Abstract
G protein-coupled receptors (GPCRs) are cell surface receptors that mediate the function of extracellular ligands. Understanding how GPCRs work at the molecular level has important therapeutic implications, as 30–40% of the drugs currently in clinical use mediate therapeutic effects by acting on GPCRs. Like many other cell types, liver function is regulated by GPCRs. More than 50 different GPCRs are predicted to be expressed in the mouse liver. However, knowledge of how GPCRs regulate liver metabolism is limited. A better understanding of the metabolic role of GPCRs in hepatocytes, the dominant constituent cells of the liver, could lead to the development of novel drugs that are clinically useful for the treatment of various metabolic diseases, including type 2 diabetes, nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). In this review, we describe the functions of multiple GPCRs expressed in hepatocytes and their role in metabolic processes.
Keywords: G protein-coupled receptor, GPCR, hepatocyte, liver, metabolism, diabetes, NAFLD, NASH
1. Introduction
The liver is the crucial orchestrator of metabolism. In humans, approximately 11,000 genes are actively transcribed in hepatocytes, and many of them are related to the metabolic functions of the liver [1]. The liver mediates numerous physiological functions, including protein synthesis, macronutrient metabolism, cholesterol and lipid homeostasis, endocrine control of growth signaling pathways, and drug detoxification [2,3,4,5,6]. Metabolic hormones, nutrients, and sympathetic and parasympathetic neural signals tightly regulate liver function [7,8]. Alterations in these signaling pathways lead to the dysfunction of liver metabolism and can cause insulin resistance, type 2 diabetes (T2D), nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and cancer [7,8,9]. The liver consists of hepatocytes, biliary epithelial cells, stellate cells, smooth muscle cells, vascular endothelial cells, various immune cells, and sinusoidal endothelial cells [10,11,12]. Each of these cell types possesses a unique function and collectively regulates liver function at multiple levels. Hepatocytes are the primary cell type present in the liver and account for 80% of the liver cell population [12].
G protein-coupled receptors (GPCRs) are cell surface receptors and mediate the function of a vast number of extracellular ligands (neurotransmitters, hormones, etc.) [13,14]. Ligands-coupled GPCRs recognize and activate heterotrimeric G proteins, which comprise Gα, Gβ, and Gγ. Gα determines the functions of a G protein through the exchange of guanosine diphosphate (GDP) with guanosine triphosphate (GTP) [15]. The Gβ and Gγ subunits are separately synthesized and form a complex to function as a single functional unit [16]. Both the Gα and Gβγ subunits activate signaling molecules and trigger cellular responses. Based on their α subunit, G proteins are classified into four families: Gs, Gi, Gq, and G12/13 (Figure 1), where s and i represent stimulation and inhibition, respectively. Receptor-activated G proteins stimulate various downstream signaling pathways; for example, Gs and Gi proteins control adenylyl cyclase activity, Gq activates phospholipase Cβ, and G12/13 stimulates guanine nucleotide exchange factors for small GTPases of the Rho family [17,18]. The human genome contains about 800 different GPCR genes, which represent 3–4% of all human genes. Importantly, 30–40% of the drugs currently used in the clinic mediate their therapeutic effects via acting on GPCRs [13]. Understanding how GPCRs work at the molecular level is, therefore, of considerable therapeutic relevance.
Figure 1.
G-proteins’ classification and downstream signals.
Similar to many other cell types, hepatocyte function is regulated by GPCRs [19,20,21,22]. More than 50 different GPCRs are predicted to be expressed by mouse liver [23]. However, our knowledge about how GPCRs regulate liver function remains incomplete. In this review, we discuss the function of various GPCRs expressed in hepatocytes and their roles in various metabolic processes. The receptors mentioned in this review were selected with reference to the data quantifying the transcriptional levels of 353 GPCRs in mouse tissues described by Regard et al. [23] and GPCR-related paper data in hepatocytes reported in PubMed. For the classification of α subunit, we referred to the IUPHAR/BPS Guide to Pharmacology [24], including those belonging to multiple families. We focused mainly on the critical GPCR functions in hepatocyte and their role in liver metabolism.
2. Role of Gs-Coupled GPCRs in Liver Metabolism
2.1. Glucagon Receptor
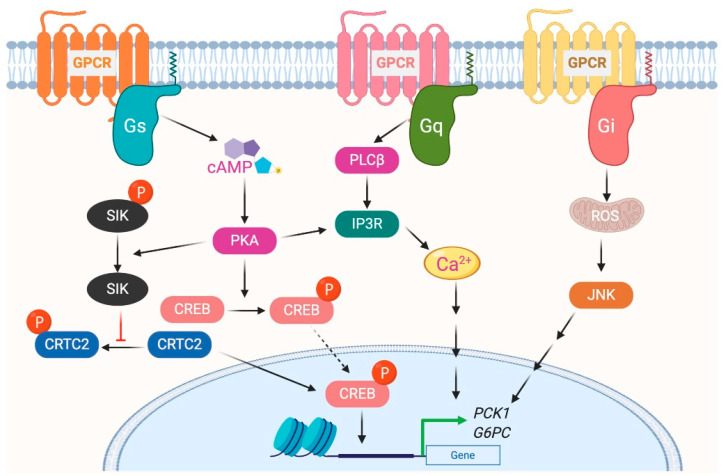
The glucagon receptor (GCGR) is abundantly expressed by hepatocytes and is the most extensively studied Gs-coupled GPCR in the liver [25]. GCGR is activated by the 29 amino acid polypeptide glucagon, which is secreted by α cells of the endocrine pancreas, in particular during the fasted state or exercise [26]. The primary function of glucagon is to prevent hypoglycemia during fasting by increasing hepatic glucose production (HGP) from hepatocytes by stimulating both glycogenolysis and gluconeogenesis [27,28]. Activation of GCGR in hepatocytes results in increased intracellular cyclic adenosine monophosphate (cAMP) levels, followed by activation of protein kinase A (PKA) and cAMP response element-binding (CREB) protein (Figure 2). Phosphorylated CREB (the active form of CREB) binds to CRE and increases the expression of essential gluconeogenic genes, including glucose 6-phosphatase (G6pc) and phosphoenolpyruvate carboxykinase (Pck1) [29]. The activated GCGR also stimulates Ca2+ release from the endoplasmic reticulum (ER) and activates calcium/calmodulin-dependent protein kinase II (CAMKII). This results in the translocation of FOXO1 to the nucleus that promotes the transcription of gluconeogenic genes [30]. In addition to transcriptional regulation of genes involved in glucose metabolism, GCGR activation also modulates the activity of enzymes involved in glucose metabolism via PKA-dependent phosphorylation. PKA phosphorylates phospho-fructokinase 2 (PFK-2), fructose 2,6-bisphosphatase (FBPase2), pyruvate kinase, and phosphorylase kinase. Phosphorylation of these enzymes increases HGP by increasing gluconeogenesis and glycogenolysis or by inhibiting glycolysis [25,31].
Figure 2.
G protein-coupled receptors-mediated glycogenesis in hepatocytes.
Kim et al. examined the effects of GCGR agonists on insulin action and glucose homeostasis [32]. The acute GCGR agonist immediately induced a hyperglycemic state, followed by improved glucose tolerance and glucose-stimulated insulin secretion. Furthermore, acute GCGR agonists improved insulin tolerance in a dose-dependent manner in both lean and obese mice. Phosphorylation of hepatic AKT at Ser473 was increased in mice treated with insulin and GCGR agonists. This effect was replicated in isolated primary mouse hepatocytes, increasing AKT kinase activity. These data suggest that hepatic GCGR activation improves glucose tolerance by enhancing the action of insulin. It has also been reported that hepatic GCGR stimulation reduces lipid content, drives glycogen flux, and improves mitochondrial turnover and function [33]. This glucagon signaling pointing mechanism has high potential applications in new drugs for NAFLD/NASH.
