Abstract
Titanium and its compounds are broadly used in both industrial and domestic products, including jet engines, missiles, prostheses, implants, pigments, cosmetics, food, and photocatalysts for environmental purification and solar energy conversion. Although titanium/titania-containing materials are usually safe for human, animals and environment, increasing concerns on their negative impacts have been postulated. Accordingly, this review covers current knowledge on the toxicity of titania and titanium, in which the behaviour, bioavailability, mechanisms of action, and environmental impacts have been discussed in detail, considering both light and dark conditions. Consequently, the following conclusions have been drawn: (i) titania photocatalysts rarely cause health and environmental problems; (ii) despite the lack of proof, the possible carcinogenicity of titania powders to humans is considered by some authorities; (iii) titanium alloys, commonly applied as implant materials, possess a relatively low health risk; (iv) titania microparticles are less toxic than nanoparticles, independent of the means of exposure; (v) excessive accumulation of titanium in the environment cannot be ignored; (vi) titanium/titania-containing products should be clearly marked with health warning labels, especially for pregnant women and young children; (vi) a key knowledge gap is the lack of comprehensive data about the environmental content and the influence of titania/titanium on biodiversity and the ecological functioning of terrestrial and aquatic ecosystems.
Keywords: titanium alloys, titania, bioavailability, photocatalysis, environmental persistence, nanomaterials
1. Introduction
Titanium (Ti) is a transition metal with silver colour, high strength and low density. The most important property of titanium is its high chemical stability, i.e., titanium is even resistant to corrosion in sea water, chlorine and aqua regia. Naturally, titanium appears widely in the Earth’s crust and lithosphere, mainly in minerals, e.g., ilmenite, rutile and titanite. Metallic titanium is extracted from mineral ores mainly by Kroll and Hunter processes, i.e., by the reduction of titanium tetrachloride with magnesium and sodium, respectively. Titanium(IV) oxide (titania) is the most common titanium compound, widely used as a pigment and a photocatalyst. Other important titanium compounds are titanium chlorides, i.e., (i) titanium(IV) chloride (TiCl4), used as smoke screens and catalysts [1], and (ii) titanium(III) chloride (TiCl3), a catalyst for polypropylene synthesis [2].
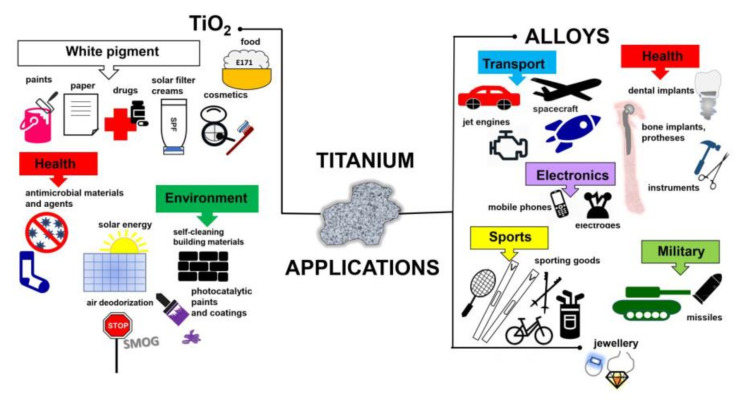
Titanium added to iron, aluminium, vanadium, molybdenum, tantalum and other metals forms lightweight and strong alloys, commonly used in aerospace (jet engines, spacecraft and missiles), metallurgy processes, dental/medical applications (prostheses, orthopedic implants, dental and endodontic instruments and files, dental implants), the car industry, agriculture, the military, sporting goods, mobile phones, jewellery and other applications [3,4,5], as shown in Figure 1. For example, Ouyang et al., (2019) have shown that Ti–Mg metal–metal composites facilitate osteoconduction and osseointegration (significantly higher around Ti-Mg than that around Ti implants) for orthopedic application [6]. Similarly, increased osseointegration has been observed on Ti35Zr28Nb alloy than that on pure titanium [7]. Moreover, Maharubin et al. [8] have proven that addition of silver (0.5–2.0 wt%) to titanium might limit post-surgery infection, one of the main causes of orthopedic implant failure. Additionally, titanium has been combined with other materials/compounds, such as organic compounds and polymers. For example, tannic acid-Ti/polysulfone membranes have been recommended for water remediation, especially for textile wastewater treatment, due to high hydrophilicity, excellent antifouling ability, powerful antimicrobial capability and good long-term stability [9]. Although, titanium and titania have already been used for various applications, their toxicity has not been addressed in detail, considering the direct and indirect impacts as well as the acute and chronic toxicity. Therefore, in this review, the toxicity of titanium/titania has been discussed thoroughly, based on recent literature reports and our own studies.
