Abstract
Simple Summary
Mesenchymal stem cells and their derivatives are used in clinical studies for their anti-apoptotic, anti-oxidant, immunomodulatory, and regenerative properties. Their use in reproductive medicine is increasing as they have been proved to be beneficial for infertility treatment. Mesenchymal stem cells can secrete factors that influence biological processes in target tissues or cells; these factors are either directly secreted by the cells or mediated through their derivatives. Although the amniotic membrane is easy to obtain and is a good source of stem cells, clinical trials using amniotic membrane-derived mesenchymal stem cells are still uncommon, especially in reproductive medicine or artificial reproductive technologies. The objective of the present study was to demonstrate the effects of conditioned medium prepared from amniotic membrane-derived stem cells on dog sperm cryopreservation. Our results showed that 10% of the conditioned medium enhanced the quality-related parameters of frozen–thawed sperm cells because of the presence of antioxidants and growth factors in the medium, which probably protected spermatozoa during the freeze–thaw process. These results suggest that conditioned media prepared from amniotic membrane-derived mesenchymal stem cells might have clinical applications in assisted reproductive technologies.
Abstract
This study investigated the effects of conditioned medium (CM) from canine amniotic membrane-derived MSCs (cAMSCs) on dog sperm cryopreservation. For this purpose, flow cytometry analysis was performed to characterize cAMSCs. The CM prepared from cAMSCs was subjected to proteomic analysis for the identification of proteins present in the medium. Sperm samples were treated with freezing medium supplemented with 0%, 5%, 10%, and 15% of the CM, and kinetic parameters were evaluated after 4–6 h of chilling at 4 °C to select the best concentration before proceeding to cryopreservation. Quality-related parameters of frozen–thawed sperm were investigated, including motility; kinetic parameters; viability; integrity of the plasma membrane, chromatin, and acrosome; and mitochondrial activity. The results showed that 10% of the CM significantly enhanced motility, viability, mitochondrial activity, and membrane integrity (p < 0.05); however, the analysis of chromatin and acrosome integrity showed no significant differences between the treatment and control groups. Therefore, we concluded that the addition of 10% CM derived from cAMSC in the freezing medium protected dog sperm during the cryopreservation process.
Keywords: sperm cryopreservation, canine, amniotic membrane, stem cells, conditioned medium, proteomics
1. Introduction
Sperm cryopreservation is used to store sperm samples from cancer patients or endangered species, and for other activities such as breeding, shipping, and research; however, the post-thaw quality obtained is low when compared with the fresh samples [1]. During cryopreservation, cold shock and crystal formation from intra- and extra-cellular water induce cryo-injuries that disturb the sperm plasma membrane integrity and lipid composition, resulting in the leakage of intracellular contents. Consequently, sperm metabolism is reduced [2] and apoptotic-like changes occur in the cells [3,4,5]. In particular, sperm cells are more sensitive to environmental changes because of their limited protein and lipid biosynthetic abilities [6] and the absence of DNA repair mechanism [7]. These events ultimately result in a weakened oxidative stress defense that exposes the sperm cells to reactive oxygen species (ROS). An increase in the ROS production during cryopreservation destroys sperm lipid matrix structures and, subsequently, causes the loss of membrane integrity, an increase in lipid peroxidation, and excessive DNA fragmentation [7,8].
To counteract the consequences of cryo-injuries, chemicals with protective properties can be added to the cells prior to or during cryopreservation. Cyto-protective agents such as cryo-protectants, anti-oxidants, or anti-apoptotic factors, act on different levels to protect cells from cryo-injuries. Anti-apoptotic factors such as the anti-cell death fibroblast-growth-factor inducible kinase (FNK) protein or curcumin prevent cell death [9,10]; cryo-protectants such as glycerol protect cells from intracellular ice formation and reduce osmotic damage [11], while anti-oxidants such as vitamin E protect the sperm ultra-structure, function, and mitochondrial DNA from oxidative stress [12,13]. Therefore, efficient and effective cryopreservation requires the addition of protective chemicals in the freezing media to increase cellular defenses and reduce ROS generation [10,14,15]. However, the commercial and homemade available freezing medium does not fully satisfy the requirements needed for the complete protection of sperm during the cryopreservation process [16]. Although Tris-egg yolk buffer is the most commonly used diluent for mammalian sperm, many studies show that the supplementation of anti-apoptotic factors, anti-oxidants, post-thaw enhancing chemicals, or novel cryo-protective agents is required to get good post-thaw results [1,5,17,18]. Thus, various molecules such as metformin [19], cholesterol [20], or α-tocopherol [21] have been successfully used in canine sperm cryopreservation to reduce oxidative stress, DNA damage [19,20], improve motility [21], and protect plasma membrane and acrosome integrity [20].
Mesenchymal stem cells (MSCs) and their derivatives are commonly used in regenerative medicine and have proved their clinical efficacy in the treatment of infertility [22,23,24]. The MSCs enhance anti-oxidant defenses in several tissues, including testis [24], through the secretion of proteins that reduce ROS production by scavenging free radicals [24,25] and enhance mitochondrial function through the Akt1 pathway [26]. They also secrete anti-inflammatory molecules and growth factors that protect cells from apoptosis when exposed to injuries [27,28]. Amniotic membrane-derived MSCs (AMSCs) have been isolated in dogs and humans [29,30]; although human AMSCs already proved to be useful in regenerative medicine [31], the use of canine AMSCs in this field have only been suggested but never applied [32,33]. In comparison with other stem cells, AMSCs are easier to obtain and isolate, which make them an ideal candidate for clinical trials [31,34].
The effects of MSCs on live tissues are mostly due to their paracrine signaling [35], since their secretome is rich in anti-oxidants and anti-apoptotic factors, which makes them a good alternative to cell therapy [36,37]. The derivatives of MSCs confer the same effects as the cells from which they originate [37], and they have regenerative and protective properties [38] that could positively affect sperm cells. In particular, conditioned medium (CM) has been studied and used in several clinical trials since it is easy to get, safer than cell-based therapies [39], and has a low immunogenicity [39], anti-oxidant and anti-apoptotic properties [40,41,42]. Moreover, CM can be used in many types of research studies since it can be manipulated more easily in comparison with cells [43] and it can also be added in solutions [44].
It is known that MSCs-derived CM obtained from starved cells consists of paracrine factors that enhance cell defense and trigger anti-apoptotic and anti-oxidative mechanisms [45]. The use of these factors may protect sperm from the detrimental effects of cryopreservation such as oxidative stress, apoptosis, DNA damage, and loss of mitochondrial activity. Therefore, we hypothesized that CM prepared from canine amniotic membrane-derived MSCs (cAMSC-CM) would have cyto-protective effects on dog sperm during the freeze–thaw process.
2. Materials and Methods
2.1. Experimental Design
Experiment 1 focused on the characterization of cAMSCs by flow cytometric analysis and pluripotency genes confirmation, the preparation of cAMSC-CM, and analysis of its components. Experiment 2 was conducted using cAMSC-CM. First, high and low ranges of cAMSC-CM concentrations were added to a freezing medium and used on dog sperm during chilling and cryopreservation process; however, high concentrations of cAMSC-CM had deleterious effects on sperm cells, and therefore, the experiments were conducted using a lower range of concentrations from 0 to 15% of cAMSC-CM. The optimal concentration of cAMSC-CM was selected by evaluating sperm kinetic parameters and viability after 4 to 6 h of chilling in the freezing medium supplemented with cAMSC-CM. Afterwards, in experiment 3, the optimal concentration of cAMSC-CM determined in Experiment 2 was used for dog sperm cryopreservation, and post-thaw quality-related parameters were evaluated and compared with the control group.
2.2. Cell Culture
Canine amniotic membrane-derived mesenchymal stem cells (cAMSC) and their culture medium were provided by Naturecell Co., Ltd. (Seoul, Korea). In brief, cAMSCs were seeded and cultured in tissue culture dishes with RCMEP media (Stem Cell Research Center, Biostar, Seoul, Korea), supplemented with serum and antibiotics. Cells were incubated in a humidified environment containing 5% CO2 at 37 °C. The cells used for the characterization of cAMSC were cultured until passage two at 90% confluency, and the cells used to make the (CM) were cultured until passage three. All chemicals, unless otherwise stated, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.3. Flow Cytometric Analysis
Fluorescence-activated cell sorting (FACS) was used to determine cAMSC immunophenotype. Cells were washed two times with phosphate-buffered saline (PBS; Thermo Fisher Scientific, Waltham, MA, USA) TrypLE™ Express (Gibco, Grand Island, NY, USA) was used to detach the cells. Cells were washed with PBS (Thermo Fisher Scientific) two times, counted, and aliquoted in a 96-well plate (1 × 105 cells/100 µL per well). In each well, 5 µL of fluorochrome-conjugated antibodies or isotype control antibodies with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)—CD29 Monoclonal Antibody-PE (Invitrogen, CA, Carlsbad, USA), CD44 Monoclonal Antibody-FITC, CD90 (Thy-1) Monoclonal Antibody-PE, CD34 Monoclonal Antibody-PE, CD45 Monoclonal Antibody-FITC, Rat IgG2a kappa Isotype Control-FITC, Rat IgG2b kappa Isotype Control-PE, Mouse IgG1 kappa Isotype Control-PE, and Rat IgG2b kappa Isotype Control-FITC (eBioscience, CA, San Diego, USA)—were added to the aliquoted cell suspensions. After 30 min of incubation at 4 °C, the cells were centrifuged (1500 rpm/3 min) and washed with PBS two times. Cells were transferred in round tubes with 5 mL of PBS, analyzed using FACSCalibur™, and Cell Quest software (BD Biosciences, CA, San Jose, USA) was used to calculate CD (Cluster of Differentiation) marker percentages. For each antibody, 10,000 cells were used.
