Abstract
Simple Summary
GATA2 deficiency is considered one of the most common cancer predisposition syndromes determining myelodysplastic syndrome in children. Little is known of this recently described syndrome, often resulting in a misdiagnosis and unclear management. In this review, we describe GATA2 deficiency clinical presentation in order to focus on phenotypes that, in patients with myelodysplastic syndrome, may be suggestive of GATA2 deficiency. Moreover, due to the lack of clear guidelines, we performed an overview on literature data regarding management of GATA2-related myelodysplastic syndrome, in order to understand the best choice of treatment for these patients.
Abstract
Myelodysplastic syndromes (MDS) are hematopoietic disorders rare in childhood, often occurring in patients with inherited bone marrow failure syndromes or germinal predisposition syndromes. Among the latter, one of the most frequent involves the gene GATA binding protein 2 (GATA2), coding for a transcriptional regulator of hematopoiesis. The genetic lesion as well as the clinical phenotype are extremely variable; many patients present hematological malignancies, especially MDS with the possibility to evolve into acute myeloid leukemia. Variable immune dysfunction, especially resulting in B- and NK-cell lymphopenia, lead to severe infections, including generalized warts and mycobacterial infection. Defects of alveolar macrophages lead to pulmonary alveolar proteinosis through inadequate clearance of surfactant proteins. Currently, there are no clear guidelines for the monitoring and treatment of patients with GATA2 mutations. In patients with MDS, the only curative treatment is allogeneic hematopoietic stem cell transplantation (HSCT) that restores normal hematopoiesis preventing the progression to acute myeloid leukemia and clears long-standing infections. However, to date, the donor type, conditioning regimen, and the optimal time to proceed to HSCT, as well as the level of chimerism needed to reverse the phenotype, remain unclear highlighting the need for consensus guidelines.
Keywords: myelodysplastic syndromes, cancer predisposition, GATA2 deficiency, childhood MDS, pediatric cancer
1. Introduction
Myelodysplastic syndromes (MDS) are clonal hematopoietic disorders, characterized by cytopenia of one or more hematopoietic lines, ineffective hematopoiesis, and a possible evolution to acute myeloid leukemia (AML). Sporadic MDS is primarily an elderly disease with an incidence of 75 per 100,000 in patients older than 65 years [1], while, during childhood, MDS are rare with an annual incidence of 1–4 cases per million. MDS account for less than 5% of all childhood hematologic malignancies, and many childhood MDS occur in the contest of germinal predisposition syndromes or Inherited Bone Marrow Failure Syndromes (IBMFS) [2].
Among germline predispositions, one of the most frequent involves the gene GATA binding protein 2 (GATA2). The first description of GATA2 mutation dates back to 2011, when four independent groups described four clinical phenotypes: Emberger syndrome (lymphedema and monosomy 7), MonoMAC syndrome (monocytopenia and Mycobacterium avium complex infection), dendritic cell, monocyte, B and natural killer (NK) lymphoid deficiency (DCML), and familial MDS/AML, all associated with a GATA2 deficiency [3,4,5]. The first description of GATA2 related MDS is due to Scott and colleagues, reporting the first description of GATA2 germline mutation in four MDS/AML families. They observed in these families the missense mutations T354M, and the 355delT mutation, absent in 659 healthy controls, such as any other variants of the GATA2 coding sequence. These mutations involve highly conserved, five consecutive threonine residues in the zinc finger 2 domain (ZF2) of the GATA2 protein, responsible for DNA-binding and homodimerization [6]. In 2016, the cooperative European Working Group on Childhood MDS (EWOG-MDS) consortium conducted a large study analyzing 508 young patients (children and adolescents), 426 with primary MDS, and 82 with secondary MDS enrolled in the prospective studies EWOG-MDS 98 and EWOG-MDS 2006 over a 15-year period. Germline GATA2 mutations were found in 7% of primary MDS cases, 15% of advanced MDS, and were never found in children with MDS secondary to aplastic anemia or previous cancer therapy. GATA2 mutations were more prevalent in advanced MDS rather than refractory childhood cytopenia; moreover, GATA2 mutated patients were older at diagnosis and presented more often with advanced MDS and monosomy 7 compared to GATA2 wild type patients [7]. These data have pointed the attention to this unique GATA2 related-MDS, but, even if the comprehension of the disease has progressed, only a few recommendations are available about the best clinical management.
2. Biological Features
GATA2 is a key transcriptional regulator of hematopoiesis involved in the development and maintenance of the stem cell pool through hematopoietic stem cell (HSC) survival and self-renewal. It is also involved in the development of monocytes, mast cells, NK cells, and megakaryocytes [8]. Even if the human syndromes related to GATA2 deficiency have been described only recently, the role of GATA2 in leukemogenesis has long been studied. This protein contains two ZF domains and a nuclear localization signal. GATA2 mutations involving the two ZF domains can be distinguished by phenotypic correlations and mutational clustering suggesting different leukemogenic mechanisms [9]. GATA2 binds to the consensus sequence W/GATA/R (W 5 A or T and R 5 A or G) in promoter/enhancer regions of target genes such as RUNX, TAL1, FLI1, SPI1 (PU.1), LMO2, regulating during embryogenesis the transformation of endothelial cells into hematopoietic cells, the formation of HSCs, and the definitive hematopoiesis [10].
Today, nearly 150 GATA2 mutations have been described, as either germline or somatic mutations. Roughly one-third of GATA2 germline mutations are inherited, while two thirds occur de novo. A wide range of mutations were described: in-frame insertions or deletions; single nucleotide variants arising amino-acid substitution in the exons 3, 4 and 5, encoding the two ZF domains; a small number of non-sense or frame-shift mutations and whole gene deletions (distributed across the ZF2 domain); splice site mutations were also described between exons 3 and 4; discrete mutations of the intron 5 enhancer have also been reported. Overall, in about two-thirds of cases reported, mutation involve the ZF domains. No mutations have been reported in the 5′ or 3′ untranslated regions, nor in the distal section of the last exon, beyond the region encoding the ZF2 domain.
