Abstract
Background
Recent evidence supports hippocampal avoidance with whole brain radiotherapy (HA-WBRT) as the recommended treatment option in patients with good prognosis and multiple brain metastases as this results in better neurocognitive preservation compared to whole brain radiotherapy. However, there is often poor tumour control with this technique due to the low doses given. Stereotactic Radiosurgery (SRS), a form of focused radiotherapy which is given to patients who have a limited number of brain metastases, delivers a higher radiation dose to the metastases resulting in better target lesion control. With improvements in radiation technology, advanced dose-painting techniques now allow a simultaneous integrated boost (SIB) dose to lesions whilst minimising doses to the hippocampus to potentially improve brain tumour control and preserve cognitive outcomes. This technique is abbreviated to HA-SIB-WBRT or HA-WBRT+SIB.
Methods
We hypothesise that the SIB in HA-SIB-WBRT (experimental arm) will result in better tumour control compared to HA-WBRT (control arm). This may also lead to better intracranial disease control as well as functional and survival outcomes. We aim to conduct a prospective randomised phase II trial in patients who have good performance status, multiple brain metastases (4–25 lesions) and a reasonable life expectancy (> 6 months). These patients will be stratified according to the number of brain metastases and randomised between the 2 arms. We aim for a recruitment of 100 patients from a single centre over a period of 2 years. Our primary endpoint is target lesion control. These patients will be followed up over the following year and data on imaging, toxicity, quality of life, activities of daily living and cognitive measurements will be collected at set time points. The results will then be compared across the 2 arms and analysed.
Discussion
Patients with brain metastases are living longer. Maintaining functional independence and intracranial disease control is thus increasingly important. Improving radiotherapy treatment techniques could provide better control and survival outcomes whilst maintaining quality of life, cognition and functional capacity. This trial will assess the benefits and possible toxicities of giving a SIB to HA-WBRT.
Trial registration
Clinicaltrials.gov identifier: NCT04452084. Date of registration 30th June 2020.
Keywords: Whole brain radiotherapy, Hippocampal-avoidance whole brain radiotherapy, Brain metastases, Study protocol
Introduction
Background
The management of brain metastases presents a significant challenge in oncology. Whole brain radiation therapy (WBRT) is widely given to improve neurological symptoms, stop brain metastasis progression and possibly prolong survival. Unfortunately, neurocognitive functional decline is reported in a significant number of patients who undergo WBRT at rates of 31–57% at 3 months and 48–89% at 1 year [1].
The hippocampus plays a key role in learning, memory and cognitive function [2–4]. The significance of hippocampi radiation in clinical studies is emerging. Gondi et al. showed that a dose of > 7.3Gy to at least 40% of the hippocampus resulted in a significant decline in delayed recall in adult brain cancer patients [5].
A recent phase 3 trial, NRG-CC001, randomised 518 patients to Hippocampal avoidance-WBRT (HA-WBRT) against standard WBRT. Cognitive endpoints were measured using a test battery of Hopkins Verbal Learning Test-Revised (HVLT-R), Controlled Oral Word Association, and Trail Making Test (TMT) Part A & B. At a median follow-up for alive patients of 7.9 months, a significant reduction in cognitive failure risk was noted (HR, 0.76; P = 0.03) in the HA-WBRT arm. This difference was most significant in the 4-month TMT Part B scores and the 6-month HVLT-R score. The median overall survival (OS) and progression free survival (PFS) did not differ and there was no increase in hippocampal relapses. However notably, OS and PFS in the HA-WBRT arm were poor at 6.3 months and 5.0 months respectively [6].
This trial introduced HA-WBRT as a new standard option in patients with multiple brain metastases and good prognosis. However, some clinicians would still favour the use of Stereotactic Radiosurgery (SRS) over HA-WBRT in patients with low volume brain metastases. The reasons could possibly be better cognitive sparing or tumoral control due to the higher ablative doses used in SRS. Several trials are underway comparing the use of HA-WBRT against SRS (ClinicalTrials.gov identifier: NCT03550391, NCT03075072 and NCT04277403).
Rationale
In the landmark RTOG 95–08 trial which compared an SRS boost vs. no boost in patients undergoing WBRT, the patients in the boost arm had improved local control rates, better functional autonomy and reduced steroid need with few toxicities [7]. OS was also improved in some patients [8, 9]. With current dose-painting radiotherapy planning techniques, we are now able to deliver a simultaneous integrated boost (SIB) dose to the lesions while giving HA-WBRT, a technique abbreviated as HA-SIB-WBRT or HA-WBRT+SIB.
