Abstract
Our aim was to evaluate the effect of dry needling alone as compared to sham needling, no intervention, or other physical interventions applied over trigger points (TrPs) related with neck pain symptoms. Randomized controlled trials including one group receiving dry needling for TrPs associated with neck pain were identified in electronic databases. Outcomes included pain intensity, pain-related disability, pressure pain thresholds, and cervical range of motion. The Cochrane risk of bias tool and the Physiotherapy Evidence Database (PEDro) score were used to assessed risk of bias (RoB) and methodological quality of the trials. The quality of evidence was assessed by using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. Between-groups mean differences (MD) and standardized mean differences (SMD) were calculated (3) Twenty-eight trials were finally included. Dry needling reduced pain immediately after (MD −1.53, 95% CI −2.29 to −0.76) and at short-term (MD −2.31, 95% CI −3.64 to −0.99) when compared with sham/placebo/waiting list/other form of dry needling and, also, at short-term (MD −0.51, 95% CI −0.95 to −0.06) compared with manual therapy. No differences in comparison with other physical therapy interventions were observed. An effect on pain-related disability at the short-term was found when comparing dry needing with sham/placebo/waiting list/other form of dry needling (SMD −0.87, 95% CI −1.60 to −0.14) but not with manual therapy or other interventions. Dry needling was effective for improving pressure pain thresholds immediately after the intervention (MD 55.48 kPa, 95% CI 27.03 to 83.93). No effect on cervical range of motion of dry needling against either comparative group was found. No between-treatment effect was observed in any outcome at mid-term. Low to moderate evidence suggests that dry needling can be effective for improving pain intensity and pain-related disability in individuals with neck pain symptoms associated with TrPs at the short-term. No significant effects on pressure pain sensitivity or cervical range of motion were observed.
Keywords: dry needling, neck pain, cervical spine, systematic review, meta-analysis
1. Introduction
Neck pain is a musculoskeletal condition that often becomes chronic and can result in high levels of disability. The point prevalence is estimated to be 20%, whereas the lifetime prevalence can reach up to 70% in the general population [1]. The Global Burden of Disease Study identified neck pain as the fourth highest condition on number of years lived with disability [2]. Physical therapy is usually the first therapeutic option requested by individuals with neck pain. Several interventions, including cervical manual therapy [3], exercises [4], and education [5], have shown to be effective for the management of neck pain. Clinical practice guidelines for physical therapy management of neck pain recommend manual therapies combined with exercises as the therapeutic strategy for the proper management of these patients [6,7]. Further, clinical practice guidelines do not recommend other treatments, such as dry needling, not because there is evidence against the particular intervention but, rather, there is a lack of studies examining its use.
The etiology of mechanical neck pain is under debate, and it seems to be multifactorial. Some authors proposed that myofascial trigger points (TrPs) can play a role in neck pain development [8]. Simons et al. [8] defined a TrP as “a hypersensitive spot located in a taut band of skeletal muscle which stimulation induces referred pain symptoms and motor phenomena”. There is evidence showing that the referred pain elicited by active TrPs from neck musculature reproduces neck pain symptoms of insidious or traumatic origin [8]. Chiarotto et al. [9] found that TrPs in the upper trapezius is the most common finding in individuals suffering from neck pain.
Among the several approaches proposed for the treatment of TrPs, dry needling has received particular attention in the last decades [8,10]. Dry needling is defined as a “skilled intervention using a thin filiform needle to penetrate the skin that stimulates myofascial TrPs, muscles, and connective tissue for the treatment of musculoskeletal pain disorders” [11].
A few previous reviews have investigated the effectiveness of dry needling for inactivating TrPs associated with neck pain. Cagnie et al. concluded that dry needling can be recommended for upper trapezius muscle TrPs treatment; however, no quantitative analysis was conducted [12]. Liu et al. concluded that TrP dry needling could be recommended for the management of neck/shoulder pain of myofascial origin at short and mid-term follow-ups [13]. This meta-analysis only included pain intensity as the outcome and considered one month as a mid-term follow-up [13]. In addition, a greater number of randomized clinical trials investigating the effectiveness of dry needling in patients with TrPs associated to neck pain symptoms have been published after the Liu et al. meta-analysis [13]. Therefore, an updated quantitative analysis of the available literature comparing the effects of dry needling vs. sham, control, or other interventions could help to further elucidate its effectiveness. The current updated meta-analysis compares the effects of dry needling against sham, control, no intervention, or other physical therapy interventions applied over muscle TrPs associated with neck pain symptoms on pain intensity, pain-related disability, pressure pain sensitivity, and cervical range of motion.
2. Experimental Section
This systematic review and metanalysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14]. The international Open Science Framework Registry link is https://doi.org/10.17605/OSF.IO/P2UWD.
2.1. Systematic Literature Search
An electronic literature search on MEDLINE, CINAHL, PubMed, PEDro, Cochrane Library, SCOPUS, and Web of Science databases was conducted from their inception to 15 July 2020. Searches were restricted to randomized clinical trials, if permitted. The reference lists of the identified papers in database searches were also searched. All search strategy was conducted with the assistance of an experienced librarian.
Population: Adults (older than 18 years) with TrPs in the cervical musculature associated with neck pain symptoms of musculoskeletal origin.
Intervention: Dry needling of muscle or tendon. Acupuncture was excluded.
Comparator: Acceptable comparators were any sham or placebo dry needling, any control group without intervention, or any other type of physiotherapy intervention. Interventions should be applied in isolation (self-stretching was permitted).
Outcomes: Pain intensity OR pain-related disability were considered as the primary outcomes. Secondary outcomes included pressure pain thresholds OR cervical range of motion.
The search strategy for each database is available in Table 1.
Table 1.
Database formulas during the literature search.
| PubMed Search Formula |
| #1 “Dry Needling” (Mesh) OR “Trigger Point Acupuncture” (Title/Abstract) OR “Needling Therapy” (Title/Abstract) OR “Intramuscular Stimulation” (Title/Abstract) |
| #2 “Placebos” (Mesh) OR “Control Groups” (Mesh) OR “Physical Therapy Modalities” (Mesh) |
| OR “Cervical Pain” (Title/Abstract) OR “Mechanical Neck Pain” (Title/Abstract) OR “Myofascial Neck Pain” (Title/Abstract) |
| #4 #1 AND #2 AND #3 |
| CINAHL/Medline (via EBSCO) Search Formula |
| #1 “Dry Needling” OR “Trigger Point Acupuncture” OR “Needling Therapy” OR “Intramuscular Stimulation” |
| #2 “Placebos” OR “Control Groups” OR “Physical Therapy Modalities” |
| #3 “Neck Pain” OR “Non-Specific Neck Pain” OR “Cervicalgia” OR “Cervical Pain” OR “Mechanical Neck Pain” OR “Myofascial Neck Pain” |
| #4 #1 AND #2 AND #3 |
| SCOPUS Search Formula |
| TITLE-ABS-KEY (“Dry Needling” OR “Trigger Point Acupuncture” OR “Needling Therapy” OR “Intramuscular Stimulation”) AND TITLE-ABS-KEY (“Placebos” OR “Control Groups” OR “Physical Therapy Modalities”) AND TITLE-ABS-KEY (“Neck Pain” OR “Non-Specific Neck Pain” OR “Cervicalgia” OR “Cervical Pain” OR “Mechanical Neck Pain” OR “Myofascial Neck Pain”) |
| PEDro Search Formula |
| Abstract & Title: Neck Pain, Myofascial Pain Syndrome |
| Therapy: Dry Needling |
| Method: Clinical trial |
| When Searching: AND |
| WOS Search Formula |
| (“Dry Needling” OR “Trigger Point Acupuncture” OR “Needling Therapy” OR “Intramuscular Stimulation”) AND (“Placebos” OR “Control Groups” OR “Physical Therapy Modalities”) AND (“Neck Pain” OR “Non-Specific Neck Pain” OR “Cervicalgia” OR “Cervical Pain” OR “Mechanical Neck Pain” OR “Myofascial Neck Pain”) |
| Cochrane Library Search Formula |
| #1 Mesh: Dry Needling |
| #2 Mesh: Placebos |
| #3 Mesh: Neck Pain |
| #4 Trigger Point Acupuncture |
| #5 Needling Therapy |
| #6 Intramuscular Stimulation |
| #7 Mesh: Control Groups |
| #8 Mesh: Physical Therapy Modalities |
| #9 Nonspecific Neck Pain |
| #10 Cervicalgia |
| #11 Cervical Pain |
| #12 Mechanical Neck Pain |
| #13 Myofascial Neck Pain |
| #14 #1 OR #4 OR #5 OR #6 |
| #15 #2 OR #7 OR #8 |
| #16 #3 OR #9 OR #10 OR #11 OR #12 OR #13 |
| #17 #14 AND #15 AND #16 |
2.2. Selection Criteria
Randomized clinical trials including at least one group receiving any form of dry needling alone in people with myofascial TrPs associated with neck pain were included in the meta-analysis. Since there is no consensus in the terminology, the diagnoses usually associated with TrPs were considered: mechanical/idiopathic neck pain, myofascial neck pain, myofascial pain syndrome, or whiplash-associated disorders.
The following inclusion criteria were considered: (1) adults older than 18 years old with at least at one active TrP in the cervical muscles associated with neck pain symptoms; (2) one group receiving muscle/tendon dry needling; (3) one comparative group including sham or placebo, a control group without intervention, or other physiotherapy intervention; and (4) neck pain intensity or pain-related disability as one of the primary outcomes of the study. Secondary outcomes included sensitivity to pressure pain (e.g., pressure pain thresholds) or cervical range of motion (e.g., as assessed with a goniometer). Exclusion criteria were: (1) trials including participants with neurological-related pain (e.g., post-stroke pain); (2) postoperative cervical pain; (3) trials not published as a full-text journal article; (4) retrospective designs or pilot studies; or (5) the use of needling interventions different than dry needling, e.g., acupuncture or wet needling (e.g., lidocaine injection).
2.3. Screening, Selection Process, and Data Extraction
Two authors reviewed the articles identified on each database for their inclusion. After removing duplicates, titles and abstracts of the remaining were screened. Finally, a full-text read of the eligible studies was conducted to determine the inclusion of the trial. The inclusion of a trial was done by consensus between both authors. If discrepancy existed, a third author participated in the process to get a consensus.
Data including study design, number of subjects, population, interventions, outcome measures, and follow-ups were extracted independently by 2 authors using a specific extraction form. Data extraction was also conducted by consensus. If disagreement occurred, a third author participated.
2.4. Assessment of Methodological Quality and Risk of Bias
The Cochrane Risk of Bias (RoB) assessment tool [15] and the Physiotherapy Evidence Database (PEDro) scale [16] were used to assess the risk of bias and methodological quality of the trials included in the meta-analysis. Methodological quality and RoB were independently assessed by two authors.
The RoB evaluated the selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias [15]. Each item was classified as low-risk, high-risk, or unclear according to the Cochrane Collaboration’s tool [15]. The PEDro score evaluated the methodological quality of a trial by assessing the random/concealed allocation, between-groups similarity at baseline, participant/ therapist/assessor blinding, dropouts, intention-to-treat analysis, between-groups comparison, point measures, and variability data [16]. A trial was considered of high quality when the PEDro score was ≥6 out of 10 points.
2.5. Level of Evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to evaluate the level of evidence [17]. The level of evidence was classified as high, moderate, low, or very low based on study limitations, indirectness of evidence, unexplained heterogeneity, imprecision of the results, and high probability of publication bias [18]. High-quality evidence was scored when all items were negative, moderate quality was scored when one item included serious risk, low quality if two items showed serious risk or one item showed very serious risk, or very low quality when three or more items had serious risk or two or more had very serious risk. This process was also performed by two authors, with the participation of a third one if disagreement occurred.
2.6. Data Synthesis and Analysis
Data analysis was performed with Review Manager statistical software (RevMan version 5.3). Data synthesis was presented by groups according to comparative groups as sham/control/placebo, manual therapy, or other physical therapy intervention and by follow-up as immediate (less than one week), short (1 to 12 weeks), and mid (12 to 24 weeks)-terms, since long-term (>24 weeks) data was not available. No other subgroup analysis was prespecified a priori.
Data extraction for the data analysis included sample size, means, and standard deviations of the outcomes. When the trial reported standard errors, they were converted to standard deviations. Mean and standard deviation were estimated from graphs when needed. If data were expressed as median and interquartile range, they were converted to mean and standard deviation as needed [19,20].
The between-groups mean difference (MD) with the 95% confidence interval (CI) was calculated for those outcomes assessed with the same instrument, e.g., pain intensity and pressure pain thresholds. Between-groups mean differences were converted to SMD when different instruments were used for the same outcome, e.g., pain-related disability. A random-effects model was used to determine the effect sizes (SMD). An effect size (SMD) of ≥0.8 was considered large, between 0.5 to 0.8 was considered moderate, and between 0.2 to 0.5 was considered small [21]. p-values < 0.05 were considered statistically significant.
Cervical range of motion was pooled for each movement, i.e., flexion, extension, lateral-flexion, and rotation. When the trial calculated the total range of motion or either side separately for lateral-flexion and rotation, the mean was used in the analysis. If different groups received dry needling with different dosages, data were pooled in just one needling group for the meta-analyses. Finally, when two subgroups included the same intervention, e.g., dry needling, the sample size was adjusted by dividing the sample size as the Cochrane textbook recommends for avoiding duplication in the overall effect [22].
The I2 statistic was applied to determine the heterogeneity between the included trials. We used the interpretation of the Cochrane group as follows: 0–40% represented no relevant heterogeneity; 30–60% represented moderate heterogeneity, 50–90% suggested substantial heterogeneity, and 75–100% suggested considerable heterogeneity [22].
The asymmetry was evaluated using funnel plots in those analyses formed by at least five trials for indicating the possible risk of publication of small studies with negative results. Funnel plots of those analyses including more than 10 trials are presented as Supplementary Files.
