Abstract
Simple Summary
Data about the efficacy of trastuzumab emtansine (T-DM1) following pertuzumab treatment is limited due to the simultaneous development of the two drugs. Thus, the aim of this study was to investigate the efficacy of T-DM1 after previous treatment with pertuzumab in a large, real-world group of patients. We showed that the progression-free survival (PFS) in patients treated with T-DM1 after pertuzumab was 3.5 months. T-DM1 was mainly administered second-line after pertuzumab. The PFS in higher therapy lines appears to be shorter than in second-line ones. In summary, this study provides evidence that T-DM1 has clinically reasonable activity after prior pertuzumab treatment, with a median PFS period of approximately 3–4 months. It appears to be recommendable to administer T-DM1 in earlier therapeutic lines.
Abstract
The approval of trastuzumab emtansine (T-DM1) was conducted without pertuzumab as previous therapy. Efficacy data on T-DM1 following pertuzumab treatment are therefore limited. This study explores this issue in a real-world setting. Within the prospective PRAEGNANT (Prospective Academic Translational Research Network for the Optimization of the Oncological Health Care Quality in the Advanced Setting) metastatic breast cancer registry (NCT02338167), patients in all therapy lines receiving any kind of treatment were eligible for inclusion. This report describes patient characteristics and progression-free survival (PFS) in human epidermal growth factor receptor 2 (HER2)-positive patients receiving T-DM1 after pertuzumab treatment. Seventy-six patients were identified, 39 of whom received T-DM1 as second-line therapy, 25 as third-line, and 12 as fourth-line therapy or higher. Pertuzumab was mostly administered as a first-line treatment (n = 61; 80.3%). The median PFS in all patients was 3.5 months (95% CI: 2.8–7.8); in second-line treatment, 7.7 months (95% CI: 2.8–11.0); in third-line, 3.4 months (95% CI: 2.3–not reached (NR)); and in fourth-line therapy or higher, 2.7 months (95% CI: 1.2–NR). T-DM1 was mainly administered second-line after pertuzumab, but also in more heavily pretreated patients. The PFS in higher therapy lines appears to be shorter than in second-line.
Keywords: advanced breast cancer, metastatic, chemotherapy, HER2 c-erbB2, HER2/neu, trastuzumab, pertuzumab, T-DM1
1. Introduction
Anti-human epidermal growth factor receptor 2 (HER2) treatments have been integrated very successfully into the treatment of patients with HER2-positive breast cancer (BC), since the discovery that HER2 amplifications have a major impact on the prognosis in BC patients [1]. The monoclonal antibody trastuzumab was the first HER2-directed agent that was approved in the European Union in 2000 for the treatment of HER2-positive, advanced BC. Since then, additional HER2-directed agents, such as the monoclonal antibody pertuzumab and the dual epidermal growth factor receptor (EGFR)/HER2 tyrosine kinase inhibitor lapatinib, have been developed to overcome resistance to trastuzumab and provide additional treatment options [2,3,4,5,6,7,8,9,10,11]. These treatments have incrementally improved the clinical outcome for patients with early and metastatic disease [12,13,14,15,16,17].
On the basis of the results of the EMILIA trial [18], trastuzumab emtansine (T-DM1) was approved by the U.S. Food and Drug Administration (FDA) in February 2013 for HER2-positive metastatic BC. T-DM1 is an antibody-drug conjugate consisting of the monoclonal antibody trastuzumab linked to the cytotoxic agent emtansine (DM1), a microtubule polymerization inhibitor, and thus represents a new generation of cytotoxic drugs. The EMILIA trial assessed the efficacy and safety of T-DM1 in comparison with capecitabine plus lapatinib in 991 women with metastatic HER2-positive disease who had disease progression at or within 6 months after receiving trastuzumab. T-DM1 led to a better progression-free survival (PFS; median = 9.6 versus 6.4 months; p < 0.001) and overall survival (OS; median = 30.9 versus 25.1 months; p < 0.001) compared with capecitabine plus lapatinib [18,19]. Following the EMILIA trial, T-DM1 was approved as a second-line treatment for HER2-positive metastatic disease after treatment with trastuzumab and a taxane. The study was conducted before efficacy data for pertuzumab became available. After FDA approval of pertuzumab in June 2012, taxane-based chemotherapy plus trastuzumab and pertuzumab became the new standard first-line therapy for HER2-positive metastatic breast cancer (mBC). T-DM1 continued to be the standard second-line treatment, but without much evidence for its efficacy after the use of pertuzumab.
The MARIANNE trial assessed T-DM1 with or without pertuzumab versus trastuzumab plus taxane as a first-line treatment for HER2-positive metastatic disease in 1095 patients. Neither T-DM1 alone nor T-DM1 in combination with pertuzumab improved the PFS in comparison with trastuzumab plus a taxane [20]. The clinical interpretation of this is unclear. However, preclinical data suggest that the treatment sequence has a strong effect on therapeutic efficacy in HER2-positive BC cells [21].
In addition, other novel substances are being developed for the treatment of HER2-positive BC patients. Neratinib, a tyrosine kinase inhibitor, has recently been approved for the extended adjuvant treatment of patients with HER2-positive early BC, due to its significant improvement of five-year disease-free survival (DFS) [22]; it was approved in the United States for the treatment of metastastic breast cancer as well, based on an improvement of PFS and time to intervention for the involvement of the central nervous system [23]. Margetuximab, a novel HER2 antibody, appears to enhance antibody-dependent, cell-mediated cytotoxicity (ADCC), while being well-tolerated [24]. Its efficacy and safety are currently being investigated in the phase 3 SOPHIA trial in patients with HER2-positive mBC who have previously been treated with trastuzumab, pertuzumab, and T-DM1 [21]. Two other novel substances (tucatinib and trastuzumab-deruxtecan) have also shown very promising activity in patients with heavily pretreated, HER2-positive, advanced breast cancer [25,26], and have been approved in the United States. Trastuzumab–deruxtecan showed in a large, one-arm, early phase study a PFS of 16.4 months (95% CI: 12.7–not reached) [26]. Tucatinib, in combination with trastuzumab and capecitabine, showed an improvement of PFS (+2.2 months) and OS (+4.5 months) compared to trastuzumab and capecitabine alone [25]. This effect has been seen also in patients with brain metastases [27].
