Abstract
Background & objectives:
Coronary artery disease (CAD), a leading cause of mortality and morbidity worldwide has multifactorial origin. Epicardial adipose tissue (EAT) has complex mechanical and thermogenic functions and paracrine actions via various cytokines released by it, which can have both pro- and anti-inflammatory actions on myocardium and adjacent coronaries. The alteration of EAT gene expression in CAD is speculated, but poorly understood. This study was undertaken to find out the difference in gene expression of epicardial fat in CAD and non-CAD patients.
Methods:
Twenty seven patients undergoing coronary artery bypass graft (CABG) and 16 controls (non-CAD patients undergoing valvular heart surgeries) were included in the study and their EAT samples were obtained. Gene expressions of uncoupling protein-1, monocyte chemoattractant protein-1 (MCP-1), adiponectin, adenosine A1 receptor (ADORA-1), vascular cell adhesion molecule-1 (VCAM-1) and tumour necrosis factor-alpha (TNF-α) were studied by real-time reverse transcription-polymerase chain reaction. Glucose, insulin, lipid profile, high-sensitivity C-reactive protein, homocysteine, vitamin D, TNF-α and leptin levels were estimated in fasting blood samples and analyzed.
Results:
Leptin levels were significantly higher in CABG group as compared to controls (P<0.05), whereas other metabolic parameters were not significantly different between the two groups. MCP-1, VCAM-1 and TNF-α were upregulated in the CABG group as compared to controls. Further, multivariate analysis showed significantly reduced adjusted odds ratio for MCP-1 [0.27; 95% confidence interval: 0.08-0.91] in the CABG group as compared to controls (P<0.05).
Interpretation & conclusions:
Our findings showed an alteration in EAT gene expression in CAD patients with significant upregulation of MCP-1. Further studies with a large sample need to be done to confirm these findings.
Keywords: Adiponectin, CAD, EAT, inflammatory biomarkers, MCP-1, paracrine
Epicardial adipose tissue (EAT) is a visceral fat depot located around heart with no fascia separating it from underlying myocardium1. EAT is vascularized by the branches of the coronary arteries. The complex physiological functioning of human epicardial fat is not completely elucidated, but is distinguished by mechanical, metabolic and endocrine/paracrine and thermogenic functions2.
Mechanically, it protects coronaries against distortion and releases free fatty acids, which are utilized by myocardium as an energy source. It acts as a sink for fatty acids in circulation, protecting heart from lipotoxicity. The expression of uncoupling protein-1 (UCP-1) and related proteins is high in EAT, indicating its role in thermogenesis in humans1,2,3,4. It is a source of multiple bioactive cytokines such as leptin, adiponectin, resistin, plasminogen activator inhibitor-1, apelin, tumour necrosis factor-alpha (TNF-α), interleukin-6 and monocyte chemoattractant protein-1 (MCP-1), which are involved in the regulation of endothelial function, coagulation and inflammation through paracrine and endocrine actions5. Growing evidence points to the role of epicardial fat involvement in the development of coronary artery disease (CAD)6. Owing to its anatomical and functional contiguity with myocardium and coronaries, epicardial fat can secrete a large number of pro-inflammatory and suppressed anti-inflammatory cytokines under pathological conditions7. The relative expression (RE) of pro- and anti-inflammatory genes by EAT may be a determinant in the development of CAD8.
CAD risk in Indians is reported to be significantly higher in the general population than in Caucasians9. Asian Indians have more total, subcutaneous and visceral fat for similar body mass index (BMI) and age, compared with Caucasians10. The amount of visceral fat in Indians is more for each BMI level when compared with Caucasians and they fit into a term described as ‘metabolically obese’ normal-weight individuals11.
Plasma inflammatory biomarkers may not adequately reflect this local tissue inflammation12. It is postulated that in the adipose tissue, hypoxia leads to increased expression of inflammatory genes and decreased expression of adiponectin13. Treatment with beta-blockers, aspirin, angiotensin converting enzyme (ACE) inhibitors and Ca2+ channel blockers does not affect the adipocytokine gene expression in the EAT13. This study was thus undertaken with the aim to find out pathological functioning of epicardial fat in patients with CAD and the difference in gene expression of EAT in CAD and non-CAD patients.