3. Results
3.1. Study Selection
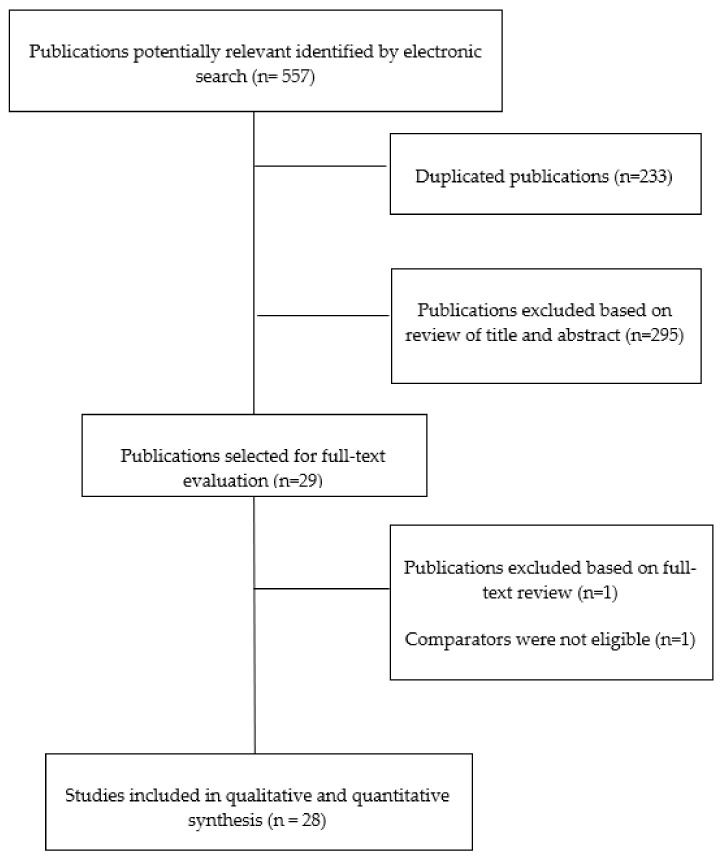
Fifty hundred and fifty-seven (n = 557) studies were initially identified. Three hundred and twenty-four (n = 324) studies remained after removing duplicates. Two hundred and ninety-five (n = 295) were excluded after the analysis of titles and/or abstracts, leaving 29 articles for final full-text review [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. One article [34] was excluded because the comparator was acupuncture intervention and the placebo used laser. Finally, a total of 28 trials [25,26,27,28,29,30,31,32,33,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] were included in the meta-analysis (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
3.2. Study Characteristics
Table 2 summarizes features of the participants on each trial. All studies targeted active TrPs (i.e., those that referred pain reproduced the patient’s symptoms) with the needle; seventeen (61%) targeted upper trapezius TrPs, seven (25%) targeted active TrPs in posterior cervical muscles, and the remaining four (14%) targeted just one muscle, e.g., levator scapulae, lower trapezius, anterior scalene, or sternocleidomastoid. Although all trials included one group receiving TrP dry needling, only 18 (65%) reported the presence of local twitch responses during the needling intervention. Fifty percent (n = 14) of the trials specified that needling intervention was applied by a physical therapist. There was heterogeneity in the comparative group, with seven trials comparing the application of dry needling against sham/control/no intervention, eight against manual therapy, and the remaining thirteen against other physiotherapy interventions ranging from high-power ultrasound to Kinesiotaping (see Table 1). All trials included pain intensity as the primary outcome, whereas twenty (72%) also assessed pain-related disability. Secondary outcomes were assessed in eighteen (pressure pain thresholds) and ten (cervical range of motion) trials. Dry needling interventions are described in Table 3.
Table 2.
Characteristics of the samples in each included trials.
| Study | Diagnosis | Group | Total (Male/Female) | Age (SD), y | Pain Duration |
|---|---|---|---|---|---|
| Ibuldu et al. 2004 [36] | Myofascial Pain Syndrome | G1: DN + Self-Stretching | 20 | 35.3 (9.2) | 38.5 (31.95) m |
| G2: Laser + Self-Stretching | 20 | 33.9 (10.35) | 32.95 (28.6) m | ||
| G3: Placebo laser + Self-Stretching | 20 | 32.35 (6.9) | 36.95 (33.65) m | ||
| Itoh et al. 2007 [33] | Chronic Neck Pain | G1: TrP-DN | 8 | 62.3 (10.1) | 2.9 (2.7) y |
| G2: Non-TrP-DN | 8 | 65.0 (10.5) | 3.3 (3.9) y | ||
| G3: Sham Acupuncture | 7 | 65.0 (10.5) | 2.3 (1.5) y | ||
| G4: Acupuncture | 8 | 62.3 (11.0) | 3.2 (3.2) y | ||
| Myburgh et al. 2012 [27] | Myofascial Pain Syndrome | G1: TrP-DN | 17 | 46.1 | NR |
| G2: TrP-SDN | 20 | 46.1 | NR | ||
| Tekin et al. 2012 [46] | Myofascial Pain Syndrome | G1: TrP-DN | 22 (5/17) | 42.9 (10.9) | 63.5 (50.7) m |
| G2: TrP-Sham DN | 17 (3/14) | 42.0 (12.0) | 57.9 (48.3) m | ||
| Llamas-Ramos et al. 2014 [32] | Mechanical Neck Pain | G1: TrP-DN | 47 (17/30) | 31 (3) | 7.4 (2.6) m |
| G2: TrP-MT | 47 (15/32) | 31 (2) | 7.1 (2.9) m | ||
| Ziaeifar et al. 2014 [35] | Myofascial Pain Syndrome | G1: TrP-DN | 16 | 30.05 (9.9) | NR |
| G2: TrP-MT | 17 | 26.5 (8.6) | NR | ||
| Mejuto-Vázquez et al. 2014 [28] | Acute Mechanical Neck Pain | G1: TrP-DN | 9 (4/5) | 24 (7) | 3.4 (0.7) d |
| G2: No intervention | 8 (4/4) | 25 (4) | 3.1 (0.8) d | ||
| Rayegani et al. 2014 [51] | Myofascial Pain Syndrome | G1: TrP-DN | 14 | 32 (10) 38.6 |
9.6 (8.4) m 9.8 (9.6) m |
| G2: Physical Therapy | 14 | (4.2) | |||
| Campa-Mran et al. 2015 [41] | Myofascial Chronic Neck Pain | G1: TrP-DN + Passive Stretching | 12 (3/9) | 53.9 (12.7) | 10.0 (2.9) m |
| G2: Soft tissue techniques | 12 (2/10) | 45.8 (15.4) | 11.8 (4.4) m | ||
| G3: MT | 12 (2/10) | 48.7 (10.2) | 14.0 (3.6) m | ||
| Pecos-Martín et al. 2015 [25] | Chronic Mechanical Neck Pain | G1: TrP-DN | 36 (6/30) | 23 (5) | 5.7 (2.6) m |
| G2: Non-TrP-DN (Sham) | 36 (8/28) | 23 (6) | 7 (2.8) m | ||
| Aridici et al. 2016 [42] | Myofascial Pain Syndrome | G1: TrP-DN | 31 (5/26) | 40.5 (10.1) | 7.5 (3.0) |
| G2: High power pain threshold ultrasound therapy | 30 (3/27) | 38.1 (11.4) | 7.75 (3.0) | ||
| Segura-Ortí et al. 2016 [50] | Myofascial Pain Syndrome | G1: TrP-DN | 12 (4/8) | 30.0 (9.5) | NR |
| G2: Strain Counter-strain Technique | 10 (3/7) | 34.1 (11.5) | NR | ||
| G3: Sham Strain Counter-strain | 12 (2/10) | 32.9 (9.5) | NR | ||
| Hayta et al. 2016 [37] | Myofascial Pain Syndrome | G1: TrP-DN | 28 (7/21) | NR | NR |
| G2: Kinesiotaping | 27 (3/24) | NR | NR | ||
| Ziaeifar et al. 2016 [23] | Myofascial Pain Syndrome | G1: TrP-DN | 14 (0/14) | 30.1 (10.4) | NR |
| G2: TrP-MT | 17 (0/17) | 26.6 (9.4) | NR | ||
| Fernández-Carnero et al. 2017 [38] | Cervical Myofascial Pain | G1: 4 LTR DN | 21 (7/14) | 29.7 (11.9) | 9.7 (17.0) m |
| G2: 6 LTR DN | 21 (5/16) | 24.25 (9.4) | 16.85 (38.5) m | ||
| G3: +6 LTR DN | 21 (5/16) | 26.45 (10.7) | 19.2 (22.15) m | ||
| G4: Non-TrP DN | 21 (4/17) | 28.2 (11.4) | 8.4 (15.4) m | ||
| De Meulemeester et al. 2017 [40] | Myofascial Neck/Shoulder Syndrome | G1: TrP-DN | 22 | 40.5 (8.3) | 3: <3m; 19: >3m: |
| G2: TrP-MT | 0 | 36.1 (10.7) | 4: <3m; 16: >3m | ||
| Sobhani et al. 2017 [49] | Chronic Mechanical Neck Pain | G1: DN + Passive stretching | 13 | 34.6 (10.5) | 12.6 (4.4) m |
| G2: MT | 13 | 35.9 (11.4) | 15.1 (7.5) m | ||
| G3: Kinesiotaping | 13 | 34.6 (9.1) | 16.1 (7.6) m | ||
| Luan et al. 2019 [31] | Myofascial Pain Syndrome | G1: DN | 32 (11/21) | 33.1 (12.8) | 8.3 (3.1) m |
| G2: Extracorporeal Shock Wave | 30 (8/22) | 32.5 (10.6) | 8.9 (2.7) m | ||
| Dogan et al. 2019 [39] | Myofascial Pain Syndrome | G1: DN | 19 | 32.4 (12.4) | 12 (4–48) m |
| G2: Kinesiotaping | 23 | 33.6 (9.1) | 12 (4–60) m | ||
| Manafnezhad et al. 2019 [30] | Non-Specific Neck Pain | G1: DN | 35 | 39.2 (7.2) | 12 (3–60) m |
| G2: Extracorporeal Shock Wave | 35 | 37 (9.1) | 12 (3–80) m | ||
| Martín-Rodríguez et al. 2019 [29] | Non-Specific Neck Pain | G1: TrP-DN | 17 (6/11) | 43.6 (12.1) | 88.5 (105.1) m |
| G2: Non-TrP- DN | 14 (4/13) | 42.5 (12.3) | 58.9 (48.5) m | ||
| Tabatabaiee et al. 2019 [47] | Myofascial Pain Syndrome | G1: Latent-TrP DN | 20 | 23.6 (1.8) | NR |
| G2: TrP-MT | 20 | 23.5 (1.6) | NR | ||
| G3: Phonophoresis with betamethasone | 20 | 23.9 (3.1) | NR | ||
| Onat et al. 2019 [26] | Neck Pain | G1: TrP-DN + Home Exercise Program | 36 (7/29) | 44.1 (14.2) | NR |
| G2: Kinesiotaping + Home Exercise Program | 36 (10/26) | 45.1 (12.5) | NR | ||
| Ziaeifar et al. 2019 [24] | Myofascial Pain Syndrome | G1: TrP-DN | 16 | 30.05 (9.9) | NR |
| G2: TrP-MT | 17 | 26.5 (8.6) | NR | ||
| Sukareechai et al. 2019 [48] | Myofascial Pain Syndrome | G1: TrP-DN | 21 (0/21) | 42.7 (12.4) | 36 (3, 120) m |
| G2: Radial Shockwave | 21 (2/19) | 38.2 (11.9) | 24 (1, 120) m | ||
| Arias-Buría et al. 2020 [43] | Mechanical Neck Pain | G1: TrP-DN | 15 (10/5) | 21 (3) | 7.5 (1.3) m |
| G2: TrP-MT | 15 (11/4) | 22 (2) | 8.0 (1.1) m | ||
| Valiente-Castrillo et al. 2020 [45] | Chronic Myofascial Neck Pain | G1: TrP-DN | 20 (4/16) | 40.3 (11.95) | 43.4 (56.55) m |
| G2: TrP-DN + pain neuroscience education | 21 (2/19) | 40.35 (8.0) | 64.95 (62.9) m | ||
| G3: Usual Care | 19 (3/16) | 42.35 (9.4) | 56.3 (67.75) m | ||
| García-de-Miguel et al. 2020 [44] | Unilateral Mechanical Neck Pain | G1: TrP-DN | 22 (9/13) | 25.45 (8.5) | >3 m |
| G2: PENS | 22 (7/15) | 24.15 (9.4) | >3 m |
TrP: trigger point, DN: dry needling, SDN: superficial dry needling, G: group, MT: manual therapy, m: months, y: years, d: days, and NR: not reported. PENS: Percutaneous Nerve Electrical Stimulation.
Table 3.
