Abstract
Microglia are the cells that comprise the innate immune system in the brain. First described more than a century ago, these cells were initially assigned a secondary role in the central nervous system (CNS) with respect to the protagonists, neurons. However, the latest advances have revealed the complexity and importance of microglia in neurodegenerative conditions such as Alzheimer’s disease (AD), the most common form of dementia associated with aging. This pathology is characterized by the accumulation of amyloid-β peptide (Aβ), which forms senile plaques in the neocortex, as well as by the aggregation of hyperphosphorylated tau protein, a process that leads to the development of neurofibrillary tangles (NFTs). Over the past few years, efforts have been focused on studying the interaction between Aβ and microglia, together with the ability of the latter to decrease the levels of this peptide. Given that most clinical trials following this strategy have failed, current endeavors focus on deciphering the molecular mechanisms that trigger the tau-induced inflammatory response of microglia. In this review, we summarize the most recent studies on the physiological and pathological functions of tau protein and microglia. In addition, we analyze the impact of microglial AD-risk genes (APOE, TREM2, and CD33) in tau pathology, and we discuss the role of extracellular soluble tau in neuroinflammation.
Keywords: Alzheimer’s disease, tauopathies, tau, Aβ, microglia, neuroinflammation, ApoE, TREM2, CD33, CX3CR1
1. Introduction
Alzheimer’s disease (AD) is recognized as the most common form of dementia associated with aging [1]. It is the main cause of dependence in older adults and the fifth cause of death worldwide, and it has a great impact on those who suffer from this condition, as well as on their families. The number of individuals with some form of dementia has doubled from 1990 (20.2 million) to the present (47 million), mainly due to population growth and aging. In 2015, dementia accrued associated costs amounting to €758 billion, equivalent to 1.1% of the global gross domestic product. It is estimated that the number of patients with dementia will reach 75 million in 2030 and the costs of this condition €1.86 trillion, a burden that may cause health care systems to collapse [2,3].
Described by Alois Alzheimer in 1906 [4,5], AD is characterized by the presence of senile plaques (comprised of amyloid-β peptide (Aβ) [6,7,8]) and neurofibrillary tangles (NFTs) (formed by the aggregation of hyperphosphorylated tau [9,10,11]) in the brain, causing marked glial activation and loss of neurons and synapses [12]. Based on a body of evidence, in 1992, John Hardy and Gerald Higgins proposed the amyloid hypothesis, stating that Aβ accumulation in the brain gives rise to AD pathogenesis, followed by the appearance of NFTs, neuronal death, and cognitive decline [13]. The rationale for this hypothesis was based on the identification of genetic modifications that altered Aβ production or the capacity of this peptide to aggregate [14].
Although genetic evidence supports the amyloid hypothesis, Aβ accumulation is believed to be a necessary, but not sufficient, condition for AD progression. In this regard, several studies have established a greater relationship between NFTs and AD [15,16,17,18]. Furthermore, other authors demonstrate that Aβ toxicity requires the presence of tau [19,20]. Contrary to what occurs in tau pathology, an extensive accumulation of Aβ can be observed in patients who do not present cognitive decline [21,22]. Likewise, individuals with tau and Aβ show higher levels of specific biomarkers compared to those with only Aβ [23,24]. All these studies suggest that although the presence of Aβ is important in AD pathogenesis, other factors such as tau accumulation or neuroinflammation may be the main causes of neurodegeneration.
2. Tau Protein
2.1. Expression, Isoforms, and Localization
Tau protein regulates the assembly and stabilization of microtubules [25]. It is expressed mainly in neurons of the central nervous system (CNS), although it is also detected to a lesser extent in oligodendrocytes [26,27,28]. In humans, tau protein is encoded by MAPT on chromosome 17q21 [29]. The adult human brain has six isoforms of this protein generated by alternative splicing, and these differ in the number of N-terminal domains and repeated binding domains [30]. Specific mutations in MAPT [31], the inhibition of the mitochondrial complex I [32], and the presence of microRNAs [33] and RNA binding proteins [34] are some of the factors that determine splicing.
The subcellular localization of tau varies throughout development. In immature neurons, this protein is distributed in the soma and neurites. Later, when axons are generated and neurons acquire polarity, tau is found mostly in the axonal region [35]. Furthermore, it is also found in dendritic spines [36] and the nucleus [37,38], and it is associated with the plasma membrane through binding to phospholipids [39,40]. Likewise, this distribution is determined by the number of tau N-terminal domains. The 0N and 2N isoforms are found primarily in the soma and axon, while the 1N isoform is generally located in the nucleus [41].
2.2. Structure and Post-Translational Modifications of Tau
The structure of tau is divided into the projection domain, which holds the N-terminal domains and is thought to regulate the space and crosslinking between microtubules [42,43], and the microtubule binding domain [44]. There is a proline-rich region between the two domains with which signaling proteins such as Fyn can interact [45].
Tau protein is subject to numerous post-translational modifications: phosphorylation, glycosylation, glycation, isomerization, nitration, ubiquitination, SUMOylation, acetylation, and methylation [46]. Of these modifications, tau phosphorylation is the most recurrent. This process is regulated throughout life, being more predominant during embryonic development than in adulthood [47,48]. However, in some pathologies such as AD, tau is hyperphosphorylated [49]. This fact plays a very important role in regulating the physiological function of tau, since it reduces the binding affinity to microtubules and promotes its aggregation, thus compromising the integrity of neurons [49,50]. Furthermore, hyperphosphorylated tau tends to accumulate in the somatodendritic compartment, where it causes AMPA receptor loss, thereby promoting synaptic dysfunction [51]. It has also been described that the hyperphosphorylation of tau can alter its degradation [52], as well as its interaction with other proteins [53,54,55].
A large number of kinases have the capacity to phosphorylate tau [56]. Glycogen-synthase kinase 3β (GSK3β), a protein involved in the regulation of glucose metabolism, has been recognized as the main tau kinase [57]. Others such as p38 (introduced in the later sections) also phosphorylate tau but at lower efficiency [58]. Furthermore, tau can be dephosphorylated by phosphatases such as PP1, PP5, PP2B, and PP2A [59]. Thus, the regulation of the activity of some of these proteins has been proposed as a possible therapeutic target to combat AD [60].
2.3. Tau Aggregation
Tau aggregation is characteristic of several neurodegenerative diseases called tauopathies, including AD [61]. These diseases can be classified into three groups depending on the isoforms detected in the aggregates: 4R (progressive supranuclear palsy, corticobasal degeneration, and argyrophilic grain disease), 3R (Pick’s disease), and 3R + 4R (AD and frontotemporal dementia with parkinsonism-17) [62]. Numerous factors promote tau aggregation, but the main trigger of this phenomenon is still unclear. The observation that tau aggregates are hyperphosphorylated [49] indicates that this post-translational modification plays an important role in this process [63,64]. In this regard, it has recently been shown that specific tau phosphorylation at Ser208 promotes the aggregation of the protein, whereas phosphorylation at Ser202 and Thr205 leads to its mislocalization to the soma and dendrites [65]. However, phosphorylation at specific sites can suppress aggregate formation [66]. Moreover, tau truncation removes some residues, thereby promoting a greater number of tau–tau than tau–tubulin interactions [67]. Additionally, the concentration of tau may be a key factor in the initiation of this process, since high concentrations of this protein favor the formation of paired helical filaments (PHFs) in vitro [68]. Some mutations that reduce the binding affinity of tau to microtubules have also been shown to accelerate aggregate formation [69]. Furthermore, several anionic molecules (glycosaminoglycans and RNA, among others) act as cofactors [70,71].
The different forms of tau presented below have been classified on the basis of their aggregation state [72]. Tau monomers are the most abundant forms in the brain [73]. They are characterized by high solubility and they normally acquire a random structure [74]. Monomers can interact with each other to form dimers, trimers, oligomers (between six and eight molecules), insoluble oligomers (approximately 40 molecules), and insoluble structures [75,76,77] with different folds [78,79,80,81,82].
2.4. Main Functions of Tau
In 1975, Weingarten et al. proposed that tau regulates microtubule assembly and stabilization [25]. This function has recently been further explored by Qiang et al., who suggest that tau is not strictly a microtubule-stabilizing protein, but that it rather allows microtubules to have long labile domains [83]. Additionally, tau plays a key role in axonal transport, since it competes with kinesin and dynein motor proteins in their binding to microtubules [84]. Tau is also very important in axonal maturation and elongation, since its silencing inhibits neurite formation [85]. Regarding the regulation of synaptic plasticity, some studies suggest that tau is involved in long-term depression (LTD) and potentiation (LTP) [86,87]. This protein is also involved in adult hippocampal neurogenesis against positive stimuli or acute stress [88], as well as in the integrity of DNA and RNA [89,90].
The Mapt−/− mouse has been widely used to study the aforementioned functions of tau. This model does not present a severe phenotype throughout its development thanks to compensation by other microtubule-stabilizing proteins [91,92]. During aging, these animals show brain atrophy, cognitive deficits, and motor disturbances [93,94]. These findings indicate that, although tau has a deleterious role in neuropathological processes, its physiological functions are especially important at advanced ages.
2.5. Mechanisms of Tau Propagation
Several in vitro [95] and in vivo studies [96,97,98,99] have revealed that tau is transmitted from one cell to another following a stereotypical pattern, thus supporting the tau prion propagation hypothesis in AD and other tauopathies [100]. Although these observations provide solid evidence of this phenomenon, the mechanisms of tau release, internalization, and processing are still being studied.
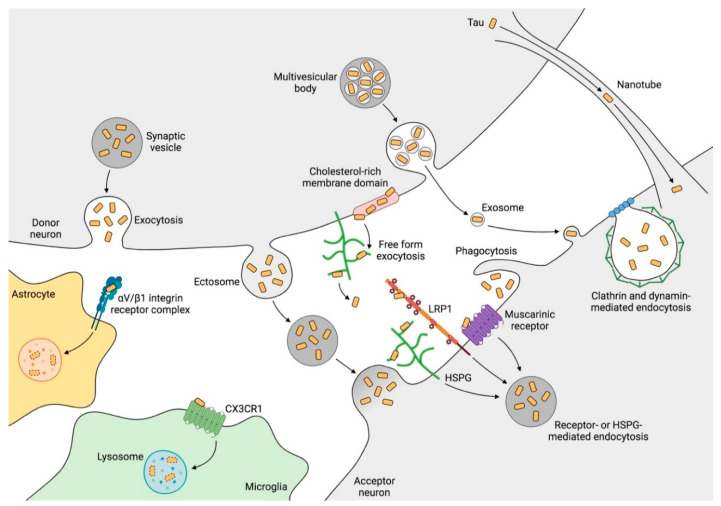
Extracellular tau was once thought to originate from axonal degeneration and neuronal death [101,102]. However, neuronal activity regulates tau release physiologically without cell death [103]. It has been suggested that the release mechanism occurs mainly in synapses [104] through synaptic vesicles [103], exosomes [105,106], ectosomes [107], or in a free form [108,109,110]. Once released, tau can be internalized by other neurons [111] through interaction with muscarinic receptors, low-density lipoprotein receptor-related protein 1 (LRP1) or heparan sulfate proteoglycans (HSPGs) [112,113,114,115], clathrin- or dynamin-mediated endocytosis [111,116], macropinocytosis [115], or direct fusion of the vesicles with the membrane. Additionally, tunneling nanotubes allow tau to be transported from one cell to another [117] (Figure 1). Propagation commonly involves the monomer and oligomer forms of tau, which are susceptible to aberrant folding and post-translational modifications such as truncation and phosphorylation [118].
Figure 1.
Tau transmission mechanisms. The processes depicted above occur commonly at synapses, where tau can be released to the extracellular space through synaptic vesicles or by direct translocation. Tau is then internalized through receptor- or heparan sulfate proteoglycan (HSPG)-mediated endocytosis, clathrin- and dynamin-mediated endocytosis, and phagocytosis. In addition, tau can be spread through extracellular vesicles (ectosomes and exosomes) that fuse to the membrane of the recipient cell. Moreover, nanotubes establish intercellular communication that serves as a bridge for tau propagation. Microglia and astrocytes also have the ability to internalize and degrade the tau present in the extracellular medium, although the mechanisms involved, especially in astrocytes, remain poorly understood. CX3CR1: fractalkine receptor, HSPG: heparan sulfate proteoglycan, LRP1: low-density lipoprotein receptor-related protein 1. Created with BioRender.com.
