as in the present study. Several studies on the influence of LED lighting on plant growth, physiology, and secondary metabolism have been reviewed [11,34]. Recently, Pedmale and co-workers reported that the quality of light affects plant growth and development by the regulation of different mechanisms, including the selective activation of light receptors, such as phytochromes by red and far-red light, cryptochromes and phototropins by blue light, and UV-B receptors by ultraviolet light [35]. In a previous study in our laboratory, LED lights affected the development of somatic embryos and callus proliferation (fresh weight) in Peucedanum japonicum Thunb [22]. Several studies have demonstrated that the quality, duration, and intensity of red, infrared, blue, and ultraviolet light can have a profound influence on plants by activating or deactivating physiological reactions and controlling their growth and development [36,37,38]. These studies confirm with many other reports that LED lights are more efficient in plant growth compared to fluorescent lamps. Similar to our results, the beneficial effects of some LED light sources on the induction of embryogenesis in Oncidium have been reported [39]. Due to these beneficial effects, LED light systems are being increasingly used to boost the horticulture industry in Taiwan and several other countries [40,41,42].
3.3. Influence of LED Lights on the Contents of Isosteroidal Alkaloids in In Vitro Cultures of F. cirrhosa
Responses of LED lights drastically varied among the four alkaloids. Out of four alkaloids tested (by LC-MS/MS) in in vitro cultures of F. cirrhosa, the most noticeable effects of eight LED lights were recorded in the case of peiminine (Table 3). The maximum peiminine in cultures (2.40 µg/g/dw) was detected under red (9R) light, followed by infrared (9IR) (2.21 µg/g/dw). Peiminine content in cultures exposed to LED lights CW-5000K, WW-2700K, 7R1G1B, and 3R3B3IR was in the range of (0.12–0.28 µg/g/dw). In in vitro-derived bulblets (3 months old), peiminine content (2.98 µg/g/dw) was detected in the sample grown under fluorescent light. However, this alkaloid could not be detected in cultures exposed to LED lights with 8R1B or in commercial bulbs (3 years old, wild type). Alkaloid sipeimine could not be detected in any in vitro culture or under any light treatment. The only material in which sipeimine in low quantities (0.6 µg/g/dw) could be detected was commercial bulbs (Table 3). Contents of another alkaloid peimisine in cultures were recorded only under two LED light treatments, 9R (3.65 µg/g/dw) followed by 9IR (3.22 µg/g/dw). Between two types of bulbs, the maximum peimisine was noted in commercial bulbs (68.4 µg/g/dw), followed by in vitro-derived bulblets (0.91 µg/g/dw). Similar to peimisine, the presence of peimine was found only under two LED light treatments, 9R (0.38 µg/g/dw) and 9IR (0.05 µg/g/dw), though the quantities were much lower compared to peimisine. Like peiminine, peimine was not detected in commercial bulbs (wild types).
Light quality affects the photochemical control of gene expression in various metabolic pathways, affecting the synthesis of nucleic acids, amino acids, organic acids, and sugars, etc., which are essential not only for cell growth and development but also for cell maintenance [43,44]. The response of plant cells to stress and their reorientation to developmental programs results in the expression of protein kinases, transcription factors, and structural genes that contribute to the adaptation [45]. Similar to the present study, the beneficial effects of LED lights on the contents of bioactive compounds in Peucedanum japonicum Thunb have been reported in our laboratory [22].
3.4. Development of Bulblets in Somatic Embryos of F. cirrhosa
Bulblet formation was observed in all three types of somatic embryos taken from cultures under LED lights CW5000K or WW2700K, as described in Section 2.3 (Table 4; Figure 6a–c; Figure 7a–b). However, the response percentage and number of bulblets varied depending upon the developmental stage of the somatic embryos. The highest response of bulblet formation (90%) was recorded with single embryos with an average of 4.7 bulblets/SE. In the case of a cluster of five embryos, the percentage of response and the average number of bulblets were 86.7 and 3.3 bulblets/SE, respectively. The lowest response (43.6%) and the least number of bulblets (1.1/SE) were recorded in SEs with cotyledonary leaf. However, in this case, some secondary somatic embryos at the base of cotyledonary leaf were observed, though cotyledonary leaf did not grow further, and withered and dried. Single embryos grew further in size and developed multiple bulblets without much callus growth. The cluster of five embryos grew in size and developed further embryogenic callus and secondary somatic embryos.