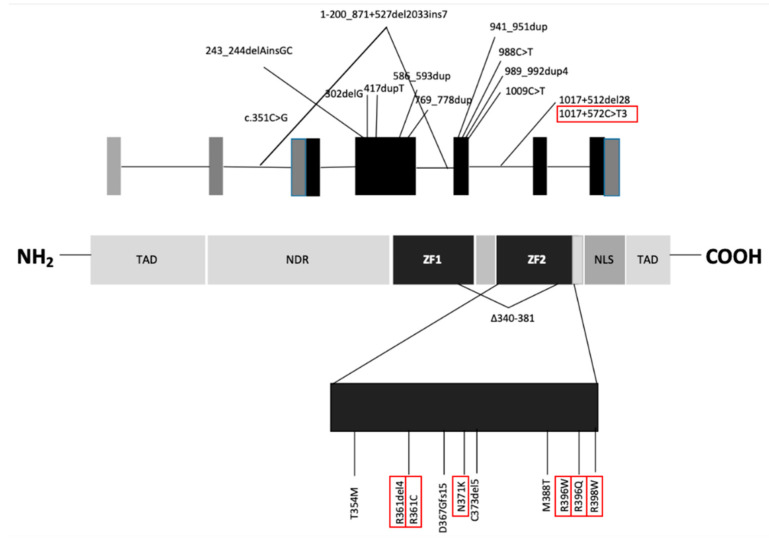
More than half of the variants described are single amino-acid substitutions that may result in the translation of mutated protein with an altered function. Gene deletions and frame-shift mutations are null alleles that may lead to the same phenotypes. Many single amino-acid substitutions impair the DNA-binding function of the ZF domains, making the protein functionally inactive [11]. However, it is also possible that the mutated protein maintains a residual function or can even act in a dominant negative fashion, as reported for T354M, or can result in a gain of function, as reported for the L359V variant, shown in two cases of blast transformation of chronic myeloid leukemia (CML) [4,12]. There are no known germ-line mutations affecting ZF1, suggesting that these kinds of mutations may be embryo-lethal [9]. Of all the known regulatory regions of GATA2, germline mutations have been reported only in the intron 5 enhancer [13]; recently, a new mutation (c.351C>G) was described in the intron 3 leading to an aberrantly spliced GATA2 transcript with a 136-bp internal deletion [14]. The most common GATA2 mutations are shown in Figure 1. Wlodarski et al. have found that GATA2 mutations can also be associated with cytogenetic abnormalities such as monosomy 7 and trisomy 8. Overall, the prevalence of GATA2 mutations reach 72% in adolescent (age 12–19) with MDS associated with monosomy 7, while its prevalence is 16% in patients with MDS and trisomy 8. Also, der (1;7) has been described in patients with MDS carrying GATA2 mutation [7].
Figure 1.
GATA2 gene and protein structure. GATA2 gene and protein structure. Mutations most frequently associated with MDS are circled [7]. TAD: transactivation domain, NRD: negative regulatory domain, ZF: zinc-finger domains, NLS: nuclear localization signal.
3. Clinical Features
GATA2 germline mutations are associated with a wide range of signs and symptoms. The proportion of asymptomatic patients carrying GATA2 germline mutations has been estimated to be 38% by age 20 and 8% by age 40. The median age at onset of the first symptoms is 18 years [15]. Among symptomatic patients, the symptoms can be extremely variable [5,8]. The most frequent clinical phenotypes are summarized below and in Table 1.
Table 1.
Clinical features of GATA2 deficiency.
| Clinical Features | Frequency (%) |
|---|---|
| Hematological features | |
| MDS | 70–84 [15,16] |
| AML | 14–19 [15,16] |
| ALL | 1.3 [15] |
| AA | 2.5 [15] |
| JMML | 1.3 [15] |
| Immunodeficit | |
| Monocytopenia | 49–78 [15,16] |
| B lymphopenia | 86–100 [15,16] |
| NK lymphopenia | 7.8–82 [15,16] |
| Neutropenia | 47 [16] |
| Infections | |
| Severe viral infections | 70 [16] |
| Disseminated NTM infections | 53 [16] |
| Severe bacterial infections | 49–56 [15,16] |
| Severe fungal infections | 16% [16] |
| Persistent EBV viremia | 11% [16] |
| Warts | |
| HPV-related | 35–40 [15,16] |
| Oncologic | 3.8 [15] |
| Lymphedema | 11–15 [15,16] |
| Pulmonary features | |
| PAPs | 3.8–18 [15,16] |
| Recurrent bacterial infections | 56 [15] |
| Pulmonary hypertension | <20% [16] |
| Thrombotic complications | 9–25 [15,16] |
| Deafness | 1.3 [15] |
| Autoimmune features | 11 [15] |
| Urinary tract malformation | 5 [15] |
| Obsetrian complications | 6.3–33 [15,16] |
| Hypothyroidism | 1.3–14 [15,16] |
3.1. Oncological and Non-Oncological Hematological Abnormalities
In patients carrying GATA2 germline mutations, the risk of developing MDS/AL is 6% at the age of 10 years, 39% at the age of 20 years, and 81% at the age of 40 years. MDS are the most common hematological malignancies and can evolve into AML. Cases of T-cell acute lymphoblastic leukemia (in addition to monosomy 7) and juvenile myelomonocytic leukemia were also described [7,15].