Once internalized, tau interacts with other tau molecules of the receptor neuron and induces their aberrant folding, thus propagating the pathology [116,119]. Propagation does not occur only in neurons as glia are also capable of engulfing extracellular tau [120,121,122,123] and therefore of contributing to tau propagation [124,125,126,127], although these cells have the capacity to degrade it [120,125,128] (Figure 1).
3. Microglia
3.1. History and Origin of Microglia
More than a century ago, Iliá Méchnikov discovered that the introduction of rose thorns into starfish larvae caused the accumulation of mobile cells around these foreign bodies. This observation led him to conclude that these cells behaved as defense agents. On the basis of this discovery, he expounded the phagocytosis theory, and he tirelessly explored the role of this phenomenon in various diseases [129]. Two decades later, in the brains of rabbits infected with rabies virus, Nicolás Achúcarro visualized cells that had the ability to phagocytize damaged neurons [130]. After the sudden death of Achúcarro, Pío del Río Hortega continued his research and improved the existing staining techniques. These advances allowed him to distinguish that a subpopulation of apolar cells, previously identified by Cajal [131], were actually microglia, making him the first scientist to describe this cell type [132,133,134,135,136,137].
Microglia are the resident macrophages of the CNS and the only cell type, along with endothelial cells, that do not have a neuroectodermal origin. The cells that give rise to microglia leave the yolk sac blood islands at E9.5 to colonize the neuroepithelium, just before the blood–brain barrier (BBB) is formed. Thereafter, this population proliferates and expands throughout the CNS [138,139,140]. However, the coupled proliferation and apoptosis of microglia imply a stable population from postnatal stages until the first signs of aging appear [141]. In fact, it is estimated that 28% of microglia in humans are renewed every year and that the half-life of these cells is 4.2 years [142].
Microglia make up 5–12% of CNS cells, and their distribution in the adult brain varies depending on the region, being more present in the hippocampus, basal ganglia, and substantia nigra compared to in the nervous tracts, cerebellum, and brain stem [143]. Moreover, transcriptomic analysis has allowed the study of the spatial and temporal heterogeneity of microglia, identifying a broad diversity of cellular subtypes depending on age, area, and pathology [144,145,146,147,148,149]. Furthermore, microglia also have sex-specific transcriptomic features. Female microglia show a neuroprotective phenotype, whereas the transcriptome of male microglia is enriched in inflammation-associated genes [150]. Hormonal cues and sex chromosomes are thought to determine these differences [151]. A very recent study has demonstrated that sex-related differences in microRNAs expressed in microglia lead to a differential response of these cells to tau pathology in males and females [152]. This observation, together with other aspects, could partially explain gender differences in the incidence of some neurodegenerative diseases [151].
3.2. Physiological Functions of Microglia
Microglia contribute to the early stages of development by establishing the neural architecture of the CNS. To this end, microglia engulf neurons that have been over-generated [153]. This phenomenon is still evident in neurogenic niches in the adult stage [154]. Furthermore, it has recently been discovered that microglia produce a set of neurogenic factors that contribute to the maintenance and correct regulation of adult hippocampal neurogenesis [155].
Furthermore, microglia shape neuronal circuits. In this regard, these cells can eliminate non-functional synapses, thus ensuring the establishment of efficient synaptic contacts [156]. Additionally, this cell type engulfs the extracellular matrix around dendritic spines and contributes to postnatal synapse formation [157,158]. While previous studies have focused on the role of microglia in synapses during the early stages of development, a very recent report demonstrates that microglial processes also maintain direct contact with the soma of neurons, thereby ensuring homeostasis [159]. In addition to their interaction with neurons, microglia support the functions of other cell types such as oligodendrocytes and endothelial cells. In this context, microglia have been shown to play a critical role in axon myelination [160] and in the vascularization of neural tissue [161].
In the adult brain, microglia show a basal motility characterized by continuous extension and retraction of their processes without movement of cell bodies. This motility allows microglia to quickly detect changes in their microenvironment and to rapidly phagocytose harmful substances [162,163]. To do so, these cells are equipped with a set of receptors that allow them to recognize pathogens, misfolded proteins, cytokines, metabolites, inorganic substances, and changes in pH [164]. The factors that regulate microglial motility and morphology are largely unknown, although some purinergic receptors [165], ion channels [166] and neurotransmitters [167] have been implicated.
3.3. Contribution of Microglia to Tau Pathology
Alois Alzheimer was the first to recognize the involvement of glia in the disease that would bear his name [168]. However, it was in the 1990s that microglia were shown to interact with Aβ and tau [169,170]. For the last thirty years, the scientific community, supported by the amyloid hypothesis, has focused on investigating the contribution of Aβ to neuroinflammation, while less attention has been paid to the association between microglia and tau pathology.
Virginia Lee’s group developed one of the most extensively used mouse models of tauopathy (P301S). They observed that treatment of this animal with an immunosuppressant inhibited microglial activation and attenuated tau pathology [171]. Those authors postulated that microgliosis precedes tau pathology. However, other studies report that tau propagation is a very early phenomenon that triggers microglial activation [172,173]. Later, it was shown that activated microglia played an important role in the propagation of tau [124]. Subsequently, it was demonstrated that tau was phagocytosed by microglia [120,121]. However, the molecular mechanism through which microglia promoted tau pathology was unknown. It has recently been described that tau induces NOD-, LRR-, and pyrin domain-containing 3 (NLRP3) inflammasome activation [174], a multimeric signaling complex that leads to the activation of inflammatory processes through IL-1β proteolysis. Similarly, NLRP3 activation is also mediated by Aβ (Figure 2). Therefore, inflammasome deletion reduces the formation of NFTs and senile plaques [174,175,176].
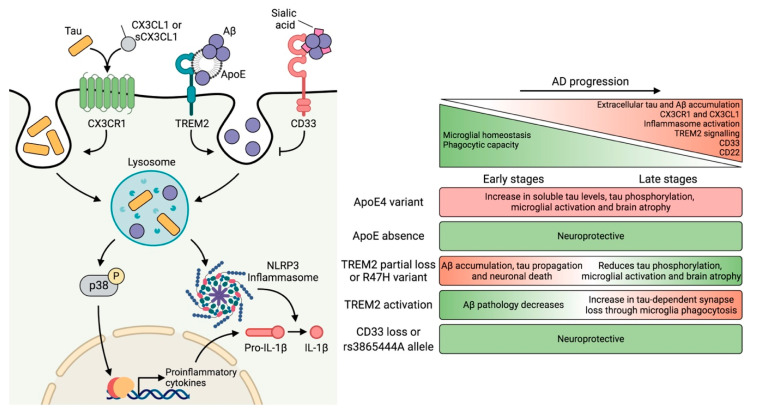
Figure 2.
Overview of microglial receptors involved in neuroinflammation. Amyloid-β peptide (Aβ) and tau accumulate in the extracellular space during Alzheimer’s disease (AD). At the same time, CX3CR1 (fractalkine receptor) and CX3CL1 (fractalkine) levels increase, which compromises this communication axis, thus altering microglial homeostasis. Moreover, the increasing levels of CD33 and CD22 reduce the phagocytic capacity of these cells. In the extracellular space, tau competes with CX3CL1 for its binding to CX3CR1, a receptor involved in tau internalization. Moreover, Aβ directly interacts with triggering receptor expressed on myeloid cells 2 (TREM2) or through apolipoprotein E (ApoE), promoting its phagocytosis. Conversely, Aβ can also bind to CD33 via sialic acid residues, thereby preventing phagocytosis. It has been described that tau binds to ApoE and that tau aggregates contain sialic acid residues. However, the interaction of tau with these receptors has not yet been described. Both Aβ and tau promote the activation of the p38 Mitogen-activated protein kinase (MAPK) pathway and the NOD-, LRR-, and pyrin domain-containing 3 (NLRP3) inflammasome, which trigger the production of proinflammatory cytokines. The table on the right summarizes the effects of genetic variants highly expressed in microglia that confer susceptibility to AD. The absence of ApoE and CD33 confers neuroprotection. In contrast, the effects of TREM2 depend on the stage of the disease. For this reason, promoting TREM2 activation is effective in the preclinical phase against Aβ pathology. However, at advanced stages, TREM2 signaling is detrimental as it facilitates the progression of the disease in the context of tau pathology. AD: Alzheimer’s disease, ApoE: apolipoprotein E, Aβ: amyloid-β, CD: cluster of differentiation, CX3CL1: fractalkine, CX3CR1: fractalkine receptor, IL-1β: interleukin-1β, NLRP3: NOD-, LRR-, and pyrin domain-containing 3, Pro-IL-1β: interleukin-1β (inactive precursor), sCX3CL1: soluble fractalkine, TREM2: triggering receptor expressed on myeloid cells 2. Created with BioRender.com.
Aging is the most important risk factor in AD [177]. In this context, microglia are believed to promote the progression of the pathology through a decrease in their neuroprotective functions, an increase in their toxicity, and alterations in their response to several stimuli, thus giving rise to a senescent phenotype [178]. These age-associated changes have been previously characterized in microglia and include alterations in cytokine secretion [179], an increase in the expression of activation markers [180], and the appearance of dystrophic morphologies [181]. It has recently been proposed that the elimination of senescent microglia and the repopulation of the CNS with new microglia, capable of supplying the functions of the former, may provide a beneficial therapeutic strategy for AD [182]. In this regard, pharmacological depletion of microglia has been shown to block tau propagation [183] and neurodegeneration [184]. Furthermore, another study demonstrated the presence of senescent markers in glia from the P301S mouse. The depletion of senescent cells in this model prevented gliosis, tau hyperphosphorylation, and neuronal degeneration, thereby preserving cognitive function [185]. Similarly, this strategy reduced senile plaque formation in an Aβ mouse model [186,187].
4. Genetic Risk Factors and Their Impact on Microglial Function
4.1. APOE
The hypothesis that activation of the innate immune system contributes to the pathogenesis of AD has been supported by genome-wide association studies, which have identified a set of genetic variants that are highly expressed in microglia. Among these, the ε4 variant of APOE is the strongest genetic risk factor in AD [188,189] and it is associated with an enhanced innate immune response [190]. In the brain, apolipoprotein E (ApoE), which is secreted primarily by microglia and astrocytes, acts as a transporter of lipoproteins between cells [27,28,191]. Several groups have reported that ApoE is also involved in Aβ metabolism [192,193,194], and subsequent studies have shown that it mediates the microglial response to amyloid pathology [195].
Although the interaction between ApoE and tau was described almost three decades ago [196], its contribution to tau pathology has been addressed only recently. In this regard, the ε4 variant in the P301S mouse increases soluble tau levels, tau phosphorylation, microglial activation, and brain atrophy. However, the absence of ApoE is neuroprotective since it prevents neurodegeneration and inflammation [197]. Additionally, a more recent study led by the same group indicates that ApoE regulates neurodegeneration in the P301S mouse by regulating the microglia-mediated innate immune response [198] (Figure 2).
Furthermore, ApoE4 binds inefficiently to LRP1 in pericytes, and the absence of signaling triggers the activation of the cyclophilin-A/NF-κB/MMP9 cascade, causing the breakdown of the BBB [199,200]. This disruption leads to the extravasation of proteins such as fibrin, which induces microglia-mediated spine elimination and cognitive decline [201].
4.2. TREM2
The triggering receptor expressed on myeloid cells 2 (TREM2) is another molecule that interacts with ApoE [202,203]. Krasemann et al. described that this interaction shifts microglia to a neurodegenerative phenotype and suggested that targeting the TREM2–ApoE pathway may restore homeostatic microglia in amyloid pathology [204].
The R47H variant confers TREM2 loss-of-function and it is the second main genetic risk factor in AD in terms of the magnitude of its effects [205]. Expressed in microglia, TREM2 is a transmembrane receptor that induces phagocytosis, modulates inflammatory signaling, and promotes survival [206]. Partial or complete TREM2 absence increases Aβ accumulation and neuronal death as this receptor modulates microglial response against these aggregates [207]. Moreover, TREM2 deficiency impedes microglia clustering around Aβ deposits, allowing senile plaques to become more diffuse and therefore increasing neuronal damage [208]. Conversely, TREM2 activation with an agonistic antibody reduces amyloid pathology [209].
