Abstract
This study aimed to characterize the in vitro antioxidant and antibacterial properties of oregano (Origanum vulgare) essential oil, as well as its chemical composition. To our best knowledge, there are few studies on oregano grown in the arid Andes region, but none on the metabolites produced and their bioactivity. This work identified fifty metabolites by Gas Chromatography–Mass Spectrometry (GC-MS)—monoterpene hydrocarbons, oxygenated monoterpenes, phenolic monoterpenes, sesquiterpene hydrocarbons, and oxygenated sesquiterpenes—present in the essential oil of oregano collected in the Atacama Desert. The main components of essential oregano oil were thymol (15.9%), Z-sabinene hydrate (13.4%), γ-terpinene (10.6%), p-cymene (8.6%), linalyl acetate (7.2%), sabinene (6.5%), and carvacrol methyl ether (5.6%). The antibacterial tests showed that the pathogenic bacteria Staphylococcus aureus and Salmonella enterica and the phytopathogenic bacteria Erwinia rhapontici and Xanthomonas campestris were the most susceptible to oregano oil, with the lowest concentrations of oil necessary to inhibit their bacterial growth. Moreover, oregano oil showed antibacterial activity against bacteria associated with food poisoning. In conclusion, O. vulgare from the arid Andean region possesses an important antibacterial activity with a high potential in the food industry and agriculture.
Keywords: antibacterial activity, antioxidant activity, essential oil, GC-MS, oregano, Putre
1. Introduction
There is a growing interest in assessing the antimicrobial and antioxidant properties of substances from natural sources that can potentially be used by the food and pharmaceutical industries. Essential oils from aromatic and medicinal plants have been known to possess biological activity [1,2]. Oregano (Origanum vulgare) is an aromatic plant with a wide distribution throughout the Mediterranean area and Asia [3]. Although oregano is an herb native to the European countries, it was also introduced to the Chilean territory more than 100 years ago [4]. In the arid Andean region, this plant is cultivated between 2800 and 3500 m above sea level. It produces a very aromatic spice with a specific quality or characteristics that are essentially due to the arid geographical environment [5,6,7]. This spice has long been used as a medicinal herb in ethnopharmacological preparations to treat various ailments such as respiratory disorders, dyspepsia, painful menstruation, rheumatoid arthritis, scrofulosis, and urinary tract disorders [8,9,10,11]. The high content of volatile essential oils is responsible for the antimicrobial activity, aroma, and flavor [12]. According to phytochemical studies on the essential oil composition of Origanum vulgare and other related species, there is a wide chemical diversity, with a considerable intraspecific qualitative and quantitative variation in constituents is found [13,14,15,16,17]. Some studies have also reported the potential of oregano essential oil to preserve food such as salmon and seaweed burgers [18], fish, and meat products [19]. Different studies on the isolation and characterization of O. vulgare essential oils from different world regions have been performed [20]. Nevertheless, there was no available information on the bioactive properties and characterization of O. vulgare essential oil cultivated in the arid Andean region. Therefore, this study aimed to describe the chemical composition of Andean O. vulgare essential oil and evaluate the antimicrobial and antioxidant activity of the oregano essential oil, as well as its potential as a food additive.
2. Results and Discussion
2.1. Physicochemical Properties
Table 1 shows the conditions, yields, and physical properties of the essential oil of oregano obtained by hydro-distillation. The density was 0.913 g/mL, and the refractive index was 1.4775; these values are similar to those reported previously for the same spice [21,22]. According to some research, the refractive index and density are proportionally related to the amount of phenols present [23]. The acidity index was 0.8345 mg of KOH/g of sample, equivalent to 0.419 g of oleic acid/100 g of oil.
Table 1.
Physicochemical characteristics of Origanum vulgare essential oil from Putre, Chile.
| Parameters | Origanum vulgare L. |
|---|---|
| Distillation time (min) | 60 |
| Yield (%) | 1.89 |
| Specific density 20 °C (g/mL) | 0.913 |
| Refractive index | 1.4775 |
| Acidity index (mg KOH/g sample) | 0.8345 |
2.2. Chemical Composition of the Essential Oil
The Origanum vulgare essential oil composition was analyzed by GC-MS. The compound identification was carried out by comparing the relative retention times and the mass spectra of oil components with authentic samples and mass spectra from the data library. In total, fifty compounds were identified in O. vulgare essential oil, accounting for 99.2% of the whole composition, and they are mostly monoterpene hydrocarbons (38.7%), oxygenated monoterpenes (38.0%), and phenolic monoterpenes (19.0%). Within the monoterpene hydrocarbons, δ-terpinene (10.6%), p-cymene (8.6%), and sabinene (6.5%) were the major compounds detected, while Z-sabinene hydrate (13.4%), linalyl acetate (7.2%), and carvacrol methyl ether (5.6%) were the most abundant oxygenated monoterpenes detected (Figure 1, Table 2). Additionally, thymol (15.9%) represented the main fraction of O. vulgare essential oil. Carvacrol was also identified, but in a lower concentration (3.1%). The phytochemical contents of oregano oil from the arid region, especially the phenolic monoterpenes, curiously exhibited a great difference compared to what was reported by other authors [14,16,24,25].
Figure 1.
Chemical structures of the main compounds present in the essential oil of O. vulgare from Putre, Chile.
Table 2.