Characteristics of the dry needling intervention of the included studies.
| Study | Group | TrP criteria | Technique Used | No. Punctures for Patient in Every Intervention | Needle Approach (Targeted Muscles or Tendon) | Gauge (mm) | Depth (mm) | Time of DN | Frequency of Incisions (Hz) | Number of Incisions in Every Needle Intervention | LTR | Therapist that Performed Intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ibuldu et al. 2004 [36] | G1: DN | Yes | NR | 1 | Upper trapezius | 0.25 × 25 | NR | NR | NR | NR | NR | Physician |
| Itoh et al. 2007 [33] | G1: DN-Trp | Yes | “sparrow pecking” technique | NR | Splenius capitis, Upper trapezius, sternocleidomastoid, scalenus, levator scapulae, suboccipital |
0.2 × 0.50 mm | 20 mm | 10 min | 1 | The manipulation was stopped when the LTR was elicited | Yes | Acupuncturist |
| G2: Acupuncture | No | “sparrow pecking” technique | 9 | GB20, GB21, BL10, BL11, S12, S13, TE5, LI4, SI3 | 0.2 × 0.40 mm | 20 mm | 10min | 1 | When the subject felt dull pain or the acupuncture sensation (de qi), the manipulation was stopped | No | Acupuncturist | |
| G3: DN-Non-TrP | Yes | “sparrow pecking” technique | NR | Splenius capitis, upper trapezius, sternocleidomastoid, scalenus, levator scapulae, suboccipital | 0.2 × 0.40 mm | 0 mm | 10 min | 1 | The manipulation was stopped when the LTR was elicited | Yes | Acupuncturis | |
| Myburgh et al. 2012 [27] | G1: DN | Yes | Repeated fanning needling insertion | 1 | Upper trapezius | 32 × 0.25 mm | No less than 10 mm | 90 sg | NR | Elicit and exhaust LTR | Yes | Clinician |
| G2: Superficial DN | Yes | The needle inserted into the epidermis until | 1 | Upper trapezius | 32 × 0.25 mm | 5 mm | 90 sg | 1 | 1 | No | Clinician | |
| Tekin et al. 2012 [46] | G1: DN | Yes | Needle moved forward until the TrP was reached | 6 | Neck and shoulder muscles | 0.25 × 0.25 mm | Until muscle | NR | 1 | 1 | No | Physician |
| G2: Sham-DN | Yes | The blunted needle for sham dry needling | 6 | Neck and shoulder muscles | 0.25 × 0.25 mm | Until skin | NR | 1 | 1 | No | Physician | |
| Llamas-Ramos et al. 2014 [32] | G1: DN | Yes | Hong | 1 | Upper trapezius | 0.30 × 30 mm | 10–15 mm | 20–30 sg | 1 | Once the first LTR was obtained, the needle was moved up and down | Yes | Physiotherapist |
| Mejuto-Vázquez et al. 2014 [28] | G1: DN | Yes | Hong | 1 | Upper trapezius | 0.30 × 30 mm | 10–15 mm | 20–30 sg | 1 | Once the first LTR was obtained, the needle was moved up and down | Yes | Physiotherapist |
| Ziaeifar et al. 2014 [35] | G1: DN | Yes | Hong | 1 | Upper trapezius | NR | NR | NR | NR | Was repeatedly needled forward and backward to the TrP until there were no more LTRs | Yes | Physiotherapist |
| Rayegani et al. 2014 [51] | G1: DN | Yes | NR | 2 | Upper trapezius | 23-gauge needle | NR | NR | NR | NR | No | Physician |
| Campa-Moran et al. 2015 [41] | G1: DN | Yes | Hong | 2 | Levator scapulae and upper trapezius muscles |
0.25 × 25 mm | Until muscle | 2 min | At least 3 times at each point | The needle insertions were repeated to achieve at least three LTR |
Yes | Physiotherapist |
| Pecos-Martín et al. 2015 [25] | G1: TrP-DN | Yes | Hong | 1 | Lower trapezius | 0.25 × 25 mm | Until muscle | NR | NR | 8-10 times | No | Physiotherapist |
| G2: Non-TrP-DN | No | Hong | 1 | Lower trapezius | 0.25 × 25 mm | 1.5cm medially from TrP | NR | NR | 8–10 times | No | Physiotherapist | |
| Aridici et al. 2016 [42] | G1: DN | Yes | Hong | 1 | Upper trapezius | 22-gauge needle and 1.5 inch | Until muscle | NR | NR | 8–10 times | Yes | Physician |
| Hayta et al. 2016 [37] | G1: DN | Yes | Manual stimulation was produced (at the TrP) by rotating the needle counterclockwise | 3 | Trapezius | 0.25 × 25 mm | Inside of muscle | 10–20 min | 1 | 1 | No | NR |
| Segura-Ortí et al. 2016 [50] | G1: DN | Yes | Hong | 1 | Upper trapezius | 0.25 × 25 mm | Inside of muscle | NR | NR | Needling at the TrP was continued until the LTR was exhausted | Yes | Physiotherapist |
| Ziaeifar et al. 2016 [23] | G1: DN | Yes | Hong | 1 | Upper trapezius | 0.30 × 50 mm | Inside of muscle | NR | NR | The procedure was repeated until there was no more LTR | Yes | Therapist |
| Fernández-Carnero et al. 2017 [38] | G1: No-LTR-DN | Yes | Hong | 1 | Upper trapezius | 0.32 × 40 mm | Inside of muscle, 1.5 cm away from TrP |
NR | NR | 1 | No | Therapist |
| G2: 4-LTR-DN | Ye | Hong | 1 | Upper trapezius | 0.32 × 40 mm | Inside TrP | NR | NR | 10 times | Yes | Therapist | |
| G3: 6-LTr-DN | Ye | Hong | 1 | Upper trapezius | 0.32 × 40 mm | Inside TrP | NR | NR | 10 times | Yes | Therapist | |
| G4: More-6-LTR-DN | Ye | Hong | 1 | Upper trapezius | 0.32 × 40 mm | Inside TrP | NR | NR | 10 times | Yes | Therapist | |
| Sobhani et al. 2017 [49] | G1: DN | Yes | NR | 2 | Upper trapezius and levator scapulae muscles | NR | NR | NR | 20 min | NR | No | Therapist |
| Dogan et al. 2019 [39] | G1: DN | Yes | Hong and the needles were kept in the TrP for ten minutes, after which they were turned counterclockwise several times | 1 | Upper trapezius | 0.20 × 40 mm | Until TrP | 10 min | NR | At least 3 insertions and 1 LTR | Yes | Physician |
| Luan et al. 2019 [31] | G1: DN | Yes | Hong | 1 | Upper trapezius | 0.30 × 50 mm | 30–35 mm | NR | NR | 10 | Yes | Physiotherapist |
| Manafnezhad et al. 2019 [30] | G1: DN | Yes | Hong | 1 | Upper trapezius | 0.30 × 0.50 mm | Until TrP | 1–2 min | NR | Until at least one or two LTR were obtained | Yes | Physiotherapist |
| Martín-Rodríguez et al. 2019 [29] | G1: DN | Yes | Hong | 1 | Sternocleidomastoid muscle | 0.25 × 0.25 mm | Until TrP | NR | NR | 8–10 | No | Physiotherapist |
| G2: DN | Yes | Hong | 1 | Sternocleidomastoid muscle | 0.25 × 0.25 mm | 1.5cm away the TrP | NR | NR | 8–10 | No | Physiotherapist | |
| Onat et al. 2019 [26] | G1: DN | Yes | Hong | 1 | The posterior muscles of the cervical spine | NR | Until TrP | NR | NR | 6–8 | No | Physician |
| Tabatabaiee et al. 2019 [47] | G1: DN | Yes | Hong | 1 | Upper trapezius | 0.25 × 40 mm | Until TrP | NR | 60 sg | Until a LTR was elicited | Yes | Physiotherapist |
| Ziaeifar et al. 2019 [24] | G1: DN | Yes | Hong | 1 | Upper trapezius | 0.30 × 50 mm | Until TrP | NR | NR | After eliciting LTR, needling was stopped. If no twitch was elicited, needling was stopped after 2-3 stellate movements | Yes | Therapist |
| Sukareechai et al. 2019 [48] | G1: DN | Yes | Multiple needle entry technique | NR | Upper trapezius, rhomboid and infraspinatus muscle | 0.25 × 50 mm | NR | NR | NR | NR | No | NR |
| Arias-Buría et al. 2020 [43] | G1: DN | Yes | Hong | 1 | Anterior scalene muscle | 0.30 × 30 mm | Until TrP | 25–30 sg | 1 | Until the first LTR was obtained | Yes | Physiotherapist |
| García-de-Miguel et al. 2020 [44] | G1: DN | Yes | Hong | 1 | Levator scapulae | 0.25 × 25 mm | Until TrP | NR | NR | 8–10 times | No | Physiotherapist |
| G2: PENS | Yes | Hong and electrostimulation asymmetric current at a 2-Hz with a pulse width of 100 us | 2 | Levator scapulae | 0.25 × 25 mm | Until TrP | 20 min | NR | 8–10 times | No | Physiotherapist | |
| Valiente-Castrillo et al. 2020 [45] | G1: DN | Yes | Hong | 4 | Upper trapezius, levator scapulae, cervical multifidus, and splenius cervicis |
032x40 mm | Until TrP | NR | NR | Until to obtain five LTR | Yes | Physiotherapist |
DN: dry needling, G: group, and LTR: local twitch response.
3.3. Methodological Quality
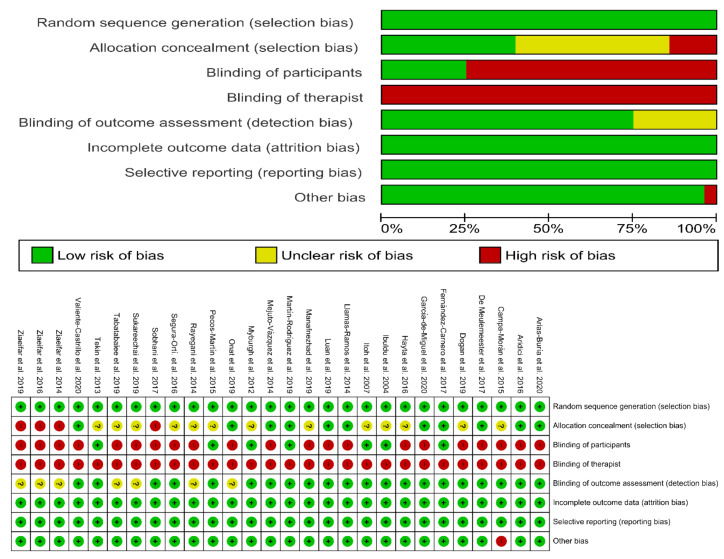
The methodological quality total score ranged from 4 to 8 (mean: 6.6; SD: 1.15) from a total of 10 points. Twenty-three studies were of high methodological quality (≥6 points), and the remaining five were of low methodological quality (<6 points). No trial was able to blind therapists. The most frequent bias was blinding participants, since only seven trials were able to do so. The methodological score of each trial is shown in Table 4.
Table 4.
Methodological quality score (Physiotherapy Evidence Database (PEDro) scale) of randomized clinical trials.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ibuldu et al. 2004 [36] | Y | N | Y | Y | N | Y | Y | N | Y | Y | 7/10 |
| Itoh et al. 2007 [33] | Y | N | Y | Y | N | Y | N | N | Y | Y | 6/10 |
| Myburgh et al. 2012 [27] | Y | N | Y | Y | N | Y | Y | N | Y | Y | 7/10 |
| Tekin et al. 2012 [46] | Y | N | Y | Y | N | Y | Y | N | Y | Y | 7/10 |
| Llamas-Ramos et al. 2014 [32] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Mejuto-Vázquez et al. 2014 [28] | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 |
| Ziaeifar et al. 2014 [35] | Y | N | Y | N | N | N | Y | N | Y | Y | 5/10 |
| Rayegani et al. 2014 [51] | Y | N | Y | N | N | N | N | N | Y | Y | 4/10 |
| Campa-Moran et al. 2015 [41] | Y | N | N | N | N | Y | Y | Y | Y | Y | 6/10 |
| Pecos-Martín et al. 2015 [25] | Y | N | Y | Y | N | Y | Y | Y | Y | Y | 8/10 |
| Aridici et al. 2016 [42] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Hayta et al. 2016 [37] | Y | N | Y | N | N | Y | Y | N | Y | Y | 6/10 |
| Segura-Ortí et al. 2016 [50] | Y | Y | Y | N | N | Y | N | N | Y | Y | 6/10 |
| Ziaeifar et al. 2016 [23] | Y | N | Y | N | N | N | Y | Y | Y | Y | 6/10 |
| Fernández-Carnero et al. 2017 [38] | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8/10 |
| Sobhani et al. 2017 [49] | Y | N | Y | N | N | Y | N | N | Y | Y | 5/10 |
| De Meulemeester et al. 2017 [40] | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 |
| Dogan et al. 2019 [39] | Y | N | Y | N | N | Y | Y | N | Y | Y | 6/10 |
| Luan et al. 2019 [31] | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 |
| Manafnezhad et al. 2019 [30] | Y | N | Y | N | N | Y | Y | N | Y | Y | 6/10 |
| Martín-Rodríguez et al. 2019 [29] | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8/10 |
| Onat et al. 2019 [26] | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7/10 |
| Tabatabaiee et al. 2019 [47] | Y | N | Y | N | N | N | Y | N | Y | Y | 5/10 |
| Ziaeifar et al. 2019 [24] | Y | N | Y | N | N | N | Y | N | Y | Y | 5/10 |
| Sukareechai et al. 2019 [48] | Y | N | Y | N | N | N | Y | Y | Y | Y | 6/10 |
| Arias-Buría et al. 2020 [43] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| García-de-Miguel et al. 2020 [44] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Valiente-Castrillo et al. 2020 [45] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
(1) Random Allocation of Participants, (2) Concealed Allocation, (3) Similarity Between Groups at Baseline, (4) Participant Blinding, (5) Therapist Blinding, (6) Assessor Blinding, (7) Fewer than 15% Dropouts, (8) Intention-to-Treat Analysis, (9) Between-Group Statistical Comparisons, and (10) Point Measures and Variability Data.
3.4. Risk of Bias
Risk of bias assessment of the included trials is summarized in Figure 2. No trial was able to blind therapists, and twenty trials had high risks of bias for blinding participants. In general, the risk of bias of the included trials in the current meta-analysis was low.
Figure 2.
Plot of the risk of bias of the included studies.