Material & Methods
Twenty seven consecutive patients meeting inclusion-exclusion criteria undergoing elective coronary artery bypass graft (CABG), at Dr. K.G. Deshpande Memorial Centre, Nagpur, Maharashtra, India, and willing to participate were recruited in the study during February 2016 to October 2017). Written informed consent was obtained from all participants (both cases and controls) before carrying out any study-related procedure. Patients with renal and hepatic insufficiency and those with psychiatric disorders and pregnancy were excluded. Proper history was taken and thorough physical examination was done. The data of echocardiography and angiography were recorded for each patient. Complete drug history and family history were also noted. Their fasting mixed venous blood sample (10 ml) was collected. The investigations performed were fasting glucose (FBS), fasting insulin (in non-diabetic patients), lipid profile, leptin, TNF-α, haemoglobin A1c (HbA1c), high-sensitivity C-reactive protein (hsCRP), homocysteine and vitamin D levels. To compare the epicardial fat expression of CAD patients with those without CAD, EAT and fasting blood samples (10 ml) were collected from non-CAD patients (confirmed by angiography) undergoing valvular surgeries (n=16). The study protocol was approved by the Institutional Ethics Committee.
RNA extraction and gene expression: The human epicardial fat tissue (0.5-1 g) biopsy was obtained during CABG. The sample was immediately frozen at −70°C till RNA was extracted. Total RNA was isolated using RNeasy Lipid Tissue Mini Kit (Qiagen, Germany) as per the manufacturer's instructions; 1 μg of total RNA was first converted to cDNA (complementary DNA) using High-Capacity Reverse Transcription kit (Applied Biosystems, USA). Resulting cDNA samples were analyzed for the expression of pro-inflammatory and anti-inflammatory chemokine genes by real-time reverse transcription-polymerase chain reaction (qRT-PCR). Relative quantification of these genes was performed on Applied Biosystems 7300 real-time PCR using EXPRESS SYBR GreenER qPCR SuperMix.
The threshold cycle (Ct) values were obtained for target as well as reference genes from both CABG and control samples. Hypoxanthine phosphoribosyltransferase (HPRT) was used as a reference gene. The target gene set was as follows: adiponectin, UCP-1, MCP-1, vascular cell adhesion molecule-1 (VCAM-1), adenosine receptor A1 (ADORA-1) and TNF-α (Table 1). The Ct values for each gene type in each group were used to determine the relative expression of that gene. The primers were synthesized by Sigma-Aldrich, USA.
Table I.
Forward and reverse primer sequences for selected genes
| Genes | Primer | Sequence | Reference |
|---|---|---|---|
| ADIN | Forward | TATGATGGCTCCACTGGTA | 14 |
| Reverse | GAGCATAGCCTTGTCCTTCT | ||
| UCP-1 | Forward | TGCCCAACTGTGCAATGAA | 15 |
| Reverse | TCGCAAGAAGGAAGGTACCAA | ||
| MCP-1 | Forward | TCGCGAGCTATAGAAGAATCA | 16 |
| Reverse | TGTTCAAGTCTTCGGAGTTTG | ||
| VCAM-1 | Forward | CCCTTGACCGGCTGGAGATT | 17 |
| Reverse | CTGGGGGCAACATTGACATAAAGTG | ||
| TNF-α | Forward | CCTCTCTCTAATCAGCCCTCTG | 18 |
| Reverse | GAGGACCTGGGAGTAGATGAG | ||
| ADORA-1 | Forward | GTCCTCATCCTCACCCAGAG | 19 |
| Reverse | CAGATTGTTCCAGCCAAACA | ||
| HPRT | Forward | ACGAAGTGTTGGATATAAGC | 20 |
| Reverse | ATAATTTTACTGGCGATGTC |
ADIN, adiponectin; UCP-1, uncoupling protein-1; MCP-1, monocyte chemoattractant protein-1; VCAM-1, vascular cell adhesion molecule-1; TNF-α, tumour necrosis factor-alpha; ADORA-1, adenosine A1 receptor; HPRT, hypoxanthine phosphoribosyl transferase
The result was expressed as ΔCt value was obtained for each targei.e., fold change in the expression of the target gene relative to reference gene. The ΔCt values from control and patients’ samples were then compared to obtain theΔΔ Ct value, which is a reliable indicator of the difference in gene expression between control and experimental samples.