Patients without hematological malignancies often show abnormal blood count with neutropenia, thrombocytopenia, and macrocytosis [15]. The mononuclear cells profiling reveals monocytes, dendritic cell, B cells, and NK deficiency [17], even though Wlodarski and colleagues described more cases of monocytosis than monocytopenia [7]. Analyzing bone marrow and peripheral blood in flow cytometry, the most distinctive feature of GATA2-related MDS seems to be the reduction of B cells, compared to healthy controls, aplastic anemia, and MDS patients without GATA2 deficiency. GATA2-deficient patients could also present a relative increase in marrow T cells with an inverted CD4:CD8 ratio and a higher proportion of plasma cells, sometimes with an atypical phenotype [18,19]. Bone marrow biopsy performed in patients without hematological malignancies often show atypical megakaryocytes that can be larger and abnormal with separated nuclear lobes, and smaller with separated nuclear lobes or micromegakaryocytes, even in the absence of overt MDS. Additionally, patients with non-tuberculous mycobacterial infections (NTM), granulomata, and mycobacteria could be found in the marrow [20]. In pediatric patients with MDS, a lack of B cells and B-cell progenitors is suggestive for GATA2 deficiency [18,19].
3.2. Infections
Patients with germline GATA2 mutation present a wide range of immune dysfunctions, leading to severe infections, primarily generalized warts and mycobacterial infection. At the age of 20, the cumulative rate of bacterial infection is 33% and at the age of 40 years is 64% [15].
This high rate of infections is related to immune defects of cell mediated and humoral immunity. The impaired T-cell mediated immunity leads to recurrent respiratory tract infection; the impaired viral clearance and the defective immunosurveillance are responsible for the higher rate of malignant transformation of HPV neoplasm. Related HPV is the most common viral infection, occurring in about two-thirds of GATA2 germline mutation carriers, presenting with warts, condyloma, and/or dysplasia. When warts are associated with monocytopenia, a GATA2 deficiency must be suspected. The lack of dendritic cells impairs recognition of viruses and intracellular pathogens contributing to disseminated herpes virus infections and mycobacterial susceptibility. Furthermore, it has been described that in the site of infection, tissue macrophages are present but organized granulomatous inflammation is defective [8,15,16].
3.3. Pulmonary Alveolar Proteinosis (PAP)
Defects of alveolar macrophages led to PAP due to inadequate clearance of surfactant proteins. Patients with GATA2 mutation who develop a PAP do not have antibodies anti-GM-CSF and respond poorly to lavage and GM-CSF therapy.
Patients without PAP can present abnormal pulmonary function such as diffusion or ventilatory defects (obstruction, restriction, or a mixed pattern) and/or structural abnormalities identified on chest CT including reticular opacities, nodules, ground glass opacities, crazy paving para-septal emphysema, and subpleural blebbing. Alveolar macrophages are abundant in bronchoalveolar lavage fluids [16].
3.4. Cardiovascular and Lymphatic
Chronic lymphedema is a common feature that could be either unilateral or bilateral, involving lower extremities and genitalia. Mouse models suggest that an intact GATA2 function is required for proper lymphatic vascular development and lymphatic valve morphogenesis [21]. Furthermore, GATA2 is involved in vascular integrity and cell adhesion, as suggested by its high expression in endothelial cells [22]. Lymphedema, particularly in an adolescent or young adult with cytopenia, is highly suggestive of GATA2 deficiency.
3.5. Other Oncological Malignancies
The majority of solid tumors are related to underlying viral infections, in particular HPV-related dysplasia and EBV-related tumors, such as leiomyosarcoma. Other cancers reported are renal cell carcinoma, pancreas adenocarcinoma, breast cancer, locally invasive desmoid tumor of the chest wall and Merkel cell carcinoma in a contest neurofibromatosis type 1 [16,23].
3.6. Deafness
Sensorineural hearing loss often occurs in patients with whole gene deletions of GATA2. Haugas and colleagues showed that, in mouse models, GATA2 is involved in ear morphogenesis. Without GATA2, the semicircular ducts fail to grow to their normal size, and the surrounding mesenchymal cells are not removed properly to generate perilymphatic space [24].
4. Phenotype-Genotype Clustering
As previously mentioned, there are more than 150 known mutations in GATA2-related disorders, but there is no evidence of a clear genotype–phenotype correlation. However, it has been noted that certain phenotypes tended to cluster within families. It has been observed from analyzing the association between phenotype and the type of GATA2 mutation that severe infections are more frequent and have an earlier onset in patients with null mutations. Lymphedema has only been observed in patients with null mutation or regulatory mutations [16]. In a survey performed among the French and Belgian patients with GATA2 mutation, the investigators found a significantly higher risk of developing leukemia in the group with missense mutations compared to the group with a non-sense or frameshift mutations [15].
In 2015, Mir and colleagues reported an association between missense mutation with a high risk of developing MDS/AML, while deletion was associated with dysmorphic features, monocytopenia, and high risk of infections [25].
The relation between genetic lesion and different clinical presentation could be explicated by the different GATA2 regulation in the biological systems; it has been noted that genetic lesions affecting certain regions of GATA2 are related to defects of certain organs and systems rather than others. Moreover, GATA2 instigated genetic networks that vary in different cellular contexts and in a stress state rather than a steady state [26]. The mechanisms sustaining the development of MDS in GATA2 deficiency are not completely understood. Clonal evolution could be associated with the development of cytogenetic clones and progression to MDS/AML. The most common somatic mutations in hematopoietic malignant clones involve the gene ASXL1, reported in 29% of case series [27]. Other somatic mutations have also been reported, including SEPTBP1, STAG2, RUNX1, CBL, EZH2, NRAS, JAK3, and PTPN11 [28]. It has been noted that GATA2 mutation is associated with a high elevation of FLT3 ligand that progressively increase with the evolution of cytopenia and clinical complications, suggesting a possible usage in clinical monitoring [17]. In vitro studies showed that GATA2 promotes the transcription and expression of IL1b and CXCL2 with positive feedback on an RAS/MAPK-GATA2-IL1b/CXCL2 axis. High expression of CXCL2 promotes leukemic cell proliferation and correlates with a poor prognosis of patients with AML [29].