Most recent trials are assessing new HER2-directed agents in patients who have previously received treatment with pertuzumab and T-DM1 [28]. However, efficacy data for T-DM1 after pertuzumab are not available from EMILIA or other trials, due to the nature of the simultaneous development of the two drugs. Recently, a few retrospective studies have investigated the efficacy of T-DM1 after pertuzumab treatment, but the sample sizes were mainly small [29,30,31].
The aim of the present study was therefore to further investigate the efficacy of T-DM1 after previous treatment with pertuzumab in a larger, real-world group of patients.
2. Results
2.1. Patient Characteristics
The patient characteristics are summarized in Table 1. A total of 76 patients with a mean age of 54.6 years (±10.8 y) were identified who could be followed until progression or death. Most patients were treated with T-DM1 as second-line treatment (n = 39; 51.3%). However, 12 patients (15.8%) were treated with T-DM1 in the fourth therapeutic line or later. Most patients (n = 62; 92.5%) had an Eastern Cooperative Oncology Group (ECOG) status of 0 or 1 at the start of T-DM1 therapy, and the most frequent metastatic pattern was visceral (n = 48; 64.9%).
Table 1.
Patient characteristics.
| Characteristic | n | % |
|---|---|---|
| Age (years) | 54.6 ± 10.8 | - |
| BMI (kg/m2) | 26.1 ± 5.7 | - |
| HER2-positive | 76 | 100.0 |
| Hormone receptor status | - | - |
| Negative | 23 | 31.5 |
| Positive | 50 | 68.5 |
| Missing | 3 | - |
| M-stage at diagnosis | - | - |
| cM0 | 44 | 57.9 |
| cM1 | 32 | 42.1 |
| Metastasis pattern | - | - |
| Brain | 17 | 23.0 |
| Visceral | 48 | 64.9 |
| Bone | 3 | 4.1 |
| Other | 6 | 8.1 |
| Missing | 2 | - |
| ECOG status | - | - |
| 0 | 37 | 55.2 |
| 1 | 25 | 37.3 |
| 2 | 3 | 4.5 |
| 3 | 2 | 3.0 |
| Missing | 9 | - |
| Lowest line of pertuzumab | - | - |
| 1 | 61 | 80.3 |
| 2 | 9 | 11.8 |
| 3 | 4 | 5.3 |
| 4+ | 2 | 2.6 |
| Lowest line of T-DM1 | - | - |
| 2 | 39 | 51.3 |
| 3 | 25 | 32.9 |
| 4+ | 12 | 15.8 |
BMI: body mass index; ECOG: Eastern Cooperative Oncology Group; T-DM1: trastuzumab emtansine; HER2: human epidermal growth factor receptor 2.
2.2. Progression-Free Survival
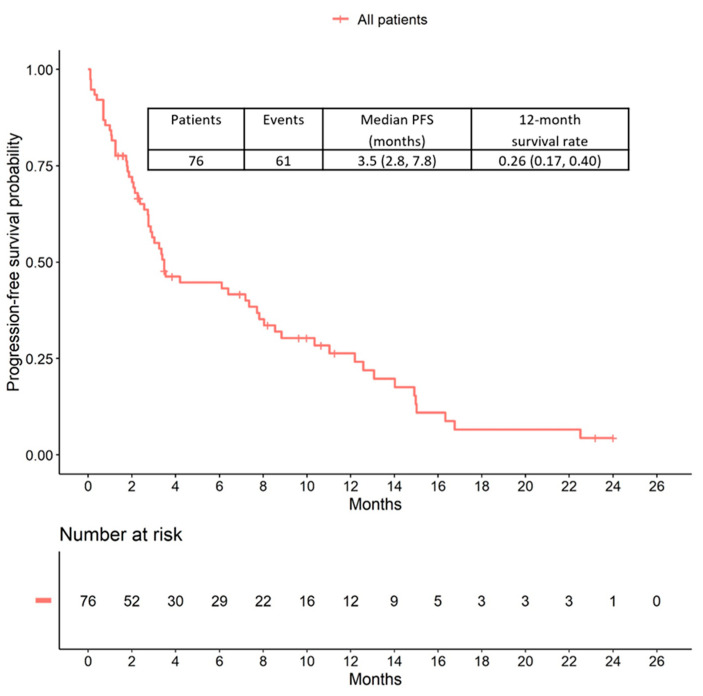
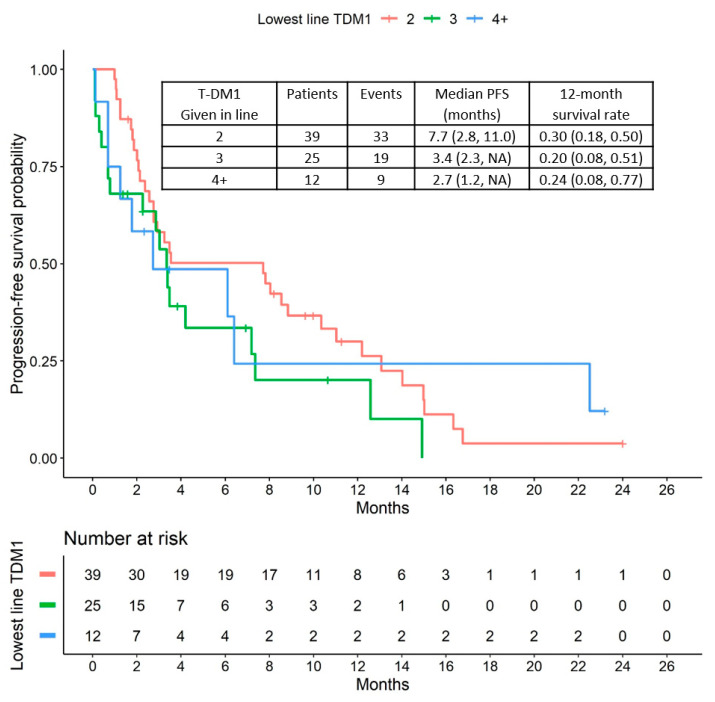
During the median follow-up period of 3.1 months (interquartile range: 1.6–8.6 months), 61 patients presented with disease progression and 30 patients died. The median progression-free survival (PFS) time was 3.5 months (95% CI: 2.8 to 7.8). The corresponding Kaplan-Meier curve is shown in Figure 1. Patients who received T-DM1 as a second-line treatment appear to have a slightly longer PFS (median PFS = 7.7 months; 95% CI: 2.8 to 11.0) than more heavily pretreated patients (third-line = 3.4 months; 95% CI: 2.3 to upper limit not reached; fourth line and higher = 2.7 months; 95% CI: 1.2 to upper limit not reached) (Figure 2).