2.4. Quantitative Polymerase Chain Reaction
Pluripotent genes expression was confirmed by quantitative polymerase chain reaction (qPCR). To summarize, RNA was extracted from cAMSC cultures at passage two using the RNeasy Mini kit (Qiagen, Hilden, Germany). Next, cDNA was synthesized by RNA reverse transcription using DiaStar 2X RT Pre-Mix (Solgent, Daejeon, Korea) and Random Hexamers (Invitrogen, Carlsbad, CA, USA). Synthesized cDNA was amplified by PCR, and real-time PCR was performed using Agilent ariaMX Real-Time PCR (Agilent, Santa Clara, CA, USA). Primers used for qPCR are displayed in Supplementary Materials Table S1 and beta-actin was used as an endogenous control. For gel electrophoresis, 10 µL of each real-time PCR product were loaded in wells and subjected to 1% agarose gel electrophoresis for 20 min.
2.5. Conditioned Medium Preparation
The cAMSCs were maintained in their culture media until they reached 80% confluency at passage three. The cells were starved by changing the media to serum-free Dulbecco’s Modified Eagle Medium. After 48 h [46], CM was retrieved, centrifuged (2000× g/30 min), and filtered with a 0.22 µm filter to remove cell debris. The CM aliquots were stored at −80 °C.
2.6. Proteomic Analysis and CM Composition
To identify and quantify cAMSC-CM protein composition, a one-dimensional electrophoresis-liquid chromatography tandem mass spectrometry (1-DE-LC-MS/MS) system coupled with a Q Exactive Plus mass spectrometer (Thermo Scientific, Waltham, MA, USA) was used. In brief, cAMSC-CM was collected and submitted to precipitation (ppt) for protein purification using ammonium sulfate (AS) saturated at 80%. Then, the CM was centrifuged at 18,000 rpm/1 h for precipitation, dissolved using 20 mM tris-HCl pH 8.0, and AS was removed using Viva spin (50kD). Protein lysates were separated using 12% sodium dodecyl–sulfate polyacrylamide gel electrophoresis (12% SDS-PAGE) followed by in-gel digestion with trypsin. MASCOT software (Matrix Science Inc., MA, Boston, USA) was used to identify the proteins, generate the exponentially modified protein abundance index (emPAI), and then, mole percentage was calculated according to emPAI values. Obtained data were analyzed using the UniProtKB (UniProt Knowledgebase) database (Canis_lupus familiaris). Identified proteins were classified by groups based on their functions, and the mole percentages for each group of proteins present in the cAMSC-CM was calculated.
2.7. Animal Use for Semen Collection
All dogs used for the study were kept in individual cages. They were fed with commercial adult dry food, and water was provided ad libitum. All the experiments and studies were conducted in accordance with the recommendations described in “The Guide for the Care and Use of Laboratory Animals” published by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University (approval numbers; SNU-180731-2-1 and SNU-200409-3). Semen was obtained from beagle dogs by using digital manipulation twice a week, and only the second fraction of the ejaculate was collected and processed. The samples with an appropriate concentration (>300 × 106 sperm/mL), motility higher than 80%, and mass motility of 4/5 at least (on a scale of 0–5) were selected and pooled to avoid individual variations. One ejaculate per male was obtained from five males, and four independent replicates were performed using the pooled semen.
2.8. Determination of CM Optimal Concentration
The pooled semen was diluted with Tris-extender 1:1 (v/v)—distilled water, tris (hydroxymethyl) aminomethane 24 g/L, citric acid 14 g/L, fructose 8 g/L, kanamycin sulfate 0.15 g/L; pH 6.6, 290 mOsm—and centrifuged at 700× g for 1 min. The supernatant was collected and centrifuged (500× g/5 min), and only the pellet was re-suspended in Tris-extender to achieve a concentration of 200 × 106 sperm cells/mL. Different concentrations of CM (0%, 5%, 10%, and 15%) were added to the freezing media—54% (v/v) Tris-extender, 40% (v/v) egg yolk, and 6% (v/v) glycerol —and the samples were chilled in it for 4–6 h at 4 °C. The sperm analysis imaging system (FSA2011 premium edition version 2011; Medical Supply, Gangwon, Korea) was used to evaluate sperm motility, curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), linearity (LIN), straightness (or VSL/VAP) (STR), amplitude of lateral head (ALH), and viability parameters. The treated group with the best results was selected for the rest of the experiments, as previously described [47,48].
2.9. Semen Cryopreservation and Thawing
The pooled semen was diluted with Tris-extender, washed, and centrifuged. The pellet was re-suspended in Tris-extender to achieve a concentration of 200 × 106 sperm cells/mL. The semen was divided into aliquots to be used for the control and the treatment groups. The freezing medium, with or without CM, was added by a multistep loading protocol [49], and 14%, 19%, 27%, and 40% of the freezing medium was added every 30 s. The samples were loaded in 0.5 mL straws (Minitube, Tiefenbach, Germany) and were kept at 4 °C for 1 h to reach equilibration. Then, they were placed horizontally to freeze at 5 cm above liquid nitrogen (LN2) for 15 min, before being transferred to LN2 tanks (−196 °C). Then, the semen straws were thawed in a water bath at 37 °C for 30 s. The samples were diluted with Tris-extender (1:5, v/v) stepwise using 14%, 19%, 27%, and 40% of the total volume at intervals of 30 s. Then, the samples were washed, and afterward, we proceeded with the analysis.
2.10. Sperm Kinetic Parameters Analysis
Sperm kinetic parameters were analyzed using a computer-assisted sperm analysis (CASA; Sperm Class Analyzer® System version 6.4.0.93, Microptic, Barcelona, Spain). The system included a Nikon Eclipse ci-L microscope (Nikon, Tokyo, Japan) with a 10× phase-contrast objective and a heating stage at 37 °C. Leja 20 µm chamber slides (Leja, Gynotec Malden, Nieuw Vennep, The Netherlands) were used for the analysis, and the frame rate was set at 25 frames/s. Various parameters such as sperm motility, progressive motility, VCL, VSL, VAP, LIN, STR, ALH, and the percentage of rapid and immotile spermatozoa were analyzed.
2.11. Eosin–Nigrosin Staining
Eosin–nigrosin staining was used to determine the percentage of sperm cells alive and tail morphology defects in each group. In brief, the frozen–thawed samples were washed, and a drop of 10 µL from the sperm pellet with an equal amount of eosin and nigrosin was mixed and smeared onto warm glass slides. The slides were then air-dried, and the sperm was evaluated afterward. For each stained smear, 200 sperms were examined with a light microscope (Eclipse Ts 2, Nikon, Tokyo, Japan) with oil immersion objective lens (1000× magnification). The unstained sperms were counted as alive, and the stained ones were counted as dead cells. The results are expressed as the percentage of live sperm cells [47]. Sperm with a coiling of the mid piece were counted as cells with a coiled tail, and sperm with a bending of the mid piece or the entire tail were counted as cells with a bent tail [50].
2.12. Aniline Blue Staining
The frozen–thawed samples were washed, and 20 µL of sperm pellet was smeared on a glass slide, air-dried, and fixed with a solution of 3% buffered glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 30 min. Then, the slides were stained with 5% aqueous aniline blue solution mixed with 4% acetic acid (pH 3.5) for 5 min. In each group, 200 sperm cells were evaluated with a light microscope (Eclipse Ts 2, Nikon, Tokyo, Japan) in oil immersion objective lens (1000× magnification). The cells with unstained nuclei were considered normal (mature chromatin), and those with blue-stained nuclei were considered abnormal (immature chromatin). The results are expressed as the percentages of aniline blue-positive sperm (abnormal) [51].
2.13. Hypo-Osmotic Swelling Test
The hypo-osmotic swelling test (HOST) was performed to evaluate the percentage of sperm cells with an intact plasma membrane. In brief, 100 µL of sperm was added to 900 µL of a hypo-osmotic solution (150–155 mOsm) and incubated at 37 °C for 30 min [52]. Then, a drop of HOST solution with sperm was placed on a warm slide and covered, and at least 100 spermatozoa were counted using a phase-contrast microscope (Eclipse Ts 2, Nikon, Tokyo, Japan). The cells with a coiled tail were counted as HOST-positive sperm.
