Abstract
The composition of essential oils of Chrysanthemum indicum and C. morifolium were comparatively studied using both Gas Chromatography/Flame ionization Detector (GC/FID) and Gas Chromatography/Mass spectrometry (GC/MS) analyses. The antiviral activity was determined using a plaque reduction assay against three common viruses namely, herpes simplex type-1 (HSV-1), hepatitis A (HAV) and vesicular stomatitis virus (VSV). The antimicrobial activity was assessed using agar diffusion and microdilution methods and the minimum inhibitory concentration (MIC) values were determined. In addition, the anti-mycobacterial evaluation was carried out using the Alamar blue assay and the effect against Helicobacter pylori was investigated. The anti-trypanosomal activity was evaluated using the resazurin method. GC investigations revealed that camphor is the major constituent of both oils accounting for 36.69 and 14.56% in the essential oils from C. indicum and C. morifolium, respectively. C. indicum was biologically more active in all experiments; it exhibited a notable antitrypanosomal activity with an IC50 value equals 45.89 μg/mL and a notable antimicrobial activity versus Streptococcus agalactiae with a MIC value of 62.5 μg/mL. It also inhibited the replication of VSV with an IC50 value of 3.14 μg/mL. Both oils revealed antioxidant potential with IC50 values of 2.21 and 2.59 mg/mL for C. indicum and C. morifolium, respectively. This study provides evidence beyond the traditional use of both Chrysanthemum indicum and C. morifolium as anti-infective agents. Thus they could be used as spices in food and can be incorporated in different food products and pharmaceutical preparations as natural preservatives possessing antioxidant potential.
Keywords: Asteraceae, antimicrobial, antioxidant, Chrysanthemum, essential oil, preservative
1. Introduction
Medicinal plants provide a large chemical library of secondary metabolites, which are widely used in phytotherapy for their antiviral, antimicrobial, anti-trypanosomal, anticancer and antioxidant activities [1]. Among them, essential oils are common in many aromatic plants. Researchers have continued to explore the chemical composition of the essential oils and their biological activities aiming to discover new bioactive and useful metabolites for the treatment of human and animal health conditions [2,3].
Based on traditional Chinese medicine and Ayurveda, the dried flower heads of many Chrysanthemum plants were widely used for the treatment of common cold [4], fever, migraine, conjunctivitis, eye irritation, hypertension, inflammation, ulcerative colitis, vertigo, ophthalmia with swelling as well as skin infections [5,6,7].
This genus, Chrysanthemum L. (Dendranthema (DC.)) belongs to the family Asteraceae, and comprises about 40 species, widely distributed in Asia, mainly in Mongolia, China and Japan, and eastern Europe. Nowadays, most of these plants are cultivated as ornamentals in the whole world [8]. C. morifolium Ramat. (Jiu Hua) and C. indicum L. (Ye Jiu Hua) are common in China [9]. They are generally perennial herbs or subshrubs with aromatic alternate, lobed or serrate leaves. Capitula are corymbs or solitary with yellow, 5-lobed corolla disc florets meanwhile ray florets are white, pink or yellowish and achenes are obvoid with 5–8 ribs [10,11].
Thorough phytochemical and biological investigations of both plants led to the isolation and identification of many bioactive secondary metabolites represented mainly by flavonoids, steroids and terpenoids [12,13,14,15,16,17]. These plants exhibit strong antibacterial, antiviral, antioxidant, antihypertension, anti-inflammatory and immunomodulatory activities [18,19,20,21,22,23,24,25,26].
The chemical compositions of C. indicum and C. morifolium essential oils have been previously studied. Camphor, borneol, camphene, α-pinene, p-cymene and 1,8 cineole are the major constituents of the oils [27,28]. In spite of the significant biological importance of these plants, their essential oils were not fully investigated, only the antimicrobial activity had been studied [28].
In this study, a detailed comparative study regarding the chemical composition and anti-infective properties of C. morifolium and C. indicum essential oils was presented. The biological activities of the essential oils against a wide range of microbes and viruses were investigated. Moreover, microorganisms of food and clinical interest have been selected as target for the antimicrobial evaluation to further consolidate the rationale beyond the traditional use of both plants for the first time as anti-infective agents aiming to use these plants and their oils as natural spices and preservatives both in food and pharmaceutical preparations.
2. Materials and Methods
2.1. Plant Material
The flower heads of Chrysanthemum morifolium Ramat. and C. indicum L. (family Asteraceae) were commercially obtained from China. DNA barcoding technique was used to authenticate the plants and it was confirmed morphologically at the Botanical Garden, Heidelberg University. The flower materials are stored at the Department of Biology, IPMB, Heidelberg University under the numbers of P6851 and P6852, respectively.
2.2. Chemicals, Reagents and Strains
All cell culture materials and media were obtained from Gibco® (Invitrogen, Karlsruhe, Germany) and Greiner Labortechnik® (Frickenhausen, Germany). Chemicals and reference drugs were bought from Sigma® (Sternheim, Germany). The solvents with analytical grade were procured from Merck® (Darmstadt, Germany). All the tested microorganisms were provided by Medical Microbiology Lab., Hygiene Institute, Heidelberg University, Germany. Mycobacterium tuberculosis (RCMB 010126) strain was obtained from RCMB, Al-Azhar University, Cairo, Egypt and Helicobacter pylori ATCC 43504 strain from the American Type Culture Collection.
2.3. Essential Oils Preparation
The air-dried flower heads of C. morifolium and C. indicum were hydro- distillated by Clevenger-type apparatus (Faculty of Bioscience, Heidelberg University glassware Lab, Heidelberg, Germany) for 6 h. Dehydrated essential oils (EO) were obtained by using anhydrous Na2SO4 to yield 0.16% and 0.18% dry weight, respectively. The essential oils were stored in amber glass with stopper vials at −30 °C until analysis.
2.4. GC/FID and GC/MS Analyses
GC 5890 II (Hewlett-Packard GmbH, Homburg, Germany) coupled with quadrupole mass spectrometer (SSQ 7000, Thermo-Finnigan, Bremen, Germany) was used for the identification of the essential oil compounds. Instrumental settings were Gas: 4He (2 mL/min); Temp: T0/45 °C, T2/isothermal, T1/300 °C, T2/isothermal, detector/300 °C, and injector/250 °C, Split ratio: 1:20. Varian 3400 (Varian GmbH, Darmstadt, Germany) was used for GC analyses with OV-5 fused bonded column (Ohio Valley, OH, USA) and a Flame Ionization Detector (FID) for quantitative determination of the components. Mean of 3 independent runs was used to calculate areas under the peaks (AUP) using an SRI Instrument (Torrance, CA, USA) and the total area was considered as 100%. Wiley Registry of Mass Spectral Data 8th edition, National Institute of Standards and Technology (NIST) Mass Spectral Library (December 2005), and the literature were used to compare and identify the active constituent of spectral data [29,30].
2.5. Cytotoxic Assay
Cytotoxicity was determined in triplicates using MTT cell viability assay [31]. A total of 2 × 104 cells/well of both cell lines CCL-81 (Vero) (ATCC 43255) and human HL-60 (human myeloid cells) (ATCC CCL-240) were incubated for 24 h at 37 °C with a humidified atmosphere of 5% CO2 in complete DMEM media and in complete RPMI media, respectively. The EOs were dissolved in DMSO, the final concentration of DMSO was <0.01% and the absorbance values of DMSO were subtracted from the sample absorbance. Several concentrations of EO (1 to 1000 μg/mL) and doxorubicin (positive control) were applied individually to the cells and incubated for further 24 h with the same conditions. The light absorption of formazan crystal that formed after incubation for 4 h with MTT (0.5 mg/mL) and solubilized by DMSO (200 µL) was measured at 570 nm using a Tecan® Safire II Reader (Männedorf, Switzerland). The percentage of cell viability of three independent experiments was calculated as follows: cell viability (%) = (OD of treated cells − OD of DMSO treated)/(OD of control cells − OD of DMSO treated) × 100%.
2.6. Antiviral Assay
Plaque reduction assay was used to determine the antiviral activity of EO as previously described [32]. Briefly, Vero cells (CCL-81) were cultivated for 24 h at 5% CO2 and 37 °C. The cells were inoculated for 1 h with individual viruses; herpes simplex type-1 virus HSV-1 (ATCC VR-1493), hepatitis A virus HAV (ATCC VR-1357) or vesicular stomatitis virus VSV (ATCC VR-1238) (1 × 101‒107 plaque-forming unit PFU/mL). The infected cells (2 × 103 PFU) were washed and incubated with several concentrations of EO as well as acyclovir (positive control) for 1 h. Then, cells were overlaid with nutrient agarose and incubated for 72 h. Formaldehyde (10%) in phosphate-buffer solution (pH 7.3) was used to fix cells for 1 h. Crystal violet, 0.5% in 20% ethanol, was used to stain cells and then plaques were counted. The percentage of viral inhibition was calculated as [1 − (Vd/Vc)] × 100, where Vd and Vc are the number of plaques in the presence and absence of test samples.