2.2. Thyrotropin-Releasing Hormone Receptor
Thyrotropin-releasing hormone (TRH) receptor (TRHR) is a Gq-coupled receptor; however, in hepatocytes, it was shown to signal through Gs [34]. TRHR is activated by TRH released from the hypothalamus. Activation of TRHR leads to increased cAMP levels and enhanced PKA activation, causing CREB activation and increased expression of gluconeogenic genes [34]. TRHR-activated PKA also phosphorylates salt-inducible kinase 2 (SIK2) and inhibits its activity [35,36]. Decreased SIK2-dependent phosphorylation of CRE2 and CREB-regulated transcription coactivator 2 (CRTC2) enables them to enter the nucleus and interact with CREB to promote the expression of the G6pc and Pck1 genes [35,36], leading to enhanced HGP.
2.3. Beta-Adrenergic Receptor
Two catecholamines, epinephrine and norepinephrine released from the sympathetic nervous system, also regulate liver metabolism [37]. These catecholamines active Gs-coupled beta-adrenergic receptors (βARs), which are expressed in both rodent and human hepatocytes [38,39]. Two subtypes of βAR (β1AR and β2AR) are predominantly expressed in the liver, and their expression is known to increase with age [38]. βAR-mediated activation of adenylyl cyclase increases hepatic glucose output and liver lipid catabolism [40,41,42]. In rat hepatocytes, activation of βARs with nonselective agonist isoproterenol increases glycogen phosphorylase activity by many folds. Similar to GCGR, activation of β2AR in hepatocytes increases the expression of Pck1, G6pc, and l-pyruvate kinase (pklr) and decreases liver glycogen levels [42]. In a recent study, it was shown that activation of βAR signaling in hepatocytes increases the activity of hormone sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) [42,43]. ATGL is the master regulator of triglyceride content in hepatocytes and it activates Sirtuin 1 (Sirt1), which can regulate expression of various lipid metabolic genes [44]. On the other hand, HSL in hepatocytes increases the activity of triglyceride hydrolase. HSL also plays a role in the hydrolysis of cholesterol esters [45]. Overall, activation of βARs in hepatocytes regulate HGP and lipid catabolism.
2.4. Prostaglandin E2 Receptor 4
The prostaglandin E2 receptor 4 (EP4) metabolic function in hepatocytes is mediated through Gs. EP4-associated protein (EPRAP) is a molecule downstream of EP4 that suppresses the inflammatory response of macrophages. [46]. In addition to macrophages, EPRAP is also expressed and detected in hepatocytes [47]. Higuchi et al. showed that Eprap-null mice fed on a high-fat sucrose diet showed significantly lower blood glucose levels compared to wild type (WT) mice when challenged with either glucose or pyruvate [47]. Furthermore, primary hepatocytes of Eprap-null mice showed reduced glucose production and reduced expression of Pck1 and G6pc genes. Lentiviral-mediated suppression of hepatic EPRAP also reduced glucose levels and glucogenic gene expression in db/db mice. In summary, the authors concluded that EPRAP regulates hypoglycemia in diabetic mice by controlling hepatic gluconeogenesis. As stated by the authors, liver-specific deletion of Eprap is necessary for further understanding the role of hepatic EPRAP in glucose metabolism.
2.5. Sphingosine-1-Phosphate Receptor 2
Recently, the role of sphingosine-1-phosphate receptor 2 (S1PR2) in bile acid-mediated hepatic lipid metabolism was clarified [48]. S1PR2-mediated signaling includes Gs, Gq, and G12/13 mechanisms. In rodent primary hepatocytes, conjugated bile acids activated S1PR2, leading to the downstream activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and AKT signaling pathways. Bile acid-mediated activation of ERK1/2 and AKT signaling pathways involves the regulation of intrahepatic glucose and lipid metabolism [49]. In primary rat hepatocytes, both insulin and bile acids similarly activated glycogen synthase activity. Infusion of taurocholate (TCA) into the chronic cholestine fistula rat activated the AKT and ERK1/2 signaling and glycogen synthase activity [50]. In addition, TCA reduced the expression of liver gluconeogenic genes, Pck1, and G6pc expression, and increased the expression of short heterodimeric partner (Shp) mRNA significantly [51]. These results suggest that TCA has an insulin-like activity to regulate hepatic glucose metabolism both in vitro and in vivo. Additionally, it has been reported that S1pr2-null mice fed a high-fat diet develop fatty liver more rapidly than wild-type mice, suggesting that S1PR2 is a vital regulator of hepatic lipid metabolism [52]. Infusion of TCA into the chronic bile fistula rat or overexpression of the gene encoding S1PR2 in mouse hepatocytes upregulated hepatic sphingosine kinase 2 (SphK2). Interestingly, the major genes encoding nuclear receptors/enzymes responsible for nutrient metabolism were downregulated in the livers of S1pr2-null and Sphk2-null mice. Consistently, overexpression of the gene encoding S1PR2 in primary mouse hepatocytes raised the mRNA levels of key genes associated with nutrient metabolism.
3. Role of Gi-Coupled GPCRs in Liver Metabolism
GPCRs that preferentially couple to Gi proteins inhibit the function of adenylyl cyclase and thereby decrease intracellular cAMP. In a recent study, to understand the role of hepatic Gi signaling on whole-body glucose metabolism, Dr. Wess’s group employed a chemo-genetic approach where a designer Gi-coupled receptor was selectively expressed in mouse hepatocytes (Hep-GiD). This designer receptor can be selectively activated by clozapine-N-oxide (CNO), an otherwise chemically inert compound [20]. As activation of the Gs-coupled GCGR increases HGP, it was expected that activation of Gi in the liver would have opposite effects. Interestingly, activation of Gi signaling in hepatocytes also increased HGP by activating c-Jun N-terminal kinases (JNK) signaling [20]. Activation of Gi signaling in mouse liver impaired glucose tolerance and stimulated gluconeogenesis and glycogenolysis. Both in vivo and in vitro studies showed that enhanced Gi signaling in hepatocytes led to a JNK-dependent increase in the expression of G6pc (Figure 2). Similar results were obtained with the human hepatocytes. Activation of Gi signaling in the liver did not affect hepatic insulin signaling [20].
3.1. Cannabinoid Receptor 1
To validate the findings obtained in the previous section with Hep-GiD mice, the authors targeted the cannabinoid receptor 1 (CB1), an endogenous Gi-coupled receptor expressed in hepatocytes. Acute activation of CB1 with anandamide in mice severely impaired glucose tolerance and increased glucose release from hepatocytes via enhanced reactive oxygen species (ROS) production, resulting in JNK activation and increased HGP [20]. A separate study by Dr. Kunos’s lab showed that activation of CB1 increased glycogen phosphorylase activity by 70%, suggesting that enhanced glycogenolysis is the primary source of the increased HGP [53]. CB1 also inhibited insulin signaling in hepatocytes by activating the Bip/PERK/eIF2α ER stress pathway [54,55]. Furthermore, another study showed that peripheral CB1 blockade in obese mice improved glycemic in control mice via the hepatic Sirt1/mTORC2/Akt pathway and increased fatty acid oxidation via LKB1/AMPK signaling [56].
