Abstract
Background & objectives:
The increase in the burden of multidrug-resistant tuberculosis (MDR-TB) is a matter of grave concern. The present study was undertaken to describe MDR-TB treatment outcome trends in Delhi and their epidemiological correlates, to assess the adequacy of treatment records and to also generate evidence towards influencing and improving practices related to the MDR-TB control programme.
Methods:
A retrospective record-based study (2009-2014) was conducted in three major drug resistance TB treatment centres of Delhi. Treatment outcomes and adverse effects were extracted from the existing programme records including patients’ treatment cards and laboratory registers.
Results:
A total of 2958 MDR-TB patients were identified from the treatment cards, of whom 1749 (59.12%) were males. The mean (±standard deviation) age was 30.56±13.5 years. Favourable treatment outcomes were reported in 1371 (53.28%) patients, but they showed a declining trend during the period of observation. On binomial logistic regression analysis, patients with age ≥35 yr, male sex and undernourishment (body mass index <18.5) at the time of treatment initiation had a significantly increased likelihood of unfavourable MDR-TB treatment outcome (P<0.001).
Interpretation & conclusions:
The study showed an increasing burden of MDR-TB patients, especially in the young population with increased risk of transmission posing a major challenge in achieving TB elimination targets.
Keywords: Drug resistance, India, MDR-TB, treatment outcome, XDR-TB
Tuberculosis (TB) is a leading cause of morbidity and mortality globally and persists as a major public health challenge. An estimated 10 million new patients of TB occurred worldwide in 20181. The TB incidence in India was estimated to be 2.7 million patients in 2018, the highest in the world2. In India, mortality due to TB declined from 56/100,000/yr in 2000 to 32/100,000/yr in 2018 as a result of the successful implementation of the landmark Revised National TB Programme [RNTCP, now renamed as National TB Elimination Programme (NTEP)] since 19922. The World Health Organization's (WHO) End-TB strategy envisages a further 95 per cent reduction in TB deaths by 20353. But the growth of multidrug-resistant TB (MDR-TB) threatens to undermine the gains and achievement of the goals for a TB-free world4,5.
It is estimated that 3.4 per cent of new TB patients and 18 per cent of previously treated TB patients have MDR-TB1. MDR-TB treatment involves prolonged treatment with injectable second-line drugs, associated with more adverse effects, suboptimal treatment outcomes and higher risks of mortality compared to patients with drug-sensitive TB and those with lesser resistant forms of TB1,6. In India, only 46 per cent MDR-TB patients initiated on treatment through programmatic management of drug-resistant TB (PMDT) had favourable treatment outcomes in 20167. In New Delhi, it was found that patients lost to follow up (for two or more consecutive months) and treatment failures in DR-TB patients further compounded the disease transmission cycle in the community8. A study in a tertiary care hospital in New Delhi also reported 20.17 per cent XDR-TB prevalence among the MDR-TB strains isolated9. The present study was conducted to describe MDR-TB treatment outcome trends in Delhi and to find out their epidemiological correlates.
Material & Methods
A retrospective record-based study was conducted among all patients registered and initiated on DR-TB treatment between January 2009 and December 2014 at three designated DR-TB Centres in Delhi. The three DR-TB centres selected included All India Institute of Medical Sciences (AIIMS), Lok Nayak Hospital (LNH) and the National Institute of TB and Respiratory Disease (NITRD). The fourth DR-TB centre of Delhi, Rajan Babu TB Hospital was not included due to operational reasons. The maximum number of MDR-TB patients were 1237 (41.81%) from LNH followed by 902 (30.49%) from NITRD and 819 (27.68%) from AIIMS.
The study was approved by the Institutional Ethics Committee, Maulana Azad Medical College and Associated Hospitals, New Delhi.
Data variables and sources of data: The records accessed included the patient treatment cards, TB registers and culture and drug susceptibility laboratory registers maintained at the centres. The extracted variables included patient age, sex, height, weight (pre- and post-treatment), adherence (missed doses), date of diagnosis, time of treatment initiation and treatment outcomes. Treatment outcomes were ascertained for all patients registered from the first quarter of 2009 until the final quarter of 2014 by following up their records over the next two years. The presumptive MDR-TB patients were defined as those TB patients with treatment failure, retreatment patients smear +ve at four months and close contacts of drug resistant TB patients10. Favourable treatment outcomes were defined as cured or treatment completed while unfavourable treatment outcomes were defined as death, treatment failure, lost to follow up, treatment stopped due to adverse reaction and a switch to an XDR-TB regimen.
