Introduction
Radiation therapy is one of the most utilized and effective treatments for cancer, though certain tissues, such as those in the central nervous system, are particularly vulnerable to being damaged in the process. For some types of cancer, such as acute lymphoblastic leukemia, whole-brain or craniospinal radiation therapy of 10 −30 Gy is used destroy and prevent the spread of tumors throughout the central nervous system. Pediatric patients are particularly susceptible to acquiring long-lasting side effects as the radiation induces damage during a critical window of brain development. Nearly half of pediatric recipients of whole-brain radiotherapy have neurocognitive problems throughout their life, thus creating a list of concerns for patients once the cancer has been effectively treated. 1,2 These problems include decreases in cognitive proficiency, processing speed, and working memory. Quality of life and overall functionality are also compromised, as these patients have been shown to have increased use of special education services, decreased high school graduation rate, and greater likelihood of developing symptoms of depression.
Children treated at a younger age and/or with higher doses of radiation showed greater incidences and degrees of cognitive impairments 3–5. The process of neurogenesis happens into adulthood when new concepts are learned. The formation of new neurons in the subgranular zone of the hippocampus is believed to be responsible for learning and the formation of memories. Once these new neurons are incorporated into the circuit of the hippocampus, a new memory is formed 6. In a study by Cheng et al, they used male mice that were exposed to 5 Gy of irradiation at either 2, 4, 6, 8, 12, or 18 months of age. After irradiation, they conducted immunostaining where they measured the levels of doublecortin in the hippocampi of these animals. They found significant decreases in doublecortin positive cells after irradiation. Interestingly, mice irradiated at older time points did not exhibit the same level of damage as did younger mice. Such that, younger mice were less radioresistant than the older mice. This suggests that age plays a major role in the susceptibility of patients to cognitive decline following cranial radiotherapy7
The hippocampus consists of several subregions— the dentate gyrus, CA1, CA2, CA3, and CA4— each of which contributes to different aspects or types of memory. For example, while CA1 and CA3 are particularly important for the processing and encoding of spatial memories, CA2 region is thought to govern object recognition and social memory 8,9. The proper function of the hippocampus relies on the rapid remodeling of postsynaptic processes known as spines. The dynamic reorganization of the number, density, and location of hippocampal dendritic spines is crucial to the processes of learning, memory, and cognition10.
Chakraborti et al, used 2 month old male mice to measure negative effects of 10 Gy of cranial radiation on hippocampal spine density and morphology in the hippocampus. They found that the spine morphology and density of the hippocampus was greatly affected by cranial radiation11. Cranial radiation has also been shown to significantly compromise neuronal morphology in the hippocampus. After 10 Gy of cranial irradiation, Parihar et al observed significant decreases in dendritic complexity. Specifically, they found significant changes in dendritic branching, area and length in irradiated mice12. Gamma-ray and proton doses spanning from 0.1 Gy to 10.0 Gy have been found to cause significant, dose-responsive reductions in dendritic complexity and spine density in the hippocampus lasting at least 1 month post-irradiation 11–13. However, the majority of these studies have focused on adult murine models. We aimed to study mouse brains subjected to irradiation on postnatal day 21, an age approximately equivalent to a juvenile child 14. Here we extend our investigation to the effects of juvenile cranial radiation on social memory and CA2 dendritic spine morphology in mice.
Results
Three-Chamber Sociability
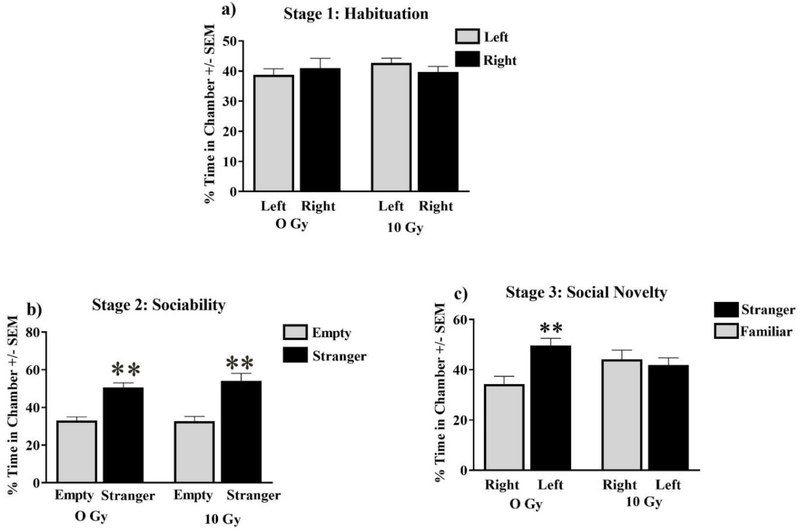
Social behavior was assessed via the three-chamber test. All mice displayed normal habituation by spending approximately equal time exploring both lateral chambers (F (5, 64) = 0.82; P = 0.54; Fig 1). All experimental groups also socialized normally, by spending significantly more time exploring a stranger (stimulus 1) than an identical chamber with an empty cage during stage 2 (F (3, 28) = 16.41; P < 0.001; Holm-Sidak multiple comparisons Stimulus vs Empty; Sham P < 0.001; 10 Gy P < 0.0001: Fig 1). Radiation affected social discrimination during the third stage where radiation eliciting an inability to discriminate between the familiar and stranger mouse, while sham animals successfully spent more time exploring the novel stranger (F (3, 28) = 4.18; P < 0.05; Holm-Sidak multiple comparisons Stimulus 1 vs Novel Stimulus; Sham P < 0.05 Fig 1).
Figure 1: Sociability.
(A) Animals of all cohorts displayed no significant chamber-exploration bias. (B) All cohorts displayed normal sociability by spending significantly more time with a novel mouse (stranger). (C) Sham-irradiated and mice were able to discriminate between a novel stranger, and a now-familiar stranger, whereas mice treated with 10 Gy failed to distinguish strangers. Average ± SEM (n = 10); **P < 0.01.