The contribution of TREM2 to tau pathology offers opposing results. One of the first reports showed that the absence of TREM2 increases tau hyperphosphorylation and aggregation (due to c-Jun N-terminal kinase (JNK), Extracellular signal-regulated kinase (ERK), p38, and GSK3β activation) and also induces microglial activation in the hTau mouse [210]. Subsequently, another study postulated that lack of TREM2 attenuates neuroinflammation and prevents neuronal death in the P301S mouse [211]. Later, the same group observed that TREM2 deficiency or the R47H variant induces tau seeding and propagation in APP/PS1 mice subjected to the stereotaxic injection of tau from AD patients. They reported that microglial impairment in these animals promotes NFT accumulation around senile plaques, a phenomenon that also occurs in patients with TREM2 mutations [212]. Nonetheless, a novel study revealed that the R47H variant has a neuroprotective role by reducing brain atrophy, synapse loss, tau phosphorylation, microglial activation, and the engulfment of postsynaptic elements in the P301S mouse. The authors of that study explained that the R47H variant attenuates microglial phagocytosis, thereby leading to a lower synapse loss and, consequently, less brain atrophy. If the R47H variant increases the risk of AD, the question arises as to why it is protective against tau pathology. They pointed out that the role of TREM2 also depends on Aβ pathology and the stage of the disease. At early phases, the R47H variant reduces microgliosis around senile plaques, thereby increasing their number, and also promotes tau propagation. However, in advanced stages of the disease, when tau pathology is conspicuous, the variant attenuates tau-dependent synapse loss by reducing microglial phagocytosis [213].
Some of the discrepancies found in these studies could be explained by the mouse model used, the differences between murine and human TREM2, and the stage of the disease. Regarding the latter, TREM2 appears to have a dual role throughout pathology, and its targeting might not be straightforward. In this regard, TREM2 function should be potentiated at early time points. However, its activity should be partially suppressed in advanced stages since complete abolishment could lead to Nasu–Hakola disease, which is characterized mainly by bone lesions [214] (Figure 2).
Additionally, soluble TREM2 (sTREM2) is one the of most reliable immune biomarkers in AD [215]. Most sTREM2 is produced by ADAM10 proteolysis, and this phenomenon has been linked to an increase in TREM2 signaling, thus suggesting that this receptor exerts a protective role [216,217]. A further report also led by Christian Haass showed that increased levels of sTREM2 are associated with tau pathology [24]. More research is still needed to establish whether sTREM2 levels correspond to the activity of this receptor. If confirmed, TREM2 cleavage emerges as an approach to reduce TREM2 levels in the membrane of microglia to halt the activation of these cells during the disease.
4.3. CD33
CD33 is another AD risk gene [218,219,220] that encodes for a transmembrane receptor expressed in microglia [27,28]. As a member of the sialic acid–binding immunoglobulin-like lectins (Siglecs) [221], CD33 recognizes sialic acid residues of proteins and lipids triggering a signaling cascade, which suppresses the immune response [222].
CD33 expression is increased in AD, thereby causing a reduction in microglial activation and Aβ clearance. This reduction occurs due to the interaction of sialic acid residues of Aβ with CD33, leading to the inhibition of Aβ internalization. However, knockout of CD33 in the APP/PS1 mouse promoted Aβ uptake by microglia, thus reducing the amyloid plaque burden [223]. This reversal was also achieved in 5×FAD mice, which also showed an improvement in cognitive function. Conversely, TREM2 deletion in these animals abrogated this protective effect. Transcriptomic data revealed that gene expression changes caused by CD33 deletion were dependent on TREM2 presence. In contrast, gene expression changes caused by TREM2 deletion occurred regardless of CD33. Therefore, the authors of that work proposed that TREM2 acts downstream of CD33 [224]. Thus, CD33 inhibition and TREM2 activation emerge as a promising strategy, at least during the early stages of AD (Figure 2).
Furthermore, NFTs, along with other histopathological hallmarks that contain hyperphosphorylated tau, also present sialic acid residues [225,226]. In this regard, it would be interesting to explore whether tau interacts with CD33, as well as to analyze the role of this receptor in tauopathies.
5. Neuron–Microglia Crosstalk: Implications of the CX3CL1–CX3CR1 Axis and Downstream Signaling
Of the cell–cell communicate strategies, the use of chemokines is one of the most highly regulated systems. Normally, a single cell type expresses the ligand for a receptor that is selectively found in another cell population, thereby conferring a high degree of specificity to the resulting signaling axis [227]. This is the case of fractalkine (CX3CL1, C-X3-C chemokine ligand 1), a chemokine produced by neurons that binds to its receptor (CX3CR1, C-X3-C chemokine receptor 1), which is specifically expressed in microglia [27,28,228,229]. CX3CL1 can be either membrane-bound or soluble (sCX3CL1) after cleavage of its N-terminal domain. The membrane-bound form acts as an adhesion molecule, while sCX3CL1 responds as a chemoattractant, promoting cell migration [230]. CX3CR1 belongs to the G protein-coupled receptor family. The inhibitory G subunit prevents cyclic adenosine monophosphate (cAMP) production, thereby giving rise to a series of second messengers known to regulate a broad repertoire of cellular functions such as transcription, migration, proliferation, and apoptosis [231,232,233].
In addition to being essential in CNS development and connectivity [233], the CX3CL1–CX3CR1 axis is in the first line of defense in response to neuronal damage and neuroinflammation, exerting neuroprotective signaling [234,235]. During AD, CX3CR1 and CX3CL1 levels increase in the brain, thereby compromising microglial homeostasis and neuronal circuits [236,237,238]. The generation of the Cx3cr1−/− mouse, together with its crossing with other AD models, has greatly contributed to our understanding of the role of the CX3CL1–CX3CR1 axis in this pathology. Moreover, CX3CR1 deficiency decreases Aβ accumulation due to an increase in the phagocytic capacity of microglia [239]. Conversely, the absence of this receptor in a mouse model of tauopathy increases tau hyperphosphorylation and aggregation and amplifies microglial activation, thereby causing IL-1β secretion [124,240]. By activating p38 in neurons, this cytokine promotes tau hyperphosphorylation, thereby worsening the pathology [241]. Subsequently, tau phagocytosis was shown to be reduced in microglia lacking CX3CR1. This observation may be due to the fact that the interaction between tau (which possesses a 37% amino acid sequence identity with CX3CL1) and CX3CR1 promotes its internalization [237] (Figure 2). In this regard, two recent studies support these results. The first one determined that Cx3cr1−/− microglia present a lower phagocytic and migratory capacity per se [242], while the second revealed that Cx3cr1−/− microglia of young animals are very similar to the wild type (WT) microglia of older animals [243]. On the basis of their findings, the authors of the latter study suggested that the absence of CX3CR1 confers a premature aging transcriptome to these cells, accompanied by a decrease in phagocytosis. These findings pose the question as to why the absence of CX3CR1 promotes the phagocytosis of Aβ and not of tau. One possibility is that the absence of CX3CR1 signaling enhances the synthesis of Aβ receptors and enzymes that degrade this peptide [244]. In turn, this pathway may block those corresponding to tau, thus having opposite effects on each pathology.
In addition, some groups have focused on analyzing the role of CX3CL1 in mouse models of tauopathy. In this regard, sCX3CL1 overexpression reduces neuroinflammation and tau pathology in the rTg4510 mouse [245]. In contrast, Bemiller et al. showed the opposite results in an hTau mouse and concluded that sCX3CL1 is not protective. The authors of that work suggested that the discrepancies between these studies are due to the experimental approach (recombinant adeno-associated virus (rAAV) vector-mediated overexpression vs. transgenic mouse), the differences in sCX3CL1 structure (presence or not of mucin stalk), and the tauopathy mouse model used (rTg4510 vs. hTau) [246]. However, the CX3CL1 intracellular fragment enhances neurogenesis, improves cognitive function, and increases the lifespan. Nevertheless, its effects on tau pathology are minimal [247,248].
In brief, all the studies mentioned above highlight the importance of the CX3CL1–CX3CR1 signaling pathway in neuron–microglia crosstalk, as well as in the progression of AD.
6. Extracellular Soluble Tau as the Main Driving Force of Toxicity
Numerous authors state that tau aggregates are not as toxic as previously believed and that their formation may suppose a neuroprotective event [72]. Added to this, it has been shown that neuronal death does not depend on the presence of NFTs [249]. Likewise, neurons bearing NFTs can survive up to 20 years [250]. Consequently, these aggregates have been found in healthy individuals and the neurons that carry them are correctly integrated into the cortical circuit [251]. Furthermore, synaptic, cognitive and behavioral alterations occur before NFTs are formed [252,253,254,255]. More recent studies have revealed that soluble tau blocks Aβ-dependent hyperactivity, thereby leading to impaired neuronal circuit function despite the presence of NFTs [256,257]. Additionally, several soluble tau species (which differ in seeding activity, phosphorylation, and oligomerization) rather than tau accumulation have been described to determine the heterogeneity in AD progression [258]. All these reports suggest that soluble tau species are the main protagonists of AD, which would explain why therapeutic strategies against Aβ or aggregated tau have failed. However, clinical trials focused solely on tau may need to be reconsidered as some authors propose the need for a deeper study of the interrelationship between Aβ and tau [259].
Other studies also support the importance of soluble dephosphorylated tau in neurodegenerative diseases. Thus, there is some evidence supporting the toxicity of dephosphorylated tau resulting from the action of extracellular phosphatases such as tissue-nonspecific alkaline phosphatase (TNAP). In this regard, previous reports showed that TNAP, whose expression and activity increases in AD, is capable of removing phosphorylated residues of tau present in the extracellular space. Afterward, tau acts as an agonist for muscarinic receptors, increasing the amount of Ca2+ and, thus, causing neuronal death. Furthermore, TNAP expression and activity increases after tau addition to a neuron culture medium, thereby creating a positive feedback loop [102,112,260,261]. Additionally, exome sequencing analysis has recently identified genetic variants of some phosphatases that confer protection against AD [262]. However, the role of these variants in tau dephosphorylation remains unexplored.
As we previously mentioned, tau also interacts with glia [120,121,122,123,126,169]. In this regard, a wide repertoire of receptors present in microglia that interact with Aβ are known [263]. In contrast, the analysis of the tau interactome has not yet identified the binding of tau to any of these receptors [264,265,266]. Nonetheless, our group described that tau interacts with CX3CR1 in microglia [237]. In addition, a more recent study has shown that tau binds to an integrin receptor in astrocytes [267] (Figure 1). In this respect, biotinylation-based proximity methods such as the one applied in that study could reveal more candidates. In fact, there is previous evidence about the interaction of tau with the plasma membrane [39]. Furthermore, the association of tau with this cell structure is regulated by its phosphorylation state [268], denoting that the dephosphorylation of tau increases its interaction with the membrane [269] and vice versa [270]. Consequently, it is plausible that membrane-bound tau interacts with receptors of the innate immune system such as Toll-like receptors (TLRs) [271], thereby reinforcing the hypothesis of the toxicity of dephosphorylated extracellular tau. In this context, we found that dephosphorylated tau activates p38 MAPK in microglia, triggering a proinflammatory response [272] (Figure 2). Interestingly, previous reports in which non-phosphorylated tau underwent a phosphorylation reaction demonstrated that phosphorylated tau is less internalized by microglia [237]. Accordingly, it could be considered that phosphorylated residues mask the binding region of tau, or alter its conformation, preventing its interaction with receptors involved in tau phagocytosis and/or p38 activation.
Therefore, these studies support the hypothesis that extracellular dephosphorylated tau has a toxic effect on surrounding cells such as microglia, whereas aggregated and/or hyperphosphorylated intracellular tau plays a key role in neuron functionality.
7. Future Perspectives
The aforementioned studies highlight that innate immune activation is a key factor in aging and AD progression. In this context, more research is needed to identify the role of other genetic risk factors, as well as the epigenetic changes that principally occur in microglia [273,274,275]. Moreover, the contribution of the adaptive immune system to aging and AD progression remains largely unexplored. In this regard, it has been found that a subpopulation of CD8+ T cells patrols the CNS [276]. These cells have also been detected in APP/PS1 mice but barely in hTau animals [277]. Therefore, it would be intriguing to investigate which specific antigen this cell population recognizes.
In addition, the study of gut microbiota in neurodegenerative diseases has attracted the attention of the scientific community. Several reports indicate that microbiota govern the maturation and function of microglia [278] and, thus, regulate Aβ phagocytosis [279,280,281]. However, the role of this assemblage of microorganisms in tau pathology is unknown.
Regarding microglial malfunction during aging, it has been shown that microglial repopulation reverses synaptic alterations and improves cognitive function in aged mice [282]. Furthermore, CD22 (whose levels increase with age) has emerged as a suppressor of microglial phagocytosis in these animals (Figure 2). Nevertheless, blockade of CD22 restores phagocytosis, and animals showed cognitive improvement [283]. Given these observations, it would be of interest to determine whether these two strategies can improve the ability of microglia to internalize tau protein.