Figure 1.
Progression-free survival (PFS) during treatment with T-DM1.
Figure 2.
PFS relative to T-DM1 treatment lines.
2.3. Overall Survival
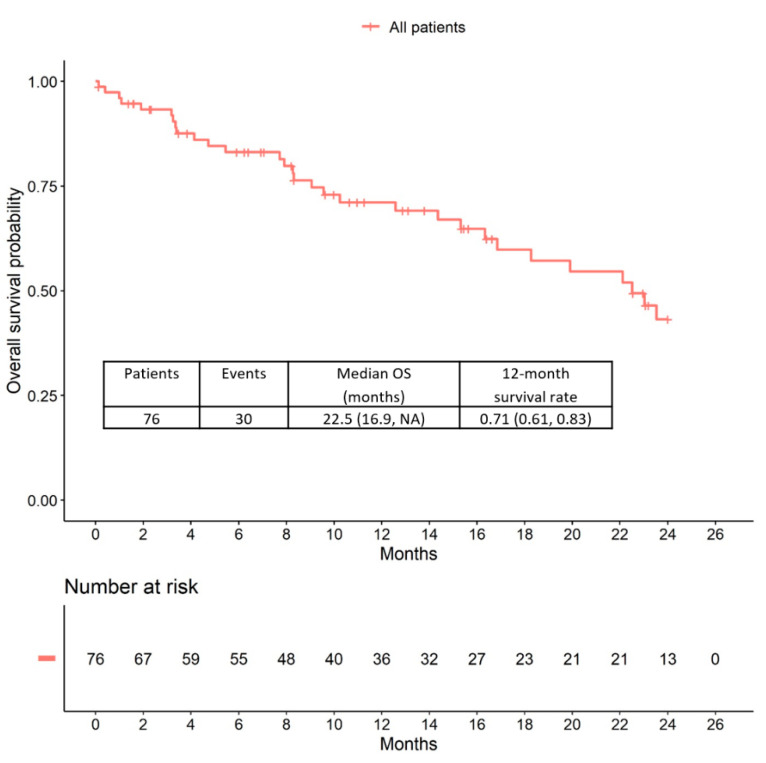
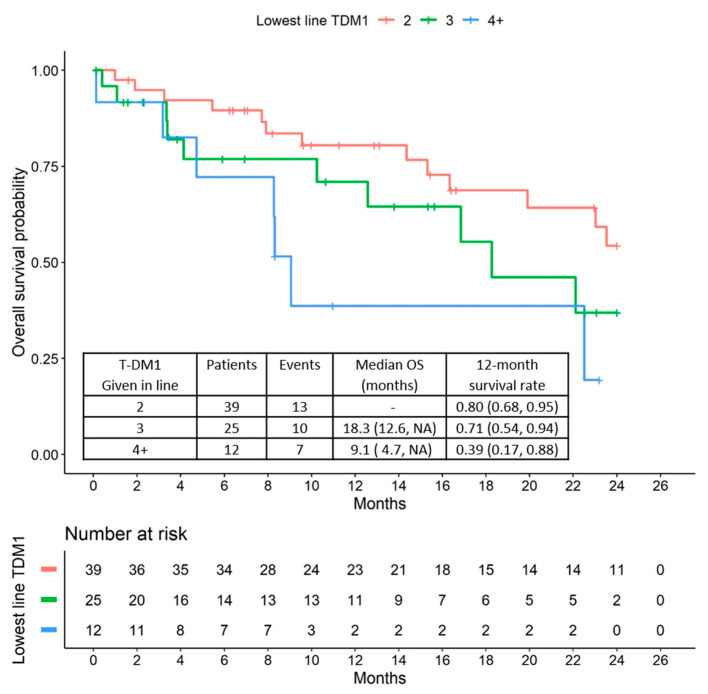
The median overall survival (OS) was 22.5 months (95% CI: 16.9 to upper limit not reached). Examination of the Kaplan-Meier curves shows that patients with higher therapy lines also appeared to have a poorer prognosis than patients with T-DM1 treatment in earlier therapy lines (Figure 3 and Figure 4).