3.2. A1 and A3 Adenosine Receptor
Another group of Gi-coupled receptors expressed in hepatocytes are the A1 and A3 adenosine receptors. Treatment of hepatocytes with the A1 receptor-selective agonist 2-chloro-N6-cyclopentyladenosine (CCPA) stimulates gluconeogenesis and glycogenolysis [57]. This receptor also regulates lipid metabolism in hepatocytes by increasing triglyceride synthesis by enhancing the expression levels of sterol regulatory element-binding protein 1 (SREBP-1) and peroxisome proliferator-activated receptor-gamma (PPARg), key regulators of genes involved in lipogenesis (Fas, Acc, Acl, stearoyl-CoA desaturase-1, and l-α-glycerophosphate acyltransferase) [58]. Similar to the A1 receptor, activation of the A3 receptor in hepatocytes increases HGP by enhancing gluconeogenesis and glycogenolysis [59,60].
3.3. GPR109A
GPR109A (HCA2, hydroxycarboxylic acid receptor 2) is a Gi-coupled receptor, and activation of GPR109A in hepatocytes by niacin decreases intracellular cAMP levels. Agonist stimulation of GPR109A in mouse hepatocytes decreases plasma high-density lipoprotein (HDL)-cholesterol by reducing the expression of the adenosine triphosphate (ATP) binding cassette transporter A1 (Abca1) and reduces the efflux of cholesterol to HDL [61,62]. Activation of either Gs or Gi signaling pathways in hepatocytes increases HGP. However, both signaling molecules use different signaling pathways. Interestingly, the A1 adenosine receptor and GPR109A Gi-coupled receptors also regulate lipid metabolism.
3.4. C-C Chemokine Receptor Types 2 and 5
The expression of Gi-coupled receptors C-C chemokine receptor type 2 (CCR2), and C-C chemokine receptor type 5 (CCR5) is increased in the livers of patients with alcoholic liver disease (ALD) [63]. Ambate et al. tested whether inhibition of CCR2/5-related signaling could inhibit ALD progression [63]. The authors used cenicriviroc (CVC), a dual inhibitor of CCR2/5, to inhibit CCR2/5 signaling in a mouse model of ALD, which reduced liver damage and steatosis. In vitro, CVC reduced expression of fatty acid synthase (Fasn) and adipose differentiation-related protein (Adrp) and increased the expression of acyl-coenzyme A oxidase 1 (Acox-1), proliferator-activated receptor gamma coactivator alpha (Pgc1α), and uncoupling protein two. These data suggested possible mechanisms for attenuated hepatocyte steatosis. Collectively, this study showed an association between alcohol-induced steatohepatitis, liver damage, and CCR2/5, indicating that inhibition of CCR2 and CCR5 may be an effective treatment for ALD.
3.5. Purinergic Receptor P2Y, G Protein-Coupled, 13
In cultured hepatocytes, activation of the Gi-coupled receptor, purinergic receptor P2Y13 (P2Y13), was reported to be essential for high-density lipoprotein (HDL) uptake [64]. The primary atheroprotective function of HDL is to promote “reverse cholesterol transport” (RCT). HDL mediates the efflux and transport of cholesterol from peripheral cells and subsequent metabolism and traffic to the liver for biliary excretion. Recently, P2Y13 has been shown to be a new target for regulating RCT [65]. This is a very relevant finding for the link between the liver and atherosclerosis, and the therapeutic application of these findings is expected.
3.6. Smoothened Receptor
The hedgehog (Hh) signaling pathway regulates the fate of hepatic progenitor cells and liver development [66]. Components of Hh signaling include the Gi- or G12/13-coupled receptor, smoothened (SMO) [67]. Activation of Hh has been observed in patients with NAFLD, but the function of hepatic Hh signaling in the pathogenesis of NAFLD was previously unknown. A recent publication examined the effects and mechanisms of hepatic Hh signaling in high fat diet (HFD)-induced NAFLD using genetic and pharmacological approaches [67]. Treatment of HFD-fed WT mice with Smo inhibitors (GDC-0449 and LED225) significantly reduced the number of activated macrophages and expression of pro-inflammatory cytokines. The SMO inhibitors were noted to have variable effects on hepatic fat accumulation. Hepatocyte-specific deletion of Smo reduced macrophage activation and inhibited the expression of pro-inflammatory cytokines but did not significantly alter fat accumulation in the liver. This paper concluded that hepatocyte Hh signaling played an important role in the progression of NAFLD by promoting hepatic inflammation via osteopontin-mediated macrophage activation, rather than a direct effect on the fat deposition. Selective inhibition of hepatocyte Hh signaling in patients with NAFLD might inhibit the progression from simple fatty liver to NASH.
4. Role of Gq-Coupled GPCRs in Liver Metabolism
Stimulation of Gq signaling leads to the activation of phospholipase Cβ, followed by an increase in intracellular levels of inositol trisphosphate, diacylglycerol, and calcium (Figure 1). Further downstream effectors of the signaling pathway are dependent on the receptor and cell type. To understand the role of hepatic Gq signaling on glucose homeostasis, Dr. Wess’s group expressed a Gq coupled, CNO-sensitive designer receptor selectively in mouse hepatocytes (Hep-GqD) [21]. Acute activation of this receptor with CNO increased blood glucose levels in a concentration-dependent manner. Hep-GqD mice showed impaired glucose and pyruvate tolerance. Impairments were predominately due to an increase in HGP [20]. Mechanistic studies confirmed an increase in the expression of various genes involved in gluconeogenesis and glycogenolysis. In particular, mRNA levels of Pck1, a rate-limiting enzyme of gluconeogenesis, were significantly increased (Figure 2). Similarly, the expression levels of genes involved in fatty acid syntheses such as Acyl, Acc, and Fas were also upregulated [20]. These data indicate that Gq signaling in hepatocytes plays a crucial role in glucose and lipid handling.
4.1. Vasopressin and Oxytocin Receptor
Hepatocytes express various Gq-coupled receptors, including arginine vasopressin receptor 1A (AVPR1A) and oxytocin (OT) [68,69,70,71]. The AVPR1A (endogenous agonist: arginine-vasopressin, AVP) is expressed predominantly in mammalian hepatocytes. Activation of AVPR1A in hepatocytes initiates glycogenolysis by phosphorylating glycogen phosphorylase (Pygl). In rat hepatocytes, activation of AVPR1A leads to increased glycogenolysis and gluconeogenesis [72,73]. Acute injection of AVP into experimental animals increases blood glucose levels, indicating that AVPR1A stimulates HGP [74,75,76].
Chronic activation of oxytocin receptors in lean mice via long-term treatment with oxytocin increased the expression of gluconeogenic genes such as G6pc, Fbp1, and Pck1. These mice also showed decreased liver glycogen levels and increased expression of hepatic Pygl [70,71,77]. In agreement with the in vivo data, the treatment of rat hepatocytes with oxytocin increased glycogen phosphorylase activity and decreased glycogen synthase activity [71]. Collectively, these data suggest that oxytocin increases net hepatic glucose oxidation.
4.2. Purinergic Receptor
Purinergic receptor P2Y receptors are activated by various extracellular nucleotides and nucleotide sugars. The presence of the Gq-coupled P2Y1, P2Y2, P2Y4, and P2Y6 receptors have been detected in hepatocytes [78]. Studies with human hepatocytes suggest that P2Y2 and P2Y4 modulate glycogen metabolism by reducing glycogen synthesis and promoting glycogenolysis [78,79]. Studies on rat hepatocytes showed that activation of P2Y1 stimulates glycogen phosphorylase [78].
4.3. Angiotensin II Type I Receptor
The Gq-coupled angiotensin II type I receptor (AT1R) is known to regulate cardiovascular function [80]. However, AT1R stimulation can also lead to hepatic stellate cell activation and fibrosis via phosphorylation of janus kinase 2 (JAK2) as well as activation of RhoA and Rho-associated kinase 1 (ROCK1). In primary rat hepatocytes, the angiotensin II analog [Sar1, Ile4, Ile8]-angiotensin II (SII Ang II) showed positive effects on insulin receptor signaling and glucose metabolism [81]. Long-term pretreatment with SII Ang II enhanced insulin-stimulated glycogen synthesis and promoted insulin-stimulated phosphorylation of Akt and GSK3a/b, but not of FOXO1 [81]. Furthermore, the effects of SII Ang II on insulin-stimulated signaling and glycogen synthesis were dependent on the activity of Src and Gaq, since inhibitors of these proteins abolished the beneficial metabolic effects of SII Ang II [81].
