Abstract
Background
The Rio Doce estuary, in Brazil, was impacted by the deposition of iron mine tailings, caused by the collapse of a dam in 2015. Based on published baseline datasets, the estuary has been experiencing chronic trace metal contamination effects since 2017, with potential bioaccumulation in fishes and human health risks. As metal and metalloid concentrations in aquatic ecosystems pose severe threats to the aquatic biota, we hypothesized that the trace metals in estuarine sediments nearly two years after the disaster would lead to bioaccumulation in demersal fishes and result in the biosynthesis of metal-responsive proteins.
Methods
We measured As, Cd, Cr, Cu, Fe, Mn, Pb, Se and Zn concentrations in sediment samples in August 2017 and compared to published baseline levels. Also, trace metals (As, Cd, Cr, Cu, Fe, Hg, Mn, Pb, Se and Zn) and protein (metallothionein and reduced glutathione) concentrations were quantified in the liver and muscle tissues of five fish species (Cathorops spixii, Genidens genidens, Eugerres brasilianus, Diapterus rhombeus and Mugil sp.) from the estuary, commonly used as food sources by local populations.
Results
Our results revealed high trace metal concentrations in estuarine sediments, when compared to published baseline values for the same estuary. The demersal fish species C. spixii and G. genidens had the highest concentrations of As, Cr, Mn, Hg, and Se in both, hepatic and muscle, tissues. Trace metal bioaccumulation in fish was correlated with the biosynthesis of metallothionein and reduced glutathione in both, liver and muscle, tissues, suggesting active physiological responses to contamination sources. The trace metal concentrations determined in fish tissues were also present in the estuarine sediments at the time of this study. Some elements had concentrations above the maximum permissible limits for human consumption in fish muscles (e.g., As, Cr, Mn, Se and Zn), suggesting potential human health risks that require further studies. Our study supports the high biogeochemical mobility of toxic elements between sediments and the bottom-dwelling biota in estuarine ecosystems.
Keywords: Rio Doce, Health risk assessment, Metalloproteins, Environmental pollution, Estuaries
Introduction
Estuaries are among the most threatened coastal ecosystems and are continually impacted by anthropogenic activities, which often increase the input of organic and inorganic pollutants to the water and sediment (Muniz et al., 2006; Hadlich et al., 2018; Lu et al., 2018; Varzim et al., 2019). Pollutants released into estuarine ecosystems include toxic metals and metalloids that are stable, and considered persistent environmental contaminants, that is, they are not biodegraded (Gómez-Parra et al., 2000; Garcia-Ordiales et al., 2018). The released contaminants typically decrease water and sediment quality, with impacts to estuarine biodiversity and productivity (Lotze et al., 2006). Therefore, understanding the fate and ecological risks of pollutants is critical to access environmental risks associated with their presence in coastal and marine ecosystems (Mayer-Pinto, Matias & Coleman, 2016).
In November 2015, the Rio Doce estuary in southeast Brazil, was severely impacted by the collapse of an iron mine tailing dam, located 600 km upstream. It is estimated that 43 million m3 of iron mine tailings were released and severely affected riverine and riparian ecosystems along the path to the estuary and Atlantic Ocean (Do Carmo et al., 2017). Although the released tailings at the breached dam were mainly composed of non-toxic minerals (Almeida et al., 2018), the high content of Fe-oxyhydroxides (goethite—FeOOH, hematite—Fe2O3) may have promoted chemical binding of metals and metalloids accumulated within the basin during decades of human impacts. As a result, the tailings exhibit high potential for element mobility, especially for Al, As, Ba, Fe, Mn, Pb and Sr, which may be potentially bound to Fe oxides (Segura et al., 2016). Once the tailings reached the estuary, in November 2015, fine sediments containing high concentrations of some elements were immediately deposited on the bottom, raising ecological concerns regarding the long term risks to the estuarine and coastal ecosystems (De Oliveira Gomes et al., 2017; Queiroz et al., 2018; Richard et al., 2020). The tailings initially impacted benthic and fish assemblages in the Rio Doce estuary (De Oliveira Gomes et al., 2017; Andrades et al., 2020). In 2017, nearly two years after the initial impact, chronic effects from trace metal contamination on benthic assemblages were evident (Bernardino et al., 2019). Large quantities of these tailings are currently deposited along upriver floodplains and river banks, which could be continually transported to the estuary and sustain high levels of contaminants. Given the potential mobility of elements between estuarine sediments and organisms (Queiroz et al., 2018), the extent of the trace metal contamination and the effects to fisheries in the estuary are still unclear.
Chemical elements often bioaccumulate in aquatic organisms, causing a range of sub-lethal effects, such as metabolism depression, diseases, and genotoxic damage (Goyer & Clarkson, 1996; Riba et al., 2005). As a result, fishes and other fishery sources may become bioindicators of metal contamination and be used as a proxy for human health risks (Carrola et al., 2014; Lavradas et al., 2014; Ahmed et al., 2015; Gusso-Choueri et al., 2016; Gu et al., 2018). Contamination effects in fishes can be immediately detected by a range of biochemical indicators (or biomarkers), which are widely applied in environmental monitoring programs (Van Der Oost, Beyer & Vermeulen, 2003; Hauser-Davis, Campos & Ziolli, 2012). Certain proteins are specific biomarkers for metal contamination in fish, which are synthesized to act in detoxification mechanisms (Atli & Canli, 2008). These biomarkers may aid in metal sequestration in tissues, subsequent detoxification, and act against oxidative stress (Forman, Zhang & Rinna, 2009; Ruttkay-Nedecky et al., 2013). Metallothioneins (MTs) are low molecular weight proteins that act in the homeostasis of essential trace elements (e.g., Cu and Zn) and in detoxification processes (e.g., As, Cd, Pb, Hg, among others; Hauser-Davis et al., 2014; Kehring et al., 2016). Metallothionein expression increases above certain trace metal contamination thresholds, as the presence of the thiol groups in cysteine residues allows MTs to bind to specific elements. This process protects the organism from trace metal toxicity through immobilization, metabolic unavailability, and subsequent detoxification, occurring mainly in the liver or organs with equivalent function (Kehring et al., 2016; Okay et al., 2016; Pacheco et al., 2017; Van Ael, Blust & Bervoets, 2017). As a result, liver tissues may exhibit high levels of elements when MTs are not efficient at detoxifying these contaminants. Another biomarker, the tripeptide reduced glutathione (GSH, γ-L-glutamyl-L-cysteinyl-glycine), is an important intracellular antioxidant and defense mechanism, which intervenes against intracellular oxidative stress-induced toxicity (Lavradas et al., 2014; Kehring et al., 2016). The sulfhydryl group (–SH), present in cysteine, is involved in protective glutathione functions (reduction and conjugation reactions; Meister, 1992), and protect cells against heavy metal ions (Singhal, Anderson & Meister, 1987).
Previous studies have showed synthesis of MTs and GSH in response to exposure of an aquatic animal to toxic elements (Lavradas et al., 2014; Souza et al., 2018). Biomarker synthesis was observed in liver, muscle, and kidney tissues that work on the capture, storage, and excretion of toxic elements in a variety of aquatic organisms including mussels, crabs, fish, and marine mammals (Lavradas et al., 2014, 2016; Souza et al., 2018; Monteiro et al., 2019). It is noteworthy that similar effects were observed in contaminated freshwater ecosystems in the Rio Doce basin following the Samarco disaster, with fish exhibiting an induction of the proteins and enzymes expression related to contamination and hepatic damage (Weber et al., 2020).
Fish is an important food source for human communities on the coast. The determination of element concentrations in fish species is crucial to improve food quality assessments, and to prevent ingestion of potentially harmful food items by vulnerable local communities (Hauser-Davis et al., 2016; Van Ael, Blust & Bervoets, 2017; Coimbra et al., 2018). Consumption of fish liver, including from Mugil sp., is considered a delicacy in some traditional communities (Hauser-Davis et al., 2016), and could highly increase contamination effects through human consumption. Demersal fishes in particular, typically used as food items by Rio Doce communities, can indicate the presence of bioavailable metals in the environment, because they are in close contact with the bottom sediments and accumulated tailings, including associated elements, and may be a key to evaluating potential threats to humans.
Given the potential of chronic bioaccumulation effects from trace metals in the Rio Doce estuary, this study has quantified trace metal contamination and the expression of two detoxification proteins on fish captured nearly two years after the tailings arrival. Our aims were: (i) to determine the trace metal concentrations in sediments, and compare them with published pre-impact reference values and with international sediment quality guidelines (SQGs); (ii) to quantify trace metal contents in the muscle and liver tissues of demersal fish species, and to compare these values with maximum residue levels (MRL) in food items; and (iii) to determine concentrations of oxidative defense and metal detoxification biomarkers in the muscle and liver tissue of five fish species to reveal active physiological responses to trace metal contamination in fish. Our hypothesis was that chronic exposure to contaminated sediments, for 1.7 years following the disaster, would lead to the assimilation of trace metals and to the expression of oxidative defenses in fish. It is well known that metal accumulation in organisms is generally higher in the liver, as it is one of the most important organs for metal detoxification (Hauser-Davis et al., 2016). The metal accumulation in muscle tissues may thus indicate high levels of environmental contamination, suggesting that the liver’s capacity for excretion was exceeded, resulting in bioaccumulation into muscle tissues. Additionally, we have compared metal and metalloid concentrations in fish to reference values from other polluted and pristine estuaries, as well as to Brazilian and international guidelines. This study provides a timely and critical assessment of bioaccumulation in fish that are used for the subsistence of villagers that rely on fisheries from the estuary, and highlights the cumulative consequences of mine tailings to aquatic systems and humans.
Materials and Methods
Study area and sampling
The Rio Doce estuary is located in the Eastern Brazil Marine Ecoregion (19° 38′–19° 45′ S and 39° 45′–39° 55′ W). The area has two well-defined seasons, a dry winter (April to September) and a rainy summer (October to March), with an average monthly rainfall of 145 mm and temperature of 25 °C (Bernardino et al., 2015; Bissoli & Bernardino, 2018). The estuary is characterized by a main channel with sand pockets that form at low tide, with water salinity ranging from 0 to 5 (De Oliveira Gomes et al., 2017). The estuary is used by local villagers as a source of subsistence through fisheries and tourism, and estuarine fishes (Mugil sp. and Eugerres brasilianus) are important food sources (Pinheiro & Joyeux, 2007).