2.7. Antimicrobial Assay
Gram positive bacteria: Bacillus subtilis (ATCC 6051), Staphylococcus aureus (ATCC 29213), Staphylococcus capitis (ATCC 35661), Staphylococcus epidermidis (ATCC 14990), Streptococcus agalactiae (ATCC 27956), Streptococcus pyogenes (ATCC 12344), Gram negative bacteria: Escherichia coli (ATCC 25922), Pseudomonas fluorescens (ATCC 13525), Salmonella typhimurium (ATCC 14028), Shigella flexneri (ATCC 700930), and fungi: Aspergillus fumigatus (ATCC 1022), Candida albicans (ATCC 90028), Geotrichum candidum (ATCC 12784), Syncephalastrum racemosum (ATCC 14831), and MDR (multidrug resistance) isolates were used to evaluate the antimicrobial activity of EOs. Bacteria and fungi were incubated for 24 h at 37 °C in Columbia media plus 5% sheep blood and for 48 h at 25 °C in CHRO-Magar® Candida, respectively (BD, Heidelberg, Germany). The agar diffusion method was used to determine the sensitivity of tested microbes to the tested EO. Pathogens 1 × 106 CFU/mL saline were incubated with Mueller Hinton agar as previously reported [33]. Pasteur pipette was used to cup wells (6 mm diameter). The EO (3.2 mg/mL), DMSO, and reference antibiotics (ampicillin, gentamycin, and clotrimazole) were applied to the experimental set. The clear zones surrounding the wells indicated the growth inhibition and the average diameters of triple wells were recorded. Micro-broth dilution method was used for the assessment of the MIC (minimum inhibition concentration) and MMC (minimum microbicidal concentration) of tested EO [34]. Several concentrations of EO were incubated with bacteria (5 × 105 CFU/mL Mueller Hinton broth) for 24 h at 37 °C and fungi (5 × 105 CFU/mL Sabouraud Dextrose broth) for 48 h at 25 °C in a 96-well plate and reference antibiotics. The MMC of EO was determined as the lowest concentration of EO that completely killed the microbe.
2.8. Anti-Mycobacterial Assay
Alamar blue assay (MABA) was used to determine the anti-TB activity of EO. Mycobacterium tuberculosis (RCMB 010126) strain was cultivated in complete 7H9-S medium in the dilution of 1:20. The inoculum (100 μL) was incubated for 7 days at 37 °C with and without several concentrations of EO and isoniazid (positive control) (100 μL) in 96-well plate. Following this, cells were incubated for further 24 h with alamar blue solution (30 μL). The alamar blue interacted with bacteria and changed from oxidized form (blue) to reduced form (pink). The color measured spectrophotometry and the inhibition % was calculated from the equation = 1 − (mean of test well/mean of blank wells) × 100. The lowest concentration of EO that prevents the color change is considered as MIC [35].
2.9. Anti-Helicobacter Pylori Assay
Helicobacter pylori (ATCC 43504) (100 μL) of 20% (v/v) bacterial suspensions were incubated with Mueller–Hinton broth for 24 h at 37 °C with 100 μL of several concentrations of EO as well as clarithromycin as positive control in 96 well plate. Then, the MTT protocol was applied using the abovementioned cytotoxicity assay [35].
2.10. Anti-Trypanosomal Assay
Trypanocidal activity of EO was determined using resazurin dye as previously described [36]. Trypanosoma b. brucei TC221 (1 × 104 cells/well) were incubated in complete Baltz medium with several concentrations of EO and diminazene (positive control) for 24 h at 5% CO2 and 37 °C. Following this, cells were incubated with resazurin (10 µL) for further 24 h. The absorbance at λ of 492 and 595 nm was measured by Tecan® plate reader and the IC50 was calculated [37].
2.11. Antioxidant Determination
2.11.1. Diphenyl-1-Picrylhydrazyl Scavenging Capacity Assay
The antioxidant potential was determined adopting the method which was reported previously by Nenadis et.al and Youssef et.al [38,39]. Briefly, 200 µL of samples at various concentration ranging between (1–100,000 μg/mL) and 3.8 mL of 60 µg/mL of DPPH methanol solution were mixed with each other. The mixture representing the reaction was maintained in the dark for 30 min at room temperature then the absorbance at 517 nm was measured. Quercetin was used as a positive control. DPPH•: free radical scavenging activity was assessed using the equation presented below:
| % Inhibition = [Ac − As/Ac] × 100, |
where Ac: absorbance of control; As: absorbance of sample.
2.11.2. Superoxide Radical Scavenging (SORS) Activity
The ability of the C. indicum and C. morifolium oil to scavenge superoxide anion radicals was evaluated according to the previously reported method [40]. In brief, several concentrations of oils and quercetin (used as a positive control) were added to SO generated solution (1 mL of Tris–HCL buffer (16 mM, pH-8), 1 mL of nitroblue tetrazolium NBT (50 µM), 1 mL of Nicotinamide adenine dinucleotide (NADH) (78 µM) solution and 1 mL of PMS (phenazine methosulfate) (10 µM). Incubation was done for 5 min at 25 °C and then the absorbance was measured at 560 nm in spectrophotometer. The percentage inhibition of O2•- generation was calculated as follows:
| % Inhibition = [Ac − As/Ac] × 100, |
where Ac: absorbance of control; As: absorbance of sample.
2.11.3. Hydroxyl Radical Scavenging (HRS) Assay
Deoxyribose degradation (HRS) assay was used to determine the ability of C. indicum and C. morifolium oils to scavenge hydroxyl radical according to the previously reported method [41]. Equal volumes of several concentrations of essential oils or quercetin (used as a positive control) were mixed with working solution of 28 mM 2-deoxy-2-ribose (2-DR), 1.04 mM EDTA, 200 µM FeCl3, and 1.0 mM H2O2. After the incubation period (1 h at 37 °C) thiobarbituric acid (TBA) and 2.8% trichloroacetic acid (TCA) (1:1 v/v) were added to the reaction mixture and incubated at 100 °C for 20 min. Absorbance was measured at 540 nm against a blank using S Jenway® 6800 UV/VIS spectrophotometer (Essex, UK). The percentage inhibition of HO•− generation was calculated as follows:
| % Inhibition = [Ac − As/Ac] × 100, |
where Ac: absorbance of control; As: absorbance of sample.
2.12. Statistical Analysis
All the assays were performed in triplicate and in three independent times. Data were expressed as the mean ± SD. The dose response curves, graphs were drawn and IC50 were calculated by four parameter logistic equation, and student’s t-test and/or the Kruskal–Wallis test were carried out by GraphPad Prism® 8.02 (GraphPad Software, Inc., San Diego, CA, USA). The p value < 0.05 was considered a significance difference between comparison groups.