Several single-arm prospective studies looked at the use of WBRT+SIB or HA-SIB-WBRT in variable patient cohorts with SIB doses of 40–52.5Gy in 10–15 fractions [10–15]. The reported intracranial PFS was noted to be as high as 13.5 months with few ≥Grade 3 toxicities (≤6.5%), making it a potentially viable option. In a propensity score-matched comparison against WBRT, Popp et al. reported significantly improved local tumour control rates, intracranial PFS, reduction in neurological deaths and even better OS [12]. This has led the group to continue to a phase II HIPPORAD Trial comparing HA-WBRT+SIB against WBRT+SIB (German Clinical Trials Registry: DRKS00004598) [16].
Given that brain metastases are being detected earlier and better systemic options have extended the survival in many patients, the need to maintain functional independence and brain disease control is increasingly important. In the management of brain metastases, there is still an unmet need for patients with good prognoses but are unable to receive SRS to have better intracranial disease control over the new standard of HA-WBRT. It is thus important to assess if the addition of a SIB will improve the outcomes of HA-WBRT.
To our knowledge, there is no prospective trial underway comparing HA-SIB-WBRT against HA-WBRT. We thus aim to test if there is a clinical benefit for the additional SIB in HA-WBRT. In preparation for this trial, we performed a planning study to test the feasibility of dosimetric targets set for HA-SIB-WBRT in 5 patients with different tumour numbers, volumes and locations as well as experimented with various treatment planning techniques [17]. From this, we were then confident in our ability to proceed with our study.
We hypothesise that HA-SIB-WBRT will be able to increase both target tumour and intracranial disease control compared with HA-WBRT alone. This, therefore, has the potential to impact on survival outcomes whilst maintaining cognitive function and quality of life.
Objectives
The purpose of the study is to assess the impact that adding SIB to standard of care treatment (HA-WBRT) has on local control, survival outcomes, cognition and other patient reported outcome measures (PROMs).
Study design
This study will be conducted as a single centre prospective randomised phase II trial in patients with multiple brain metastases and good prognosis (> 6 months). The patients will be compared between the 2 arms: HA-WBRT (control) vs. HA-SIB-WBRT (experimental). The target recruitment is 100 patients over 2 years.
Methods: participants, interventions and outcomes
Study setting
The trial will be conducted at the National Cancer Centre Singapore, which is the largest cancer centre in the country. The study workflow is depicted in Fig. 1.
Fig. 1.
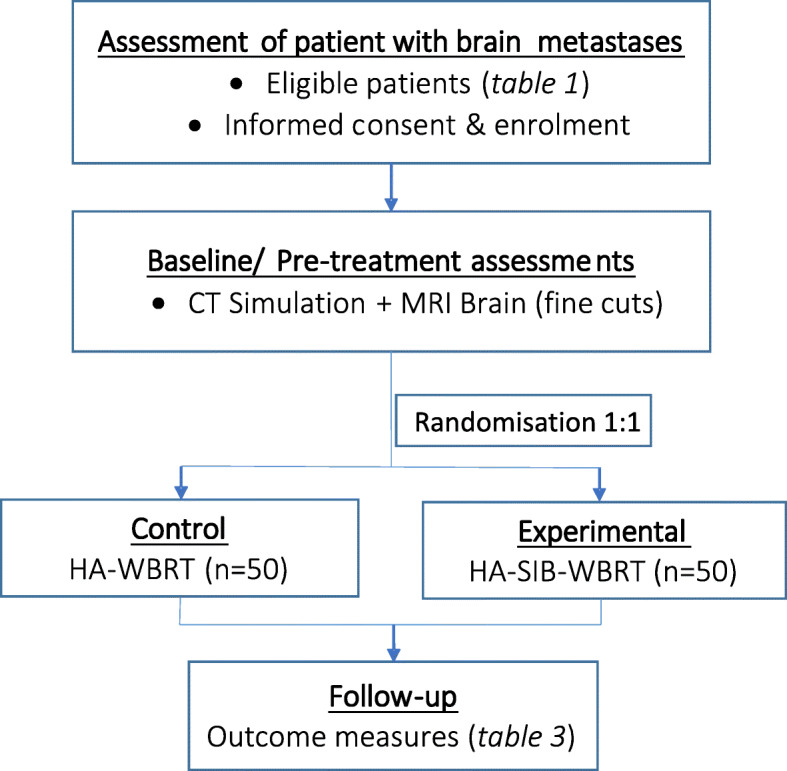
Project Schema
Eligibility criteria
Participants will be recruited if they meet the criteria set out in Table 1 below.