In a recent report published in 2018 by McReynolds, the authors selected 25 subjects (probands or parents) with a known GATA2 deficiency and performed a panel sequence of 49 genes commonly mutated in AML or MDS. Variants were found in 73% (8/11) of patients with MDS, 71% (5/7) of patients with GATA2 deficiency related bone marrow and immunodeficiency disorder (G2BMID), and only in one subject with normal blood cells count and bone marrow histology. These additional somatic mutations affected genes involved in trascriptional regulation, DNA modification, chromatin regulation, cell–cell or cell-matrix cohesion, and cell signaling. The most frequent somatic mutations involve ASXL1, that were seen in 6 of 18 patients with MDS or G2BMID (33%). In the MDS group, another frequent mutation involved the gene STAG2, seen in 3 of 11 MDS patients. Most patients had normal bone marrow cytogenetics (68%, 17/25) (Figure 2). The most common abnormality in this cohort was isolated trisomy 8 (5/8), in contrast to another report that describes an association with monosomy 7 [7,30].
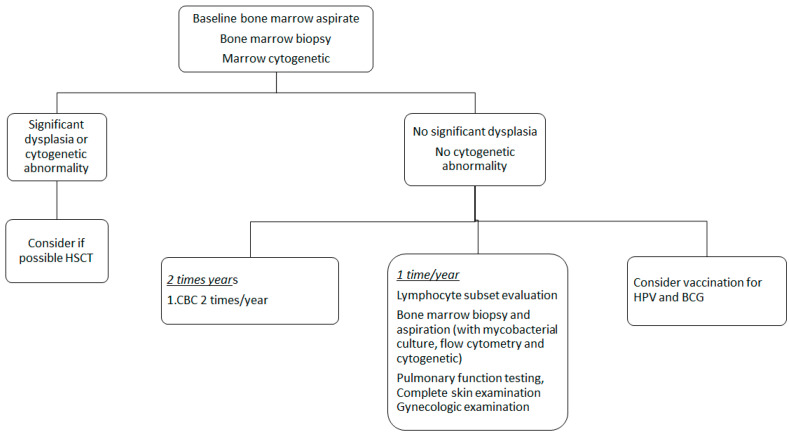
Figure 2.
Possible algorithm for management of patients with GATA2 deficiency.
Concerning the differences among races, it should be noted that most of literature reports concerned the Caucasian race. Few pieces of data are available about GATA2 germline mutation in Asiatic patients; in particular, one case report described a Japanese patient with a GATA2 mutation c.988C>T affected by immunodeficiency, who developed MDS [31]; recently, a case series of pediatric patients with hematological malignancies with monosomy 7 and germline GATA2 mutation have been described [32]. In a study performed in Taiwan on de novo AML, the authors found a GATA2 mutation in 43 patients; most were missense mutations, as reported in the most study on Caucasians [33]. These data were confirmed in the cohort study reported by Spinner and colleagues, in which 54% of patients were Caucasian, with no differences in the type of genetic abnormalities among Caucasians, Hispanics, African Americans, and Asians [16].
5. Management
Currently, there are no clear guidelines for the monitoring and treatment of patients with GATA2 mutations. The first critical issue is the diagnosis of the GATA2 deficiency. Although an exceedingly high number of mutations in the GATA2 locus have been described, the complete coverage of the GATA2 gene is technically cumbersome. Some authors suggest that, when disease is highly suspected and the sequencing of exons and intron 5 is negative, a copy number variation analysis and mRNA sequencing should be performed [28].
In patients with MDS, a GATA2 deficiency should be suspected in case of suggestive clinical features, such as monocytopenia, NTM infections, recurrent HPV infections, and lymphedema; the report of the European Working Group of MDS in children and adolescent patients with a high-risk MDS (often presenting other cytogenetic abnormalities) should be considered for GATA2 deficiency [7]. Screening tests searching for healthy carriers should be performed in families with almost one case of GATA2 deficiency; however, the best management for healthy carriers is not well established.
Once identified as a healthy carrier, experts suggest performing a baseline bone marrow aspirate and cytogenetic to identify GATA2 related alterations. There is no complete agreement in the indications for bone marrow biopsy; however, most international societies including EWOG-MDS recommend this evaluation at diagnosis. Complete blood count with differential should be evaluated at least twice yearly, while lymphocyte subset assessment, bone marrow aspiration (with flow cytometry, cytogenetic and mycobacterial culture), pulmonary function testing, complete skin examination, and gynecologic examination should be performed once yearly [20].
Some authors suggest daily azithromycin prophylaxis for NTM, but clear guidelines for antibiotic prophylaxis are missing. Routine childhood vaccinations should be performed, including an HPV vaccination such as BCG vaccination. Patients with normal blood counts seem to tolerate these vaccinations well, and no data are available about tolerance of live virus vaccinations in patients with cytopenia [20]. BCG is generally well tolerated, but data available in literature are very limited and thus there is no agreement in recommending it [34].
Good oral hygiene and regular dental visits are recommended, especially for HPV-related oral disease surveillance, as well as periodic gynecological visits for women with GATA2 deficiency [20].
Recommendations are shown in Figure 2.
In GATA2 mutation, carriers who develop MDS, allogeneic hematopoietic stem cell transplantation (HSCT) represent the only curative option. HSCT restores normal hematopoiesis, resolves MDS (or AML), and clears long-standing infections. Moreover, despite the limitation of data, HSCT has been described in clinical cases associated with a regression of PAP and pulmonary hypertension [35] and the resolution of condylomas and cervical cancer in situ [36,37].
Currently, the best choice of conditioning regimen, donor source, and graft-versus-host disease (GVHD) prophylaxis remains unclear. Regarding the best time to proceed to HSCT, both myeloablative and reduced intensity conditioning regimens have been successfully used as well as different donor types: peripheral blood stem cells from matched related or unrelated donors, bone marrow stem cells from matched or haploidentical donor, and umbilical cord blood [20,37]. However, HSCT remains challenging because of many comorbidities presented in the GATA2 related syndromes, such as the previously mentioned infections from Mycobacterium avium complex and PAP.