Acknowledgments
The authors thank Tanya Yates for a critical reading of the manuscript and reviewers for their contribution to the peer review of this work.
Author Contributions
Conceptualization, J.R.P., M.B., and J.A.; investigation, J.R.P., M.B. and J.A.; writing—original draft preparation, J.R.P., M.B. and J.A.; writing—review and editing, J.R.P., M.B. and J.A.; visualization, J.R.P., M.B. and J.A.; supervision, M.B. and J.A.; project administration, M.B. and J.A.; funding acquisition, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Science and Innovation (PGC2018-096177-B-00), the Network Center for Biomedical Research on Neurodegenerative Diseases, and Fundación Ramón Areces and Banco de Santander to the Centro de Biología Molecular “Severo Ochoa” institutional grants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Katzman R. The prevalence and malignancy of Alzheimer disease. A major killer. Arch. Neurol. 1976;33:217–218. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- 2.Wimo A., Guerchet M., Ali G.-C., Wu Y.-T., Prina A.M., Winblad B., Jönsson L., Liu Z., Prince M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017;13:1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols E., Szoeke C.E.I., Vollset S.E., Abbasi N., Abd-Allah F., Abdela J., Aichour M.T.E., Akinyemi R.O., Alahdab F., Asgedom S.W., et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer A. Über einen eigenartigen schweren Erkrankungsprozeß der Hirnrinde. Neurol. Zentralblatt. 1906;23:1129–1136. [Google Scholar]
- 5.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Zeitschrift fur Psychiatr. und Psych. Medizin. 1907;64:146–148. [Google Scholar]
- 6.Masters C.L., Simms G., Weinman N.A., Multhaup G., McDonald B.L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarrett J.T., Berger E.P., Lansbury P.T. The C-terminus of the beta protein is critical in amyloidogenesis. Ann. N. Y. Acad. Sci. 1993;695:144–148. doi: 10.1111/j.1749-6632.1993.tb23043.x. [DOI] [PubMed] [Google Scholar]
- 8.Maggio J.E., Stimson E.R., Ghilardi J.R., Allen C.J., Dahl C.E., Whitcomb D.C., Vigna S.R., Vinters H.V., Labenski M.E., Mantyh P.W. Reversible in vitro growth of Alzheimer disease beta-amyloid plaques by deposition of labeled amyloid peptide. Proc. Natl. Acad. Sci. USA. 1992;89:5462–5466. doi: 10.1073/pnas.89.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidd M. Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature. 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- 10.Crowther R.A., Wischik C.M. Image reconstruction of the Alzheimer paired helical filament. EMBO J. 1985;4:3661–3665. doi: 10.1002/j.1460-2075.1985.tb04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Bergen M., Friedhoff P., Biernat J., Heberle J., Mandelkow E.M., Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc. Natl. Acad. Sci. USA. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy J.A., Higgins G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 14.Tcw J., Goate A.M. Genetics of β-Amyloid Precursor Protein in Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2017;7:a024539. doi: 10.1101/cshperspect.a024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/WNL.42.3.631. [DOI] [PubMed] [Google Scholar]
- 16.Bierer L.M., Hof P.R., Purohit D.P., Carlin L., Schmeidler J., Davis K.L., Perl D.P. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch. Neurol. 1995;52:81–88. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- 17.Giannakopoulos P., Herrmann F.R., Bussière T., Bouras C., Kövari E., Perl D.P., Morrison J.H., Gold G., Hof P.R. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.WNL.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 18.La Joie R., Visani A.V., Baker S.L., Brown J.A., Bourakova V., Cha J., Chaudhary K., Edwards L., Iaccarino L., Janabi M., et al. Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. Sci. Transl. Med. 2020;12:eaau5732. doi: 10.1126/scitranslmed.aau5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapoport M., Dawson H.N., Binder L.I., Vitek M.P., Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. USA. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.-Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 21.Mintun M.A., Larossa G.N., Sheline Y.I., Dence C.S., Lee S.Y., Mach R.H., Klunk W.E., Mathis C.A., DeKosky S.T., Morris J.C. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 22.Aizenstein H.J., Nebes R.D., Saxton J.A., Price J.C., Mathis C.A., Tsopelas N.D., Ziolko S.K., James J.A., Snitz B.E., Houck P.R., et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer P.-F., Savard M., Poirier J., Labonté A., Rosa-Neto P., Weitz T.M., Town T., Breitner J. Alzheimer’s Disease Neuroimaging Initiative; PREVENT-AD Research Group Bi-directional Association of Cerebrospinal Fluid Immune Markers with Stage of Alzheimer’s Disease Pathogenesis. J. Alzheimers. Dis. 2018;63:577–590. doi: 10.3233/JAD-170887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suárez-Calvet M., Morenas-Rodríguez E., Kleinberger G., Schlepckow K., Araque Caballero M.Á., Franzmeier N., Capell A., Fellerer K., Nuscher B., Eren E., et al. Early increase of CSF sTREM2 in Alzheimer’s disease is associated with tau related-neurodegeneration but not with amyloid-β pathology. Mol. Neurodegener. 2019;14:1. doi: 10.1186/s13024-018-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weingarten M.D., Lockwood A.H., Hwo S.Y., Kirschner M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LoPresti P., Szuchet S., Papasozomenos S.C., Zinkowski R.P., Binder L.I. Functional implications for the microtubule-associated protein tau: Localization in oligodendrocytes. Proc. Natl. Acad. Sci. USA. 1995;92:10369–10373. doi: 10.1073/pnas.92.22.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O’Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N., et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Sloan S.A., Clarke L.E., Caneda C., Plaza C.A., Blumenthal P.D., Vogel H., Steinberg G.K., Edwards M.S.B., Li G., et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neve R.L., Harris P., Kosik K.S., Kurnit D.M., Donlon T.A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986;387:271–280. doi: 10.1016/0169-328X(86)90033-1. [DOI] [PubMed] [Google Scholar]
- 30.Andreadis A. Tau gene alternative splicing: Expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim. Biophys. Acta. 2005;1739:91–103. doi: 10.1016/j.bbadis.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 32.Bruch J., Xu H., De Andrade A., Höglinger G. Mitochondrial complex 1 inhibition increases 4-repeat isoform tau by SRSF2 upregulation. PLoS ONE. 2014;9:e113070. doi: 10.1371/journal.pone.0113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith P.Y., Delay C., Girard J., Papon M.-A., Planel E., Sergeant N., Buée L., Hébert S.S. MicroRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy. Hum. Mol. Genet. 2011;20:4016–4024. doi: 10.1093/hmg/ddr330. [DOI] [PubMed] [Google Scholar]
- 34.Orozco D., Tahirovic S., Rentzsch K., Schwenk B.M., Haass C., Edbauer D. Loss of fused in sarcoma (FUS) promotes pathological Tau splicing. EMBO Rep. 2012;13:759–764. doi: 10.1038/embor.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binder L.I., Frankfurter A., Rebhun L.I. The distribution of tau in the mammalian central nervous system. J. Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B.C., Christie M.J., Napier I.A., et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 37.Loomis P.A., Howard T.H., Castleberry R.P., Binder L.I. Identification of nuclear tau isoforms in human neuroblastoma cells. Proc. Natl. Acad. Sci. USA. 1990;87:8422–8426. doi: 10.1073/pnas.87.21.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rady R.M., Zinkowski R.P., Binder L.I. Presence of tau in isolated nuclei from human brain. Neurobiol. Aging. 1995;16:479–486. doi: 10.1016/0197-4580(95)00023-8. [DOI] [PubMed] [Google Scholar]
- 39.Brandt R., Léger J., Lee G. Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J. Cell Biol. 1995;131:1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ait-Bouziad N., Lv G., Mahul-Mellier A.-L., Xiao S., Zorludemir G., Eliezer D., Walz T., Lashuel H.A. Discovery and characterization of stable and toxic Tau/phospholipid oligomeric complexes. Nat. Commun. 2017;8:1678. doi: 10.1038/s41467-017-01575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C., Götz J. Profiling murine tau with 0N, 1N and 2N isoform-specific antibodies in brain and peripheral organs reveals distinct subcellular localization, with the 1N isoform being enriched in the nucleus. PLoS ONE. 2013;8:e84849. doi: 10.1371/journal.pone.0084849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J., Kanai Y., Cowan N.J., Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- 43.Frappier T.F., Georgieff I.S., Brown K., Shelanski M.L. Tau regulation of microtubule-microtubule spacing and bundling. J. Neurochem. 1994;63:2288–2294. doi: 10.1046/j.1471-4159.1994.63062288.x. [DOI] [PubMed] [Google Scholar]
- 44.Lee G., Neve R.L., Kosik K.S. The microtubule binding domain of tau protein. Neuron. 1989;2:1615–1624. doi: 10.1016/0896-6273(89)90050-0. [DOI] [PubMed] [Google Scholar]
- 45.Lee G., Newman S.T., Gard D.L., Band H., Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 1998;111:3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- 46.Tapia-Rojas C., Cabezas-Opazo F., Deaton C.A., Vergara E.H., Johnson G.V.W., Quintanilla R.A. It’s all about tau. Prog. Neurobiol. 2019;175:54–76. doi: 10.1016/j.pneurobio.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ercan E., Eid S., Weber C., Kowalski A., Bichmann M., Behrendt A., Matthes F., Krauss S., Reinhardt P., Fulle S., et al. A validated antibody panel for the characterization of tau post-translational modifications. Mol. Neurodegener. 2017;12:87. doi: 10.1186/s13024-017-0229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanemaru K., Takio K., Miura R., Titani K., Ihara Y. Fetal-type phosphorylation of the tau in paired helical filaments. J. Neurochem. 1992;58:1667–1675. doi: 10.1111/j.1471-4159.1992.tb10039.x. [DOI] [PubMed] [Google Scholar]
- 49.Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biernat J., Gustke N., Drewes G., Mandelkow E.M., Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: Distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. doi: 10.1016/0896-6273(93)90279-Z. [DOI] [PubMed] [Google Scholar]
- 51.Hoover B.R., Reed M.N., Su J., Penrod R.D., Kotilinek L.A., Grant M.K., Pitstick R., Carlson G.A., Lanier L.M., Yuan L.-L., et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickey C.A., Kamal A., Lundgren K., Klosak N., Bailey R.M., Dunmore J., Ash P., Shoraka S., Zlatkovic J., Eckman C.B., et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Investig. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhaskar K., Yen S.-H., Lee G. Disease-related modifications in tau affect the interaction between Fyn and Tau. J. Biol. Chem. 2005;280:35119–35125. doi: 10.1074/jbc.M505895200. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds C.H., Garwood C.J., Wray S., Price C., Kellie S., Perera T., Zvelebil M., Yang A., Sheppard P.W., Varndell I.M., et al. Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cgamma1, Grb2, and Src family kinases. J. Biol. Chem. 2008;283:18177–18186. doi: 10.1074/jbc.M709715200. [DOI] [PubMed] [Google Scholar]
- 55.Ittner L.M., Ke Y.D., Götz J. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J. Biol. Chem. 2009;284:20909–20916. doi: 10.1074/jbc.M109.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanger D.P. Tau Phosphorylation Sites. [(accessed on 15 July 2020)]; Available online: https://docs.google.com/spreadsheets/d/1hGYs1ZcupmTnbB7n6qs1r_WVTXHt1O7NBLyKBN7EOUQ/edit#gid=0.