Spine Morphology
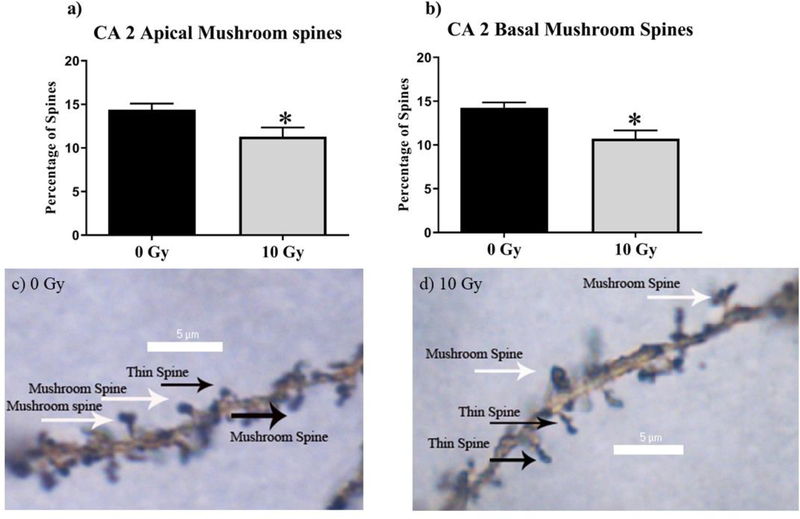
To investigate the effects of radiation on hippocampal physiology, we performed spine-density analyses between cohorts (Table 1). CA2 was a found to have reduced mushroom-spine density in the apical CA2 at (Sham vs. 10 Gy, P < 0.05 Fig 2) and in the basal CA2 (Sham vs. 10 Gy, P < 0.05; Fig 2).
Table 1.
Morphological Analysis of Apical and Basal Dendrites in CA2
| Cell types and measurements | O Gya | 10 Gya | P value |
|---|---|---|---|
| CA2 apical | |||
| Thin spines (no.) | 56.07 ± 1.62 | 54.08 ±2.09 | P = 0.58 |
| Stubby spines (no.) | 29.54 ± 1.39 | 30.74 ± 1.47 | P = 0.67 |
| Mushroom spines (no.) | 14.40 ± 0.69 | 11.31 ± 1.03 | P < 0.05 |
| Overall density (no.) | 18.15 ± 0.60 | 18.18± 0.51 | P = 0.96 |
| CA2 basal | |||
| Thin spines (no.) | 52.39 ± 1.60 | 55.89 ±1.74 | P = 0.25 |
| Stubby spines (no.) | 33.36 ±1.75 | 33.39 ± 1.26 | P = 0.99 |
| Mushroom spines (no.) | 14.26 ±0.59 | 10.72 ± 0.93 | P < 0.05 |
| Overall density (no.) | 16.89 ±0.58 | 18.16 ± 0.39 | P < 0.05 |
Mean ± SEM.
Values in boldface are significant.
Figure 2: 10 Gy reduces mushroom spine density in the CA 2 hippocampus.
(A)The Apical CA2 underwent reduced mushroom spine density (B) The basal CA2 mushroom spine density was significantly decreased by Irradiation. (C-D) Representative Images. Average ± SEM (n = 5); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Proteomics
Identification of proteins differentially expressed relative to neurocognitive deficits
Identification of specific proteins and pathways associated with neurocognitive dysfunction in 10 Gy-treated C57BL/6 male mice as well as putative candidates that may underlie the behavioral and neuronal changes observed in cranial irradiation treatment, 10 Gy-treated, hippocampal sample cell lysates were analyzed using proteomic analysis relative to a control. A set of 2,076 proteins out of a total of 4,560 identified proteins were differentially expressed between the 10 Gy-treated animals and control animals with a p-value of < 0.05 and a fold change >2 (Table 3, Supplementary 1). Of these 14/2076 were highlighted for their association oxidative stress, neuronal function, and a significant log ratio fold change greater or equal to +/− 2.
Table 3.
Top five IPA protein networks associated with 10 Gy treatment
| Network 1 | Associated network functions: Cell Morphology, Cellular Assembly and Organization, Cell-To-Cell Signaling and Interaction Number of “focus molecules” contained in the network: 31 IPA p-score: 44 Network proteins: CACNB4,CCDC136,CCDC47,CDIPT,CEP170B,CKAP4,CLASP1,CNP,CTNND2,CTTN BP2,DSG1,EMC1,EMC2,GHITM,GRIA2,LRRC59,MAT2B,MTFR1L,NT5C3A,PGP,PI4 KA,PTPase,Ppp2c,SHANK1,SHANK2,SHISA7,SMAP2,SRC (family),SRCIN1,STRN,STRN4,Sod,TECR,TNIK,TSPAN7 |
| Network 2 | Associated network functions: Cellular Assembly and Organization, Cellular Function and Maintenance, Molecular Transport Number of “focus molecules” contained in the network: 30 IPA p-score: 42 Network proteins: AP- 3,AP3D1,AP3S1,ARL8B,BLVRB,Bvr,C1QTNF4,CLIP2,DAD1,DDOST,DIRAS1,DIRAS 2,DMXL2,LMAN1,MCAT,NFkB |
| Network 3 | Associated network functions: Developmental Disorder, Neurological Disease, Organismal Injury and Abnormalities Number of “focus molecules” contained in the network: 30 IPA p-score: 42 Network proteins: 14-3-3(α, ε, ζ) ,ACTB,ATAT1,C2orf72,CD81,DCPS,EEF1A1,EMD,Gm10358,HIST1H1C,HTRA2,IFN |
| Network 4 | Associated network functions: Protein Synthesis, Cellular Compromise, Inflammatory Response Number of “focus molecules” contained in the network: 29 IPA p-score: 39 Network proteins: ARSB,CPPED1,CROCC,CST3,CTSA,CTSZ,Cathepsin,ENDOD1,ERGIC1,Ephb,FIS1,GL B1,Glycoprotein |
| Network 5 | Associated network functions: Cellular Movement, Molecular Transport, Developmental Disorder Number of “focus molecules” contained in the network: 29 IPA p-score: 39 Network proteins: 14-3-3 (α,β,η, δ, ζ, 14-3-3(δ, η, ζ),ANXA3,ATAD3A,CA2,CA4,CADM3,CALB1,CLNS1A,CYFIP2,EPB41L2,EPB41L3,F N3KRP,IDI1,IGSF21,IMPA1,Integrin alpha 3 beta 1,KCNMA1,LIN7A,MARK1,MPC1,MPC2,MPP1,MPP2,NPL,PLLP,PLPP3,SARNP,SER PINB6,SLC4A4,TLR7/8,Vegf,WDR45,YWHAH,c-Src |
Using a proprietary algorithm, IPA generates these networks and adds proteins not contained within the original dataset. Additionally, IPA calculates a p-score, which indicates the probability of finding a given number of focus molecules in a given network selected randomly from IPA’s Global Molecular Network. The p-score (−log10 (p-value)) is calculated by the Fisher’s exact test.