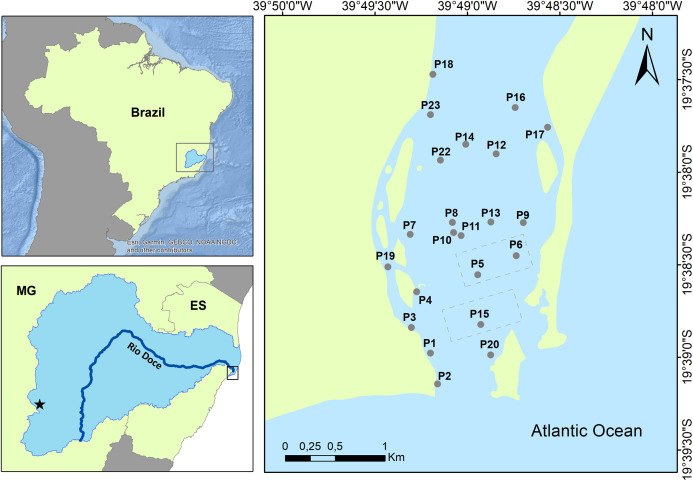
The great magnitude of the disaster made it practically impossible to establish control sampling sites, because the entire estuary was highly impacted by the mining tailings. However, evidence indicates increased trace metal contamination in the estuarine sediments, associated with the tailings arrival in November 2015 and subsequent negative effects on both benthic and fish assemblages (De Oliveira Gomes et al., 2017; Queiroz et al., 2018; Andrades et al., 2020; Richard et al., 2020). In August 2017, sediment trace metal concentrations were still significantly above baseline levels in the estuary, with measurable effects on benthic assemblages (Bernardino et al., 2019). Herein, we used the same sediment sampling stations as Bernardino et al. (2019) to determine estuarine contamination nearly two years after the disaster, with additional fish sampling in common fishing areas in the estuary to evaluate potential bioaccumulation effects (Fig. 1).
Figure 1. Map of the sampling stations in the Rio Doce estuary, Brazil in August 2017.
Fundão dam failure area (star), sediment sampling (circles), and fish sampling areas (rectangle).
Qualitative fish sampling was conducted at two sites in the estuary, using a gillnet (5 cm internodes) submerged for 12 h. Estuarine Cathorops spixii (n = 15), Genidens genidens (n = 18), Eugerres brasilianus (n = 18), Diapterus rhombeus (n = 9), and Mugil sp. (n = 11) specimens were captured, cryoanesthetized, and stored at 4 °C until laboratory processing. After dissection, fish muscle and liver tissues were separated and stored at −80 °C until analysis. All sampled fish were adults (Table S1 in Supplemental Material) and contained active gonadal maturation, both spawning and post-spawning individuals. All fish included in this study are demersal species with predominant benthic foraging and are typical food items for local villagers (Andrades et al., 2020). Field campaigns were conducted under Sisbio/ICMBio license number 57819-4, with assistance from local villagers.
Surface sediment (0–5 cm) was sampled at 17 random stations along the Rio Doce estuary using a Van-Veen Grab (Fig. 1) and stored in previously decontaminated (30% HNO3) containers for processing. These sampling sites are part of a long-term (4-year) monitoring of pre- and post-impacts in the Rio Doce estuary, following the disaster in 2015 (De Oliveira Gomes et al., 2017; Bernardino et al., 2019; Gabriel et al., 2020).
Sediment analysis
Trace metals were determined in the estuarine sediment samples by tri-acid digestion, using HNO3, HF and H3BO3, in a microwave oven, according to the EPA 3052 method (United States Environmental Protection Agency (EPA), 1996). The analysis included two-gram aliquots (wet weight) of sediment. Digestion was performed using nine mL of HNO3, three mL of HF (1 mol L−1) and 5 mL of H3BO3 (5%). Vessels containing the subsamples were shaken and heated at 110 °C for 4 hours. Subsequently, samples were diluted to 40 mL with deionized water. Finally, 0.1 mL aliquots were analyzed on an ICP-OES spectrometer (iCAP 6200; Thermo Scientific, Waltham, MA, USA). The analyses were performed in triplicate. To guarantee quality control, standard solutions were prepared from dilution of certified standard solutions and certified reference materials (NIST SRM 2709a) and used for comparison to measured and certified values (Table S2). Trace metal concentrations in sediments were then compared to baseline values for the estuary (De Oliveira Gomes et al., 2017) and to sediment quality guidelines (Table 1). The determined metals and metalloid (As, Cd, Cr, Cu, Mn, Pb, Se and Zn) were selected due to their significant increase in concentration post-disaster (Queiroz et al., 2018) and their relevance from a toxicological and biogeochemical perspective. In addition, metal concentrations were compared to local reference values calculated from pre-impact assessment (between 11 and 2 days before the arrival of tailings; De Oliveira Gomes et al., 2017) and sediment quality guidelines (SQGs) for the protection of aquatic life as determined by the National Oceanic and Atmospheric Administration (NOAA as Threshold Effect Level—TEL (limit under which no adverse effects on the biological community is observed) and Probable Effect Level—PEL (probable level where adverse effects in the biological community would occur, Threshold effect concentrations—TEC and Probable effect concentrations—PEC (NOAA, 2008).
Table 1. Comparison between mean elements values in sediments with the local reference values (LRV) and sediment quality guidelines (SQGs).
| Element | This study | LRV | Sediment quality guidelines (SQGs) | ||||
|---|---|---|---|---|---|---|---|
| Min–max | Mean ± SD | TEL | PEL | TEC | PEC | ||
| Cr | 18–71.1 | 47.4 ± 15.2 | 3.6 | 52.3 | 160 | 43 | 110 |
| Zn | 18.2–78.1 | 38.7 ± 14.5 | 1.6 | 124 | 271 | 120 | 460 |
| Mn | 148.7–1,002.7 | 540.8 ± 220 | 231 | – | – | 460 | 1,100 |
| As | <LQ–28.8 | 7.8 ± 7.7 | 3.3 | 7.24 | 41.6 | 9.8 | 33 |
| Cu | 3–15.0 | 9.4 ± 4.0 | 1.3 | 18.7 | 108 | 32 | 150 |
| Pb | 5.6–192.9 | 100.2 ± 47.5 | 4.7 | 30.2 | 112 | 36 | 130 |
| Cd | 0.6–7.1 | 3.6 ± 1.4 | 0.01 | 7.24 | 41.6 | 0.99 | 5 |
| Se | <LQ–13.6 | 5.9 ± 4.3 | 1.0 | – | – | – | – |
Note:
Threshold effect level (TEL), Probable effect level (PEL), Threshold effect concentrations (TEC) and Probable effect concentrations (PEC). All values are reported as mg kg−1. Local reference values were calculated from pre-impact assessment in the Rio Doce estuary by De Oliveira Gomes et al. (2017). LOQ for Se and As = 0.01 mg kg−1.
Metals and metalloid in fish
Approximately 100 mg of wet sample (muscle and liver) were weighed in sterile polypropylene tubes, followed by the addition of 1.0 mL of bidistilled HNO3. Method accuracy was established by the parallel analyses of procedural blanks (containing only 1.0 mL of bidistilled HNO3) and the certified reference material (CRM, DORM-4, dogfish muscle tissue, National Research Council of Canada, Canada), in triplicate. Observed and certified values (mg kg−1) for the DORM-4 certified reference material and recovery efficiencies (%) for each element and their respective LOQ (mg kg−1) were determined (Table S3). Recovery values were considered adequate for this method, according to Eurachem standards (Eurachem, 1998; Ishak et al., 2015). The samples, blanks and CRM were left for approximately 12 hours overnight, then heated the following morning on a digester block for 4 h at approximately 100 °C. The closed vessels were monitored hourly with manual pressure relief, as necessary. After heating, the samples, CRM and blanks were left to cool at room temperature and made up to appropriate volumes with ultra-pure water (resistivity> 18 MΩ cm). Element quantification was performed by ICP-MS using an ELAN DRC II ICP-MS (Perkin-Elmer Sciex, Norwalk, CT, USA). 103Rh was used as the internal standard at 20 µg L−1.
Metallothionein (MT) and reduced glutathione (GSH) in fish
Samples for MT extraction were prepared according to the protocol proposed by Erk et al. (2002). Briefly, muscle and liver samples (50 mg) were homogenized for 3 min in a 300 µL solution with 20 mmol L−1 Tris-HCl pH 8.6, phenylmethanesulphonyl fluoride 0.5 mmol L−1 as the antiproteolytic agent and β-mercaptoethanol 0.01 % as the reducing agent. The samples were then centrifuged at 20,000 rpm at 4 °C for 60 min. The resulting supernatants were separated from the pellets and placed in new microtubes. Proteins in the samples were denatured by heating the semi-purified supernatants for 10 min at 70 °C, followed by centrifugation for 30 min in the same conditions. Finally, the supernatants containing MT were transferred to new microtubes and frozen at −80 °C until analysis.
Metallothionein quantification via sulfhydryl content determination was performed by UV-Vis spectrophotometry through Ellman’s reaction (Ellman, 1959). The samples were treated with a mixture of 1 mol L−1 HCl, 4 mol L−1 EDTA and 2 mol L−1 NaCl containing 5.5 dithiobis (2-nitrobenzoic acid) buffered in 0.2 mol L−1 sodium phosphate, pH 8.0. After incubation for 30 minutes, sample absorbances were determined at 412 nm on a UV-Vis spectrophotometer. Metallothionein concentrations were estimated using an analytical curve plotted with GSH as an external standard and transformed to metallothionein through the known stoichiometric relationship between metallothionein and reduced glutathione, 1:20; GSH contains 1 mole of cysteine per molecule and metallothionein, 20 moles.
The reduced glutathione analysis followed the protocol proposed by Beutler (1975), with modifications introduced by Wilhelm-Filho et al. (2005). Twenty-five milligrams of tissue (liver and muscle) were weighed and homogenized in 350 µL of 0.1 mol L−1 sodium phosphate buffer pH 6.5 containing 0.25 mol L−1 sucrose. The samples were then centrifuged at 11,000 rpm for 30 minutes at 4 °C. The supernatants were transferred to microtubes and treated with 0.1 mol L−1 DTNB at pH 8.0 with a 1:1 ratio. After incubation for 15 minutes in the dark, sample absorbance was determined at 412 nm on a UV-Vis spectrophotometer. Reduced glutathione concentrations were estimated using an analytical curve plotted with GSH as an external standard (Monteiro et al., 2006).
References for fish consumption
Trace metal levels in fish muscle and liver tissues were compared to maximum permissible levels for consumption, according to the Brazilian Health Regulatory Agency (BRASIL, 1965), the Food and Agriculture Organization of the United Nations (FAO/WHO, 1997), the American Food and Drug Administration (U.S. Food and Drug Administration, 1993), the Environmental Protection Agency (United States Environmental Protection Agency (EPA), 2007), the British Ministry of Forestry, Agriculture and Fisheries (MAFF, 1995), and European Community legislation (European Commission, 2001; Table 2).
Table 2. National and international maximum permissible levels (mg kg−1) for the ingestion of fish products worldwide.
| Agency | Zn | Cu | Cd | Pb | Hg | As | Se | Cr | Mn |
|---|---|---|---|---|---|---|---|---|---|
| ANVISA | 50 | 30 | 1 | 2 | 0.5 | 1 | 0.3 | 0.1 | – |
| FAO/WHO | 30 | 30 | 1 | 2 | 0.5 | – | – | – | 0.5 |
| US FDA | NA | NA | 3.7 | – | – | – | – | – | – |
| US EPA | 10–30 | 1–20 | >2 | – | – | – | – | – | – |
| MAFF | 50 | 20 | 0.2 | 2 | 0.3 | – | – | – | – |
| EC | – | – | 0.05 | 0.2 | 0.5 | – | – | – | – |
Note:
NA = Not Available.