- 57.Hanger D.P., Hughes K., Woodgett J.R., Brion J.P., Anderton B.H. Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: Generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci. Lett. 1992;147:58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- 58.Buée-Scherrer V., Goedert M. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases in intact cells. FEBS Lett. 2002;515:151–154. doi: 10.1016/S0014-5793(02)02460-2. [DOI] [PubMed] [Google Scholar]
- 59.Liu F., Grundke-Iqbal I., Iqbal K., Gong C.-X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 60.Hanger D.P., Anderton B.H., Noble W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Lee V.M.-Y., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 62.Dickson D.W., Kouri N., Murray M.E., Josephs K.A. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau) J. Mol. Neurosci. 2011;45:384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alonso A., Zaidi T., Novak M., Grundke-Iqbal I., Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haase C., Stieler J.T., Arendt T., Holzer M. Pseudophosphorylation of tau protein alters its ability for self-aggregation. J. Neurochem. 2004;88:1509–1520. doi: 10.1046/j.1471-4159.2003.02287.x. [DOI] [PubMed] [Google Scholar]
- 65.Xia Y., Prokop S., Gorion K.-M.M., Kim J.D., Sorrentino Z.A., Bell B.M., Manaois A.N., Chakrabarty P., Davies P., Giasson B.I. Tau Ser208 phosphorylation promotes aggregation and reveals neuropathologic diversity in Alzheimer’s disease and other tauopathies. Acta Neuropathol. Commun. 2020;8:88. doi: 10.1186/s40478-020-00967-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider A., Biernat J., von Bergen M., Mandelkow E., Mandelkow E.-M. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Garg S., Mandelkow E.-M., Mandelkow E. Proteolytic processing of tau. Biochem. Soc. Trans. 2010;38:955–961. doi: 10.1042/BST0380955. [DOI] [PubMed] [Google Scholar]
- 68.Montejo de Garcini E., Avila J. In vitro conditions for the self-polymerization of the microtubule-associated protein, tau factor. J. Biochem. 1987;102:1415–1421. doi: 10.1093/oxfordjournals.jbchem.a122187. [DOI] [PubMed] [Google Scholar]
- 69.Hong M., Zhukareva V., Vogelsberg-Ragaglia V., Wszolek Z., Reed L., Miller B.I., Geschwind D.H., Bird T.D., McKeel D., Goate A., et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 70.Goedert M., Jakes R., Spillantini M.G., Hasegawa M., Smith M.J., Crowther R.A. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 71.Kampers T., Friedhoff P., Biernat J., Mandelkow E.M., Mandelkow E. RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett. 1996;399:344–349. doi: 10.1016/S0014-5793(96)01386-5. [DOI] [PubMed] [Google Scholar]
- 72.Cowan C.M., Mudher A. Are tau aggregates toxic or protective in tauopathies? Front. Neurol. 2013;4:114. doi: 10.3389/fneur.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mirbaha H., Chen D., Morazova O.A., Ruff K.M., Sharma A.M., Liu X., Goodarzi M., Pappu R.V., Colby D.W., Mirzaei H., et al. Inert and seed-competent tau monomers suggest structural origins of aggregation. Elife. 2018;7:e36584. doi: 10.7554/eLife.36584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schweers O., Schönbrunn-Hanebeck E., Marx A., Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J. Biol. Chem. 1994;269:24290–24297. [PubMed] [Google Scholar]
- 75.Wille H., Drewes G., Biernat J., Mandelkow E.M., Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J. Cell Biol. 1992;118:573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sahara N., Maeda S., Murayama M., Suzuki T., Dohmae N., Yen S.-H., Takashima A. Assembly of two distinct dimers and higher-order oligomers from full-length tau. Eur. J. Neurosci. 2007;25:3020–3029. doi: 10.1111/j.1460-9568.2007.05555.x. [DOI] [PubMed] [Google Scholar]
- 77.Maeda S., Sahara N., Saito Y., Murayama M., Yoshiike Y., Kim H., Miyasaka T., Murayama S., Ikai A., Takashima A. Granular tau oligomers as intermediates of tau filaments. Biochemistry. 2007;46:3856–3861. doi: 10.1021/bi061359o. [DOI] [PubMed] [Google Scholar]
- 78.Fitzpatrick A.W.P., Falcon B., He S., Murzin A.G., Murshudov G., Garringer H.J., Crowther R.A., Ghetti B., Goedert M., Scheres S.H.W. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547:185–190. doi: 10.1038/nature23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Falcon B., Zhang W., Murzin A.G., Murshudov G., Garringer H.J., Vidal R., Crowther R.A., Ghetti B., Scheres S.H.W., Goedert M. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature. 2018;561:137–140. doi: 10.1038/s41586-018-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Falcon B., Zivanov J., Zhang W., Murzin A.G., Garringer H.J., Vidal R., Crowther R.A., Newell K.L., Ghetti B., Goedert M., et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019;568:420–423. doi: 10.1038/s41586-019-1026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang W., Tarutani A., Newell K.L., Murzin A.G., Matsubara T., Falcon B., Vidal R., Garringer H.J., Shi Y., Ikeuchi T., et al. Novel tau filament fold in corticobasal degeneration. Nature. 2020;580:283–287. doi: 10.1038/s41586-020-2043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arakhamia T., Lee C.E., Carlomagno Y., Duong D.M., Kundinger S.R., Wang K., Williams D., DeTure M., Dickson D.W., Cook C.N., et al. Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains. Cell. 2020;180:633–644. doi: 10.1016/j.cell.2020.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiang L., Sun X., Austin T.O., Muralidharan H., Jean D.C., Liu M., Yu W., Baas P.W. Tau Does Not Stabilize Axonal Microtubules but Rather Enables Them to Have Long Labile Domains. Curr. Biol. 2018;28:2181–2189. doi: 10.1016/j.cub.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 84.Dixit R., Ross J.L., Goldman Y.E., Holzbaur E.L.F. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caceres A., Kosik K.S. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990;343:461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- 86.Kimura T., Whitcomb D.J., Jo J., Regan P., Piers T., Heo S., Brown C., Hashikawa T., Murayama M., Seok H., et al. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014;369:20130144. doi: 10.1098/rstb.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahmed T., Van der Jeugd A., Blum D., Galas M.-C., D’Hooge R., Buee L., Balschun D. Cognition and hippocampal synaptic plasticity in mice with a homozygous tau deletion. Neurobiol. Aging. 2014;35:2474–2478. doi: 10.1016/j.neurobiolaging.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Pallas-Bazarra N., Jurado-Arjona J., Navarrete M., Esteban J.A., Hernández F., Ávila J., Llorens-Martín M. Novel function of Tau in regulating the effects of external stimuli on adult hippocampal neurogenesis. EMBO J. 2016;35:1417–1436. doi: 10.15252/embj.201593518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sultan A., Nesslany F., Violet M., Bégard S., Loyens A., Talahari S., Mansuroglu Z., Marzin D., Sergeant N., Humez S., et al. Nuclear tau, a key player in neuronal DNA protection. J. Biol. Chem. 2011;286:4566–4575. doi: 10.1074/jbc.M110.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Violet M., Delattre L., Tardivel M., Sultan A., Chauderlier A., Caillierez R., Talahari S., Nesslany F., Lefebvre B., Bonnefoy E., et al. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front. Cell. Neurosci. 2014;8:84. doi: 10.3389/fncel.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harada A., Oguchi K., Okabe S., Kuno J., Terada S., Ohshima T., Sato-Yoshitake R., Takei Y., Noda T., Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- 92.van Hummel A., Bi M., Ippati S., van der Hoven J., Volkerling A., Lee W.S., Tan D.C.S., Bongers A., Ittner A., Ke Y.D., et al. No Overt Deficits in Aged Tau-Deficient C57Bl/6.Mapttm1(EGFP)Kit GFP Knockin Mice. PLoS ONE. 2016;11:e0163236. doi: 10.1371/journal.pone.0163236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei P., Ayton S., Finkelstein D.I., Spoerri L., Ciccotosto G.D., Wright D.K., Wong B.X.W., Adlard P.A., Cherny R.A., Lam L.Q., et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- 94.Lei P., Ayton S., Moon S., Zhang Q., Volitakis I., Finkelstein D.I., Bush A.I. Motor and cognitive deficits in aged tau knockout mice in two background strains. Mol. Neurodegener. 2014;9:29. doi: 10.1186/1750-1326-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frost B., Jacks R.L., Diamond M.I. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clavaguera F., Bolmont T., Crowther R.A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A.K., Beibel M., Staufenbiel M., et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Calignon A., Polydoro M., Suárez-Calvet M., William C., Adamowicz D.H., Kopeikina K.J., Pitstick R., Sahara N., Ashe K.H., Carlson G.A., et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DeVos S.L., Corjuc B.T., Oakley D.H., Nobuhara C.K., Bannon R.N., Chase A., Commins C., Gonzalez J.A., Dooley P.M., Frosch M.P., et al. Synaptic Tau Seeding Precedes Tau Pathology in Human Alzheimer’s Disease Brain. Front. Neurosci. 2018;12:267. doi: 10.3389/fnins.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wegmann S., Bennett R.E., Delorme L., Robbins A.B., Hu M., McKenzie D., Kirk M.J., Schiantarelli J., Tunio N., Amaral A.C., et al. Experimental evidence for the age dependence of tau protein spread in the brain. Sci. Adv. 2019;5:eaaw6404. doi: 10.1126/sciadv.aaw6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duyckaerts C., Clavaguera F., Potier M.-C. The prion-like propagation hypothesis in Alzheimer’s and Parkinson’s disease. Curr. Opin. Neurol. 2019;32:266–271. doi: 10.1097/WCO.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 101.Blennow K., Wallin A., Agren H., Spenger C., Siegfried J., Vanmechelen E. Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer disease? Mol. Chem. Neuropathol. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 102.Gómez-Ramos A., Díaz-Hernández M., Cuadros R., Hernández F., Avila J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006;580:4842–4850. doi: 10.1016/j.febslet.2006.07.078. [DOI] [PubMed] [Google Scholar]
- 103.Pooler A.M., Phillips E.C., Lau D.H.W., Noble W., Hanger D.P. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14:389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sokolow S., Henkins K.M., Bilousova T., Gonzalez B., Vinters H.V., Miller C.A., Cornwell L., Poon W.W., Gylys K.H. Pre-synaptic C-terminal truncated tau is released from cortical synapses in Alzheimer’s disease. J. Neurochem. 2015;133:368–379. doi: 10.1111/jnc.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simón D., García-García E., Royo F., Falcón-Pérez J.M., Avila J. Proteostasis of tau. Tau overexpression results in its secretion via membrane vesicles. FEBS Lett. 2012;586:47–54. doi: 10.1016/j.febslet.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 106.Saman S., Kim W., Raya M., Visnick Y., Miro S., Saman S., Jackson B., McKee A.C., Alvarez V.E., Lee N.C.Y., et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dujardin S., Bégard S., Caillierez R., Lachaud C., Delattre L., Carrier S., Loyens A., Galas M.-C., Bousset L., Melki R., et al. Ectosomes: A new mechanism for non-exosomal secretion of tau protein. PLoS ONE. 2014;9:e100760. doi: 10.1371/journal.pone.0100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chai X., Dage J.L., Citron M. Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol. Dis. 2012;48:356–366. doi: 10.1016/j.nbd.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 109.Katsinelos T., Zeitler M., Dimou E., Karakatsani A., Müller H.-M., Nachman E., Steringer J.P., Ruiz de Almodovar C., Nickel W., Jahn T.R. Unconventional Secretion Mediates the Trans-cellular Spreading of Tau. Cell Rep. 2018;23:2039–2055. doi: 10.1016/j.celrep.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 110.Merezhko M., Brunello C.A., Yan X., Vihinen H., Jokitalo E., Uronen R.-L., Huttunen H.J. Secretion of Tau via an Unconventional Non-vesicular Mechanism. Cell Rep. 2018;25:2027–2035. doi: 10.1016/j.celrep.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 111.Wu J.W., Herman M., Liu L., Simoes S., Acker C.M., Figueroa H., Steinberg J.I., Margittai M., Kayed R., Zurzolo C., et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem. 2013;288:1856–1870. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gómez-Ramos A., Díaz-Hernández M., Rubio A., Miras-Portugal M.T., Avila J. Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol. Cell. Neurosci. 2008;37:673–681. doi: 10.1016/j.mcn.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 113.Morozova V., Cohen L.S., Makki A.E.-H., Shur A., Pilar G., El Idrissi A., Alonso A.D. Normal and Pathological Tau Uptake Mediated by M1/M3 Muscarinic Receptors Promotes Opposite Neuronal Changes. Front. Cell. Neurosci. 2019;13:403. doi: 10.3389/fncel.2019.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rauch J.N., Luna G., Guzman E., Audouard M., Challis C., Sibih Y.E., Leshuk C., Hernandez I., Wegmann S., Hyman B.T., et al. LRP1 is a master regulator of tau uptake and spread. Nature. 2020;580:381–385. doi: 10.1038/s41586-020-2156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holmes B.B., DeVos S.L., Kfoury N., Li M., Jacks R., Yanamandra K., Ouidja M.O., Brodsky F.M., Marasa J., Bagchi D.P., et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA. 2013;110:E3138-47. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Calafate S., Flavin W., Verstreken P., Moechars D. Loss of Bin1 Promotes the Propagation of Tau Pathology. Cell Rep. 2016;17:931–940. doi: 10.1016/j.celrep.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 117.Tardivel M., Bégard S., Bousset L., Dujardin S., Coens A., Melki R., Buée L., Colin M. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol. Commun. 2016;4:117. doi: 10.1186/s40478-016-0386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brunello C.A., Merezhko M., Uronen R.-L., Huttunen H.J. Mechanisms of secretion and spreading of pathological tau protein. Cell. Mol. Life Sci. 2019 doi: 10.1007/s00018-019-03349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Flavin W.P., Bousset L., Green Z.C., Chu Y., Skarpathiotis S., Chaney M.J., Kordower J.H., Melki R., Campbell E.M. Endocytic vesicle rupture is a conserved mechanism of cellular invasion by amyloid proteins. Acta Neuropathol. 2017;134:629–653. doi: 10.1007/s00401-017-1722-x. [DOI] [PubMed] [Google Scholar]