Chemical composition of volatiles in the Origanum vulgare essential oil from Putre, Chile.
| N° | Compounds | RI 1 | Ref RI 2 | % | Identification |
|---|---|---|---|---|---|
| 1 | α-Thujene | 927 | 924 | 1.2 | a |
| 2 | α-Pinene | 936 | 932 | 0.7 | a,b |
| 3 | Camphene | 951 | 946 | tr | a,b |
| 4 | Sabinene | 971 | 974 | 6.5 | a |
| 5 | β-Pinene | 978 | 976 | 0.3 | a,b |
| 6 | 1-Octen-3-ol | 978 | 979 | 0.2 | a,b |
| 7 | Eucalyptol | 988 | 988 | tr | a |
| 8 | β-Myrcene | 991 | 988 | 1.9 | a,b |
| 9 | α-Phellandrene | 994 | 1001 | 0.5 | a |
| 10 | 3-Octanol | 993 | 991 | tr | a |
| 11 | δ-3−Carene | 1008 | 1008 | tr | a |
| 12 | α-Terpinene | 1020 | 1015 | 3.5 | a |
| 13 | Z-β-Ocimene | 1027 | 1032 | 0.9 | a |
| 14 | p-Cymene | 1028 | 1024 | 8.6 | a,b |
| 15 | Limonene | 1033 | 1027 | 0.9 | a,b |
| 16 | E-β-Ocimene | 1047 | 1044 | 0.2 | a |
| 17 | α-Terpinolene | 1053 | 1053 | 0.8 | a |
| 18 | γ-Terpinene | 1062 | 1058 | 10.6 | a,b |
| 19 | Z-Sabinene hydrate | 1073 | 1066 | 13.4 | a |
| 20 | Z-Linalool oxide | 1075 | 1067 | tr | a |
| 21 | E-Linalool oxide | 1082 | 1084 | tr | a |
| 22 | E-Sabinene hydrate | 1105 | 1098 | 2.0 | a |
| 23 | β-Thujone | 1114 | 1112 | 2.2 | a |
| 24 | E-p-2-Menthen-1-ol | 1124 | 1129 | 0.4 | a |
| 25 | endo-Borneol | 1160 | 1165 | 0.2 | a |
| 26 | p-Cymen-8-ol | 1180 | 1179 | 0.1 | a |
| 27 | 4-Terpineol | 1179 | 1177 | 4.4 | a |
| 28 | p-Cymen-7-ol | 1181 | 1181 | tr | a |
| 29 | α-Terpineol | 1186 | 1186 | 1.8 | a |
| 30 | E-Piperitol | 1193 | 1207 | 0.3 | a |
| 31 | Z-Piperitol | 1207 | 1195 | 0.3 | a |
| 32 | Nerol | 1229 | 1227 | tr | a |
| 33 | Thymol methyl ether | 1237 | 1232 | 2.3 | a |
| 34 | Carvacrol methyl ether | 1245 | 1241 | 5.6 | a |
| 35 | Geraniol | 1250 | 1249 | tr | a |
| 36 | Linalyl acetate | 1256 | 1257 | 7.2 | a |
| 37 | Bornyl acetate | 1289 | 1284 | tr | a |
| 38 | Thymol | 1293 | 1289 | 15.9 | a,b |
| 39 | Carvacrol | 1300 | 1298 | 3.1 | a,b |
| 40 | Eugenol | 1357 | 1356 | tr | a |
| 41 | Geranyl acetate | 1380 | 1379 | 0.1 | a |
| 42 | β-Caryophyllene | 1420 | 1418 | 1.2 | a,b |
| 43 | Aromadendrene | 1442 | 1439 | tr | a |
| 44 | α-Humulene | 1454 | 1452 | 0.4 | a |
| 45 | Bicyclogermacrene | 1500 | 1494 | 1.5 | a |
| 46 | δ-Cadinene | 1524 | 1522 | tr | a |
| 47 | Ledene | 1552 | 1552 | tr | a |
| 48 | Spathulenol | 1573 | 1577 | 0.1 | a,b |
| 49 | Caryophyllene oxide | 1581 | 1582 | 0.1 | a,b |
| 50 | Isospathulenol | 1704 | 1704 | tr | a |
| Monoterpene hydrocarbons | 38.7 | ||||
| Oxygenated monoterpenes | 38.0 | ||||
| Phenolic monoterpenes | 19.0 | ||||
| Sesquiterpene hydrocarbons | 3.1 | ||||
| Oxygenated sesquiterpenes | 0.2 | ||||
| Others | 0.2 | ||||
| Total identified | 99.2 | ||||
a Comparison of mass spectra with MS libraries and retention times. b Comparison with authentic compounds. tr: concentration lower than 0.05%. Compounds are listed in order of their elution on the HP-5MS column. 1 Retention index on the HP-5MS column relative to C8–C24 n-alkanes. 2 Retention index from the literature [26].
In most cases, carvacrol constituted the major component (12.6–88.7%) of the oil, while the sum of the two phenolic monoterpenes (carvacrol and thymol) and their biosynthetic precursors p-cymene and γ-terpinene [27] represented approximately 75% of each essential oil [28,29]. Other compounds have also been reported as important essential oil components, such as caryophyllene, spathulenol, and germacrene-D [30,31]. However, the Origanum composition depends on the climate, altitude, time of recollection, and the stage of growth [32]. In this context, previous works on the phytochemical content of oregano oil grown in the northwestern Himalayas at 3200 m above sea level reported low values for carvacrol (1.1%) [33]. These results are in agreement with our observations on oregano grown at 3000 m above sea level. One of the arid Andean region’s main environmental characteristics is its high radiation levels, which would favor the concentration of some phenolic monoterpenes in the essential oil, as reported by Naghdi Badi [34].
On the other hand, a study in the semi-arid zones of North Africa shows that the essential oil from oregano grown in this region is mainly composed of thymol, p-cymene, γ-terpinene, and carvacrol [13]. It has also been reported that the concentration of p-cymene and γ-terpinene is high only in the respective poor oils of carvacrol and thymol, such as the essential oil of Satureja thymbra [29]. In short, we have made it clear that the biosynthesis of secondary metabolites of the essential oil of oregano grown in the arid Andean region studied is strongly affected by environmental factors. Agricultural practices also have a critical effect on the quantitative and qualitative characteristics of plant-derived metabolites.