3.5. Dry Needling and Neck Pain Intensity
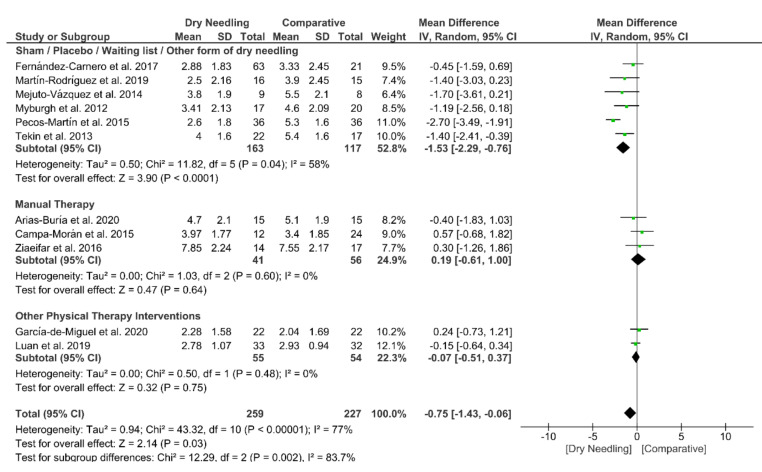
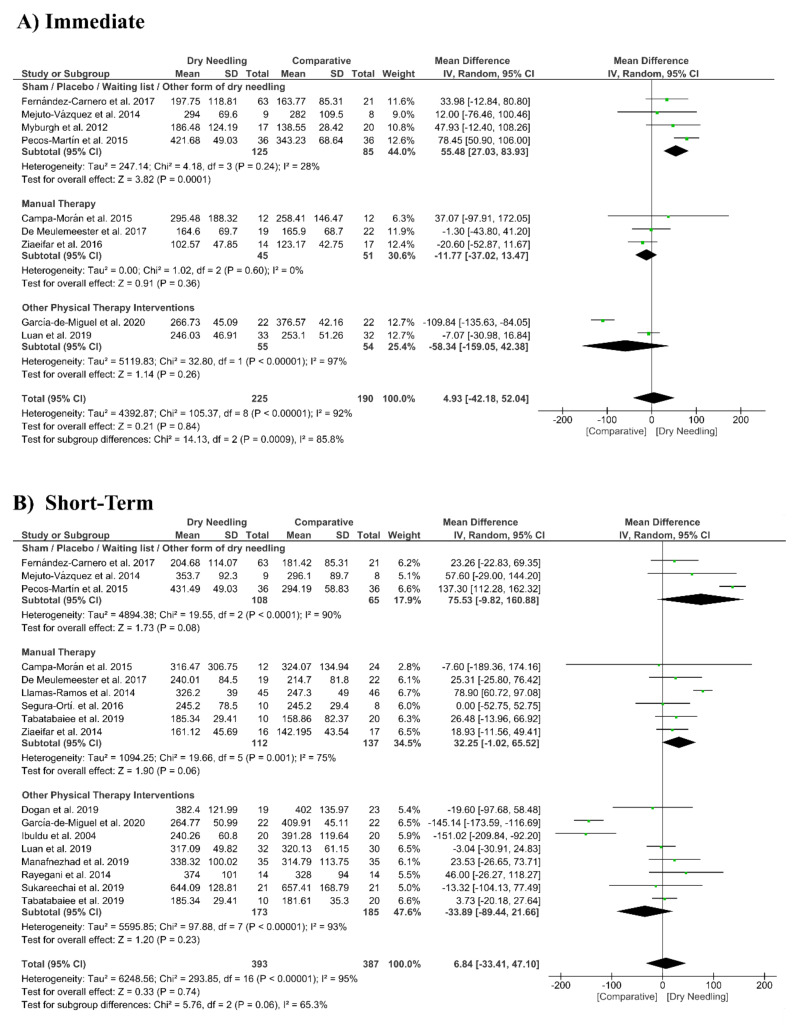
Dry needling exhibited a small overall significant effect (MD −0.75, 95% CI −1.43 to −0.06; p = 0.03 Z = 2.14, N = 486, n = 11 trials) for reducing neck pain immediately after the intervention vs. a comparison group but with substantial heterogeneity (I2 = 77%) between the trials (Figure 3). A significant effect (MD −1.53, 95% CI −2.29 to −0.76, p < 0.001) was found for the grouping analysis (p = 0.002) being significant comparing dry needling vs. sham/placebo/waiting list/other forms of dry needling (MD −1.53, 95% CI −2.29 to −0.76, p = 0.04). The funnel plot did not present potential publication bias (Figure S1).
Figure 3.
Mean differences (MD) comparing the immediate effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on pain intensity.
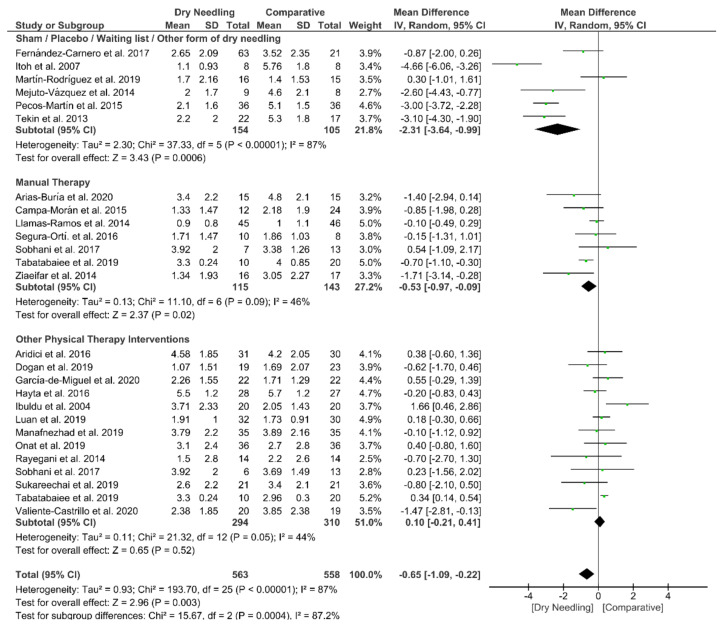
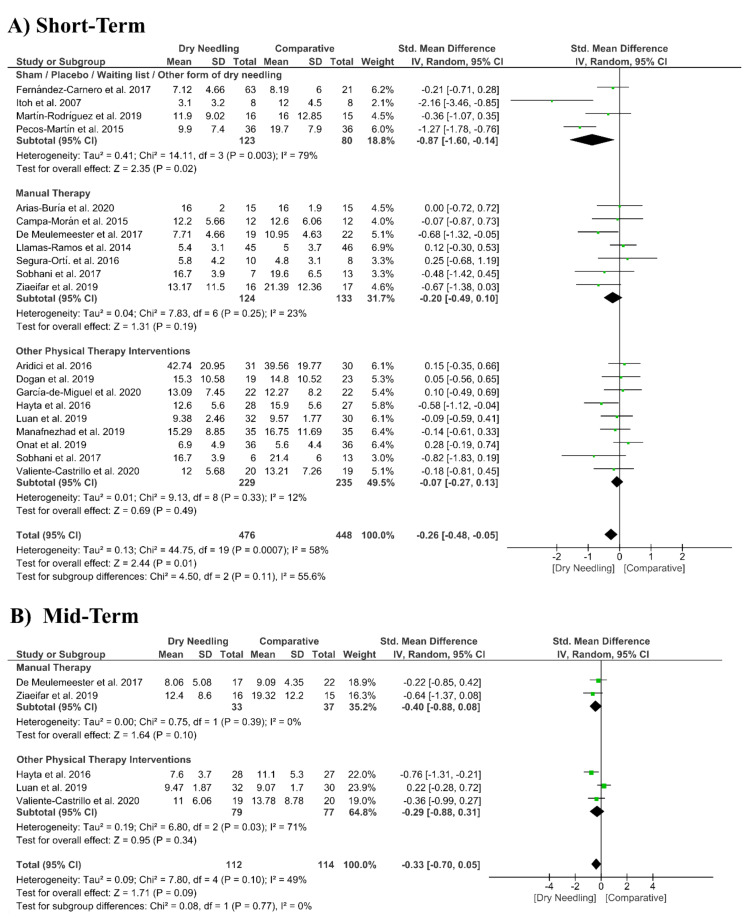
Dry needling also showed a significant overall short-term effect (MD −0.65, 95% CI −1.09 to −0.22; p = 0.003, Z = 2.96, N = 1121, n = 24 trials) for reducing the intensity of neck pain as compared to a comparative group but, also, with considerable heterogeneity (I2 = 87%) between the trials (Figure 4). Significant subgroup differences (p = 0.0004, I2 = 87.2%) were observed when comparing dry needling with sham/placebo/waiting list/other forms of dry needling (MD −2.31, 95% CI −3.64 to −0.99, p < 0.001) and with manual therapy (MD −0.53, 95% CI −0.97 to −0.09, p = 0.02), but not when comparing with other physical therapy interventions (MD 0.10, 95% CI −0.21 to 0.41, p = 0.52). The funnel plot did not present a potential publication bias (Figure S2).
Figure 4.
Mean differences (MD) comparing the short-term effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy. SD: standard deviation; CI: confidence interval.
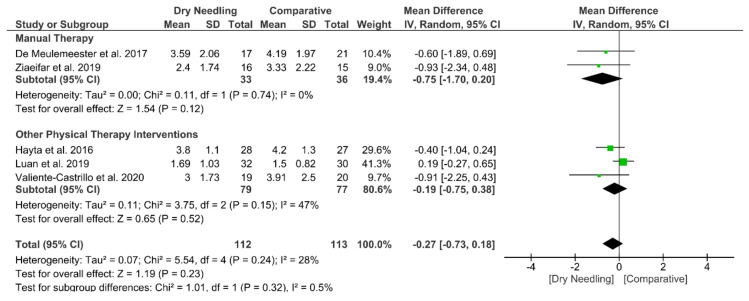
At mid-term, dry needling did not exhibit a significant overall effect (MD −0.27, 95% CI −0.73 to 0.18, p = 0.23, Z = 1.19, N = 225, n = 5 trials) for decreasing neck pain intensity when compared with a comparative group, with no significant heterogeneity (I2 = 28%) between the studies (Figure 5). No significant subgroup differences (p = 0.32, I2 = 0.5%) were observed. Table S1 summarizes the main results of the included studies.
Figure 5.
Mean differences (MD) comparing the mid-term effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy. SD: standard deviation; CI: confidence interval.
3.6. Dry Needling and Pain-Related Disability
Dry needling had a significant overall small effect size (SMD −0.26, 95% CI −0.48 to −0.05, p = 0.001, Z = 2.44, N = 924, n = 20 trials) for improving pain-related disability at the short-term when compared with a comparative group but with moderate heterogeneity (I2 = 58%) among trials (Figure 6A). Significant differences were found when comparing dry needing with sham/placebo/waiting list/other forms of dry needling (SMD −0.87, 95% CI −1.60 to −0.14, p = 0.003) but not when compared with manual therapy (SMD −0.20, 95% CI −0.49 to 0.10, p = 0.19) or other physical therapy interventions (SMD −0.07, 95% CI −0.27 to 0.13, p = 0.49). The funnel plot presented asymmetry and publication bias (Supplementary Figure S3).
Figure 6.
Standardized mean differences (SMD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on pain-related disability at the (A) short- and (B) mid-terms. SD:standard deviation; CI: confidence interval.
At mid-term follow-up, dry needling did not exhibit a significant overall effect (SMD −0.33, 95% CI −0.70 to 0.05, p = 0.09, Z = 1.71, N = 226, n = 5 trials) for reducing pain related-disability as compared to a comparative group, with moderate heterogeneity (I2 = 49%) among the trials (Figure 6B). No significant subgroup differences were found (p = 0.77, I2 = 0%). Table S1 summarizes the main results of the included studies.
3.7. Dry Needling and Pressure Pain Sensitivity (Pressure Pain Thresholds)
Dry needling did not show a significant overall effect immediately after (MD 4.93 kPa, 95% CI −42.18 to 52.04, n = 415, Z = 0.21, p = 0.84, Figure 7A) and at short-term (MD 6.84 kPa, 95% CI −33.41 to 47.10, n = 780, Z = 0.33, p = 0.74, Figure 7B) for increasing the pressure pain thresholds vs. a comparative group. The funnel plot did not present a potential publication bias (Supplementary Figure S4).
Figure 7.
Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the pressure pain thresholds (kPa) (A) immediately after and (B) at the short-term. SD: standard deviation; CI: confidence interval.
The analysis also revealed considerable heterogeneity (I2 > 95%) between the studies. Only the subgroup comparing dry needling with sham/placebo/waiting list/other forms of dry needling had a significant immediate effect (MD 55.48 kPa, 95% CI 27.03 to 83.93, p < 0.001, Figure 7A).