- 120.Luo W., Liu W., Hu X., Hanna M., Caravaca A., Paul S.M. Microglial internalization and degradation of pathological tau is enhanced by an anti-tau monoclonal antibody. Sci. Rep. 2015;5:11161. doi: 10.1038/srep11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bolós M., Llorens-Martín M., Jurado-Arjona J., Hernández F., Rábano A., Avila J. Direct Evidence of Internalization of Tau by Microglia In Vitro and In Vivo. J. Alzheimers. Dis. 2015;50:77–87. doi: 10.3233/JAD-150704. [DOI] [PubMed] [Google Scholar]
- 122.Piacentini R., Li Puma D.D., Mainardi M., Lazzarino G., Tavazzi B., Arancio O., Grassi C. Reduced gliotransmitter release from astrocytes mediates tau-induced synaptic dysfunction in cultured hippocampal neurons. Glia. 2017;65:1302–1316. doi: 10.1002/glia.23163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perea J.R., López E., Díez-Ballesteros J.C., Ávila J., Hernández F., Bolós M. Extracellular Monomeric Tau Is Internalized by Astrocytes. Front. Neurosci. 2019;13:442. doi: 10.3389/fnins.2019.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maphis N., Xu G., Kokiko-Cochran O.N., Jiang S., Cardona A., Ransohoff R.M., Lamb B.T., Bhaskar K. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015;138:1738–1755. doi: 10.1093/brain/awv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hopp S.C., Lin Y., Oakley D., Roe A.D., DeVos S.L., Hanlon D., Hyman B.T. The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer’s disease. J. Neuroinflammation. 2018;15:269. doi: 10.1186/s12974-018-1309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martini-Stoica H., Cole A.L., Swartzlander D.B., Chen F., Wan Y.-W., Bajaj L., Bader D.A., Lee V.M.Y., Trojanowski J.Q., Liu Z., et al. TFEB enhances astroglial uptake of extracellular tau species and reduces tau spreading. J. Exp. Med. 2018;215:2355–2377. doi: 10.1084/jem.20172158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Narasimhan S., Changolkar L., Riddle D.M., Kats A., Stieber A., Weitzman S.A., Zhang B., Li Z., Roberson E.D., Trojanowski J.Q., et al. Human tau pathology transmits glial tau aggregates in the absence of neuronal tau. J. Exp. Med. 2020;217:e20190783. doi: 10.1084/jem.20190783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Behrendt A., Bichmann M., Ercan-Herbst E., Haberkant P., Schöndorf D.C., Wolf M., Fahim S.A., Murolo E., Ehrnhoefer D.E. Asparagine endopeptidase cleaves tau at N167 after uptake into microglia. Neurobiol. Dis. 2019;130:104518. doi: 10.1016/j.nbd.2019.104518. [DOI] [PubMed] [Google Scholar]
- 129.Metschnikoff E. Lecture on Phagocytosis and Immunity. Br. Med. J. 1891;1:213–217. doi: 10.1136/bmj.1.1570.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Achúcarro N. Cellules allongées et Stäbchenzellen: Cellules neurogliques et cellules granulo-adipeuses à la corne dámmon du lapin. Trab. Lab. Investig. Biológica la Univ. Complut. Madrid. 1909;4:2–15. [Google Scholar]
- 131.Ramón y Cajal S. Contribución al conocimiento de la neuroglía del cerebro humano. Trab. Lab. Investig. Biológica Univ. Complut. Madrid. 1913;XI:215–315. [Google Scholar]
- 132.Río-Hortega P. El “tercer elemento” de los centros nerviosos. I. La microglía en estado normal. Boletín Soc. Española Biol. 1919;VIII:67–82. [Google Scholar]
- 133.Río-Hortega P. El “tercer elemento” de los centros nerviosos. II. Intervención de la microglía en los procesos patológicos (células en bastoncito y cuerpos gránulo-adiposos) Boletín Soc. Española Biol. 1919;VIII:91–103. [Google Scholar]
- 134.Río-Hortega P. El “tercer elemento” de los centros nerviosos. III. Naturaleza probable de la microglía. Boletín Soc. Española Biol. 1919;VIII:108–121. [Google Scholar]
- 135.Río-Hortega P. El “tercer elemento” de los centros nerviosos. IV. Poder fagocitario y movilidad de la microglía. Boletín Soc. Española Biol. 1919;VIII:154–171. [Google Scholar]
- 136.Tremblay M.-È., Lecours C., Samson L., Sánchez-Zafra V., Sierra A. From the Cajal alumni Achúcarro and Río-Hortega to the rediscovery of never-resting microglia. Front. Neuroanat. 2015;9:1–10. doi: 10.3389/fnana.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sierra A., Paolicelli R.C., Kettenmann H. Cien Años de Microglía: Milestones in a Century of Microglial Research. Trends Neurosci. 2019;42:778–792. doi: 10.1016/j.tins.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 138.Alliot F., Godin I., Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. Dev. Brain Res. 1999;117:145–152. doi: 10.1016/S0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 139.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ajami B., Bennett J.L., Krieger C., Tetzlaff W., Rossi F.M. V Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 141.Askew K., Li K., Olmos-Alonso A., Garcia-Moreno F., Liang Y., Richardson P., Tipton T., Chapman M.A., Riecken K., Beccari S., et al. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep. 2017;18:391–405. doi: 10.1016/j.celrep.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Réu P., Khosravi A., Bernard S., Mold J.E., Salehpour M., Alkass K., Perl S., Tisdale J., Possnert G., Druid H., et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017;20:779–784. doi: 10.1016/j.celrep.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lawson L.J., Perry V.H., Dri P., Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-W. [DOI] [PubMed] [Google Scholar]
- 144.Grabert K., Michoel T., Karavolos M.H., Clohisey S., Baillie J.K., Stevens M.P., Freeman T.C., Summers K.M., McColl B.W. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Masuda T., Sankowski R., Staszewski O., Böttcher C., Amann L., Scheiwe C., Nessler S., Kunz P., van Loo G., Coenen V.A., et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566:388–392. doi: 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 146.Böttcher C., Schlickeiser S., Sneeboer M.A.M., Kunkel D., Knop A., Paza E., Fidzinski P., Kraus L., Snijders G.J.L., Kahn R.S., et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 2019;22:78–90. doi: 10.1038/s41593-018-0290-2. [DOI] [PubMed] [Google Scholar]
- 147.Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 2017;169:1276–1290. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 148.Colonna M., Brioschi S. Neuroinflammation and neurodegeneration in human brain at single-cell resolution. Nat. Rev. Immunol. 2020;20:81–82. doi: 10.1038/s41577-019-0262-0. [DOI] [PubMed] [Google Scholar]
- 149.Hammond T.R., Dufort C., Dissing-Olesen L., Giera S., Young A., Wysoker A., Walker A.J., Gergits F., Segel M., Nemesh J., et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity. 2019;50:253–271. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Villa A., Gelosa P., Castiglioni L., Cimino M., Rizzi N., Pepe G., Lolli F., Marcello E., Sironi L., Vegeto E., et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018;23:3501–3511. doi: 10.1016/j.celrep.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yanguas-Casás N. Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunol. Neuroinflamm. 2020 doi: 10.20517/2347-8659.2019.31. [DOI] [Google Scholar]
- 152.Kodama L., Guzman E., Etchegaray J.I., Li Y., Sayed F.A., Zhou L., Zhou Y., Zhan L., Le D., Udeochu J.C., et al. Microglial microRNAs mediate sex-specific responses to tau pathology. Nat. Neurosci. 2020;23:167–171. doi: 10.1038/s41593-019-0560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Peri F., Nüsslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 154.Sierra A., Encinas J.M., Deudero J.J.P., Chancey J.H., Enikolopov G., Overstreet-Wadiche L.S., Tsirka S.E., Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Diaz-Aparicio I., Paris I., Sierra-Torre V., Plaza-Zabala A., Rodríguez-Iglesias N., Márquez-Ropero M., Beccari S., Huguet P., Abiega O., Alberdi E., et al. Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. J. Neurosci. 2020;40:1453–1482. doi: 10.1523/JNEUROSCI.0993-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 157.Miyamoto A., Wake H., Ishikawa A.W., Eto K., Shibata K., Murakoshi H., Koizumi S., Moorhouse A.J., Yoshimura Y., Nabekura J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 2016;7:12540. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nguyen P.T., Dorman L.C., Pan S., Vainchtein I.D., Han R.T., Nakao-Inoue H., Taloma S.E., Barron J.J., Molofsky A.B., Kheirbek M.A., et al. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell. 2020;182:388–403. doi: 10.1016/j.cell.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cserép C., Pósfai B., Lénárt N., Fekete R., László Z.I., Lele Z., Orsolits B., Molnár G., Heindl S., Schwarcz A.D., et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020;367:528–537. doi: 10.1126/science.aax6752. [DOI] [PubMed] [Google Scholar]
- 160.Wlodarczyk A., Holtman I.R., Krueger M., Yogev N., Bruttger J., Khorooshi R., Benmamar-Badel A., de Boer-Bergsma J.J., Martin N.A., Karram K., et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 2017;36:3292–3308. doi: 10.15252/embj.201696056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fantin A., Vieira J.M., Gestri G., Denti L., Schwarz Q., Prykhozhij S., Peri F., Wilson S.W., Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davalos D., Grutzendler J., Yang G., Kim J.V., Zuo Y., Jung S., Littman D.R., Dustin M.L., Gan W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 163.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 164.Hickman S.E., Kingery N.D., Ohsumi T.K., Borowsky M.L., Wang L., Means T.K., El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Haynes S.E., Hollopeter G., Yang G., Kurpius D., Dailey M.E., Gan W.-B., Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 166.Madry C., Kyrargyri V., Arancibia-Cárcamo I.L., Jolivet R., Kohsaka S., Bryan R.M., Attwell D. Microglial Ramification, Surveillance, and Interleukin-1β Release Are Regulated by the Two-Pore Domain K+ Channel THIK-1. Neuron. 2018;97:299–312. doi: 10.1016/j.neuron.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Noda M., Nakanishi H., Nabekura J., Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J. Neurosci. 2000;20:251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Alzheimer A. Histologische und histopathologische Arbeiten über Die Grosshirnrinde mit Besonderer Berücksichtigung der pathologischen Anatomie der Geisteskrankheiten. Gustav Fischer; Jena, Germany: 1910. Beiträge zur Kenntnis der pathologischen Neuroglia und ihrer Beziehungen zu den Abbauvorgängen im Nervengewebe; pp. 401–562. [Google Scholar]
- 169.Cras P., Kawai M., Siedlak S., Perry G. Microglia are associated with the extracellular neurofibrillary tangles of Alzheimer disease. Brain Res. 1991;558:312–314. doi: 10.1016/0006-8993(91)90783-R. [DOI] [PubMed] [Google Scholar]