Table 1.
Eligibility Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
• 21–80 years old patients • Radiological confirmed brain metastases (4–25 lesions) • Histologically proven malignancy • ECOG performance status ≤2 • Maximum lesion or cavity size ≤5 cm o For patients with large (≥3 cm) lesions, a neurosurgical consult is recommended due to the increased risk of cerebral oedema o If brain surgery or other invasive procedures are performed, the treatment should begin at least 2-weeks post-procedure • Life expectancy of at least 6 months • Negative serum pregnancy test within 14 days prior to registration for women of childbearing potential • Women of childbearing potential and male participants who are sexually active must agree to use a medically effective means of birth control throughout protocol treatment • Not suitable for or does not want SRS • Agrees to be randomised to either HA-WBRT or HA-SIB-WBRT |
• Prior whole brain radiotherapy. o Prior SRS is not an exclusion. Details of treatment must be recorded. • Diffuse leptomeningeal disease • Extensive extracranial disease, not controlled by systemic treatment • Major medical or psychiatric illness, which in the investigator’s opinion would interfere with the completion of therapy and follow up • Dementia, ongoing psychotic episodes or moderate-severe depression (PHQ-9). • Recent stroke in the past 3 months • Symptomatic brain metastases limiting ADLs • Rapid progression of brain lesion • Patients unable to give informed consent • Total tumour planning target volume (PTV) > 60 cc • Radiological evidence of hydrocephalus • Contraindication to Gadolinium contrast-enhanced MRI brain • Patients with diagnoses of small cell carcinoma, lymphoma or primary brain tumour |
Interventions
The control arm in this study is HA-WBRT. This will be given 30Gy in 10 fractions.
The experimental procedure is HA-SIB-WBRT. 40-45Gy in 10 fractions will be used for the SIB boost depending on the location of the lesion. 45Gy was selected as it has a similar biological effective dose (BED 65.2Gy; α/β = 10) to the 21Gy SRS dose. 40Gy (EQD2 60Gy; α/β = 2) was selected for tumours within/ close to organs at risk (OARs) to meet the recommended dose limits of common OARs. Any post-operative cavity will be contoured using consensus guidelines [18]. The cavity will be given a boost of 40Gy due to the high risk of microscopic residual disease and tumour seeding. Any gross residual tumour should be boosted to 45Gy. Further radiotherapy dose guidance is described in Table 2.
Table 2.
Radiotherapy Planning targets, Organs at Risk and Dose Guidance
| Volume | Description | Dose Target/ Limits | Variation Acceptable | Notes |
|---|---|---|---|---|
| GTV_45Gyb | Contoured using fused contrast enhanced MRI images. |
45Gy 98% covered by 100% dose Hotspot > 110% within GTV |
95% covered by 95% dose |
Lesion visible on at least 2 scan slices. Coverage to be comprised to meet OAR constraints |
| PTV_45Gyb | GTV + 0-2 mm isotropic margins |
45Gy 98% covered by 95% dose |
90% covered by 90% dose | Coverage to be comprised to meet OAR constraints |
| GTV_40Gyb | Tumour volume within/ ≤5 mm from OAR |
40Gy 95% covered by 95% dose Hotspot > 110% within GTV |