Figure 3.
Overall survival (OS) in all patients receiving T-DM1 therapy.
Figure 4.
OS relative to T-DM1 treatment line.
3. Discussion
Since the approval of T-DM1 and pertuzumab, treatment with T-DM1 has been the standard of care after failure of chemotherapy plus trastuzumab/pertuzumab. However, there is a lack of large trials assessing the efficacy of sequential administration, due to the nature of the simultaneous development of the two drugs. This study assessed the efficacy of T-DM1 in a population of patients who had previously received pertuzumab for HER2-positive mBC. The median PFS was 3.5 months in the overall cohort. The PFS appeared to be shorter when T-DM1 was given in later therapeutic lines.
The efficacy data obtained in this group of patients are consistent with previously published reports. Dzimitrowicz et al. analyzed data from 78 patients who received T-DM1 after prior pertuzumab. The median period of therapy was 4.0 months, with one-third of the patients receiving treatment with T-DM1 for >6 months. T-DM1 was discontinued due to disease progression in 84% of the patients, or due to toxicity in 10%. The study did not show an increase in the median overall survival, but Kaplan-Meier estimates of overall survival did result in numbers similar to those in the present study [31].
A retrospective study in Italy included 250 patients who were treated with T-DM1. Forty-seven patients had previously received pertuzumab [30]. The patient characteristics were similar to those in the present study, and the median PFS in patients with prior pertuzumab was 4 months. The median OS was reported to be 17 months [30]. No differences in the efficacy of T-DM1 treatment were observed between patients with and without prior pertuzumab, although due to small patient numbers, the study might not have had the power to draw firm conclusions.
Data for patients treated with T-DM1 after pertuzumab in the CLEOPATRA and PHEREXA studies have been analyzed [32]. The period on T-DM1 therapy was 7.1 months in 32 patients after prior pertuzumab in the CLEOPATRA trial, and 4.2 months in 43 patients after prior pertuzumab in the PHEREXA study [32]. Median overall survival times were not reached after 2 years [32].
The largest study reporting on the PFS after pertuzumab is the PERNETTA study [29]. Patients received either dual blockage with trastuzumab/pertuzumab alone or in combination with chemotherapy (paclitaxel or vinorelbine). Patients in both study arms received T-DM1 as second-line treatment after disease progression. The PFS for a total of 101 of these patients was recently reported. The median PFS was 7.1 months (95% CI: 4.3 to 11.3) for patients with prior pertuzumab + trastuzumab (n = 59), and 5.3 months (95% CI: 4.0 to 10.3) for patients previously treated with chemotherapy and trastuzumab + pertuzumab (n = 42) [29].
The present study now adds additional evidence consistent with the previously published data and increasing the total number of patients with reported efficacy data for T-DM1 after prior pertuzumab to 276.
As previously reported in other studies, the PFS times observed in this study were shorter for patients receiving treatment in higher therapeutic lines. Although this is a general prognostic pattern in patients with advanced BC, it might be beneficial to administer T-DM1 in earlier lines rather than in later ones.
In comparison with the EMILIA trial, with a median PFS of 9.3 months, the PFS in retrospective studies was shorter after pertuzumab treatment; however, caution is warranted when interpreting these results. It has been shown that treatment effects are usually smaller in retrospective or prospective real-world studies. Particularly in BC studies, the effects observed in real-world data may be lower than in those in randomized controlled trials (RCTs). A systematic comparison of the treatment effects in 21 oncological RCTs and the corresponding effects in real-world datasets shows that the real-world treatment benefit was 16% lower than that observed in the RCTs with surrogate end points, such as PFS [33]. The effects in relation to OS appeared to be similar when RCTs were compared with real-world data. However, in the four RCTs examining BC patients, RCTs appeared to overestimate the effect by up to 46.5% (95% CI: 19.5% to 79.8%).
On the other hand, the possibility cannot be excluded that T-DM1 is less effective after treatment with pertuzumab. The half-life of pertuzumab is 18 days, and it cannot be ruled out that relevant concentrations of pertuzumab may still be active if the pertuzumab → T-DM1 sequence is administered in a timely fashion. In a neoadjuvant study, the combination of pertuzumab and T-DM1 was associated with lower pCR rates and a lower event-free survival rate in comparison with chemotherapy, trastuzumab, and pertuzumab [34,35]. The sample size in the present study is too small for an analysis of subgroups of patients who received T-DM1 immediately after pertuzumab, or with longer time intervals between the two therapies.
Since T-DM1 is nowadays mostly administered after progression in patients who are receiving chemotherapy plus dual blockage for HER2-positive mBC, there is a strong clinical need to further evaluate its therapeutic efficacy in this setting.
The main limitation of this study is the small sample size of 76 patients. Therefore, for example, the associations between prognosis and therapy line are only explorative, as they were not adjusted for commonly-established confounders. Nevertheless, since T-DM1 after prior pertuzumab is a relatively new standard of care, larger real-world studies may be expected in the near future.
4. Materials and Methods
4.1. The PRAEGNANT Research Network
The PRAEGNANT study (Prospective Academic Translational Research Network for the Optimization of the Oncological Health Care Quality in the Adjuvant and Advanced/Metastatic Setting; NCT02338167 [36]) is an ongoing, prospective BC registry with a documentation system similar to that of a clinical trial. The aims of PRAEGNANT are to assess treatment patterns and quality of life, and to identify patients who may be eligible for clinical trials or specific targeted treatments [36,37,38,39]. Patients can be included at any time point during the course of their disease. All of the patients included in the present study provided informed consent, and the study was approved by the relevant ethics committees (ethics approval number: 234/2014BO1, first approval on June 17, 2014; approval of Amendment 1 on June 11, 2015; approval of Amendment 2 on March 18, 2019; Ethical Committee of the Medical Faculty, University of Tübingen, Tübingen, Germany).