- 170.El Khoury J., Hickman S.E., Thomas C.A., Cao L., Silverstein S.C., Loike J.D. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 171.Yoshiyama Y., Higuchi M., Zhang B., Huang S.-M., Iwata N., Saido T.C., Maeda J., Suhara T., Trojanowski J.Q., Lee V.M.-Y. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 172.Holmes B.B., Furman J.L., Mahan T.E., Yamasaki T.R., Mirbaha H., Eades W.C., Belaygorod L., Cairns N.J., Holtzman D.M., Diamond M.I. Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. USA. 2014;111:E4376–E4385. doi: 10.1073/pnas.1411649111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.van Olst L., Verhaege D., Franssen M., Kamermans A., Roucourt B., Carmans S., Ytebrouck E., van der Pol S.M.A., Wever D., Popovic M., et al. Microglial activation arises after aggregation of phosphorylated-tau in a neuron-specific P301S tauopathy mouse model. Neurobiol. Aging. 2020;89:89–98. doi: 10.1016/j.neurobiolaging.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 174.Ising C., Venegas C., Zhang S., Scheiblich H., Schmidt S.V., Vieira-Saecker A., Schwartz S., Albasset S., McManus R.M., Tejera D., et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575:669–673. doi: 10.1038/s41586-019-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Heneka M.T., Kummer M.P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T.-C., et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Barnes D.E., Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet. Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Luo X.-G., Ding J.-Q., Chen S.-D. Microglia in the aging brain: Relevance to neurodegeneration. Mol. Neurodegener. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sierra A., Gottfried-Blackmore A.C., McEwen B.S., Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 180.Perry V.H., Matyszak M.K., Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- 181.Streit W.J., Sammons N.W., Kuhns A.J., Sparks D.L. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- 182.Han J., Zhu K., Zhang X.-M., Harris R.A. Enforced microglial depletion and repopulation as a promising strategy for the treatment of neurological disorders. Glia. 2019;67:217–231. doi: 10.1002/glia.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Asai H., Ikezu S., Tsunoda S., Medalla M., Luebke J., Haydar T., Wolozin B., Butovsky O., Kügler S., Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mancuso R., Fryatt G., Cleal M., Obst J., Pipi E., Monzón-Sandoval J., Ribe E., Winchester L., Webber C., Nevado A., et al. CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain. 2019;142:3243–3264. doi: 10.1093/brain/awz241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bussian T.J., Aziz A., Meyer C.F., Swenson B.L., van Deursen J.M., Baker D.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sosna J., Philipp S., Albay R., Reyes-Ruiz J.M., Baglietto-Vargas D., LaFerla F.M., Glabe C.G. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol. Neurodegener. 2018;13:11. doi: 10.1186/s13024-018-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Spangenberg E., Severson P.L., Hohsfield L.A., Crapser J., Zhang J., Burton E.A., Zhang Y., Spevak W., Lin J., Phan N.Y., et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 2019;10:3758. doi: 10.1038/s41467-019-11674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., Boland A., Vronskaya M., van der Lee S.J., Amlie-Wolf A., et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Strittmatter W.J., Saunders A.M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G.S., Roses A.D. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gale S.C., Gao L., Mikacenic C., Coyle S.M., Rafaels N., Murray Dudenkov T., Madenspacher J.H., Draper D.W., Ge W., Aloor J.J., et al. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J. Allergy Clin. Immunol. 2014;134:127–134. doi: 10.1016/j.jaci.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mahley R.W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 192.Bales K.R., Verina T., Dodel R.C., Du Y., Altstiel L., Bender M., Hyslop P., Johnstone E.M., Little S.P., Cummins D.J., et al. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat. Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 193.Jiang Q., Lee C.Y.D., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T.M., Collins J.L., et al. ApoE Promotes the Proteolytic Degradation of Aβ. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Castellano J.M., Kim J., Stewart F.R., Jiang H., DeMattos R.B., Patterson B.W., Fagan A.M., Morris J.C., Mawuenyega K.G., Cruchaga C., et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ulrich J.D., Ulland T.K., Mahan T.E., Nyström S., Nilsson K.P., Song W.M., Zhou Y., Reinartz M., Choi S., Jiang H., et al. ApoE facilitates the microglial response to amyloid plaque pathology. J. Exp. Med. 2018;215:1047–1058. doi: 10.1084/jem.20171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Strittmatter W.J., Saunders A.M., Goedert M., Weisgraber K.H., Dong L.M., Jakes R., Huang D.Y., Pericak-Vance M., Schmechel D., Roses A.D. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: Implications for Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1994;91:11183–11186. doi: 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shi Y., Yamada K., Liddelow S.A., Smith S.T., Zhao L., Luo W., Tsai R.M., Spina S., Grinberg L.T., Rojas J.C., et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shi Y., Manis M., Long J., Wang K., Sullivan P.M., Remolina Serrano J., Hoyle R., Holtzman D.M. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J. Exp. Med. 2019;216:2546–2561. doi: 10.1084/jem.20190980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bell R.D., Winkler E.A., Singh I., Sagare A.P., Deane R., Wu Z., Holtzman D.M., Betsholtz C., Armulik A., Sallstrom J., et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Montagne A., Nation D.A., Sagare A.P., Barisano G., Sweeney M.D., Chakhoyan A., Pachicano M., Joe E., Nelson A.R., D’Orazio L.M., et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Merlini M., Rafalski V.A., Rios Coronado P.E., Gill T.M., Ellisman M., Muthukumar G., Subramanian K.S., Ryu J.K., Syme C.A., Davalos D., et al. Fibrinogen Induces Microglia-Mediated Spine Elimination and Cognitive Impairment in an Alzheimer’s Disease Model. Neuron. 2019;101:1099–1108. doi: 10.1016/j.neuron.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Atagi Y., Liu C.-C., Painter M.M., Chen X.-F., Verbeeck C., Zheng H., Li X., Rademakers R., Kang S.S., Xu H., et al. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) J. Biol. Chem. 2015;290:26043–26050. doi: 10.1074/jbc.M115.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bailey C.C., DeVaux L.B., Farzan M. The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E. J. Biol. Chem. 2015;290:26033–26042. doi: 10.1074/jbc.M115.677286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., Beckers L., O’Loughlin E., Xu Y., Fanek Z., et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity. 2017;47:566–581. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A.I., Lah J.J., et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ulland T.K., Colonna M. TREM2—A key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 2018;14:667–675. doi: 10.1038/s41582-018-0072-1. [DOI] [PubMed] [Google Scholar]
- 207.Wang Y., Cella M., Mallinson K., Ulrich J.D., Young K.L., Robinette M.L., Gilfillan S., Krishnan G.M., Sudhakar S., Zinselmeyer B.H., et al. TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang Y., Ulland T.K., Ulrich J.D., Song W., Tzaferis J.A., Hole J.T., Yuan P., Mahan T.E., Shi Y., Gilfillan S., et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 2016;213:667–675. doi: 10.1084/jem.20151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang S., Mustafa M., Yuede C.M., Salazar S.V., Kong P., Long H., Ward M., Siddiqui O., Paul R., Gilfillan S., et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bemiller S.M., McCray T.J., Allan K., Formica S.V., Xu G., Wilson G., Kokiko-Cochran O.N., Crish S.D., Lasagna-Reeves C.A., Ransohoff R.M., et al. TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol. Neurodegener. 2017;12:74. doi: 10.1186/s13024-017-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Leyns C.E.G., Ulrich J.D., Finn M.B., Stewart F.R., Koscal L.J., Remolina Serrano J., Robinson G.O., Anderson E., Colonna M., Holtzman D.M. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. USA. 2017;114:11524–11529. doi: 10.1073/pnas.1710311114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Leyns C.E.G., Gratuze M., Narasimhan S., Jain N., Koscal L.J., Jiang H., Manis M., Colonna M., Lee V.M.Y., Ulrich J.D., et al. TREM2 function impedes tau seeding in neuritic plaques. Nat. Neurosci. 2019;22:1217–1222. doi: 10.1038/s41593-019-0433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gratuze M., Leyns C.E.G., Sauerbeck A.D., St-Pierre M.-K., Xiong M., Kim N., Serrano J.R., Tremblay M.-È., Kummer T.T., Colonna M., et al. Impact of TREM2R47H variant on tau pathology–induced gliosis and neurodegeneration. J. Clin. Investig. 2020;130:4954–4968. doi: 10.1172/JCI138179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Paloneva J., Manninen T., Christman G., Hovanes K., Mandelin J., Adolfsson R., Bianchin M., Bird T., Miranda R., Salmaggi A., et al. Mutations in Two Genes Encoding Different Subunits of a Receptor Signaling Complex Result in an Identical Disease Phenotype. Am. J. Hum. Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Brosseron F., Kolbe C., Santarelli F., Carvalho S., Antonell A., Castro-Gomez S., Tacik P., Namasivayam A.A., Mangone G., Schneider R., et al. Multicenter Alzheimer’s and Parkinson’s disease immune biomarker verification study. Alzheimers. Dement. 2020;16:292–304. doi: 10.1016/j.jalz.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 216.Ewers M., Franzmeier N., Suárez-Calvet M., Morenas-Rodriguez E., Caballero M.A.A., Kleinberger G., Piccio L., Cruchaga C., Deming Y., Dichgans M., et al. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci. Transl. Med. 2019;11:eaav6221. doi: 10.1126/scitranslmed.aav6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ewers M., Biechele G., Suárez-Calvet M., Sacher C., Blume T., Morenas-Rodriguez E., Deming Y., Piccio L., Cruchaga C., Kleinberger G., et al. Higher CSF sTREM2 and microglia activation are associated with slower rates of beta-amyloid accumulation. EMBO Mol. Med. 2020:1–14. doi: 10.15252/emmm.202012308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bertram L., Lange C., Mullin K., Parkinson M., Hsiao M., Hogan M.F., Schjeide B.M.M., Hooli B., DiVito J., Ionita I., et al. Genome-wide Association Analysis Reveals Putative Alzheimer’s Disease Susceptibility Loci in Addition to APOE. Am. J. Hum. Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J.-C., Carrasquillo M.M., Abraham R., Hamshere M.L., Pahwa J.S., Moskvina V., et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Naj A.C., Jun G., Beecham G.W., Wang L.-S., Vardarajan B.N., Buros J., Gallins P.J., Buxbaum J.D., Jarvik G.P., Crane P.K., et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Freeman S., Kelm S., Barber E., Crocker P. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85:2005–2012. doi: 10.1182/blood.V85.8.2005.bloodjournal8582005. [DOI] [PubMed] [Google Scholar]
- 222.Pillai S., Netravali I.A., Cariappa A., Mattoo H. Siglecs and Immune Regulation. Annu. Rev. Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., Hooli B., Choi S.H., Hyman B.T., Tanzi R.E. Alzheimer’s Disease Risk Gene CD33 Inhibits Microglial Uptake of Amyloid Beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Griciuc A., Patel S., Federico A.N., Choi S.H., Innes B.J., Oram M.K., Cereghetti G., McGinty D., Anselmo A., Sadreyev R.I., et al. TREM2 Acts Downstream of CD33 in Modulating Microglial Pathology in Alzheimer’s Disease. Neuron. 2019;103:820–835. doi: 10.1016/j.neuron.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liu F., Zaidi T., Iqbal K., Grundke-Iqbal I., Merkle R.K., Gong C.-X. Role of glycosylation in hyperphosphorylation of tau in Alzheimer’s disease. FEBS Lett. 2002;512:101–106. doi: 10.1016/S0014-5793(02)02228-7. [DOI] [PubMed] [Google Scholar]
- 226.Nagamine S., Yamazaki T., Makioka K., Fujita Y., Ikeda M., Takatama M., Okamoto K., Yokoo H., Ikeda Y. Hypersialylation is a common feature of neurofibrillary tangles and granulovacuolar degenerations in Alzheimer’s disease and tauopathy brains. Neuropathology. 2016;36:333–345. doi: 10.1111/neup.12277. [DOI] [PubMed] [Google Scholar]
- 227.Zlotnik A., Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hatori K., Nagai A., Heisel R., Ryu J.K., Kim S.U. Fractalkine and fractalkine receptors in human neurons and glial cells. J. Neurosci. Res. 2002;69:418–426. doi: 10.1002/jnr.10304. [DOI] [PubMed] [Google Scholar]
- 229.Harrison J.K., Jiang Y., Chen S., Xia Y., Maciejewski D., McNamara R.K., Streit W.J., Salafranca M.N., Adhikari S., Thompson D.A., et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bazan J.F., Bacon K.B., Hardiman G., Wang W., Soo K., Rossi D., Greaves D.R., Zlotnik A., Schall T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 231.Al-Aoukaty A., Rolstad B., Giaid A., Maghazachi A.A. MIP-3alpha, MIP-3beta and fractalkine induce the locomotion and the mobilization of intracellular calcium, and activate the heterotrimeric G proteins in human natural killer cells. Immunology. 1998;95:618–624. doi: 10.1046/j.1365-2567.1998.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chandrasekar B., Mummidi S., Perla R.P., Bysani S., Dulin N.O., Liu F., Melby P.C. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem. J. 2003;373:547–558. doi: 10.1042/bj20030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Paolicelli R.C., Bisht K., Tremblay M.-È. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zujovic V., Benavides J., Vigé X., Carter C., Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. doi: 10.1002/(SICI)1098-1136(20000215)29:4<305::AID-GLIA2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 235.Cardona A.E., Pioro E.P., Sasse M.E., Kostenko V., Cardona S.M., Dijkstra I.M., Huang D., Kidd G., Dombrowski S., Dutta R., et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 236.Lastres-Becker I., Innamorato N.G., Jaworski T., Rábano A., Kügler S., Van Leuven F., Cuadrado A. Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain. 2014;137:78–91. doi: 10.1093/brain/awt323. [DOI] [PubMed] [Google Scholar]