4.4. Free Fatty Acid Receptor 4 and GPR40
Free fatty acid receptor 4 (FFA4, GPR120) is a known Gq-coupled receptor and has been suggested as a target of omega-3 polyunsaturated fatty acids (n-3 PUFAs) [82]. Kang et al. evaluated the functional role of FFA4 in hepatic steatosis using an in vitro model of liver X receptor (LXR)-mediated hepatic steatosis [82]. The expression of FFA4 in Hep3B and HepG2 human hepatocytes was first confirmed. T0901317 (a specific LXR activator) induced lipid accumulation and docosahexaenoic acid (DHA, one of the n-3 PUFAs) inhibited lipid accumulation. This DHA-induced inhibition was blocked by small interfering RNA (siRNA)-mediated FFA4 knockdown or treatment with FFA4 antagonist, AH7614. SREBP-1c (a vital transcription factor for adipogenesis) expression was induced by treatment with T0901317, and DHA inhibited SREBP-1c at the mRNA and protein levels. Furthermore, DHA inhibited T0901317-induced lipid accumulation in primary hepatocytes of WT mice, but not in primary hepatocytes of FFA4-deficient mice. These results suggest that FFA4, Gq/11 protein, and SREBP-1c suppression are sequentially involved in liver fat deposition.
On the other hand, there are conflicting views on the receptors that DHA operates on. The various bioactivities of n-3 PUFAs depend not only on the FFA4, but also on their agonistic effects on GPR40 (free fatty acid receptor 1, FFA1). On et al. evaluated whether the anti-lipogenic effects of DHA in hepatocytes were mediated through FFA4 or GPR40 using specific agonists (compound A for FFA4 and AMG-1638 for GPR40), hepatocytes from FFA4 knockout (KO) mice, and a GPR40 selective antagonist (GW1100) [83]. Compound A did not reduce SREBP-1 and FAS protein expression in hepatocytes exposed to T0901317 or high glucose with insulin. Furthermore, DHA reduced the expression of lipogenic enzymes in FFA4-null hepatocytes. On the contrary, AMG-1638 decreased SREBP-1 and stearoyl-coenzyme A denaturase 1 (SCD-1) protein levels. Moreover, GW1100, a GPR40 antagonist, reversed the anti-lipogenic effects of DHA. Based on these results, the authors concluded that DHA reduced the expression of SREBP-1-mediated lipid-metabolizing enzymes via GPR40 but not FFA4.
The protective effect of n-3 PUFAs on NAFLD patients has been reported [84,85]. Tanaka et al. tested the efficacy of eicosapentaenoic acid (EPA), one of the major members of the n-3 PUFAs, against NASH [85]. In this study, 23 patients with biopsy-proven NASH were treated with high-purity EPA (2700 mg/d) for 12 months, and their efficacy was assessed by biochemical parameters and liver histological examination. After 12 months, serum alanine aminotransferase levels, serum-free fatty acids, plasma soluble tumor necrosis factor receptor levels, and serum ferritin and thioredoxin levels, which may reflect hepatic oxidative stress, were significantly decreased. Seven of the 23 patients underwent post-treatment liver biopsies, and six patients had liver steatosis and fibrosis. Liver improvement in hepatocyte ballooning and lobular inflammation was observed. Although this was a small-scale study and the authors did not conclude that EPA is a specific treatment for NASH, this study suggested a positive impact of EPA on NASH pathogenesis due to its anti-inflammatory and antioxidant effects. There was a recent review of clinical trials conducted on n-3 PUFAs and NAFLD/NASH [84]. Very interestingly, 13 of 17 published studies reported that n-3 PUFAs supplementation decreased liver fat, liver enzymes, or markers of inflammation; four studies suggested a reduction in ballooning and two studies suggested a reduction in fibrosis. Results also indicated that DHA was more effective than EPA in the treatment of NAFLD. Caloric restriction and supplementation with n-3 PUFAs were additive in decreasing hepatic steatosis.
4.5. Prostaglandin E Receptor 1
Prostaglandin E receptor 1 (EP1) is also a Gq-coupled receptor, with a few metabolic studies on hepatocytes. Prostaglandin E has been reported both to stimulate glycogen-phosphorylase activity (glycogenolytic effect) and to inhibit the glucagon-stimulated glycogen-phosphorylase activity (anti-glycogenolytic effect) in rat hepatocytes [86]. This conflicting event has been reported to be caused via EP1 and prostaglandin E receptor 3 (EP3, Gi-coupled receptor), respectively [86]. However, there are still many unresolved aspects of these signal analyses. The functions of EP1/3 receptors in liver metabolism require further investigation.
4.6. Serotonin Receptor
In addition to the role as a neurotransmitter in the regulation of central nervous system function, serotonin (5-hydroxytryptamine, 5-HT) has metabolic functions in the periphery [87]. The action of 5-HT is dependent on the type of 5-HT receptors (types 1–7 5-HT receptors). Serotonin receptor 2A (5-HT2A) and serotonin receptor 2B (5-HT2B) are distributed in the liver [88]. Hepatic synthetic 5-HT acting on the serotonin receptor 2 (5-HT2) has been shown to regulate lipid-induced lipid over synthesis [88]. The combination of antagonists of both 5-HT synthesis and 5-HT2 significantly inhibited hepatic steatosis, inflammation, fibrosis, hyperglycemia, and dyslipidemia in diabetic mice. Thus, activation of the 5-HT system in hepatocytes plays a vital role in the induction of diabetes-related hepatic dysfunction and may be a potential therapeutic target.
5. Role of G12/13 Signaling in Liver Metabolism
5.1. Gα12
The G12/13 family comprises two members: Gα12 and Gα13. Kim et al. examined the regulatory role of G protein α12 (Gα12) on hepatic lipid metabolism and systemic energy expenditure [89]. Hepatic levels of Gα12 were increased by fasting in the livers of mice. The fasting-induced hepatic fat accumulation was significantly enhanced in Gna12-KO mice. The cDNA microarray analysis of Gna12-KO mouse livers demonstrated that the Gα12 signaling pathway regulates SIRT1 and PPARα, both of which are involved in mitochondrial respiration. SIRT1 was poorly induced in the livers of Gna12-KO mice during fasting, but Gα12 overexpression in lentivirus-mediated hepatocytes reversed this expression. Gα12 stabilized the SIRT1 protein by inducing transcription of ubiquitin-specific peptidase 22 (USP22) via increased HIF-1a. Gna12-KO mice fed a high-fat diet were more prone to diet-induced hepatic steatosis and obesity due to reduced energy expenditure. Furthermore, as an impactful result, liver biopsies of NAFLD patients had significantly reduced Gα12 levels. These results indicate that Gα12 regulates SIRT1-dependent mitochondrial respiration via HIF-1α-dependent USP22 induction, suggesting that Ga12 is an upstream molecule that contributes to the regulation of mitochondrial energy expenditure.
Kim et al. further argued that the adenosine receptor, which was also referred to above, is relevant to Gα12 signaling [89]. In embryonic fibroblasts, treatment of WT mice-derived cells with an agonist for adenosine significantly increased SIRT1 levels, whereas SIRT levels were lower in Gα12-KO mice-derived cells. Similar results were obtained using AML12 cells, stably expressing shRNA for Gα12. In addition, as evidence of hepatocyte involvement, primary hepatocytes exposed to adenosine receptor agonists showed a significant increase in SIRT1 and USP22. In addition, serum adenosine levels in mice were significantly increased by fasting. No change was observed in liver homogenates. Taken together, increased circulating adenosine concentrations, activation of adenosine receptors, and increased Gα12 may amplify SIRT1 induction and maintain systemic energy homeostasis.