3. Results and Discussion
3.1. Volatile Oil Constituents of Chrysanthemum indicum and C. morifolium Flower Heads
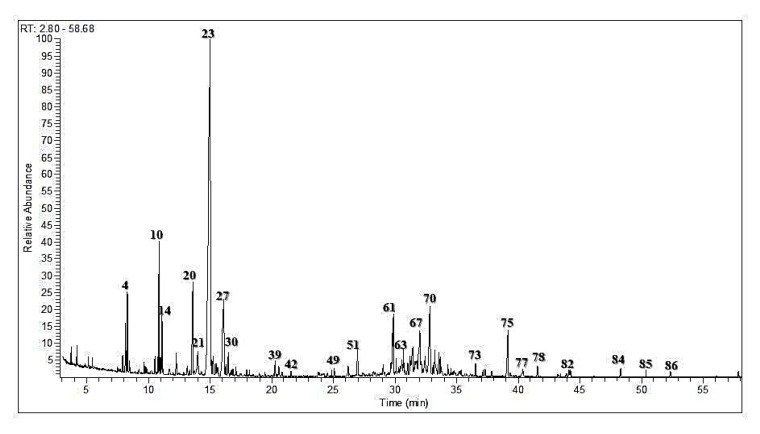
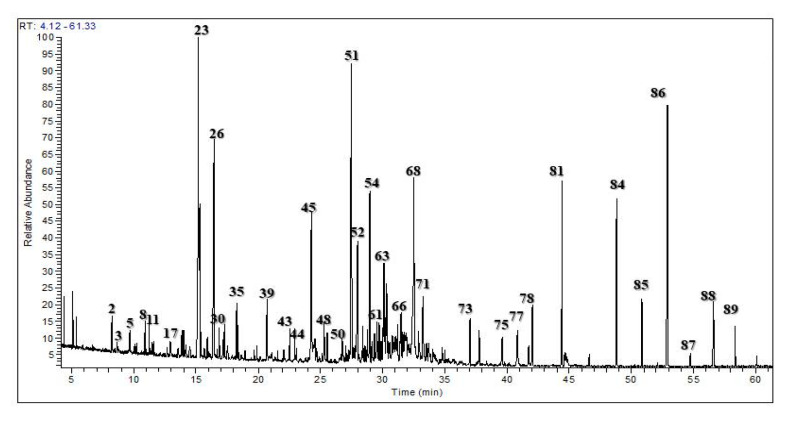
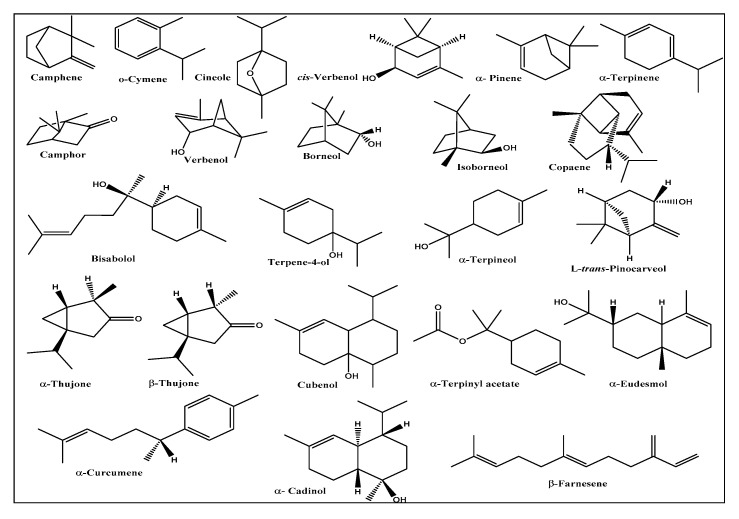
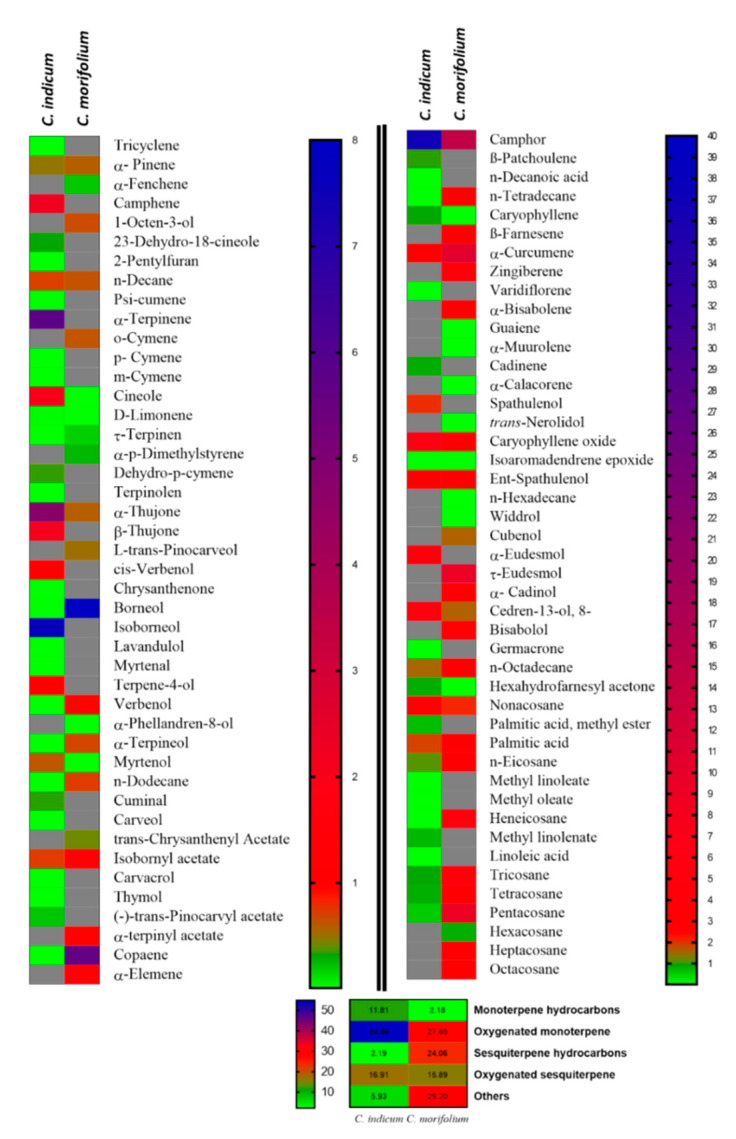
The essential oils from Chrysanthemum indicum and C. morifolium flower heads were comparatively studied using both GC/FID and GC/MS analyses. The aromatic essential oil from C. indicum flower heads has a blue color (0.16% v/w yield), whereas the aromatic oil from C. morifolium flower heads is greenish blue (yield 0.18% v/w). GC profiling revealed the presence of 89 metabolites representing 91.70 and 98.98% of the total essential oil composition of C. indicum and C. morifolium flower heads, respectively (Figure 1 and Figure 2). Sixty-four compounds were tentatively identified in C. indicum with camphor (36.69%) as the major constituent followed by isoborneol (7.64%), α-terpinene (5.73%), and caryophyllene oxide (5.46%). For C. morifolium fifty-five compounds were determined; similar to C. indicum camphor (14.56%) represented the prevailing compound followed by curcumene (10.50%), τ-eudesmol (8.92%), pentacosane (8.65%), borneol (7.95%) and copaene (5.61%) (Table 1). The major constituents present in both oils are illustrated in Figure 3. Apparently, oxygenated monoterpenes, represented mainly by camphor predominate in both oils. Moreover, monoterpenes and oxygenated sesquiterpenes exist in considerable amounts in the essential oil of C. morifolium, but in minor abundance in C. indicum oil. In contrast, monoterpenes form only 2.18% of the total identified oil components of C. morifolium essential oil. The differences in the composition of both oils were further illustrated using the heat map analysis, which is a type of graphical cluster analysis to better visualize the differences (Figure 4). The conditional formatting was applied using color code for each value. Dark grey coded for “non-detected compound”, green “for low levels content” from 0.01 to 0.9% meanwhile red coded from 1 to 8%, and blue for compounds showing % above 8%. Noteworthy to mention that the chemical composition of the essential oil obtained from the flowers of C. morifolium growing in Nigeria is quite different; its main constituent of the volatile oil is cis chrysanthenyl acetate (21.6%) followed by octadecanoic acid (19.5%) and borneol (15.5%) [42]. However, the essential oil obtained from the Korean C. indicum flower heads revealed nearly the same major constituents in which camphor represents the major component [43]. This greatly highlighted the impact of geographical distribution on the volatile oil compositions.
Figure 1.
Gas Liquid Chromatography (GLC) profile of the volatile constituents in the essential oil of C. indicum flower heads.
Figure 2.
Gas Liquid Chromatography (GLC) profile of the volatile constituents in the essential oil of C. morifolium flower heads.
Table 1.
Volatile oil compositions of C. indicum and C. morifolium flower heads.