Statistical analyses
Sediment metal concentrations were averaged across sampling stations and expressed as means (three replicated per site) and standard deviation (SD). Before statistical tests, data distribution was verified by the Shapiro-Wilk test. Because the data was normally distributed, parametric tests were applied. One-way ANOVAs were used to test variations of each element concentration (Zn, Cu, Cd, Pb, Hg, As, Se, Cr and Mn) and biomarkers (MT and GSH) between muscle and liver tissues for each fish species. Pearson’s correlation test was used to verify the existence of significant correlations between trace metal concentrations and metallothionein and reduced glutathione data. A one-way ANOVA test was applied to verify differences between biometric data across fish species. As no statistically significant differences were observed between fish size, weight, and sex, the groups were treated homogeneously without a weight/size stratification range or sex separation.
A Canonical Analysis of Principal Coordinates (CAP; Anderson & Willis, 2003) complemented by multidimensional scaling (Anderson, 2001; McArdle & Anderson, 2001; Oksanen et al., 2018) was performed to evaluate the metal trace metal contamination and stress protein expression. Data was square-root transformed prior to CAP. CAP was then used to identify the metal or group of elements that best explained the variation in stress protein expression among species and to determine the protein that contributed most to the differences among samples. All statistical tests used an α = 0.05 significance level. Graphical and analytical processing was performed in Numbers (Apple Inc.) and R project (R Development Core Team, 2005) using the ‘stats’ and ‘vegan’ packages (Oksanen et al., 2018).
Results
Sediment contamination and quality assessments
Overall, the mean As, Cd, Cr, Cu, Mn, Pb, Se and Zn concentrations in sediments were higher than the reference values (pre-impact) for the estuary (De Oliveira Gomes et al., 2017), indicating an accumulation of these elements since 2015 (Table 1). The concentrations of Cd, Cr, Pb, and Zn reached 3.6 ± 1.4 mg kg−1, 38.7 ± 14.5 mg kg−1, 100.2 ± 47.5 mg kg−1 and 47.4 ± 15.2 mg kg−1, respectively. These values were 35,900%, 2,319%, 2,031% and 1,217% higher, respectively, than the baseline concentrations reported by De Oliveira Gomes et al. (2017). When compared to sedimentary quality guidelines (NOAA, 2008), sedimentary Pb concentrations were higher than the threshold effect level (TEL) at 94% of the sampled stations (min. 5.6 and max. 192.9 mg kg−1). Pb sediment values were also above probable effect level (PEL) at 47% of the sampled stations, above the threshold effect concentrations (TEC) over at 82% of the sampled stations, and above probable effect concentrations (PEC) over at 23% of the sampled sites. Sedimentary Cr and As were higher than the TEL and PEC in 53-23% of the samples. Mn was higher than the TEC in 59% and Cd higher than both the TEC and PEC in 94 and 12% of the samples, respectively. These results suggest that the observed concentrations of As, Cd, Cr, Mn and Pb in the estuarine sediments may cause harmful effects to living organisms. On a toxicity risk scale, the Pb and Cd PECs exceeded sediment concentrations.
Metal accumulation and biomarkers in fish
We detected higher trace metal concentrations in the liver tissues, when compared to muscle tissues, in fish sampled from the Rio Doce estuary. Zinc concentrations were significantly higher in the liver tissue from all species (ANOVA, DF = 99, F = 22.9; p < 0.0001), and Mn concentrations were higher only in the liver tissue of E. brasilianus (ANOVA, DF = 19, F = 30.51; p = 0.0309). Cd concentrations were below the limit of quantification (LOQ = 0.0255 mg kg−1) in muscle tissues from all species, while Pb displayed the same behavior only in Mugil sp. (Table 3).
Table 3. Elements concentrations in liver and muscle tissues and percentage (number/total samples) of samples that exceeded maximum permissible levels allowed by Brazilian and international guidelines.
| Species | Tissue | As | Cd | Cr | Cu | Hg | Mn | Pb | Se | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
|
Cathorops spixii N = 15 |
Muscle | 7.46 ± 10.46 | <LOQ | 0.38 ± 0.14 | 0.30 ± 0.32 | 0.22 ± 0.02 | 0.48 ± 0.97 | 0.03 ± 0.02 | 0.53 ± 0.20 | 8.21 ± 12.60 |
| (0.17–39.31) 73% |
<LOQ 0% |
(0.25–0.66) 80% |
(0.14–1.27) 13% |
(0.02–0.07) 20% |
(0.11–3.16) 53% |
(0.02–0.07) 0% |
(0.29–0.85) 87% |
(3.58–46.64) 33% |
||
| Liver | 0.31 ± 1.24 | 0.26 ± 0.17 | 0.40 ± 0.11 | 15.13 ± 37.87 | 0.45 ± 0.52 | 3.27 ± 1.84 | 0.18 ± 0.11 | 4.13 ± 3.42 | 94.61 ± 203.05a | |
| (0.13–3.64) 40% |
(0.16–0.76) 93% |
(0.31–0.70) 93% |
(0.66–129.10) 93% |
(0.03–1.81) 73% |
(0.17–5.86) 93% |
(0.06–0.47) 13% |
(0.34–11.08) 100% |
(18.50–693.80) 100% |
||
|
Genidens genidens N = 18 |
Muscle | 0.23 ± 4.12 | <LOQ | 0.34 ± 0.11 | 0.29 ± -0.13 | 0.26 ± 0.15 | 0.43 ± 0.19 | 0.13 ± 0.07 | 0.38 ± 0.08 | 14,00 ± 5.74 |
| (0.10–15.51) 33% |
<LOQ 0% |
(0.25–0.58) 100% |
(0.11–0.61) 0% |
(0.11–0.61) 39% |
0.14–0.94 33% |
(0.02–0.19) 0% |
(0.32–0.61) 100% |
(6.61–95.41) 67% |
||
| Liver | 0.21 ± 0.35 | 0.41 ± 0.49 | 0.41 ± 0.17 | 8.94 ± 9.48 | 0.75 ± 0.66 | 1.49 ± 1.87 | 0.29 ± 0.40 | 3.91 ± 1.60 | 571.43 ± 320.2a | |
| (0.12–1.35) 11% |
(0.03–2.36) 94% |
(0.30–0.62) 89% |
(1.89–46.20) 100% |
(0.14–3.02) 89% |
(0.89–9.00) 100% |
(0.03–1.91) 72% |
(2.37–9.75) 100% |
(126.00–1310.90) 100% |
||
|
Eugerres brasilianus N = 18 |
Muscle | 0.20 ± 0.08 | <LOQ | 0.39 ± 0.09 | 0.32 ± 0.40 | 0.19 ± 0.07 | 0.35 ± 0.91 | 0.05 ± 0.08 | 0.69 ± 0.34 | 3.22 ± 4.61 |
| (0.11–0.33) 0% |
<LOQ 6% |
(0.26–0.51) 44% |
(0.13–1.71) 11% |
(0.07–0.30) 6% |
(0.08–4.22) 17% |
(0.02–0.23) 6% |
(0.33–1.90) 100% |
(1.75–22.97) 6% |
||
| Liver | 0.51 ± 0.23 | 0.19 ± 0.25 | 0.42 ± 0.11 | 2.36 ± 0.75 | 0.11 ± 0.02 | 7.20 ± 4.10 a | 0.05 ± 0.04 | 2.14 ± 0.84 | 27.27 ± 25.70a | |
| (0.09–0.91) 0% |
(0.04–0.96) 83% |
(0.25–0.67) 78% |
(0.32–3.66) 94% |
(0.06–0.15) 0% |
(0.28–17.25) 94% |
(0.02–0.14) 0% |
(0.58–3.86) 100% |
(3.41–119.56) 94% |
||
|
Diapterus rhombeus N = 9 |
Muscle | 0.52 ± 0.56 | <LOQ | 0.21 ± 0.04 | 0.16 ± 0.03 | 0.08 ± 0.05 | 0.30 ± 0.53 | 0.02 ± 0.01 | 0.78 ± 0.23 | 3.81 ± 1.34 |
| (0.15–1.73) 22% |
<LOQ 0% |
(0.17–0.27) 100% |
(0.14–0.22) 0% |
(0.05–0.23) 0% |
(0.10–1.67) 33% |
(0.02–0.03) 0% |
(0.45–1.11) 100% |
(2.50–7.10) 0% |
||
| Liver | 0.97 ± 0.62 | 0.05 ± 0.26 | 0.34 ± 0.11 | 1.67 ± 0.82 | 0.10 ± 0.03 | 9.01 ± 8.29 | 0.06 ± 0.02 | 1.26 ± 0.79 | 32.9 ± 28.68a | |
| (0.26–2.37) 44% |
(0.04–0.81) 44% |
(0.14–0.58) 100% |
(0.16–3.04) 89% |
(0.06–0.16) 0% |
(0.53–31.90) 100% |
(0.03–0.08) 0% |
(0.17–2.96) 89% |
(1.54–99.79) 89% |
||
|
Mugil sp. N = 11 |
Muscle | 0.25 ± 0.53 | <LOQ | 0.32 ± 0.06 | 0.30 ± 0.28 | 0.07 ± 0.06 | 0.20 ± 0.18 | <LOQ | 0.50 ± 0.21 | 3.21 ± 2.74 |
| (0.17–2.02) 9% |
<LOQ 0% |
(0.27–0.42) 36% |
(0.15–0.73) 9% |
(0.05–0.24) 0% |
(0.07–0.57) 18% |
<LOQ 0% |
(0.17–0.97) 82% |
(1.81–12.18) 9% |
||
| Liver | 1.73 ± 1.99 | 0.11 ± 0.35 | 0.46 ± 0.17 | 47.30 ± 150.60 | 0.16 ± 0.40 | 1.85 ± 2.14 | 0.04 ± 0.30 | 5.81 ± 9.33 | 71.71 ± 370.92a | |
| (0.24–7.34) 73% |
(0.03–1.17) 82% |
(0.30–0.75) 73% |
(2.83–539.90) 100% |
(0.03–1.23) 36% |
(0.93–8.78) 100% |
(0.02–0.89) 18% |
(1.16–33.38) 100% |
(23.80–1351.00) 100% |
Note:
LOQ for Cd = 0.0255 and Pb = 0.0126 mg kg−1.