Statistical analysis: The data were entered in MS Excel, cleaned and then analyzed using SPSS v16.0 (SPSS Inc., Chicago, IL, USA). Results were expressed in frequency and proportions. The chi-square test was used to find the difference in proportions between groups. A binary logistic regression analysis was conducted to find variables independently associated with unfavourable treatment outcomes.
Results & Discussion
A total of 2958 MDR-TB patients were identified from the treatment records, of whom 1749 (59.12%) were males and 1209 (40.87%) females. The mean (±standard deviation) age of the patients was 30.56±13.5 years. There were 1662 (56.18%) patients in the age group of 15-29 yr, 1144 (38.67%) in 30-59 yr and the geriatric (≥60) age group comprised only 152 (5.13%) patients. A majority of the MDR-TB patients belonged to the economically productive age groups (14-50 yr), a finding consistent with other studies11,12. More than half (59.12%) of the patients were male indicating a higher risk for them developing MDR-TB; but the prior evidence relating gender with the disease is equivocal11,12,13,14. It was observed that male MDR-TB patients were 1.4 times more likely to have unfavourable treatment outcomes compared to female patients which corroborated the evidence from previous studies12,15. Younger patients below the age of 35 yr were two times more likely to have a favourable treatment outcome compared to older patients, a finding also consistent with the global evidence16,17,18.
Only 111 (4.12%) patients were transferred out to other health institutions indicating that most of the patients were residents of the city. Smear positive-retreatment patients (n=731, 26.99%) were observed to be the most common group needing MDR-TB treatment initiation Table I. Further, presumptive MDR-TB comprised 18.46 per cent of the patients. Treatment outcome in these patients is reported in Table II. After excluding the patients who were transferred out (n=111) or had treatment stopped (n=6), it was observed that of the 2690 patients 1108 (41.18%) patients were cured and 263 (9.77%) had treatment completed status showing a total favourable outcome in 1371 (53.28%) patients. The favourable treatment outcome in the MDR-TB patients also showed a decreasing trend from 2009 (58.13%) to 2014 (38.74%) while unfavourable outcome showed an increasing trend from 2009 (40.69%) to 2014 (50.86%) (Figure). The proportion of female patients who were cured was higher and the proportion of female patients that were lost to follow up was lower compared to male patients (Table II). However, compared to WHO targets which envisage a 75-90 per cent cure rate for TB-patients19, the proportion of favourable treatment outcomes observed in our study was lower.
Table I.
Distribution of type of suspected multidrug-resistant (MDR)-TB patients by sex (n=2958)
| Type of MDR suspect | Total patients (n=2708)† n (%) | Males (n=1609) n (%) | Females (n=1099) n (%) |
|---|---|---|---|
| Treatment failure | 374 (13.81) | 227 (14.10) | 147 (13.37) |
| Retreatment case smear +ve at four month | 93 (3.43) | 59 (3.66) | 34 (3.09) |
| Contact of known MDR-TB case | 33 (1.21) | 16 (0.99) | 17 (1.54) |
| Smear +ve at diagnosis, retreatment case | 731 (26.99) | 450 (27.96) | 281 (25.56) |
| Any follow up smear +ve | 260 (9.60) | 151 (9.38) | 109 (9.91) |
| Smear –ve at diagnosis, retreatment case | 53 (1.95) | 23 (1.42) | 30 (2.72) |
| HIV-TB comorbidity | 7 (0.25) | 2 (0.12) | 5 (0.45) |
| Relapse | 486 (17.94) | 280 (17.40) | 206 (18.74) |
| Treatment after loss to follow up | 284 (10.48) | 188 (11.68) | 96 (8.73) |
| Others | 97 (3.58) | 48 (2.98) | 49 (4.45) |
| New patients | 290 (10.70) | 165 (10.25) | 125 (11.37) |
†Data not available for 250 patients (140 males and 110 females)
Table II.