| Compound | RI | Content [%] | Identification Method | |||
|---|---|---|---|---|---|---|
| Cal. | Rep. | C. indicum | C. morifolium | |||
| 1. | Tricyclene | 904 | 905 | tr | - | MS, RI |
| 2. | α- Pinene | 917 | 917 | 0.49 | 0.57 | MS, RI, AU |
| 3. | α-Fenchene | 930 | 934 | - | 0.20 | MS, RI |
| 4. | Camphene | 933 | 933 | 2.21 | - | MS, RI, AU |
| 5. | 1-Octen-3-ol | 961 | 964 | - | 0.63 | MS, RI |
| 6. | 2,3-Dehydro-1,8-cineole | 972 | 972 | 0.32 | - | MS, RI |
| 7. | 2-Pentylfuran | 975 | 972 | tr | - | MS, RI |
| 8. | n-Decane | 1000 | 1000 | 0.67 | 0.61 | MS, RI |
| 9. | Psi-cumene | 1002 | 1004 | tr | - | MS, RI |
| 10. | α-Terpinene | 1006 | 1006 | 5.73 | - | MS, RI, AU |
| 11. | o-Cymene | 1008 | 1006 | - | 0.60 | MS, RI |
| 12. | p-Cymene | 1009 | 1006 | tr | - | MS, RI |
| 13. | m-Cymene | 1011 | 1010 | tr | - | MS, RI |
| 14. | Cineole | 1015 | 1015 | 2.02 | tr | MS, RI, AU |
| 15. | D-Limonene | 1017 | 1017 | 0.02 | tr | MS, RI, AU |
| 16. | τ-Terpinene | 1046 | 1046 | tr | 0.18 | MS, RI, AU |
| 17. | α-p-Dimethylstyrene | 1070 | 1070 | - | 0.26 | MS, RI |
| 18. | Dehydro-p-cymene | 1070 | 1071 | 0.35 | - | MS, RI |
| 19. | Terpinolene | 1075 | 1075 | tr | - | MS, RI |
| 20. | α-Thujone | 1082 | 1087 | 4.73 | 0.57 | MS, RI, AU |
| 21. | β-Thujone | 1093 | 1097 | 2.07 | - | MS, RI, AU |
| 22. | L-trans-Pinocarveol | 1119 | 1119 | - | 0.51 | MS, RI |
| 23. | Camphor | 1120 | 1124 | 36.69 | 14.56 | MS, RI, AU |
| 24. | cis-Verbenol | 1126 | 1131 | 0.97 | - | MS, RI, AU |
| 25. | Chrysanthenone | 1133 | 1131 | tr | - | MS, RI |
| 26. | Borneol | 1136 | 1130 | tr | 7.95 | MS, RI, AU |
| 27. | Isoborneol | 1149 | 1154 | 7.64 | - | MS, RI, AU |
| 28. | Lavandulol | 1152 | 1152 | tr | - | MS, RI |
| 29. | Myrtenal | 1155 | 1151 | tr | - | MS, RI, AU |
| 30. | Terpene-4-ol | 1159 | 1159 | 0.93 | - | MS, RI |
| 31. | Verbenol | 1166 | 1165 | tr | 0.87 | MS, RI, AU |
| 32. | α-Phellandren-8-ol | 1167 | 1167 | - | tr | MS, RI |
| 33. | α-Terpineol | 1169 | 1169 | tr | 0.65 | MS, RI, AU |
| 34. | Myrtenol | 1176 | 1178 | 0.59 | tr | MS, RI, AU |
| 35. | n-Dodecane | 1199 | 1200 | tr | 0.68 | MS, RI |
| 36. | Cuminal | 1206 | 1207 | 0.34 | - | MS, RI |
| 37. | Carveol | 1215 | 1217 | tr | - | MS, RI |
| 38. | trans-Chrysanthenyl acetate | 1243 | 1243 | - | 0.42 | MS, RI |
| 39. | Isobornyl acetate | 1266 | 1268 | 0.69 | 1.23 | MS, RI |
| 40. | Carvacrol | 1274 | 1275 | tr | - | MS, RI, AU |
| 41. | Thymol | 1282 | 1285 | tr | - | MS, RI |
| 42. | (-)-trans-Pinocarvyl acetate | 1303 | 1297 | 0.21 | - | MS, RI |
| 43. | α-terpinyl acetate | 1331 | 1325 | - | 0.89 | MS, RI |
| 44. | Copaene | 1367 | 1367 | tr | 5.61 | MS, RI |
| 45. | β-Elemene | 1382 | 1382 | - | 1.19 | MS, RI |
| 46. | β -Patchoulene | 1369 | 1370 | 0.34 | - | MS, RI |
| 47. | n-Decanoic acid | 1380 | 1380 | tr | - | MS, RI |
| 48. | n-Tetradecane | 1399 | 1400 | tr | 1.27 | MS, RI |
| 49. | Caryophyllene | 1406 | 1406 | 0.32 | tr | MS, RI, AU |
| 50. | β-Farnesene | 1447 | 1446 | - | 1.06 | MS, RI |
| 51. | α-Curcumene | 1467 | 1464 | 1.23 | 10.50 | MS, RI |
| 52. | Zingiberene | 1486 | 1486 | - | 4.33 | MS, RI, AU |
| 53. | Varidiflorene | 1498 | 1499 | tr | - | MS, RI |
| 54. | α-Bisabolene | 1500 | 1500 | - | 1.37 | MS, RI |
| 55. | Guaiene | 1506 | 1500 | - | tr | MS, RI |
| 56. | α-Muurolene | 1511 | 1508 | - | tr | MS, RI |
| 57. | Cadinene | 1509 | 1508 | 0.3 | - | MS, RI |
| 58. | α-Calacorene | 1523 | 1523 | - | tr | MS, RI |
| 59. | Spathulenol | 1535 | 1537 | 0.73 | - | MS, RI |
| 60. | trans-Nerolidol | 1548 | 1549 | - | tr | MS, RI |
| 61. | Caryophyllene oxide | 1562 | 1562 | 5.46 | 1.27 | MS, RI |
| 62. | Isoaromadendrene epoxide | 1583 | 1579 | tr | tr | MS, RI |
| 63. | Ent-Spathulenol | 1588 | 1578 | 1.38 | 1.27 | MS, RI |
| 64. | n-Hexadecane | 1599 | 1600 | - | tr | MS, RI |
| 65. | Widdrol | 1604 | 1606 | - | tr | MS, RI |
| 66. | Cubenol | 1608 | 1609 | - | 0.56 | MS, RI |
| 67. | α-Eudesmol | 1634 | 1630 | 4.4 | - | MS, RI |
| 68. | τ-Eudesmol | 1637 | 1638 | - | 8.92 | MS, RI |
| 69. | α-Cadinol | 1651 | 1651 | - | 1.05 | MS, RI |
| 70. | Cedren-13-ol, 8- | 1664 | 1668 | 4.94 | 0.56 | MS, RI |
| 71. | Bisabolol | 1665 | 1667 | - | 2.26 | MS, RI |
| 72. | Germacrone | 1691 | 1691 | tr | - | MS, RI |
| 73. | n-Octadecane | 1799 | 1800 | 0.54 | 1.06 | MS, RI |
| 74. | Hexahydrofarnesyl acetone | 1827 | 1832 | 0.30 | tr | MS, RI |
| 75. | Nonacosane | 1903 | 1900 | 2.75 | 0.75 | MS, RI |
| 76. | Palmitic acid, methyl ester | 1910 | 1910 | 0.24 | - | MS, RI |
| 77. | Palmitic acid | 1951 | 1951 | 0.66 | 1.54 | MS, RI, AU |
| 78. | n-Eicosane | 1998 | 2000 | 0.38 | 1.32 | MS, RI |
| 79. | Methyl linoleate | 2072 | 2078 | tr | - | MS, RI |
| 80. | Methyl oleate | 2082 | 2082 | tr | - | MS, RI |
| 81. | Heneicosane | 2103 | 2100 | tr | 4.88 | MS, RI |
| 82. | Methyl linolenate | 2113 | 2099 | 0.26 | - | MS, RI |
| 83. | Linoleic acid | 2118 | 2113 | tr | - | MS, RI, AU |
| 84. | Tricosane | 2304 | 2300 | 0.32 | 4.18 | MS, RI |
| 85. | Tetracosane | 2397 | 2400 | 0.29 | 1.34 | MS, RI |
| 86. | Pentacosane | 2503 | 2500 | 0.19 | 8.65 | MS, RI |
| 87. | Hexacosane | 2605 | 2600 | - | 0.29 | MS, RI |
| 88. | Heptacosane | 2705 | 2700 | - | 1.43 | MS, RI |
| 89. | Octacosane | 2799 | 2800 | - | 0.94 | MS, RI |
| Monoterpene hydrocarbons | 11.81 | 2.18 | ||||
| Oxygenated monoterpens | 54.86 | 27.65 | ||||
| Sesquiterpene hydrocarbons | 2.19 | 24.06 | ||||
| Oxygenated sesquiterpens | 16.91 | 15.89 | ||||
| Others | 5.93 | 29.20 | ||||
| Total identified components | 91.70 | 98.98 | ||||
Figure 3.
Major constituents of C. indicum and C. morifolium essential oils.
Figure 4.
A heatmap comparison based on abundance of individual component of C. indicum and C. morifolium; gray color indicated non-detectable levels of identified component.
3.2. Antiviral Activity of Essential Oils of Chrysanthemum Indicum and C. morifolium Flower Heads
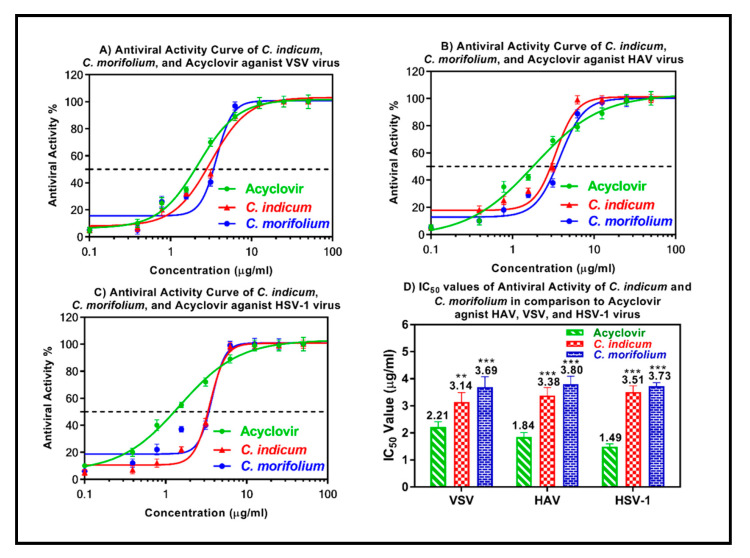
The antiviral activity of the essential oils obtained from the flower heads of C. indicum and C. morifolium was assessed using the plaque reduction assay on Vero cells (CCL-81). Nothing was previously reported in literature about the antiviral potential of the oils of interest. However, it was interesting to us that our previous work on aqueous extracts of both plants showed no activity against both hepatitis B virus (HBV), and bovine viral diarrhea virus (BVDV) [44]. Generally, most of the oils derived from the plant origin are evaluated against HSV-1 or rhinovirus. No reports could be traced regarding evaluation most of the oils against HAV or VSV. Both oils lack measurable cytotoxicity on Vero cells at the selected doses with IC50 values > 100 μg/mL being 125.81 and 145.35 μg/mL for C. indicum and C. morifolium oils, respectively as shown by the MTT assay. However, both oils in general and the C. indicum essential oil in particular, showed substantial antiviral potential in a dose dependent manner against VSV, HAV and HSV-1 as demonstrated in Figure 5. However, VSV was more sensitive to the antiviral activity of the C. indicum essential oil comparable to HAV and HSV-1 showing IC50 values of 3.14, 3.38 and 3.51 μg/mL towards the three previously mentioned viruses, respectively. It is worthy to mention that the activity of the Chrysanthemum oil is more potent than both clove and Eucalyptus oils which have a potent antiviral activity against HSV-1 [45]. Standard antiviral drug, acyclovir, displayed IC50 values of 2.21, 1.84 and 1.49 μg/mL towards VSV, HAV and HSV-1, respectively. The substantial antiviral activity of C. indicum essential oil could be interpreted in virtue of the presence of camphor, isoborneol and other major constituents. Camphor was previously reported to exert a remarkable virucidal potential versus herpes simplex virus-1 [46]. Additionally, isoborneol was previously reported to inhibit herpes simplex virus-1 replication via a specific inhibition in the glycosylation of its polypeptides exerting no cytotoxic effect on Vero cells (CCL-81) [47]. It is noteworthy to highlight that evaluation of cytotoxicity is crucially important in the assessment of the antiviral activity as beneficial oil should selectively inhibits viral replication, growth and other process with no or rare effects on different cellular metabolic process [48]. In general, lipophilic secondary metabolites interfere with the lipid membrane envelope of viruses. Therefore, they are not very specific and usually attack free viral particles and not intracellular viruses [49].