90% covered by 95% dose |
Lesion visible on at least 2 scan slices. Coverage to be comprised to meet OAR constraints |
| PTV_40Gyb | PTV_40Gy = GTV_40Gy; i.e. no expansion |
40Gy 95% covered by 95% dose |
90% covered by 90% dose | Coverage to be comprised to meet OAR constraints |
| GTVall_5mmb | All GTVs + 5 mm isotropic margins | – | – | For plan optimisation |
| Left & Right Hippocampus | Contoured using fused MRI as per RTOG contouring atlas (http://www.rtog.org//corelab/contouringatlases/hippocampalsparing.aspx), excluding any GTV |
D100% (Dose to 100% volume) ≤ 9Gy Dmax ≤16Gy Dmax ≤33Gya |
D100% ≤ 10Gy Dmax ≤17Gy Dmax ≤44Gyab |
Dmax to 0.03 cc |
| Hippocampal Avoidance Zone (HAZ) | Hippocampus + 5 mm isotropic margins | – | – | For plan optimization |
| Left & Right Optic chiasm | – | Dmax ≤33Gy |
Dmax ≤37.5Gyb Dmax ≤40Gyab |
Dmax to 0.03 cc |
| Left & Right Optic nerve | – | Dmax ≤33Gy | Dmax ≤35Gyb | Dmax to 0.03 cc |
| Left & Right Orbits | – | Dmax ≤33Gy | Dmax ≤35Gyb | Dmax to 0.03 cc |
| Left & Right Lens | – | Dmax ≤6Gy | Dmax ≤10Gyb | Dmax to 0.03 cc |
| Brain Stem | – | Dmax ≤33Gy |
Dmax ≤37.5Gyb Dmax ≤40Gyab |
Dmax to 0.03 cc |
|
Left & Right Cochlear |
– | Dmax ≤33Gy | Dmax ≤35Gya | Dmax to 0.03 cc |
| Brain | Brain parenchyma down to cranial margin of dens | 30Gy | – | – |
| PTV_Brain | (Brain + 3 mm isotropic margins) – (GTVall_5mmb + HAZ) |
95% of volume covered by 30Gy D2% ≤ 37.5Gy D98% (Dose to 98% of volume) ≥ 25Gy |
90% of volume covered by 30Gy D98% ≥ 22.5Gyb D2% ≤ 40Gyb |
– |
aApplicable if tumours within/ ≤5 mm from OARsbOnly applicable to HA-SIB-WBRT plans
Patients whose plans are unable to meet the recommended constraints will not be eligible for the trial and will be dropped out and undergo WBRT or HA-WBRT as standard of care treatment. Patients can also voluntarily or involuntarily drop-out of the trial at any time after enrolment. These patients will not be replaced. The reason for drop-out must be recorded.
All treatment plans in the experimental arm will be reviewed and approved at the weekly Neuro-radiation oncology team audits prior to the start of treatment. Immobilisation will be performed in all patients. Daily image verification by image-guided radiotherapy is required.
All concomitant treatments should be documented. The use of concurrent cytotoxic systemic treatments is not allowed as this could cause additional or unexpected neuro-toxicities. If the participant is on systemic treatments, a treatment break of at least 7 days for immunotherapy or chemotherapy and 3 days for targeted therapy is recommended before and after radiotherapy. Interruptions should be discussed with the patient’s prescribing medical oncologist.
The use of dexamethasone during radiotherapy is not mandatory but is recommended for use if the patient with symptomatic brain metastases, significant cerebral oedema, large tumours or posterior cranial fossa lesions. The concurrent use of memantine for cognitive protection is recommended but not mandated.
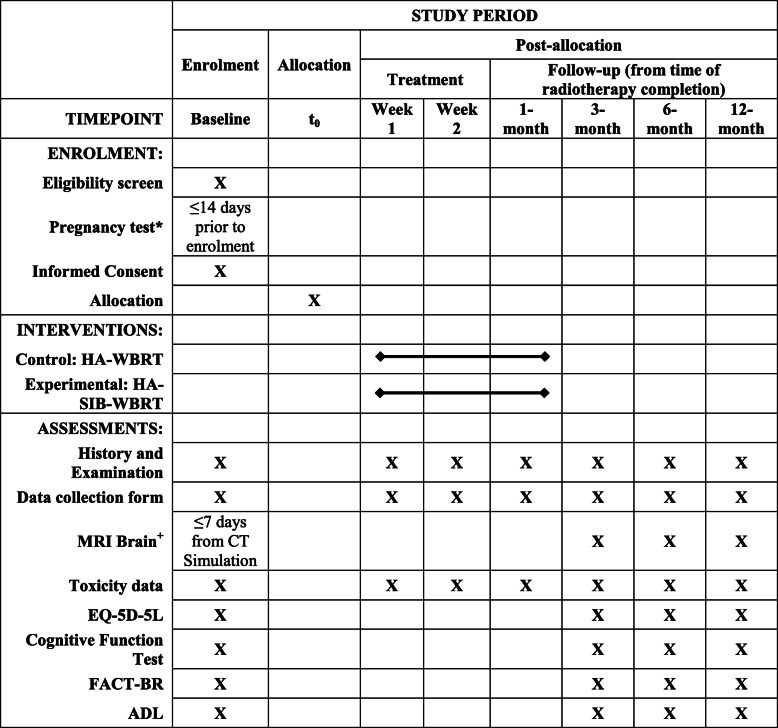
Outcomes and timeline
Primary endpoint
-
Target lesion control
▪ Response to treated lesions will be rated based on RANO-Criteria [19] or RECIST 1.1-Criteria.