4.2. Patients
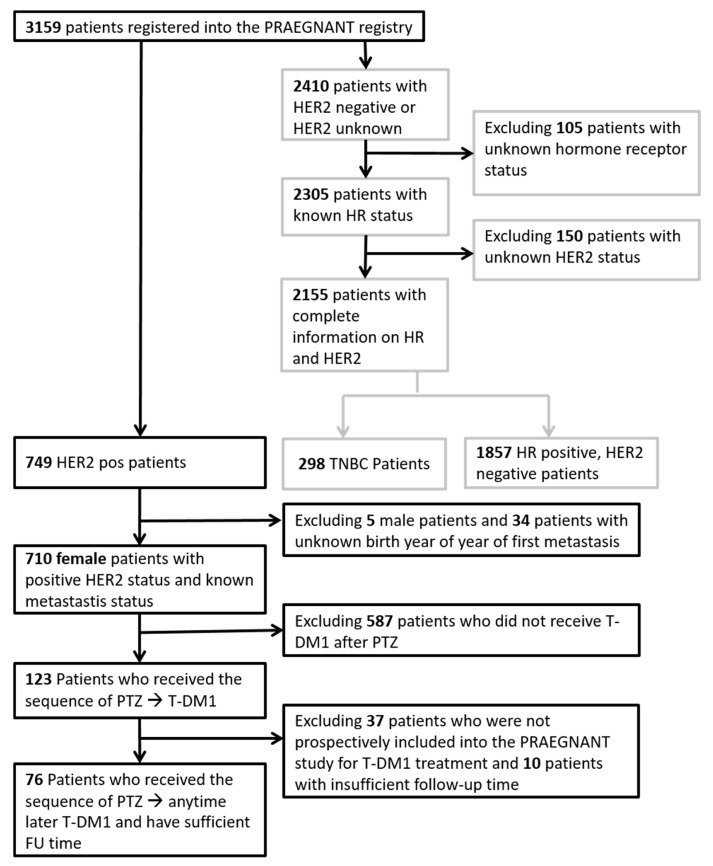
At the time of database closure (June 2019), a total of 3159 patients were registered in the PRAEGNANT registry. Among them, 710 had a known positive HER2 status and documented therapies with follow-up information. A total of 587 patients had to be excluded because they did not receive T-DM1 after pertuzumab for advanced breast cancer, with T-DM1 administered prospectively in the PRAEGNANT registry. Another 47 patients had to be excluded because there was no follow-up available for them. This resulted in 76 patients who were followed up prospectively in this study, and received T-DM1 after treatment with pertuzumab. The patient flow chart is shown in Figure 5.
Figure 5.
Flow chart. Black fields and arrows represent HER2 positive patient selection; grey fields and arrows represent HER2 negative or unknown patient selection; HER2: human epidermal growth factor receptor 2; HR: hormone receptor; pos: positive; TNBC: triple negative breast cancer; PTZ: pertuzumab; T-DM1: trastuzumab emtansine; FU time: follow-up time.
4.3. Data Collection
Data were collected by trained staff and documented in an electronic case report form [36]. Data were monitored using automated plausibility checks and on-site monitoring. Data that are not usually documented as part of routine clinical work are collected prospectively using structured questionnaires completed on paper. These consist of epidemiological data, such as family history, cancer risk factors, quality of life, nutrition and lifestyle items, and psychological health. Supplementary Table S1 provides an overview of the data collected.
4.4. Definition of Hormone Receptors, HER2 Status, Grading, and Metastatic Patterns
The definition of hormone receptor status, HER2 status, and grading has been described previously [37]. Briefly, if a biomarker assessment of the metastatic site was available, this receptor status was used for the analysis. If there was no information for metastases, the latest biomarker results from the primary tumor were used. Additionally, all patients who received estrogen therapy in the metastatic setting were assumed to be hormone receptor-positive, and all patients who had ever received anti-HER2 therapy were assumed to be HER2-positive. There was no central review of biomarkers. The study protocol recommended assessing estrogen receptor and progesterone receptor status as positive if ≥1% was stained. A positive HER2 status required an immunohistochemistry score of 3+ or positive fluorescence during in situ hybridization/competitive in situ hybridization (FISH/CISH). The site of metastasis was classified into four discrete categories in the following hierarchical order—brain (additional locations allowed), viscera (additional locations except brain allowed), bone only, and other—based on the presence or absence of tumors at these locations.
4.5. Statistical Considerations
Progression-free survival (PFS) was defined as the period from the start of therapy to the earliest date of disease progression (distant metastasis, local recurrence, or death of any cause) or the last date on which the patient was known to be progression-free. It was censored at 2 years. Overall survival was defined as the time interval between the first dose of T-DM1 and death.
Survival rates with 95% confidence intervals (CIs) and median survival time were estimated for all patients and for patient subgroups defined by the T-DM1 therapy line, using the Kaplan-Meier product limit method. The 95% CIs for median survival time were computed using the Brookmeyer and Crowley method [40].
All tests were two-sided, and a p value <0.05 was regarded as statistically significant. Calculations were carried out using the R system for statistical computing (version 3.6.1; R Development Core Team, Vienna, Austria, 2019).
5. Conclusions
In summary, this study adds evidence that T-DM1 has clinically reasonable activity after prior pertuzumab, with a median PFS period of approximately 3–4 months. It appears to be recommendable to administer T-DM1 in earlier therapeutic lines. A combined meta-analysis of the available studies could help substantiate experience with this therapeutic sequence.