Figure 5.
Antiviral activity of the essential oils obtained from C. indicum and C. morifolium flower heads versus vesicular stomatitis virus (VSV) (A), hepatitis A (HAV) (B) and herpes simplex type-1 (HSV-1) (C) using acyclovir as a standard antiviral drug. The means with different stars are significantly different (p < 0.05).
3.3. Antimicrobial Activity of C. indicum and C. morifolium Essential Oils
The antibacterial activity of essential oils obtained from the flower heads of C. indicum and C. morifolium was evaluated in vitro using the agar diffusion method against different standard Gram-positive and Gram-negative bacteria via measuring the mean diameter of inhibition zones (DIZ) and determining the minimum inhibition concentrations (MIC) values as well. Both oils exerted a moderate antibacterial activity against the tested Gram-positive bacteria with C. indicum essential oil being more potent comparable to C. morifolium oil displaying MIC of 62.5 µg/mL against each of Bacillus subtilis, Streptococcus agalactiae and Streptococcus pyogenes. However, both oils exerted weak activity versus the examined Gram-negative bacteria and fungal strains with MICs > 500 µg/mL. The mean diameter of inhibition zones (DIZ) and the minimum inhibition concentrations (MIC) of both oils versus all the tested bacterial and fungal strains are illustrated in Table 2. The results reported herein were in accordance with previously reported data for the essential oils from many Chrysanthemum species that exhibited antibacterial and antifungal potential versus many fungal and bacterial strains [28,50]. C. indicum essential oil flower heads growing in Korea exhibited significant antimicrobial potential versus all tested oral bacteria (showing MIC values between 0.1 and 1.6 mg/mL and Minimum Bactericidal Concentrations (MBCs) between 0.2 and 3.2 mg/mL) [51]. The existence of considerable amounts of monoterpenes and oxygenated monoterpenes could greatly explain the high potency versus Gram-positive bacteria via diffusing and destabilizing the structure of cell membrane. Thus, terpenoid rich essential oils are more effective against Gram-positive bacteria than to Gram-negative ones. Besides, a synergism could exist between major and minor compounds might probably contribute to the moderate antibacterial effect [52,53,54].
Table 2.
Minimum inhibitory concentrations (MIC) in (µg/mL) and mean diameter of inhibition zones (DIZ) in (mm) of C. indicum and C. morifolium essential oils against different pathogens determined by microdilution and agar diffusion method.
| Microorganisms | C. indicum | C. morifolium | Positive Control * | |||
|---|---|---|---|---|---|---|
| DIZ (mm) |
MIC (µg/mL) |
DIZ (mm) |
MIC (µg/mL) |
DIZ (mm) |
MIC (µg/mL) |
|
| Gram Positive Bacteria | ||||||
| Bacillus subtilis ATCC 6051 | 20.0 ± 0.2 | 62.5 | 15.0 ± 0.7 | 125 | 31.6 ± 0.3 | 0.08 |
| Staphylococcus aureus ATCC 29213 | 20.4 ± 0.5 | 250 | 19.5 ± 0.3 | 250 | 30.1 ± 0.6 | 0.16 |
| Staphylococcus capitis ATCC 35661 | 19.9 ± 0.4 | 125 | 16.1 ± 0.5 | 250 | 29.2 ± 0.1 | 0.08 |
| Staphylococcus epidermidis ATCC 14990 | 19.6 ± 0.7 | 250 | 17.4 ± 0.8 | 250 | 26.4 ± 0.2 | 0.16 |
| Streptococcus agalactiae ATCC 27956 | 19.2 ± 0.8 | 62.5 | 14.4 ± 0.6 | 125 | 30.1 ± 0.1 | 0.08 |
| Streptococcus pyogenes ATCC 12344 | 21.9 ± 0.9 | 62.5 | 17.5 ± 0.3 | 125 | 29.7 ± 0.0 | 0.08 |
| Gram Negative Bacteria | ||||||
| Escherichia coli ATCC 25922 | 13.8 ± 0.2 | >500 | 13.7 ± 0.8 | 500 | 32.1 ± 0.0 | 0.06 |
| Pseudomonas fluorescens ATCC 13525 | 12.3 ± 0.1 | >500 | 11.7 ± 0.1 | >500 | 27.3 ± 0.1 | 0.24 |
| Salmonella typhimurium ATCC 14028 | 12.1 ± 0.5 | >500 | 11.5 ± 0.1 | >500 | 24.6 ± 0.2 | 0.96 |
| Shigella flexneri ATCC 700930 | 11.2 ± 0.9 | >500 | 10.9 ± 0.5 | >500 | 21.2 ± 0.0 | 0.96 |
| Fungi | ||||||
| Aspergillus fumigatus ATCC 1022 | 15.8 ± 0.5 | >500 | 14.5 ± 0.5 | 500 | 26.3 ± 0.1 | 0.12 |
| Candida albicans ATCC 90028 | 13.6 ± 0.7 | >500 | 12.6 ± 0.2 | >500 | 24.8 ± 0.7 | 0.24 |
| Geotrichum candidum ATCC 12784 | 14.0 ± 0.5 | 500 | 13.3 ± 0.4 | >500 | 23.2 ± 0.3 | 0.48 |
| Syncephalastrum racemosum ATCC 14831 | 11.9 ± 0.6 | >500 | 11.2 ± 0.7 | >500 | 21.4 ± 0.5 | 0.48 |
Data are presented as means ± S.D. n = 3; * The positive control was taken as ampicillin for Gram-positive bacteria, gentamycin for Gram-negative bacteria and clotrimazole for fungi; all assays consisted of 30 mg essential oil in 1 mL DMSO, and 100 μL were applied. DIZ, diameter of inhibition zone is measured in (mm) by the agar diffusion method.