5.2. Gα13
Kim et al. also explored differences in the number of proteins regulated by Gα13 in the liver using a proteomics-based approach [90]. Among the secreted proteins enriched in the liver, they identified inter-alpha-trypsin inhibitor heavy chain 1 (ITIH1) as a critical molecule associated with metabolic abnormalities. ITIH1 is a hyaluronan-binding protein that is predominantly expressed in hepatocytes under diabetic conditions. ITIH1 levels were reduced in patients with liver fibrosis, and circulating ITIH1 mRNA levels were increased in rats with D-galactosamine-induced liver injury. Hepatic Gα13 levels were reduced under diabetic conditions, in which ITIH1 is a major driver of cross talk between organs regulated by the liver. Hepatic Gα13 increased ITIH1 overexpression via O-linked β-N-acetylglucosamine transferase catalyzed O-GlcNAcylation. Overexpressed ITIH1 in the absence of hepatic Gα13 was secreted into the circulation and bound directly to adipose tissue and skeletal muscle hyaluronan, stabilizing their respective integrity and exacerbating peripheral insulin resistance. These results indicate that a liver Gα13-mediated signaling cascade is important for systemic glucose metabolism.
6. GPCR Signaling in Relation to Metformin, PPARγ Agonist, and Statins
The relationship between GPCR signaling and drugs already widely used for diabetes and dyslipidemia, such as metformin, PPARγ agonist, and statins, should also be discussed. Although these drugs are not direct GPCR stimulators, indirect effects on GPCR signaling can be occurring.
We recently reported that activation of Gq-coupled receptors enhanced AMPK-mediated glucose uptake in muscle [91]. Whether there is a synergistic effect between hepatic AMPK activation by metformin and Gq receptor activation in the muscle (and other tissues, including liver) is a subject to be examined. Additionally, Paschoal et al. showed that GPR120 and PPARγ agonists functionally interact and markedly improve insulin resistance by using adipocytes and macrophages [92]. Also, chronic cholesterol depletion by statins in HEK cells has been shown to switch the serotonin 1A receptor (5-HT1A) endocytosis pathway from clathrin-mediated endocytosis to caveolin-mediated endocytosis [93]. Clarifying the interactions of these agents with GPCRs in the liver is an interesting topic for future research.
7. Closing Remarks
In addition to the receptors described Below (Table 1), many other GPCRs are present in hepatocytes, including many orphan receptors, but their potential roles in regulating hepatic glucose fluxes remain to be explored. An improved understanding of the metabolic functions of hepatocyte GPCRs may lead to the development of novel drugs that could prove clinically useful for the treatment of various metabolic disorders, including T2D, NAFLD, and NASH.
Table 1.
G protein-coupled receptors involved in metabolic function of hepatocytes.
| Gene Symbol (Human) | Receptor Name | Family | Transduction Mechanisms | |||
|---|---|---|---|---|---|---|
| GCGR | Glucagon receptor (GCGR) | Glucagon receptors | Gs | |||
| TRHR | Thyrotropin-releasing hormone receptor (TRH) | Thyrotropin-releasing hormone receptors | Gs | Gq | ||
| ADRB1 | β1-adrenoceptor (B1AR) | Adrenoceptors | Gs | Gi | ||
| ADRB2 | β2-adrenoceptor (B2AR) | Adrenoceptors | Gs | Gi | ||
| PTGER4 | Prostaglandin E receptor 4 (EP4) | Prostanoid receptors | Gs | Gi | Gq | |
| S1PR2 | Sphingosine-1-phosphate receptor 2 (S1PR2) | Lysophospholipid (S1P) receptors | Gs | Gq | G12/13 | |
| CNR1 | Cannabinoid receptor 1 (CB1) | Cannabinoid receptors | Gi | Gs | ||
| ADORA1 | Adenosine A1 receptor (A1) | Adenosine receptors | Gi | Gq | Gs | G12/13 |
| ADORA3 | Adenosine A3 receptor (A3) | Adenosine receptors | Gi | G12/13 | ||
| HCAR2 | Hydroxycarboxylic acid receptor 2 (HCA2, GPR109A) | Hydroxycarboxylic acid receptors | Gi | |||
| CCR2 | C-C chemokine receptor type 2 (CCR2) | Chemokine receptors | Gi | |||
| CCR5 | C-C chemokine receptor type 5 (CCR5) | Chemokine receptors | Gi | |||
| P2RY13 | Purinergic receptor P2Y13 (P2Y13) | P2Y receptors | Gi | |||
| SMO | Smoothened receptor (SMO) | Class Frizzled GPCRs | Gi | G12/13 | ||
| AVPR1A | Arginine vasopressin receptor 1A (AVPR1A) | Vasopressin and oxytocin receptors | Gq | |||
| OXTR | Oxytocin receptor (OT) | Vasopressin and oxytocin receptors | Gq | Gi | ||
| P2RY1 | Purinergic receptor P2Y1 (P2Y1) | P2Y receptors | Gq | Gi | ||
| P2RY2 | Purinergic receptor P2Y2 (P2Y2) | P2Y receptors | Gq | Gi | G12/13 | |
| P2RY4 | Purinergic receptor P2Y4 (P2Y3) | P2Y receptors | Gq | |||
| P2RY6 | Purinergic receptor P2Y6 (P2Y6) | P2Y receptors | Gq | G12/13 | ||
| AGTR1 | Angiotensin II receptor type 1 (AT1) | Angiotensin receptors | Gq | Gi | ||
| FFAR4 | Free fatty acid receptor 4 (FFA4, CPR120) | Free fatty acid receptors | Gq | |||
| FFAR1 | Free fatty acid receptor 1 (FFA1, GPR40) | Free fatty acid receptors | Gq | Gs | Gi | |
| PTGER1 | Prostaglandin E receptor 1 (EP1) | Prostanoid receptors | Gq | Gi | ||
| HTR2A | 5-hydroxytryptamine receptor 2A (5-HT2A) | 5-Hydroxytryptamine receptors | Gq | Gi | ||
| HTR2B | 6-hydroxytryptamine receptor 2B (5-HT2B) | 5-Hydroxytryptamine receptors | Gq | |||
Acknowledgments
We sincerely thank Jurgen Wess for his mentorship.
Author Contributions
S.P.P. and T.K. contributed equally to the conception, writing, reviewing, and editing of this manuscript. J.P. and N.T. supervised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
T.K. was supported by JSPS research fellowship for Japanese biomedical and behavioral researchers at NIH (T.K., Number 71704).