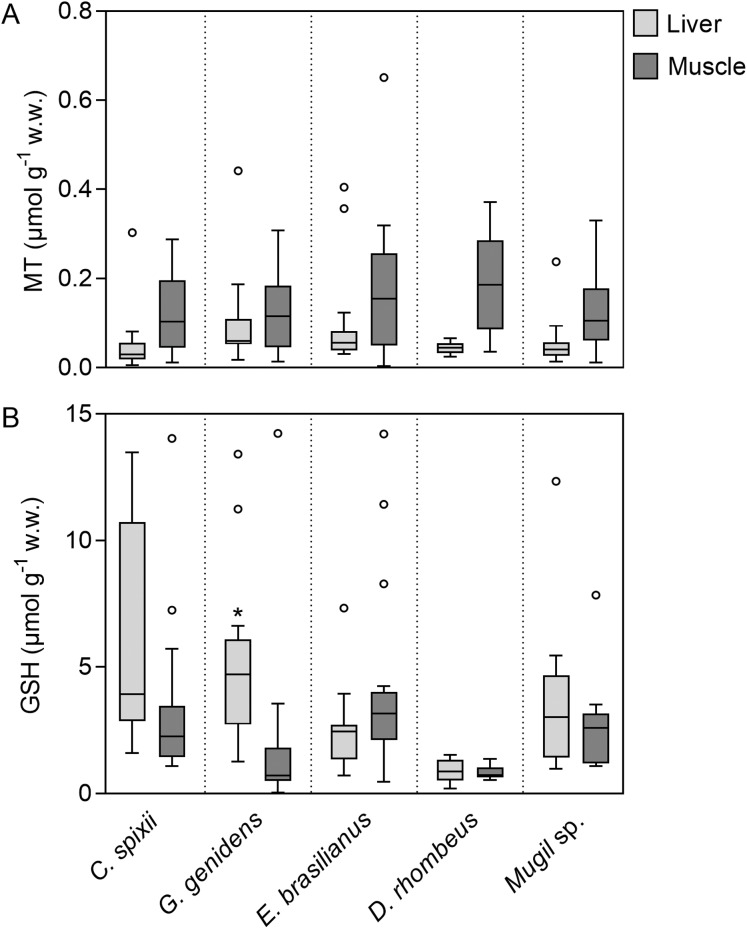
Contrary to higher metal concentrations (Zn, Mn) in liver tissues, MT levels in the muscle and liver tissues were similar in all sampled fish species (ANOVA, F = 2.816; p > 0.05; Fig. 2A). In contrast, GSH concentrations were higher in the liver tissue of C. spixii, G. genidens, D. rhombeus and Mugil sp. in comparison to their muscle tissue (Fig. 2B), whereas muscle tissues of E. brasilianus exhibited higher GSH concentrations (Fig. 2B). A significant difference in GSH levels was observed between liver and muscle tissues for G. genidens, with a higher concentration in the liver (ANOVA, F = 6.874; p < 0.0001; Fig. 2B).
Figure 2. Total metallothionein and reduced glutathione concentrations (μmol g−1 wet weight) in estuarine fishes liver and muscle tissues in the Rio Doce estuary.
Box plots indicate minimum, maximum, median, quartiles, and outliers (cicle). The asterisk indicates significance at p < 0.05.
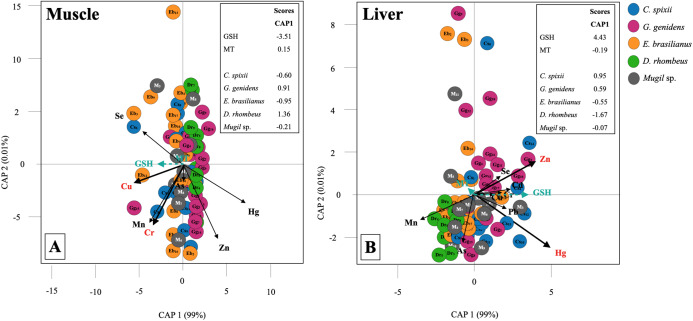
The CAP analysis indicated a significant association between trace metal concentrations in muscle and liver tissues and the expression of stress proteins in fish (muscle F = 2.68, p = 0.016, liver F = 3.94, p = 0.003; Table 4; Fig. 3). In the liver, GSH expression was positively correlated to Zn and Hg concentrations mainly for C. spixii and G. genidens (Zn F = 12.44, p = 0.003, Hg F = 12.42, p = 0.002; Table 4; Fig. 3B). In muscle tissues of C. spixii and E. brasilianus, Cu and Cr contributed mostlymost to GSH expression (Cu; F = 7.12, p = 0.012, Cr; F = 5.11, p = 0.028; Table 4; Fig. 3A). In general, differences in protein expression were the highest in D. rhombeus individuals and lowest for Mugil sp.
Table 4. Results of the canonical analysis of principal coordinates to evaluate the contribution of elements contamination and the variations in the expression of antioxidant biomarkers in estuarine fishes.
| Muscle | Liver | |||||||
|---|---|---|---|---|---|---|---|---|
| CAP 1 (0.99%) | CAP 2 (0.01%) | F | p | CAP 1 (99%) | CAP 2 (0.01%) | F | p | |
| Zn | 0.28 | −0.69 | 1.61 | 0.200 | 0.59 | 0.22 | 12.44 | 0.003 |
| Cu | −0.52 | −0.14 | 7.12 | 0.012 | 0.18 | −0.002 | 1.61 | 0.203 |
| Cd | – | – | – | – | 0.33 | 0.04 | 0.29 | 0.613 |
| Pb | – | – | – | – | 0.31 | −0.09 | 2.76 | 0.097 |
| Hg | 0.58 | −0.40 | 3.03 | 0.084 | 0.74 | −0.37 | 12.42 | 0.002 |
| As | −0.29 | −0.12 | 0.51 | 0.490 | −0.11 | −0.34 | 2.29 | 0.153 |
| Se | −0.49 | 0.31 | 0.48 | 0.503 | 0.24 | 0.12 | 0.22 | 0.631 |
| Cr | −0.43 | −0.58 | 5.11 | 0.028 | 0.21 | 0.02 | 0.14 | 0.690 |
| Mn | −0.50 | −0.56 | 0.93 | 0.334 | −0.54 | −0.18 | 3.32 | 0.076 |
Note:
Spearman correlation values for each metal are described for CAP axis 1–2. Note: proportions of variability explained by CAP axes are between parentheses ‘()’, Fisher test statistic, significant results (p < 0.05) are in bold. Muscle: F = 2.68, p = 0.016; Liver: F = 3.94, p = 0.003.
Figure 3. Canonical analysis of principal coordinates (CAP) indicating differences in expression of antioxidant biomarkers and the contribution of elemental contamination in estuarine fishes.
Vectors are based on Spearman correlation values > 0.5 (p < 0.05) for elements and scores for protein concentration and species (mean score among sampled). The proportions of data explained by axis 1 and 2 are in parentheses.
Fish contamination and human health
The trace metal concentrations on fish tissues from the Rio Doce estuary were compared to the maximum residue level (MRL) standards. When using the most restrictive guideline for each element, concentrations of As, Cd, Cr, Cu, Hg, Mn, Pb, Se and Zn in fish liver of all species analyzed exceeded the MRL guidelines. The MRL standards were not exceeded for Pb and Hg in D. rhombeus and E. brasilianus (Table 3). Liver tissue concentrations of Zn, Se and Mn exceeded guidelines for most specimens (>89%). The concentrations of As exceeded the guidelines for C. spixii in 73% of the analyzed specimens, and in G. genidens for 33% of the muscle tissue samples. Cr concentrations exceeded guidelines in the muscle tissue of all analyzed specimens. Se concentrations also exceeded guidelines in all tissues of G. genidens and E. brasilianus specimens, in C. spixii and Mugil sp. liver tissues, and in D. rhombeus muscle. Pb and Hg concentrations exceeded guidelines in C. spixii and G. genidens liver and E. brasilianus muscle.
Discussion
This study revealed a previously unreported bioaccumulation of metals and metalloids in edible fish of the Rio Doce estuary nearly two years after a mine tailing disaster. Trace metal concentrations in fish tissues were associated overall to the expression of biomarkers of oxidative stress in several demersal fish species, suggesting active incorporation of toxic contaminants in the Rio Doce estuary at the time of sampling. Our results support the hypothesis of fish trace metal contamination, which were markedly augmented after the arrival of mine tailings in 2015, and the consequent lower health of aquatic ecosystems in the Rio Doce estuary. Observed increases in the sediment’s trace metal concentrations were above 1000% for most elements studied (Cd, Cr, Pb and Zn), supporting chronic effects of trace metal contamination on the estuarine fauna (Bernardino et al., 2019). The estuarine fish sampled, including Genidens genidens, Diapterus rhombeus and Mugil sp., are typically associated to bottom sediments and often ingest food items buried within sediment matrices (invertebrates; Chaves & Otto, 1998; Chaves & Vendel, 1996; Seixas et al., 2005), which make them especially vulnerable to chemical contamination. Our results suggest that this ingestion may be continuous and active, which is supported by the high trace metal contents in the liver tissues, the primary detoxification organ in fishes (Hauser-Davis, Campos & Ziolli, 2012). In addition, trace metal contamination was observed in bottom-dwelling fish species, typically consumed by local human populations, with potential health implications to villagers that rely on fish as the main protein source for their subsistence. Although baseline trace metal levels are not available for the fish from the estuary, it is very unlikely that the fish sampled 1.7 years after the disaster survived the acute impacts from the tailing in 2015 and would therefore exhibit inherited chemical contamination from the estuary. Hence, our findings suggest a rapid transfer (<2 years) of trace metals and metalloids from sediments to the estuarine biota.
The released mine tailings 600 km upstream of the estuary were initially characterized by low metal concentrations with non-hazardous residues (Almeida et al., 2018). However, the deposited tailings in estuarine soils had significant concentrations of Fe oxides with a high adsorption potential for trace metals that were scavenged during their downstream riverine transport until reaching the estuary (Queiroz et al., 2018). Queiroz et al. (2018) hypothesized that the trace metals bound to Fe oxy-hydroxides would become bioavailable upon Fe reduction in estuarine soils, leading to high ecological risks to the estuarine biota. These risks were evident in the analysis of significant trace metal levels in the estuarine sediments within 2 years after the initial impact (Gabriel et al., 2020). The mobilization of trace metals from tailings have occurred in aquatic riverine ecosystems downstream of the ruptured mining dam (Ferreira et al., 2020; Weber et al., 2020), which supports that, not only the tailings are harmful in aquatic sub-oxic environments, but that toxic trace metals can be adsorbed into these sediments. We detected a relatively rapid bioaccumulation in fish from the Rio Doce estuary, which confirms our previous hypothesis of bioavailability of trace metals that are accumulated in sub-oxic estuarine sediments. Our results reveal that biogeochemical conditions in estuarine sediments may promote bioavailability of trace metals bound to Fe from tailing in the sediments for an extremely long period, as the estuarine ecosystem is a major depocenter of pollutants in coastal zones. If this is confirmed, the fish in the Rio Doce estuary will be contaminated as long as the deposited tailings and associated toxic elements are gradually supplied from upstream transport, and remain deposited on the bottom.
The physiological responses of fish revealed significant correlations of MT and GSH to chemical elements, supporting that the assessed species were under sublethal contamination (or physiological stress) effects at the time of sampling. Although fish sampling can bring stress conditions to fish and lead to biomarker synthesis, the biomarkers (MT and GSH) were significantly correlated to trace metal contamination in both, liver and muscle, tissues. This is also a key advantage of the use of these biomarkers, given that most contaminated areas, including our study, lack baseline metal values in fish. We observed a marked response of biomarker synthesis to Cr, Cu, Hg, and Zn tissue concentrations, which are major contaminants that increased significantly in sediments of the Rio Doce estuary, after the tailings arrival (Queiroz et al., 2018). Therefore, the observed biomarker responses are an additional support for the indication of chronic trace metal contamination effects in this ecosystem, and for fish contamination by bioavailable toxic elements (Gusso-Choueri et al., 2018; Bernardino et al., 2019). The antioxidant function of MTs and GSH and their positive correlations with trace metals proved to be sufficiently sensitive for an impact assessment of the Rio Doce estuary, and thus should be continued during monitoring programs. The trace metal and biomarker concentrations detected in the tissues of these fish seem to reflect the level of contamination of the sediment and its biota.