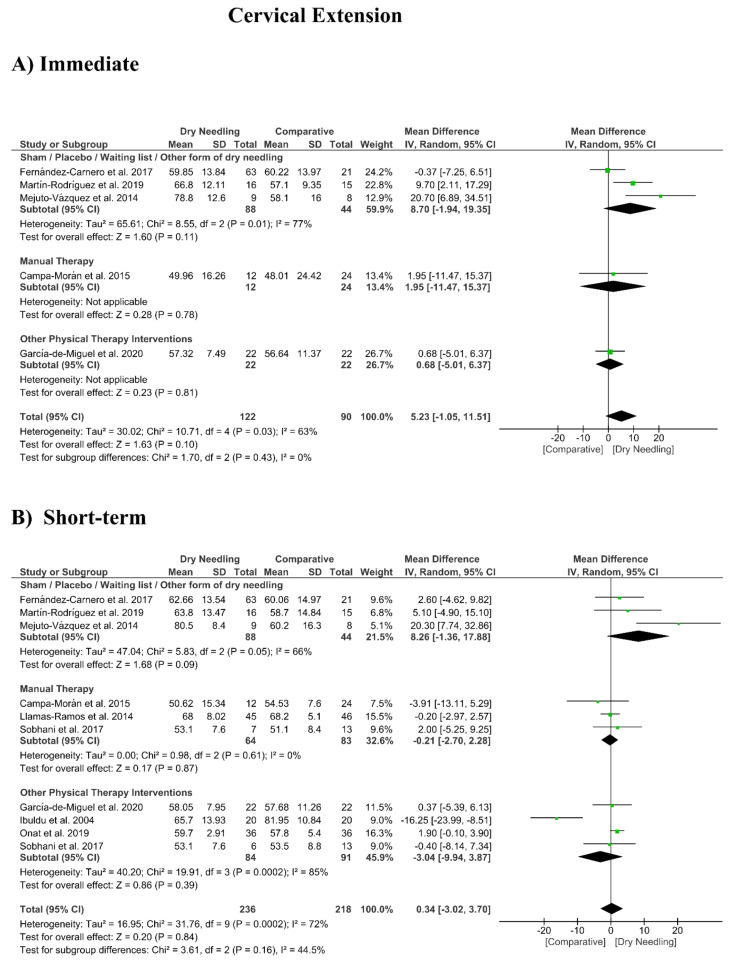
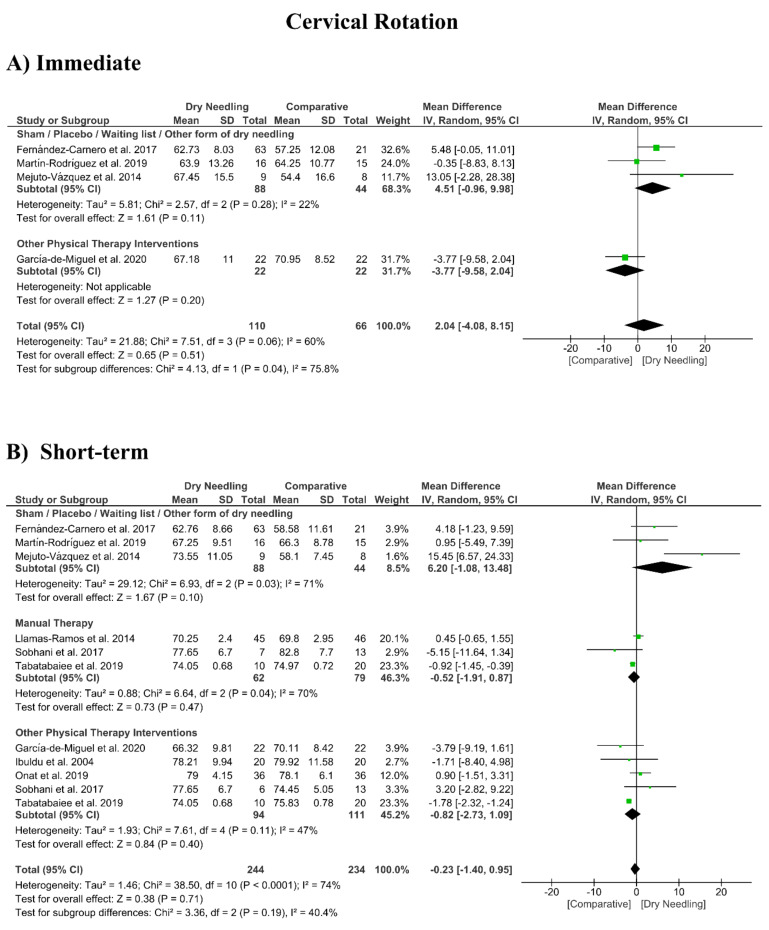
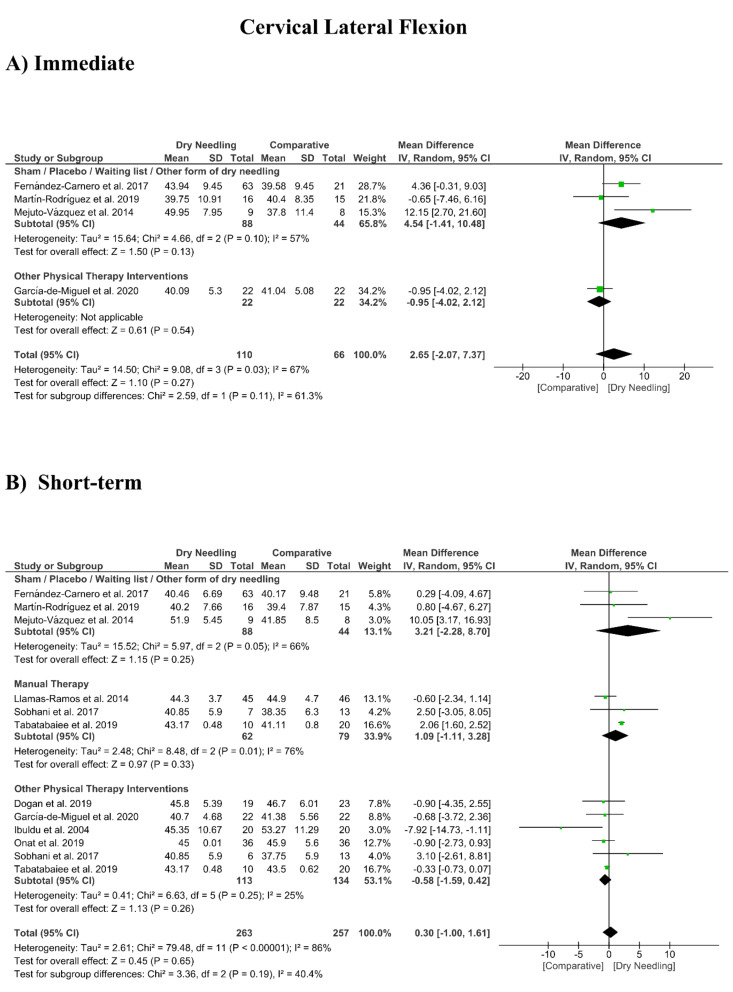
3.8. Dry Needling and Cervical Range of Motion
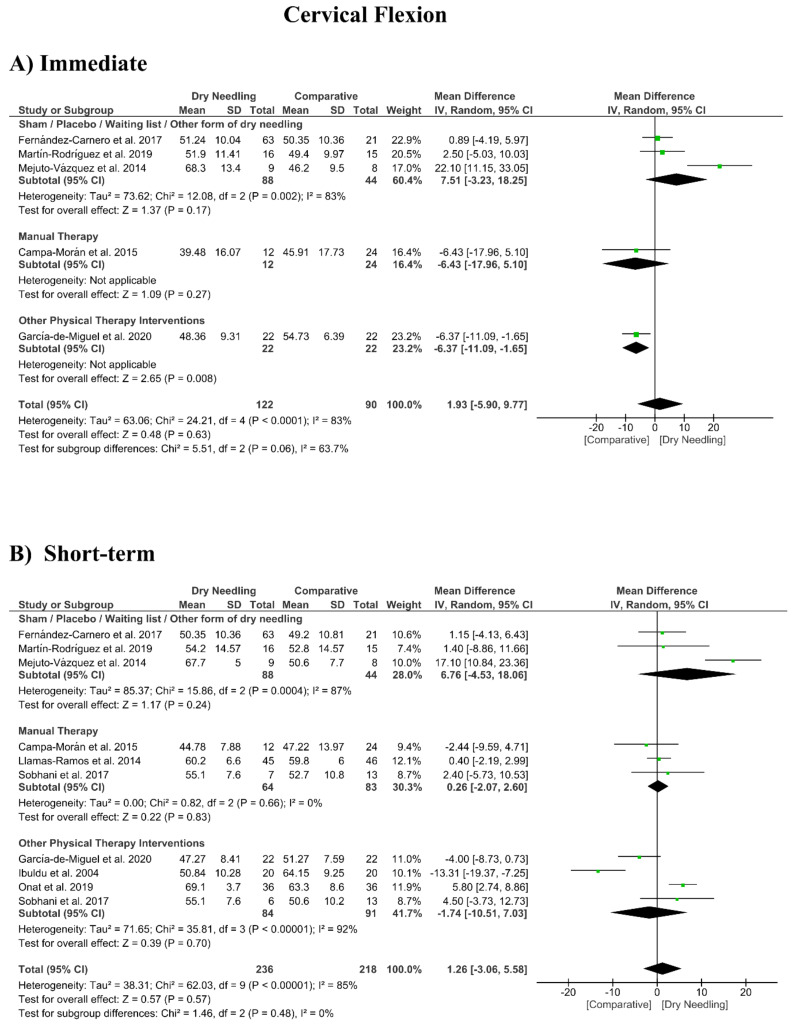
No significant overall effects of dry needling immediately after on the cervical range of motion when compared with a comparison group were observed: flexion (MD 1.93°, 95% CI −5.90° to 9.77°, n = 212, Z = 0.48, p = 0.63, Figure 8A), extension (MD 5.23°, 95% CI −1.05° to 11.51°, n = 212, Z = 1.63, p = 0.10, Figure 9A), rotation (MD 2.04°, 95% CI −4.08° to 8.15°, n = 176, Z = 0.65, p = 0.51, Figure 10A), and lateral-flexion (MD 2.65°, 95% CI −2.07° to 7.37°, n = 176, Z = 1.10, p = 0.27, Figure 11A). Similarly, no significant overall short-term effect of dry needling on cervical flexion (MD 1.26°, 95% CI −3.06° to 5.58°, n = 458, Z = 0.57, p = 0.57, Figure 8B), extension (MD 0.34°, 95% CI −3.02° to 3.70°, n = 454, Z = 0.20, p = 0.84, Figure 9B), rotation (MD −0.23°, 95% CI −1.40° to 0.95°, n = 478, Z = 0.38, p = 0.71, Figure 10B), and lateral-flexion (MD 0.30°, 95% CI −1.00° to 1.61°, n = 520, Z = 0.45, p = 0.65, Figure 11B) was found. All group analyses showed substantial heterogeneity. Table 3 summarizes the main results of the included studies.
Figure 8.
Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the cervical range of motion in flexion (A) immediately after and (B) at the short-term. SD: standard deviation; CI: confidence interval.
Figure 9.
Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the cervical range of motion in extension (A) immediately after and (B) at the short-term. SD: standard deviation; CI: confidence interval.
Figure 10.
Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the cervical range of motion in rotation (A) immediately after and (B) at the short-term. SD: standard deviation; CI: confidence interval.
Figure 11.
Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the cervical range of motion in lateral-flexion (A) immediately after and (B) at the short-term. SD: standard deviation; CI: confidence interval.
3.9. Adverse Events
Fifteen trials (53%, n = 15/28) reported information about adverse effects, with all of them reporting just minor events, and none reported any serious adverse effects [27,28,29,30,31,32,33,38,39,40,41,43,45,50]. Post-needling soreness was the most common adverse event and was reported in 53% (8/15) of the trials [27,28,32,38,40,43,45,48] and resolved spontaneously in 24–48h without further treatment. Thirteen (47%, n = 13/28) of the included studies [23,24,25,30,35,36,42,44,46,47,48,49,51] did not report any information about adverse events (Table 5).
Table 5.
Adverse events described in the included studies.
| Ibuldu et al. 2004 [36] | No data about adverse events were provided. |
| Itoh et al. 2007 [33] | One patient in the sham group was excluded due to deterioration of symptoms. No adverse events were observed during treatment. |
| Myburgh et al. 2012 [27] | Within the DN group, 5 patients (29.4%) perceived post-needling soreness, and 8 patients (47.1%) perceived muscle strength soreness (diffuse muscle fatigue) 48 hours postintervention. Within the sham needling group, 9 patients (45%) experienced post-needling soreness. |
| Tekin et al. 2012 [46] | No data about adverse events were provided. |
| Rayegani et al. 2014 [51] | No data about adverse events were provided. |
| Llamas-Ramos et al. 2014 [32] | Twenty-six patients (55%) assigned to DN group experienced post-needling soreness. Eleven patients assigned to manual therapy group experienced muscle fatigue. All minor adverse events resolved spontaneously within 24-48 h without further treatment. |
| Ziaeifar et al. 2014 [35] | No data about adverse events were provided. |
| Mejuto-Vázquez et al. 2014 [28] | Eighty-eight percent (88%) of patients assigned in the DN group experienced post-needling soreness. This minor adverse event resolved spontaneously within 24-36 h without further treatment. |
| Campa-Moran et al. 2015 [41] | No adverse effect was registered after the needling application. |
| Pecos-Martín et al. 2015 [25] | No data about adverse events were provided. |
| Aridici et al. 2016 [42] | No data about adverse events were provided. |
| Segura-Ortí et al. 2016 [50] | Two subjects assigned to the DN group dropped out due to aversion to needles. No other adverse event was observed. |
| Hayta et al. 2016 [37] | No data about adverse events were provided. |
| Ziaeifar et al. 2016 [23] | No data about adverse events were provided. |
| Sobhani et al. 2017 [49] | No data about adverse events were provided. |
| Fernández-Carnero et al. 2017 [38] | Ninety-one percent (91%) of the patients reported post-needling soreness. No other adverse effects were reported |
| De Meulemeester et al. 2017 [40] | Post-needling soreness. No other adverse effects were reported. |
| Luan et al. 2019 [31] | No adverse effects were observed during the study. |
| Dogan et al. 2019 [39] | No adverse effects were observed during the study. |
| Manafnezhad et al. 2019 [30] | No data about adverse events were provided. |
| Martín-Rodríguez et al. 2019 [29] | Within the non-trigger point DN group, three patients (17.6%) experimented contralateral side pain, 4 patients (23.5%) suffered headache, one patient (5.9%) earache, and one (5.9%) hematoma. Within the trigger point DN group, three patients (17.6%) experimented contralateral side pain and one patient (2.9%) post-needling soreness. |
| Tabatabaiee et al. 2019 [47] | No data about adverse events were provided. |
| Onat et al. 2019 [26] | Three patients (8.3%) in the DN group experienced an increase in neck pain after dry needling, and 2 patients (5.5%) in the Kinesiotaping group showed cutaneous irritation. |
| Ziaeifar et al. 2019 [24] | No data about adverse events were provided. |
| Sukareechai et al. 2019 [48] | Some participants experienced soreness after dry needling therapy. |
| Arias-Buría et al. 2020 [43] | Six patients assigned to the DN experienced post-needling soreness, but it resolved spontaneously. |
| Valiente-Castrillo et al. 2020 [45] | Ninety percent (90%) patients presented post-needling soreness after DN, but it resolved spontaneously. |
| García-de-Miguel et al. 2020 [44] | No data about adverse events were provided. |
DN: Dry Needling.