Conflicts of Interest
The authors declare no conflict of interest with this paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramskold D., Wang E.T., Burge C.B., Sandberg R. An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoS Comput. Biol. 2009;5:e1000598. doi: 10.1371/journal.pcbi.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rui L. Energy metabolism in the liver. Compr. Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hers H.G. The control of glycogen metabolism in the liver. Annu. Rev. Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- 4.Rines A.K., Sharabi K., Tavares C.D., Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2016;15:786–804. doi: 10.1038/nrd.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postic C., Dentin R., Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004;30:398–408. doi: 10.1016/S1262-3636(07)70133-7. [DOI] [PubMed] [Google Scholar]
- 6.Grant D.M. Detoxification pathways in the liver. J. Inherit. Metab. Dis. 1991;14:421–430. doi: 10.1007/BF01797915. [DOI] [PubMed] [Google Scholar]
- 7.Trefts E., Gannon M., Wasserman D.H. The liver. Curr. Biol. 2017;27:R1147–R1151. doi: 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordillo M., Evans T., Gouon-Evans V. Orchestrating liver development. Development. 2015;142:2094–2108. doi: 10.1242/dev.114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuppan D., Afdhal N.H. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenne C.N., Kubes P. Immune surveillance by the liver. Nat. Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 11.Ding C., Li Y., Guo F., Jiang Y., Ying W., Li D., Yang D., Xia X., Liu W., Zhao Y., et al. A Cell-type-resolved Liver Proteome. Mol. Cell Proteom. 2016;15:3190–3202. doi: 10.1074/mcp.M116.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kmiec Z. Cooperation of liver cells in health and disease. Adv. Anat. Embryol. Cell Biol. 2001;161 doi: 10.1007/978-3-642-56553-3. [DOI] [PubMed] [Google Scholar]
- 13.Lagerstrom M.C., Schioth H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 14.Lefkowitz R.J. Historical review: A brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Denis C., Sauliere A., Galandrin S., Senard J.M., Gales C. Probing heterotrimeric G protein activation: Applications to biased ligands. Curr. Pharm. Des. 2012;18:128–144. doi: 10.2174/138161212799040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syrovatkina V., Alegre K.O., Dey R., Huang X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016;428:3850–3868. doi: 10.1016/j.jmb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tesmer J.J. The quest to understand heterotrimeric G protein signaling. Nat. Struct. Mol. Biol. 2010;17:650–652. doi: 10.1038/nsmb0610-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jastrzebska B. GPCR: G protein complexes--the fundamental signaling assembly. Amino Acids. 2013;45:1303–1314. doi: 10.1007/s00726-013-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng W.T., Sun W.Y., Li X.R., Sun J.C., Du J.J., Wei W. Emerging Roles of G Protein-Coupled Receptors in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2018;19:1366. doi: 10.3390/ijms19051366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi M., Zhu L., McMillin S.M., Pydi S.P., Jain S., Wang L., Cui Y., Lee R.J., Cohen A.H., Kaneto H., et al. Hepatic Gi signaling regulates whole-body glucose homeostasis. J. Clin. Investig. 2018;128:746–759. doi: 10.1172/JCI94505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J.H., Jain S., McMillin S.M., Cui Y., Gautam D., Sakamoto W., Lu H., Jou W., McGuinness O.P., Gavrilova O., et al. A novel experimental strategy to assess the metabolic effects of selective activation of a G(q)-coupled receptor in hepatocytes in vivo. Endocrinology. 2013;154:3539–3551. doi: 10.1210/en.2012-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husted A.S., Trauelsen M., Rudenko O., Hjorth S.A., Schwartz T.W. GPCR-Mediated Signaling of Metabolites. Cell Metab. 2017;25:777–796. doi: 10.1016/j.cmet.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Regard J.B., Sato I.T., Coughlin S.R. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UPHAR/BPS Guide to Pharmacology. [(accessed on 14 September 2020)]; Available online: https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=694.
- 25.Janah L., Kjeldsen S., Galsgaard K.D., Winther-Sorensen M., Stojanovska E., Pedersen J., Knop F.K., Holst J.J., Wewer Albrechtsen N.J. Glucagon Receptor Signaling and Glucagon Resistance. Int. J. Mol. Sci. 2019;20:3314. doi: 10.3390/ijms20133314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wewer Albrechtsen N.J., Pedersen J., Galsgaard K.D., Winther-Sorensen M., Suppli M.P., Janah L., Gromada J., Vilstrup H., Knop F.K., Holst J.J. The Liver-alpha-Cell Axis and Type 2 Diabetes. Endocr. Rev. 2019;40:1353–1366. doi: 10.1210/er.2018-00251. [DOI] [PubMed] [Google Scholar]
- 27.Ramnanan C.J., Edgerton D.S., Kraft G., Cherrington A.D. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes. Metab. 2011;13(Suppl. 1):118–125. doi: 10.1111/j.1463-1326.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felig P., Wahren J., Sherwin R., Hendler R. Insulin, glucagon, and somatostatin in normal physiology and diabetes mellitus. Diabetes. 1976;25:1091–1099. doi: 10.2337/diab.25.12.1091. [DOI] [PubMed] [Google Scholar]
- 29.Berglund E.D., Lee-Young R.S., Lustig D.G., Lynes S.E., Donahue E.P., Camacho R.C., Meredith M.E., Magnuson M.A., Charron M.J., Wasserman D.H. Hepatic energy state is regulated by glucagon receptor signaling in mice. J. Clin. Investig. 2009;119:2412–2422. doi: 10.1172/JCI38650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozcan L., Wong C.C., Li G., Xu T., Pajvani U., Park S.K., Wronska A., Chen B.X., Marks A.R., Fukamizu A., et al. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metab. 2012;15:739–751. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galsgaard K.D., Pedersen J., Knop F.K., Holst J.J., Wewer Albrechtsen N.J. Glucagon Receptor Signaling and Lipid Metabolism. Front. Physiol. 2019;10:413. doi: 10.3389/fphys.2019.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim T., Holleman C.L., Nason S., Arble D.M., Ottaway N., Chabenne J., Loyd C., Kim J.A., Sandoval D., Drucker D.J., et al. Hepatic Glucagon Receptor Signaling Enhances Insulin-Stimulated Glucose Disposal in Rodents. Diabetes. 2018;67:2157–2166. doi: 10.2337/db18-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boland M.L., Laker R.C., Mather K., Nawrocki A., Oldham S., Boland B.B., Lewis H., Conway J., Naylor J., Guionaud S., et al. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat. Metab. 2020;2:413–431. doi: 10.1038/s42255-020-0209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Wang L., Zhou L., Song Y., Ma S., Yu C., Zhao J., Xu C., Gao L. Thyroid stimulating hormone increases hepatic gluconeogenesis via CRTC2. Mol. Cell Endocrinol. 2017;446:70–80. doi: 10.1016/j.mce.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Yang S., Chen J., Su Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. 2018;9:802. doi: 10.3389/fendo.2018.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh J.H., Sieglaff D.H., Zhang A., Xia X., Cvoro A., Winnier G.E., Webb P. SIRT1 is a direct coactivator of thyroid hormone receptor beta1 with gene-specific actions. PLoS ONE. 2013;8:e70097. doi: 10.1371/journal.pone.0070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dax E.M., Partilla J.S., Pineyro M.A., Gregerman R.I. Beta-adrenergic receptors, glucagon receptors, and their relationship to adenylate cyclase in rat liver during aging. Endocrinology. 1987;120:1534–1541. doi: 10.1210/endo-120-4-1534. [DOI] [PubMed] [Google Scholar]