It is important to note that a myeloablative conditioning regimen followed by HSCT is the standard approach in pediatric patients with myelodysplasia, particularly in patients with high-risk features such as monosomy 7 (often found in patients with GATA2 related MDS). Otherwise, patients with GATA2 deficiency present variable grade of immunodeficiency and other comorbidity that could threaten the outcome of HSCT with a high rate of transplant related toxicity. Interestingly, different case series reported thrombotic complications after HSCT in GATA2 deficient patients, sometimes with a fatal outcome [36,38,39].
In the analysis of MDS in children by the European Working Group, 34 GATA2 mutation carriers have been transplanted. In this group, the 5-year OS (66% vs. 69%) and EFS (60% for both) after HSCT were comparable to a GATA2 wild type group. The relapse rate, TRM, and the rate of infectious complications did not significantly differ between both groups. Specifically, the rate of infectious complications (cytomegalovirus reactivation, cytomegalovirus disease, Epstein–Barr virus infections/post-transplant lymphoproliferative disorder, adenovirus infections, viral, bacterial, fungal, and parasitic infections) was 66% in the GATA2 deficient patients and 61% in the control group [7].
Hofmann et al. analyzed a cohort of 15 pediatric patients with GATA2-related MDS undergoing HSCT after a myeloablative conditioning regimen. They compared this cohort of patients to patients with a BMF/MDS and patients with acute leukemia, and found a comparable rate of graft failure, graft versus host disease, and transplant related mortality. Interestingly, patients with GATA2 deficiency showed a higher incidence of neurological complication and thrombotic events [39], questioning the safety of myeloablative condition regimen in GATA2 deficient patients.
Considering the ideal time to proceed to HSCT, a better prognosis was seen if HSCT was performed in an early phase without evidence of disease progression, but the EWOG-MDS 2017 guidelines on MDS- refractory cytopenia of childhood (RCC) recommended watchful waiting in case of absence of high-risk genetic alterations and stable blood counts. Therefore, it is not possible to state if HSCT is recommended in patients with GATA2 deficiency without MDS with high-risk features. In the analysis performed by EWOG-MDS working group, a stable disease was rarely reported in GATA2-related MDS. Among the 57 children with GATA2-related MDS, seven patients did not undergo allogenic stem cell transplantation, three of these patients with stable, non-transfusion dependent RCC remained alive and without severe infections 5 to 16 years from diagnosis, the other four patients died for disease progression or infection after AML-like chemotherapy [7]. These data support the idea that a watch and wait strategy could not be safe in patients with GATA2 related MDS.
Different managements of treatment have been described in literature, considering the presence or not of additional cytogenetic or molecular abnormalities, hematological features, and other clinical presentation. A brief summary is reported in Table 2.
Table 2.
Cases described of GATA2 related MDS/AML. * patients with GATA2 synonymous mutations.
| No. of Patients with GATA2 Related MDS/AML | MDS Type | Additional Cytogenetic Abnormality | Median Age at MDS Diagnosis (Range) | HSCT | Outcome | Reference |
|---|---|---|---|---|---|---|
| 3 | AML(33%) MDS (66%) |
t(1;21) (33%) | 27.7 (10–38) | 3/3 | Relapse (33%) Alive (66%) |
Mir, 2015 [25] |
| 28 | MDS-RAEB-1 (7%) MDS-RAEB-2 (4%) MDS-RCMD (89%) |
Monosomy 7 (14%) Trisomy 8 (25%) Der(1;7) (4%) |
35.4 (12–73) | n.a. | n.a. | Ganapathi [19] |
| 7 | MDS-RCMD (29%) MDS-RAEB-2 (29%) AML (43%) |
Trisomy 8 (14%) | 16.8 (13–25) | 1/7 | Alive (57%) Dead (43%) |
Churpek [42] |
| 5 | MDS n.s. (80%) MDS-RCMD (20%) |
Trisomy 8 (60%) Monosomy 7 (40%) Der(1;7) (40%) |
n.a. | 4/5 | Alive (60%) Dead (40%) |
Wang [43] |
| 5 | Marrow failure (100%) | Trisomy 8 (40%) | 16.0 (12–22) | 1/5 | n.a. | Zahng [44] |
| 57 | RCC (54%) RAEB (35%) RAEB-t (11%) |
Monosomy 7 (68%) Trisomy 8 (9%) Der(1;7) (7%) |
12.0 (3–19) | 50/57 | Died (28%) Relapse (5%) Alive (67%) |
Wlodarski [7] |
| 11 | RCC (73%) RCMD (9%) RAEB (18%) |
Monosomy 7 (73%) Trisomy 8 (18%) |
14.7 (4–21) | 9/11 | Alive (73%) Dead (27%) Relapse (9%) |
Novakova [18] |
| 5 | MDS n.s. (100%) | n.a. | 26.0 (7–60) | n.a. | n.a. | Schlums [45] |
| 5 | RCC (100%) | Monosomy 7 (80%) | 9.8 (5–15) | 5/5 | Alive (80%) Dead (20%) |
Fisher [46] |
| 11 | MDS n.s. (100%) | Trisomy 8 (45%) | 33.5 (23–53) | n.a. | n.a. | McReynolds [30] |
| 8 * | RCC (75%) RAEB (13%) MDS-MLD (13%) |
Monosomy 7 (50%) | 11.6 (3–24) | 6/8 | Alive (88%) Dead (13%) |
Kozyra [47] |
| 3 | AML (33%) MDS (66%) |
n.a. | 19.0 (13–27) | 2/3 | Alive (66%) Dead after HSCT (33%) |
Bogaert [36] |
| 1 | MDS n.s. (100%) | Monosomy 7 (50%) | 22 (19–25) | 1/2 | Alive (50%) Dead (50%) |
Fox [48] |
| 6 | AML (50%) RAEB (17%) RCC (33%) |
Monosomy 7 (100%) Trisomy 8 (17%) |
10.5 (5–15) | 6/6 | Alive (83%) Dead (17%) |
Yoshida [33] |
Evaluating risks and benefits of both strategies (to transplant or not to transplant) in patients with stable disease, it could be suitable to distinguish GATA2 deficient patients who undergo HSCT for bone marrow failure or immunodeficiency from patients who undergo HSCT for high-risk MDS or acute leukemia.