3.10. Quality of Evidence (GRADE)
Table 6 summarizes the RoB, inconsistency of the results, indirectness of evidence, imprecision of results, and high probability of publication bias for determining the level of evidence according to GRADE assessment. The serious/very serious inconsistency of the results (heterogeneity) and the serious/very serious impression downgraded the evidence level of dry needling to low or moderate.
Table 6.
Level of Evidence (Grading of Recommendations Assessment, Development, and Evaluation (GRADE)) for dry needling on pain intensity, pressure pain sensitivity, and cervical range of motion in patients with neck pain.
| Number of Studies | Risk of Bias | Inconsistency | Indirectness of Evidence | Imprecision | Publication Bias | Quality of Evidence | MD or SMD (95% CI) |
|---|---|---|---|---|---|---|---|
| Dry Needling vs. Sham/Control vs. Physical Therapy Modalities on Neck Pain Intensity | |||||||
| Immediate Follow-Up (less than 1 week after single session) | |||||||
| Overall effect (n = 11) | No | Serious (I2 = 77%) | No | No | No | Moderate | MD −0.75 (−1.43 to −0.06) * |
| Sham/Placebo/Waiting list/Other form of Dry Needling (n = 6) | No | Serious (I2 = 58%) | No | Serious | No | Low | MD −1.53 (−2.29 to −0.76) * |
| Manual Therapy (n = 3) | No | No (I2 = 0%) | No | Very Serious | No | Low | MD 0.19 (−0.61 to 1.00) |
| Other Physical Therapy Intervention (n = 2) | No | No (I2 = 0%) | No | Very Serious | No | Low | MD −0.07 (−0.51 to 0.37) |
| Short-term Follow-Up (1 to 12 weeks after intervention) | |||||||
| Overall effect (n = 24) | No | Very Serious (I2 = 87%) | No | No | No | Low | MD −0.65 (−1.09 to −0.22) * |
| Sham/Placebo/waiting list/Other form of Dry Needling (n = 6) | No | Very Serious (I2 = 87%) | No | No | No | Low | MD −2.31 (−3.64 to −0.99) * |
| Manual Therapy (n = 7) | No | Serious (I2 = 46%) | No | No | No | Moderate | MD −0.53 (−0.97 to −0.09) * |
| Other Physical Therapy Intervention (n = 13) | No | Serious (I2 = 44%) | No | No | No | Moderate | MD 0.10 (−0.21 to 0.41) |
| Mid-term Follow-Up (more than 12 weeks after intervention) | |||||||
| Overall effect (n = 5) | No | No (I2 = 28%) | No | Very Serious | No | Low | MD −0.27 (−0.73 to 0.18) |
| Manual Therapy (v = 2) | No | No (I2 = 0%) | No | Very Serious | No | Low | MD −0.75 (−1.70 to 0.20) |
| Other Physical Therapy Intervention (n = 3) | No | Serious (I2 = 47%) | No | Very Serious | No | Very Low | MD −0.19 (−0.75 to 0.38) |
| Dry Needling vs. Sham/Control vs. Physical Therapy Modalities on Pain-Related Disability | |||||||
| Short-term Follow-Up (1 to 12 weeks after intervention) | |||||||
| Overall effect (n = 20) | No | Serious (I2 = 58%) | No | No | Yes | Low | SMD −0.26 (−0.48 to −0.05) * |
| Sham/Placebo/Waiting list/Other form of Dry Needling (n = 5) | No | Serious (I2 = 79%) | No | No | No | Moderate | SMD −0.87 (−1.60 to −0.14) * |
| Manual Therapy (n = 7) | No | No (I2 = 23%) | No | No | No | High | SMD −0.20 (−0.49 to 0.10) |
| Other Physical Therapy Intervention (n = 9) | No | No (I2 = 12%) | No | No | No | High | SMD −0.07 (−0.27 to 0.13) |
| Mid-term Follow-Up (more than 12 weeks after intervention) | |||||||
| Overall effect (n = 5) | No | Serious (I2 = 48%) | No | Very Serious | No | Very Low | SMD −0.33 (−0.70 to 0.05) |
| Manual Therapy (n = 2) | No | No (I2 = 0%) | No | Very Serious | No | Low | SMD −0.40 (−0.88 to 0.08) |
| Other Physical Therapy Intervention (n = 3) | No | Serious (I2 = 71%) | No | Very Serious | No | Very Low | SMD −0.29 (−0.88 to 0.31) |
| Dry Needling vs. Sham/Control vs. Physical Therapy Modalities on Pressure Pain Thresholds | |||||||
| Immediate Follow-Up (less than 1 week after single session) | |||||||
| Overall effect (n = 9) | No | Very Serious (I2 = 92%) | No | No | No | Low | MD 4.93 (−42.18 to 52.04) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 4) | No | No (I2 = 28%) | No | Serious | No | Moderate | MD 55.48 (27.03 to 83.93) * |
| Manual Therapy (n = 3) | No | No (I2 = 0%) | No | Very Serious | No | Low | MD −11.77 (−37.02 to 13.47) |
| Other Physical Therapy Intervention (n = 2) | No | Very Serious (I2 = 97%) | No | Very Serious | No | Very Low | MD −58.34 (−159.05 to 42.38) |
| Short-term Follow-Up (1 to 12 weeks after intervention) | |||||||
| Overall effect (n = 17) | No | Very Serious (I2 = 95%) | No | No | No | Low | MD 6.84 (−33.41 to 47.12) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 3) | No | Very Serious (I2 = 90%) | No | Very Serious | No | Very Low | MD 75.53 (−9.82 to 160.88) |
| Manual Therapy (n = 6) | No | Serious (I2 = 75%) | No | No | No | Moderate | MD 32.25 (−1.02 to 65.52) |
| Other Physical Therapy Intervention (n = 8) | No | Very Serious (I2 = 93%) | No | No | No | Low | MD −33.89 (−89.44 to 21.66) |
| Number of studies | Risk of bias | Inconsistency | Indirectness of evidence | Imprecision | Publication bias | Quality of evidence | MD or SMD (95% CI) |
| Dry Needling vs. Sham/Control vs. Physical Therapy Modalities on Cervical Range of Motion | |||||||
| Cervical Flexion (Immediate Follow-Up, less than 1 week after single session) | |||||||
| Overall effect (n = 5) | No | Very Serious (I2 = 83%) | No | Very Serious | No | Very Low | MD 1.93 (−5.90, 9.77) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 3) | No | Very Serious (I2 = 83%) | No | Very Serious | No | Very Low | MD 7.51 (−3.23, 18.25) |
| Manual Therapy (n = 1) | No | No | No | Very Serious | No | Low | MD −6.43 (−17.96, 5.10) |
| Other Physical Therapy Intervention (n = 1) | No | No | No | Very Serious | No | Low | MD −6.37 (−11.09, −1.65) |
| Cervical Flexion (Short-term Follow-Up, 1 to 12 weeks after intervention) | |||||||
| Overall effect (n = 10) | No | Very Serious (I2 = 85%) | No | No | No | Low | MD 1.26 (−3.06, 5.58) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 3) | No | Very Serious (I2 = 87%) | No | Very Serious | No | Very Low | MD 6.76 (−4.53, 18.06) |
| Manual Therapy (n = 3) | No | No (I2 = 0%) | No | Very Serious | No | Low | MD 0.26 (−2.07, 2.60) |
| Other Physical Therapy Intervention (n = 4) | No | Very Serious (I2 = 92%) | No | Very Serious | No | Very Low | MD −1.74 (−10.51, 7.03) |
| Cervical Extension (Immediate Follow-Up, less than 1 week after single session) | |||||||
| Overall effect (n = 5) | No | Serious (I2 = 63%) | No | Very Serious | No | Very Low | MD 5.23 (−1.05, 11.51) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 3) | No | Serious (I2 = 77%) | No | Very Serious | No | Very Low | MD 8.70 (−1.94, 19.35) |
| Manual Therapy (n = 1) | No | No | No | Very Serious | No | Low | MD 1.95 (−11.47, 15.37) |
| Other Physical Therapy Intervention (n = 1) | No | No | No | Very Serious | No | Low | MD 0.68 (−5.01, 6.37) |
| Cervical Extension (Short-term Follow-Up, 1 to 12 weeks after intervention) | |||||||
| Overall effect (n = 10) | No | Serious (I2 = 72%) | No | No | Yes | Low | MD 0.34 (−3.02, 3.70) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 3) | No | Serious (I2 = 66%) | No | Very Serious | No | Very Low | MD 8.26 (−1.36, 17.88) |
| Manual Therapy (n = 3) | No | No (I2 = 0%) | No | Very Serious | No | Low | MD −0.21 (−2.70, 2.28) |
| Other Physical Therapy Intervention (n = 4) | No | Very Serious (I2 = 85%) | No | Very Serious | No | Very Low | MD −3.04 (−9.94, 3.87) |
| Cervical Lateral-Flexion (Immediate Follow-Up, less than 1 week after single session) | |||||||
| Overall effect (n = 4) | No | Serious (I2 = 67%) | No | Very Serious | No | Very Low | MD 2.65 (−2.07, 7.37) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 3) | No | Serious (I2 = 57%) | No | Very Serious | No | Very Low | MD 4.54 (−1.41, 10.48) |
| Other Physical Therapy Intervention (n = 1) | No | No | No | Very Serious | No | Low | MD −0.95 (−2.07, 7.37) |
| Cervical Lateral-Flexion (Short-term Follow-Up, 1 to 12 weeks after intervention) | |||||||
| Overall effect (n = 10) | No | Very Serious (I2 = 86%) | No | No | No | Low | MD 0.30 (−1.00, 1.61) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 3) | No | Serious (I2 = 66%) | No | Very Serious | No | Very Low | MD 3.21 (−2.28, 8.70) |
| Manual Therapy (n = 3) | No | Serious (I2 = 77%) | No | Very Serious | No | Very Low | MD 1.09 (−1.11, 3.28) |
| Other Physical Therapy Intervention (n = 6) | No | No (I2 = 25%) | No | No | No | High | MD −0.58 (−1.59, 0.42) |
| Cervical Rotation (Immediate Follow-Up, less than 1 week after single session) | |||||||
| Overall effect (n = 4) | No | Serious (I2 = 60%) | No | Very Serious | No | Very Low | MD 2.04 (−4.08, 8.15) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 3) | No | No (I2 = 22%) | No | Very Serious | No | Low | MD 4.51 (−0.96, 9.98) |
| Other Physical Therapy Intervention (n = 1) | No | No | No | Very Serious | No | Low | MD −3.77 (−9.58, 2.04) |
| Cervical Rotation (Short-term Follow-Up, 1 to 12 weeks after intervention) | |||||||
| Overall effect (n = 9) | No | Serious (I2 = 74%) | No | No | Yes | Low | MD −0.23 (−1.40, 1.09) |
| Sham/Placebo/Waiting list/Other form of dry needling (n = 3) | No | Serious (I2 = 71%) | No | Very Serious | No | Very Low | MD 6.20 (−1.08, 13.48) |
| Manual Therapy (n = 3) | No | Serious (I2 = 70%) | No | Very Serious | No | Very Low | MD −0.52 (−1.91, 0.87) |
| Other Physical Therapy Intervention (n = 5) | No | Serious (I2 = 47%) | No | Very Serious | No | Very Low | MD −0.82 (−2.73, 1.09) |
* Statistically significant (p < 0.05). Risk of bias: No: Most information is from results at a low risk of bias. Serious: Crucial limitation for one criterion, or some limitations for multiple criteria, sufficient to lower confidence in the estimate of the effect. and Very Serious: Crucial limitation for one or more criteria sufficient to substantially lower the confidence in the estimate of the effect. Inconsistency: Serious: I2 > 40% and Very Serious: I2 > 80%. Indirectness of Evidence: No indirectness of evidence was found in any study. Imprecision (based on the sample size): Serious: n < 250 subjects and Very Serious: n < 250 and the estimated effect is little or absent. Publication bias (based on funnel plots): Funnel plots are shown as Supplementary Files in those analyses with more than 10 trials. MD: mean differences and SMD: standardized mean differences.
4. Discussion
4.1. Trigger Point Dry Needling and Neck Pain
This meta-analysis aimed to compare the effects of dry needling alone against any comparative group, e.g., sham, control, no intervention, or other physical therapy interventions applied over TrPs associated with neck pain symptoms. We found moderate-to-low evidence supporting the effectiveness of dry needling for improving pain intensity and related-disability as compared with a comparative group immediately after and at short-, but not at mid-, term follow-ups. The effects were observed when dry needling was compared with sham, placebo, or a waiting list. No significant effect on pressure pain sensitivity or cervical range of motion was found. The RoB of the included trials was relatively low, but the inconsistency (heterogeneity) or the imprecision of the results downgraded the evidence level according to the GRADE.
This is an updated meta-analysis analyzing the effectiveness of the application of dry needling alone on the pain intensity, related-disability, pressure pain sensitivity, and cervical range of motion in patients with myofascial TrPs associated with neck pain symptoms. Liu et al. [13] concluded that dry needling was effective immediately after (SMD −1.91, 95% CI −3.10 to −0.73) and at four weeks (SMD −1.07, 95% CI −1.87 −0.27) when compared with the control or sham. The current updated meta-analysis also observed that dry needling was more effective than sham/placebo/waiting list/other forms of dry needling immediately after (MD −1.53, 95% CI −2.29 to −0.76) and at short-term (MD −2.31 points, 95% CI −3.64 to −0.99).