Statistical analysis: Data on parameters such as age, gender, BMI, behavioural habits and biochemical parameters were obtained for patients undergoing CABG as well as other valvular surgeries, referred to as control group in this study. Descriptive statistics such as mean, standard deviation, frequencies and percentages were obtained. Variables measured on numeric scale were compared between the two groups using t test for independent samples, whereas categorical variables were compared using Pearson's Chi-square test.
The Ct values were obtained for target and reference genes for each patient from CABG and control groups and expressed in terms of mean and standard deviation. The expression of target gene was normalized to reference gene expression level to obtained ΔCt values: ΔCt= Ct (target gene) − Ct (reference gene).
Further, ΔΔCt value was obtained for each target gene as ΔΔCt = ΔCt (CABG sample) − ΔCt (control sample). Finally, the fold change, also known as relative expression in qRT-PCR, of target gene expression in CABG sample in comparison with control sample, after normalizing with reference gene was obtained as 2−DDCt, considering a uniform PCR amplification efficiency of 100 per cent across all samples. The significance of difference in the mean normalized expression levels of target genes between the two groups was determined using t test of independent samples and the P values were adjusted for multiple testing correction using Benjamin and Hochberg (BH) method21. The multivariate logistic regression analysis was performed with groups as dependent variable and demographic and biochemical parameters as independent variables. Hosmer-Lemeshow test22 was used to decide the goodness of fit of the model. All the analyses were performed in R-3.2.1 programming tool (R Core Team, Vienna, Austria).
Results
The data on demographic and personal characteristics, metabolic parameters and marker expression were obtained on 27 CABG patients and 16 controls. Table II provides the descriptive statistics for parameters and their comparison between the groups. The mean age of patients in CABG group was significantly higher than that of control group (P<0.01). Gender bias, mean BMI and smoking habit pattern were not significantly different between the two groups. All the patients from CABG group were hypertensive unlike control group, showing significant difference (P <0.001). Dyslipidaemia was also predominantly observed in CABG patients as compared to controls (P <0.001). Mean arterial pressure (MAP) was significantly higher in CABG group than control group (P <0.001). Other biochemical and haematological parameters differed insignificantly between the two groups. Among markers of interest, the mean leptin level was significantly higher in the CABG group as compared to the control group (P<0.05).
Table II.