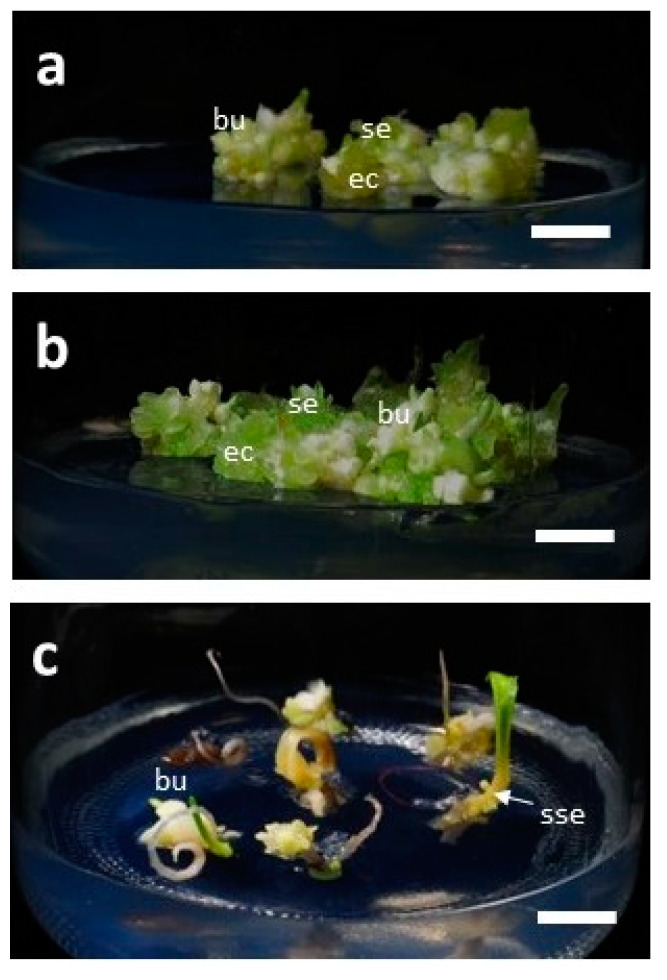
Figure 6.
Development of bulblets in somatic embryos of F. cirrhosa D. Don (photographs taken after three months of incubation): (a) Bulblets from a single embryo, (b) bulblets in a cluster of five embryos, (c) bulblets in embryo with cotyledon leaf. bu: bulblet; se: somatic embryo; sse: secondary somatic embryo; ec: embryogenic callus. Bar = 0.8 cm.
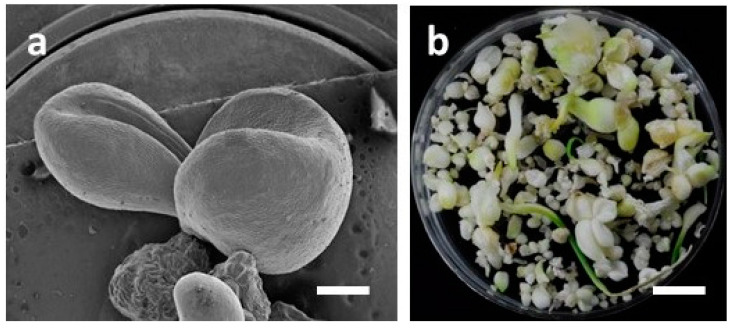
Figure 7.
Development of bulblets from somatic embryos of F. cirrhosa D. Don: (a) Scanning electron microscopy (SEM) of bulblets (bar = 0.77 mm), (b) different stages of growth of culture-derived bulblets of F. cirrhosa (bar = 1.0 cm).