Proteins, pathways, and protein networks affected by radiation treatment
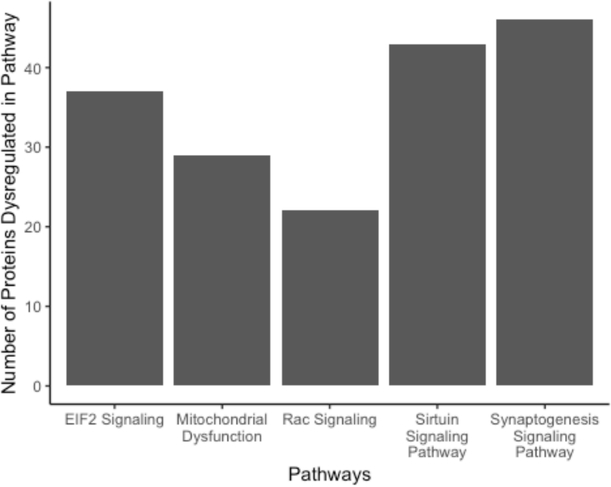
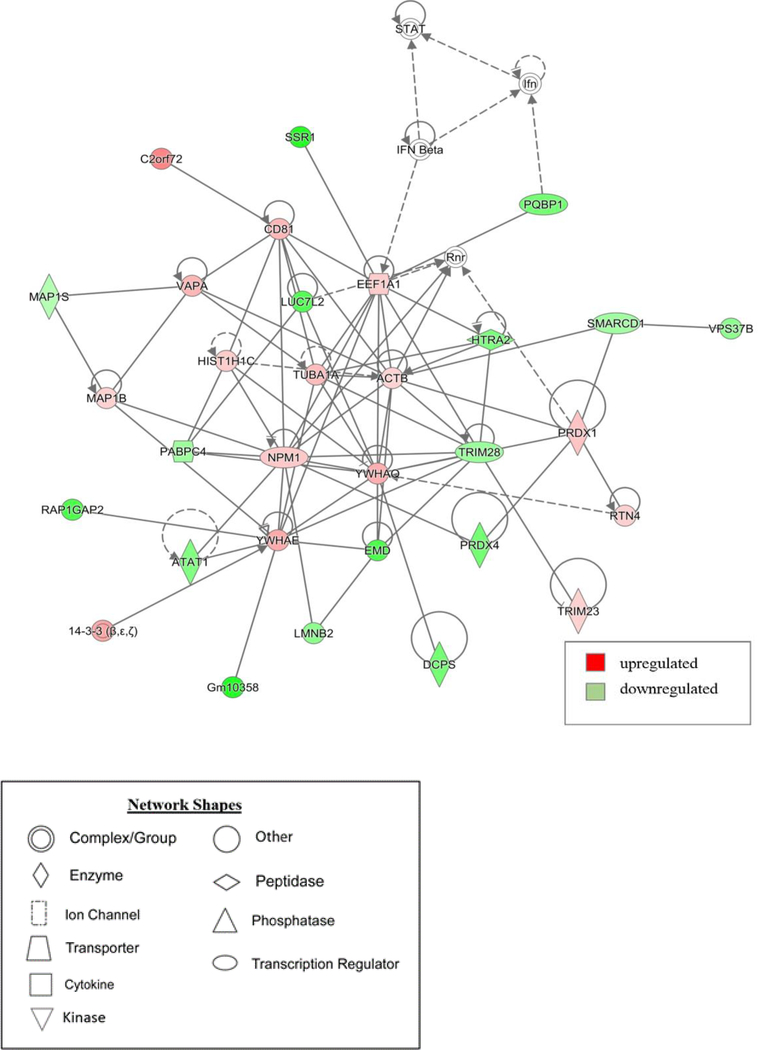
To gain insight on the potential pathways and networks involved in mediating irradiation-induced neurocognitive dysfunction IPA analysis was conducted on all 2,076 proteins that were identified to be differentially expressed between the 10 Gy-treated and control animals. IPA is a web-based software application that is used to extract from biological meaningful information from our list of differentially expressed proteins. This analysis was able to determine the top 5 canonical pathways (Synaptogenesis Signaling Pathway, Sirtuin Signaling Pathway, EIF2 Signaling, Mitochondrial Dysfunction, and Rac Signaling), which are predictions of pathways that are changing based on gene expression due to 10 Gy radiation treatment (Table 2). The number of proteins within each of these pathways thought to be disrupted is represented in (Fig 3). Interestingly, each of these pathways have associations with neurocognitive disorders15–18. Additionally, IPA provided non-directional gene interaction maps in the form of networks with associated functions (Table 3). These IPA networks provide information on how proteins with similar functions interact with each other as well as predictions on which direction each of the proteins within the network are dysregulated. For example, in this analysis network 1 has associated functions with cell morphology, cell assembly and organization, and cell to cell signaling and interaction (Fig 4). Similarly, network 3 which has associated networks functions of developmental disorders, neurological disease, and organismal injury and abnormalities (Fig 5). Additional canonical pathways and protein networks disrupted due to 10 Gy radiation treatment can be found in the supplementary material.
Table 2.
Top five pathways associated with memory deficits in 10 Gy treated mice. Overlapping proteins that met statistical significance for differential expression in 10 Gy treated animals (10 Gy vs. 0Gy) comparisons were uploaded into Ingenuity Pathway Analysis (IPA) to identify enriched pathways.
| Top Canonical Pathways | P-value |
|---|---|
| Synaptogenesis Signaling Pathway | 1.23e-15 |
| Sirtuin Signaling Pathway | 1.11e-14 |
| EIF2 Signaling | 2.12e-14 |
| Mitochondrial Dysfunction | 7.15e-12 |
| Rac Signaling | 1.08e-10 |
Figure 3.