Descriptive statistics of patient characteristics in the two groups
| Characteristics | Levels | CABG (n=27) | Control (n=16) | P |
|---|---|---|---|---|
| Age (yr) | - | 61.30±9.18 | 52.19±9.93 | <0.01† |
| Gender, n (%) | Male | 20 (74.1) | 10 (62.5) | 0.649* |
| Female | 7 (25.9) | 6 (37.5) | ||
| BMI (kg/m2), mean±SD | - | 24.58±4.06 | 23.02±2.71 | 0.14† |
| Smoker, n (%) | Yes | 11 (40.7) | 5 (31.3) | 0.41* |
| No | 16 (59.26) | 11 (68.8) | ||
| HTN, n (%) | Yes | 27 (100.0) | 6 (37.5) | <0.001* |
| No | 0 | 10 (62.5) | ||
| Diabetes, n (%) | Yes | 14 (51.9) | 6 (37.5) | 0.55* |
| No | 13 (48.2) | 10 (62.5) | ||
| Dyslipidaemia, n (%) | Yes | 26 (96.3) | 7 (43.8) | <0.001* |
| No | 1 (3.7) | 9 (56.3) | ||
| MAP (mm Hg), mean±SD | - | 102.03±9.12 | 86.71±10.76 | <0.001† |
| Platelet count (×105/µl), mean±SD | - | 2.66±0.63 | 2.45±0.80 | 0.36† |
| Homocysteine (μmol/l) | - | 21.96±11.83 | 22.09±8.31 | 0.96† |
| hsCRP (nmol/l) | - | 50.48±45.90 | 53.05±33.90 | 0.83† |
| Vitamin D (nmol/l) | - | 43.32±28.62 | 59.80±40.27 | 0.16† |
| CKMB (μg/l) | - | 21.25±14.41 | 17.64±6.72 | 0.27† |
| FBS (mmol/l) | - | 6.18±1.59 | 5.54±1.25 | 0.15† |
| Serum insulin (mIU/l), mean±SD‡ | - | 29.36±20.92 | 20.34±14.95 | 0.26† |
| Homostatic model assessment of insulin resistance (HOMA-IR), mean±SD | - | 6.77±5.63 | 4.42±3.23 | 0.24† |
| Glycosylated haemoglobin, HbA1c (%), mean±SD‡ | - | 7.31±1.76 | 6.97±2.56 | 0.64† |
| TNF-α (pg/ml) | - | 17.42±34.12 | 19.14±20.72 | 0.84† |
| Leptin (μg/l) | - | 3.88±4.66 | 1.43±1.16 | <0.05† |
| Non-alcoholic steatohepatitis, n (%) | Yes | 13 (48.2) | 3 (18.8) | 0.10* |
| No | 14 (51.9) | 13 (81.3) |
*Using Chi-square test; †using independent t test; ‡only of diabetic patients (n=14) in cases and (n=6) in controls BMI, body mass index; SD, standard deviation; hsCRP, high-sensitivity C-reactive protein; MAP, mean arterial pressure; HTN, hypertension; CKMB, creatinine kinase muscle/brain; FBS, fasting blood sugar; CABG, coronary artery bypass graft
The relative expression or fold change of six target genes was obtained based on Ct values as given in Table III. It was evident that RE for MCP-1 was 2.7132 indicating upregulation of this gene in CABG patients as compared to controls. This was followed by upregulation of VCAM-1, TNF-α and UCP-1. Adiponectin showed downregulation in CABG patients. The (Figure) shows the mean ΔCt values for these genes in CABG group through column chart. The difference between the means of CABG and control groups for MCP-1 was significant (P <0.05) after BH correction. The Figure also shows the relative expression for different genes. MCP-1 and VCAM-1 were focused in the downstream functional analysis, although the difference for later was not significant.
Table III.
Descriptive statistics for gene expression and relative expression of target genes
| Genes | Control (n=16) | CABG (n=27) | Control-HPRT | CABG patients-HPRT | ΔCt(Ref) | ΔCt(target) | ΔΔCt | RE |
|---|---|---|---|---|---|---|---|---|
| TNF-α | 28.70±1.97 | 28.25±1.62 | 30.11±0.57 | 30.02±0.79 | 0.09 | 0.45 | −0.36 | 1.2834 |
| UCP-1 | 29.40±1.70 | 29.00±1.67 | 30.11±0.57 | 30.02±0.79 | 0.09 | 0.40 | −0.31 | 1.2397 |
| MCP-1 | 21.82±1.60 | 20.29±1.29 | 30.11±0.57 | 30.02±0.79 | 0.09 | 1.53 | −1.44 | 2.7132 |
| VCAM-1 | 24.87±4.94 | 24.16±4.66 | 30.11±0.57 | 30.02±0.79 | 0.09 | 0.71 | −0.62 | 1.5369 |
| ADORA 1 | 30.55±2.32 | 30.27±1.53 | 30.11±0.57 | 30.02±0.79 | 0.09 | 0.28 | −0.19 | 1.1408 |
| ADIN | 22.37±1.36 | 22.66±1.15 | 30.11±0.57 | 30.02±0.79 | 0.09 | −0.29 | 0.38 | 0.7684 |
Values given as mean±SD. RE, relative expression
Figure.