Some authors suggested the use of a non-myeloablative regimen for patients transplanted earlier with a proliferative disadvantage of the bone marrow and the use of a higher dose regimen when either the development of clonal progression has occurred or the patient has a hypercellular marrow [40].
However, to date, the donor type, the conditioning regimen, and the optimal time to proceed to HSCT as well as the level of chimerism needed to reverse the phenotype remain unclear [41], underlining the need for consensus guidelines. It is important to note that potential family donors before stem cell donation should be tested for GATA2 abnormalities. In the United States, there is an ongoing clinical trial of the National Institute of Health Clinical Center with the aim to determine whether an allogeneic HSCT approach results in engraftment and restores normal hematopoiesis by day + 100 in patients with GATA2 mutations (ClinicalTrials.gov Identifier: NCT01861106).
6. Conclusions
In summary, the somatic mutation landscape significantly differs between children and adults with MDS. A peculiarity of childhood MDS is represented by the frequent association with genetic (i.e., germinal) predisposition or with IBMFS. Specifically, with the increasing use of molecular test in clinical practice, more children diagnosed with MDS are likely to be diagnosed as having a genetic predisposition syndrome. Since advanced MDS and monosomy 7 are highly overrepresented in GATA2-related MDS, we suggest that monosomy 7 could be used as a diagnostic indicator for GATA2 deficiency in adolescents, given the high prevalence of mutations in this subgroup of age. In particular, GATA2 analysis has to be included in the workup of all young adults and children above the age of four diagnosed with MDS associated with monosomy 7, trisomy 8, or der(1;7), regardless of the presence of a clinical phenotype suggestive to GATA2-deficency or the family history. For children with GATA2 mutations and MDS, the ideal time for HSCT seems to be during the hypo cellular phase of the disease and before serious complications (i.e., invasive infections) or progression to advanced MDS occur.
Acknowledgments
The authors are thankful to Megan Eckley for her support in English grammar proofreading.
Author Contributions
A.B., D.L., R.M., L.S., K.G., M.A., G.D.B., F.L., and A.M. contributed to the manuscript and were involved in revisions and proof-reading. All authors have read and agreed to the published version of the manuscript.
Funding
The researcher received no funding.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Cogle C.R., Craig B.M., Rollison D.E., List A.F. Incidence of the myelodysplastic syndromes using a novel claims-based algorithm: High number of uncaptured cases by cancer registries. Blood. 2011;117:7121–7125. doi: 10.1182/blood-2011-02-337964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locatelli F., Strahm B. How I Treat Myelodysplastic Syndromes of Childhood. Blood. 2018;131:1406–1414. doi: 10.1182/blood-2017-09-765214. [DOI] [PubMed] [Google Scholar]
- 3.Hsu A.P., Sampaio E.P., Khan J., Calvo K.R., Lemieux J.E., Patel S.Y., Frucht D.M., Vinh D.C., Auth A.F., Freeman K.N., et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn C.N., Chong C.E., Carmichael C.L., Wilkins E.J., Brautigan P.J., Li X.C., Babic M., Lin M., Carmagnac A., Lee Y.K., et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011;43:1012. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostergaard P., Simpson M.A., Connell F.C., Steward C.G., Brice G., Woollard W.J., Dafou D., Kilo T., Smithson S., Lunt P., et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat. Genet. 2011;43:929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- 6.Scott H.S., Hahn C.N., Carmichael C.L., Wilkins E.J., Chong C.-E., Brautigan P.J., Babic M., Lin M., Carmagnac A., Lee Y.K., et al. GATA2 is a New Predisposition Gene for Familial Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML) Blood. 2010;116 doi: 10.1182/blood.V116.21.LBA-3.LBA-3. [DOI] [Google Scholar]
- 7.Wlodarski M.W., Hirabayashi S., Pastor V., Starý J., Hasle H., Masetti R., Dworzac M., Schmugge M., van den Heuvel-Eibrink M., Ussowicz M., et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127:1387–1397. doi: 10.1182/blood-2015-09-669937. [DOI] [PubMed] [Google Scholar]
- 8.Collin M., Dickinson R., Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. Br. J. Haematol. 2015;169:173–187. doi: 10.1111/bjh.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leubolt G., Redondo Monte E., Greif P.A. GATA2 mutations in myeloid malignancies: Two zinc fingers in many pies. IUBMB Life. 2020;72:151–158. doi: 10.1002/iub.2204. [DOI] [PubMed] [Google Scholar]