3.4. Anti-Mycobacterial and Anti-Helicobacter Pylori Activity of the Essential Oils
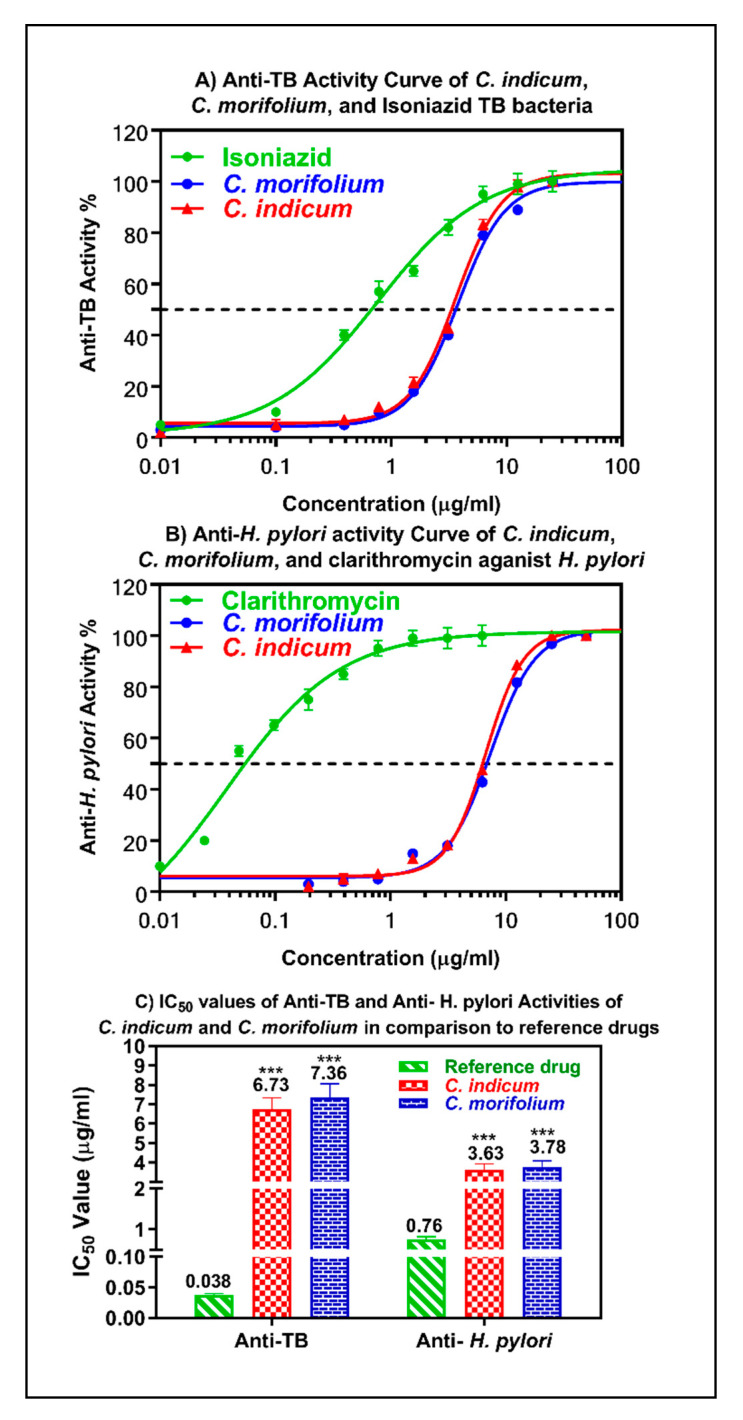
Additionally, both oils showed a substantial anti-mycobacterial activity against M. tuberculosis with IC50 values of 6.73 and 7.36 µg/mL for C. indicum and C. morifolium, respectively, albeit lower than the positive control isoniazid (IC50 = 0.038 µg/mL). Nothing was previously reported in literature about the anti-mycobacterial and anti-Helicobacter pylori of the oils. Furthermore, both oils exhibited a good activity against Helicobacter pylori with IC50 values of 3.63 and 3.78 µg/mL for C. indicum and C. morifolium, respectively approaching that of clarithromycin (Figure 6). These results represent very good candidates for further studies taking into consideration that very limited work was done on the anti-Helicobacter pylori (only MICs were calculated and no IC50 could be found) [55,56]. The observed anti-mycobacterial and anti-Helicobacter pylori potential of the essential oils is mainly attributed to the presence of terpenes and fatty acids that account for the lipophilicity and greater penetration through the cell membrane triggering a massive disturbance in the oxidative phosphorylation and the electron transport chain as well resulting in a serious interference in the production of energy with concomitant evolution of auto-oxidation and peroxidation degradation compounds and bacterial lysis [35,57].
Figure 6.
Anti-mycobacterial and anti-Helicobacter pylori activity of the essential oils obtained from C. indicum and C. morifolium flower heads versus reference drugs. The means with different stars are significantly different (p < 0.05).
3.5. Anti-Trypanosomal Activity of Essential Oils
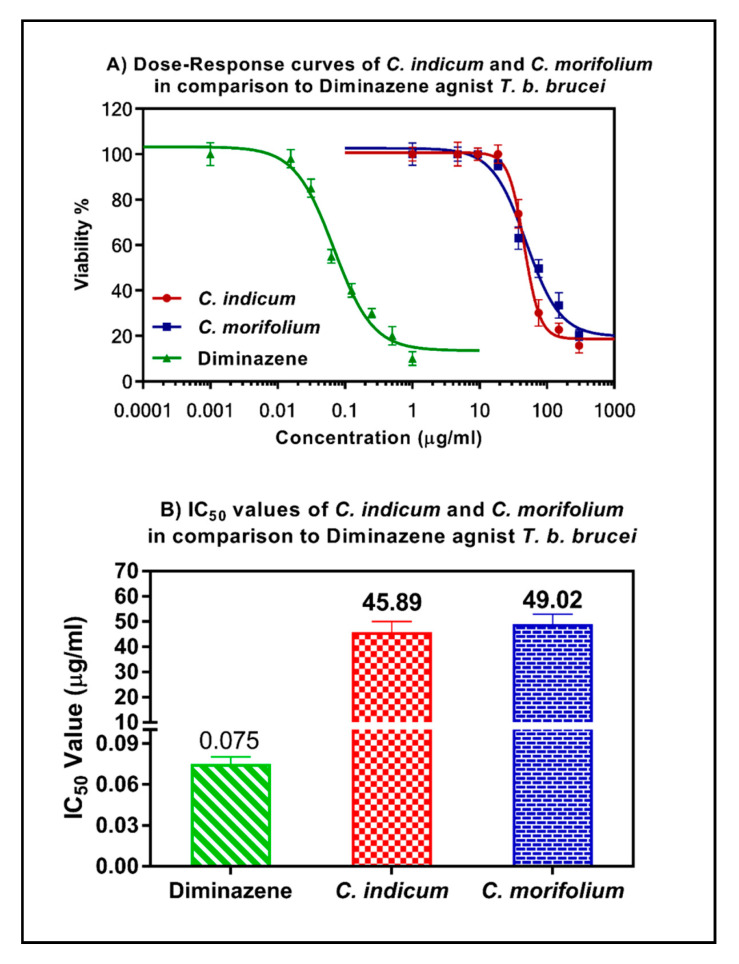
Both essential oils showed a moderate anti-trypanosomal activity with IC50 values of 49.02 and 45.89 μg/mL, albeit lower than diminazene (positive control; IC50 = 0.075 μg/mL) (Figure 7). The selectivity indexes of the two oils were calculated as 1.8 and 1.5, respectively, indicating low selectivity against trypanosomes compared to their cytotoxicity versus HL-60 cells (88.24 and 68.84 μg/mL, respectively). It is worthy to highlight that nothing was traced in literature regarding the assessment of the anti-trypanosomal potency of both essential oils. However, it was noticed that both methanol and chloroform extracts of the same plants showed higher potency against T. brucei. C. indicum showed IC50s of 16 and 15.3 μg/mL for chloroform and methanol extracts, respectively [44]. In addition, this activity is better than our previously tested Citrus oils which exhibited lower activity with IC50 value = 60–72 μg/mL [33,58]. The activity of both oils is caused by their lipophilic terpenes which effectively interact with membrane lipids and proteins causing membrane disruption and ultimate cell death [59].
Figure 7.
Anti-trypanosomal activity of the essential oils obtained from C. indicum and C. morifolium flower heads versus reference drugs.
3.6. Antioxidant Activity of Essential Oils
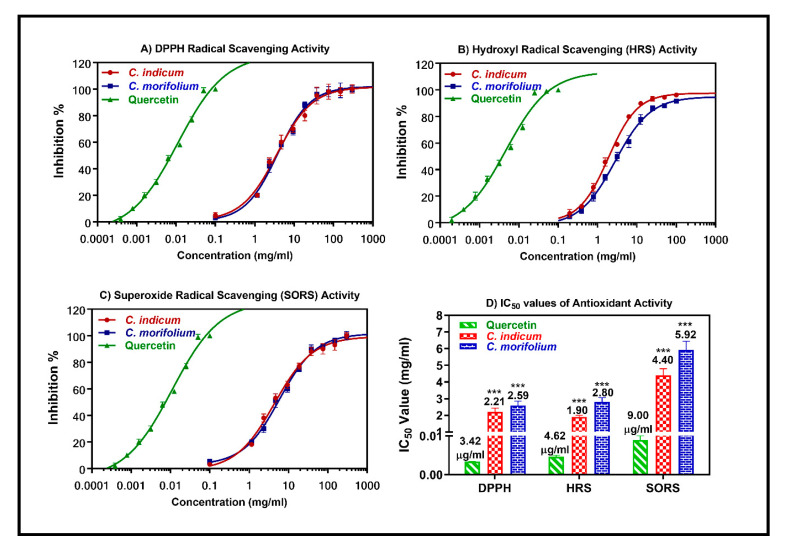
The antioxidant activity was assessed using DPPH, HRS, and SORS assays. The essential oils obtained from the flower heads of C. indicum and C. morifolium revealed substantial antioxidant potential with IC50 values of 2.21 and 2.59 mg/mL, in the DPPH assay, respectively, 1.90 and 2.89 mg/mL in HRS assay whereas they showed IC50 values of 4.40 and 5.92 mg/mL in SORS assay, respectively (Figure 8). The antioxidant activity can be interpreted by the synergistic interactions between all of the essential oil components in addition to their action as pro-oxidants. Inhibition of free radical chain reaction, destruction of peroxides, prompt clearance of free radical and hydrogen are familiar among the mode of action of many essential oils and thus could be the probable mode of action by which the tested oils exerted their effect.