The general trend of higher MT concentrations in the fish muscle observed in the present study may be associated with trace metal overload in the liver and other excretory organs (e.g., kidneys), with the excess accumulated in the muscle (Pacheco et al., 2017; Souza et al., 2018), suggesting high exposure to the assessed contaminants. However, further monitoring of biomarker expression in fish and trace metal concentrations in fish muscle are required to confirm this hypothesis. GSH expression in liver of C. spixii, E. brasilianus, Mugil sp. and D. rhombeus was correlated with Cd, Cr, Hg, Mn and Zn, suggesting that metals create oxidative stress in those individuals (Di Giulio et al., 1995; Atli & Canli, 2008; Monteiro et al., 2008; Sharma & Langer, 2014). Although GSH levels may vary among fish species, the species captured in the Rio Doce estuary exhibited higher GSH expression when compared to fish from uncontaminated freshwater ecosystems upstream (Weber et al., 2020). In addition, fish species from contaminated freshwater ecosystems upstream exhibited similar oxidative stress effects, with higher biomarker levels in addition to internal tissue degeneration (Weber et al., 2020; Macêdo et al., 2020). The GSH expression in fish captured in the estuary suggests that local estuarine ecosystem health has also been severely compromised by trace metal contamination, with possible sub-optimal conditions for the development of fish species (Andrades et al., 2020).
Tissue accumulation of Cd, Cr, Cu, Mn, Pb, Se and Zn was higher in the liver, which is the primary trace metal detoxification organ (Hauser-Davis, Campos & Ziolli, 2012). The liver is rapidly contaminated by toxic metals through the bloodstream, after absorption, so liver trace metal concentrations are assumed to closely resemble those present in the environment (Dural, Göksu & Özak, 2007; De Souza Lima Junior et al., 2002; Bosco-Santos & Luiz-Silva, 2019). Increased trace metal concentrations in muscle tissue from the species C. spixii and G. genidens may suggest a saturation response for trace metal contamination (Lu et al., 2018; Souza et al., 2018). Several trace metals with high concentrations in fish muscle, including Cu, Zn, Cd and Hg, were also observed at high concentrations in the mine tailings deposited in the estuary (Gabriel et al., 2020). Comparing the concentrations of trace metals in fish from the present study with those found in other studies on the Brazilian coast, those in fish from the Rio Doce were similar or higher than strongly polluted estuaries in Brazil (Table 5). The transfer of bioavailable trace metals from contaminated sediments in coastal ecosystems has been widely reported (Zhu et al., 2015; Hauser-Davis et al., 2016; Gusso-Choueri et al., 2018, Mason et al., 2019), supporting that contaminated sediments in the Rio Doce estuary were the source of the observed trace metals and subsequent physiological effects in fish.
Table 5. Elements concentrations (mg kg−1) in muscle and liver tissues of fish from the Rio Doce estuary compared with other polluted and pristine estuaries and coastal bays in Brazil.
| Species | Location | Tissue | As | Cd | Cr | Cu | Hg | Mn | Pb | Se | Zn | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathorops spixii | Cananéia estuary (Near-pristine) | Muscle | 0.007 | 0.067 | 0.157 | 0.059 | 10.95 | Azevedo, Hortellani & Sarkis (2012) | ||||
| Santos-São Vicente estuary (Polluted) | Muscle | 0.005 | 0.351 | 0.268 | <LOD | 10.66 | Azevedo, Hortellani & Sarkis (2012) | |||||
| Santos-São Vicente estuary (Polluted) | Muscle | 0.008 | 0.494 | 0.096 | 0.018 | 6.30 | Azevedo, Hortellani & Sarkis (2012) | |||||
| Cananéia estuary (Near-pristine) | Liver | 0.25 | 12.7 | 1.11 | Azevedo et al. (2009) | |||||||
| Santos Bay (Polluted) | Muscle | 0.06 | Azevedo et al. (2009) | |||||||||
| Santos Bay (Polluted) | Liver | 0.33 | 15.4 | 261 | Azevedo et al. (2009) | |||||||
| Paranaguá Bay (Polluted) | Muscle | 3.24 | 0.12 | 0.22 | 5.98 | Angeli et al. (2013) | ||||||
| Rio Doce estuary | Muscle | 7.46 | <LOQ | 0.38 | 0.30 | 0.22 | 0.48 | 0.03 | 0.53 | 8.21 | This study | |
| Rio Doce estuary | Liver | 0.31 | 0.26 | 0.40 | 15.13 | 0.45 | 3.27 | 0.18 | 4.13 | 94.61 | This study | |
| Genidens genidens | Santos-São Vicente estuary (Polluted) | Muscle | 0.007 | 0.177 | 0.392 | 0.01 | 12.12 | Azevedo, Hortellani & Sarkis (2012) | ||||
| Santos-São Vicente estuary (Polluted) | Muscle | 0.009 | 0.280 | 0.105 | 0.018 | 9.396 | Azevedo, Hortellani & Sarkis (2012) | |||||
| Cananéia estuary (Near-pristine) | Muscle | 0.006 | 0.037 | 0.209 | 0.057 | 11.67 | Azevedo, Hortellani & Sarkis (2012) | |||||
| Paranaguá Bay (Polluted) | Muscle | 0.91 | 0.1 | 0.22 | 6.93 | Angeli et al. (2013) | ||||||
| Morrão River estuary (Polluted) | Muscle | 0.003 | 1.29 | 0.07 | 80.5 | Bosco-Santos & Luiz-Silva (2019) | ||||||
| Morrão River estuary (Polluted) | Liver | 0.24 | 26.1 | 7.80 | 1,201 | Bosco-Santos & Luiz-Silva (2019) | ||||||
| Rio Doce estuary | Muscle | 0.23 | <LOQ | 0.34 | 0.29 | 0.26 | 0.43 | 0.13 | 0.38 | 14.00 | This study | |
| Rio Doce estuary | Liver | 0.21 | 0.41 | 0.41 | 8.94 | 0.75 | 1.49 | 0.29 | 3.91 | 571.43 | This study | |
| Eugerres brasilianus | Babitonga Bay (Polluted) | Muscle | 0.06 | 9.28 | Bonatti et al. (2004) | |||||||
| Rio Doce estuary | Muscle | 0.20 | <LOQ | 0.39 | 0.32 | 0.19 | 0.35 | 0.05 | 0.69 | 3.22 | This study | |
| Rio Doce estuary | Liver | 0.51 | 0.19 | 0.42 | 2.36 | 0.11 | 7.20 | 0.05 | 2.14 | 27.27 | This study | |
| Diapterus rhombeus | Todos os Santos Bay (Polluted) | Muscle | <LOD | 0.22 | 4.59 | <LOD | 0.61 | <LOD | 6.74 | Santana et al. (2017) | ||
| Morrão River estuary (Polluted) | Muscle | 0.002 | 0.79 | 0.80 | 25.2 | Bosco-Santos & Luiz-Silva (2019) | ||||||
| Morrão River estuary (Polluted) | Liver | 0.25 | 10.9 | 0.34 | 138 | Bosco-Santos & Luiz-Silva (2019) | ||||||
| Rio Doce estuary | Muscle | 0.52 | <LOQ | 0.21 | 0.16 | 0.08 | 0.30 | 0.02 | 0.78 | 3.81 | This study | |
| Rio Doce estuary | Liver | 0.97 | 0.05 | 0.34 | 1.67 | 0.10 | 9.01 | 0.06 | 1.26 | 32.9 | This study | |
| Mugil liza | Itaipu - Guanabara Bay (Near-pristine) | Muscle | <LOQ | <LOQ | 1.18 | 3.57 | Hauser-Davis et al. (2016) | |||||
| Itaipu - Guanabara Bay (Near-pristine) | Liver | 0.13 | 0.81 | 2.43 | 64.61 | Hauser-Davis et al. (2016) | ||||||
| Ipiranga - Guanabara Bay (Polluted) | Muscle | <LOQ | <LOQ | <LOQ | <LOQ | Hauser-Davis et al. (2016) | ||||||
| Ipiranga - Guanabara Bay (Polluted) | Liver | 0.06 | <LOQ | 0.59 | 74.31 | Hauser-Davis et al. (2016) | ||||||
| Morrão River estuary (Polluted) | Muscle | 0.001 | 0.79 | 0.06 | 11.8 | Bosco-Santos & Luiz-Silva (2019) | ||||||
| Morrão River estuary (Polluted) | Liver | 0.26 | 176 | 1.13 | 188 | Bosco-Santos & Luiz-Silva (2019) | ||||||
| Mugil curema | Itaguaré River (Pristine) | Muscle | <LOD | 0.27 | 0.51 | 6.68 | Carmo, Abessa & Machado-Neto (2012) | |||||
| São Vicente estuary (Polluted) | Muscle | 1.25 | 0.01 | 0.35 | 7.33 | Carmo, Abessa & Machado-Neto (2012) | ||||||
| São Vicente estuary (Polluted) | Muscle | 1.70 | 0.02 | 0.25 | 6.75 | Carmo, Abessa & Machado-Neto (2012) | ||||||
| Mugil platanus | Babitonga Bay (Polluted) | Muscle | 0.06 | 4.40 | Bonatti et al. (2004) | |||||||
| Mugil sp. | Vitória Bay (Polluted) | Muscle | 0.030 | 0.151 | 0.214 | 0.27 | 3.26 | Joyeux, Campanha Filho & de Jesus (2004) | ||||
| Rio Doce estuary | Muscle | 0.25 | <LOQ | 0.32 | 0.30 | 0.07 | 0.20 | <LOQ | 0.50 | 3.21 | This study | |
| Rio Doce estuary | Liver | 1.73 | 0.11 | 0.46 | 47.30 | 0.16 | 1.85 | 0.04 | 5.81 | 71.71 | This study |
Trace metal concentrations varied between fish species and between liver and muscle tissues, probably as a result of varied physiological responses and exposure to contaminants (Shah & Altindağ, 2005). The inter- and intra -specific differences in protein expression suggest that fish sampled were either exposed to variable contaminant levels or that some fish species or individuals are less adapted to these stress levels. This hypothesis requires further investigation in the Rio Doce estuary. The fishes sampled in the Rio Doce estuary display demersal behavior, feeding on benthic invertebrates and other food sources, enabling possible direct ingestion of contaminated sediments and other pollutants (Dantas et al., 2019; Andrades et al., 2020). In addition, fish behavior may increase exposure to contaminants in sediments during active search for food on the bottom, leading to resuspension of contaminants and their intake through gills (Cline, 1994; Bustamante et al., 2003). The demersal catfish species of this region deserve special attention as they showed the highest trace metal concentration in both liver and muscle tissues and may increase human health risks when consumed. Differences in trace metal concentrations among species can also be associated to age, differences in metabolism or the presence of migratory behavior (Rodrigues et al., 2010). Fish age, in particular, may also reflect exposure periods in the environment and consequently influence bioaccumulation. Although we did not sample separate fish cohorts in our study, trace metal contamination and population patterns deserve future consideration as they could support decisions on fish consumption by vulnerable populations affected by the disaster.