We also found low-quality evidence supporting a small positive overall effect (SMD −0.26, 95% CI −0.48 to −0.05) of dry needling for improving related disability when compared with a comparison group at the short-term. The effects were only observed comparing dry needling against sham/placebo/waiting list/other forms of dry needling. Based on the current evidence, it seems that the application alone of dry needling targeting active TrP may be effective for the treatment of neck pain (low-to-moderate evidence); however, the effects were mostly observed at the short-term (2–12 weeks after treatment) and vs. sham/placebo/waiting list/other forms of dry needling but not against manual therapy or physical therapy interventions. In fact, the topic of a proper sham needling approach is questioned, since sham needling interventions used in the current literature are highly diverse, limiting the comparability of blinding effectiveness across current studies [52]. It has been supported that sham needling could also have a potential therapeutic effect, probably related to cognitive factors, such as expectative or placebo [52].
It is important to consider if the observed changes on pain intensity were clinically relevant. We reported an overall mean decrease of pain intensity of −0.75 points (95% CI −1.43 to −0.06) immediately after and of −0.65 points, 95% CI −1.09 to −0.22 at the short-term after applying dry needling alone. These between-groups mean differences did not reach the minimal clinically important difference (MCID) of 2.1 points specifically described for patients with mechanical neck pain [53] or the general MCID of 1.4 points determined by Bijur et al. [54]. Nevertheless, comparing dry needling vs. sham/placebo/waiting list, changes observed immediately after (−1.53 points, 95% CI −2.29 to −0.76) and at the short-term (−2.31 points, 95% CI −3.64 to −0.99) were slightly superior to the MCID reported by Bijur et al. [54] and Cleland et al. [53], respectively. Nevertheless, the lower-bound estimate of the confidence intervals did not surpass the MCID.
We did not find significant differences for the application of dry needling or other interventions on the pressure pain sensitivity and cervical range of motion. The results suggest that dry needling has similar effects on these outcomes than manual therapy or other physical therapy interventions, although this conclusion should be considered with caution (very low evidence). Current results would agree with recent theories supporting a common neurophysiological mechanism for manual therapy [55] or needling approaches [56], explaining the hypoalgesic effects and improvements in range of motion observed. In such a scenario, clinicians could choose the application of an intervention according to the individual clinical presentation of each patient based on his/her beliefs, preferences, or expectative.
Although our meta-analysis could be considered an updated version of the Liu et al. [13] paper, several differences can be observed: (1) Liu et al. [13] included trials analyzing wet needling, whereas we included only dry needling; (2) Liu et al. [13] only included pain intensity as the outcome in their quantitative analysis, whereas our study included other outcomes such as related-disability, pressure pain sensitivity, and neck range of motion; (3) Liu et al. [13] considered 9–28 days after the intervention as a mid-term follow-up period, when it is more appropriate to be considered as a short-term; and (4) Liu et al. [13] included trials conducted on post-stroke patients presenting with shoulder pain [57], whereas we included patients with neck pain of musculoskeletal origin associated to TrPs. Therefore, it seems that this meta-analysis represents the most updated information about the effects of dry needling on patients with TrPs associated with neck pain of musculoskeletal origin.
4.2. Adverse Events Associated to Trigger Point Dry Needling
The safety of dry needling is under debate in the current literature due to the presence of potential adverse events. Carlesso et al. [58] defined an adverse event “as a sequela of medium-term duration with any symptom perceived as unacceptable to the patient and requiring further treatment”.
Two previous studies investigating the presence of adverse events after the application of dry needling reported that bleeding (16%), bruising (7.7%), and pain during/after treatment (5.9%) were the most prevalent adverse events [59,60]. All these events were considered as minor [59,60]. Fifty percent of the trials included in our meta-analysis reported the presence of post-needling soreness as the main minor adverse event, supporting that dry needling is a potentially safe intervention. However, major adverse events, e.g., pneumothorax, have been also reported in some cases, although their rate is less than 0.1% (1 per 1024 needling treatments) and depend on the anatomical location. In fact, case reports describing pneumothorax after dry needling have applied the intervention over the thoracic, and not cervical spine, muscles [61,62]. Although dry needling could be considered a safe treatment if properly applied, potential risks associated with its application on each body area where it is applied should be taken into account. In fact, recent studies have proposed different positions [63] or the use of echography [64] for improving the safety of dry needling application.
4.3. Strengths and Limitations
The results of this meta-analysis should be considered according to its potential strengths and limitations. Potential strengths include the comprehensive literature search, rigorous statistical analysis, and the inclusion of randomized controlled trials of high methodological quality. Among the limitations, first, dry needling interventions were highly heterogeneous in the number of sessions, the frequency of application, presence or absence of local twitch responses, or musculature receiving the treatment. In addition, it should be noted that current results come from including all dry needling protocols in the same group, i.e., we compared the application of dry needling for 10 min or 90 s during a single session or different sessions with heterogeneous protocols of manual therapy or other physiotherapy interventions (e.g., 10 sessions over four weeks). Second, the heterogeneity and imprecision of the results of the trials were serious; therefore, the results should be considered with caution at this stage. Nevertheless, this heterogeneity led to the use of a random-effects model rather than the use of a fixed-effects model [65]. Third, the number of trials analyzing mid-term effects was small (n = 3), and no long-term data were available. Therefore, a greater number of high-quality clinical trials investigating mid- and long-term effects of dry needling could lead to different results.
4.4. Clinical and Research Implications
Considering that this is the most updated meta-analysis evaluating the effectiveness of applying dry needling in isolation in patients with neck pain associated to muscle TrPs, several questions need to be elucidated in future trials. First, most studies investigated immediate or short-term effects, with just a small number of studies investigating mid- and long-term follow-ups. Second, trials in this meta-analysis investigated the isolated application of dry needling without any other intervention, which does not represent common clinical practice.
Future high-quality clinical trials examining the long-term effects of the inclusion of dry needling into multimodal physical therapy programs is more effective than not including them. Additionally, since neck pain is characterized by motor control changes, it would be interesting to investigate if the inclusion of dry needling could lead to changes in muscle strength outcomes. In fact, a recent meta-analysis reported medium effect sizes for dry needling to enhance the force production in those with neck pain (moderate evidence), although this analysis was based on just two studies [66].
Finally, it should be noted that only 50% (n = 14) of the trials included in this study specified that the dry needling intervention was applied by a physical therapist. This would be a relevant topic to research, since the clinical reasoning behind the application of needling interventions, e.g., traditional Chinese medicine vs. Western occidental reasoning, may potentially modify the procedure and the outcomes. In fact, the meta-analysis by Gattie et al. [67] investigated the effects of dry needling applied just by physical therapists, although further research is clearly needed.
5. Conclusions
This systematic review and meta-analysis found moderate-to-low evidence suggesting that dry needling can be effective for improving neck pain intensity and related disability when compared with a comparative group immediately after and at short-, but not at mid-, term follow-ups in people with myofascial TrPs associated with neck pain symptoms. The effects were mostly observed when dry needling was compared with sham/placebo/waiting list/other forms of dry needling but not against other physical therapy interventions. No significant effects on the pressure pain sensitivity or cervical range of motion were found. The RoB of the clinical trials included was relatively low, but the inconsistency (heterogeneity) and imprecision of the results downgraded the level of evidence.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/10/3300/s1: Figure S1: Funnel Plot of the trials (n = 11) investigating the immediate effects of dry needling on pain intensity. The funnel plot showed small asymmetry not associated to potential publication bias. Figure S2: Funnel Plot of the trials (n = 11) investigating the immediate effects of dry needling on pain intensity. The funnel plot showed small asymmetry not associated to potential publication bias. Figure S3: Funnel Plot of the trials (n = 20) investigating the short-term effects of dry needling on pain-related disability. The funnel plot showed asymmetry due to the study by Itoh et al 2007 [33], therefore, it was associated to potential publication bias. The exclusion of this study would tend to a symmetric funnel plot. Figure S4: Funnel Plot of the trials (n = 17) investigating the short-term effects of dry needling on pressure pain thresholds. The funnel plot showed small asymmetry not associated to potential publication bias. Table S1: Main results and raw data of the included studies
Author Contributions
Conceptualization: all authors; methodology: M.J.N.-S. and J.S.-I.; software and formal analysis: M.J.N.-S. and C.F.-d.-l.-P.; writing—original draft: J.A.C.; C.F.-d.-l.-P.; P.M.-C., and G.P.-M.; writing—review and editing: all authors; visualization: all authors; and supervision: C.F.-d.-l.-P.; Patricia Martín-Casas, and G.P.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoy D., March L., Woolf A., Blyth F., Brooks P., Smith E. The global burden of neck pain: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1309–1315. doi: 10.1136/annrheumdis-2013-204431. [DOI] [PubMed] [Google Scholar]
- 2.Vos T., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., Abdulkader R.S., Abdulle A.M., Abebo T.A., Abera S.F. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross A., Langevin P., Burnie S.J., Bédard-Brochu M., Empey B., Dugas E., Faber-Dobrescu M., Andres C., Graham N., Goldsmith C.H. Manipulation and mobilisation for neck pain contrasted against an inactive control or another active treatment. Cochrane Database Syst. Rev. 2015;9:CD004249. doi: 10.1002/14651858.CD004249.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross A., Kay T.M., Paquin J., Blanchette S., Lalonde P., Christie T., Dupont G., Graham N., Burnie S.J., Gelley G. Exercises for mechanical neck disorders. Cochrane Database Syst. Rev. 2015;1:CD004250. doi: 10.1002/14651858.CD004250.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross A., Forget M., St George K., Fraser M.M.H., Graham N., Perry L., Burnie S.J., Goldsmith C.H., Haines T., Brunarski D. Patient education for neck pain. Cochrane Database Syst. Rev. 2012;3:CD005106. doi: 10.1002/14651858.CD005106.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bier J.D., Scholten-Peeters W.G.M., Staal J.B., Pool J., van Tulder M.W., Beekman E., Knoop J., Meerhoff G., Verhagen A.P. Clinical practice guideline for physical therapy assessment and treatment in patients with nonspecific neck pain. Phys. Ther. 2018;98:162–171. doi: 10.1093/ptj/pzx118. [DOI] [PubMed] [Google Scholar]
- 7.Blanpied P.R., Gross A.R., Elliott J.M., Devaney L.L., Clewley D., Walton D.M., Sparks C., Robertson E.K., Altman R.D., Beattie P. Neck pain: Revision 2017: Clinical practice guidelines linked to the international classification of functioning, disability and health from the orthopaedic section of the American Physical Therapy Association. J. Orthop. Sport. Phys. Ther. 2017;47:A1–A83. doi: 10.2519/jospt.2017.0302. [DOI] [Google Scholar]
- 8.Simons D.G., Travell J.G. Myofascial Pain and Dysfunction: The Trigger Point Manual. 3rd ed. Wolters Kluwer; Philadelphia, PA, USA: 2019. [Google Scholar]
- 9.Chiarotto A., Clijsen R., Fernandez-De-Las-Penas C., Barbero M. Prevalence of myofascial trigger points in spinal disorders: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2016;97:316–337. doi: 10.1016/j.apmr.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Dommerholt J., Fernandez-de-las Peñas C. Trigger Point Dry Needling: An Evidence and Clinical-Based Approach. 2nd ed. Elsevier; London, UK: 2019. [Google Scholar]
- 11.American Physical Therapy Association (APTA) Description of Dry Needling in Clinical Practice: An EducAtional Resource Paper. APTA; Alexandria, VA, USA: 2013. [Google Scholar]
- 12.Cagnie B., Castelein B., Pollie F., Steelant L., Verhoeyen H., Cools A. Evidence for the use of ischemic compression and dry needling in the management of trigger points of the upper trapezius in patients with neck pain: A systematic review. Am. J. Phys. Med. Rehabil. 2015;94:573–583. doi: 10.1097/PHM.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 13.Liu L., Huang Q.-M., Liu Q.-G., Ye G., Bo C.-Z., Chen M.-J., Li P. Effectiveness of dry needling for myofascial trigger points associated with neck and shoulder pain: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2015;96:944–955. doi: 10.1016/j.apmr.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro Scale for Rating Quality of Randomized. Phys. Ther. 2003;83:713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 17.Schünemann H.J., Oxman A.D., Brozek J., Glasziou P., Bossuyt P., Chang S., Muti P., Jaeschke R., Guyatt G.H. GRADE: Assessing the quality of evidence for diagnostic recommendations. BMJ Evid.-Based Med. 2008;13:162–163. doi: 10.1136/ebm.13.6.162-a. [DOI] [PubMed] [Google Scholar]