2.3. Antioxidant Activity and Total Polyphenols Content
Because the antioxidant capacity cannot be fully described by a single method, for this study, the antioxidant capacities of O. vulgare essential oil were assayed with three different assays. Free radical scavenging capacities were measured using DPPH radical and ABTS radical cation. The values found are given in Table 3. The DPPH radical scavenging activity of the oregano oil from the arid region was very low (4750 ± 91.11 μmol Trolox/g); however, our result agrees with other authors’ observations on oregano oil ranging from 1509 μg/mL to 8900 μg/mL [24,30]. The value found for ABTS (1252.74 μmol Trolox/g) is higher than that reported by Sarikurkcu for the same species cultivated at 372 m above sea level [8]. Major components of the oregano oils may explain these differences in free radical scavenging capacities [16,35,36].
Table 3.
Antioxidant activity of Origanum vulgare essential oil from Putre, Chile.
| Species | DPPH a | ABTS b | FRAP b | TPC c |
|---|---|---|---|---|
| O. vulgare | 4750 ± 91.11 | 1252.74 ± 47.10 | 270.53 ± 3.52 | 102.71± 3.87 |
| Quercetin * | 6.99 ± 0.02 | - | - | - |
| Trolox * | 20.99 ± 1.24 | - | - | - |
a Antiradical DPPH activities are expressed as IC50 in µg/mL for sample and compounds; b Expressed as μmol Trolox/g sample; c Total phenolic content (TPC) expressed as mg GAE/g sample; * Used as standard antioxidants.
The reducing power of an essential oil can serve as a significant indicator of its potential antioxidant activity. For this reason, the reducing power of ferric ions and total polyphenols content were examined (Table 3). The ferric reducing antioxidant power (FRAP) of the essential oil in this study exhibited a weak reducing power compared to the values obtained in other works [8], but the total phenolic content (102.71 ± 3.87 mg GAE/g sample) was higher than those reported for the same species (4.1–17.7 mg GAE/g sample) [13,37,38] and slightly less than those reported for wild oregano populations from Sicily, Italy [39]. The lack of correlation between total polyphenol content and antioxidant capacity could be attributed to the fact that the Folin–Ciocalteu method reflects the presence of all reducing substances in a matrix, not just polyphenolics. Thus, the presence of non-phenolic substances cannot be ruled out. Therefore, interpretations of total phenolic content (TPC) results must consider these potential limitations. Despite these limitations, the TPC and antioxidant methods are widely used [40].
2.4. Antibacterial Activity
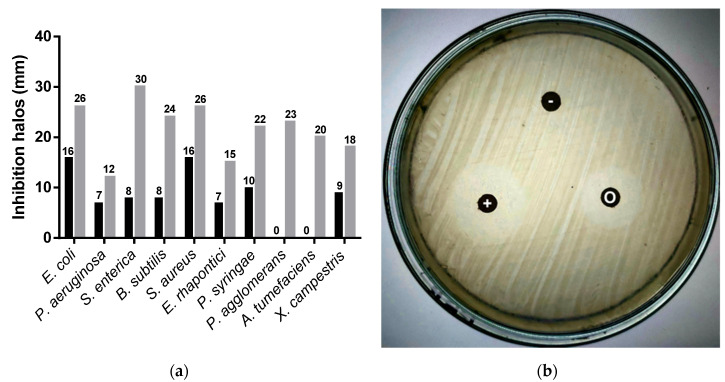
Figure 2 shows the inhibitory diameters for the oregano essential oil against five common foodborne pathogens and five phytopathogenic bacteria (Figure 2). This technique is recognized as a useful semi-quantitative method to determine the sensitivity of microorganisms to certain compounds. Disks impregnated in ethanol were used as a negative control. These disks were dried under the biosafety chamber’s flow to evaporate the ethanol and avoid its antimicrobial effect. The essential oil showed inhibitory activity against these pathogens, with diameters between 7 and 16 mm, classified as moderate/mild inhibitory activity compared to those reported by other authors [30,41,42]. As observed, almost all bacteria were sensitive to the inhibitory effect of oregano essential oil, except Pantoea agglomerans and Agrobacterium tumefaciens. It presented an inhibitory effect against Pseudomonas aeruginosa, contrary to what has been reported by other authors [31,42].
Figure 2.
(a) Inhibitory activity (mm) of O. vulgare essential oil against pathogens and five phytopathogenic bacteria. (■) oregano; (■) kanamycin positive control. (b) Paper disk diffusion assay of oregano essential oil against Escherichia coli. Disk O: oregano oil; disk +: kanamycin positive control; disk −: ethanol negative control.