This is, to the best of our knowledge, the first report on MT and GSH associations with metals in these fish species, except for Mugil sp., with a potential protective role against toxic elements. Their assessment could be an effective tool in the evaluation of metal contamination, once the expression of detoxifying biomarkers indicates current exposure to trace metals in aquatic ecosystems. Based on high trace metal contents in fish muscle, the consumption of demersal fish species poses risks to human health and should be prohibited in this estuarine region. In addition, estuarine health is probably compromised by chronic contamination, likely to be sub-optimal for fish development and fisheries production, both of which are important indirect effects neglected in management actions.
Conclusions
Significant tissue bioaccumulation and oxidative stress defenses in fish were observed in response to contamination of the Rio Doce estuary by iron mine tailings. High concentrations of potentially toxic trace metals in the liver of the demersal species G. genidens and C. spixii, and their respective protein synthesis correlations, indicate chronic sublethal effects, while higher metallothionein levels in muscle tissues suggests metal overload in excretion organs. Trace metal concentrations in both liver and muscle tissue were above Brazilian and international guidelines for Maximum Residue Limits in foods for As, Cd, Cr, Cu, Mn, Pb and Zn, indicating potentially high human risks if consumed by communities near the impacted areas. Although our study evaluated these effects nearly 2 years after the disaster, these effects are likely to continue as long as the tailings are still being deposited in the estuarine ecosystem, which will also likely offer sub-optimal conditions for the development of fish species. Our study supports the use of demersal fish species used in the present study in an environmental biomonitoring context, which may improve the performance of current and future short and long-term impact assessment studies.
Supplemental Information
Data is displayed as means ± SD.
Certified reference material: NIST SRM 2709a.
Funding Statement
This work was financially supported by the Fundação de Amparo a Pesquisa e Inovação do Espírito Santo (FAPES), Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support granted to the Soil Benthos Rio Doce Network Project (FAPES 77683544/17) and the doctoral scholarship of Fabrício  Gabriel. Angelo F Bernardino, Tiago O Ferreira and Tatiana D Saint Pierre were also supported by CNPq PQ grants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Rachel A. Hauser-Davis and Angelo F Bernardino are Academic Editors for PeerJ.
Author Contributions
Fabrício Â. Gabriel conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Rachel Ann Hauser-Davis conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Lorena Soares conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Ana Carolina A. Mazzuco analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Rafael Christian Chavez Rocha performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Tatiana D. Saint Pierre performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Enrico Saggioro conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Fabio Verissimo Correia conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Tiago O. Ferreira performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Angelo F. Bernardino conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Instituto Chico Mendes de Conservação da Biodiversidade provided full approval for this research (57819-4).
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The field sampling was conducted under SISBIO/ICMBio license number 57819-4.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Ahmed et al. (2015).Ahmed MK, Baki MA, Islam MS, Kundu GK, Habibullah-Al-Mamun M, Sarkar SK, Hossain MM. Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environmental Science and Pollution Research. 2015;22(20):15880–15890. doi: 10.1007/s11356-015-4813-z. [DOI] [PubMed] [Google Scholar]
- Almeida et al. (2018).Almeida CA, De Oliveira AF, Pacheco AA, Lopes RP, Neves AA, Queiroz MELR. Characterization and evaluation of sorption potential of the iron mine waste after Samarco dam disaster in Doce river basin—Brazil. Chemosphere. 2018;209:411–420. doi: 10.1016/j.chemosphere.2018.06.071. [DOI] [PubMed] [Google Scholar]
- Anderson (2001).Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. doi: 10.1111/j.1442-9993.2001.01070. [DOI] [Google Scholar]
- Anderson & Willis (2003).Anderson MJ, Willis TJ. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology. 2003;84(2):511–525. doi: 10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2. [DOI] [Google Scholar]
- Andrades et al. (2020).Andrades R, Guabiroba HC, Hora MSC, Martins RF, Rodrigues VLA, Vilar CC, Giarrizzo T, Joyeux J-C. Early evidences of niche shifts in estuarine fishes following one of the T world’s largest mining dam disasters. Marine Pollution Bulletin. 2020;154:111073. doi: 10.1016/j.marpolbul.2020.111073. [DOI] [PubMed] [Google Scholar]
- Angeli et al. (2013).Angeli JLF, Trevizani TH, Ribeiro A, Machado EC, Figueira RCL, Barkert B, Fraenzle S, Wuenschmann S. Arsenic and other trace elements in two catfish species from Paranaguá Estuarine Complex, Paraná, Brazil. Environmental Monitoring and Assessment. 2013;185(10):8333–8342. doi: 10.1007/s10661-013-3176-5. [DOI] [PubMed] [Google Scholar]
- Atli & Canli (2008).Atli G, Canli M. Responses of metallothionein and reduced glutathione in a freshwater fish Oreochromis niloticus following metal exposures. Environmental Toxicology and Pharmacology. 2008;25(1):33–38. doi: 10.1016/j.etap.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Azevedo et al. (2009).Azevedo JS, Fernandez WS, Farias LA, Fávaro DTI, Braga ES. Use of Cathorops spixii as bioindicator of pollution of trace metals in the Santos Bay, Brazil. Ecotoxicology. 2009;18(5):577–586. doi: 10.1007/s10646-009-0315-4. [DOI] [PubMed] [Google Scholar]
- Azevedo, Hortellani & Sarkis (2012).Azevedo JS, Hortellani MA, Sarkis JES. Accumulation and distribution of metals in the tissues of two catfish species from Cananéia and Santos-São Vicente estuaries. Brazilian Journal of Oceanography. 2012;60(4):463–472. doi: 10.1590/S1679-87592012000400005. [DOI] [Google Scholar]
- Bernardino et al. (2015).Bernardino AF, Netto SA, Pagliosa PR, Barros F, Christofoletti RA, Rosa Filho JS, Colling A, Lana PC. Predicting ecological changes on benthic estuarine assemblages through decadal climate trends along Brazilian marine ecoregions. Estuarine, Coastal and Shelf Science. 2015;166:74–82. doi: 10.1016/j.ecss.2015.05.021. [DOI] [Google Scholar]
- Bernardino et al. (2019).Bernardino AF, Pais FS, Oliveira LS, Gabriel FA, Ferreira TO, Queiroz HM, Mazzuco AC. Chronic trace metals effects of mine tailings on estuarine assemblages revealed by environmental DNA. PeerJ. 2019;7(7):e8042. doi: 10.7717/peerj.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler (1975).Beutler E. Red cell metabolism: a manual of biochemical methods. Second Edition. New York: Grune and Stratton; 1975. p. 546. [Google Scholar]
- Bissoli & Bernardino (2018).Bissoli LB, Bernardino AF. Benthic macrofaunal structure and secondary production in tropical estuaries on the Eastern Marine Ecoregion of Brazil. PeerJ. 2018;6(3):e4441. doi: 10.7717/peerj.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatti et al. (2004).Bonatti M, Furlan S, Manente S, Perin G. Study of the toxicity of marine sediments of Babitonga bay—Brazil. Journal of Coastal Research. 2004;21:39. [Google Scholar]
- Bosco-Santos & Luiz-Silva (2019).Bosco-Santos A, Luiz-Silva W. Implications of sediment geochemistry and diet habitats in fish metal levels and human health risk. Applied Geochemistry with Case Studies on Geological Formations, Exploration Techniques and Environmental Issues. 2019 doi: 10.5772/intechopen.89872. [DOI] [Google Scholar]
- BRASIL (1965).BRASIL ANVISA, Agência Nacional de Vigilância Sanitária. 1965. http://legis.senado.leg.br/norma/478463/publicacao/15642284. http://legis.senado.leg.br/norma/478463/publicacao/15642284 Decreto no. 55.871, 26 de março de 1965. [DOI] [PMC free article] [PubMed]
- Bustamante et al. (2003).Bustamante P, Bocher P, Chérel Y, Miramand P, Caurant F. Distribution of trace elements in the tissues of benthic and pelagic fish from the Kerguelen Islands. Science of the Total Environment. 2003;313(1–3):25–39. doi: 10.1016/S0048-9697(03)00265-1. [DOI] [PubMed] [Google Scholar]
- Carmo, Abessa & Machado-Neto (2012).Carmo CA, Abessa DMS, Machado-Neto JG. Metals in muscles of mullet (Mugil curema) from a contaminated estuary: evidences of potential risks to public health. Natural Resources. 2012;2(2):81–94. doi: 10.6008/ESS2237-9290.2012.002.0007. [DOI] [Google Scholar]
- Carrola et al. (2014).Carrola J, Santos N, Rocha MJ, Fontainhas-Fernandes A, Pardal MA, Monteiro RA, Rocha E. Frequency of micronuclei and of other nuclear abnormalities in erythrocytes of the grey mullet from the Mondego, Douro and Ave estuaries—Portugal. Environmental Science and Pollution Research. 2014;21(9):6057–6068. doi: 10.1007/s11356-014-2537-0. [DOI] [PubMed] [Google Scholar]
- Chaves & Otto (1998).Chaves PTC, Otto G. Aspectos biológicos de Diapterus rhombeus (Cuvier)d(Teleostei, Gerreidae) na Baía de Guaratuba, Paraná, Brasil. Revista Brasileira de Zoologia. 1998;15(2):289–295. doi: 10.1590/S0101-81751998000200002. [DOI] [Google Scholar]
- Chaves & Vendel (1996).Chaves PTC, Vendel AL. Aspectos da alimentação de Genidens genidens (Valenciennes) (Siluriformes, Ariidae) na baía de Guaratuba, Paraná. Revista Brasileira de Zoologia. 1996;13(3):669–675. doi: 10.1590/S0101-81751996000300016. [DOI] [Google Scholar]
- Cline (1994).Cline JM. Fish interactions with the sediment-water interface. Hydrobiologia. 1994;275/276:301–311. doi: 10.1007/978-94-017-2460-9_27. [DOI] [Google Scholar]
- Coimbra et al. (2018).Coimbra ESC, Mascarenhas MS, Saraiva VB, Santos CR, Lopes RM, Hauser-Davis RA, Oliveira VPS, Molisani MM, Almeida MG, Rezende CE, Carvalho CEV, Oliveira MM. Metal loads and biomarker suite responses in a tropical carnivorous fish indicative of anthropogenic impacts in a Southeastern Brazilian lagoon. Environmental Monitoring and Assessment. 2018;190(9):564. doi: 10.1007/s10661-018-6910-1. [DOI] [PubMed] [Google Scholar]