- 10.Crispino J.D., Horwitz M.S. GATA factor mutations in hematologic disease. Blood. 2017;129:2103–2110. doi: 10.1182/blood-2016-09-687889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson R.E., Griffin H., Bigley V., Reynard L.N., Hussain R., Haniffa M., Lakey J.H., Rahman T., Wang X.-N., McGovern N., et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S.J., Ma L.Y., Huang Q.H., Li G., Gu B.W., Gao X.D., Shi J.Y., Wang Y.Y., Gao L., Cai X., et al. Gain-of-function mutation of GATA-2 in acute myeloid transformation of chronic myeloid leukemia. Proc. Natl. Acad. Sci. USA. 2008;105:2076–2081. doi: 10.1073/pnas.0711824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson K.D., Hsu A.P., Ryu M.J., Wang J., Gao X., Boyer M.E., Liu Y., Lee Y., Calvo K.R., Keles S., et al. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J. Clin. Investig. 2012;122:3692–3704. doi: 10.1172/JCI61623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wehr C., Grotius K., Casadei S., Bleckmann D., Bode S.F.N., Frye B.C., Seidls M., Gulsuner S., King M.C., Percival M.B., et al. A novel disease-causing synonymous exonic mutation in GATA2 affecting RNA splicing. Blood. 2018;132:1211–1215. doi: 10.1182/blood-2018-03-837336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donadieu J., Lamant M., Fieschi C., de Fontbrune F.S., Caye A., Ouachee M., Beaupain B., Baustamante J., Poirel H.A., Isidor B., et al. Natural history of GATA2 deficiency in a survey of 79 French and Belgian patients. Haematologica. 2018;103:1278–1287. doi: 10.3324/haematol.2017.181909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinner M.A., Sanchez L.A., Hsu A.P., Shaw P.A., Zerbe C.S., Calvo K.R., Arthur D.C., Gu W., Gould C.M., Brewer C.C., et al. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson R.E., Milne P., Jardine L., Zandi S., Swierczek S.I., McGovern N., Cookson S., Ferozepurwalla Z., Langridge A., Pagan S., et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123:863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nováková M., Žaliová M., Suková M., Wlodarski M., Janda A., Froňková E., Campr V., Lejhancová K., Zapletal O., Pospíšilová D., et al. Loss of B cells and their precursors is the most constant feature of GATA-2 deficiency in childhood myelodysplastic syndrome. Haematologica. 2016;101:707–716. doi: 10.3324/haematol.2015.137711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganapathi K.A., Townsley D.M., Hsu A.P., Arthur D.C., Zerbe C.S., Cuellar-Rodriguez J., Hickstein D.D., Rosenzweing S.D., Braylan R.C., Young N.S., et al. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood. 2015;125:56–70. doi: 10.1182/blood-2014-06-580340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McReynolds L.J., Calvo K.R., Holland S.M. Germline GATA2 Mutation and Bone Marrow Failure. Hematol. Oncol. Clin. N. Am. 2018;32:713–728. doi: 10.1016/j.hoc.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim K.C., Hosoya T., Brandt W., Ku C.J., Hosoya-Ohmura S., Camper S.A., Yamamoto M., Engel J.D. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J. Clin. Investig. 2012;122:3705–3717. doi: 10.1172/JCI61619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linnemann A.K., O’Geen H., Keles S., Farnham P.J., Bresnick E.H. Genetic framework for GATA factor function in vascular biology. Proc. Natl. Acad Sci. USA. 2011;108:13641–13646. doi: 10.1073/pnas.1108440108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crall C., Morley K.W., Rabinowits G., Schmidt B., Dioun Broyles A., Huang J.T. Merkel cell carcinoma in a patient with GATA2 deficiency: A novel association with primary immunodeficiency. Br. J. Dermatol. 2016;174:169–171. doi: 10.1111/bjd.14062. [DOI] [PubMed] [Google Scholar]
- 24.Haugas M., Lilleväli K., Hakanen J., Salminen M. Gata2 is required for the development of inner ear semicircular ducts and the surrounding perilymphatic space. Dev. Dyn. 2010;239:2452–2469. doi: 10.1002/dvdy.22373. [DOI] [PubMed] [Google Scholar]
- 25.Mir M.A., Kochuparambil S.T., Abraham R.S., Rodriguez V., Howard M., Hsu A.P., Jackson A.E., Holland S.M., Patnaik M.M. Spectrum of myeloid neoplasms and immune deficiency associated with germline GATA2 mutations. Cancer Med. 2015;4:490–499. doi: 10.1002/cam4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bresnick E.H., Jung M.M., Katsumara K.R. Human GATA2 mutations and hematologic disease: How many paths to pathogenesis? Blood Adv. 2020;4:4584–4592. doi: 10.1182/bloodadvances.2020002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West R.R., Hsu A.P., Holland S.M., Cuellar-Rodriguez J., Hickstein D.D. Acquired ASXL1 mutations are common in patients with inherited GATA2 mutations and correlate with myeloid transformation. Haematologica. 2014;99:276–281. doi: 10.3324/haematol.2013.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastor Loyola V.B., Hirabayashi S., Pohl S., Kozyra E.J., Catala A., De Moerloose B., Hasle H., Masetti R., Schmugge M., Smith O., et al. Somatic Genetic and Epigenetic Architecture of Myelodysplastic Syndromes Arising from GATA2 Deficiency. Blood. 2015;126:299. doi: 10.1182/blood.V126.23.299.299. [DOI] [Google Scholar]
- 29.Katsumara K.R., Ong I.M., DeVilbiss A.W., Sanalkumar R., Bresnick E.H. GATA Factor-Dependent Positive-feedback Circuit in Acute Myeloid Leukemia cells. Cell Rep. 2016;16:2428–2441. doi: 10.1016/j.celrep.2016.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McReynolds L.J., Yang Y., Wong H.Y., Tang J., Zhang Y., Mulé M.P., Dub J., Palmer C., Foruraghi L., Liu Q., et al. MDS-associated mutations in germline GATA2 mutated patients with hematologic manifestations. Leuk. Res. 2019;76:70–75. doi: 10.1016/j.leukres.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujiwara T., Fukuhara N., Funavama R., Nariai N., Kamata M., Nagashima T., Kojima K., Onishi Y., Sasahara Y., Ishizawa K., et al. Identification of acquired mutations by whole-genome sequencing in GATA-2 deficiency evolving into myelodysplasia and acute leukemia. Ann. Hematol. 2014;93:1515–1522. doi: 10.1007/s00277-014-2090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida M., Tanase-Nakao K., Shima H., Shirai R., Yoshida K., Osumi T., Deguchi T., Mori M., Arakawa Y., Takagi M., et al. Prevalence of Germline GATA2 and SAMD9/9L Variants in Paediatric Haematological Disorders with Monosomy 7. Br. J. Haematol. 2020 doi: 10.1111/bjh.17006. [DOI] [PubMed] [Google Scholar]