Secondary endpoints
-
Intracranial Progression
▪ Target lesion or distal brain lesion
▪ Symptomatic or asymptomatic brain lesion- PFS
-
OS▪ Neurological or non-neurological death▪ Cancer or non-cancer related death
-
Cognitive Function▪ HVLT-R (immediate recall, delayed recall, and total recall)▪ Colour Trail Test (CTT) – a language-free version of the TMT
-
Quality of Life (QoL)▪ Functional Assessment of Cancer Therapy with Brain Subscale (FACT-BR)▪ Euro QOL – 5 Dimension – 5 Level (EQ-5D-5L)
-
Activities of Daily Living (ADL)▪ Barthel Index of ADLs
-
Toxicity▪ Scored using Common Terminology Criteria for Adverse Events (CTCAE) ver5.0 criteria▪ Presence of radiation necrosis (asymptomatic or symptomatic)
All time points will be taken from the time of randomisation to event. If response assessment of the target lesion(s) is uncertain or equivocal, this should be reassessed by a multi-disciplinary team for clarification. When needed, this may be followed up with advanced imaging or histology.
Cognitive function, ADL and QoL will be assessed using patient-directed questionnaires that have been validated, available in local languages and used in the trials mentioned earlier for ease of comparison. These tests are optional but encouraged for all patients and will be conducted by a trained physician or clinical research associate at specified time points (Table 3). The total duration of the combined test is estimated to be 30 min. In the analysis of cognitive function, ADL and QoL, each patient will serve as his/her own control. The test results at each follow-up (post-treatment) time point will be compared to the baseline (pre-treatment) test result.
Table 3.
Schedule of Enrolment, Interventions, and Assessments Recommended Follow-up Schedule Protocol^
^The follow-up schedule is based on current recommended routine practice. A window period of +/− 2 weeks is allowed for the 1st month follow up visit. +/− 1 month is allowed for the 3rd, 6th and 12th month follow-up visit. It is advised that this be followed for standardisation of records, however individual deviations will be allowed. +MRI Brain imaging if performed following surgery should be done 2-weeks post op. MRI is recommended for follow-up brain imaging but contrasted-CT scan is allowed
*Only in females with child-bearing potential
Sample size
Assuming a 6-month target lesion control rate of 50% in the HA-WBRT arm, we will need 60 subjects (30 per arm) to detect a hazard ratio of 0.27 [9] between the 2 arms with 80% power using a 2-sided log-rank test at 5% significance level. A hazard ratio of 0.27 means the 6-month target lesion control rate in the HA-SIB-WBRT arm is expected to be 83%. These calculations assume that subjects will be followed up till target lesion progression, or for a minimum of 6 months. Assuming a 40% non-evaluable rate, an estimated total sample size of 100 subjects will be required.
Assignment of interventions
Patients recruited will be stratified by the number of brain metastases < 10 vs. ≥10. Within each stratum, patients will be randomized in a 1:1 ratio to control vs. experimental arms. Simple randomization will be performed using a computer-generated random sequence allocation. This will be performed by the research coordinator after informed consent has been taken. The allocation sequence is concealed from the investigators. Patients and treating radiation oncologist will not be blinded to the intervention as it was opined that our patients would want to be aware of the treatment received and that unblinding would not significantly affect our primary objective measure.
Data collection and management
Data management
Data (soft-copy) will be kept in a password protected database “SingHealth REDCap”. All documented (hard copy) data including consent forms, data collection forms and neurocognitive, functional and ADL assessment forms will be stored in a secured research folder. Research coordinators will help ensure per-protocol follow-up and filling-in of data targets. 3-monthly random checks on the data will be performed by the investigators to ensure integrity and quality.
Statistical methods
Statistical analysis plans
Efficacy analyses will be performed using the intention-to-treat principle. Per-protocol analyses may be performed as secondary analyses. Safety analyses will include all patients who started treatment and will be performed according to the actual treatment received.
The primary endpoint, time to target lesion progression, will be defined as the time from randomisation to target lesion progression. Patients who pass away before documented target lesion progression will be censored at the last brain imaging assessment. Time to target lesion progression will be compared between the 2 treatment groups using a 2-sided log-rank test.