2.14. Acrosome Assessment Test
The sperm acrosome membrane was analyzed using fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA) as described previously. In brief, semen was smeared on glass slides, air-dried, fixed in absolute methanol, stained, and mounted with anti-fade mounting medium (VECTASHIELD®, Vector Laboratories, Burlingame, CA, USA). The integrity of sperm acrosome membrane was analyzed using an epifluorescence phase-contrast microscope (Eclipse Ts 2, Nikon, Tokyo, Japan) and classified as intact acrosome (strong green fluorescence) or non-intact acrosome (partial or no fluorescence) [53].
2.15. Mitochondria Activity Assessment
The percentage of live sperm cells with functional mitochondria was assessed using a combination of fluorescent stains Rhodamine 123 (R123) (Molecular Probes, OR, Eugene, USA) and propidium iodide (PI) as described previously. In each slide, 200 spermatozoa were examined under an epifluorescence phase-contrast microscope (Eclipse Ts 2, Nikon, Tokyo, Japan) at 600× magnification, equipped with an excitation/barrier filter of 490/515 nm for R123 (blue excitation), excitation/barrier filter of 545/590 nm for PI (green excitation), and a digital camera (Olympus DP 11, Tokyo, Japan). The sperm cells displaying green fluorescence in the mid-piece region and no red fluorescence in the head were considered viable with functional mitochondria, whereas cells exhibiting red fluorescence in the head were counted as dead [54].
2.16. Statistical Analysis
Prior to analysis, D’Agostino and Pearson omnibus test was performed. Optimal concentration data were analyzed using one-way analysis of variance (ANOVA) following by a Tukey’s multiple comparison test. For the control and 10% CM-treated groups, the independent sample t-test was used. For each experiment, four replicates were performed and the statistical analysis was performed using GraphPad Prism 5.0 (GraphPad, CA, San Diego, USA). The values are expressed as mean ± standard error of the mean (SEM), and the values less than p < 0.05 were considered statistically significant.
3. Results
3.1. cAMSC and cAMSC-CM Characterization
3.1.1. Confirmation of the Surface Markers
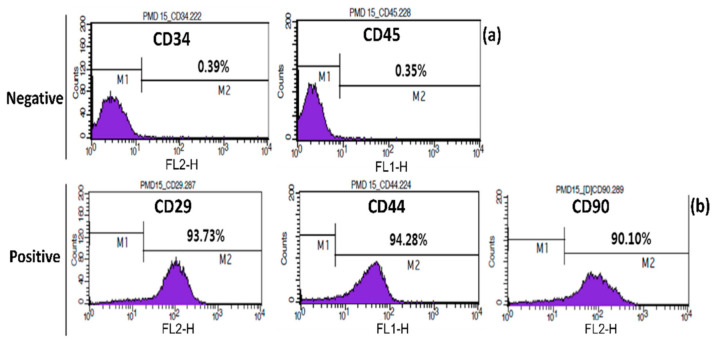
Flow cytometry analysis of cAMSC showed that the expression of CD29, CD44, and CD90 markers was high (93.73, 94.28, and 90.10%, respectively), whereas the expression of CD34 and CD45 markers was low (0.39 and 0.35%, respectively) (Figure 1). Therefore, based on the presence of these surface markers, we could infer that these cells were MSCs.
Figure 1.
Confirmation of surface markers of canine amniotic membrane-derived mesenchymal stem cells (cAMSC) using fluorescence-activated cell sorting (FACS); (a) negative surface markers CD34, CD45; (b) cAMSC-positive surface markers CD29, CD44, and CD90 surface makers analyzed by FACS.
3.1.2. Confirmation of Pluripotency Genes Expression
The qPCR analysis was performed to analyze the expression of pluripotency genes. qPCR results confirmed the expression of pluripotency genes Oct3/4, Sox2, and Nanog in cAMSC (Figure S1), which proves that cAMSCs exhibit pluripotency potential.
3.1.3. cAMSC-CM Proteome
The proteomic analysis showed the presence of 86 proteins (Table S2) and was expressed in mole percentage. Intermediate filaments (26%), other types of proteins involved in cell metabolism (21%), growth factors (18%), extracellular matrix components (15%), anti-oxidants (13%), and enzymes (7%) were found in the cAMSC-CM (Table 1).
Table 1.
Mole percentages of canine amniotic membrane-derived mesenchymal stem cells CM components from proteomics analysis.
| Type of Proteins | Total Mole Percentage by Type of Proteins (%) |
|---|---|
| Intermediate filaments | 26 |
| Cell metabolism | 21 |
| Growth factors | 18 |
| Extra-cellular matrix components | 15 |
| Anti-oxidants | 13 |
| Enzymes | 7 |
3.2. Determination of cAMSC-CM Optimal Concentration
Sperm viability and VCL were higher in the treated groups, 5%, 10%, and 15% CM (5% CM, 75.4 ± 6.7% and 83.9 ± 2.8%; 10% CM, 87.2 ± 8.1% and 90.1 ± 2.8%; 15% CM, 75.4 ± 6.7% and 86.8 ± 3.5%, respectively), and the group treated with 10% CM was significantly higher (p < 0.05) in comparison with the control group (74.2 ± 4.4% and 80.8 ± 2.0%, respectively). The 10% CM-treated group showed significantly higher motility and ALH (79.2 ± 2.6% and 4.8 ± 0.3 µm) than the other groups (control, 67.3 ± 2.5% and 4.1 ± 0.1 µm; 5% CM, 72.4 ± 2.5% and 4.1 ± 0.1 µm; 15% CM, 72.1 ± 3.9% and 4.1 ± 0.3 µm) (p < 0.05), (Table 2). Therefore, the 10% CM-treated group was selected for the rest of the experiment.
Table 2.
Motility and velocity parameters of 4 h chilled sperm using different concentrations of canine amniotic membrane-derived mesenchymal stem cells CM.
| Concentration of CM (%) | Motility (%) | Viability (%) | 1 VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | ALH (µm) |
|---|---|---|---|---|---|---|---|---|
| 0 | 67.3 ± 2.5 b | 80.8 ± 2.0 b | 74.2 ± 4.4 b | 21.4 ± 1.3 | 45.8 ± 2.0 | 29.0 ± 1.3 | 47.4 ± 1.7 | 4.1 ± 0.1 b |
| 5 | 72.4 ± 2.5 b | 83.9 ± 2.8 ab | 75.4 ± 6.7 ab | 20.4 ± 1.0 | 46.8 ± 3.1 | 29.0 ± 1.5 | 44.1 ± 1.4 | 4.2 ± 0.3 b |
| 10 | 79.2 ± 2.6 a | 90.1 ± 2.8 a | 87.2 ± 8.1 a | 23.8 ± 1.8 | 54.0 ± 4.0 | 31.2 ± 1.6 | 44.5 ± 1.2 | 4.8 ± 0.3 a |
| 15 | 72.1 ± 3.9 b | 86.8 ± 3.5 ab | 75.4 ± 6.7 ab | 24.6 ± 3.7 | 46.7 ± 4.0 | 31.6 ± 2.1 | 46.4 ± 2.3 | 4.1 ± 0.3 b |
1 VCL, average curvilinear velocity; VSL, straight-line velocity; VAP, average path velocity; LIN, linearity (average ratio of VSL/VCL); STR, straightness (average value of the ratio VSL/VAP); ALH, amplitude of lateral head. All results show means ± SEM. Values within marked with the letters “a” or “b” are significantly different (p < 0.05, n = 4).
3.3. cAMSC-CM Effects on Sperm Cryopreservation
3.3.1. Motility and Velocity Parameters
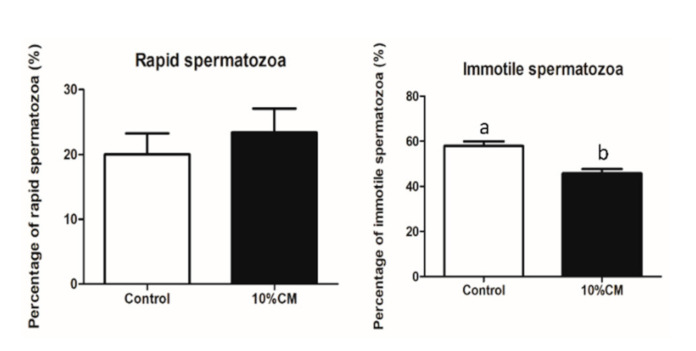
The CASA system results showed that 10% CM treatment significantly enhanced (p < 0.05) motility and LIN (54.3 ± 1.9% and 50.3 ± 3.1%, respectively) of sperm compared to that of the control group (42.1 ± 2.1% and 47.0 ± 3.4%, respectively). The percentage of immotile spermatozoa was significantly reduced in the treatment group (45.7 ± 1.9%) when compared with the control group (57.9 ± 2.1%) (p < 0.05), (Figure 2). The rest of the parameters showed no significant differences between the groups (p < 0.05) (Table 3).