Figure 8.
Antioxidant activities of the essential oils obtained from C. indicum and C. morifolium flower heads versus quercetin as a reference drug in DPPH (A), hydroxyl radical scavenging (HRS) (B), and superoxide radical scavenging (SORS) (C) assays. The means with different stars are significantly different (p < 0.05).
4. Conclusions
Both essential oils exhibited antimicrobial, antiviral, anti-mycobacterial, anti-Helicobacter pylori, anti-trypanosomal and antioxidant activities with C. indicum being more effective. This study provides for the first time scientific consolidation beyond the traditional use of both Chrysanthemum indicum and C. morifolium as anti-infective agents. Thus, they could be used as spices in food and can be incorporated in different food products and pharmaceutical preparations as natural preservatives possessing antioxidant potential. However, further studies should be conducted to test their efficacy against other life-threatening microorganisms comprising the novel coronavirus (COVID-19) that threatens the worldwide. Moreover, in vivo studies are greatly recommended to further confirm the obtained results that should be proceeded by toxicity tests to guarantee the safety of the essential oils.
Author Contributions
F.S.Y.; Identification of the essential oil compounds, writing the manuscript; S.Y.E. and M.Z.E.-R.; performing the biological activities and writing this section; E.A.; performing the antioxidant activity and revising the manuscript; M.L.A.; collection of the plants, isolation of the essential oil samples and writing the manuscript; M.W.; supervising the study and revising the whole manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-Track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El Mokni R., Youssef F.S., Jmii H., Khmiri A., Bouazzi S., Jlassi I., Jaidane H., Dhaouadi H., Ashour M.L., Hammami S. The essential oil of Tunisian Dysphania ambrosioides and its antimicrobial and antiviral properties. J. Essent. Oil Bear. Plants. 2019;22:282–294. doi: 10.1080/0972060X.2019.1588171. [DOI] [Google Scholar]
- 2.Mamadalieva N.Z., Youssef F.S., Ashour M.L., Sasmakov S.A., Tiezzi A., Azimova S.S. Chemical composition, antimicrobial and antioxidant activities of the essential oils of three Uzbek Lamiaceae species. Nat. Prod. Res. 2018;33:2394–2397. doi: 10.1080/14786419.2018.1443088. [DOI] [PubMed] [Google Scholar]
- 3.Mamadalieva N.Z., Youssef F.S., Ashour M.L., Akramov D.K., Sasmakov S.A., Ramazonov N.S., Azimova S.S. A comparative study on chemical composition and antimicrobial activity of essential oils from three Phlomis species from Uzbekistan. Nat. Prod. Res. 2019:1–6. doi: 10.1080/14786419.2019.1591400. [DOI] [PubMed] [Google Scholar]
- 4.Shahrajabian M.H. A review of Chrysanthemum, the eastern queen in traditional Chinese medicine with healing power in modern pharmaceutical sciences. Appl. Ecol. Environ. Res. 2019;17:13355–13369. doi: 10.15666/aeer/1706_1335513369. [DOI] [Google Scholar]
- 5.Wiart C. Ethnopharmacology of Medicinal Plants: Asia and the Pacific. Volume 12. Humana Press; Totowa, NJ, USA: 2006. p. 228. [Google Scholar]
- 6.Khare C.P. Indian Medicinal Plants: An Illustrated Dictionary. Volume 10. Springer Reference; Springer; Berlin, Germany: 2007. p. 32. [Google Scholar]
- 7.Van Wyk B.-E., Wink M. Medicinal Plants of the World: An Illustrated Scientific Guide to Important Medicinal Plants and Their Uses. 1st ed. Timber Press; Portland, OR, USA: 2004. p. 480. [Google Scholar]
- 8.Mabberley D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses. 3rd ed. Cambridge University Press; New York, NY, USA: 2008. p. 1021. [Google Scholar]
- 9.Li T.S.C. Chinese and Related North. American Herbs: Phytopharmacology and Therapeutic Values. CRC Press; Boca Raton, FL, USA: 2002. p. 598. [Google Scholar]
- 10.WHO . Medicinal Plants in China: A Selection of 150 Commonly Used Species. Volume 7. World Health Organization, Regional Office for the Western Pacific; Manila, Phillippines: 1997. p. 331. (WHO Regional Publications. Western Pacific Series). [Google Scholar]
- 11.Schmid R., Kubitzki K., Kadereit J.W. The families and genera of vascular plants. TAXON. 2005;54:574. doi: 10.2307/25065407. [DOI] [Google Scholar]
- 12.Yoshikawa M., Morikawa T., Toguchida I., Harima S., Matsuda H. Medicinal flowers. Inhibitors of nitric oxide production and absolute stereostructures of five new germacrane-type sesquiterpenes, kikkanols D, D monoacetate, E, F, and F monoacetate from the flowers of Chrysanthemum indicum L. Chem. Pharm. Bull. 2000;48:651–656. doi: 10.1248/cpb.48.651. [DOI] [PubMed] [Google Scholar]
- 13.Ukiya M., Akihisa T., Yasukawa K., Kasahara Y., Kimura Y., Koike K., Nikaido T., Takido M. Constituents of compositae plants. 2. Triterpene diols, triols, and their 3-o-fatty acid esters from edible chrysanthemum flower extract and their anti-inflammatory effects. J. Agric. Food Chem. 2001;49:3187–3197. doi: 10.1021/jf010164e. [DOI] [PubMed] [Google Scholar]
- 14.Mladenova K., Tsankova E., Van Hung D. New Sesquiterpenoids from Chrysanthemum indicum var. tuneful. Planta Med. 1988;54:553–555. doi: 10.1055/s-2006-962548. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda H., Morikawa T., Toguchida I., Harima S., Yoshikawa M. Medicinal flowers. Absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L.: Their inhibitory activities for rat lens aldose reductase. Chem. Pharm. Bull. 2002;50:972–975. doi: 10.1248/cpb.50.972. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.S., Kim H.J. A new anti-HIV flavonoid glucuronide from Chrysanthemum morifolium. Planta Med. 2003;69:859–861. doi: 10.1055/s-2003-43207. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee A., Sarkar S., Saha S.K. Acacetin 7-O-[beta]-galactopyranoside from Chrysanthemum indicum. Phytochemistry. 1981;20:1760–1761. doi: 10.1016/S0031-9422(00)98580-7. [DOI] [Google Scholar]
- 18.Yoshikawa M., Morikawa T., Murakami T., Toguchida I., Harima S., Matsuda H. Medicinal flowers. I. Aldose reductase inhibitors and three new eudesmane-type sesquiterpenes, Kikkanols A, B, and C, from the flowers of Chrysanthemum indicum L. Chem. Pharm. Bull. 1999;47:340–345. doi: 10.1248/cpb.47.340. [DOI] [PubMed] [Google Scholar]
- 19.Ukiya M., Akihisa T., Tokuda H., Suzuki H., Mukainaka T., Ichiishi E., Yasukawa K., Kasahara Y., Nishino H. Constituents of Compositae plants. Anti-tumor promoting effects and cytotoxic activity against human cancer cell lines of triterpene diols and triols from edible Chrysanthemum flowers. Cancer Lett. 2002;177:7–12. doi: 10.1016/S0304-3835(01)00769-8. [DOI] [PubMed] [Google Scholar]
- 20.Miyazawa M., Hisama M. Antimutagenic activity of flavonoids from Chrysanthemum morifolium. Biosci. Biotechnol. Biochem. 2003;67:2091–2099. doi: 10.1271/bbb.67.2091. [DOI] [PubMed] [Google Scholar]
- 21.Lee D.Y., Choi G., Yoon T., Cheon M.S., Kil Choo B., Kim H.K. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2009;123:149–154. doi: 10.1016/j.jep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Kim H.J., Lee Y.S. Identification of new dicaffeoylquinic acids from Chrysanthemum morifolium and their antioxidant activities. Planta Med. 2005;71:871–876. doi: 10.1055/s-2005-873115. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H., Xia Q., Xu W., Zheng M. Chrysanthemum morifolium attenuated the reduction of contraction of isolated rat heart and cardiomyocytes induced by ischemia/reperfusion. Die Pharm. 2004;59:565–567. [PubMed] [Google Scholar]
- 24.Hu C.Q., Chen K., Shi Q., Kilkuskie R.E., Cheng Y.-C., Lee K.H. Anti-AIDS agents, 10. Acacetin-7-O-beta-D-galactopyranoside, an anti-HIV principle from Chrysanthemum morifolium and a structure-activity correlation with some related flavonoids. J. Nat. Prod. 1994;57:42–51. doi: 10.1021/np50103a006. [DOI] [PubMed] [Google Scholar]