Time to intracranial progression, time to symptomatic brain progression, progression-free survival and overall survival will be defined as the time from randomisation to intracranial progression, symptomatic brain progression, overall progression and death from any cause respectively. All time-to-event endpoints will be summarized using the Kaplan-Meier method. Treatment arms will be compared using log-rank tests and Cox proportional hazards models, adjusting for the stratification variable (number of brain metastases). Other prognostic variables such as histology, ECOG status and disease-specific GPA class may also be included in the Cox models.
Adverse events will be recorded according to CTCAE version 5.0 and summarised by treatment arm.
Cognitive test (HVLT-R and CTT) scores will be standardised based on published norms: (Patient value – Published-norm mean value) ÷ Published-norm standard deviation value. Cognitive deterioration will be defined as a decline of at least 1 SD in score from baseline. FACT-BR scores will be transformed to a 0- to 100- point scale. A 10-point decrease will be considered clinically significant. Deterioration in functional independence will be defined as a decline of at least 10% in the Barthel ADL index from baseline.
The proportions of patients with cognitive deterioration, QoL deterioration and functional independence deterioration will be compared between treatment arms using Fisher’s exact test. A sensitivity analysis will be conducted assuming patients who had not completed the neurocognitive assessment or had passed away prior to the assessment time-point, had cognitive deterioration. Change from baseline values will be compared using 2-sample t-tests and repeated measures linear regression, adjusting for the stratification variable (number of brain metastases).
All analyses will use a 2-sided 0.05 level of significance. There will be no adjustment for multiple comparisons.
There is no planned interim analysis for this study.
Oversight and monitoring
Safety
There is no independent Data Safety Monitoring Committee for this study. Any ≥Grade 3 serious adverse events must be reviewed by the co-investigators to determine if this toxicity is treatment related. All serious treatment related toxicities must be reported to the institutional review board (IRB). If any ≥Grade 4 toxicities are noted, the study will stop for review of safety before continuation, cessation or amendment of trial.
The principal investigator and co-investigators will be responsible for monitoring patient recruitment, toxicities, observed results and the evaluation of data quality. This will be done every 3 months.
Ethics and dissemination
Research ethics
This study will be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with the Good Clinical Practice and the applicable regulatory requirements.
The Clinical Trial Protocol, including the Participant Information Sheet and Consent Form, has been approved by the SingHealth Centralised Institutional Review Board No. 2019/2407 prior to enrolment of any patient into the study.
Only the study team radiation oncology investigators will be allowed to take inform consent from potential trial participants.
Confidentiality
Only the Principal investigator and study coordinators will have access to the research data. At the completion of the study, participant identifiers will be removed from stored data and anonymized. Re-identification of participants will be kept by a 3rd party person not involved in the study project. The participants’ name will not be used in any public report of the study.
Discussion
The optimal management of patients with multiple brain metastases is multifaceted and depends on several factors including tumour type, volume and number of brain metastases, available brain penetrating drugs, prognosis and performance status. Traditionally, the treatment was limited to WBRT. Fortunately, improvements in technology and pharmacology have expanded the therapeutic options for these patients.
SRS which is often given in preference over WBRT in limited brain metastases (≤3–4 lesions) has shown better learning and memory preservation in several randomised trials [20]. In patients with > 4 brain lesions, WBRT remains the standard of care although the role of SRS is fast emerging. A prospective observational study and a large retrospective multi-institutional study have shown that the total number of brain metastases (up to 15 lesions) treated with SRS did not seem to affect the survival outcome and could be considered instead of WBRT [21, 22]. Despite this, there remains some controversy in giving SRS for multiple brain metastases. One would expect that with more brain metastases, the risk of brain micrometastases and intracranial failure is higher and thus giving SRS instead of WBRT would mean the need for early salvage treatment thus negating the clinical benefit [20, 23]. The previous SRS trials also did not perform comparisons against newer neurocognitive protecting strategies like HA-WBRT and the use of neuroprotective agents like memantine. Several randomised phase III trials are thus underway that will hopefully provide further clarity on the role of SRS in the setting of multiple brain metastases.