Figure 2.
Percentages of rapid and immotile in frozen–thawed sperm. (a) Percentage of rapid sperm cells; (b) Percentage of immotile sperm cells. Bars with the letters “a” or “b” are values with a statistically significant difference (p < 0.05, n = 4).
Table 3.
Motility and velocity parameters of frozen–thawed sperm in control and 10% of canine amniotic membrane-derived mesenchymal stem cells conditioned media (CM) treatment groups.
| Concentration of CM (%) | Motility (%) | Progressive Motility (%) | 1 VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | ALH (µm) |
|---|---|---|---|---|---|---|---|---|
| 0 | 42.1 ± 2.1 b | 22.8 ± 3.4 | 81.5 ± 6.4 | 49.4 ± 5.6 | 57.2 ± 5.6 | 47.0 ± 3.4 b | 68.1 ± 2.4 | 3.1 ± 0.3 |
| 10 | 54.3 ± 1.9 a | 26.2 ± 4.2 | 74.5 ± 7.8 | 46.3 ± 7.1 | 53.3 ± 7.2 | 50.3 ± 3.1 a | 70.0 ± 2.2 | 2.8 ± 0.2 |
1 VCL, average curvilinear velocity; VSL, straight-line velocity; VAP, average path velocity; LIN, linearity (average ratio of VSL/VCL); STR, straightness (average value of the ratio VSL/VAP); ALH, amplitude of lateral head. Values are presented as means ± standard error of the mean (SEM). Values within columns marked with the letters “a” or “b” are significantly different (p < 0.05, n = 4).
3.3.2. Live/ Dead Count and Morphology Assessment
The percentage of live sperm cells was higher in the 10% CM-treated group (55.2 ± 3.0%) than that of the control group (43.9 ± 4.3%), and the percentage of spermatozoa with a bent tail was lower in the treatment group (1.8 ± 0.6%) than the control group (3.1 ± 0.7%) (p < 0.05), (Table 3). However, the percentage of cells with a coiled tail was not significantly different between the control and the 10% CM-treated groups (3.0 ± 1.2% and 3.0 ± 1.8%, respectively). These results suggest that cAMSC-CM probably has an anti-apoptotic effect during sperm cryopreservation.
3.3.3. Chromatin Integrity
The percentage of spermatozoa with abnormal chromatin condensation-stained nuclei was not significantly different in both the groups (control, 34.0 ± 2.9%; 10% CM, 31.0 ± 3.1%) (p < 0.05), (Table 4). These results suggest that cAMSC-CM has no protective effect on DNA integrity.
Table 4.
Live sperm percentage and morphological defects of frozen–thawed sperm in control and 10% of canine amniotic membrane-derived mesenchymal stem cells CM treatment groups.
| Concentration of CM (%) | Live Sperm Cells (%) | Coiled Tail (%) | Bent Tail (%) |
|---|---|---|---|
| 0 | 43.9 ± 4.3 a | 3.0 ± 1.2 | 3.1 ± 0.7 a |
| 10 | 55.2 ± 3.0 b | 3.0 ± 1.8 | 1.8 ± 0.6 b |
Values are presented as means ± standard error of the mean (SEM). Values within columns marked with the letters “a” or “b” are significantly different (p < 0.05, n = 4).
3.3.4. Acrosome and Membrane Integrity Assessment
The percentage of sperm cells with intact plasma membrane was significantly higher (p < 0.05) in the treatment group (66.5 ± 2.3%) than the control group (54.5 ± 2.9%). The FITC-PNA test revealed no significant difference (p < 0.05) in the percentage of spermatozoa with an intact acrosome between the two groups (control, 74.0 ± 4.3%; 10% CM, 76.6 ± 4.0%) (Table 5).
Table 5.
Percentage of frozen–thawed sperm with an abnormal chromatin condensation in control and 10% of canine amniotic membrane-derived mesenchymal stem cells conditioned media (CM) treatment groups.
| Concentration of CM (%). | Aniline Blue Positive Spermatozoa (%) |
|---|---|
| 0 | 34.0 ± 2.9 |
| 10 | 31.0 ± 3.1 |
All results show means ± SEM (n = 4).
3.3.5. Mitochondria Activity Assessment
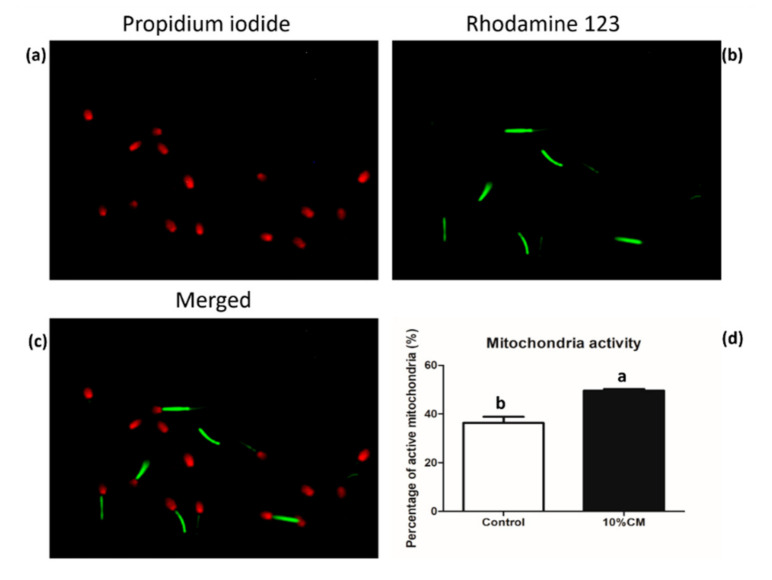
The R123 dye was used to assess mitochondrial activity, and PI was used to stain dead sperm cells. Both the data were used to calculate the percentage of live sperm with active mitochondria in each group. The 10% CM-treated group showed significantly enhanced (p < 0.05) mitochondrial activity (49.6 ± 0.7%) compared with the control group (36.4 ± 2.5%) (Figure 3).
Figure 3.
Mitochondria activity in frozen–thawed sperm using rhodamine 123 (R123) and propidium iodide (PI) dual staining; (a) dead sperm stained with PI; (b) live sperm with R123-stained mitochondria; (c) merged channels showing both PI and R123 stained sperm; (d) percentage of sperm with active mitochondria. Bars with the letters “a” or “b” are values with a statistically significant difference (p < 0.05, n = 4).
4. Discussion
Since the isolation of MSCs from amniotic tissues, researchers have displayed a growing interest in the study of their characteristics, properties, and possible applications [31,34,55]. Thus, they have become an interesting alternative to embryonic stem cells, as they can be obtained by non-invasive methods [31], and the ethical issues associated with the use of amniotic tissues-derived stem cells are minor [34], since they are obtained from the placenta that is usually discarded after caesarian sections [29]. They are non-teratogenic [55], pluripotent, and also have regenerative properties and low immunogenicity [31,34,55]. Canine MSCs derived from the amniotic membrane have recently been isolated and proved to have the same characteristics, biosafety, and properties as other MSCs, which make them a good candidate for clinical trials [32,56]. The composition and potential use of cAMSC-CM have not been studied yet, but since CM contains cell paracrine factors [37], the application of cAMSC-CM in clinical studies seems to be promising. In our study, cAMSC phenotype was confirmed through FACS analysis and showed a high expression of CD29, CD44, and CD90 surface markers, whereas CD34 and CD45 expression, which are associated with hematopoietic stem cells [57,58], was low (Figure 1). This phenotype has also been found in other studies using canine MSCs [59,60,61], and CD29, CD44, and CD90 surface antigens are found on adipose, bone marrow, and umbilical cord blood-derived mesenchymal stem cells [62,63]. Pluripotency is an important factor in MSCs, since it is involved in regenerative pathways [64]. The analysis of pluripotency gene expression (Oct3/4, Nanog, and Sox2) also confirmed the MSC phenotype (Figure S1). The analyzed genes are essential for the self-renewal of MSCs, determination of pluripotency and maintenance of cells’ undifferentiated state [65,66]. Their expression by cAMSCs is an indicator that cAMSCs could be used in regenerative medicine.
Stem cells-derived CM contains growth factors and anti-apoptotic factors, and it has been used in cell-free therapies to mediate stem cells paracrine effects on tissues [39,41,42]. Each CM has a different composition depending on the cells of origin, cell state, and the microenvironment surrounding them [35]. Previously, canine MSC-derived CM was used for different clinical applications and showed good results in xenogeneic tissues wound healing [67], laryngotracheal stenosis healing [68], and stem cells’ survival and differentiation [69]. However, no study has unveiled the effects of cAMSC-CM and its proteomic analysis. In this study, the composition of cAMSC-CM was investigated, and the proteomic analysis revealed the presence of intermediate filaments, extra-cellular matrix components (ECM), anti-oxidants, growth factors, enzymes, and other proteins involved in the cell metabolism (Table S2, Table 1). Our results corroborate those of previous studies showing that two of the main components of CMs were growth factors and anti-oxidants [39]. Furthermore, an amniotic membrane stem cells proteome from another study [70] showed the presence of ECM components involved in cellular processes through the focal adhesion kinase signaling pathway such as lumican, collagen, and fibronectin, which were also found in cAMSC-CM in our study (Table S2, Table 1). Apolipoproteins, especially Apolipoprotein A-1, which is involved in sperm capacitation and motility [71]; and Apolipoprotein E, which has an anti-oxidant activity [72], were also found in cAMSC-CM (Table S2). Some of the growth factors found in our study include thioredoxin, which has an anti-oxidant activity and plays a role in fertility [73,74,75], and serum albumin, which might also play an important role in fertility improvement [76] and sperm cryopreservation, by enhancing post-thaw motility and protecting sperm morphology, and the integrity of the plasma membrane, acrosome, and DNA [77,78].