- Dantas et al. (2019).Dantas DV, Ribeiro CIR, Frischknecht CCA, Machado R, Farias EGG. Ingestion of plastic fragments by the Guri sea catfish Genidens genidens (Cuvier, 1892) in a subtropical coastal estuarine system. Environmental Science and Pollution Research. 2019;26(8):8344–8351. doi: 10.1007/s11356-019-04244-9. [DOI] [PubMed] [Google Scholar]
- De Oliveira Gomes et al. (2017).De Oliveira Gomes LE, Correa LB, Sa F, Neto RR, Bernardino AF. The impacts of the Samarco mine tailing spill on the Rio Doce estuary, Eastern Brazil. Marine Pollution Bulletin. 2017;120(1–2):28–36. doi: 10.1016/j.marpolbul.2017.04.056. [DOI] [PubMed] [Google Scholar]
- De Souza Lima Junior et al. (2002).De Souza Lima Junior RG, Araújo FG, Maia MF, Da Silveira Braz Pinto AS. Evaluation of heavy metals in fish of the Sepetiba and Ilha Grande Bays, Rio de Janeiro, Brazil. Environmental Research. 2002;89(2):171–179. doi: 10.1006/enrs.2002.4341. [DOI] [PubMed] [Google Scholar]
- Di Giulio et al. (1995).Di Giulio RT, Benson WH, Sanders BM, Van Veld PA, Rand GM. Fundamentals of Aquatic Toxicology Effects, Environmental Fate, and Risk Assessment. London: Taylor and Francis; 1995. pp. 523–561. [Google Scholar]
- Do Carmo et al. (2017).Do Carmo FF, Kamino LHY, Tobias Junior R, Campos IC, Carmo FF, Silvino G, Castro KJSX, Mauro ML, Rodrigues NUA, Miranda MPS, Pinto CEF. Fundão tailings dam failures: the environment tragedy of the largest technological disaster of Brazilian mining in global context. Perspectives in Ecology and Conservation. 2017;15(3):145–151. doi: 10.1016/j.pecon.2017.06.002. [DOI] [Google Scholar]
- Dural, Göksu & Özak (2007).Dural M, Göksu MZL, Özak AA. Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chemistry. 2007;102(1):415–421. doi: 10.1016/j.foodchem.2006.03.001. [DOI] [Google Scholar]
- European Commission (2001).European Commission Commission regulation no. 466/2001 of 8 March (2001), 1.77/1. 2001. https://op.europa.eu/en/publication-detail/-/publication/52b2484d-39e0-4aa9-ba19-4b13a887bb1c https://op.europa.eu/en/publication-detail/-/publication/52b2484d-39e0-4aa9-ba19-4b13a887bb1c
- Ellman (1959).Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Erk et al. (2002).Erk M, Ivanković D, Raspor B, Pavicić J. Evaluation of different purification procedures for the electrochemical quantification of mussel metallothioneins. Talanta. 2002;57(6):1211–1218. doi: 10.1016/S0039-9140(02)00239-4. [DOI] [PubMed] [Google Scholar]
- Eurachem (1998).Eurachem . The fitness for purpose of analytical methods. First Edition. Teddington: 1.0. LGC Ltd; 1998. Eurachem Guide. [Google Scholar]
- FAO/WHO (1997).FAO/WHO Joint FAO/WHO food standards programme Codex Committee on contaminants in foods 1-89. 1997 . http://www.fao.org/3/W5979E/w5979e00.htm#Contents http://www.fao.org/3/W5979E/w5979e00.htm#Contents
- Ferreira et al. (2020).Ferreira FF, Freitas MBD, Szinwelski N, Vicente N, Medeiros LCC, Schaefer CEGR, Dergam JÁ, Sperber CF. Impacts of the Samarco tailing dam collapse on metals and arsenic concentration in freshwater fish muscle from Doce River, Southeastern Brazil. Integrated Environmental Assessment and Management. 2020;16(5):622–630. doi: 10.1002/ieam.4289. [DOI] [PubMed] [Google Scholar]
- Forman, Zhang & Rinna (2009).Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Molecular Aspects of Medicine. 2009;30(1–2):1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel et al. (2020).Gabriel FA, Silva AG, Queiroz HM, Ferreira TO, Hauser-Davis RA, Bernardino AF. Ecological risks of metal and metalloid contamination in the Rio Doce estuary. Integrated Environmental Assessment and Management. 2020;16(5):655–660. doi: 10.1002/ieam.4250. [DOI] [PubMed] [Google Scholar]
- Garcia-Ordiales et al. (2018).Garcia-Ordiales E, Covelli S, Rico JM, Roqueñí N, Fontolan G, Flor-Blanco G, Cienfuegos P, Loredo J. Occurrence and speciation of arsenic and mercury in estuarine sediments affected by mining activities (Auturias, northern Spain) Chemosphere. 2018;198:281–289. doi: 10.1016/j.chemosphere.2018.01.146. [DOI] [PubMed] [Google Scholar]
- Goyer & Clarkson (1996).Goyer RA, Clarkson TW. Toxic effects of metals. Casarett and Doull’s Toxicology: The Basic Science of Poisons. 1996;5:696–698. [Google Scholar]
- Gu et al. (2018).Gu YG, Huang HH, Liu Y, Gong XY, Liao XL. Non-metric multidimensional scaling and human risks of heavy metal concentrations in wild marine organisms from the Maowei Sea, the Beibu Gulf, South China Sea. Environmental Toxicology and Pharmacology. 2018;59:119–124. doi: 10.1016/j.etap.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Gusso-Choueri et al. (2018).Gusso-Choueri PK, Araújo GS, Cruz ACF, Stremel TRO, Campos SX, Abessa DMS, Oliveira Ribeiro CA, Choueri RB. Metals and arsenic in fish from a Ramsar site under past and present human pressures: consumption risk factors to the local population. Science of the Total Environment. 2018;628–629:621–630. doi: 10.1016/j.scitotenv.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Gusso-Choueri et al. (2016).Gusso-Choueri PK, Choueri RB, Santos GS, De Araújo GS, Cruz ACF, Stremel T, De Campos SX, Cestari MM, Ribeiro CAO, De Sousa Abessa DM. Assessing genotoxic effects in fish from a marine protected area influenced by former mining activities and other stressors. Marine Pollution Bulletin. 2016;104(1–2):229–239. doi: 10.1016/j.marpolbul.2016.01.025. [DOI] [PubMed] [Google Scholar]
- Gómez-Parra et al. (2000).Gómez-Parra A, Forja JM, DelValls TA, Saenz I, Riba I. Early contamination by heavy metals of the Guadalquivir estuary after the Aznalcóllar mining spill (SW Spain) Marine Pollution Bulletin. 2000;40(12):1115–1123. doi: 10.1016/S0025-326X(00)00065-5. [DOI] [Google Scholar]
- Hadlich et al. (2018).Hadlich HL, Venturini N, Martins CC, Hatje V, Tinelli P, De Oliveira Gomes LE, Bernardino AF. Multiple biogeochemical indicators of environmental quality in tropical estuaries reveal contrasting conservation opportunities. Ecological Indicators. 2018;95(1):21–31. doi: 10.1016/j.ecolind.2018.07.027. [DOI] [Google Scholar]
- Hauser-Davis et al. (2014).Hauser-Davis RA, Bastos FF, Tuton B, Chávez Rocha R, Saint’ Pierre T, Ziolli RL, Arruda MA. Bile and liver metallothionein behavior in copper-exposed fish. Journal of Trace Elements in Medicine and Biology. 2014;28(1):70–74. doi: 10.1016/j.jtemb.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Hauser-Davis et al. (2016).Hauser-Davis RA, Bordon ICAC, Oliveira TF, Ziolli RL. Metal bioaccumulation in edible target tissues of mullet (Mugil liza) from a tropical bay in Southeastern Brazil. Journal of Trace Elements in Medicine and Biology. 2016;36:38–43. doi: 10.1016/j.jtemb.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Hauser-Davis, Campos & Ziolli (2012).Hauser-Davis RA, Campos RC, Ziolli RL. Fish metalloproteins as biomarkers of environmental contamination. Reviews of Environmental Contamination and Toxicology. 2012;218:101–123. doi: 10.1007/978-1-4614-3137-4_2. [DOI] [PubMed] [Google Scholar]
- Ishak et al. (2015).Ishak I, Rosli FD, Mohamed J, Mohd Ismail MF. Comparison of digestion methods for the determination of trace elements and heavy metals in human hair and nails. Malaysian Journal of Medical Sciences. 2015;22(6):11–20. [PMC free article] [PubMed] [Google Scholar]
- Joyeux, Campanha Filho & de Jesus (2004).Joyeux JC, Campanha Filho EA, de Jesus HC. Trace metal contamination in estuarine fishes from Vitória bay, ES, Brazil. Brazilian Archives of Biology and Technology. 2004;47(5):765–774. doi: 10.1590/S1516-89132004000500012. [DOI] [Google Scholar]
- Kehring et al. (2016).Kehring HA, Hauser-Davis RA, Seixas TG, Pinheiro AB, Di Beneditto APM. Mercury species, selenium, metallothioneins and glutathione in two dolphins from the southeastern Brazilian coast: mercury detoxification and physiological differences in diving capacity. Environmental Pollution. 2016;213:785–792. doi: 10.1016/j.envpol.2016.03.041. [DOI] [PubMed] [Google Scholar]
- Lavradas et al. (2014).Lavradas R, Hauser-Davis RA, Lavandier RC, Rocha RC, Saint’ Pierre TD, Seixas T, Kehrig HA, Moreira I. Metal, mellothionein and glutathione levels in blue crab (Callinectes sp.) specimens from southeastern Brazil. Ecotoxicology and Environmental Safety. 2014;107:55–60. doi: 10.1016/j.ecoenv.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Lavradas et al. (2016).Lavradas RT, Rocha RCC, Bordon ICAC, Saint’Pierre TD, Godoy JM, Hauser-Davis RA. Differential metallothionein, reduced glutathione and metal levels in Perna perna mussels in two environmentally impacted tropical bays in southeastern Brazil. Ecotoxicology and Environmental Safety. 2016;129:75–84. doi: 10.1016/j.ecoenv.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Lotze et al. (2006).Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, Kidwell SM, Kirby MX, Peterson CH, Jackson JBC. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 2006;312(5781):1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2018).Lu Y, Yuan J, Lu X, Su C, Zhang Y, Wang C, Cao X, Li Q, Su J, Ittekkot V, Garbutt RA, Bush S, Fletcher S, Wagey T, Kachur A, Sweijd N. Major threats of pollution and climate change to global coastal ecosystems and enhanced management for sustainability. Environmental Pollution. 2018;239:670–680. doi: 10.1016/j.envpol.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Macêdo et al. (2020).Macêdo AKS, Dos Santos KPE, Brighenti LS, Windmöller CC, Barbosa FAR, De Azambuja Ribeiro RIM, Dos Santos HB, Thomé RG. Histological and molecular changes in gill and liver of fish (Astyanax lacustris Lütken, 1875) exposed to water from the Doce basin after the rupture of a mining tailings dam in Mariana, MG. Brazil Science of the Total Environment. 2020;735:139505. doi: 10.1016/j.scitotenv.2020.139505. [DOI] [PubMed] [Google Scholar]
- MAFF (1995).MAFF . Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea. Lowestoft: Directorate of Fisheries Research; 1995. p. 44. [Google Scholar]