Table 4. shows the inhibitory and bactericidal effects of oregano oil, tested on five species of bacteria of clinical importance (Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica, Bacillus subtilis, and Staphylococcus aureus) and five species of plant-pathogenic bacteria (Erwinia rhapontici, Pseudomonas syringae, Pantoea agglomerans, Agrobacterium tumefaciens, and Xanthomonas campestris). The different bacterial species tested showed varying susceptibility to the effects of the essential oil, which was expected since bacterial susceptibility to antimicrobial compounds is variable even at the strain level. This variation is due to specific differences in the composition of the different macromolecules and structures that these microorganisms possess. From Table 4, it can be observed that almost all microorganisms were susceptible to the action of oregano essential oil, with a variation in the MIC values from 0.04% v/v to 0.63% v/v and MBC values from 0.08% v/v to 1.25% v/v. P. agglomerans and A. tumefaciens showed resistance to the antimicrobial activity, and P. aeruginosa presented the highest resistance (0.63% v/v MIC and 1.25% v/v MBC). Kanamycin (50 µg) was used as a positive control of bacterial inhibition. E. rhapontici (0.04% v/v MIC and 0.08% v/v MBC) and X. campestris (0.08% v/v MIC and 0.08% v/v MBC) were the most susceptible phytopathogenic bacteria to oregano oil, with the lowest concentrations necessary to inhibit bacterial growth. These results could indicate a potential use of this essential oil in agriculture and agribusiness [43]. On the other hand, among the bacteria of clinical importance, S. aureus and S. enterica (0.08% v/v MIC and 0.08% v/v MBC) were the most susceptible to oregano oil. These results represent a promising strategy for controlling common foodborne pathogens.
Table 4.
Antibacterial activity of Origanum vulgare essential oil from Putre, Chile.
| Bacteria | MIC 1 (%) | MBC 2 (%) |
|---|---|---|
| Pathogenic | ||
| Escherichia coli (ATCC 23716) | 0.16 | 0.16 |
| Pseudomonas aeruginosa (ATCC 19429) | 0.63 | 1.25 |
| Salmonella enterica (ATCC 13311) | 0.08 | 0.08 |
| Bacillus subtilis (ATCC 6051) | 0.08 | 0.32 |
| Staphylococcus aureus (ATCC 29737) | 0.08 | 0.08 |
| Phytopathogenic | ||
| Erwinia rhapontici (MK883065) | 0.04 | 0.08 |
| Pseudomonas syringae (MF547632) | 0.16 | 0.32 |
| Pantoea agglomerans (MK883087) | ND | ND |
| Agrobacterium tumefaciens (ATCC 19358) | ND | ND |
| Xanthomonas campestris (MH885473) | 0.08 | 0.08 |
The high sensitivity of all of the tested strains to the essential oil of O. vulgare is of particular interest. This antimicrobial effect could be attributed to an alteration in the lipidic components of the bacterial plasma membrane, resulting in the leakage of intracellular contents [44]. Furthermore, O. vulgare essential oil’s higher antimicrobial activity could be explained by the amount of phenolic oxygenated monoterpenes—especially thymol, p-cymene, and carvacrol, which were found in the ratio of 27.6% and they could show a synergistic effect causing membrane destabilization. Some studies ensure that the combination of carvacrol and thymol at low concentrations could be used as a reference to apply this combination in foods to control S. aureus, to maintain the organoleptic properties, and to extend the shelf-life of them [45]. Other studies have reported the synergic potential of oregano essential oil to preserve food, such as salmon and seaweed burgers [18], beef meatballs [46], fish and meat products [19]. In this context, the activity found in essential oil from the arid Andean region revealed its potential as a food additive to combat common foodborne pathogens.
3. Materials and Methods
3.1. Preparation of O. vulgare Essential Oil
Aerial parts of Origanum vulgare were collected cultivated in Socoroma (Putre, Chile) (18°15’4.67’’ S; 69°36’7.44’’ W, 2945 masl). The collection was carried out as described by Mechergui et al. [13], and Bisht et al. [33], with some modifications. Each collection consisted of 20 plants at the bloom stage in November 2019 from four different estates in the same village. The samples were selected to provide a homogenous group based on harvest, color, size, and freshness according to visual analysis. This sample was identified and deposited in the Herbarium from the Botany Department of Universidad de Concepción, Chile (voucher specimen 184934). The essential oil was obtained from dry plant material (50 g) by hydrodistillation for 60 min using a modified Clevenger system. Subsequently, the essential oil was dried through anhydrous sodium sulfate, and the yield was 5.3% (v/d.w.) of the plants collected in 2019. The essential oil was stored at −20 °C.
3.2. Chemicals
Phosphate buffer, trichloroacetic acid, ferric chloride, hydrochloric acid, ascorbic acid, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), Folin–Ciocalteu reagent, gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), potassium hexacyanoferrate(III), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and dimethylsulfoxide were from Sigma-Aldrich (Santiago, Chile); ferrous sulfate, sodium acetate, sodium carbonate, sodium persulfate, sodium sulfate anhydrous, and ethanol were from Merck (Santiago, Chile).
3.3. Gas Chromatography–Mass Spectrometry Analysis
GC-MS analysis was carried out as described by Teixeira et al., with some modifications [24]. The essential oil was analyzed using an Agilent 5975 gas chromatograph coupled to an Agilent 5973N mass-selective detector (Agilent Technologies, Palo Alto, CA., USA). It was fitted with an HP-5MS capillary column (30 m length × 0.32 mm internal diameter × 0.25 µm film thickness). The oven temperature was programmed at 45 °C for 1 min, raised to 250 °C at 5 °C min−1, and maintained at 250 °C for 5 min. Helium was used as carrier gas at 30 cm s−1, and the injection volume was 1 µL. The identity of each compound was assigned by comparing their retention index relative to a standard mixture of n-alkanes, as well as by comparison with the mass spectra characteristic features obtained from the Wiley data bank (Wiley 7N Edition [Agilent Part No. G1035B]: Wiley Registry of Mass Spectral Data, 7th Edition), whenever possible, co-injections with authentic samples.
3.4. Determination of Physicochemical Characteristics
Physicochemical characteristics were evaluated as described by Tellez-Monzón et al. [21], with some modifications. The fresh essential oil’s specific density was measured using a 10 mL pycnometer at 20 °C. The weight of the empty pycnometer was recorded. The pycnometer was filled with the essential oil and weighed again. The experiment was carried out in triplicate. The density of the essential oils at room temperature was calculated using the formula:
| (1) |
The refractive index of the essential oils at room temperature was measured using an ABBE REF-1 refractometer (PCE Instruments, Meschede, Germany). The acidity index was determined through a potentiometric titration with a 0.02 N NaOH solution and expressed as a percentage of oleic acid.