- 33.Tien F.M., Hou H.A., Tsai C.H., Tang J.L., Chiu Y.C., Chen C.Y., Kuo Y.Y., Tseng M.H., Peng Y.L., Liu M.C., et al. GATA2 zinc finger 1 mutations are associated with distinct clinico-biological features and outcomes different from GATA2 zinc finger 2 mutations in adult acute myeloid leukemia. Blood Cancer J. 2018;8:87. doi: 10.1038/s41408-018-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jesus Nunes-Santos C., Sergio D. Rosenzweig. Bacille Calmette-Guerin Complications in Newly Described Primary Immunodeficiency Diseases: 2010–2017. Front. Immunol. 2018;9:1423. doi: 10.3389/fimmu.2018.01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuellar-Rodriguez J., Gea-Banacloche J., Freeman A.F., Hsu A.P., Zerbe C.S., Calvo K.R., Wilder J., Kurlander R., Olivier K.N., Holland S.M., et al. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011;118:3715–3720. doi: 10.1182/blood-2011-06-365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lübking A., Vosberg S., Konstandin N.P., Dufour A., Graf A., Krebs S. Young woman with mild bone marrow dysplasia, GATA2 and ASXL1 mutation treated with allogeneic stem cell transplantation. Leuk. Res. Rep. 2015;4:72–75. doi: 10.1016/j.lrr.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeurer M., Magalhaes I., Andersson J., Ljungman P., Sandholm E., Uhlin M., Mattsson J., Ringdén O. Allogeneic Hematopoietic Cell Transplantation for GATA2 Deficiency in a Patient with Disseminated Human Papillomavirus Disease. Transplantation. 2014;98:e95-6. doi: 10.1097/TP.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 38.Bogaert D.J., Laureys G., Naesens L., Mazure D., De Bruyne M., Hsu A.P., Bordon V., Wouters E., Tavernier S.J., Lambrecht B.N., et al. GATA2 deficiency and haematopoietic stem cell transplantation: Challenges for the clinical practitioner. Br. J. Haematol. 2020;188:768–773. doi: 10.1111/bjh.16247. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann I., Avagyan S., Stetson A., Guo D., Al-Sayegh H., London W.B., Lehman L. Comparison of Outcomes of Myeloablative Allogeneic Stem Cell Transplantation for Pediatric Patients with Bone Marrow Failure, Myelodysplastic Syndrome and Acute Myeloid Leukemia with and without Germline GATA2 Mutations. Biol. Blood Marrow. Transplant. 2020;26:1124–1130. doi: 10.1016/j.bbmt.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parta M., Shah N.N., Baird K., Rafei H., Calvo K.R., Hughes T., Cole K., Kenyon M., Schuver B.B., Cuellar-Rodriguez J., et al. Allogeneic Hematopoietic Stem Cell Transplantation for GATA2 Deficiency Using a Busulfan-Based Regimen. Biol. Blood Marrow. Transplant. 2018;24:1250–1259. doi: 10.1016/j.bbmt.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickstein D. HSCT for GATA2 deficiency across the pond. Blood. 2018;131:1272–1274. doi: 10.1182/blood-2018-02-826461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Churpek J.E., Pyrtel K., Kanchi K.L., Shao L., Koboldt D., Miller C.A., Shen D., Fulton R., O’Laughlin M., Fronick C., et al. Genomic Analysis of Germ Line and Somatic Variants in Familial Myelodysplasia/Acute Myeloid Leukemia. Blood. 2015;126:2484–2490. doi: 10.1182/blood-2015-04-641100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X., Muramatsu H., Okuno Y., Sakaguchi H., Yoshida K., Kawashima N., Xu Y., Shiraishi Y., Chiba K., Tanaka H., et al. GATA2 and Secondary Mutations in Familial Myelodysplastic Syndromes and Pediatric Myeloid Malignancies. Haematologica. 2015;100:e398–e401. doi: 10.3324/haematol.2015.127092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M.Y., Keel S.B., Walsh T., Lee M.K., Gulsuner S., Watts A.C., Pritchard C.C., Salipante S.J., Jeng M.R., Hofmann I., et al. Genomic analysis of bone marrow failure and myelodysplastic syndromes reveals phenotypic and diagnostic complexity. Haematologica. 2015;100:42–48. doi: 10.3324/haematol.2014.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlums H., Jung M., Han H., Theorell J., Bigley V., Chiang S.C.C., Allan D.S.J., Davidson-Moncada J.K., Dickinson R.E., Holmes T.D., et al. Adaptive NK Cells Can Persist in Patients with GATA2 Mutation Depleted of Stem and Progenitor Cells. Blood. 2017;129:1927–1939. doi: 10.1182/blood-2016-08-734236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher K.E., Hsu A.P., Williams C.L., Sayeed H., Merritt B.Y., Elghetany M.T., Holland S.M., Bertuch A.A., Gramatges M.M. Somatic Mutations in Children with GATA2-Associated Myelodysplastic Syndrome Who Lack Other Features of GATA2 Deficiency. Blood Adv. 2017;1:443–448. doi: 10.1182/bloodadvances.2016002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozyra E.J., Pastor V.B., Lefkopoulos S., Sahoo S.S., Busch H., Voss R.K., Erlacher M., Lebrecht D., Szvetnik E.A., Hirabayashi S., et al. Synonymous GATA2 Mutations Result in Selective Loss of Mutated RNA and Are Common in Patients with GATA2 Deficiency. Leukemia. 2020 doi: 10.1038/s41375-020-0899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox L.C., Tan M., Brown A.L., Arts P., Thompson E., Ryland G.L., Lickiss J., Scott H.S., Poplawski N.K., Phillips K., et al. A Synonymous GATA2 Variant Underlying Familial Myeloid Malignancy with Striking Intrafamilial Phenotypic Variability. Br. J. Haematol. 2020;190:e297–e301. doi: 10.1111/bjh.16819. [DOI] [PubMed] [Google Scholar]