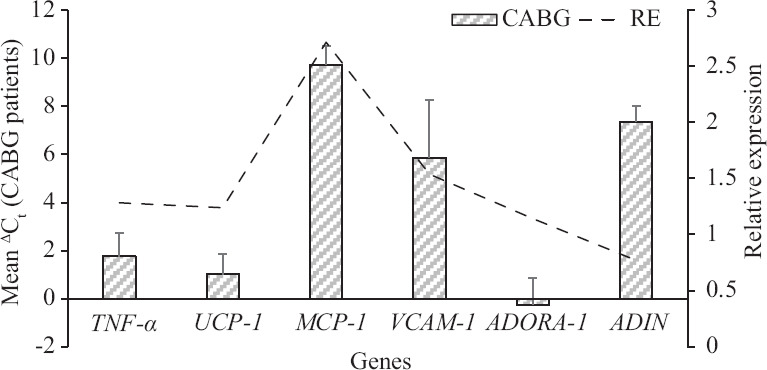
Showing mean (±SD) of ΔCt values for target genes in coronary artery bypass graft group (n=27) and the relative expression. TNF-α, tumour necrosis factor-alpha; UCP-1, uncoupling protein-1; MCP-1, monocyte chemoattractant protein-1; VCAM-1, vascular cell adhesion molecule-1; ADORA-1, adenosine A1 receptor; ADIN; adiponectin.
Further, the risk of CAD associated with metabolic parameters of interest after adjusting for covariates was obtained (Table IV). The odds ratio associated with homocysteine, creatinine kinase muscle/brain (CKMB) and TNF-α was close to 1.0, indicating marginal effect of unit increase in these parameters on CAD. Increase in the leptin levels increased the risk of CAD, although not significant. The unit increase in Ct values for MCP-1 decreased the odds of CAD significantly (P<0.05). In other words, decrease in Ct values of MCP-1 (upregulation of MCP-1) increased the risk of CAD.
Table IV.
Risk of coronary artery disease associated with different factors
| Factors | OR | 95% confidence interval (CI) | P* | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (yr) | 1.16 | 0.96 | 1.41 | 0.11 |
| Gender (reference:female) | 5.71 | 0.19 | 173.8 | 0.32 |
| BMI (kg/m2) | 1.29 | 0.86 | 1.96 | 0.22 |
| Dyslipidaemia (reference:no) | 12.32 | 0.05 | 312.2 | 0.37 |
| hsCRP (nmol/l) | 0.94 | 0.68 | 1.29 | 0.70 |
| Glycosylated haemoglobin (%) | 1.43 | 0.68 | 3.03 | 0.35 |
| Homocysteine | 0.98 | 0.85 | 1.14 | 0.85 |
| CKMB (μg/l) | 0.99 | 0.89 | 1.10 | 0.91 |
| TNF-α (pg/ml) | 0.98 | 0.93 | 1.03 | 0.42 |
| Leptin (μg/l) | 1.92 | 0.61 | 6.09 | 0.27 |
| MCP-1 | 0.27 | 0.08 | 0.91 | <0.05 |
*Obtained using multivariate logistic regression. OR, odds ratio
Discussion
The key findings of the study were upregulation and higher expression of pro-inflammatory chemokines MCP-1, TNF-α and VCAM-1 in EAT of patients with CAD as compared to controls. The expression of anti-inflammatory chemokines, adiponectin, ADORA-1 and UCP-1 which are cardioprotective, was downregulated in CAD patients as compared to controls. However, upregulation of MCP-1 expression was observed after adjusting for all confounders. The plasma levels of inflammatory biomarkers such as hsCRP, homocysteine, insufficient vitamin D levels and TNF-α were comparable in both groups. This indicates that EAT may have a larger role to play in the development of CAD. Earlier we have reported that epicardial fat mass correlates positively with diastolic dysfunction1. Higher levels of MCP-1, TNF-α and some other chemokines have been reported in different studies7,13,23. Lower levels of adiponectin in the EAT in CABG patients as compared with non-CAD patients have also been shown24,25.