- 38.Katz M.S., McNair C.L., Hymer T.K., Boland S.R. Emergence of beta adrenergic-responsive hepatic glycogenolysis in male rats during post-maturational aging. Biochem. Biophys. Res. Commun. 1987;147:724–730. doi: 10.1016/0006-291X(87)90990-9. [DOI] [PubMed] [Google Scholar]
- 39.Arner P., Engfeldt P., Hellstrom L., Lonnqvist F., Wahrenberg H., Sonnenfeld T., Bronnegard M. Beta-adrenoreceptor subtype expression in human liver. J. Clin. Endocrinol. Metab. 1990;71:1119–1126. doi: 10.1210/jcem-71-5-1119. [DOI] [PubMed] [Google Scholar]
- 40.Aggerbeck M., Ferry N., Zafrani E.S., Billon M.C., Barouki R., Hanoune J. Adrenergic regulation of glycogenolysis in rat liver after cholestasis. Modulation of the balance between alpha 1 and beta 2 receptors. J. Clin. Investig. 1983;71:476–486. doi: 10.1172/JCI110792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh P.M., Shu Z.J., Zhu B., Lu Z., Ikeno Y., Barnes J.L., Yeh C.K., Zhang B.X., Katz M.S., Kamat A. Role of beta-adrenergic receptors in regulation of hepatic fat accumulation during aging. J. Endocrinol. 2012;213:251–261. doi: 10.1530/JOE-11-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erraji-Benchekroun L., Couton D., Postic C., Borde I., Gaston J., Guillet J.G., Andre C. Overexpression of beta2-adrenergic receptors in mouse liver alters the expression of gluconeogenic and glycolytic enzymes. Am. J. Physiol. Endocrinol. Metab. 2005;288:E715–E722. doi: 10.1152/ajpendo.00113.2004. [DOI] [PubMed] [Google Scholar]
- 43.Ong K.T., Mashek M.T., Bu S.Y., Greenberg A.S., Mashek D.G. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan S.A., Sathyanarayan A., Mashek M.T., Ong K.T., Wollaston-Hayden E.E., Mashek D.G. ATGL-catalyzed lipolysis regulates SIRT1 to control PGC-1alpha/PPAR-alpha signaling. Diabetes. 2015;64:418–426. doi: 10.2337/db14-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reid B.N., Ables G.P., Otlivanchik O.A., Schoiswohl G., Zechner R., Blaner W.S., Goldberg I.J., Schwabe R.F., Chua S.C., Jr., Huang L.S. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasui M., Tamura Y., Minami M., Higuchi S., Fujikawa R., Ikedo T., Nagata M., Arai H., Murayama T., Yokode M. The Prostaglandin E2 Receptor EP4 Regulates Obesity-Related Inflammation and Insulin Sensitivity. PLoS ONE. 2015;10:e0136304. doi: 10.1371/journal.pone.0136304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higuchi S., Fujikawa R., Nakatsuji M., Yasui M., Ikedo T., Nagata M., Mishima K., Irie K., Matsumoto M., Yokode M., et al. EP4 receptor-associated protein regulates gluconeogenesis in the liver and is associated with hyperglycemia in diabetic mice. Am. J. Physiol. Endocrinol. Metab. 2019;316:E410–E417. doi: 10.1152/ajpendo.00035.2018. [DOI] [PubMed] [Google Scholar]
- 48.Kwong E., Li Y., Hylemon P.B., Zhou H. Bile acids and sphingosine-1-phosphate receptor 2 in hepatic lipid metabolism. Acta Pharm. Sin. B. 2015;5:151–157. doi: 10.1016/j.apsb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Studer E., Zhou X., Zhao R., Wang Y., Takabe K., Nagahashi M., Pandak W.M., Dent P., Spiegel S., Shi R., et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang Y., Studer E., Mitchell C., Grant S., Pandak W.M., Hylemon P.B., Dent P. Conjugated bile acids regulate hepatocyte glycogen synthase activity in vitro and in vivo via Galphai signaling. Mol. Pharmacol. 2007;71:1122–1128. doi: 10.1124/mol.106.032060. [DOI] [PubMed] [Google Scholar]
- 51.Cao R., Cronk Z.X., Zha W., Sun L., Wang X., Fang Y., Studer E., Zhou H., Pandak W.M., Dent P., et al. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(alpha)i-protein-coupled receptors and the AKT pathway. J. Lipid Res. 2010;51:2234–2244. doi: 10.1194/jlr.M004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagahashi M., Takabe K., Liu R., Peng K., Wang X., Wang Y., Hait N.C., Wang X., Allegood J.C., Yamada A., et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015;61:1216–1226. doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J., Zhou L., Xiong K., Godlewski G., Mukhopadhyay B., Tam J., Yin S., Gao P., Shan X., Pickel J., et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142:1218–1228. doi: 10.1053/j.gastro.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bazwinsky-Wutschke I., Zipprich A., Dehghani F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int. J. Mol. Sci. 2019;20:2516. doi: 10.3390/ijms20102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Mariscal I., Krzysik-Walker S.M., Doyle M.E., Liu Q.R., Cimbro R., Santa-Cruz Calvo S., Ghosh S., Ciesla L., Moaddel R., Carlson O.D., et al. Human CB1 Receptor Isoforms, present in Hepatocytes and beta-cells, are Involved in Regulating Metabolism. Sci. Rep. 2016;6:33302. doi: 10.1038/srep33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J., Godlewski G., Jourdan T., Liu Z., Cinar R., Xiong K., Kunos G. Cannabinoid-1 Receptor Antagonism Improves Glycemic Control and Increases Energy Expenditure Through Sirtuin-1/Mechanistic Target of Rapamycin Complex 2 and 5’Adenosine Monophosphate-Activated Protein Kinase Signaling. Hepatology. 2019;69:1535–1548. doi: 10.1002/hep.30364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buxton D.B., Fisher R.A., Robertson S.M., Olson M.S. Stimulation of glycogenolysis and vasoconstriction by adenosine and adenosine analogues in the perfused rat liver. Biochem. J. 1987;248:35–41. doi: 10.1042/bj2480035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez-Benitez E., Guinzberg R., Diaz-Cruz A., Pina E. Regulation of glycogen metabolism in hepatocytes through adenosine receptors. Role of Ca2+ and cAMP. Eur. J. Pharmacol. 2002;437:105–111. doi: 10.1016/S0014-2999(02)01299-2. [DOI] [PubMed] [Google Scholar]
- 59.Dixon A.K., Gubitz A.K., Sirinathsinghji D.J., Richardson P.J., Freeman T.C. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salvatore C.A., Jacobson M.A., Taylor H.E., Linden J., Johnson R.G. Molecular cloning and characterization of the human A3 adenosine receptor. Proc. Natl. Acad. Sci. USA. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X., Millar J.S., Brownell N., Briand F., Rader D.J. Modulation of HDL metabolism by the niacin receptor GPR109A in mouse hepatocytes. Biochem. Pharmacol. 2010;80:1450–1457. doi: 10.1016/j.bcp.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koupenova M., Ravid K. Adenosine, adenosine receptors and their role in glucose homeostasis and lipid metabolism. J. Cell Physiol. 2013 doi: 10.1002/jcp.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambade A., Lowe P., Kodys K., Catalano D., Gyongyosi B., Cho Y., Iracheta-Vellve A., Adejumo A., Saha B., Calenda C., et al. Pharmacological Inhibition of CCR2/5 Signaling Prevents and Reverses Alcohol-Induced Liver Damage, Steatosis, and Inflammation in Mice. Hepatology. 2019;69:1105–1121. doi: 10.1002/hep.30249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacquet S., Malaval C., Martinez L.O., Sak K., Rolland C., Perez C., Nauze M., Champagne E., Terce F., Gachet C., et al. The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell Mol. Life Sci. 2005;62:2508–2515. doi: 10.1007/s00018-005-5194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fabre A.C., Malaval C., Ben Addi A., Verdier C., Pons V., Serhan N., Lichtenstein L., Combes G., Huby T., Briand F., et al. P2Y13 receptor is critical for reverse cholesterol transport. Hepatology. 2010;52:1477–1483. doi: 10.1002/hep.23897. [DOI] [PubMed] [Google Scholar]