Number of proteins determined to be dysregulated within each of top 5 pathways associated with memory deficits in 10 Gy-treated mice.
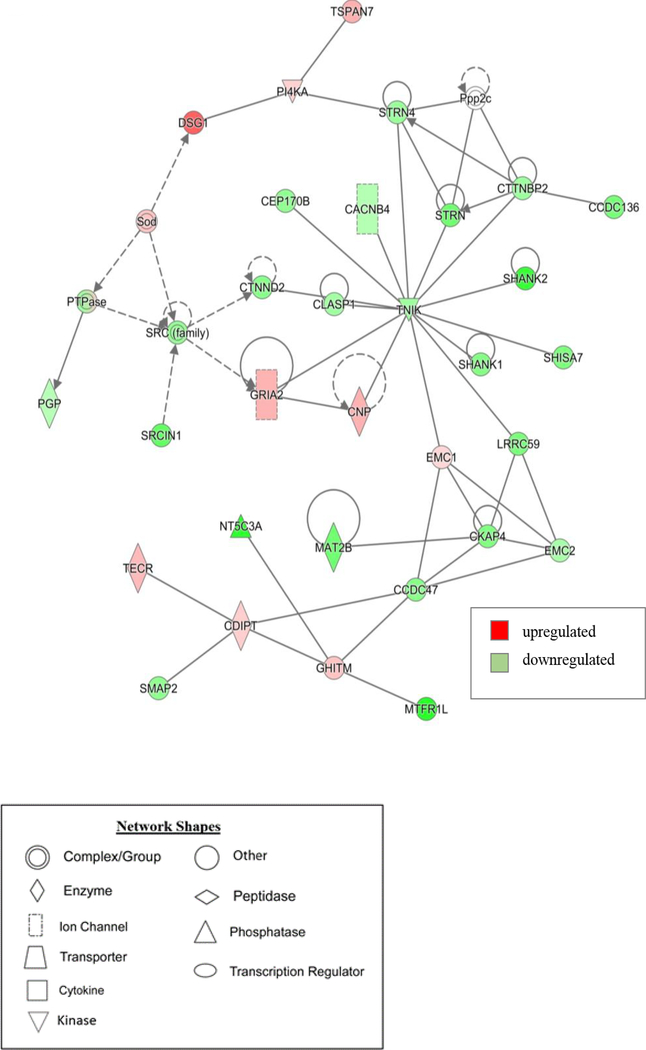
Figure 4. Graphic representation of mouse hippocampus protein network 1, identified by IPA as being affected by 10 Gy-treatment.
The color of the node depicts differential expression. Red represents upregulated proteins. Green represents downregulated proteins. The intensity of the color denotes the degree of regulation where brighter colors are more regulated. Gray node color reflects proteins that were found in the data set but were insignificant expression wise. Uncolored nodes were not identified as differentially expressed in our data, but were incorporated into the computational network based on evidence stored in the Ingenuity Knowledge Base. Known direct and indirect interactions between network proteins, as well as the direction of the interaction, are indicated by arrows or blocked lines.
Figure 5. Graphic representation of mouse hippocampus protein network 3, identified by IPA as being affected by 10 Gy-treatment.
Green indicates downregulation and red indicates. The color of the node depicts differential expression. Red represents upregulated proteins. Green represents downregulated proteins. The intensity of the color denotes the degree of regulation where brighter colors are more regulated. Gray node color reflects proteins that were found in the data set but were insignificant expression wise. Uncolored nodes were not identified as differentially expressed in our data, but were incorporated into the computational network based on evidence stored in the Ingenuity Knowledge Base. Known direct and indirect interactions between network proteins, as well as the direction of the interaction, are indicated by arrows or blocked lines
Discussion
Though cranial radiation is a necessary therapy for some types of cancer, the treatment itself can induce long-term neurocognitive deficits, particularly in children. In this study we investigated how cranial radiation in juvenile mice affected a specific aspect of neurocognition—social recognition. We then explore the radiation-induced changes in neuroanatomy and cellular function of the CA2 of the hippocampus, a brain region known to be important for social memory. Social recognition, which includes the ability to recognize familiar versus novel individuals of the same species, is a major component of social memory. In our study we assay for differences in social cognitive abilities in sham versus irradiated mice by using the three-chamber sociability paradigm, previously established by Dr. Jaqueline Crawley, which has been employed to study social affiliation and social memory 19. In order to habituate the mice to the experimental apparatus, mice are first placed in the middle chamber, then are allowed to freely explore the flanking compartments. Given that both the left and right compartments are devoid of a stimulus, mice tend to spend equal amounts of time in each. In the next stage of experiments, sociability is tested by giving the subject a choice between an unfamiliar mouse in one chamber and a novel object in the other. As social mammals, the subjects spend more time with the mouse than the object 19, a trend that was observed in both our sham and our irradiated mice. Sociability includes the choice to engage in social behaviors, but is not an implicit component of social memory. Social novelty, however, can be used an indicator of social recognition. When given a choice between a familiar mouse versus a novel one, mice prefer to explore the novel individual19,20. We found that 10 Gy-irradiated mice failed to show a preference between a familiar individual and a stranger, perhaps due to an inability to actually recognize the familiar mouse.
The Cornu Ammonis 2 (CA2) of the hippocampus has emerged as an important region for social memory formation. Social recognition memory in animals is essential for social hierarchy, territorial defense, interspecies recognition21. Silencing of the CA2, leads to a pronounced deficit in social memory, without impairment in sociability or other hippocampal-dependent behaviors, such as contextual or spatial memory22,23. In this study, we identified a decrease in overall spine mushroom spine density within CA 2 apical and basal dendrites, but there were no changes in thin spine and stubby spines apical dendrites. Spines are activity-dependent, as high-frequency stimulation induces spine development in hippocampal neurons 24. Thin spines, or “learning” spines, are transient and mobile; mushroom spines, or “memory” spines, are more stable and persist for months 25,26. Changes in spine density or length are thought to represent a morphological correlate of altered brain functions associated with learning and memory 27. Alterations in spine morphology can disrupt synapse formation and/or stability, which ultimately can lead to neurological and cognitive disorders, such as autism spectrum disorders, Alzheimer’s disease, schizophrenia, anxiety, and depression 28.