3.5. Determination of Total Phenols (Folin-Ciocalteu)
Total polyphenol content was determined using the Folin-Ciocalteu method, with some modifications [38]. From the 50 g/L solution, one was prepared at 0.5 g/L, where an aliquot of the solution (1000 μL) was mixed with 500 μL of Folin-Ciocalteu reagent (50% v/v) and 5000 μL of ethanol at 80% v/v. After 5 min of reaction, 250 µL of 5% w/v Na2CO3 was added and brought to a final volume of 8000 µL with 80% v/v ethanol. This was then allowed to incubate for 30 min at room temperature. Each sample was measured at 760 nm and compared to a calibration curve using gallic acid as the standard.
3.6. Antioxidant Activity Assays
3.6.1. Ferric Reducing Antioxidant Power (FRAP)
The determination of the ferric reducing antioxidant power or the ferric reducing capacity (FRAP test) was carried out as described by Parra et al. [47], with some modifications. The FRAP reagent was prepared by mixing 25 mL of acetate buffer (300 mmol/L, pH 3.6), 2.5 mL of TPTZ solution (10 mmol/L) in hydrochloric acid (40 mmol/L), and 2.5 mL of FeCl3·6H2O (20 mmol/L). For measurement, 25 µL of diluted extract (0.5 g/L) was mixed with 175 µL of FRAP reagent in a 96-well microplate. The mixture was incubated for 40 min at 25 °C, and then absorption was recorded at 593 nm in a Synergy ™ HTX multimodal microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). To quantify each solution’s antioxidant capacity, Trolox was used as standard and ethanol 80% v/v as blank.
3.6.2. Free Radical Scavenging (DPPH)
The radical DPPH methodology was used with some modifications [38]. In an analytical balance, 0.0197 g of material was ground and brought to a final volume of 50 mL in 80% ethanol. A 100 µL aliquot of 1 mM DPPH was then mixed with 100 µL of gallic acid standard (15–120 ppm) and solution of essential oil from 2 g/L to 50 g/L. The absorbance was fixed at 517 nm, with shaking for 5 min and incubation for 30 min at 36 °C. The percentage of radical inhibition DPPH was calculated according to the following formula:
| (2) |
Subsequently, a curve of percent inhibitory activity of DPPH versus concentration of the extract was drawn, and the IC50 value was calculated.
3.6.3. ABTS Method
The ABTS assay was performed by bleaching the cationic radical ABTS•+ as described by Soto et al. [48]. For the preparation of the radical ABTS•+, 2.5 mL of the 7 mM ABTS solution was mixed with 2.5 mL of 2.45 μM sodium persulfate for 12 h in the dark at 4 °C. Then, the resulting solution was diluted with absolute ethanol until an initial absorbance of approximately 0.70 ± 0.03 was obtained at 734 nm. The radical discoloration was initiated by adding 50 µL of the extract to 150 µL of the ABTS•+ solution. After 15 min of incubation at 25 °C, the absorbance was measured at 734 nm and compared with a calibration curve using Trolox as standard and ethanol as blank. Results were expressed as millimoles of Trolox equivalents per gram of dry sample (mmol TE/g).
3.7. Antibacterial Activity
3.7.1. Strain and Growth Conditions
Oregano oil was used to determine antibacterial activity against the human pathogenic bacteria Escherichia coli (ATCC 23716), Pseudomonas aeruginosa (ATCC 19429), Salmonella enterica (ATCC 13311), Bacillus subtilis (ATCC 6051), and Staphylococcus aureus (ATCC 29737); and the phytopathogenic bacteria Erwinia rhapontici (MK883065), Pseudomonas syringae (MF547632), Pantoea agglomerans (MK883087), Agrobacterium tumefaciens (ATCC 19358), and Xanthomonas campestris (MH885473). Bacteria were inoculated into nutrient broth containing 5.0 g/L peptone and 3.0 g/L meat extract and incubated at 25 °C (plant pathogens) or 35 °C (human pathogens) for 18 h at 150 rpm using an incubator with orbital shaking LOM-80 (MRC Lab, London, UK).
3.7.2. Paper Disk Diffusion Method
The antibacterial activity of oregano oil was determined by the method described by Klančnik et al., with modifications [49]. A stock solution of 10% (v/v) oregano oil in ethanol was prepared. An aliquot of 15 μL of the stock solution was impregnated on 5 mm sterile cellulose filter paper disks. The same procedure was repeated with disks impregnated only with ethanol, which were used as a negative control. The impregnated disks were dried under the biosafety chamber flow to evaporate ethanol and avoid its antimicrobial effect. Disks containing 50 μg kanamycin were used as a positive control of bacterial inhibition. Fresh inocula of each bacterial species were prepared as previously described, diluted to 0.5 of McFarland standard, and inoculated uniformly on plates containing nutrient broth supplemented with 12 g/L of agar. The dried and impregnated disks were arranged equidistant from each other. The plates were incubated for 24 h at 25 °C or 35 °C, as appropriate. Bacterial growth inhibition diameter was recorded after the incubation step, which is observed as a transparent halo without growth around each disk. The tests were carried out in triplicate.