Since bulbs constitute the most critical parts of Fritillaria plants and are the primary source of isosteroidal alkaloids in Fritillaria species used in traditional Chinese medicine (TCM), the production of bulbs in F. cirrhosa by tissue culture technology is highly desirable. The objective of the present study was to investigate the effects of LED lights on the growth and development of embryogenic callus and the analysis of alkaloid contents in cultures; however, the development of bulblets in somatic embryos is an important observation in the study because in natural conditions, one bulb typically develops into a single seedling and it takes about 5–6 years to grow into an appropriate size [46]. It has also been reported that isosteroidal alkaloids in F. cirrhosa bulbs are greatly influenced by environmental conditions, plant age, and harvest times [47]. Recently, Chang and co-workers in our laboratory reported an efficient micropropagation method of bulblet production in F. cirrhosa and also the presence of some isosteroidal alkaloids in tissue culture-derived bulblets and callus [10].
4. Conclusions
Eight LED lights influenced the growth and development, and morphological features of somatic embryos of F. cirrhosa D. Don. Embryogenic calli exposed to different LED lights developed somatic embryos in different developmental stages from early globular to mature SEs with cotyledonary leaves. The average number of somatic embryos that developed varied depending upon the wavelength of light emitted by the LEDs. The maximum number of SEs was recorded under red light spectra, followed by infrared and a combination of red/blue/infrared light spectra, respectively. Concerning alkaloids, the most significant effects were recorded with red and infrared light spectra in which peimisine, peimine, and peiminine were recorded. Sipeimine was not detected in any culture. Results obtained in the study indicate that red and infrared light spectra may be useful to obtain peimisine, peimine, and peiminine from callus cultures of F. cirrhosa D. Don. However, further research is needed to boost the quantities of these compounds in cultures and also for optimization to scale up production in bioreactors for commercial feasibility. Further research is also required for the optimization of large qualities of bulblet production in cultures. The production of important and precious alkaloids under a laboratory set up may reduce our dependence on natural materials from the wild and may help in the conservation of vulnerable plant species, such as F. cirrhosa D. Don.
Acknowledgments
Financial support by the Ministry of Science and Technology, grant number 106-2313-B-324-001-MY2, 108-2313-B-324-001, and 109-2628-B-324 -001 is gratefully acknowledged.
Author Contributions
Conceptualization, H.-C.C.; methodology, C.-C.C. and H.-J.K.; software, C.-C.C. and H.-J.K.; validation, H.-S.T., C.-R.W. and M.-R.L.; formal analysis, H.-C.C.; investigation, C.-C.C.; resources, H.-C.C. and H.-M.X.; data curation, H.-C.C.; writing—Original draft preparation, D.C.A.; writing—Review and editing, D.C.A. and H.-C.C.; visualization, C.-C.C.; supervision, H.-C.C.; project administration, H.-C.C.; funding acquisition, H.-C.C. All authors have read and agreed to publish this version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (MOST), Taiwan. Grant numbers 106-2313-B-324-001-MY2, 108-2313-B-324-001, and 109-2628-B-324 -001.
Conflicts of Interest
The authors declare that they have no competing interests among them.
References
- 1.Lin G., Li P., Li S.L., Chan S.W. Chromatographic analysis of Fritillaria isosteroidal alkaloids, the active ingredients of Beimu, the antitussive traditional Chinese medicinal herb. J. Chromatogr. A. 2001;935:321–338. doi: 10.1016/S0021-9673(01)01258-4. [DOI] [PubMed] [Google Scholar]