MCP-1 initiates macrophage infiltration of adipose tissue, a hallmark of many studies which have reported presence of inflammatory state of EAT in CAD patients7,13,22,26,27,28. Hirata et al26 have reported the presence of greater number of M1 macrophages (inflammatory) as compared to M2 (inactive) in epicardial fat in CAD patients. The fact that atherosclerotic lesions develop in those parts of coronary arteries, which are surrounded completely by epicardial fat and the amount of fat and macrophage infiltration correlate with atherosclerotic plaque size and composition, further emphasizes the inflammatory role of EAT29. Expression of MCP-1 by EAT is high as compared with subcutaneous adipose tissue (SAT) and omental fat26,30. The parts of coronaries, which are free of atherosclerotic lesions are also free of adipose tissue31.
The secretion of inflammatory chemokines such as TNF-α and MCP-1 induces inflammatory cell influx into arterial wall, coronary vasospasm affecting arterial homeostasis, inducing plaque instability and apoptosis13,32. Whole genome analysis of EAT from 29 CAD and 15 non-CAD (undergoing valvular surgeries) patients through microarrays has shown complex overactivation of inflammatory cascades in EAT of CAD patients, whereas negative modulators of inflammation were found to be downregulated in them at transcriptional level33. Various pro-inflammatory adipokines released from the EAT increase the expression of VCAM-1, which mediates vascular endothelial inflammation via oxidative stress-dependent NFκB activation34. In our study, we found upregulation of VCAM-1 gene from EAT in CAD patients but significant upregulation was seen with MCP-1.
The finding that inflammatory markers are overexpressed in EAT after adjusting for all confounders including hypertension, diabetes, dyslipidaemia, age, gender and BMI signifies the independent role played by EAT in the development of CAD. The expression of adipokines correlates well with the EAT thickness. EAT can be a potential therapeutic target. Drugs such as sodium glucose transporter 2 (SGLT-2) inhibitors reduce EAT thickness. Epicardial fat decreases after very low calorie diet, aerobic exercise and even after bariatric surgery-induced weight loss35,36,37.
Our study had certain limitations. The sample size for the study was modest. This was a single-centre study and there were constraints in getting age- and sex-matched controls as the mean age of valvular heart disease without CAD was low.
In conclusion, the upregulation of expression of inflammatory markers such as MCP-1, TNF-α and VCAM-1 with downregulation of protective molecules such as adiponectin and ADORA-1 remained independent after adjusting for various confounders, indicating a pathological functioning of EAT in the development of CAD. MCP-1 expression was significantly higher in CAD patients as compared with non-CAD patients.
Acknowledgment
Authors acknowledge the contribution of Dr Dhananjay Raje, MDS Bio-Analytics, Nagpur, for data management and statistical analysis.
Footnotes
Financial support & sponsorship: Funding for gene expression study was provided by the Council of Scientific & Industrial Research (CSIR) to CSIR-National Environmental Engineering Research Institute, Nagpur.
Conflicts of Interest: None.