Although sperm cells have the ability to adapt to osmotic changes [79], freezing medium does not fully protect them from the osmotic stress happening during freezing and thawing that leads to an increase in ROS production, apoptosis-like changes, DNA damage, and an increase in tail defects [80,81,82]. Therefore, we hypothesized that the addition of cAMSC-CM to the freezing medium would increase sperm tolerance to osmotic changes, oxidative defence, and help protect their ultra-structure, because it contains proteins with anti-oxidant, regenerative, and anti-apoptotic effects (Table S2). To date, no study has depicted the addition of CM in sperm cryopreservation, and the range of CM concentrations added to the freezing medium remains unknown. Here, we evaluated the optimal concentration of CM starting from a low range of concentrations, from 0 to 15% of the CM, among which 10% of the CM revealed to be the best concentration (Table 2). We assumed that a concentration higher than 15% would influence sperm homeostasis, as it would probably change the osmolarity of the milieu. In fact, during our preliminary study, we observed that the use of a higher range of concentrations (from 25 to 75%) negatively impacted the quality-related parameters of frozen–thawed sperms (data not shown). Nevertheless, from the low range of concentrations, the supplementation of 10% CM in the freezing medium was found to be the optimal one and was further used for cryopreservation.
Our results showed that the percentage of live sperm was significantly increased when cAMSC-CM was added to the freezing medium (Table 4), which could be explained by the regenerative and anti-apoptotic effects of cAMSC-CM proteins. When CM is prepared, the components released from the starvation phase resulting from the MSC survival state activation causes the release of protective factors that protects cells from apoptosis and oxidative stress [45]. These factors probably improved the anti-oxidant defence and anti-apoptotic mechanisms in sperm. However, they were not sufficient to protect chromatin integrity, as aniline blue test results showed no significant differences between the control and cAMSC-CM treated groups. Aniline blue dye binds to nucleoproteins and allows researchers to evaluate chromatin integrity; however, DNA denaturation and fragmentation induced by freezing and thawing are not always immediately apparent. A study showed that canine sperm further incubated after thawing showed an increase in the DNA fragmentation index [83].
The cryopreservation process leads to an increase in sperm tail abnormalities [80,84], altered acrosome and plasma membrane integrity [85], and a decrease in the mitochondrial activity [4,82] and motility [80,82]. In our study, we found that the percentage of coiled tail sperm was the same in both groups, but the percentage of sperm with a bent tail was significantly reduced in the 10% CM-treated group in comparison with the control group (Table 4). The proteins present in seminal plasma can interact with sperm at the surface and repair plasma membranes and mitochondrial DNA [86]. In our study, some of the proteins found in cAMSC-CM, including collagen, olfactory receptors, zinc finger protein, vitamin D binding protein, fibronectin, and serum albumin (Table S2), have also been found in the seminal plasma collected from fertile men [87], boar [88,89], alpaca, and camels [89]. These proteins, along with the protective nature of other cAMSC-CM factors, might explain the positive effects on sperm ultra-structural characteristics (Table 4). Moreover, the positive correlation between some of these proteins and fertility [76,87] and their role in mitochondrial DNA repair [86] might explain post-thaw improved sperm motility, LIN (Table 3), and the enhanced mitochondrial activity (Figure 3). Mitochondrial activity is essential for sperm motility, and a decrease in its activity results in increased apoptosis [90]. Furthermore, the addition of 10% cAMSC-CM reduced the percentage of immotile sperm (45.7 ± 1.9%) (Figure 2). We can hypothesize that proteins in cAMSC-CM might have reduced ROS production and have had a positive role in mitochondrial DNA repair. However, the percentage of sperm with an intact acrosome was high in both the groups (74.0 ± 4.3% and 76.6 ± 4.0% respectively), but there was no significant difference between them (Table 6). A study showed that acrosome integrity is above 60% in canine sperm at 0 h after thawing, when 6–8% glycerol is used [91]. In our freezing medium, 6% glycerol was used, and the acrosome integrity test was performed within few minutes after thawing. This might explain the high percentage of intact acrosomes observed in both groups.
Table 6.
Percentages of intact acrosome and membrane in frozen–thawed sperm in control and 10% of canine amniotic membrane-derived mesenchymal stem cells CM treatment groups.
| Concentration of CM (%) | Intact Acrosome (%) | Intact Membrane (%) |
|---|---|---|
| 0 | 74.0 ± 4.3 | 54.5 ± 2.9 b |
| 10 | 76.6 ± 4.0 | 66.5 ± 2.3 a |
Values are presented as means ± standard error of the mean (SEM). Values within columns marked with the letters “a” or “b” are significantly different (p < 0.05, n = 4).
The preservation of sperm membrane integrity and mitochondrial function during the freeze–thaw process is important for successful fertilization, but the commercial freezing media do not protect sperm from the loss of these functions [16]. The addition of cyto-protective factors in the freezing medium can prevent the deleterious effects of cryopreservation [92], because they can protect cells from the negative effects of ROS on sperm motility, mitochondrial activity, and DNA integrity [93]. In our study, the preservation of membrane integrity was more effective in the 10% CM-treated group (66.5 ± 2.3%) in comparison with the control group (54.5 ± 2.9%). This might be due to the proteins present in cAMSC-CM, especially apolipoproteins (Table S2). ROS targets polyunsaturated fatty acids and cholesterol [94], which disturbs the integrity of the plasma membrane that is essential to sperm homeostasis [95]. In particular, cholesterol is important for membrane stability and can determine sperm freezability, as a disturbed cholesterol to phospholipid ratio can negatively impact the outcome of cryopreservation [96]. Apolipoproteins play a crucial role in cholesterol homeostasis in the epididymis [97], and they are involved in lipid exchange, capacitation, and membrane remodeling [71,98]. In addition, fibronectin, an ECM component that acts as a growth factor [99], was found in the cAMSC-CM, which is also involved in protecting membrane integrity (Table S2 and Table 1). Qamar et al. [100] showed that fibronectin was expressed in adipose-derived MSCs and that plasma membrane integrity was protected when adipose-derived MSCs were added to the freezing media. This suggests that cAMSC-CM protected plasma membrane integrity in the present study (Table 6).
5. Conclusions
In conclusion, our results showed that cAMSC-CM contained proteins, including growth factors, anti-oxidants, enzymes, and ECM components, which protect sperm functions and ultra-structure characteristics during cryopreservation. The addition of cAMSC-CM in the freezing medium enhanced sperm motility and viability, membrane integrity, and mitochondrial activity. This was the first study to reveal the composition of cAMSC-CM and its effect on sperm cryopreservation. Further in vitro studies to evaluate cAMSC-CM effects on sperm capacitation and fertilizing ability should be conducted. In vivo studies should also be conducted to reveal the effects of cAMSC-CM proteins on fertility, along with a much deeper study on the proteins implicated in canine sperm fertility.
Acknowledgments
The authors would like to thank the Korea Basic Science Institute (KBSI) for their help with the proteomic analysis. We appreciate the assistance and help of the staff from laboratory of theriogenology and biotechnologies, especially Ye Seol Yun, Hyo Kyung Yoo, and Do Yeon Kim. We would also like to thank Kayla Orta for her help in English revision.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/10/10/1899/s1, Figure S1: Confirmation of pluripotent genes expressions in all cAMSC cell lines (n = 11) using quantitative polymerase chain reaction (qPCR); Table S1. List of primers used for quantitative polymerase chain reaction (qPCR); Table S2. Proteins found in canine amniotic membrane-derived mesenchymal stem cells (AMSC) derived conditioned medium.