Figure 1.
Schematic drawing showing miscellaneous applications of titanium-containing materials.
2. Toxicity of Titanium and Its Alloys
Titanium (Ti) has been widely used for building materials, parts of vehicles, and consumer goods (e.g., glass, camera and watches), cosmetics, drugs and dental/medical implants, due to its stability, low-density, mechanical strength, corrosion resistance and biocompatibility. Although some metals are essential biological elements, titanium has not played a biological role inside cells [10]. Moreover, it is widely known that titanium rarely causes allergic reactions in comparison to other metals. Osseointegration (binding between bone and titanium implant) without rejection was first reported by Branemark in 1983 [11]. Since this great discovery, titanium implant therapy has been developed intensively, e.g., for dental implantation, artificial joints and bones. It has been proposed that the formation of a passive film on the surface of titanium, due to the instantaneous binding of oxygen is the main reason for its lower allergic effect (lower ion release) than in the case of other metals [12,13,14].
The interest in Ti properties has been continuously growing, because of its use as an inert bio-implant material for medical and dental applications [15,16,17,18,19]. The evidence of titanium toxicity has not been reported for many years, and thus titanium has been considered as an inert material with high biocompatibility. However, in rare cases, allergic symptoms, caused by titanium (alloy) implants, have been suggested, e.g., irritation, inflammation, erythema, lichenoid reactions and so on [20,21,22,23]. Additionally, it has been reported that titanium could corrode under some conditions, e.g., low pH, in the presence of fluorine, or in the contact with other metals [14,20,23]. Interestingly, Hanawa (2004) has suggested that a release of metal ions does not necessarily damage the human body, however, their binding to biomolecules could be toxic [13]. It is known that Ti ions exhibit high activity, reacting with hydroxyl radicals and anions immediately, and thus the trace amount of Ti ions might react with biomolecules, inducing body injury [13]. Indeed, the titanium release from hip-replacement components has resulted in titanium accumulation in serum and hair of patients with titanium alloy implants [24], probably because of the long-distance “travelled” by titanium [25]. Additionally, it has been suggested that the released titanium ions show high affinity for serum transferrin, binding the protein through metal binding sites [26].
There are many indications that titanium might cause some problems, e.g., “yellow nail syndrome” (YNS), allergic and autoimmune reactions. In view of this, it is possible and even necessary to discuss the toxicity and the allergy caused by titanium and its alloys [19]. Although numerous papers on titanium have been published, the chronic or sub-chronic effects on organs and various types of tissue, the dose-response correlations, and models of action have not been fully elucidated. Due to widespread use of titanium implants in prosthodontics and orthopedics, the most valuable data could be found in respective medical papers. It has been well known that titanium implants are in direct contact with body fluids (saliva) that contain various inorganic and organic compounds. In addition, the implant surfaces can be inhabited by bacteria, which might initiate the corrosion [27]. Although, titanium alloys are generally considered as passive under normal physiological conditions, some exceptions, such as oxide layer disruption or oral implant corrosion, have been reported [15,17]. For example, low pH, high concentration of fluoride (dental implants) and the presence of oxidizing agents are considered as the main factors inducing corrosion [28,29,30]. Moreover, it has been found that the toxicity of titanium alloys depends on the alloy composition [16,31,32,33,34,35], and thus the careful selection of the material should be performed [18]. The first-generation titanium alloys, which contain Cr, Ni, Be and Co, are very toxic, whereas those with Al and V exhibit little toxicity and slight allergic effects. On the other hand, new titania alloys containing Nb show favourable osteoconductive and osteoinductive properties due to the formation of an apatite layer on their surfaces, when exposed to an acidic environment [36]. Moreover, other cations (e.g., Ag, Cu, Zn and Ce) might present additional therapeutic effects, e.g., angiogenesis that is essential for cicatrize process, and antimicrobial properties [18,37]. According to Ikarashi et al. [38], titanium–zirconium (Ti–Zr) alloy-implants exhibit the best biocompatibility, improved properties (in respect to pure Ti) and a low level of fretting corrosion. Nowadays, toxicological effects, related to antibacterial properties of noble metals’ ions, such as Ag+ and Au+, which might be released by titanium alloys, have been a growing matter of concern [39]. Similarly, nanostructures/compounds containing antibiotics with the antibacterial, anti-infective and anti-inflammatory properties, have been under consideration. Although antibiotics are used to control invading organisms (mainly bacteria and protozoa) on the surface of implants, very often they cause some problems, including cell toxicity, allergic response, impairment of osteogenic activity and antibiotic-induced adverse reactions, e.g., superinfections and hypersensitivity [40,41,42].