In order to understand the molecular mechanisms contributing to hippocampal dependent cognitive impairment after 10 Gy irradiation treatment a comparative proteomic analysis was conducted. Currently there are few studies that have used proteomic technology to explore the molecular underpinnings behind radiation-induced cognitive impairment. One study found that juvenile mice 7 months after receiving low doses of radiation (≤1.0 Gy) treatment there were significant disruptions in key signaling pathways, such as Ephrin B, Rho Family GTPases, RhoGDI, Axonal Guidance, and Ephrin Receptor signaling 29. A similar study was conducted on both 10 week and 10 day old mice 6 months after receiving low/moderate (0.1 and 2 Gy) cranial radiation. In their proteomic analysis, they saw significant dysregulation in pathways such as superoxide radicals degradation, oxidative phosphorylation, and mitochondrial dysfunction for both 10-week-old and 10 day old mice30.
In our study, we implemented a comparative proteomic analysis on 21 day old mice hippocampal tissue 30 days after receiving radiation treatment (10 Gy). Interestingly, in our study which demonstrated short term effects at higher dose of radiation revealed some key similarities to the prior studies discussed. Like previous studies our IPA revealed that one of the top five canonical mechanisms by which 10 Gy cranial irradiation contributes to hippocampal dysfunction is mitochondrial dysfunction (Table 3). These pathways each contain a number of proteins that have been dysregulated as a result of 10 Gy treatment (Figure 4). It has been determined that radiation can damage mitochondria resulting in mitochondrial-induce cellular damage particularly within the central nervous system. The extent of mitochondrial-induced damage on the central nervous system has been characterized as dosage dependent with low doses inducing via antioxidant defenses and higher dosages inducing damage through oxidative stress and neuroinflammation31. Also, in this analysis several significantly differentially disrupted proteins were found in 10-Gy hippocampal samples associated with mitochondrial dysfunction (Table 4). For example, subunits of both the cytochrome b (Cytochrome b-c1 complex subunit 6, mitochondrial) and c complex (Cytochrome c oxidase subunit 5A, mitochondrial) were determined to be significantly upregulated with fold changes of 6.74 and 4.90 respectively.
Table 4.
Top up regulated proteins differentially expressed associated with cognitive decline. A comparison of 10 Gy-treated mice versus control mice identified 2076 proteins as meeting statistical significance for differential expression.
| Protein | Description | Location | Type | Fold Change (log ratio) |
|---|---|---|---|---|
| P99028 | Cytochrome b-c1 complex subunit 6, mitochondrial | Mitochondrion | Other | 6.747313809 |
| B1ARW4 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 5;NADH dehydrogenase [ubiquinone] iron-sulfur protein 5, N-terminally processed | Mitochondrion | Other | 4.914809526 |
| P12787 | Cytochrome c oxidase subunit 5A, mitochondrial | Mitochondrion | Other | 4.909787013 |
| Q56A15 | Cytochrome c, somatic | Mitochondrion | Other | 5.001447813 |
| Q9CQB4 | Cytochrome b-c1 complex subunit 7 | Mitochondrion | Other | 4.529687357 |
| A2RSV8 | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial | Mitochondrion | Other | 3.750787159 |
| Q06185 | ATP synthase subunit e, mitochondrial | Mitochondrion | Other | 5.050258482 |
| Q9WVA2 | Mitochondrial import inner membrane translocase subunit Tim8 A | Mitochondrion | Other | 4.163148008 |
| Q9CQ54 | NADH dehydrogenase [ubiquinone] 1 subunit C2 | Mitochondrion | Other | 3.629549791 |
| Q9CQZ5 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 6 | Mitochondrion | Other | 3.500211383 |
| Q9ERS2 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 13 | Mitochondrion | Other | 3.288135065 |
| Q9D6J6 | NADH dehydrogenase [ubiquinone] flavoprotein 2, mitochondrial | Mitochondrion | Other | 3.940287473 |
| P20108 | Thioredoxin-dependent peroxide reductase, mitochondrial | Mitochondrion | Enzyme | 3.217668799 |
Upregulation of mitochondrial proteins after radiation have been characterized in other tissues as well. One study found that irradiation of lung carcinoma cells resulted in an increase of mitochondria abundance and an increase of electron transport chain activity after 3.9 Gy irradiation32. What we can gather from these findings is that low to moderate doses of irradiation seems to consistently disrupt mitochondrial function both in long term and in the short-term post irradiation. This suggests evidence for a radiation response in hippocampal tissue that results in mitochondrial damage and subsequently an increase in reactive oxygen species tissue damage. Thus, oxidative stress is a likely candidate mechanism for the social behavioral deficits we see in these juvenile 10 Gy-irradiated mice (Fig 1). Additionally, IPA identified other disrupted canonical pathways such as EIF2, Rac, Sirutin, and Synaptogenesis signaling pathways, which have all have been implicated in neurocognitive disorders 33–36.
Another feature of the proteomic analysis was the identification of top disrupted protein networks by the IPA-network functions tool (Table 3). These networks provide non-directional predictions of how significantly differentially expressed proteins identified in the initial analysis interact with each other as well as their relative expression levels. For example, network 1 from this analysis has associated functions with cell morphology, cellular assembly and organization, and cell to cell signaling and interaction (Table 3). This network contains dysregulated central node proteins such as GRIA2 (Glutamate ionotropic receptor AMPA type subunit 2), TNIK (TRAF2-and NCK interacting kinase), and STRN (Striatin) all generally related to dendritic organization, synaptic activity, and spine morphology (Fig 4). GRIA2 is a glutamate ionotropic receptor often expressed on the surface of spines and is responsible for mediating synaptic transmission in the central nervous system thus having a key role in processes like learning and memory37. TNIK has a role in organizing dendritic spines, critical for glutamatergic signaling, and regulation of synaptic strength38. STRN is part of a family of proteins that is largely localized in dendritic spines and has been implicated in dendritic outgrowth and remodeling 42. Network 3 from this analysis also contains proteins like Tuba1a (Tubulin alpha-1a chain), which is a major constituent of microtubules and is associated with regulating synapse organization as well as spine development (Fig 5) 35,39. These results suggest that disruption of these regulators of dendritic spine and synaptic morphology could be molecular candidates to explain the decreases in mushroom spines density observed after 10 Gy treatment (Fig 2). Interestingly, mitochondria have also been implicated in having a role in dendritic spine morphology and synaptic function.