Acknowledgments
The PRAEGNANT network is supported by grants from Pfizer, Hexal, Celgene, Daiichi-Sankyo, Roche, Merrimack, Eisai, AstraZeneca, and Novartis. These companies did not have any involvement in the study design, in the collection, analysis, or interpretation of the data, in the writing of the report, or in the decision to submit this article for publication. The authors also acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding program Open Access Publishing.
Abbreviations
| ADCC | Antibody-dependent, cell-mediated cytotoxicity |
| BC | Breast cancer |
| CI | Confidence interval |
| CISH | Competitive in Situ Hybridization |
| DFS | Disease-free survival |
| ECOG | Eastern Cooperative Oncology Group |
| EGFR | Epidermal growth factor receptor |
| FDA | U.S. Food and Drug Administration |
| FISH | Fluorescence in Situ Hybridization |
| mBC | Metastatic breast cancer |
| OS | Overall survival |
| PFS | Progression-free survival |
| RCT | Randomized controlled trial |
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/10/3021/s1, Supplementary Table S1: Data categories recorded in the PRAEGNANT study.
Author Contributions
Conceptualization, P.A.F., A.S., and T.N.F.; methodology, L.L.M., A.D.H., and L.H.; formal analysis, P.A.F. and L.H. ; investigation and resources, L.L.M., A.D.H., H.-C.K., P.H., H.H., H.T., J.E., D.L., M.W., V.M., M.W.B., P.W., C.H., C.M., C.K., R.W., M.U., F.O., J.H., W.J., F.-A.T., M.P.L., D.W., S.Y.B., A.S., and T.N.F.; data curation, E.B. and B.V.; writing—original draft preparation, P.A.F, E.B., H.H., T.N.F., and L.L.M.; writing—review and editing, all authors; visualization, L.H.; supervision, P.A.F and T.N.F.; project administration, E.B.; funding acquisition, all authors. All authors have read, revised, and agreed to the published version of the manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the interpretation of data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
E.B. has received honoraria from Novartis, Celgene, EISAI, Daiichi Sankyo, Merrimack, AstraZeneca, Riemser, Pfizer, Hexal, Amgen, and onkowissen.de for consulting, clinical research management, or medical education activities. J.E. has received honoraria from Roche, Celgene, Novartis, Pfizer, Lilly, Pierre Fabre, Teva, and Tesaro, as well as travel support from Celgene, Novartis, Lilly, Pfizer, Teva, and Tesaro. P.A.F. has received honoraria from Roche, Pfizer, Novartis, and Celgene; his institution conducts research for Novartis. A.D.H. has received honoraria from Teva, Genomic Health, Celgene, AstraZeneca, Novartis, Pfizer, and Roche. C.H. has received honoraria from Amgen, Celgene, Oncovis, Roche, and Pfizer. J.H. has received honoraria from Novartis, Roche, Celgene, Teva, and Pfizer, as well as travel support from Roche, Celgene, and Pfizer. C.K. has received honoraria from Amgen, Roche, Teva, Novartis, MSD, Axios, and Riemser. H.C.K. has received honoraria from Carl Zeiss meditec, Teva, Theraclion, Novartis, Amgen, AstraZeneca, Pfizer, Janssen-Cilag, GSK, LIV Pharma, Roche, MSD, SurgVision, Onkowissen, and Genomic Health. M.P.L. has received honoraria from Lilly, Pfizer, Roche, MSD, Hexal, Novartis, AstraZeneca, Eisai, and medac for advisory boards, lectures, and travel support. V.M. has received speaker honoraria from Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Pfizer, Pierre-Fabre, Novartis, Roche, Teva, and Janssen–Cilag, and consultancy honoraria from Genomic Health, Roche, Pierre Fabre, Amgen, Daiichi-Sankyo, and Eisai. F.O. has received speaker and consultancy honoraria from Amgen, AstraZeneca, Bayer, BMS, Boehringer, Celgene, Chugai, Eisai, Gilead, Hexal, Ipsen, Janssen, Merck, MSD, Novartis, Novonordisc, Riemser, Roche, Servier, Shire, Tesaro, and Teva. P.H. has received honoraria, unrestricted educational grants, and research funding from Amgen, AstraZeneca, Eli Lilly, MSD, Novartis, Pfizer, and Roche. A.S. has received honoraria from Roche, Celgene, AstraZeneca, Novartis, Pfizer, Zuckschwerdt Verlag GmbH, Georg Thieme Verlag, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH, and promedicis GmbH. H.T. has received honoraria from Novartis, Roche, Celgene, Teva, and Pfizer, as well as travel support from Roche, Celgene, and Pfizer. M.W. received speaker honoraria from AstraZeneca, Celgene, Roche, MSD and Novartis. R.W. has received honoraria from Roche, Celgene, Novartis, Pfizer, Teva, MSD, Eisai, Genomic Health, Agendia, Prosigna, Amgen, Pierre Fabre, and AstraZeneca. F.A.T. has received honoraria from AstraZeneca, Genomic Health, Novartis, Roche, Tesaro, and Teva, as well as travel support from Novartis and Tesaro. P.W. has received honoraria from Roche, Novartis, Amgen, AstraZeneca, Pfizer, MSD, Clovis, Tesaro, Celgene, Teva, Eisai, and Eli Lilly. D.L. received honoraria from Amgen, Loreal, Pfizer, Novartis, Eli Lilly, Samsung, Celgene, Astra Zeneca, Teva and GSK. All remaining authors have declared that they have no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Geyer C.E., Forster J., Lindquist D., Chan S., Gilles Romieu C., Pienkowski T., Jagiello-Gruszfeld A., Crown J., Chan A., Kaufman B., et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 3.Cameron D., Casey M., Press M., Lindquist D., Pienkowski T., Gilles Romieu C., Chan S., Jagiello-Gruszfeld A., Kaufman B., Crown J., et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 4.Schneeweiss A., Chia S., Hickish T., Harvey V., Eniu A., Hegg R., Tausch C., Seo J.H., Tsai Y.