Author Contributions
Conceptualization, F.Y.M. and M.J.K.; methodology, F.Y.M., A.Y.Q., and M.J.K.; validation, F.Y.M. and M.J.K.; formal analysis, F.Y.M., J.C.R., S.H.K., E.J.J.; investigation, F.Y.M.; resources, M.J.K.; data curation, F.Y.M. and J.W.K.; writing—original draft preparation, F.Y.M.; writing—review and editing, F.Y.M., A.Y.Q. and M.J.K.; visualization, F.Y.M., J.W.K. and M.J.K.; supervision, M.J.K.; project administration, M.J.K.; funding acquisition, M.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by RDA (CCAR, PJ013954022019, PJ014786012020) and The Research Institute for Veterinary Science.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bencharif D., Dordas-Perpinya M. Canine semen cryoconservation: Emerging data over the last 20 years. Reprod. Domest. Anim. 2020;55(Suppl. 2):61–65. doi: 10.1111/rda.13629. [DOI] [PubMed] [Google Scholar]
- 2.Desrosiers P., Legare C., Leclerc P., Sullivan R. Membranous and structural damage that occur during cryopreservation of human sperm may be time-related events. Fertil. Steril. 2006;85:1744–1752. doi: 10.1016/j.fertnstert.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Pena F.J., Nunez-Martinez I., Moran J.M. Semen technologies in dog breeding: An update. Reprod. Domest. Anim. 2006;41:21–29. doi: 10.1111/j.1439-0531.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- 4.Martin G., Sabido O., Durand P., Levy R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 2004;71:28–37. doi: 10.1095/biolreprod.103.024281. [DOI] [PubMed] [Google Scholar]
- 5.Ezzati M., Shanehbandi D., Hamdi K., Rahbar S., Pashaiasl M. Influence of cryopreservation on structure and function of mammalian spermatozoa: An overview. Cell Tissue Bank. 2020;21:1–15. doi: 10.1007/s10561-019-09797-0. [DOI] [PubMed] [Google Scholar]
- 6.Hammerstedt R.H., Parks J.E. Changes in sperm surfaces associated with epididymal transit. J. Reprod. Fertil. Suppl. 1987;34:133–149. doi: 10.1530/biosciprocs.9.011. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Marin C., Gosalvez J., Roy R. Types, Causes, Detection and Repair of DNA Fragmentation in Animal and Human Sperm Cells. Int. J. Mol. Sci. 2012;13:14026–14052. doi: 10.3390/ijms131114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicherle C.C., de Souza F.F., Freitas-Dell’Aqua C.P., Mothe G.B., Padovani C.R., Papa F.O., Lopes M.D. Effects of the cryopreservation process on dog sperm integrity. Anim. Reprod. 2020;17:e20190081. doi: 10.21451/1984-3143-AR2019-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudo K., Asoh S., Ohsawa I., Ozaki D., Yamagata K., Ito H., Ohta S. The anti-cell death FNK protein protects cells from death induced by freezing and thawing. Biochem. Biophys. Res. Commun. 2005;330:850–856. doi: 10.1016/j.bbrc.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 10.Kanitkar M., Bhonde R.R. Curcumin treatment enhances islet recovery by induction of heat shock response proteins, Hsp70 and heme oxygenase-1, during cryopreservation. Life Sci. 2008;82:182–189. doi: 10.1016/j.lfs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Storey B.T., Noiles E.E., Thompson K.A. Comparison of glycerol, other polyols, trehalose, and raffinose to provide a defined cryoprotectant medium for mouse sperm cryopreservation. Cryobiology. 1998;37:46–58. doi: 10.1006/cryo.1998.2097. [DOI] [PubMed] [Google Scholar]
- 12.Cummins J.M., Jequier A.M., Kan R. Molecular biology of human male infertility: Links with aging, mitochondrial genetics, and oxidative stress? Mol. Reprod. Dev. 1994;37:345–362. doi: 10.1002/mrd.1080370314. [DOI] [PubMed] [Google Scholar]
- 13.Silva S.V., Soares A.T., Batista A.M., Almeida F.C., Nunes J.F., Peixoto C.A., Guerra M.M. Vitamin E (Trolox) addition to Tris-egg yolk extender preserves ram spermatozoon structure and kinematics after cryopreservation. Anim. Reprod. Sci. 2013;137:37–44. doi: 10.1016/j.anireprosci.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Wang A.W., Zhang H., Ikemoto I., Anderson D.J., Loughlin K.R. Reactive oxygen species generation by seminal cells during cryopreservation. Urology. 1997;49:921–925. doi: 10.1016/S0090-4295(97)00070-8. [DOI] [PubMed] [Google Scholar]
- 15.Sieme H., Oldenhof H., Wolkers W.F. Mode of action of cryoprotectants for sperm preservation. Anim. Reprod. Sci. 2016;169:2–5. doi: 10.1016/j.anireprosci.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Miguel-Jimenez S., del Alamo M.M.R., Alvarez-Rodriguez M., Hidalgo C.O., Pena A.I., Muino R., Rodriguez-Gil J.E., Mogas T. In vitro assessment of egg yolk-, soya bean lecithin- and liposome-based extenders for cryopreservation of dairy bull semen. Anim. Reprod. Sci. 2020;215:106315. doi: 10.1016/j.anireprosci.2020.106315. [DOI] [PubMed] [Google Scholar]
- 17.Ugur M.R., Saber Abdelrahman A., Evans H.C., Gilmore A.A., Hitit M., Arifiantini R.I., Purwantara B., Kaya A., Memili E. Advances in Cryopreservation of Bull Sperm. Front. Vet. Sci. 2019;6:268. doi: 10.3389/fvets.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A., Prasad J., Srivastava N., Ghosh S.J.B. Strategies to minimize various stress-related freeze–thaw damages during conventional cryopreservation of mammalian spermatozoa. Biopreserv. Biobank. 2019;17:603–612. doi: 10.1089/bio.2019.0037. [DOI] [PubMed] [Google Scholar]
- 19.Grandhaye J., Partyka A., Ligocka Z., Dudek A., Nizanski W., Jeanpierre E., Estienne A., Froment P. Metformin Improves Quality of Post-Thaw Canine Semen. Animals. 2020;10:287. doi: 10.3390/ani10020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan J., Tahir M.Z., Khalid A., Sattar A., Ahmad N. Effect of cholesterol-loaded cyclodextrins on cryosurvival of dog spermatozoa. Reprod. Domest. Anim. 2017;52(Suppl. 2):265–268. doi: 10.1111/rda.12893. [DOI] [PubMed] [Google Scholar]
- 21.Belala R., Fatmi S., Kaidi R., Iguer-Ouada M. Benefits of cholesterol and α-tocopherol loaded cyclodextrins in dog semen cryopreservation. Revue. Méd. Vét. 2016;167:22–27. [Google Scholar]
- 22.Cakici C., Buyrukcu B., Duruksu G., Haliloglu A.H., Aksoy A., Isik A., Uludag O., Ustun H., Subasi C., Karaoz E. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: The sperm generation. Biomed. Res. Int. 2013;2013:529589. doi: 10.1155/2013/529589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrabani D., Hassanshahi M.A., Tamadon A., Zare S., Keshavarz S., Rahmanifar F., Dianatpour M., Khodabandeh Z., Jahromi I., Tanideh N., et al. Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats. J. Hum. Reprod. Sci. 2015;8:103–110. doi: 10.4103/0974-1208.158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan A.I., Alam S.S. Evaluation of mesenchymal stem cells in treatment of infertility in male rats. Stem Cell Res. Ther. 2014;5:131. doi: 10.1186/scrt521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stavely R., Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020 doi: 10.1002/sctm.19-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeSantiago J., Bare D.J., Banach K. Ischemia/Reperfusion injury protection by mesenchymal stem cell derived antioxidant capacity. Stem Cells Dev. 2013;22:2497–2507. doi: 10.1089/scd.2013.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y.S., Joo H.W., Park I.H., Shen G.Y., Lee Y., Shin J.H., Kim H., Kim K.S. Bone marrow mesenchymal stem cell-derived vascular endothelial growth factor attenuates cardiac apoptosis via regulation of cardiac miRNA-23a and miRNA-92a in a rat model of myocardial infarction. PLoS ONE. 2017;12:e0179972. doi: 10.1371/journal.pone.0179972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson S.M., Kalamegam G., Pushparaj P.N., Matta C., Memic A., Khademhosseini A., Mobasheri R., Poletti F.L., Hoyland J.A., Mobasheri A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69–80. doi: 10.1016/j.ymeth.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Park S.B., Seo M.S., Kim H.S., Kang K.S. Isolation and characterization of canine amniotic membrane-derived multipotent stem cells. PLoS ONE. 2012;7:e44693. doi: 10.1371/journal.pone.0044693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao P., Ise H., Hongo M., Ota M., Konishi I., Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79:528–535. doi: 10.1097/01.TP.0000149503.92433.39. [DOI] [PubMed] [Google Scholar]