3.7.3. Minimum Inhibitory Concentration (MIC)
The minimum concentration of oregano oil necessary to inhibit bacterial growth was carried out using the method described by Andrews [50]. The final oregano oil concentrations used were 0 to 10% (v/v). The final working volume for each concentration was 200 µL. Each dilution was inoculated with the different bacteria to be tested in 96-well plates at 25 °C or 35 °C, as appropriate. As controls, nutrient broth without compound inoculated with each bacterium (growth control), and nutrient broth with 0–10% (v/v) of oregano oil without bacterial inoculation (negative control of growth and sterility of the compound) were used. An assay using 0–90% ethanol was performed to estimate the ethanol MIC of each bacterium. Concentrations higher than 20% ethanol were shown to be inhibitory for all bacteria. For this reason, a 40% (v/v) oregano oil was prepared as a stock solution to avoid the inhibitory effect of ethanol during MIC assays. After 24 h of incubation, we determined the lowest concentration of compound at which no bacterial growth was observed.
3.7.4. Minimum Bactericidal Concentration (MBC)
The bactericidal capacity of oregano oil was determined from the last three wells that showed no bacterial growth in the MIC assay, as described by Taylor et al. [51]. For this, 100 µL of these cultures were taken and plated in nutrient broth plates supplemented with agar. As a growth control, a culture that did show microbial growth in the MIC test was used. The plates were incubated for 24 h at the corresponding temperature, after which the concentration of oregano oil where no growth was observed (MBC) was determined for each microorganism.
4. Conclusions
This work determined the chemical composition of the essential oil of Origanum vulgare grown in the arid Andean region (Putre, Chile). The main components of the essential oregano oil were thymol (15.9%), Z-sabinene hydrate (13.4%), γ-terpinene (10.6%), and p-cymene (8.6%). The antioxidant activity found was not related to its chemical composition. We also investigated the use of oregano essential oil as an antimicrobial agent against several bacteria. In this context, O. vulgare oil is a natural source of antibacterial compounds and has strong potential as a food additive or for agricultural applications. Nonetheless, safety and toxicity issues of this essential oil still need to be evaluated.
In summary, this study expands the knowledge of O. vulgare species cultivated under environmental conditions typical of the Atacama Desert’s Andean region. This knowledge could help to conduct more research on O. vulgare and strengthen its potential use as a natural source of bioactive compounds that could drive the development of local, regional, and international trade as a vehicle for rural economic growth in this region.
Acknowledgments
The authors wish to express their gratitude to the Rectoría of Universidad de Tarapacá for their financial and administrative support.
Author Contributions
Conceptualization, M.J.S. and C.P.; methodology, F.P., D.B., J.L., and P.M.; validation, M.J.S., C.P., and H.E.; formal analysis, F.P., D.B., J.L., and P.M.; investigation, C.P. and P.M.; resources, H.E.; writing—original draft preparation, C.P. and P.M.; writing—review and editing, H.E. and M.J.S.; supervision, C.P.; project administration, C.P.; funding acquisition, H.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UTA-MAYOR, grant number 9731-20. M.J.S. acknowledges Fondecyt 1180059. D.B. acknowledges Fondef VIU18E0097.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baser K.H.C., Buchbauer G. Handbook of Essential Oils: Science, Technology, and Applications. CRC Press; Boca Raton, FL, USA: 2015. p. 235. [Google Scholar]
- 2.Tajkarimi M., Ibrahim S., Cliver D. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- 3.Wei H.-K., Chen G., Wang R.-J., Peng J. Oregano essential oil decreased susceptibility to oxidative stress-induced dysfunction of intestinal epithelial barrier in rats. J. Funct. Foods. 2015;18:1191–1199. doi: 10.1016/j.jff.2015.02.035. [DOI] [Google Scholar]
- 4.Quitral V., Donoso M.L., Ortiz J., Herrera M.V., Araya H., Aubourg S.P. Chemical changes during the chilled storage of Chilean jack mackerel (Trachurus murphyi): Effect of a plant-extract icing system. LWT. 2009;42:1450–1454. doi: 10.1016/j.lwt.2009.03.005. [DOI] [Google Scholar]
- 5.Eguillor Recabarren P. Indicaciones Geográficas: Una Herramienta de Diferenciación. Oficina de Estudios y Políticas Agrarias, ODEPA; Santiago, Chile: 2014. p. 7. [Google Scholar]
- 6.Pulgar S.L. Reglamento de uso y control—Indicación Geográfica “Orégano de la Precordillera de Putre”. Biotecnología Agropecuaria S.A.; Arica, Chile: 2015. p. 16. [Google Scholar]
- 7.Goykovic V. Pot marjoram (Origanum vulgare L.) production in the andean precordillera in the Province of Parinacota. Idesia (Arica) 1995;14:10. [Google Scholar]
- 8.Sarikurkcu C., Zengin G., Oskay M., Uysal S., Ceylan R., Aktumsek A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crop. Prod. 2015;70:178–184. doi: 10.1016/j.indcrop.2015.03.030. [DOI] [Google Scholar]
- 9.Gulluce M., Karadayi M., Guvenalp Z., Ozbek H., Arasoglu T., Baris O. Isolation of some active compounds from Origanum vulgare L. ssp. vulgare and determination of their genotoxic potentials. Food Chem. 2012;130:248–253. doi: 10.1016/j.foodchem.2011.07.024. [DOI] [Google Scholar]