Treatment outcome in multidrug-resistant tuberculosis patients related to age and sex (n=2958)
| Treatment outcomes | Total patients (n=2690)† n (%) | Male (n=1606) n (%) | Female (n=1084) n (%) | Age group (yr) | ||||
|---|---|---|---|---|---|---|---|---|
| 0-14 (n=93) n (%) | 15-29 (n=1414), n (%) | 30-49 (n=849) n (%) | 50-60 (n=196) n (%) | ≥60 (n=138) n (%) | ||||
| Cured | 1108 (41.18) | 592 (36.86) | 516 (47.60) | 46 (49.46) | 629 (44.48) | 332 (39.10) | 61 (31.12) | 40 (28.98) |
| Treatment completed | 263 (9.77) | 143 (8.90) | 120 (11.07) | 14 (15.05) | 153 (10.82) | 73 (8.59) | 14 (7.14) | 9 (6.52) |
| Died | 483 (17.95) | 278 (17.31) | 205 (18.91) | 22 (23.65) | 219 (15.48) | 144 (16.96) | 54 (27.55) | 44 (31.88) |
| Failed | 91 (3.38) | 56 (3.48) | 35 (3.22) | 2 (2.15) | 54 (3.81) | 30 (3.53) | 3 (1.53) | 2 (1.44) |
| Lost to follow up | 553 (20.55) | 405 (25.21) | 148 (13.65) | 7 (7.52) | 244 (17.25) | 214 (25.20) | 51 (26.02) | 37 (26.81) |
| Transferred out | 111 (4.12) | 79 (4.91) | 32 (2.95) | 1 (1.07) | 65 (4.59) | 35 (4.12) | 5 (2.55) | 5 (3.62) |
| Treatment stopped due to adverse drug reaction | 1 (0.03) | 1 (0.06) | 0 | 0 | 1 (0.07) | 0 | 0 | 0 |
| Treatment stopped due to other reason | 5 (0.18) | 4 (0.24) | 1 (0.09) | 0 | 3 (0.21) | 2 (0.23) | 0 | 0 |
| Switched to regimen for XDR-TB | 75 (2.78) | 48 (2.98) | 27 (2.49) | 1 (1.07) | 46 (3.25) | 19 (2.23) | 8 (4.08) | 1 (0.72) |
†Data not recorded for 268 patients (143 males and 125 females). XDR-TB, extensively drug-resistant tuberculosis
Figure.
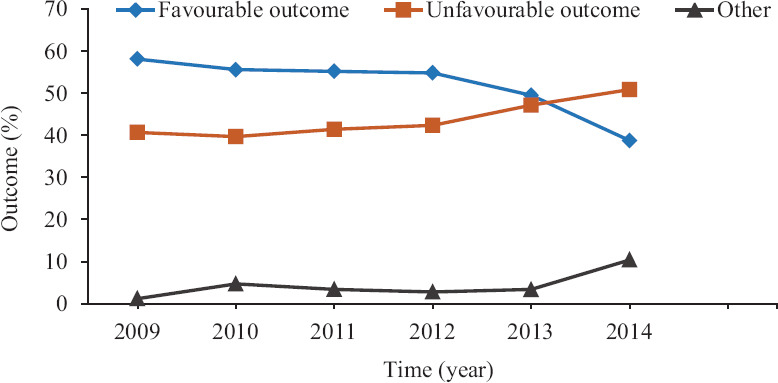
Trend in treatment outcomes in multidrug-resistant tuberculosis patients (2009-2014).
A total of more than seven missed doses indicating poor treatment adherence was recorded in 185 (6.25%) patients during the intensive phase and among 339 (11.46%) during the continuation phase. However, 1508 (50.98%) and 1554 (52.53%) patient records (n=2958) during the intensive and continuation phases, respectively, had missing observations relating to treatment adherence. Based on available data (n=1697), it was found that before treatment initiation, more than two-third (n=1189, 70.06%) patients were underweight [body mass index (BMI) <18.5 kg/m2]. The height variable was missing in 1251 of the 2958 (42.29%) patients. The body weight significantly increased from 43.63±10.41 at pre-treatment to 48±11.2 kg post-treatment, and there was significant improvement irrespective of gender (P<0.001). However, the pre-treatment body weight was not recorded in 103 and post-treatment body weight in 863 patients.
A binomial logistic regression was performed to ascertain the effects of age, sex and pre-treatment BMI on the likelihood that participants have unfavourable MDR-TB treatment outcomes. The logistic regression model was significant (P<0.001). This model correctly classified 59.10 per cent patients, sensitivity was 27.10 per cent and specificity was 84.90 per cent. It was observed that the model estimating the data was acceptable by the application of the Hosmer-Lemeshow goodness of fit test (P=0.916). Patients aged ≥35 yr, of male sex and being undernourished (BMI <18.5 kg/m2) at the time of treatment initiation had a significantly increased risk of unfavourable MDR-TB treatment outcomes (P<0.001) (Table III). It is well-established that among TB patients, there exists a high burden of undernutrition (BMI< 18.5 kg/m2)20. Our study showed that in MDR-TB patients, underweight status at the time of treatment initiation was significantly associated with unfavourable treatment outcomes.