Mitochondria in dendrites are often localized in dendritic shafts and enhance the activity of both spines and synapses. Reduction of mitochondrial content in dendrites has been shown to directly decrease both spine and synaptic abundance40. Thus, it is possible that dysregulation of these regulators of spine and synaptic morphology may work mechanistically in tandem with mitochondrial dysfunction to induce the changes in hippocampal physiology seen after 10 Gy-irradiation treatment. Altogether, we conclude that these cognitive deficits and changes in hippocampal physiology after 10 Gy treatment are indicative of both mitochondrial damage and disruption molecular regulators of dendritic morphology. Further validation of these highlighted proteins in this analysis will be needed to further support these findings.
MATERIALS AND METHODS
Animals and Irradiation
Animals
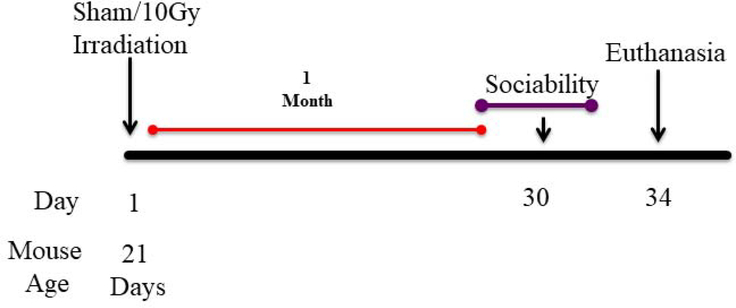
Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), 21 days old (n = 20), were used in this study. Mice were irradiated (Schematic). Behavior testing was conducted when mice were 2 months old. The mice were housed under a constant 12-h light/12-h dark cycle and were cared for in compliance with the UAMS Institutional Animal Care and Use Committee.
Schematic diagram showing experimental design.
21 day old C57BL/6J male mice received head only irradiation with the SARRP at UAMS. 30 Days post irradiation behavioral testing was performed.
Small-Animal Radiation Research Platform
The male mice were irradiated with the use of the SARRP. They were anaesthetized with 1% isoflurane inhalation for the duration of the radiation exposure at 0 Gy (n = 10) 10 Gy (n = 10). Each mouse was placed prone on the horizontal bed in the SARRP. A cone beam computed tomographic image of each mouse was obtained to deliver image-guided radiation targeted to the brain at 60 kVp and 0.8 mA; reconstruction was accomplished with the use of 720 projections. From Merislice, image precision targeting of the brain was determined to avoid the eyes and frontal lobe. One cohort received 1 round of 2 × 5-Gy x-ray irradiation from 90° and −90° gantry angles with an isocenter tissue depth of 5 mm. The brain-targeted radiation doses were delivered with a 0.5-mm copper filter with a 5 × 5 mm collimator using 220 kVp and 13 mA. All sham-irradiated mice were anaesthetized and placed in the SARRP for an equivalent amount time as the irradiated mice.
Three-Chamber Sociability
Next, mice underwent testing for social memory via the three-chamber sociability paradigm, in which mice freely explore three adjacent chambers for 10 minutes in three consecutive trials. The first trial consists of habituation, allowing mice to explore the 3 empty chambers. Trial 2 involves social familiarization, where a previously unknown animal (stimulus 1) of the same sex, strain, and age is placed in a small vertical cage on one of the lateral chambers, and the other lateral chamber contains an identical, but empty cage. For trial 3, another previously unknown animal (stimulus 2) of the same sex, strain, and age is placed in a small vertical cage on the opposite lateral chamber. Chamber selections for trials 2 and 3 are randomized to ensure there is no location bias. Strangers were randomly selected on a non-consecutive basis for each subject. Strangers were naïve animals housed in separate facilities and had no contact with test animals prior to behavioral testing, and were not aggressive. The apparatus is made of an aluminum floor, transparent acrylic walls, and an open ceiling. Each adjacent chamber has a dimension of (40 × 20 × 23 cm). Cages are made of aluminum and plastic. The tracking software was programmed to track animal nose-points during all trials.
Golgi Staining
The day after behavioral testing, animals were anesthetized with isoflurane, their brains were subsequently collected and dissected along the midsagittal plane. The Golgi method of staining has long proven to be a reliable method for assessing dendrite and dendritic spine dynamics due to various conditions, because of its resistance to fading or photobleaching over time.41,42 We adapted a staining protocol and used the reagents contained in the superGolgi kit (Bioenno Tech).43 Right hemispheres were immediately impregnated in a potassium dichromate solution for two weeks (n = 5). Next, sections were immersed for at least 48 hours in a post-impregnation buffer. Samples were sectioned at 200 μm in 1X PBS along the coronal plane. Samples were then transferred into wells and washed with 0.01 M PBS buffer (pH 7.4) with Triton X-100 (0.3%) (PBS-T). Immediately after washing, samples were stained with ammonium hydroxide and then immersed in a post-staining buffer. Sections were again washed in PBS-T, mounted on 1% gelatin-coated slides, and allowed to dry. Sections were finally dehydrated with ethanol solutions, followed by cleaning in xylene, and coverslipped with Permount™ (Thermo Fisher Scientific).
Dendritic Morphology Quantification
Dendritic spines were also analyzed using Golgi-stained brain sections featuring the hippocampus. The criteria for neurons chosen for analysis was the following: 1. non-truncated dendrites; 2. consistent Golgi staining along dendrites; 3. relative isolation from other neurons to avoid interference 44. The neurons were traced using Neurolucida software version 11. Six to seven neurons per brain with 5 dendritic segments (at least 20 nm long) per neuron were analyzed 45.