- 25.Cheon M.S., Yoon T., Lee do Y., Choi G., Moon B.C., Lee A.Y., Choo B.K., Kim H.K. Chrysanthemum indicum Linne extract inhibits the inflammatory response by suppressing NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2009;122:473–477. doi: 10.1016/j.jep.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Cheng W., Li J., You T., Hu C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linné. J. Ethnopharmacol. 2005;101:334–337. doi: 10.1016/j.jep.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Yang X.-W. GC-MS analysis of essential oil of the flower of the Chrysanthemum morifolium by the different processing methods. China J. Chin. Mater. Med. 2006;31:456–459. [PubMed] [Google Scholar]
- 28.Shunying Z., Yang Y., Huaidong Y., Yue Y., Guolin Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J. Ethnopharmacol. 2005;96:151–158. doi: 10.1016/j.jep.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Youssef F.S., Hamoud R., Ashour M.L., Singab A.N.B., Wink M. Volatile oils from the aerial parts of Eremophila maculata and their antimicrobial activity. Chem. Biodivers. 2014;11:831–841. doi: 10.1002/cbdv.201300366. [DOI] [PubMed] [Google Scholar]
- 30.Ayoub I.M., Youssef F.S., El-Shazly M., Ashour M.L., Singab A.N.B., Wink M. Volatile constituents of Dietes bicolor (Iridaceae) and their antimicrobial activity. Z. Naturforsch. C. 2015;70:217–225. doi: 10.1515/znc-2015-0164. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.Abou-Karam M., Shier W.T. A simplified plaque reduction assay for antiviral agents from plants. Demonstration of frequent occurrence of antiviral activity in higher plants. J. Nat. Prod. 1990;53:340–344. doi: 10.1021/np50068a011. [DOI] [PubMed] [Google Scholar]
- 33.Ashour M.L., El-Readi M., Youns M., Mulyaningsih S., Sporer F., Efferth T., Wink M. Chemical composition and biological activity of the essential oil obtained from Bupleurum marginatum (Apiaceae) J. Pharm. Pharmacol. 2009;61:1079–1087. doi: 10.1211/jpp.61.08.0012. [DOI] [PubMed] [Google Scholar]
- 34.Mulyaningsih S., Sporer F., Reichling J., Wink M. Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm. Biol. 2011;49:893–899. doi: 10.3109/13880209.2011.553625. [DOI] [PubMed] [Google Scholar]
- 35.El-Din M.I.G., Youssef F.S., Ashour M.L., Eldahshan O.A., Singab A.N.B. Comparative analysis of volatile constituents of Pachira aquatica Aubl. and Pachira glabra Pasq., their anti-mycobacterial and anti-helicobacter pylori activities and their metabolic discrimination using chemometrics. J. Essent. Oil Bear. Plants. 2018;21:1550–1567. doi: 10.1080/0972060X.2019.1571950. [DOI] [Google Scholar]
- 36.Nibret E., Ashour M.L., Rubanza C.D., Wink M. Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phytother. Res. 2009;24:945–947. doi: 10.1002/ptr.3066. [DOI] [PubMed] [Google Scholar]
- 37.Huber W., Koella J.C. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993;55:257–261. doi: 10.1016/0001-706X(93)90083-N. [DOI] [PubMed] [Google Scholar]
- 38.Nenadis N., Lazaridou A.O., Tsimidou M.Z. Use of reference compounds in antioxidant activity assessment. J. Agric. Food Chem. 2007;55:5452–5460. doi: 10.1021/jf070473q. [DOI] [PubMed] [Google Scholar]
- 39.Youssef F.S., Ashour M.L., Sobeh M., El-Beshbishy H.A., Singab A.N.B., Wink M. Eremophila maculate-Isolation of a rare naturally-occurring lignan glycoside and the hepatoprotective activity of the leaf extract. Phytomedicine. 2016;23:1484–1493. doi: 10.1016/j.phymed.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Nishikimi M., Rao N.A., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 41.Aruoma O.I. Deoxyribose Assay for Detecting Hydroxyl Radicals. Meth. Enzymol. 1994;233:57–66. [Google Scholar]
- 42.Lawal O.A., Ogunwande I.A., Olorunloba O.F., Opoku A.R. The essential oils of Chrysanthemum morifolium Ramat. from Nigeria. Am. J. Essent. Oil Nat. Prod. 2014;2:63–66. [Google Scholar]
- 43.Choi H.-S., Kim G.-H. Volatile flavor composition of gamguk (Chrysanthemum indicum) flower essential oils. Food Sci. Biotechnol. 2011;20:319–325. doi: 10.1007/s10068-011-0045-2. [DOI] [Google Scholar]
- 44.Herrmann F., Romero M.R., Blazquez A.G., Kaufmann D., Ashour M.L., Kahl S., Marin J.J., Efferth T., Wink M. Diversity of pharmacological properties in Chinese and European medicinal plants: Cytotoxicity, antiviral and antitrypanosomal screening of 82 herbal drugs. Diversity. 2011;3:547–580. doi: 10.3390/d3040547. [DOI] [Google Scholar]
- 45.Schnitzler P., Schön K., Reichling J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Die Pharm. 2001;56:343–347. [PubMed] [Google Scholar]
- 46.Chen W., Vermaak I., Viljoen A.M. Camphor-A fumigant during the Black Death and a coveted fragrant wood in Ancient Egypt and Babylon-A review. Molecules. 2013;18:5434–5454. doi: 10.3390/molecules18055434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armaka M., Papanikolaou E., Sivropoulou A., Arsenakis M., Armaka M. Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1. Antivir. Res. 1999;43:79–92. doi: 10.1016/S0166-3542(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 48.Ben Sassi A., Harzallah-Skhiri F., Chraief I., Bourgougnon N., Hammami M., Aouni M. Chemical composition and antimicrobial activities of the essential oil of (Tunisian) Chrysanthemum trifurcatum (Desf.) Batt. and Trab. flowerheads. Comptes Rendus Chim. 2008;11:324–330. doi: 10.1016/j.crci.2007.09.006. [DOI] [Google Scholar]
- 49.Wink M. Diversity of plant secondary metabolites with antiviral activities. Potential of DNA intercalating alkaloids against infections with the Corona virus SARS-CoV-2 causing COVID-19. Diversity. 2020;12:175. doi: 10.3390/d12050175. [DOI] [Google Scholar]
- 50.Kim Y.-H., Yu H.-H., Cha J.-D., You Y.-O., Kim K.-J., Jeong S.-I., Kil B.-S. Antibacterial activity and chemical composition of essential oil of Chrysanthemum boreale. Planta Med. 2003;69:274–277. doi: 10.1055/s-2003-38479. [DOI] [PubMed] [Google Scholar]
- 51.Rippey E., Rowland B. Coastal Plants: Perth and the South-West. Region. UWA Publishing; Perth, Australia: 2004. [Google Scholar]
- 52.Thabet A.A., Youssef F.S., El-Shazly M., Singab A.N.B. GC-MS and GC-FID analyses of the volatile constituents of Brachychiton rupestris and Brachychiton discolor, their biological activities and their differentiation using multivariate data analysis. Nat. Prod. Res. 2018;34:590–594. doi: 10.1080/14786419.2018.1490908. [DOI] [PubMed] [Google Scholar]
- 53.Middleton J.E., Drzewiecki G. Naturally occurring flavonoids and human basophil histamine release. Int. Arch. Allergy Immunol. 1985;77:155–157. doi: 10.1159/000233771. [DOI] [PubMed] [Google Scholar]
- 54.Eftekhar F., Nariman F., Yousefzadi M., Hadian J., Ebrahimi S.N. Anti-Helicobacter pylori activity and essential oil composition of Thymus caramanicus from Iran. Nat. Prod. Commun. 2009;4:1139–1142. doi: 10.1177/1934578X0900400825. [DOI] [PubMed] [Google Scholar]
- 55.Menghini L., Leporini L., Tirillini B., Epifano F., Genovese S. Chemical composition and inhibitory activity against Helicobacter pylori of the essential oil of Apium nodiflorum (Apiaceae) J. Med. Food. 2010;13:228–230. doi: 10.1089/jmf.2009.0010. [DOI] [PubMed] [Google Scholar]
- 56.Van Wyk B.-E., Wink M. Medicinal Plants of the World. CABI; London, UK: 2017. [Google Scholar]
- 57.Hamdan D., El-Readi M.Z., Nibret E., Sporer F., Farrag N., El-Shazly A., Wink M. Chemical composition of the essential oils of two Citrus species and their biological activities. Die Pharm. 2010;65:141–147. [PubMed] [Google Scholar]
- 58.Hamdan D., Ashour M.L., Mulyaningsih S., El-Shazly A., Wink M. Chemical composition of the essential oils of variegated pink-fleshed lemon (Citrus x limon L. Burm. f.) and their anti-inflammatory and antimicrobial activities. Z. Naturforsch. C. 2013;68:275–284. doi: 10.1515/znc-2013-7-804. [DOI] [PubMed] [Google Scholar]
- 59.Zengin H., Baysal A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19:17773–17798. doi: 10.3390/molecules191117773. [DOI] [PMC free article] [PubMed] [Google Scholar]