-F., Ratnayake J., et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann. Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 5.Schneeweiss A., Chia S., Hickish T., Harvey V., Eniu A., Waldron-Lynch M., Eng-Wong J., Kirk S., Cortés J. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur. J. Cancer. 2018;89:27–35. doi: 10.1016/j.ejca.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Gianni L., Pienkowski T., Im Y.-H., Roman L., Tseng L.M., Liu M.C., Lluch A., Staroslawska E., De la Haba-Rodriguez J., Im S.A., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 7.Gianni L., Pienkowski T., Im Y.-H., Tseng L.M., Liu M.C., Lluch A., Starosławska E., De la Haba-Rodriguez J., Im S.A., Luiz Pedrini J., et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 8.Von Minckwitz G., Procter M., De Azambuja E., Zardavas D., Benyunes M., Viale G., Suter T., Arahmani A., Rouchet N., Clark E., et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., Fleming T., Eiermann W., Wolter J., Pegram M., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Welslau M., Hartkopf A.D., Muller V., Wöckel A., Lux M.P., Janni W., Ettl J., Lüftner D., Belleville E., Schutz F., et al. Update Breast Cancer 2019 Part 5—Diagnostic and Therapeutic Challenges of New, Personalised Therapies in Patients with Advanced Breast Cancer. Geburtshilfe Frauenheilkd. 2019;79:1090–1099. doi: 10.1055/a-1001-9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schutz F., Fasching P.A., Welslau M., Hartkopf A.D., Wöckel A., Lux M.P., Janni W., Ettl J., Lüftner D., Belleville E., et al. Update Breast Cancer 2019 Part 4—Diagnostic and Therapeutic Challenges of New, Personalised Therapies for Patients with Early Breast Cancer. Geburtshilfe Frauenheilkd. 2019;79:1079–1089. doi: 10.1055/a-1001-9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneeweiss A., Lux M.P., Janni W., Hartkopf A.D., Nabieva N., Taran F.A., Overkamp F., Kolberg H.C., Hadji P., Tesch H., et al. Update Breast Cancer 2018 (Part 2)—Advanced Breast Cancer, Quality of Life and Prevention. Geburtshilfe Frauenheilkd. 2018;78:246–259. doi: 10.1055/s-0044-101614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taran F.A., Schneeweiss A., Lux M.P., Janni W., Hartkopf A.D., Nabieva N., Overkamp F., Kolberg H.C., Hadji P., Tesch H., et al. Update Breast Cancer 2018 (Part 1)—Primary Breast Cancer and Biomarkers. Geburtshilfe Frauenheilkd. 2018;78:237–245. doi: 10.1055/s-0044-101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lux M.P., Janni W., Hartkopf A.D., Taran F.A., Overkamp F., Kolberg H.C., Hadji P., Tesch H., Ettl J., Huober J.B., et al. Update Breast Cancer 2017—Implementation of Novel Therapies. Geburtshilfe Frauenheilkd. 2017;77:1281–1290. doi: 10.1055/s-0043-122885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Untch M., Huober J., Jackisch C., Schneeweiss A., Brucker S.Y., Dall P., Denkert C., Fasching P.A., Fehm T., Gerber B., et al. Initial Treatment of Patients with Primary Breast Cancer: Evidence, Controversies, Consensus: Spectrum of Opinion of German Specialists at the 15th International St. Gallen Breast Cancer Conference (Vienna 2017) Geburtshilfe Frauenheilkd. 2017;77:633–644. doi: 10.1055/s-0043-111601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swain S.M., Kim S.B., Cortes J., Ro J., Semiglazov V., Campone M., Ciruelos E., Ferrero J.M., Schneeweiss A., Knott A., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swain S.M., Baselga J., Kim S.B., Ro J., Semiglazov V., Campone M., Ciruelos E., Ferrero J.M., Schneeweiss A., Heeson S., et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J., Pegram M., Oh D.Y., Diéras V., Guardino E., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieras V., Miles D., Verma S., Pegram M., Welslau M., Baselga J., Krop I.E., Blackwell K., Hoersch S., Xu J., et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): A descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez E.A., Barrios C., Eiermann W., Toi M., Im Y.-H., Conte P., Martin M., Pienkowski T., Pivot X., Burris H. 3rd.; et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J. Clin. Oncol. 2017;35:141–148. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 22.Deeks E.D. Neratinib: First Global Approval. Drugs. 2017;77:1695–1704. doi: 10.1007/s40265-017-0811-4. [DOI] [PubMed] [Google Scholar]
- 23.Saura C., Oliveira M., Feng Y.H., Dai M.S., Chen S.W., Hurvitz S.A., Kim S.B., Moy B., Delaloge S., Gradishar W., et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With >/= 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020;38:3138–3149. doi: 10.1200/JCO.20.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang Y.J., Giaccone G., Im S.A., Oh D.Y., Bauer T.M., Nordstrom J.L., Li H., Chichili G.R., Moore P.A., Hong S., et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 2017;28:855–861. doi: 10.1093/annonc/mdx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy R.K., Loi S., Okines A., Paplomata E., Hamilton E., Hurvitz S.A., Lin N.U., Borges V., Abramson V., Anders C., et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020;13:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 26.Modi S., Saura C., Yamashita T., Park Y.H., Kim S.B., Tamura K., Andre F., Iwata H., Ito Y., Tsurutani J., et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020;13:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin N.U., Murthy R.K., Anders C.K., Murthy R.K., Paplomata E., Hamilton E., Hurvitz S., Loi S., Okines A., Abramson V., et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with previously treated HER2+ metastatic breast cancer with brain metastases (HER2CLIMB) J. Clin. Oncol. 2020;38:1005. doi: 10.1200/JCO.2020.38.15_suppl.1005. [DOI] [Google Scholar]