References
- 1.Sahasrabuddhe AV, Pitale SU, Dhoble SJ, Shivalkar J, Sagdeo MM. Cardiac diastolic dysfunction and regional body fat distribution in insulin resistant peripubertal obese males. J Assoc Physicians India. 2016;64:20–6. [PubMed] [Google Scholar]
- 2.Wu Y, Zhang A, Hamilton DJ, Deng T. Epicardial fat in the maintenance of cardiovascular health. Methodist Debakey Cardiovasc J. 2017;13:20–4. doi: 10.14797/mdcj-13-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iacobellis G, Pond CM, Sharma AM. Different “weight” of cardiac and general adiposity in predicting left ventricle morphology. Obesity (Silver Spring) 2006;14:1679–84. doi: 10.1038/oby.2006.192. [DOI] [PubMed] [Google Scholar]
- 4.Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: Epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611–5. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 5.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–43. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 6.Yagi S, Hirata Y, Ise T, Kusunose K, Yamada H, Fukuda D, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9:78. doi: 10.1186/s13098-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, et al. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: Changes associated with pioglitazone. Diabetes Care. 2011;34:730–3. doi: 10.2337/dc10-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekhri T, Kanwar RS, Wilfred R, Chugh P, Chhillar M, Aggarwal R, et al. Prevalence of risk factors for coronary artery disease in an urban Indian population. BMJ Open. 2014;4:E005346. doi: 10.1136/bmjopen-2014-005346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366–71. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 11.Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutr. 1981;34:1617–21. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 12.Iacobellis G, Bianco AC. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–7. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Chen H, Wu Y, Liu B, Li Z, Wang Z. Adiponectin exerts antiproliferative effect on human placenta via modulation of the JNK/c-Jun pathway. Int J Clin Exp Pathol. 2014;7:2894–904. [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JY, Takahashi N, Yasubuchi M, Kim YI, Hashizaki H, Kim MJ, et al. Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am J Physiol Cell Physiol. 2012;302:C463–72. doi: 10.1152/ajpcell.00010.2011. [DOI] [PubMed] [Google Scholar]
- 16.Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-κB ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem. 2005;280:16163–9. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- 17.Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis. 2007;193:328–34. doi: 10.1016/j.atherosclerosis.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura Y, Kano R, Hasegawa A, Watanabe S. Interleukin-8 and tumor necrosis factor alpha production in human epidermal keratinocytes induced by Trichophyton mentagrophytes. Clin Diagn Lab Immunol. 2002;9:935–7. doi: 10.1128/CDLI.9.4.935-937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans BA, Elford C, Pexa A, Francis K, Hughes AC, Deussen A, et al. Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J Bone Miner Res. 2006;21:228–36. doi: 10.1359/JBMR.051021. [DOI] [PubMed] [Google Scholar]
- 20.Daiwile AP, Sivanesan S, Izzotti A, Bafana A, Naoghare PK, Arrigo P, et al. Noncoding RNAs: Possible players in the development of fluorosis. Biomed Res Int. 2015;2015:274852. doi: 10.1155/2015/274852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. Hoboken (NJ): John Wiley & Sons, Inc; 2000. [Google Scholar]
- 23.Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: Possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91:4620–7. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 24.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32:268–74. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 25.Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–6. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 26.Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C, et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol. 2011;58:248–55. doi: 10.1016/j.jacc.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verhagen SN, Vink A, van der Graaf Y, Visseren FL. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study. Atherosclerosis. 2012;225:99–104. doi: 10.1016/j.atherosclerosis.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–21. [PubMed] [Google Scholar]
- 31.Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab. 2012;303:E937–49. doi: 10.1152/ajpendo.00061.2012. [DOI] [PubMed] [Google Scholar]
- 32.Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, et al. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol. 2000;20:2351–8. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- 33.Vacca M, Di Eusanio M, Cariello M, Graziano G, D’Amore S, Petridis FD, et al. Integrative miRNA and whole-genome analyses of epicardial adipose tissue in patients with coronary atherosclerosis. Cardiovasc Res. 2016;109:228–39. doi: 10.1093/cvr/cvv266. [DOI] [PubMed] [Google Scholar]
- 34.Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:T17–36. doi: 10.1530/JOE-12-0232. [DOI] [PubMed] [Google Scholar]
- 35.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 2008;16:1693–7. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 36.Willens HJ, Byers P, Chirinos JA, Labrador E, Hare JM, de Marchena E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol. 2007;99:1242–5. doi: 10.1016/j.amjcard.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 37.Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol ( 1985;2009(106):5–11. doi: 10.1152/japplphysiol.90756.2008. [DOI] [PubMed] [Google Scholar]