The risk assessment of titanium and titanium alloys requires the quantification of unintended effects associated with a release of particular components. Obviously, this is not an easy task since very often contradictory data have been provided. For example, Rae [43] has postulated that pure titanium and titanium alloy (Ti-6Al-4V) do not affect human fibroblast cultures because of the relatively-low solubility of Ti ions [43]. By contrast, the corrosion products of titanium implants have been identified in serum and bone marrow, then being transported through the bloodstream and/or lymph to hair, lungs, spleen, liver and kidneys [44,45,46]. Accordingly, titanium has been detected in inner organs, including lungs, kidneys and liver, five months after a dental implant placement because of the translocation mechanisms [47]. Therefore, an estimation of changes in the content of Ti in the blood exposed to bone and dental implants, has been proposed as one of the toxicity indicators. Unfortunately, the changes of titanium content in the blood do not correlate with the implant-bone contact area, implants’ number and gender [48,49].
Other symptoms of implants’ corrosion include periprosthetic osteolysis, implant loosening and increased expression of proinflammatory mediators such as interleukins, prostaglandins, monocyte chemotactic proteins and macrophage colony stimulating factors [50,51,52,53]. Moreover, the particles of dental implants have been considered as initiators of destructive inflammatory processes, affecting tissues that surround dental implants—peri-implantitis [54,55,56]. For example, 100–300 ppm of titanium has been detected in trigger tissues [57]. The contact allergy to titanium might lead to pain, eczema, atopic dermatitis, swelling, erythema, urticaria and weakening of implants [15,57,58,59,60]. However, a difficulty in assessment of Ti allergy, because of uncertainty of the detection methods, seems to be the main problem [16]. In order to prevent implant failure, attention should be paid to a patient’s medical history to indicate the multiple allergies, e.g., to metals and jewellery [61].
A few studies indicate a possible connection between titanium and YNS [62,63,64]. The YNS or lymphedema associated with yellow nails is an uncommon and rare medical syndrome [63], characterized by slow nails’ growth, their yellow discoloration, lymphedema and tract involvement. In 2011, Berglund found a correlation between the titanium content in nail clippings and the yellowness and/or thickness of the nails. An excessive exposure to titanium from orthopedic implants along with ingestion of some drugs and foods (e.g., chewing gums, candies, chocolates) might be given as a probable cause of YNS. It seems extremely likely that the synergistic effect of chronic subthreshold is relevant. Moreover, fluoride-containing toothpastes and fluoride gels used for oral hygiene might exacerbate YSN. Berglund [62] has shown that titanium implants are a source of titanium ions, which are released from implants because of the galvanic action of dental gold and amalgam or oxidative reaction with fluorides [62]. Interestingly, the symptoms disappeared after stopping the galvanic reactions of titanium with other metals, and thus an exposure to titanium. Moreover, YNS might be dependent on underlying genetic and immunological disposition [63,64]. The experiments on animals have confirmed the titanium release from dental and orthopedic implants [27,56,65,66]. Furthermore, it has been found that the surface roughness of a metal insert is the most important factor of titanium release from the implant surface, i.e., the rougher the surface is, the higher is the coefficient of friction, and thus titanium release. In contrast, total area and diameter of implants are less important [66].
Titanium plasma-sprayed (TPS) implants should be considered as a special case, due to gradual and passive dissolution of their surface, which results in a decrease in the size of titanium particles with an increase of the distance from the implant surface. For example, titanium particles, released from TPS implants, have been detected at the average distance of 200–250 µm (till 500 µm) from the implants’ surface [67]. The analysis of histological sections has shown the presence of titanium at the distance from 0.4 mm up to 4.0 mm [27]. Generally, the Ti particles’ size, found in animal and human tissues, ranges from 10 nm to 54 µm [56]. It has been reported that Ti particles have a cytotoxicity effect through reduction of bone marrow stem cells (BMSCs) viability and proliferation, increase of p53 protein level, disruption of cell homeostasis and induction of DNA damage [68]. For example, Gomes et al. [69] showed (through an in vitro study) that Ti-6Al-4V alloy, widely used in medical and odonatological implants, presents a cytotoxic effect, i.e., the DNA damage (breaking of DNA strands) and mitotic spindle, leading to loss of whole chromosomes during cell division. However, the model of action is still unknown.
Two mechanisms of metal ions’ interactions with DNA have been considered: (1) direct and (2) indirect actions [70,71,72,73]. (1) Titanium as a transition metal (d-block metal) has incomplete d-orbital, and thus can bind directly to the DNA bases (N7 of purine or N3 of pyrimidine atom at G-C sites). On the other hand, (2) titanium has low-energy d orbitals, which suggests that indirect mechanism is more probable, i.e., based on increased formation of reactive oxygen species (ROS), and formation of hydrogen bonds between the coordinated ligands and negatively charged phosphate groups in DNA structure [38,70].