Tissue Preparation
The hippocampus was removed and placed in 400 μl of RIPA lysis buffer (10 mM Tris-Cl pH 8.0, 1 mM EDTA, 0.5 M EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl). The tissue was homogenized on ice, incubated for 30 min on ice, and then centrifuged at 20,000 × g for 10 min at 4 °C. The supernatant was transferred to a new microcentrifuge tube and stored at −80 °C until processing. The Compat-Able™ Protein Assay Preparation Reagent Kit (Thermo Scientific ™) was used to eliminate EGTA as an interfering substance for the BCA Pierce TM BCA Protein Assay Kit (Thermo Scientific ™). Protein was separated by SDS-PAGE using the 4–15% Criterion™ TGX™ Precast Midi Protein Gel, 18-well, 30 uL (Bio-Rad) ran at 120V for 75 min. The gel was stained using Coomassie Blue staining (Bio-Rad). The samples were then sent to the UAMS Proteomics Core for further processing for mass spectrometry.
GeLC-MS/MS Analysis
Each SDS-PAGE gel lane was sectioned into 12 segments of equal volume. Each segment was subjected to in-gel trypsin digestion as follows. Gel slices were destained in 50% methanol (Fisher), 100 mM ammonium bicarbonate (Sigma-Aldrich), followed by reduction in 10 mM Tris[2-carboxyethyl]phosphine (Pierce) and alkylation in 50 mM iodoacetamide (Sigma-Aldrich). Gel slices were then dehydrated in acetonitrile (Fisher), followed by addition of 100 ng porcine sequencing grade modified trypsin (Promega) in 100 mM ammonium bicarbonate (Sigma-Aldrich) and incubation at 37oC for 12–16 hours. Peptide products were then acidified in 0.1% formic acid (Pierce). Tryptic peptides were separated by reverse phase XSelect CSH C18 2.5 um resin (Waters) on an in-line 150 × 0.075 mm column using a nanoAcquity UPLC system (Waters). Peptides were eluted using a 30 min gradient from 97:3 to 67:33 buffer A: B ratio. [Buffer A = 0.1% formic acid, 0.5% acetonitrile; buffer B = 0.1% formic acid, 99.9% acetonitrile.] Eluted peptides were ionized by electrospray (2.15 kV) followed by MS/MS analysis using higher-energy collisional dissociation (HCD) on an Orbitrap Fusion Tribrid mass spectrometer (Thermo) in top-speed data-dependent mode. MS data were acquired using the FTMS analyzer in profile mode at a resolution of 240,000 over a range of 375 to 1500 m/z. Following HCD activation, MS/MS data were acquired using the ion trap analyzer in centroid mode and normal mass range with precursor mass-dependent normalized collision energy between 28.0 and 31.0.
Data Analysis
Proteins were identified and quantified by searching the UniprotKB database restricted to Mus musculus using MaxQuant (version 1.6.5.0, Max Planck Institute). The database search parameters included selecting the MS1 reporter type, trypsin digestion with up to two missed cleavages, fixed modifications for carbamidomethyl of cysteine, variable modifications for oxidation on methionine and acetyl on N-terminus, the precursor ion tolerance of 5 ppm for the first search and 3 ppm for the main search, and label-free quantitation with iBAQ normalized intensities. Peptide and protein identifications were accepted using the 1.0% false discovery rate identification threshold. Protein probabilities were assigned by the Protein Prophet algorithm 46.
MaxQuant iBAQ intensities for each sample were median-normalized so the medians were equal to the sample with the maximum median. Median-normalized iBAQ intensities were then imported into Perseus (version 1.6.1.3, Max Planck Institute) to perform log2 transformation and impute the missing values using a normal distribution with a width of 0.3 and a downshift of 2 standard deviations. The Linear Models for Microarray Data (limma) Bioconductor package was used to calculate differential expression among the experimental conditions using the lmFit and eBayes functions 46,47. Proteins were considered to be significantly different with a fold change > 2 and an FDR adjusted p-value < 0.05. Differentially expressed proteins were analyzed using Ingenuity Pathway Analysis (IPA, QIAGEN Redwood City, www.qiagen.com/ingenuity) to identify pathways and networks.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [1] partner repository with the dataset identifier PXD017596 and 10.6019/PXD017596”.
Supplementary Material
Funding
This work was supported by under NIH P20 GM109005 (ARA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interest: None of the authors has competing financial interests or other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldsby RE et al. Late-occurring neurologic sequelae in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 324–331, doi: 10.1200/JCO.2009.22.5060 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH & Howard SC Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol 9, 257–268, doi: 10.1016/S1470-2045(08)70070-6 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Kahalley LS, Winter-Greenberg A, Stancel H, Ris MD & Gragert M Utility of the General Ability Index (GAI) and Cognitive Proficiency Index (CPI) with survivors of pediatric brain tumors: Comparison to Full Scale IQ and premorbid IQ estimates. J Clin Exp Neuropsychol 38, 1065–1076, doi: 10.1080/13803395.2016.1189883 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulsifer MB et al. Cognitive and Adaptive Outcomes After Proton Radiation for Pediatric Patients With Brain Tumors. International journal of radiation oncology, biology, physics 102, 391–398, doi: 10.1016/j.ijrobp.2018.05.069 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Yoo HJ et al. Neurocognitive Function and Health-Related Quality of Life in Pediatric Korean Survivors of Medulloblastoma. J Korean Med Sci 31, 1726–1734, doi: 10.3346/jkms.2016.31.11.1726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kee N, Teixeira CM, Wang AH & Frankland PW Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nature neuroscience 10, 355–362, doi: 10.1038/Nn1847 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Cheng Z, Li Y-Q & Wong CS Effects of aging on hippocampal neurogenesis after irradiation. International Journal of Radiation Oncology• Biology• Physics 94, 1181–1189 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Anand KS & Dhikav V Hippocampus in health and disease: An overview. Ann Indian Acad Neurol 15, 239–246, doi: 10.4103/0972-2327.104323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang CC, Kiecker C, O’Brien JT, Noble W & Chang RC Ammon’s Horn 2 (CA2) of the Hippocampus: A Long-Known Region with a New Potential Role in Neurodegeneration. Neurosicientist 25, 167–180, doi: 10.1177/1073858418778747 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Chidambaram SB et al. Dendritic spines: Revisiting the physiological role. Prog Neuropsychopharmacol Biol Psychiatry 92, 161–193, doi: 10.1016/j.pnpbp.2019.01.005 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Chakraborti A, Allen A, Allen B, Rosi S & Fike JR Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PloS one 7, e40844, doi: 10.1371/journal.pone.0040844 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parihar VK & Limoli CL Cranial irradiation compromises neuronal architecture in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America 110, 12822–12827, doi: 10.1073/pnas.1307301110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parihar VK et al. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct. Func. In Press (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semple BD, Blomgren K, Gimlin K, Ferriero DM & Noble-Haeusslein LJ Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progress in neurobiology 106–107, 1–16, doi: 10.1016/j.pneurobio.2013.04.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma T et al. Suppression of eIF2alpha kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nature neuroscience 16, 1299–1305, doi: 10.1038/nn.3486 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae JR & Kim SH Synapses in neurodegenerative diseases. BMB Rep 50, 237–246, doi: 10.5483/bmbrep.2017.50.5.038 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jesko H, Wencel P, Strosznajder RP & Strosznajder JB Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem Res 42, 876–890, doi: 10.1007/s11064-016-2110-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johri A & Beal MF Mitochondrial dysfunction in neurodegenerative diseases. The Journal of pharmacology and experimental therapeutics 342, 619–630, doi: 10.1124/jpet.112.192138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moy SS et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3, 287–302, doi: 10.1111/j.1601-1848.2004.00076.x (2004). [DOI] [PubMed] [Google Scholar]
- 20.Clapcote SJ et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 54, 387–402, doi: 10.1016/j.neuron.2007.04.015 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Ferguson JN, Young LJ & Insel TR The neuroendocrine basis of social recognition. Front Neuroendocrinol 23, 200–224, doi: 10.1006/frne.2002.0229 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Hitti FL & Siegelbaum SA The hippocampal CA2 region is essential for social memory. Nature 508, 88–92, doi: 10.1038/nature13028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J & Young WS Targeted activation of the hippocampal CA2 area strongly enhances social memory. Molecular psychiatry 21, 1137–1144, doi: 10.1038/mp.2015.189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maletic-Savatic M, Malinow R & Svoboda K Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Bourne J & Harris KM Do thin spines learn to be mushroom spines that remember? Current opinion in neurobiology 17, 381–386, doi: 10.1016/j.conb.2007.04.009 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N & Nakahara H Structure-stability-function relationships of dendritic spines. Trends in neurosciences 26, 360–368, doi: 10.1016/S0166-2236(03)00162-0 (2003). [DOI] [PubMed] [Google Scholar]
- 27.von Bohlen und Halbach O, Zacher C, Gass P & Unsicker K Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. Journal of neuroscience research 83, 525–531, doi: 10.1002/jnr.20759 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni VA & Firestein BL The dendritic tree and brain disorders. Molecular and cellular neurosciences 50, 10–20, doi: 10.1016/j.mcn.2012.03.005 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Kempf SJ et al. The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol Neurodegener 9, 57, doi: 10.1186/1750-1326-9-57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casciati A et al. Age-related effects of X-ray irradiation on mouse hippocampus. Oncotarget 7, 28040–28058, doi: 10.18632/oncotarget.8575 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betlazar C, Middleton RJ, Banati RB & Liu GJ The impact of high and low dose ionising radiation on the central nervous system. Redox biology 9, 144–156, doi: 10.1016/j.redox.2016.08.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamori T et al. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free radical biology & medicine 53, 260–270, doi: 10.1016/j.freeradbiomed.2012.04.033 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Marei H & Malliri A Rac1 in human diseases: The therapeutic potential of targeting Rac1 signaling regulatory mechanisms. Small GTPases 8, 139–163, doi: 10.1080/21541248.2016.1211398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herskovits AZ & Guarente L Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res 23, 746–758, doi: 10.1038/cr.2013.70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gartz Hanson M et al. Novel alpha-tubulin mutation disrupts neural development and tubulin proteostasis. Dev Biol 409, 406–419, doi: 10.1016/j.ydbio.2015.11.022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohno M Roles of eIF2alpha kinases in the pathogenesis of Alzheimer’s disease. Front Mol Neurosci 7, 22, doi: 10.3389/fnmol.2014.00022 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chater TE & Goda Y The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci 8, 401, doi: 10.3389/fncel.2014.00401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burette AC et al. Organization of TNIK in dendritic spines. J Comp Neurol 523, 1913–1924, doi: 10.1002/cne.23770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu J, Firestein BL & Zheng JQ Microtubules in dendritic spine development. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 12120–12124, doi: 10.1523/JNEUROSCI.2509-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todorova V & Blokland A Mitochondria and Synaptic Plasticity in the Mature and Aging Nervous System. Curr Neuropharmacol 15, 166–173, doi: (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao ZH et al. Low-level Gestational Lead Exposure Alters Dendritic Spine Plasticity in the Hippocampus and Reduces Learning and Memory in Rats. Scientific reports 8, 3533, doi: 10.1038/s41598-018-21521-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikolaenko I, Rao LM, Roberts RC, Kolb B & Jinnah HA A Golgi study of neuronal architecture in a genetic mouse model for Lesch-Nyhan disease. Neurobiology of disease 20, 479–490, doi: 10.1016/j.nbd.2005.04.005 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Groves TR, Wang J, Boerma M & Allen AR Assessment of Hippocampal Dendritic Complexity in Aged Mice Using the Golgi-Cox Method. Journal of visualized experiments : JoVE, doi: 10.3791/55696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Titus ADJ et al. Hypobaric hypoxia-induced dendritic atrophy of hippocampal neurons is associated with cognitive impairment in adult rats. Neuroscience 145, 265–278, doi: 10.1016/j.neuroscience.2006.11.037 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Kiffer F et al. Late Effects of 16O-Particle Radiation on Female Social and Cognitive Behavior and Hippocampal Physiology. Radiation Research 191, 278, doi: 10.1667/rr15092.1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nesvizhskii AI, Keller A, Kolker E & Aebersold R A Statistical Model for Identifying Proteins by Tandem Mass Spectrometry. Analytical Chemistry 75, 4646–4658, doi: 10.1021/ac0341261 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Ritchie ME et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47, doi: 10.1093/nar/gkv007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.