Table III.
Distribution of factors associated with multidrug-resistant tuberculosis treatment outcome (n=2958)
| Variables | Total (n=2573)† n (%) | Unfavourable treatment outcome (n=1202), n (%) | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| Age (yr) | ||||
| <18 | 344 (13.36) | 128 (37.20) | 1 | <0.001 |
| 18-34 | 1356 (52.70) | 591 (43.58) | 1.19 (0.86-1.65) | |
| ≥35 | 873 (33.92) | 483 (55.32) | 2.1 (1.47-3) | |
| Sex | ||||
| Male | 1522 (59.15) | 787 (51.70) | 1.4 (1.12-1.75) | 0.002 |
| Female | 1051 (40.84) | 415 (39.48) | 1 | |
| Pre-treatment BMI | (n=1493)‡ | |||
| Undernourished | 1051 (70.39) | 505 (48.04) | 1.88 (1.48-2.38) | <0.001 |
| Normal/overweight | 442 (29.60) | 160 (36.19) | 1 |
†Outcome not recorded in 268 patients and 117 patients transferred out; ‡height variable missing in 1080 patients OR, odds ratio; CI, confidence interval; BMI, body mass index
Adverse reactions due to the MDR-TB treatment regimen were recorded in only 185 (6.25%) patients. The most common adverse effects were related to psychological (behaviour change and insomnia), gastrointestinal (vomiting, jaundice and loss of appetite), ototoxicity, musculoskeletal (joint pains) and CNS involvement (giddiness, numbness, neuropathy, weakness, etc.) reported in 47, 40, 27, 25 and 12 patients, respectively.
The present study had certain limitations. The data were collected retrospectively by accessing medical records of MDR-TB patients. However, more than half the records did not report treatment adherence and 42.29 per cent records did not have patient height. The large volume of missing data limited the generalizability of the study findings. Moreover, there exist a possibility of bias in the estimation of our study parameters since the analysis was conducted on the residual data applying a pair-wise deletion technique after assuming all our missing data to be ‘missing at random’ type. The consequent bias was not tested by analyzing whether the profile of the missing (excluded) patients significantly differed from the non-missing or included patients, since age and gender were the only major socio-demographic variables recorded on the treatment cards.
There was no information regarding tobacco smoking status and the harmful use of alcohol which can adversely influence MDR-TB treatment outcomes21,22. Certain other variables such as the individual pattern of drug resistance, the efficacy of drug combinations used and the quality of treatment monitoring and supervision can also influence treatment outcomes of drug-resistant TB patients, but the retrospective study design precluded their assessment.
Our study findings have indicated that the treatment outcomes in drug-resistant TB patients may be influenced by the factors affecting their drug adherence including socio-economic status and the presence of adverse drug reactions and these variables need to be accurately recorded23. Since undernutrition (low pre-treatment BMI) was found to be a predictor of unfavourable drug-resistant TB treatment outcomes, BMI variable (height and weight) should be correctly recorded in the treatment cards.
Footnotes
Financial support & sponsorship: This project was funded by the Revised National Tuberculosis Control Programme (now renamed as National TB Elimination Programme), New Delhi.
Conflicts of Interest: None.