The NRG-CC001 trial which proved the benefit of HA-WBRT over WBRT had a poor intracranial PFS of 5.0 months [6]. This was not surprising, given the inherent low doses utilised in standard HA-WBRT. Several studies have shown that higher doses to target lesions could improve on local control rates and potentially reduce intracranial failure [9, 20, 23]. In turn, improving intracranial control could possibly result in improved performance status and reduced neurological death [8, 9, 12]. It would thus seem reasonable to consider giving tumours a simultaneous higher dose during HA-WBRT using HA-SIB-WBRT. The preliminary evidence for this has been promising. However, there is still a lack of high-level evidence on the benefit and safety it has over standard HA-WBRT. This trial thus sets out to explore the magnitude of these benefits including control rates, toxicities, survival outcomes, cognition and other PROMs. To the best of our knowledge, this is the only prospective, randomised trial comparing HA-WBRT against HA-SIB-WBRT. We, therefore, believe that this trial is significant as it will provide the evidence required to support its use.
Trial status
The first patient was recruited on June 2020. The trial is currently ongoing and recruiting patients. The trial is registered on ClinicalTrials.gov under NCT04452084.
Acknowledgements
Not applicable.
Abbreviations
- ADL
Activities of daily living
- BED
Radiation biological effective dose
- CT
Computer tomography
- CTCAE
Common terminology criteria for adverse events
- CTT
Colour trail test
- ECOG
Eastern cooperative oncology group performance status
- EQD2
Radiation equivalent dose in 2gy fractions
- EQ-5D-5L
Euro QOL – 5 dimension – 5 level questionnaire
- FACT-BR
Functional assessment of cancer therapy with brain subscale
- GPA
Graded prognostic assessment prognostic index
- GTV
Gross tumour volume
- Gy
Gray
- HAZ
Hippocampal avoidance zone.
- HA-WBRT
Hippocampal avoidance whole brain radiotherapy
- HA-SIB-WBRT
Hippocampal avoidance with simultaneous integrated boost whole brain radiotherapy
- HVLT-R
Hopkins verbal learning test-revised
- IRB
Institutional review board
- MRI
Magnetic resonance imaging
- OAR
Organs at risk
- OS
Overall survival
- PFS
Progression free survival
- PROMs
Patient report outcome measures
- PTV
Planning target volume
- QoL
Quality of life
- RANO
Response assessment in neuro-oncology
- RECIST 1.1
Response evaluation criteria in solid tumours version 1.1
- SIB
Simultaneous integrated boost
- SRS
Stereotactic radiosurgery
- TMT
Trail making test
- WBRT
Whole brain radiotherapy
- WBRT+SIB
Whole brain radiotherapy with simultaneous integrated boost
Authors’ contributions
BSHC and JYL contributed equally in the initial conception, design and coordination of this study. All authors provided input and guidance in the revision and development of the study. BSHC, ETC, GK, FYW, KLMC, ALKO and MLKC will be responsible for the recruitment of patients and will perform the radiotherapy planning, treatment and follow-up care of patients in this study. Neurocognitive and psychological testing and training are supported by JYL, SHP and TSL. Statistical support in preparing the study was provided by CL. The manuscript was drafted by BSHC and JYL. All authors have read and approved the final manuscript.
Funding
We received funding for this trial from the SingHealth Oncology Academic Clinical Programme Fund (Grant ID: 08/FY2019/P2/13-A67). This organization has not been involved in any part of the design of the study or writing the manuscript.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
The study, Protocol version 1.5 dated 2 March 2020, Participant Information Sheet and Consent Form, have been approved by the SingHealth Centralised Institutional Review Board No. 2019/2407 in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with the Good Clinical Practice and the applicable regulatory requirements. All included patients have to give their written consent before entering the study. Informed consent will be obtained by study investigators.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tallet AV, Azria D, Barlesi F, Spano JP, Carpentier AF, Gonçalves A, et al. Neurocognitive function impairment after whole brain radiotherapy for brain metastases: actual assessment. Radiat Oncol. 2012;7:77. doi: 10.1186/1748-717X-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/S0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler SM, McLelland VC, Sheard E, McAndrews MP, Rovet JF. Hippocampal functioning and verbal associative memory in adolescents with congenital hypothyroidism. Front Endocrinol (Lausanne) 2015;6:163. doi: 10.3389/fendo.