- 66.Omenetti A., Choi S., Michelotti G., Diehl A.M. Hedgehog signaling in the liver. J. Hepatol. 2011;54:366–373. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwon H., Song K., Han C., Chen W., Wang Y., Dash S., Lim K., Wu T. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease. Hepatology. 2016;63:1155–1169. doi: 10.1002/hep.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howl J., Ismail T., Strain A.J., Kirk C.J., Anderson D., Wheatley M. Characterization of the human liver vasopressin receptor. Profound differences between human and rat vasopressin-receptor-mediated responses suggest only a minor role for vasopressin in regulating human hepatic function. Pt 1Biochem. J. 1991;276:189–195. doi: 10.1042/bj2760189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X., Luo G., Jiang J., Ma T., Lin X., Jiang L., Cheng J., Tao R. Signaling through hepatocyte vasopressin receptor 1 protects mouse liver from ischemia-reperfusion injury. Oncotarget. 2016;7:69276–69290. doi: 10.18632/oncotarget.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Altirriba J., Poher A.L., Caillon A., Arsenijevic D., Veyrat-Durebex C., Lyautey J., Dulloo A., Rohner-Jeanrenaud F. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology. 2014;155:4189–4201. doi: 10.1210/en.2014-1466. [DOI] [PubMed] [Google Scholar]
- 71.Arino J., Bosch F., Gomez-Foix A.M., Guinovart J.J. Oxytocin inactivates and phosphorylates rat hepatocyte glycogen synthase. Biochem. J. 1989;261:827–830. doi: 10.1042/bj2610827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Combettes L., Tran D., Tordjmann T., Laurent M., Berthon B., Claret M. Ca(2+)-mobilizing hormones induce sequentially ordered Ca2+ signals in multicellular systems of rat hepatocytes. Pt 2Biochem. J. 1994;304:585–594. doi: 10.1042/bj3040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keppens S., de Wulf H. The activation of liver glycogen phosphorylase by vasopressin. FEBS Lett. 1975;51:29–32. doi: 10.1016/0014-5793(75)80848-9. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura K., Velho G., Bouby N. Vasopressin and metabolic disorders: Translation from experimental models to clinical use. J. Intern. Med. 2017;282:298–309. doi: 10.1111/joim.12649. [DOI] [PubMed] [Google Scholar]
- 75.Zelena D., Mergl Z., Makara G.B. The role of vasopressin in diabetes mellitus-induced hypothalamo-pituitary-adrenal axis activation: Studies in Brattleboro rats. Brain Res. Bull. 2006;69:48–56. doi: 10.1016/j.brainresbull.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 76.Sharma R.J., Rodrigues L.M., Whitton P.D., Hems D.A. Control mechanisms in the acceleration of hepatic glycogen degradation during hypoxia. Biochim. Biophys. Acta. 1980;630:414–424. doi: 10.1016/0304-4165(80)90290-1. [DOI] [PubMed] [Google Scholar]
- 77.Ding C., Leow M.K., Magkos F. Oxytocin in metabolic homeostasis: Implications for obesity and diabetes management. Obes. Rev. 2019;20:22–40. doi: 10.1111/obr.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dixon C.J., Hall J.F., Webb T.E., Boarder M.R. Regulation of rat hepatocyte function by P2Y receptors: Focus on control of glycogen phosphorylase and cyclic AMP by 2-methylthioadenosine 5’-diphosphate. J. Pharmacol. Exp. Ther. 2004;311:334–341. doi: 10.1124/jpet.104.067744. [DOI] [PubMed] [Google Scholar]
- 79.Tackett B.C., Sun H., Mei Y., Maynard J.P., Cheruvu S., Mani A., Hernandez-Garcia A., Vigneswaran N., Karpen S.J., Thevananther S. P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G1073–G1087. doi: 10.1152/ajpgi.00092.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dasgupta C., Zhang L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov. Today. 2011;16:22–34. doi: 10.1016/j.drudis.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanni S.J., Lyngso C., Gammeltoft S., Hansen J.L. [Sar1, Ile4, Ile8]-angiotensin II Potentiates Insulin Receptor Signalling and Glycogen Synthesis in Hepatocytes. Basic Clin. Pharmacol. Toxicol. 2018;122:460–469. doi: 10.1111/bcpt.12937. [DOI] [PubMed] [Google Scholar]
- 82.Kang S., Huang J., Lee B.K., Jung Y.S., Im E., Koh J.M., Im D.S. Omega-3 polyunsaturated fatty acids protect human hepatoma cells from developing steatosis through FFA4 (GPR120) Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:105–116. doi: 10.1016/j.bbalip.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 83.On S., Kim H.Y., Kim H.S., Park J., Kang K.W. Involvement of G-Protein-Coupled Receptor 40 in the Inhibitory Effects of Docosahexaenoic Acid on SREBP1-Mediated Lipogenic Enzyme Expression in Primary Hepatocytes. Int. J. Mol. Sci. 2019;20:2625. doi: 10.3390/ijms20112625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelley N.S. Treatment of Nonalcoholic Fatty Liver Disease with Long-Chain n-3 Polyunsaturated Fatty Acids in Humans. Metab. Syndr. Relat. Disord. 2016;14:417–430. doi: 10.1089/met.2016.0051. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka N., Sano K., Horiuchi A., Tanaka E., Kiyosawa K., Aoyama T. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2008;42:413–418. doi: 10.1097/MCG.0b013e31815591aa. [DOI] [PubMed] [Google Scholar]
- 86.Puschel G.P., Kirchner C., Schroder A., Jungermann K. Glycogenolytic and antiglycogenolytic prostaglandin E2 actions in rat hepatocytes are mediated via different signalling pathways. Eur. J. Biochem. 1993;218:1083–1089. doi: 10.1111/j.1432-1033.1993.tb18468.x. [DOI] [PubMed] [Google Scholar]
- 87.Berger M., Gray J.A., Roth B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu J., Li C., Zhang G., Tong X., Zhang H., Ding J., Ma Y., Cheng R., Hou S., An S., et al. Crucial Roles of 5-HT and 5-HT2 Receptor in Diabetes-Related Lipid Accumulation and Pro-Inflammatory Cytokine Generation in Hepatocytes. Cell Physiol. Biochem. 2018;48:2409–2428. doi: 10.1159/000492656. [DOI] [PubMed] [Google Scholar]
- 89.Kim T.H., Yang Y.M., Han C.Y., Koo J.H., Oh H., Kim S.S., You B.H., Choi Y.H., Park T.S., Lee C.H., et al. Galpha12 ablation exacerbates liver steatosis and obesity by suppressing USP22/SIRT1-regulated mitochondrial respiration. J. Clin. Investig. 2018;128:5587–5602. doi: 10.1172/JCI97831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim T.H., Koo J.H., Heo M.J., Han C.Y., Kim Y.I., Park S.Y., Cho I.J., Lee C.H., Choi C.S., Lee J.W., et al. Overproduction of inter-alpha-trypsin inhibitor heavy chain 1 after loss of Galpha13 in liver exacerbates systemic insulin resistance in mice. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aan4735. [DOI] [PubMed] [Google Scholar]
- 91.Bone D.B.J., Meister J., Knudsen J.R., Dattaroy D., Cohen A., Lee R., Lu H., Metzger D., Jensen T.E., Wess J. Skeletal Muscle-Specific Activation of Gq Signaling Maintains Glucose Homeostasis. Diabetes. 2019;68:1341–1352. doi: 10.2337/db18-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paschoal V.A., Walenta E., Talukdar S., Pessentheiner A.R., Osborn O., Hah N., Chi T.J., Tye G.L., Armando A.M., Evans R.M., et al. Positive Reinforcing Mechanisms between GPR120 and PPARgamma Modulate Insulin Sensitivity. Cell Metab. 2020;31:1173–1188.e5. doi: 10.1016/j.cmet.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar G.A., Chattopadhyay A. Statin-Induced Chronic Cholesterol Depletion Switches GPCR Endocytosis and Trafficking: Insights from the Serotonin1A Receptor. ACS Chem. Neurosci. 2020;11:453–465. doi: 10.1021/acschemneuro.9b00659. [DOI] [PubMed] [Google Scholar]