- 2.The State Pharmacopoeia Commission of P.R. China . Pharmacopoeia of the People’s Republic of China. 10th ed. China Medical Science Press; Beijing, China: 2015. p. 37. [Google Scholar]
- 3.Li H.J., Jiang Y., Li P. Chemistry, bioactivity and geographical diversity of steroidal alkaloids from the Liliaceae family. Nat. Prod. Rep. 2006;23:735–752. doi: 10.1039/b609306j. [DOI] [PubMed] [Google Scholar]
- 4.Li K., Wu W., Zheng Y., Dai Y., Xiang L., Liao K. Genetic diversity of Fritillaria from Sichuan province based on ISSR. China J. Chin. Mater. Med. 2009;34:2149–2154. [PubMed] [Google Scholar]
- 5.Ding K., Lin G., Ho Y.P., Cheng T.Y., Li P. Prederivatization and high-performance liquid chromatographic analysis of alkaloids of bulbs of Fritillaria. J. Pharm. Sci. 1996;85:1174–1179. doi: 10.1021/js960211v. [DOI] [PubMed] [Google Scholar]
- 6.Xu J., Zhao W., Pan L., Zhang A., Chen Q., Xu K., Lu H., Chen Y. Peimine, a main active ingredient of Fritillaria, exhibits anti-inflammatory and pain suppression properties at the cellular level. Fitoterapia. 2016;111:1–6. doi: 10.1016/j.fitote.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Liu S., Yang T., Ming T.W., Gaun T.K.W., Zhou T., Wang S., Ye B. Isosteroid alkaloids from Fritillaria cirrhosa bulbs as inhibitors of cigarette smoke-induced oxidative stress. Fitoterapia. 2020;140:104434. doi: 10.1016/j.fitote.2019.104434. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham A.B., Brinckmann J.A., Pei S.J., Luo P., Schippmann U., Long X., Bi Y.F. High altitude species, high profits: Can the trade in wild harvested Fritillaria cirrhosa (Liliaceae) be sustained? J. Ethnopharmacol. 2018;223:142–151. doi: 10.1016/j.jep.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D., Gao L., Yang Y. Genetic diversity and structure of a traditional Chinese medicinal plant species, Fritillaria cirrhosa (Liliaceae) in southwest China and implications for its conservation. Biochem. Syst. Ecol. 2010;38:236–242. doi: 10.1016/j.bse.2009.12.029. [DOI] [Google Scholar]
- 10.Chang H.C., Xie H.M., Lee M.R., Lin C.Y., Yip M.K., Agrawal D.C., Tsay H.S. In vitro propagation of bulblets and LC–MS/MS analysis of isosteroidal alkaloids in tissue culture derived materials of Chinese medicinal herb Fritillaria cirrhosa D. Don. Bot. Stud. 2020;61:9. doi: 10.1186/s40529-020-00286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batista D.S., Felipe S.H.S., Silva T.D., de Castro K.M., Mamedes-Rodrigues T.C., Miranda N.A., Ríos-Ríos A.M., Faria D.V., Fortini E.A., Chagas K., et al. Light quality in plant tissue culture: Does it matter? In Vitro Cell. Dev. Biol.-Plant. 2018;54:195–215. doi: 10.1007/s11627-018-9902-5. [DOI] [Google Scholar]
- 12.Kozai T. Why LED lighting for urban agriculture? In: Kozai T., Fujiwara K., Runkle E., editors. LED Lighting for Urban Agriculture. 1st ed. Springer; Singapore: 2016. pp. 3–18. [Google Scholar]
- 13.Xue Y., Gu H.L. Determination of peimine and peiminine in Fritillaria thunbergii by HPLC-ELSD. Acta Pharm. Sin. 2005;40:550–552. [PubMed] [Google Scholar]
- 14.Ruan X., Yang L., Cui W.X., Zhang M.X., Li Z.H., Liu B., Wan Q. Optimization of supercritical fluid extraction of total Alkaloids, Peimisine, Peimine and Peiminine from the Bulb of Fritillaria thunbergii Miq, and evaluation of antioxidant activities of the extracts. Materials. 2016;9:524. doi: 10.3390/ma9070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu K., Mo C., Xiao H., Jiang Y., Ye B., Wang S. Imperialine and verticinone from bulbs of Fritillaria wabuensis inhibit pro-inflammatory mediators in LPS stimulated RAW 264.7 macrophages. Planta Med. 2015;81:821–829. doi: 10.1055/s-0035-1546170. [DOI] [PubMed] [Google Scholar]
- 16.Luo Z., Zheng B., Jiang B., Xue X., Xue E., Zhou Y. Peiminine inhibits the IL-1β induced inflammatory response in mouse articular chondrocytes and ameliorates murine osteoarthritis. Food Funct. 2019;10:2198–2208. doi: 10.1039/C9FO00307J. [DOI] [PubMed] [Google Scholar]