- 28.Lux M.P., Nabieva N., Hartkopf A.D., Huober J., Volz B., Taran F.A., Overkamp F., Kolberg H.C., Hadji P., Tesch H., et al. Therapy Landscape in Patients with Metastatic HER2-Positive Breast Cancer: Data from the PRAEGNANT Real-World Breast Cancer Registry. Cancers. 2018;11:10. doi: 10.3390/cancers11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huober J., Weder P., Veyret C., Thürlimann B., Xyrafas A., Vanlemmens L., Guiu S., Brain E., Grenier J., Dalenc F., et al. PERNETTA – A non comparative randomized open label phase II trial of pertuzumab (P) + trastuzumab (T) with or without chemotherapy both followed by T-DM1 in case of progression, in patients with HER2 positive metastatic breast cancer (MBC): (SAKK 22/10 / UNICANCER UC-0140/1207) Ann. Oncol. 2018:29. doi: 10.1093/annonc/mdy272. [DOI] [Google Scholar]
- 30.Vici P., Pizzuti L., Michelotti A., Sperduti I., Natoli C., Mentuccia L., Di Lauro L., Sergi D., Marchetti P., Santini D., et al. A retrospective multicentric observational study of trastuzumab emtansine in HER2 positive metastatic breast cancer: A real-world experience. Oncotarget. 2017;8:56921–56931. doi: 10.18632/oncotarget.18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzimitrowicz H., Berger M., Vargo C., Hood A., Abdelghany O., Raghavendra A.S., Tripathy D., Valero V., Hatzis C., Pusztai L., et al. T-DM1 Activity in Metastatic Human Epidermal Growth Factor Receptor 2-Positive Breast Cancers That Received Prior Therapy With Trastuzumab and Pertuzumab. J. Clin. Oncol. 2016;34:3511–3517. doi: 10.1200/JCO.2016.67.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urruticoechea A., Im S.A., Munoz M., Baselga J., Yardley D.A., Heeson S., Jones S., Knott A., Douthwaite H., Badovinac Crnjevic T., et al. Efficacy of trastuzumab emtansine (T-DM1) in patients (pts) with HER2+metastatic breast cancer (MBC) previously treated with pertuzumab (P) J. Clin. Oncol. 2017;35:1023. doi: 10.1200/JCO.2017.35.15_suppl.1023. [DOI] [Google Scholar]
- 33.Lakdawalla D.N., Shafrin J., Hou N., Peneva D., Vine S., Park J., Zhang J., Brookmeyer R., Figlin R.A. Predicting Real-World Effectiveness of Cancer Therapies Using Overall Survival and Progression-Free Survival from Clinical Trials: Empirical Evidence for the ASCO Value Framework. Value Health. 2017;20:866–875. doi: 10.1016/j.jval.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Hurvitz S.A., Martin M., Symmans W.F., Jung K.H., Huangm C.S., Thompson A.M., Harbeck N., Valero V., Stroyakovskiy D., Wildiers H., et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115–126. doi: 10.1016/S1470-2045(17)30716-7. [DOI] [PubMed] [Google Scholar]
- 35.Hurvitz S.A., Martin M., Jung K.H., Huang C.S., Harbeck N., Valero V., Stroyakovskiy D., Wildiers H., Campone M., Boileau J.F., et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. J. Clin. Oncol. 2019;37:2206–2216. doi: 10.1200/JCO.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fasching P.A., Brucker S.Y., Fehm T.N., Overkamp F., Janni W., Wallwiener M., Hadji P., Belleville E., Häberle L., Taran F.A., et al. Biomarkers in Patients with Metastatic Breast Cancer and the PRAEGNANT Study Network. Geburtshilfe Frauenheilkd. 2015;75:41–50. doi: 10.1055/s-0034-1396215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartkopf A.D., Huober J., Volz B., Nabieva N., Taran F.A., Schwitulla J., Overkamp F., Kolberg H.C., Hadji P., Tesch H., et al. Treatment landscape of advanced breast cancer patients with hormone receptor positive HER2 negative tumors—Data from the German PRAEGNANT breast cancer registry. Breast. 2018;37:42–51. doi: 10.1016/j.breast.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Muller V., Nabieva N., Haberle L., Taran F.A., Hartkopf A.D., Volz B., Overkamp F., Brandl A.L., Kolberg H.C., Hadji P., et al. Impact of disease progression on health-related quality of life in patients with metastatic breast cancer in the PRAEGNANT breast cancer registry. Breast. 2018;37:154–160. doi: 10.1016/j.breast.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Hein A., Gass P., Walter C.B., Taran F.A., Hartkopf A., Overkamp F., Kolberg H.C., Hadji P., Tesch H., Ettl J., et al. Computerized patient identification for the EMBRACA clinical trial using real-time data from the PRAEGNANT network for metastatic breast cancer patients. Breast Cancer Res. Treat. 2016;158:59–65. doi: 10.1007/s10549-016-3850-8. [DOI] [PubMed] [Google Scholar]
- 40.Brookmeyer R., Crowley J. A Confidence-Interval for the Median Survival-Time. Biometrics. 1982;38:29–41. doi: 10.2307/2530286. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.