- 18.Austin T.M., Richter R.R., Sebelski C.A. Introduction to the GRADE approach for guideline development: Considerations for physical therapist practice. Phys. Ther. 2014;94:1652–1659. doi: 10.2522/ptj.20130627. [DOI] [PubMed] [Google Scholar]
- 19.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:1–13. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan G.M., Feinn R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012;4:279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Churchill R., Chandler J., Cumpston M.S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (Updated June 2017) Cochrane; London, UK: 2017. [(accessed on 1 August 2020)]. Analyzing data and undertaking metanalyses. Available online: www.training.cochrane.org/handbook. [Google Scholar]
- 23.Ziaeifar M., Arab A.M., Nourbakhsh M.R. Clinical effectiveness of dry needling immediately after application on myofascial trigger point in upper trapezius muscle. J. Chiropr. Med. 2016;15:252–258. doi: 10.1016/j.jcm.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziaeifar M., Arab A.M., Mosallanezhad Z., Nourbakhsh M.R. Dry needling versus trigger point compression of the upper trapezius: A randomized clinical trial with two-week and three-month follow-up. J. Man. Manip. Ther. 2019;27:152–161. doi: 10.1080/10669817.2018.1530421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pecos-Martín D., Montañez-Aguilera F.J., Gallego-Izquierdo T., Urraca-Gesto A., Gómez-Conesa A., Romero-Franco N., Plaza-Manzano G. Effectiveness of dry needling on the lower trapezius in patients with mechanical neck pain: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015;96:775–781. doi: 10.1016/j.apmr.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Onat S.S., Polat C.S., Bicer S., Sahin Z., Tasoglu O. Effect of dry needling injection and kinesiotaping on pain and quality of life in patients with mechanical neck pain. Pain Physician. 2019;22:583–589. [PubMed] [Google Scholar]
- 27.Myburgh C., Hartvigsen J., Aagaard P., Holsgaard-Larsen A. Skeletal muscle contractility, self-reported pain and tissue sensitivity in females with neck/shoulder pain and upper Trapezius myofascial trigger points- a randomized intervention study. Chiropr. Man. Ther. 2012;20:36. doi: 10.1186/2045-709X-20-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mejuto-Vázquez M.J., Salom-Moreno J., Ortega-Santiago R., Truyols-Domínguez S., Fernández-De-Las-peñas C. Short- Term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2014;44:252–260. doi: 10.2519/jospt.2014.5108. [DOI] [PubMed] [Google Scholar]
- 29.Martín-Rodríguez A., Sáez-Olmo E., Pecos-Martín D., Calvo-Lobo C. Effects of dry needling in the sternocleidomastoid muscle on cervical motor control in patients with neck pain: A randomised clinical trial. Acupunct. Med. 2019;37:151–163. doi: 10.1177/0964528419843913. [DOI] [PubMed] [Google Scholar]
- 30.Manafnezhad J., Salahzadeh Z., Salimi M., Ghaderi F., Ghojazadeh M. The effects of shock wave and dry needling on active trigger points of upper trapezius muscle in patients with non-specific neck pain: A randomized clinical trial. J. Back Musculoskelet. Rehabil. 2019;32:811–818. doi: 10.3233/BMR-181289. [DOI] [PubMed] [Google Scholar]
- 31.Luan S., Zhu Z.M., Ruan J.L., Lin C.N., Ke S.J., Xin W.J., Liu C.C., Wu S.L., Ma C. Randomized Trial on Comparison of the Efficacy of Extracorporeal Shock Wave Therapy and Dry Needling in Myofascial Trigger Points. Am. J. Phys. Med. Rehabil. 2019;98:677–684. doi: 10.1097/PHM.0000000000001173. [DOI] [PubMed] [Google Scholar]
- 32.Llamas-Ramos R., Pecos-Martín D., Gallego-Izquierdo T., Llamas-Ramos I., Plaza-Manzano G., Ortega-Santiago R., Cleland J., Fernández-de-las-Peñas C. Comparison of the short-term outcomes between trigger point dry needling and trigger point manual therapy for the management of chronic mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2014;44:852–861. doi: 10.2519/jospt.2014.5229. [DOI] [PubMed] [Google Scholar]
- 33.Itoh K., Katsumi Y., Hirota S., Kitakoji H. Randomised trial of trigger point acupuncture compared with other acupuncture for treatment of chronic neck pain. Complement. Ther. Med. 2007;15:172–179. doi: 10.1016/j.ctim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Irnich D., Behrens N., Gleditsch J.M., Stör W., Schreiber M.A., Schöps P., Vickers A.J., Beyer A. Immediate effects of dry needling and acupuncture at distant points in chronic neck pain: Results of a randomized, double-blind, sham-controlled crossover trial. Pain. 2002;99:83–89. doi: 10.1016/S0304-3959(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 35.Ziaeifar M., Arab A.M., Karimi N., Nourbakhsh M.R. The effect of dry needling on pain, pressure pain threshold and disability in patients with a myofascial trigger point in the upper trapezius muscle. J. Bodyw. Mov. Ther. 2014;18:298–305. doi: 10.1016/j.jbmt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Ibuldu E., Cakmak A., Disci R., Aydin R. Comparison of laser, dry needling, and placebo laser treatments in myofascial pain syndrome. Photomed. Laser Surg. 2004;22:306–311. doi: 10.1089/pho.2004.22.306. [DOI] [PubMed] [Google Scholar]
- 37.Hayta E., Umdu N.M. A randomized trial to study the comparison of trigger point dry needling versus Kinesio Taping technique in myofascial pain syndrome during a 3-month follow up. Int. J. Physiother. 2016;3 doi: 10.15621/ijphy/2016/v3i5/117436. [DOI] [Google Scholar]
- 38.Fernández-Carnero J., Gilarranz-De-Frutos L., León-Hernández J.V., Pecos-Martin D., Alguacil-Diego I., Gallego-Izquierdo T., Martín-Pintado-Zugasti A. Effectiveness of different deep dry needling dosages in the treatment of patients with cervical myofascial pain: A pilot RCT. Am. J. Phys. Med. Rehabil. 2017;96:726–733. doi: 10.1097/PHM.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 39.Doǧan N., Şengül I., Akçay-Yalbuzdaǧ Ş., Kaya T. Kinesio taping versus dry needling in the treatment of myofascial pain of the upper trapezius muscle: A randomized, single blind (evaluator), prospective study. J. Back Musculoskelet. Rehabil. 2019;32:819–827. doi: 10.3233/BMR-181162. [DOI] [PubMed] [Google Scholar]
- 40.De Meulemeester K.E., Castelein B., Coppieters I., Barbe T., Cools A., Cagnie B. Comparing Trigger Point Dry Needling and Manual Pressure Technique for the Management of Myofascial Neck/Shoulder Pain: A Randomized Clinical Trial. J. Manipulative Physiol. Ther. 2017;40:11–20. doi: 10.1016/j.jmpt.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Campa-Moran I., Rey-Gudin E., Fernández-Carnero J., Paris-Alemany A., Gil-Martinez A., Lerma Lara S., Prieto-Baquero A., Alonso-Perez J.L., La Touche R. Comparison of Dry Needling versus Orthopedic Manual Therapy in Patients with Myofascial Chronic Neck Pain: A Single-Blind, Randomized Pilot Study. Pain Res. Treat. 2015;2015:1–15. doi: 10.1155/2015/327307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aridici R., Yetisgin A., Boyaci A., Tutoglu A., Bozdogan E., Sen Dokumaci D., Kilicaslan N., Boyaci N. Comparison of the Efficacy of Dry Needling and High-Power Pain Threshold Ultrasound Therapy with Clinical Status and Sonoelastography in Myofascial Pain Syndrome. Am. J. Phys. Med. Rehabil. 2016;95:e149–e158. doi: 10.1097/PHM.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 43.Arias-Buría J.L., Monroy-Acevedo Á., Fernández-de-las-Peñas C., Gallego-Sendarrubias G.M., Ortega-Santiago R., Plaza-Manzano G. Effects of dry needling of active trigger points in the scalene muscles in individuals with mechanical neck pain: A randomized clinical trial. Acupunct. Med. 2020 doi: 10.1177/0964528420912254. [DOI] [PubMed] [Google Scholar]
- 44.García-de-Miguel S., Pecos-Martín D., Larroca-Sanz T., Sanz-de-Vicente B., Garcia-Montes L., Fernandez-Matias R., Gallego-Izquierdo T. Short-term effects of PENS versus dry needling in subjects with unilateral mechanical neck pain and active myofascial trigger points in levator scapulae muscle: A randomized controlled trial. J. Clin. Med. 2020;9:1665. doi: 10.3390/jcm9061665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valiente-Castrillo P., Martín-Pintado-Zugasti A., Calvo-Lobo C., Beltran-Alacreu H., Fernández-Carnero J. Effects of pain neuroscience education and dry needling for the management of patients with chronic myofascial neck pain: A randomized clinical trial. Acupunct. Med. 2020 doi: 10.1177/0964528420920300. [DOI] [PubMed] [Google Scholar]
- 46.Tekin L., Akarsu S., Durmuş O., Çakar E., Dinçer Ü., Kiralp M.Z. The effect of dry needling in the treatment of myofascial pain syndrome: A randomized double-blinded placebo-controlled trial. Clin. Rheumatol. 2013;32:309–315. doi: 10.1007/s10067-012-2112-3. [DOI] [PubMed] [Google Scholar]
- 47.Tabatabaiee A., Ebrahimi-Takamjani I., Ahmadi A., Sarrafzadeh J., Emrani A. Comparison of pressure release, phonophoresis and dry needling in treatment of latent myofascial trigger point of upper trapezius muscle. J. Back Musculoskelet. Rehabil. 2019;32:587–594. doi: 10.3233/BMR-181302. [DOI] [PubMed] [Google Scholar]
- 48.Sukareechai C., Sukareechai S. Comparison of radial shockwave and dry needling therapies in the treatment of myofascial pain syndrome. Int. J. Ther. Rehabil. 2019;26:1–8. doi: 10.12968/ijtr.2016.0072. [DOI] [Google Scholar]
- 49.Sobhani V., Shamsoddini A., Khatibi-aghda A., Mazloum V., Hesari H., Kazem M., Meybodi E. Differences Among Effectiveness of Dry Needling, Manual Therapy, and Kinesio Taping® Methods for the Management of Patients with Chronic Myofascial Neck Pain: A Single-Blind Clinical Trial. Trauma Mon. 2017;22:1–8. [Google Scholar]
- 50.Segura-Ortí E., Prades-Vergara S., Manzaneda-Piña L., Valero-Martínez R., Polo-Traverso J.A. Trigger point dry needling versus strain-counterstrain technique for upper trapezius myofascial trigger points: A randomised controlled trial. Acupunct. Med. 2016;34:171–177. doi: 10.1136/acupmed-2015-010868. [DOI] [PubMed] [Google Scholar]
- 51.Rayegani S.M., Bayat M., Bahrami M.H., Raeissadat S.A., Kargozar E. Comparison of dry needling and physiotherapy in treatment of myofascial pain syndrome. Clin. Rheumatol. 2014;33:859–864. doi: 10.1007/s10067-013-2448-3. [DOI] [PubMed] [Google Scholar]
- 52.Braithwaite F.A., Walters J.L., Li L.S.K., Moseley G.L., Williams M.T., McEvoy M.P. Blinding Strategies in Dry Needling Trials: Systematic Review and Meta-Analysis. Phys. Ther. 2019;99:1461–1480. doi: 10.1093/ptj/pzz111. [DOI] [PubMed] [Google Scholar]
- 53.Cleland J.A., Childs J.D., Whitman J.M. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch. Phys. Med. Rehabil. 2008;89:69–74. doi: 10.1016/j.apmr.2007.08.126. [DOI] [PubMed] [Google Scholar]
- 54.Bijur P.E., Latimer C.T., Gallagher E.J. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad. Emerg. Med. 2003;10:390–392. doi: 10.1197/aemj.10.4.390. [DOI] [PubMed] [Google Scholar]
- 55.Bialosky J.E., Beneciuk J.M., Bishop M.D., Coronado R.A., Penza C.W., Simon C.B., George S.Z. Unraveling the Mechanisms of Manual Therapy: Modeling an Approach. J. Orthop. Sport. Phys. Ther. 2017;48:1–31. doi: 10.2519/jospt.2018.7476. [DOI] [PubMed] [Google Scholar]
- 56.Fernández-De-Las-Peñas C., Nijs J. Trigger point dry needling for the treatment of myofascial pain syndrome: Current perspectives within a pain neuroscience paradigm. J. Pain Res. 2019;12:1899–1911. doi: 10.2147/JPR.S154728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiLorenzo L., Traballesi M., Morelli D., Pompa A., Brunelli S., Buzzi M.G., Formisano R. Hemiparetic shoulder pain syndrome treated with deep dry needling during early rehabilitation: A prospective, open-label, randomized investigation. J. Musculoskelet. Pain. 2004;12:25–34. doi: 10.1300/J094v12n02_04. [DOI] [Google Scholar]
- 58.Carlesso L.C., MacDermid J.C., Santaguida L.P. Standardization of adverse event terminology and reporting in orthopaedic physical therapy: Application to the cervical spine. J. Orthop. Sports Phys. Ther. 2010;40:455–463. doi: 10.2519/jospt.2010.3229. [DOI] [PubMed] [Google Scholar]
- 59.Brady S., McEvoy J., Dommerholt J., Doody C. Adverse events following trigger point dry needling: A prospective survey of chartered physiotherapists. J. Man. Manip. Ther. 2014;22:134–140. doi: 10.1179/2042618613Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyce D., Wempe H., Campbell C., Fuehne S., Zylstra E., Smith G., Wingard C., Jones R. Adverse events aossciated with therapeutic dry needling. Int. J. Sports Phys. Ther. 2020;15:103–113. doi: 10.26603/ijspt20200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cummings M., Ross-Marrs R., Gerwin R. Pneumothorax complication of deep dry needling demonstration. Acupunct. Med. 2014;32:517–519. doi: 10.1136/acupmed-2014-010659. [DOI] [PubMed] [Google Scholar]
- 62.Uzar T., Turkmen I., Menekse E.B., Dirican A., Ekaterina P., Ozkaya S. A case with iatrogenic pneumothorax due to deep dry needling. Radiol. Case Rep. 2018;13:1246–1248. doi: 10.1016/j.radcr.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitchell U.H., Johnson A.W., Larson R.E., Seamons C.T. Positional changes in distance to the pleura and in muscle thickness for dry needling. Physiotherapy. 2019;105:362–369. doi: 10.1016/j.physio.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Folli A., Schneebeli A., Ballerini S., Mena F., Soldini E., Fernández-de-las-Peñas C., Barbero M. Enhancing trigger point dry needling safety by ultrasound skin-to-rib measurement: An inter-rater reliability study. J. Clin. Med. 2020;9:1958. doi: 10.3390/jcm9061958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higgins J., Green S. Cochrane Handbook for Systematic Review Interventions. [(accessed on 31 July 2020)];2011 Available online: http://handbook.cochrane.org.
- 66.Mansfield C.J., Vanetten L., Willy R., di Stasi S., Magnussen R., Briggs M. The Effects of Needling Therapies on Muscle Force Production: A Systematic Review and Meta-analysis. J. Orthop. Sports Phys. Ther. 2019;49:154–170. doi: 10.2519/jospt.2019.8270. [DOI] [PubMed] [Google Scholar]
- 67.Gattie E., Cleland J.A., Snodgrass S. The Effectiveness of Trigger Point Dry Needling for Musculoskeletal Conditions by Physical Therapists: A Systematic Review and Meta-analysis. J. Orthop. Sport. Phys. Ther. 2017;47:133–149. doi: 10.2519/jospt.2017.7096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.