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Katz JS, Wright AA. The hippocampus and memory of verbal and pictorial material. Learn Mem. 2002;9:99–104. doi: 10.1101/lm.44302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal Dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol. 2012;83:e487–e493. doi: 10.1016/j.ijrobp.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020;38:1019–1029. doi: 10.1200/JCO.19.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 8.Sperduto PW, Shanley R, Luo X, Andrews D, Werner-Wasik M, Valicenti R, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA) Int J Radiat Oncol Biol Phys. 2014;90:526–531. doi: 10.1016/j.ijrobp.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev. 2017. 10.1002/14651858.CD006121.pub4. [DOI] [PMC free article] [PubMed]
- 10.Awad R, Fogarty G, Hong A, Kelly P, Ng D, Santos D, et al. Hippocampal avoidance with volumetric modulated arc therapy in melanoma brain metastases – the first Australian experience. Radiat Oncol. 2013;8:62. doi: 10.1186/1748-717X-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oehlke O, Wucherpfennig D, Fels F, Frings L, Egger K, Weyerbrock A, et al. Ganzhirnbestrahlung mit Hippocampusschonung und Dosiseskalation bei multiplen Hirnmetastasen: Lokale Tumorkontrolle und Überleben. Strahlentherapie Onkol. 2015;191:461–469. doi: 10.1007/s00066-014-0808-9. [DOI] [PubMed] [Google Scholar]
- 12.Popp I, Rau S, Hintz M, Schneider J, Bilger A, Fennell JT, et al. Hippocampus-avoidance whole-brain radiation therapy with a simultaneous integrated boost for multiple brain metastases. Cancer. 2020;126:2694–2703. doi: 10.1002/cncr.32787. [DOI] [PubMed] [Google Scholar]
- 13.Kim KH, Cho BC, Lee CG, Kim HR, Suh YG, Kim JW, et al. Hippocampus-sparing whole-brain radiotherapy and simultaneous integrated boost for multiple brain metastases from lung adenocarcinoma. Technol Cancer Res Treat. 2016;15:122–129. doi: 10.1177/1533034614566993. [DOI] [PubMed] [Google Scholar]
- 14.Westover KD, Mendel JT, Dan T, Kumar K, Gao A, Pulipparacharuv S, et al. Phase II trial of hippocampal-sparing whole brain irradiation with simultaneous integrated boost for metastatic cancer. Neuro-Oncology. 2020. 10.1093/neuonc/noaa092. [DOI] [PMC free article] [PubMed]
- 15.Zhong J, Waldman AD, Kandula S, Eaton BR, Prabhu RS, Huff SB, et al. Outcomes of whole-brain radiation with simultaneous in-field boost (SIB) for the treatment of brain metastases. J Neuro-Oncol. 2020;147:117–123. doi: 10.1007/s11060-020-03405-y. [DOI] [PubMed] [Google Scholar]
- 16.Grosu AL, Frings L, Bentsalo I, Oehlke O, Brenner F, Bilger A, et al. Whole-brain irradiation with hippocampal sparing and dose escalation on metastases: neurocognitive testing and biological imaging (HIPPORAD) - a phase II prospective randomized multicenter trial (NOA-14, ARO 2015-3, DKTK-ROG) BMC Cancer. 2020;20:532. doi: 10.1186/s12885-020-07011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seng Hup Chia B, Li Kuan Ong A, Master Z. RTHP-26. Dosimetric feasibility study using hippocampal avoidance with simultaneous integrated boost whole brain radiotherapy. (HA-SIB-WBRT) Neuro Oncol. 2019;21(Supplement_6):vi215. doi: 10.1093/neuonc/noz175.897. [DOI] [Google Scholar]
- 18.Soliman H, Ruschin M, Angelov L, Brown PD, Chiang VLS, Kirkpatrick JP, et al. Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2018;100:436–442. doi: 10.1016/j.ijrobp.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Lin N, Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, et al. Response assessment criteria for brain metastases: proposal from the RANO group 2015. doi:10.1016/S1470-2045(15)70057-4. [DOI] [PubMed]
- 20.Tsao MN, Xu W, Wong RK, Lloyd N, Laperriere N, Sahgal A, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2018;1:CD003869. doi: 10.1002/14651858.CD003869.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 22.Hughes RT, Masters AH, McTyre ER, Farris MK, Chung C, Page BR, et al. Initial SRS for patients with 5 to 15 brain metastases: results of a multi-institutional experience. Int J Radiat Oncol Biol Phys. 2019;104:1091–1098. doi: 10.1016/j.ijrobp.2019.03.052. [DOI] [PubMed] [Google Scholar]
- 23.McTyre E, Ayala-Peacock D, Contessa J, Corso C, Chiang V, Chung C, et al. Multi-institutional competing risks analysis of distant brain failure and salvage patterns after upfront radiosurgery without whole brain radiotherapy for brain metastasis. Ann Oncol. 2018;29:497–503. doi: 10.1093/annonc/mdx740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.