- 31.Kim E.Y., Lee K.B., Kim M.K. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014;47:135–140. doi: 10.5483/BMBRep.2014.47.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borghesi J., Ferreira Lima M., Mario L.C., de Almeida da Anunciacao A.R., Silveira Rabelo A.C., Giancoli Kato Cano da Silva M., Assunpcao Fernandes F., Miglino M.A., Oliveira Carreira A.C., Oliveira Favaron P. Canine amniotic membrane mesenchymal stromal/stem cells: Isolation, characterization and differentiation. Tissue Cell. 2019;58:99–106. doi: 10.1016/j.tice.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Pall E., Pop R.A., Ciupe S., Cenariu M., Groza I.S. “Agriculture for Life, Life for Agriculture” Conference Proceedings. Sciendo; Warsaw, Poland: 2018. Canine Amniotic Membrane Derived Mesenchymal Stem Cells-Potential Sources for Regenerative Medicine; pp. 461–464. [Google Scholar]
- 34.Fauza D. Amniotic fluid and placental stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004;18:877–891. doi: 10.1016/j.bpobgyn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Kusuma G.D., Carthew J., Lim R., Frith J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017;26:617–631. doi: 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- 36.Kim W.S., Park B.S., Kim H.K., Park J.S., Kim K.J., Choi J.S., Chung S.J., Kim D.D., Sung J.H. Evidence supporting antioxidant action of adipose-derived stem cells: Protection of human dermal fibroblasts from oxidative stress. J. Dermatol. Sci. 2008;49:133–142. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Yuan Q.l., Zhang Y.g., Chen Q. Mesenchymal Stem Cell (MSC)-Derived Extracellular Vesicles: Potential Therapeutics as MSC Trophic Mediators in Regenerative Medicine. Anat. Rec. 2020;303:1735–1742. doi: 10.1002/ar.24186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green E.M., Lee R.T. Proteins and small molecules for cellular regenerative medicine. Physiol. Rev. 2013;93:311–325. doi: 10.1152/physrev.00005.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunawardena T.N.A., Rahman M.T., Abdullah B.J.J., Abu Kasim N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019;13:569–586. doi: 10.1002/term.2806. [DOI] [PubMed] [Google Scholar]
- 40.R Ra K., Oh H.J., Kim G.A., Kang S.K., Ra J.C., Lee B.C. High Frequency of Intravenous Injection of Human Adipose Stem Cell Conditioned Medium Improved Embryo Development of Mice in Advanced Maternal Age through Antioxidant Effects. Animals. 2020;10:978. doi: 10.3390/ani10060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y.X., Zeng Z.C., Sun J., Zeng H.Y., Huang Y., Zhang Z.Y. Mesenchymal stem cell-conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J. Radiat. Res. 2015;56:700–708. doi: 10.1093/jrr/rrv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi S., Shibata R., Yamamoto N., Nishikawa M., Hibi H., Tanigawa T., Ueda M., Murohara T., Yamamoto A. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci. Rep. 2015;5:16295. doi: 10.1038/srep16295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Santo S., Yang Z., von Ballmoos M.W., Voelzmann J., Diehm N., Baumgartner I., Kalka C. Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korkmaz-Icöz S., Li K., Loganathan S., Ding Q., Ruppert M., Radovits T., Brlecic P., Sayour A.A., Karck M., Szabó G. Brain-dead donor heart conservation with a preservation solution supplemented by a conditioned medium from mesenchymal stem cells improves graft contractility after transplantation. Am. J. Transplant. 2020;20:2847–2856. doi: 10.1111/ajt.15843. [DOI] [PubMed] [Google Scholar]
- 45.Ferro F., Spelat R., Shaw G., Duffy N., Islam M.N., O’Shea P.M., O’Toole D., Howard L., Murphy J. Survival/Adaptation of Bone Marrow-Derived Mesenchymal Stem Cells after Long-Term Starvation through Selective Processes. Stem Cells. 2019;37:813–827. doi: 10.1002/stem.2998. [DOI] [PubMed] [Google Scholar]
- 46.Ando Y., Matsubara K., Ishikawa J., Fujio M., Shohara R., Hibi H., Ueda M., Yamamoto A. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone. 2014;61:82–90. doi: 10.1016/j.bone.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 47.Qamar A.Y., Fang X., Kim M.J., Cho J. Improved Post-Thaw Quality of Canine Semen after Treatment with Exosomes from Conditioned Medium of Adipose-Derived Mesenchymal Stem Cells. Animals. 2019;9:865. doi: 10.3390/ani9110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdillah D.A., Setyawan E.M.N., Oh H.J., Ra K., Lee S.H., Kim M.J., Lee B.C. Iodixanol supplementation during sperm cryopreservation improves protamine level and reduces reactive oxygen species of canine sperm. J. Vet. Sci. 2019;20:79–86. doi: 10.4142/jvs.2019.20.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Setyawan E.M., Kim M.J., Oh H.J., Kim G.A., Jo Y.K., Lee S.H., Choi Y.B., Lee B.C. Maintaining canine sperm function and osmolyte content with multistep freezing protocol and different cryoprotective agents. Cryobiology. 2015;71:344–349. doi: 10.1016/j.cryobiol.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Brito L.F. Evaluation of stallion sperm morphology. Clin. Tech. Equine Pract. 2007;6:249–264. doi: 10.1053/j.ctep.2007.09.004. [DOI] [Google Scholar]
- 51.Sati L., Huszar G. Spermatogenesis. Springer; Berlin/Heidelberg, Germany: 2013. Methodology of Aniline Blue Staining of Chromatin and the Assessment of the Associated Nuclear and Cytoplasmic Attributes in Human Sperm; pp. 425–436. [DOI] [PubMed] [Google Scholar]
- 52.Pinto C.R., Kozink D.M. Simplified hypoosmotic swelling testing (HOST) of fresh and frozen-thawed canine spermatozoa. Anim. Reprod. Sci. 2008;104:450–455. doi: 10.1016/j.anireprosci.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Ren F., Fang Q., Feng T., Li Y., Wang Y., Zhu H., Hu J. Lycium barbarum and Laminaria japonica polysaccharides improve Cashmere goat sperm quality and fertility rate after cryopreservation. Theriogenology. 2019;129:29–36. doi: 10.1016/j.theriogenology.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Fraser L., Dziekonska A., Strzezek R., Strzezek J. Dialysis of boar semen prior to freezing-thawing: Its effects on post-thaw sperm characteristics. Theriogenology. 2007;67:994–1003. doi: 10.1016/j.theriogenology.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Petsche Connell J., Camci-Unal G., Khademhosseini A., Jacot J.G. Amniotic fluid-derived stem cells for cardiovascular tissue engineering applications. Tissue Eng. Part B Rev. 2013;19:368–379. doi: 10.1089/ten.teb.2012.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Oliveira Pinheiro A., Lara V.M., Souza A.F., Casals J.B., Bressan F.F., Fantinato Neto P., Oliveira V.C., Martins D.S., Ambrosio C.E. Characterization and Immunomodulation of Canine Amniotic Membrane Stem Cells. Stem Cells Cloning. 2020;13:43–55. doi: 10.2147/SCCAA.S237686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J., Zhao H.P., Lin G., Xie C.Q., Nie D.S., Wang Q.R., Lu G.X. In vitro hematopoietic differentiation of human embryonic stem cells induced by co-culture with human bone marrow stromal cells and low dose cytokines. Cell Biol. Int. 2005;29:654–661. doi: 10.1016/j.cellbi.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 58.Park K.S., Pang B., Park S.J., Lee Y.G., Bae J.Y., Park S., Kim I., Kim S.J. Identification and functional characterization of ion channels in CD34(+) hematopoietic stem cells from human peripheral blood. Mol. Cells. 2011;32:181–188. doi: 10.1007/s10059-011-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takemitsu H., Zhao D., Yamamoto I., Harada Y., Michishita M., Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet. Res. 2012;8:150. doi: 10.1186/1746-6148-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vieira N.M., Brandalise V., Zucconi E., Secco M., Strauss B.E., Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transpl. 2010;19:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- 61.Trindade Hill A., Therrien J., Garcia J., Smith L. Mesenchymal-like stem cells in canine ovary show high differentiation potential. Cell Proliferat. 2017;50:e12391. doi: 10.1111/cpr.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kern S., Eichler H., Stoeve J., Kluter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 63.Zeng G., Wang G., Guan F., Chang K., Jiao H., Gao W., Xi S., Yang B. Human amniotic membrane-derived mesenchymal stem cells labeled with superparamagnetic iron oxide nanoparticles: The effect on neuron-like differentiation in vitro. Mol. Cell Biochem. 2011;357:331–341. doi: 10.1007/s11010-011-0904-4. [DOI] [PubMed] [Google Scholar]
- 64.Greenow K., Clarke A.R. Controlling the stem cell compartment and regeneration in vivo: The role of pluripotency pathways. Physiol. Rev. 2012;92:75–99. doi: 10.1152/physrev.00040.2010. [DOI] [PubMed] [Google Scholar]