- 17.Wang D., Zhu J., Wang S., Wang X., Ou Y., Wei D., Li X. Antitussive, expectorant and anti-inflammatory alkaloids from Bulbus Fritillariae Cirrhosae. Fitoterapia. 2011;82:1290–1294. doi: 10.1016/j.fitote.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Lin Q., Qu M., Patra H.K., He S., Wang L., Hua X., Xiao L., Fu Y., Gong T., He Q., et al. Mechanistic and therapeutic study of novel anti-tumor function of natural compound imperialine for treating non-small cell lung cancer. J. Ethnopharmacol. 2020;247:112283. doi: 10.1016/j.jep.2019.112283. [DOI] [PubMed] [Google Scholar]
- 19.Yin Z., Zhang J., Guo Q., Chen L., Zhang W., Kang W. Pharmacological effects of verticine: Current status. Evid. Based Complement. Altern. Med. 2019;2019:2394605. doi: 10.1155/2019/2394605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., Cui M., Chen S. Identification of the molecular mechanisms of peimine in the treatment of cough using computational target fishing. Molecules. 2020;25:1105. doi: 10.3390/molecules25051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco culture. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 22.Chen C.C., Agrawal D.C., Lee M.R., Lee R.J., Kuo C.L., Wu C.R., Tsay H.S., Chang H.C. Influence of LED light spectra on in vitro somatic embryogenesis and LC–MS analysis of chlorogenic acid and rutin in Peucedanum japonicum Thunb.: A medicinal herb. Bot. Stud. 2016;57:9. doi: 10.1186/s40529-016-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsiao H.H., Hsieh H.Y., Chou C.C., Lin S.Y., Wang A.H.J., Khoo K.H. Concerted experimental approach for sequential mapping of peptides and phosphopeptides using C18-functionalized magnetic nanoparticles. J. Proteome Res. 2007;6:1313–1324. doi: 10.1021/pr0604817. [DOI] [PubMed] [Google Scholar]
- 24.Yu X., Liu H., Diao J., Sun Y., Wang Y. Magnetic molecularly imprinted polymer nanoparticles for separating aromatic amines from azo dyes–Synthesis, characterization and application. Sep. Purif. Technol. 2018;204:213–219. doi: 10.1016/j.seppur.2018.04.081. [DOI] [Google Scholar]
- 25.Agrawal D.C., Chang H.C., Chen C.C., Kuo C.L., Tsay H.S. Biotechnology of medicinal plants and fungi in Taiwan—Production of bioactive secondary metabolites in vitro culture systems. In: Agrawal D.C., Chang H.C., Chen C.C., Kuo C.L., Tsay H.S., editors. Medicinal Plants and Fungi: Recent Advances in Research and Development. 1st ed. Springer Nature; Singapore: 2017. pp. 459–483. [Google Scholar]
- 26.Wu C.T., Vanisree M., Satish M.N., Chen C.L., Yang T.F., Tsay H.S. Isolation and quantitative analysis of cryptotanshinone, an active quinoid diterpene formed in the callus of Salvia miltiorrhiza Bunge. Biol. Pharm. Bull. 2003;26:845–848. doi: 10.1248/bpb.26.845. [DOI] [PubMed] [Google Scholar]
- 27.Kuo C.L., Agrawal D.C., Chang H.C., Chiu Y.T., Huang C.P., Chen Y.L., Huang S.H., Tsay H.S. In vitro culture and production of syringin and rutin in Saussurea involucrata (Kar. et Kir.)—An endangered medicinal plant. Bot. Stud. 2015;56:12. doi: 10.1186/s40529-015-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efferth T. Biotechnology applications of plant callus cultures. Engineering. 2019;5:50–59. doi: 10.1016/j.eng.2018.11.006. [DOI] [Google Scholar]
- 29.Higuchi Y., Hisamatsu T. Light acts as a signal for regulation of growth and development. In: Kozai T., Fujiwara K., Runkle E., editors. LED Lighting for Urban Agriculture. 1st ed. Springer; Singapore: 2016. pp. 57–73. [Google Scholar]
- 30.Kim S.J., Hahn E.J., Heo J.W., Paek K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004;101:143–151. doi: 10.1016/j.scienta.2003.10.003. [DOI] [Google Scholar]
- 31.Li H.M., Xu Z.G., Tang C.M. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult. 2010;103:155–163. doi: 10.1007/s11240-010-9763-z. [DOI] [Google Scholar]