- 10.Ocaña-Fuentes A., Gutiérrez E.A., Señoráns F.J., Reglero G. Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: Anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem. Toxicol. 2010;48:1568–1575. doi: 10.1016/j.fct.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Albano S.M., Miguel M. Biological activities of extracts of plants grown in Portugal. Ind. Crop. Prod. 2011;33:338–343. doi: 10.1016/j.indcrop.2010.11.012. [DOI] [Google Scholar]
- 12.Coccimiglio J., Alipour M., Jiang Z.-H., Gottardo C., Suntres Z. Antioxidant, Antibacterial, and Cytotoxic Activities of the Ethanolic Origanum vulgare Extract and Its Major Constituents. Oxidative Med. Cell. Longev. 2016;2016:1404505. doi: 10.1155/2016/1404505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mechergui K., Jaouadi W., Coelho J., Khouja M.L. Effect of harvest year on production, chemical composition and antioxidant activities of essential oil of oregano (Origanum vulgare subsp glandulosum (Desf.) Ietswaart) growing in North Africa. Ind. Crop. Prod. 2016;90:32–37. doi: 10.1016/j.indcrop.2016.06.011. [DOI] [Google Scholar]
- 14.Lukas B., Schmiderer C., Novak J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae) Phytochem. 2015;119:32–40. doi: 10.1016/j.phytochem.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Béjaoui A., Chaabane H., Jemli M., Boulila A., Boussaid M. Essential Oil Composition and Antibacterial Activity of Origanum vulgare subsp. glandulosum Desf. at Different Phenological Stages. J. Med. Food. 2013;16:1115–1120. doi: 10.1089/jmf.2013.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Mastro G., Tarraf W., Verdini L., Brunetti G., Ruta C. Essential oil diversity of Origanum vulgare L. populations from Southern Italy. Food Chem. 2017;235:1–6. doi: 10.1016/j.foodchem.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Morshedloo M.R., Salami S.A., Nazeri V., Maggi F., Craker L. Essential oil profile of oregano (Origanum vulgare L.) populations grown under similar soil and climate conditions. Ind. Crop. Prod. 2018;119:183–190. doi: 10.1016/j.indcrop.2018.03.049. [DOI] [Google Scholar]
- 18.Dolea D., Rizo A., Fuentes A., Barat J.M., Fernández-Segovia I. Effect of thyme and oregano essential oils on the shelf life of salmon and seaweed burgers. Food Sci. Technol. Int. 2018;24:394–403. doi: 10.1177/1082013218759364. [DOI] [PubMed] [Google Scholar]
- 19.Van Haute S., Raes K., Van Der Meeren P., Sampers I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control. 2016;68:30–39. doi: 10.1016/j.foodcont.2016.03.025. [DOI] [Google Scholar]
- 20.Leyva-López N., Gutiérrez-Grijalva E.P., Vazquez-Olivo G., Heredia J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules. 2017;22:989. doi: 10.3390/molecules22060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tellez-Monzón L.A., Nolazco-Cama D.M. Estudio de la composición química del aceite esencial de orégano (Origanum vulgare spp.) de Tacna. Ingeniería Industrial. 2017;2017:195. doi: 10.26439/ing.ind2017.n035.1801. [DOI] [Google Scholar]
- 22.Plaus E.A., Flores G.S., Ataucusi S.G. Composición química y actividad antibacteriana del aceite esencial del Origanum vulgare (orégano) Rev. Medica Hered. 2013;12:16. doi: 10.20453/rmh.v12i1.660. [DOI] [Google Scholar]
- 23.Başer K.H.C., Demirci F. In: Chemistry of Essential Oils. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability. Berger R.G., editor. Springer; New York, NY, USA: 2007. pp. 43–86. [Google Scholar]
- 24.Teixeira B., Marques A., Ramos C., Serrano C., Matos O., Neng N.R., Nogueira J., Saraiva J.A., Nunes M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare ) extracts and essential oil. J. Sci. Food Agric. 2013;93:2707–2714. doi: 10.1002/jsfa.6089. [DOI] [PubMed] [Google Scholar]
- 25.Prieto J.M., Iacopini P., Cioni P., Chericoni S. In vitro activity of the essential oils of Origanum vulgare, Satureja montana and their main constituents in peroxynitrite-induced oxidative processes. Food Chem. 2007;104:889–895. doi: 10.1016/j.foodchem.2006.10.064. [DOI] [Google Scholar]
- 26.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Volume 456 Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 27.Mancini E., Camele I., Elshafie H.S., De Martino L., Pellegrino C., Grulova D., De Feo V. Chemical composition and biological activity of the essential oil of Origanum vulgare ssp. hirtum from different areas in the Southern Apennines (Italy) Chem. Biodivers. 2014;11:639–651. doi: 10.1002/cbdv.201300326. [DOI] [PubMed] [Google Scholar]
- 28.Khan M., Khan S.T., Khan M., Mousa A.A., Mahmood A., Alkhathlan H.Z. Chemical diversity in leaf and stem essential oils of Origanum vulgare L. and their effects on microbicidal activities. AMB Express. 2019;9:176. doi: 10.1186/s13568-019-0893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chorianopoulos N., Kalpoutzakis E., Aligiannis N., Mitaku S., Nychas G., Haroutounian S.A. Essential Oils ofSatureja, Origanum, andThymusSpecies: Chemical Composition and Antibacterial Activities Against Foodborne Pathogens. J. Agric. Food Chem. 2004;52:8261–8267. doi: 10.1021/jf049113i. [DOI] [PubMed] [Google Scholar]
- 30.Sahin F., Güllüce M., Daferera D., Sökmen A., Sökmen M., Polissiou M., Agar G., Ozer H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control. 2004;15:549–557. doi: 10.1016/j.foodcont.2003.08.009. [DOI] [Google Scholar]