- Mason et al. (2019).Mason RP, Baumann Z, Hansen G, Yao KM, Coulibaly M, Coulibaly S. An assessment of the impact of artisanal and commercial gold mining on mercury and methylmercury levels in the environment and fish in Cote d’Ivoire. Science of the Total Environment. 2019;665:1158–1167. doi: 10.1016/j.scitotenv.2019.01.393. [DOI] [PubMed] [Google Scholar]
- Mayer-Pinto, Matias & Coleman (2016).Mayer-Pinto M, Matias MG, Coleman RA. The interplay between habitat structure and chemical contaminants on biotic responses of benthic organisms. PeerJ. 2016;4:e1985. doi: 10.7717/peerj.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle & Anderson (2001).McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 2001;82(1):290–297. doi: 10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2. [DOI] [Google Scholar]
- Meister (1992).Meister A. Biosynthesis and functions of glutathione, an essential biofactor. Journal of Nutritional Science and Vitaminology. 1992;38:1–6. doi: 10.3177/jnsv.38.Special_1. [DOI] [PubMed] [Google Scholar]
- Monteiro et al. (2008).Monteiro SM, Almeida JA, Rantin FT, Kalinin AL. Quantitative histopathology of Oreochromis niloticus gills after copper exposure. Journal of Fish Biology. 2008;73(6):1376–1392. doi: 10.1111/j.1095-8649.2008.02009.x. [DOI] [Google Scholar]
- Monteiro et al. (2006).Monteiro DA, De Almeida JÁ, Rantin FT, Kalinin AL. Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus exposed to organophosphorus insecticide Folisuper 600 (methyl parathion) Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2006;143(2):141–149. doi: 10.1016/j.cbpc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Monteiro et al. (2019).Monteiro F, Lemos LS, De Moura JF, Rocha RCC, Moreira I, Di Beneditto AP, Kehrig HA, Bordon ICAC, Siciliano S, Saint’Pierre TD, Hauser-Davis RA. Subcellular metal distributions and metallothionein associations in rough-toothed dolphins (Steno bredanensis) from Southeastern Brazil. Marine Pollution Bulletin. 2019;146:263–273. doi: 10.1016/j.marpolbul.2019.06.038. [DOI] [PubMed] [Google Scholar]
- Muniz et al. (2006).Muniz P, Pires-Vanin AMS, Martins CC, Montone RC, Bícego MC. Trace metals and organic compounds in the benthic environment of a subtropical embayment (Ubatuba Bay, Brazil) Marine Pollution Bulletin. 2006;52(9):1098–1105. doi: 10.1016/j.marpolbul.2006.05.014. [DOI] [PubMed] [Google Scholar]
- NOAA (2008).NOAA Screening quick reference tables (SQuiRTs) NOAA OR&R report 08-1, National Oceanic and Atmospheric Administration, Office of Response and Restoration, Seattle. 2008. https://repository.library.noaa.gov/view/noaa/9327 https://repository.library.noaa.gov/view/noaa/9327
- Okay et al. (2016).Okay OS, Ozmen M, Güngördü A, Yilmaz A, Yakan SD, Karacik B, Tutak B, Schramm KW. Heavy metal pollution in sediments and mussels: assessment by using pollution indices and metallothionein levels. Environmental Monitoring and Assessment. 2016;188(6):352. doi: 10.1007/s10661-016-5346-8. [DOI] [PubMed] [Google Scholar]
- Oksanen et al. (2018).Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. vegan: community ecology package. R package version 20–10https://cran.r-project.org/web/packages/vegan/index.html 2018
- Pacheco et al. (2017).Pacheco CSV, Da Silva EG, Hauser-Davis RA, Dias F, Amorim FA, Jesus RM, Novaes CG, Santos AM, Saint’ Pierre TD. Determination and evaluation of metallothionein and metals in Mugil cephalus (Mullet) from Pontal Bay, Brazil. Bulletin of Environmental Contamination and Toxicology. 2017;98(1):84–90. doi: 10.1007/s00128-016-1959-4. [DOI] [PubMed] [Google Scholar]
- Pinheiro & Joyeux (2007).Pinheiro HT, Joyeux J-C. Pescarias multi-específicas na região da foz do Rio Doce, ES, Brasil: características, problemas e opções para um futuro sustentável. Brazilian Journal of Aquatic Science and Technology. 2007;11(2):15–23. doi: 10.14210/bjast.v11n2.p15-23. [DOI] [Google Scholar]
- Queiroz et al. (2018).Queiroz HM, Nóbrega GN, Ferreira TO, Almeida LS, Romero TB, Santaella ST, Bernardino AF, Otero XL. The Samarco mine tailing disaster: a possible time-bomb for heavy metals contamination? Science of the Total Environment. 2018;637–638:498–506. doi: 10.1016/j.scitotenv.2018.04.370. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2005).R Development Core Team . Vienna: R Foundation for Statistical Computing; 2005. [Google Scholar]
- Riba et al. (2005).Riba I, Blasco J, Jiménez-Tenorio N, DelValls TÁ. Heavy metal bioavailability and effects: I. Bioaccumulation caused by mining activities in the Gulf of Cádiz (SW, Spain) Chemosphere. 2005;58(5):659–669. doi: 10.1016/j.chemosphere.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Richard et al. (2020).Richard EC, Estrada GCD, Bechtold J-P, Duarte HA, Maioli BG, Freitas AHA, Warner KE, Figueiredo LHM. Water and sediment quality in the coastal zone around the mouth of Doce River after the Fundão tailings dam failure. Integrated Environmental Assessment and Management. 2020;16(5):643–654. doi: 10.1002/ieam.4309. [DOI] [PubMed] [Google Scholar]
- Rodrigues et al. (2010).Rodrigues APC, Carvalheira RG, Cesar RG, Bidone ED, Castilhos ZC, Almosny NRP. Mercury bioaccumulation in distinct tropical fish species from estuarine ecosystems in Rio de Janeiro state, Brazil. Anuário do Instituto de Geociências. 2010;33(2010)):54–62. [Google Scholar]
- Ruttkay-Nedecky et al. (2013).Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R. The role of metallothionein in Oxidative Stress. International Journal of Molecular Sciences. 2013;14(3):6044–6066. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana et al. (2017).Santana CO, De Jesus TB, Ahuiar WM, Franca-Rocha WJS, Soares CAC. Trace elements in muscle of three fish species from Todos os Santos bay, Bahia state, Brazil. Environmental Monitoring and Assessment. 2017;189(123):1–8. doi: 10.1007/s10661-016-5706-4. [DOI] [PubMed] [Google Scholar]
- Segura et al. (2016).Segura FR, Nunes EA, Paniz FP, Paulelli ACC, Rodrigues GB, Braga GÚ.L, Pedreira Filho WR, Barbosa F, Jr, Cerchiaro G, Silva FF, Batista BL. Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil) Environmental Pollution. 2016;218:813–825. doi: 10.1016/j.envpol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Seixas et al. (2005).Seixas TG, Kehrig HA, Moreira I, Malm O. Selênio em tecidos de quatro organismos marinhos da Baía de Guanabara-RJ. Tropical Oceanography. 2005;33(2):207–222. doi: 10.5914/tropocean.v33i2.5063. [DOI] [Google Scholar]
- Shah & Altindağ (2005).Shah SL, Altindağ A. Effects of heavy metal accumulation on the 96-h LC50 values in tench Tinca tinca L., 1758. Turkish Journal of Veterinary & Animal Sciences. 2005;29:139–144. [Google Scholar]
- Sharma & Langer (2014).Sharma J, Langer S. Effect of manganese on haematological parameters of fish, Garra gotyla gotyla. Journal of Entomology and Zoology Studies. 2014;2(3):77–81. [Google Scholar]
- Singhal, Anderson & Meister (1987).Singhal RK, Anderson ME, Meister A. Glutathione; a first line of defense against cadmium toxicity. Faseb Journal. 1987;1(3):220–223. doi: 10.1096/fasebj.1.3.2887478. [DOI] [PubMed] [Google Scholar]
- Souza et al. (2018).Souza EC, Morozesk M, Bonomo MM, Azevedo VC, Sakuragui MM, Elliott M, Matsumoto ST, Wunderlin DA, Baroni MV, Monferrán MV, Fernandes MN. Differential biochemical responses to metal/metalloid accumulation in organs of an edible fish (Centropomus parallelus) from Neotropical estuaries. Ecotoxicology and Environmental Safety. 2018;161:260–269. doi: 10.1016/j.ecoenv.2018.05.068. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (EPA) (1996).United States Environmental Protection Agency (EPA) Method 3052: microwave assisted acid digestion of siliceous and organically based matrices. Usepa 1–20. 1996. https://www.epa.gov/sites/production/files/2015-12/documents/3052.pdf https://www.epa.gov/sites/production/files/2015-12/documents/3052.pdf
- United States Environmental Protection Agency (EPA) (2007).United States Environmental Protection Agency (EPA) Risk based concentration table. 2007. https://www.epa.gov/risk/risk-assessment-guidelines https://www.epa.gov/risk/risk-assessment-guidelines
- U.S. Food and Drug Administration (1993).U.S. Food and Drug Administration . Guidance document for metals in shellfish. Washington, D.C.: DHHS/PHS/FDA/CFSAN/Office of Seafood; 1993. [Google Scholar]
- Van Ael, Blust & Bervoets (2017).Van Ael E, Blust R, Bervoets L. Metals in the scheldt estuary: from environmental concentrations to bioaccumulation. Environmental Pollution. 2017;228:82–91. doi: 10.1016/j.envpol.2017.05.028. [DOI] [PubMed] [Google Scholar]
- Van Der Oost, Beyer & Vermeulen (2003).Van Der Oost R, Beyer J, Vermeulen NP. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology. 2003;13(2):57–149. doi: 10.1016/S1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Varzim et al. (2019).Varzim CS, Hadlich HL, Andrades R, Mazzuco ACA, Bernardino AF. Tracing pollution in estuarine benthic organisms and its impacts on food webs of the Vitoria Bay estuary. Estuarine, Coastal and Shelf Science. 2019;229:106410. doi: 10.1016/j.ecss.2019.106410. [DOI] [Google Scholar]
- Weber et al. (2020).Weber AA, Sales CF, Faria FS, Melo RMC, Bazzoli N, Rizzo E. Effects of metal contamination on liver in two fish species from a highly impacted neotropical river: a case study of the Fundão dam, Brazil. Ecotoxicology and Environmental Safety. 2020;190:110–165. doi: 10.1016/j.ecoenv.2020.110165. [DOI] [PubMed] [Google Scholar]
- Wilhelm-Filho et al. (2005).Wilhelm-Filho D, Torres MA, Zaniboni-Filho E, Pedrosa RC. Effect of different oxygen tensions on weight gain, feed conversion, and antioxidant status in piapara, Leporinus elongatus (Valenciennes, 1847) Aquaculture. 2005;244(1–4):349–357. doi: 10.1016/j.aquaculture.2004.11.024. [DOI] [Google Scholar]
- Zhu et al. (2015).Zhu F, Qu L, Fan W, Wang A, Hao H, Li X, Yao S. Study on heavy metal levels and its health risk assessment in some edible fishes from Nansi Lake, China. Environmental Monitoring and Assessment. 2015;187(4):161. doi: 10.1007/s10661-015-4355-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data is displayed as means ± SD.
Certified reference material: NIST SRM 2709a.
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.