References
- 1.World Health Organization. Global Tuberculosis Report 2019. Geneva: WHO; 2019. [Google Scholar]
- 2.Central TB Division. India TB Report 2019. New Delhi: Ministry of Health & Family Welfare, Government of India; 2019. [Google Scholar]
- 3.World Health Organization. The END-TB Strategy. [accessed on October 21, 2019]. Available from: https://wwwwhoint/tb/post2015_strategy/en/
- 4.Rifat M, Hall J, Oldmeadow C, Husain A, Hinderaker SG, Milton AH. Factors related to previous tuberculosis treatment of patients with multidrug-resistant tuberculosis in Bangladesh. BMJ Open. 2015;5:E008273. doi: 10.1136/bmjopen-2015-008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A, Hill A, Kurbatova E, van der Walt M, Kvasnovsky C, Tupasi TE, et al. Estimating the future burden of multidrug-resistant and extensively drug-resistant tuberculosis in India, the Philippines, Russia, and South Africa: A mathematical modelling study. Lancet Infect Dis. 2017;17:707–15. doi: 10.1016/S1473-3099(17)30247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan T, Ben Amor Y. What's in a name? The future of drug-resistant tuberculosis classification. Lancet Infect Dis. 2013;13:373–6. doi: 10.1016/S1473-3099(12)70318-3. [DOI] [PubMed] [Google Scholar]
- 7.Central TB Division. National strategic plan for tuberculosis elimination 2017-2025. New Delhi: Ministry of Health & Family Welfare, Government of India; 2018. [Google Scholar]
- 8.Central TB Division. TB India 2012. New Delhi: Ministry of Health & Family Welfare, Government of India; 2012. [Google Scholar]
- 9.Myneedu VP, Visalakshi P, Verma AK, Behera D, Bhalla M. Prevalence of XDR TB cases-a retrospective study from a tertiary care TB hospital. Indian J Tuberc. 2011;58:54–9. [PubMed] [Google Scholar]
- 10.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 11.Dzeyie KA, Basu S, Dikid T, Bhatnagar AK, Chauhan LS, Narain JP. Epidemiological and behavioural correlates of drug-resistant tuberculosis in a Tertiary Care Centre, Delhi, India. Indian J Tuberc. 2019;66:331–6. doi: 10.1016/j.ijtb.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt G, Vyas S, Trivedil K. An epidemiological study of multi drug resistant tuberculosis cases registered under Revised National Tuberculosis Control Programme of Ahmedabad city. Indian J Tuberc. 2012;59:18–27. [PubMed] [Google Scholar]
- 13.Lomtadze N, Aspindzelashvili R, Janjgava M, Mirtskhulava V, Wright A, Blumberg HM, et al. Prevalence and risk factors for multidrug-resistant tuberculosis in the Republic of Georgia: A population-based study. Int J Tuberc Lung Dis. 2009;13:68–73. [PMC free article] [PubMed] [Google Scholar]
- 14.Ejaz M, Siddiqui AR, Rafiq Y, Malik F, Channa A, Mangi R, et al. Prevalence of multi-drug resistant tuberculosis in Karachi, Pakistan: Identification of at risk groups. Trans R Soc Trop Med Hyg. 2010;104:511–7. doi: 10.1016/j.trstmh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Nair D, Velayutham B, Kannan T, Tripathy JP, Harries AD, Natrajan M, et al. Predictors of unfavourable treatment outcome in patients with multidrug-resistant tuberculosis in India. Public Health Action. 2017;7:32–8. doi: 10.5588/pha.16.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ettehad D, Schaaf HS, Seddon JA, Cooke GS, Ford N. Treatment outcomes for children with multidrug-resistant tuberculosis: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12:449–56. doi: 10.1016/S1473-3099(12)70033-6. [DOI] [PubMed] [Google Scholar]
- 17.Pratt RH, Winston CA, Kammerer JS, Armstrong LR. Tuberculosis in older adults in the United States, 1993-2008. J Am Geriatr Soc. 2011;59:851–7. doi: 10.1111/j.1532-5415.2011.03369.x. [DOI] [PubMed] [Google Scholar]
- 18.Ananthakrishnan R, Kumar K, Ganesh M, Kumar AM, Krishnan N, Swaminathan S, et al. The profile and treatment outcomes of the older (aged 60 years and above) tuberculosis patients in Tamilnadu, South India. PLoS One. 2013;8:E67288. doi: 10.1371/journal.pone.0067288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. The global plan to stop TB 2011-2015: Transforming the fight towards elimination of tuberculosis - reprinted with changes. Geneva: WHO; 2011. [Google Scholar]
- 20.Padmapriyadarsini C, Shobana M, Lakshmi M, Beena T, Swaminathan S. Undernutrition tuberculosis in India: Situation analysis the way forward. Indian J Med Res. 2016;144:11–20. doi: 10.4103/0971-5916.193278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zetola NM, Modongo C, Kip EC, Gross R, Bisson GP, Collman RG. Alcohol use and abuse among patients with multidrug-resistant tuberculosis in Botswana. Int J Tuberc Lung Di s. 2012;16:1529–34. doi: 10.5588/ijtld.12.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balabanova Y, Radiulyte B, Davidaviciene E, Hooper R, Ignatyeva O, Nikolayevskyy V, et al. Survival of drug resistant tuberculosis patients in Lithuania: Retrospective national cohort study. BMJ Open. 2011;1:E000351. doi: 10.1136/bmjopen-2011-000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson C, Stagg HR, Doshi A, Pan D, Sinha A, Batra R, et al. Tuberculosis treatment outcomes among disadvantaged patients in India. Public Health Action. 2017;7:134–40. doi: 10.5588/pha.16.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]