- 31.Sivropoulou A., Papanikolaou E., Nikolaou C., Kokkini S., Lanaras T., Arsenakis M. Antimicrobial and Cytotoxic Activities ofOriganumEssential Oils. J. Agric. Food Chem. 1996;44:1202–1205. doi: 10.1021/jf950540t. [DOI] [Google Scholar]
- 32.Arcila-Lozano C.C., Loarca-Piña G., Lecona-Uribe S., González de Mejía E. El orégano: Propiedades, composición y actividad biológica de sus componentes. Archivos Latinoamericanos de nutrición. 2004;54:100–111. [PubMed] [Google Scholar]
- 33.Bisht D., Chanotiya C., Rana M., Semwal M. Variability in essential oil and bioactive chiral monoterpenoid compositions of Indian oregano (Origanum vulgare L.) populations from northwestern Himalaya and their chemotaxonomy. Ind. Crop. Prod. 2009;30:422–426. doi: 10.1016/j.indcrop.2009.07.014. [DOI] [Google Scholar]
- 34.Naghdi Badi H., Abdollahi M., Mehrafarin A., Ghorbanpour M., Tolyat M., Qaderi A., Ghiaci Yekta M. An overview on two valuable natural and bioactive compounds, thymol and carvacrol, in medicinal plants. J. Med. Plant. 2017;3:1–32. [Google Scholar]
- 35.Quiroga P.R., Asensio C.M., Nepote V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2014;95:471–479. doi: 10.1002/jsfa.6744. [DOI] [PubMed] [Google Scholar]
- 36.Ruberto G., Baratta M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69:167–174. doi: 10.1016/S0308-8146(99)00247-2. [DOI] [Google Scholar]
- 37.Mechergui K., Coelho J., Serra M.C., Lamine S.B., Boukhchina S., Khouja M.L. Essential oils of Origanum vulgare L. subsp. glandulosum (Desf.) Ietswaart from Tunisia: Chemical composition and antioxidant activity. J. Sci. Food Agric. 2010;90:1745–1749. doi: 10.1002/jsfa.4011. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira B., Marques A., Ramos C., Neng N.R., Nogueira J., Saraiva J.A., Nunes M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crop. Prod. 2013;43:587–595. doi: 10.1016/j.indcrop.2012.07.069. [DOI] [Google Scholar]
- 39.Tuttolomondo T., La Bella S., Licata M., Virga G., Leto C., Saija A., Trombetta D., Tomaino A., Speciale A., Napoli E., et al. Biomolecular Characterization of Wild Sicilian Oregano: Phytochemical Screening of Essential Oils and Extracts, and Evaluation of Their Antioxidant Activities. Chem. Biodivers. 2013;10:411–433. doi: 10.1002/cbdv.201200219. [DOI] [PubMed] [Google Scholar]
- 40.López-Alarcón C., DeNicola A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 41.Lofa A., Velasco V., Gerding M., López M.D., Vallejos D., Bonilla A.M., Logue C.M. Antibiotic-resistant Staphylococcus aureus strains of swine origin: Molecular typing and susceptibility to oregano (Origanum vulgare L.) essential oil and maqui (Aristotelia chilensis (Molina) Stuntz) extract. J. Appl. Microbiol. 2019;127:1048–1056. doi: 10.1111/jam.14393. [DOI] [PubMed] [Google Scholar]
- 42.Busatta C., Mossi A.J., Rodrigues M.R.A., Oliveira J.V., Cansian R.L. Evaluation of Origanum vulgare essential oil as antimicrobial agent in sausage. Braz. J. Microbiol. 2007;38:610–616. doi: 10.1590/S1517-83822007000400006. [DOI] [Google Scholar]
- 43.Huang H.C., Hsieh T.F., Erickson R.S. Biology and epidemiology of Erwinia rhapontici, causal agent of pink seed and crown rot of plants. Plant Pathol. Bull. 2003;12:69–76. [Google Scholar]
- 44.Rodriguez-Garcia I., Silva-Espinoza B.A., Ortega-Ramirez L.A., Leyva J.M., Siddiqui M.W., Cruz-Valenzuela M.R., González-Aguilar G.A., Ayala-Zavala J.F. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2015;56:1717–1727. doi: 10.1080/10408398.2013.800832. [DOI] [PubMed] [Google Scholar]
- 45.Rúa J., Del Valle P., De Arriaga D., Fernández-Álvarez L., García-Armesto M.R. Combination of Carvacrol and Thymol: Antimicrobial Activity Against Staphylococcus aureus and Antioxidant Activity. Foodborne Pathog. Dis. 2019;16:622–629. doi: 10.1089/fpd.2018.2594. [DOI] [PubMed] [Google Scholar]
- 46.Pesavento G., Calonico C., Bilia A., Barnabei M., Calesini F., Addona R., Mencarelli L., Carmagnini L., Di Martino M., Nostro A.L. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control. 2015;54:188–199. doi: 10.1016/j.foodcont.2015.01.045. [DOI] [Google Scholar]
- 47.Parra C., Soto E., León G., Salas C., Heinrich M., Echiburú-Chau C. Nutritional composition, antioxidant activity and isolation of scopoletin from Senecio nutans: Support of ancestral and new uses. Nat. Prod. Res. 2017;32:719–722. doi: 10.1080/14786419.2017.1335726. [DOI] [PubMed] [Google Scholar]
- 48.Soto E., Diaz-Gonzalez R., Simirgiotis M.J., Parra C. Potential of Baccharis alnifolia Meyen & Walpan (Chilka) from northern Chile used as a medicinal infusion. Ciência Rural. 2019;49 doi: 10.1590/0103-8478cr20190428. [DOI] [Google Scholar]
- 49.Klančnik A., Piskernik S., Jeršek B., Možina S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods. 2010;81:121–126. doi: 10.1016/j.mimet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Andrews J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 51.Taylor P.C., Schoenknecht F.D., Sherris J.C., Linner E.C. Determination of minimum bactericidal concentrations of oxacillin for Staphylococcus aureus: Influence and significance of technical factors. Antimicrob. Agents Chemother. 1983;23:142–150. doi: 10.1128/AAC.23.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]