- 237.Bolós M., Llorens-Martín M., Perea J., Jurado-Arjona J., Rábano A., Hernández F., Avila J. Absence of CX3CR1 impairs the internalization of Tau by microglia. Mol. Neurodegener. 2017;12:59. doi: 10.1186/s13024-017-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fuster-Matanzo A., Jurado-Arjona J., Benvegnù S., García E., Martín-Maestro P., Gómez-Sintes R., Hernández F., Ávila J. Glycogen synthase kinase-3β regulates fractalkine production by altering its trafficking from Golgi to plasma membrane: Implications for Alzheimer’s disease. Cell. Mol. Life Sci. 2017;74:1153–1163. doi: 10.1007/s00018-016-2408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lee S., Varvel N.H., Konerth M.E., Xu G., Cardona A.E., Ransohoff R.M., Lamb B.T. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am. J. Pathol. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., Lamb B.T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li Y., Liu L., Barger S.W., Griffin W.S.T. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J. Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Castro-Sánchez S., García-Yagüe Á.J., Kügler S., Lastres-Becker I. CX3CR1-deficient microglia shows impaired signalling of the transcription factor NRF2: Implications in tauopathies. Redox Biol. 2019;22:101118. doi: 10.1016/j.redox.2019.101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gyoneva S., Hosur R., Gosselin D., Zhang B., Ouyang Z., Cotleur A.C., Peterson M., Allaire N., Challa R., Cullen P., et al. Cx3cr1-deficient microglia exhibit a premature aging transcriptome. Life Sci. Alliance. 2019;2:e201900453. doi: 10.26508/lsa.201900453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hickman S.E., Allison E.K., Coleman U., Kingery-Gallagher N.D., El Khoury J. Heterozygous CX3CR1 Deficiency in Microglia Restores Neuronal β-Amyloid Clearance Pathways and Slows Progression of Alzheimer’s Like-Disease in PS1-APP Mice. Front. Immunol. 2019;10:2780. doi: 10.3389/fimmu.2019.02780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nash K.R., Lee D.C., Hunt J.B., Morganti J.M., Selenica M.-L., Moran P., Reid P., Brownlow M., Guang-Yu Yang C., Savalia M., et al. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol. Aging. 2013;34:1540–1548. doi: 10.1016/j.neurobiolaging.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bemiller S.M., Maphis N.M., Formica S.V., Wilson G.N., Miller C.M., Xu G., Kokiko-Cochran O.N., Kim K.-W., Jung S., Cannon J.L., et al. Genetically enhancing the expression of chemokine domain of CX3CL1 fails to prevent tau pathology in mouse models of tauopathy. J. Neuroinflamm. 2018;15:278. doi: 10.1186/s12974-018-1310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fan Q., He W., Gayen M., Benoit M.R., Luo X., Hu X., Yan R. Activated CX3CL1/Smad2 Signals Prevent Neuronal Loss and Alzheimer’s Tau Pathology-Mediated Cognitive Dysfunction. J. Neurosci. 2020;40:1133–1144. doi: 10.1523/JNEUROSCI.1333-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Winter A.N., Subbarayan M.S., Grimmig B., Weesner J.A., Moss L., Peters M., Weeber E., Nash K., Bickford P.C. Two forms of CX3CL1 display differential activity and rescue cognitive deficits in CX3CL1 knockout mice. J. Neuroinflamm. 2020;17:157. doi: 10.1186/s12974-020-01828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bondareff W., Mountjoy C.Q., Roth M., Hauser D.L. Neurofibrillary degeneration and neuronal loss in Alzheimer’s disease. Neurobiol. Aging. 1989;10:709–715. doi: 10.1016/0197-4580(89)90007-9. [DOI] [PubMed] [Google Scholar]
- 250.Morsch R., Simon W., Coleman P.D. Neurons may live for decades with neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 1999;58:188–197. doi: 10.1097/00005072-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 251.Kuchibhotla K.V., Wegmann S., Kopeikina K.J., Hawkes J., Rudinskiy N., Andermann M.L., Spires-Jones T.L., Bacskai B.J., Hyman B.T. Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc. Natl. Acad. Sci. USA. 2014;111:510–514. doi: 10.1073/pnas.1318807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Andorfer C., Acker C.M., Kress Y., Hof P.R., Duff K., Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J. Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Santacruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M., Guimaraes A., DeTure M., Ramsden M., McGowan E., et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sydow A., Van der Jeugd A., Zheng F., Ahmed T., Balschun D., Petrova O., Drexler D., Zhou L., Rune G., Mandelkow E., et al. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J. Neurosci. 2011;31:2511–2525. doi: 10.1523/JNEUROSCI.5245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Van der Jeugd A., Hochgräfe K., Ahmed T., Decker J.M., Sydow A., Hofmann A., Wu D., Messing L., Balschun D., D’Hooge R., et al. Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol. 2012;123:787–805. doi: 10.1007/s00401-012-0987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Busche M.A., Wegmann S., Dujardin S., Commins C., Schiantarelli J., Klickstein N., Kamath T.V., Carlson G.A., Nelken I., Hyman B.T. Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo. Nat. Neurosci. 2019;22:57–64. doi: 10.1038/s41593-018-0289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pickett E.K., Herrmann A.G., McQueen J., Abt K., Dando O., Tulloch J., Jain P., Dunnett S., Sohrabi S., Fjeldstad M.P., et al. Amyloid Beta and Tau Cooperate to Cause Reversible Behavioral and Transcriptional Deficits in a Model of Alzheimer’s Disease. Cell Rep. 2019;29:3592–3604. doi: 10.1016/j.celrep.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dujardin S., Commins C., Lathuiliere A., Beerepoot P., Fernandes A.R., Kamath T.V., De Los Santos M.B., Klickstein N., Corjuc D.L., Corjuc B.T., et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 2020;26:1256–1263. doi: 10.1038/s41591-020-0938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Busche M.A., Hyman B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020 doi: 10.1038/s41593-020-0687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Díaz-Hernández M., Gómez-Ramos A., Rubio A., Gómez-Villafuertes R., Naranjo J.R., Miras-Portugal M.T., Avila J. Tissue-nonspecific alkaline phosphatase promotes the neurotoxicity effect of extracellular tau. J. Biol. Chem. 2010;285:32539–32548. doi: 10.1074/jbc.M110.145003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Vardy E.R.L.C., Kellett K.A.B., Cocklin S.L., Hooper N.M. Alkaline phosphatase is increased in both brain and plasma in Alzheimer’s disease. Neurodegener. Dis. 2012;9:31–37. doi: 10.1159/000329722. [DOI] [PubMed] [Google Scholar]
- 262.Curtis D., Bakaya K., Sharma L., Bandyopadhyay S. Weighted burden analysis of exome-sequenced late-onset Alzheimer’s cases and controls provides further evidence for a role for PSEN1 and suggests involvement of the PI3K/Akt/GSK-3β and WNT signalling pathways. Ann. Hum. Genet. 2020:1–12. doi: 10.1111/ahg.12375. [DOI] [PubMed] [Google Scholar]
- 263.Doens D., Fernández P.L. Microglia receptors and their implications in the response to amyloid β for Alzheimer’s disease pathogenesis. J. Neuroinflamm. 2014;11:48. doi: 10.1186/1742-2094-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gunawardana C.G., Mehrabian M., Wang X., Mueller I., Lubambo I.B., Jonkman J.E.N., Wang H., Schmitt-Ulms G. The Human Tau Interactome: Binding to the Ribonucleoproteome, and Impaired Binding of the Proline-to-Leucine Mutant at Position 301 (P301L) to Chaperones and the Proteasome. Mol. Cell. Proteomics. 2015;14:3000–3014. doi: 10.1074/mcp.M115.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Stefanoska K., Volkerling A., Bertz J., Poljak A., Ke Y.D., Ittner L.M., Ittner A. An N-terminal motif unique to primate tau enables differential protein-protein interactions. J. Biol. Chem. 2018;293:3710–3719. doi: 10.1074/jbc.RA118.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Luck K., Kim D.-K., Lambourne L., Spirohn K., Begg B.E., Bian W., Brignall R., Cafarelli T., Campos-Laborie F.J., Charloteaux B., et al. A reference map of the human binary protein interactome. Nature. 2020 doi: 10.1038/s41586-020-2188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang P., Ye Y. An integrin receptor complex mediates filamentous Tau-induced activation of primary astrocytes. bioRxiv. 2020;.95 doi: 10.1101/2020.05.07.083295. [DOI] [Google Scholar]
- 268.Pooler A.M., Usardi A., Evans C.J., Philpott K.L., Noble W., Hanger D.P. Dynamic association of tau with neuronal membranes is regulated by phosphorylation. Neurobiol. Aging. 2012;33:431–e27. doi: 10.1016/j.neurobiolaging.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 269.Arrasate M., Pérez M., Avila J. Tau dephosphorylation at tau-1 site correlates with its association to cell membrane. Neurochem. Res. 2000;25:43–50. doi: 10.1023/A:1007583214722. [DOI] [PubMed] [Google Scholar]
- 270.Ait-Bouziad N., Chiki A., Limorenko G., Xiao S., Eliezer D., Lashuel H.A. Phosphorylation of the overlooked tyrosine 310 regulates the structure, aggregation, and microtubule- and lipid-binding properties of Tau. J. Biol. Chem. 2020;295 doi: 10.1074/jbc.RA119.012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Momtazmanesh S., Perry G., Rezaei N. Toll-like receptors in Alzheimer’s disease. J. Neuroimmunol. 2020;348:577362. doi: 10.1016/j.jneuroim.2020.577362. [DOI] [PubMed] [Google Scholar]
- 272.Perea J.R., Ávila J., Bolós M. Dephosphorylated rather than hyperphosphorylated Tau triggers a pro-inflammatory profile in microglia through the p38 MAPK pathway. Exp. Neurol. 2018;310:14–21. doi: 10.1016/j.expneurol.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 273.Gjoneska E., Pfenning A.R., Mathys H., Quon G., Kundaje A., Tsai L.-H., Kellis M. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature. 2015;518:365–369. doi: 10.1038/nature14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Adsera C.B., Park Y.P., Meuleman W., Kellis M. Integrative analysis of 10,000 epigenomic maps across 800 samples for regulatory genomics and disease dissection. bioRxiv. 2019:810291. doi: 10.1101/810291. [DOI] [Google Scholar]
- 275.Wendeln A.-C., Degenhardt K., Kaurani L., Gertig M., Ulas T., Jain G., Wagner J., Häsler L.M., Wild K., Skodras A., et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556:332–338. doi: 10.1038/s41586-018-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gate D., Saligrama N., Leventhal O., Yang A.C., Unger M.S., Middeldorp J., Chen K., Lehallier B., Channappa D., De Los Santos M.B., et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577:399–404. doi: 10.1038/s41586-019-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Unger M.S., Li E., Scharnagl L., Poupardin R., Altendorfer B., Mrowetz H., Hutter-Paier B., Weiger T.M., Heneka M.T., Attems J., et al. CD8+ T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain. Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.05.070. [DOI] [PubMed] [Google Scholar]
- 278.Erny D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Minter M.R., Zhang C., Leone V., Ringus D.L., Zhang X., Oyler-Castrillo P., Musch M.W., Liao F., Ward J.F., Holtzman D.M., et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Minter M.R., Hinterleitner R., Meisel M., Zhang C., Leone V., Zhang X., Oyler-Castrillo P., Zhang X., Musch M.W., Shen X., et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1ΔE9 murine model of Alzheimer’s disease. Sci. Rep. 2017;7:10411. doi: 10.1038/s41598-017-11047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Dodiya H.B., Kuntz T., Shaik S.M., Baufeld C., Leibowitz J., Zhang X., Gottel N., Zhang X., Butovsky O., Gilbert J.A., et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 2019;216:1542–1560. doi: 10.1084/jem.20182386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Elmore M.R.P., Hohsfield L.A., Kramár E.A., Soreq L., Lee R.J., Pham S.T., Najafi A.R., Spangenberg E.E., Wood M.A., West B.L., et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell. 2018;17:e12832. doi: 10.1111/acel.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pluvinage J.V., Haney M.S., Smith B.A.H., Sun J., Iram T., Bonanno L., Li L., Lee D.P., Morgens D.W., Yang A.C., et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature. 2019;568:187–192. doi: 10.1038/s41586-019-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]