- 32.Nhut D.T., Takamura T., Watanabe H., Okamoto K., Tanaka M. Responses of strawberry plantlets cultured in vitro under super bright red and blue light-emitting diodes (LEDs) Plant Cell Tissue Organ Cult. 2003;73:43–52. doi: 10.1023/A:1022638508007. [DOI] [Google Scholar]
- 33.Saebo A., Krekling T., Appelgren M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995;41:177–185. doi: 10.1007/BF00051588. [DOI] [Google Scholar]
- 34.Olle M., Viršile A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013;22:223–234. doi: 10.23986/afsci.7897. [DOI] [Google Scholar]
- 35.Pedmale U.V., Huang S.C., Zander M., Cole B.J., Hetzel J., Ljung K., Reis P.A.B., Sridevi P., Nito K., Nery J.R., et al. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell. 2016;164:233–245. doi: 10.1016/j.cell.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briggs W.R., Olney M.A. Photoreceptors in plant photomorphogenesis to date, five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol. 2001;125:85–88. doi: 10.1104/pp.125.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briggs W.R., Beck C.F., Cashmore A.R., Christie J.M., Hughes J., Jarillo J.A., Kagawa T., Kanegae H., Liscum E., Nagatani A., et al. The phototropin family of photoreceptors. Plant Cell. 2001;13:993–997. doi: 10.1105/tpc.13.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clouse S.D. Integration of light and brassinosteroid signals in etiolated seedling growth. Trends Plant Sci. 2001;6:443–445. doi: 10.1016/S1360-1385(01)02102-1. [DOI] [PubMed] [Google Scholar]
- 39.Chung J.P., Huang C.Y., Dai T.E. Spectral effects on embryogenesis and plantlet growth of Oncidium ‘Gower Ramsey’. Sci. Hortic. 2010;124:511–516. doi: 10.1016/j.scienta.2010.01.028. [DOI] [Google Scholar]
- 40.Agarwal A., Gupta S.D. Impact of light-emitting diodes (LEDs) and its potential on plant growth and development in controlled environment plant production system. Curr. Biotechnol. 2016;5:28–43. doi: 10.2174/2211550104666151006001126. [DOI] [Google Scholar]
- 41.Bello-Bello J.J., Pérez-Sato J.A., Cruz-Cruz C.A., Martínez-Estrada E. Light-emitting diodes: Progress in plant micropropagation. In: Jacob-Lopes E., Zepka L.Q., Queiroz M.I., editors. Chlorophyll. InTech; Rijeka, Croatia: 2017. pp. 93–103. [Google Scholar]
- 42.Fang W., Chen C.C., Lee Y.Y., Chang M.Y. Development of LED lids for tissue culture lighting. Acta Hortic. 2011;907:397–402. doi: 10.17660/ActaHortic.2011.907.67. [DOI] [Google Scholar]
- 43.Cepauskas D., Miliute I., Staniene G., Gelvonauskiene D., Stanys V., Jesaitis A.J., Baniulis D. Characterization of apple NADPH oxidase genes and their expression associated with oxidative stress in shoot culture in vitro. Plant Cell Tissue Organ Cult. 2016;124:621–633. doi: 10.1007/s11240-015-0920-2. [DOI] [Google Scholar]
- 44.Li C.X., Xu Z.G., Dong R.Q., Chang S.X., Wang L.Z., Khalil-Ur-Rehman M., Tao J.M. An RNA-Seq analysis of grape plantlets grown in vitro reveals different responses to blue, green, red led light, and white fluorescent light. Front. Plant Sci. 2017;8:78. doi: 10.3389/fpls.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neelakandan A.K., Wang K. Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Rep. 2012;31:597–620. doi: 10.1007/s00299-011-1202-z. [DOI] [PubMed] [Google Scholar]
- 46.Ruan X., Cui W.X., Yang L., Li Z.H., Liu B., Wang Q. Extraction of total alkaloids, peimine and peiminine from the flower of Fritillaria thunbergii Miq using supercritical carbon dioxide. J. CO2 Util. 2017;18:283–293. doi: 10.1016/j.jcou.2017.01.024. [DOI] [Google Scholar]
- 47.Konchar K., Li X.L., Yang Y.P., Emshwiller E. Phytochemical variation in Fritillaria cirrhosa D. Don (Chuan Bei Mu) in relation to plant reproductive stage and timing of harvest. Econ. Bot. 2011;65:283. doi: 10.1007/s12231-011-9170-3. [DOI] [Google Scholar]