- 65.Han S.M., Han S.H., Coh Y.R., Jang G., Ra J.C., Kang S.K., Lee H.W., Youn H.Y. Enhanced proliferation and differentiation of Oct4-and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp. Mol. Med. 2014;46:e101. doi: 10.1038/emm.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu T.M., Wu Y.N., Guo X.M., Hui J.H., Lee E.H., Lim B. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev. 2009;18:1013–1022. doi: 10.1089/scd.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ansari M.M., Sreekumar T., Chandra V., Dubey P., Kumar G., Amarpal S. Therapeutic potential of canine bone marrow derived mesenchymal stem cells and its conditioned media in diabetic rat wound healing. J. Stem Cell Res. Ther. 2013;3:2. doi: 10.4172/2157-7633.1000141. [DOI] [Google Scholar]
- 68.Iravani K., Sobhanmanesh A., Ashraf M.J., Hashemi S.B., Mehrabani D., Zare S. The Healing Effect of Conditioned Media and Bone Marrow-Derived Stem Cells in Laryngotracheal Stenosis: A Comparison in Experimental Dog Model. World J. Plast. Surg. 2017;6:190–197. [PMC free article] [PubMed] [Google Scholar]
- 69.Nakamura M., Nishida H., Yoshizaki K., Akiyoshi H., Hatoya S., Sugiura K., Inaba T. Canine mesenchymal stromal cell-conditioned medium promotes survival and neurite outgrowth of neural stem cells. J. Vet. Med. Sci. 2020;82:668–672. doi: 10.1292/jvms.19-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baharvand H., Heidari M., Ebrahimi M., Valadbeigi T., Salekdeh G.H. Proteomic analysis of epithelium-denuded human amniotic membrane as a limbal stem cell niche. Mol. Vis. 2007;13:1711–1721. [PubMed] [Google Scholar]
- 71.Jha K.N., Shumilin I.A., Digilio L.C., Chertihin O., Zheng H., Schmitz G., Visconti P.E., Flickinger C.J., Minor W., Herr J.C. Biochemical and structural characterization of apolipoprotein AI binding protein, a novel phosphoprotein with a potential role in sperm capacitation. Endocrinology. 2008;149:2108–2120. doi: 10.1210/en.2007-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pham T., Kodvawala A., Hui D.Y. The receptor binding domain of apolipoprotein E is responsible for its antioxidant activity. Biochemistry. 2005;44:7577–7582. doi: 10.1021/bi0472696. [DOI] [PubMed] [Google Scholar]
- 73.Arner E.S.J., Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 74.Gasdaska J.R., Berggren M., Powis G. Cell growth stimulation by the redox protein thioredoxin occurs by a novel helper mechanism. Cell Growth Differ. 1995;6:1643–1650. [PubMed] [Google Scholar]
- 75.Kuribayashi Y., Gagnon C. Effect of catalase and thioredoxin addition to sperm incubation medium before in vitro fertilization on sperm capacity to support embryo development. Fertil. Steril. 1996;66:1012–1017. doi: 10.1016/S0015-0282(16)58699-3. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y., Foote R.H., Brockett C.C. Effect of sucrose, trehalose, hypotaurine, taurine, and blood serum on survival of frozen bull sperm. Cryobiology. 1993;30:423–431. doi: 10.1006/cryo.1993.1042. [DOI] [PubMed] [Google Scholar]
- 77.Uysal O., Bucak M.N. Effects of oxidized glutathione, bovine serum albumin, cysteine and lycopene on the quality of frozen-thawed ram semen. Acta Vet. Brno. 2007;76:383–390. doi: 10.2754/avb200776030383. [DOI] [Google Scholar]
- 78.Sarıözkan S., Türk G., Cantürk F., Yay A., Eken A., Akçay A. The effect of bovine serum albumin and fetal calf serum on sperm quality, DNA fragmentation and lipid peroxidation of the liquid stored rabbit semen. Cryobiology. 2013;67:1–6. doi: 10.1016/j.cryobiol.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Yeung C.H., Anapolski M., Setiawan I., Lang F., Cooper T.G. Effects of putative epididymal osmolytes on sperm volume regulation of fertile and infertile c-ros transgenic Mice. J. Androl. 2004;25:216–223. doi: 10.1002/j.1939-4640.2004.tb02781.x. [DOI] [PubMed] [Google Scholar]
- 80.Raad G., Lteif L., Lahoud R., Azoury J., Azoury J., Tanios J., Hazzouri M., Azoury J. Cryopreservation media differentially affect sperm motility, morphology and DNA integrity. Andrology. 2018;6:836–845. doi: 10.1111/andr.12531. [DOI] [PubMed] [Google Scholar]
- 81.Ball B.A. Oxidative stress, osmotic stress and apoptosis: Impacts on sperm function and preservation in the horse. Anim. Reprod. Sci. 2008;107:257–267. doi: 10.1016/j.anireprosci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 82.Martins A.D., Agarwal A., Henkel R. In Vitro Fertilization. Springer; Berlin/Heidelberg, Germany: 2019. Sperm Cryopreservation; pp. 625–642. [Google Scholar]
- 83.Koderle M., Aurich C., Schafer-Somi S. The influence of cryopreservation and seminal plasma on the chromatin structure of dog spermatozoa. Theriogenology. 2009;72:1215–1220. doi: 10.1016/j.theriogenology.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 84.Woolley D.M., Richardson D.W. Ultrastructural injury to human spermatozoa after freezing and thawing. J. Reprod. Fertil. 1978;53:389–394. doi: 10.1530/jrf.0.0530389. [DOI] [PubMed] [Google Scholar]
- 85.Ozkavukcu S., Erdemli E., Isik A., Oztuna D., Karahuseyinoglu S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J. Assist. Reprod. Genet. 2008;25:403–411. doi: 10.1007/s10815-008-9232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bernardini A., Hozbor F., Sanchez E., Fornes M.W., Alberio R.H., Cesari A. Conserved ram seminal plasma proteins bind to the sperm membrane and repair cryopreservation damage. Theriogenology. 2011;76:436–447. doi: 10.1016/j.theriogenology.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 87.Milardi D., Grande G., Vincenzoni F., Messana I., Pontecorvi A., De Marinis L., Castagnola M., Marana R. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil. Steril. 2012;97:67–73.e1. doi: 10.1016/j.fertnstert.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Gonzalez-Cadavid V., Martins J.A.M., Moreno F.B., Andrade T.S., Santos A.C.L., Monteiro-Moreira A.C.O., Moreira R.A., Moura A.A. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology. 2014;82:697–707. doi: 10.1016/j.theriogenology.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 89.Druart X., Rickard J.P., Mactier S., Kohnke P.L., Kershaw-Young C.M., Bathgate R., Gibb Z., Crossett B., Tsikis G., Labas V., et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteom. 2013;91:13–22. doi: 10.1016/j.jprot.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 90.Durairajanayagam D., Singh D., Agarwal A., Henkel R. Causes and consequences of sperm mitochondrial dysfunction. Andrologia. 2020:e13666. doi: 10.1111/and.13666. [DOI] [PubMed] [Google Scholar]
- 91.Pena A.I., Barrio F., Quintela L.A., Herradon P.G. Effect of different glycerol treatments on frozen-thawed dog sperm longevity and acrosomal integrity. Theriogenology. 1998;50:163–174. doi: 10.1016/S0093-691X(98)00122-8. [DOI] [PubMed] [Google Scholar]
- 92.Gungor S., Ata A., Inanc M.E., Kastelic J.P. Effect of various antioxidants and their combinations on bull semen cryopreservation. Turk. J. Vet. Anim. Sci. 2019;43:590–595. doi: 10.3906/vet-1907-39. [DOI] [Google Scholar]
- 93.Chai R.R., Chen G.W., Shi H.J., Wai-Sum O., Martin-DeLeon P.A., Chen H. Prohibitin involvement in the generation of mitochondrial superoxide at complex I in human sperm. J. Cell Mol. Med. 2017;21:121–129. doi: 10.1111/jcmm.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lenzi A., Picardo M., Gandini L., Dondero F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update. 1996;2:246–256. doi: 10.1093/humupd/2.3.246. [DOI] [PubMed] [Google Scholar]
- 95.Hossain M.S., Johannisson A., Wallgren M., Nagy S., Siqueira A.P., Rodriguez-Martinez H. Flow cytometry for the assessment of animal sperm integrity and functionality: State of the art. Asian J. Androl. 2011;13:406–419. doi: 10.1038/aja.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leahy T., Gadella B.M. New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J. Androl. 2015;17:561–567. doi: 10.4103/1008-682x.153309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saez F., Ouvrier A., Drevet J.R. Epididymis cholesterol homeostasis and sperm fertilizing ability. Asian J. Androl. 2011;13:11–17. doi: 10.1038/aja.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Argraves W.S., Morales C.R. Immunolocalization of cubilin, megalin, apolipoprotein J, and apolipoprotein A-I in the uterus and oviduct. Mol. Reprod. Dev. 2004;69:419–427. doi: 10.1002/mrd.20174. [DOI] [PubMed] [Google Scholar]
- 99.Bitterman P.B., Rennard S.I., Adelberg S., Crystal R.G. Role of Fibronectin as a Growth-Factor for Fibroblasts. J. Cell Biol. 1983;97:1925–1932. doi: 10.1083/jcb.97.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qamar A.Y., Fang X., Kim M.J., Cho J. Improved viability and fertility of frozen-thawed dog sperm using adipose-derived mesenchymal stem cells. Sci. Rep. UK. 2020;10:1–10. doi: 10.1038/s41598-020-61803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.