Considering the methods of toxicity evaluation, both Ti (alloy) particles and Ti ions have been investigated in vitro, ex vivo and in vivo, i.e., on DNA/RNA, protein, lipids, cells and animals. Accordingly, the cellular incorporation of titanium has been well studied, e.g., the cellular uptake efficiency is higher for titanium nanoparticles (NPs < 100 nm) than titanium microparticles (<44 μm). Moreover, only NPs have been observed in the nucleus, as shown in Figure 2, with 352 times higher cytotoxicity than microparticles [73]. However, it should be mentioned that large titanium particles could be incorporated into cells by phagocytosis [74]. Evans has evaluated the effect of titanium (mean size of 49 μm), ground titanium (14 μm) and titanium alloy (Ti90/Al6/V4, 8.9 μm) on the cell viability using two experimental conditions, i.e., (1) in the direct contact with cells, and (2) separated from them [75]. Although large titanium does not cause a decrease in the cell number under both conditions, small titanium significantly reduces the number of cells when they are in contact with titanium. Moreover, titanium alloy causes a higher reduction of cell number than ground titanium when in contact with cells. Accordingly, it has been proposed that small particles (5–10 μm) could induce cell damage by direct contact. The size-dependent cytotoxic effect of titanium particles/ions on neutrophils has also been shown, i.e., the fine titanium particles (1–3 μm) are incorporated into cells by phagocytosis causing the cytotoxicity [74,76], whereas Ti ions stimulate neutrophils and increase the quantity of released superoxide anions [74]. Moreover, it has been shown that the intraperitoneal injection of titania suspension induces the uptake of titanium by the blood cells (macrophages and phagocytic mononuclear cells) and its further dissemination to organs, such as liver, spleen and lungs via cells [44].
Figure 2.
Laser scanning confocal microscopy image of: (a) a periodontal ligament human telomerase reverse transcriptase (PDL-hTERT) cell after exposure to titanium nanoparticles (Ti-NPs, 28 μg/ml) for 24 h (z-stack slices merged into one image). Ti-NPs: pink, cell plasma membrane: blue, nucleus: green, yellow arrow: Ti-NPs in the nucleus; (b) the nucleus of the same cell in (a). Ti-NPs: pink, nucleus: green, yellow arrow: Ti-NPs in the nucleus. Adapted from reference [73] with permission from Elsevier, 2015.
The cellular response to titanium particles/ions has been investigated mainly for oral mucosa cells. For example, it has been found that the exposure of mouse osteoblast-like MC3T3-E1 cells to Ti ions inhibits cell proliferation (in dependence on the concentration and time), and induces nuclear expression of Yes-associated protein YAP (a key transcription co-activator, the activity of which is inhibited by the Hippo signaling pathway) in osteoblasts, resulting in down regulation of osteogenic differentiation of MC3T3-E1 cells [77]. According to the in vivo study on detection of lactate dehydrogenase (LDH), interleukin (IL) and activated nuclear factor-kappa B (NF-κB), inflammatory reaction (high content of IL-6) and activated NF-κB have been detected around a titanium implant [78]. Moreover, it has been proposed that TNF-α, IL-1β, and IL-6 might induce osteoclastogenesis and inhibit osteoblastogenesis through the RANK–RANKL (receptor activator of nuclear factor kappa-Β–receptor activator of nuclear factor kappa-Β ligand) pathway [79]. Therefore, it has been concluded that titanium might induce inflammation. Moreover, it has been proposed that cells’ exposure to titanium might also influence the content of proteins and lipids. Indeed, titanium has caused a decrease in total protein content and some types of lipids, e.g., cholesterol ester and phosphatidylethanolamine, inducing the potential damage of tissues [80].
López-Jornet et al. [81] evaluated the DNA damage by dental implants in gingival cells, collected from patients with implants. The concentration of titanium (Ti47) in these cells was significantly higher than that in control cells (from patients without implants). The frequencies of micronuclei and binucleated cells, and nuclear buds in the “implant” group, have been higher than those in the control group, but without statistically significant differences. Moreover, during the study on the effect of Ti ions on osteoblast, Liao et al. [82] have revealed that the equal or higher concentration of Ti ions than 10 ppm inhibits cell proliferation. Additionally, it has been found that Ti ions: (i) reduce the expression of osteonectin and osteopontin mRNAs, (ii) delay the development of alkaline phosphatase mRNA expression, and (iii) decrease the enzyme activity.
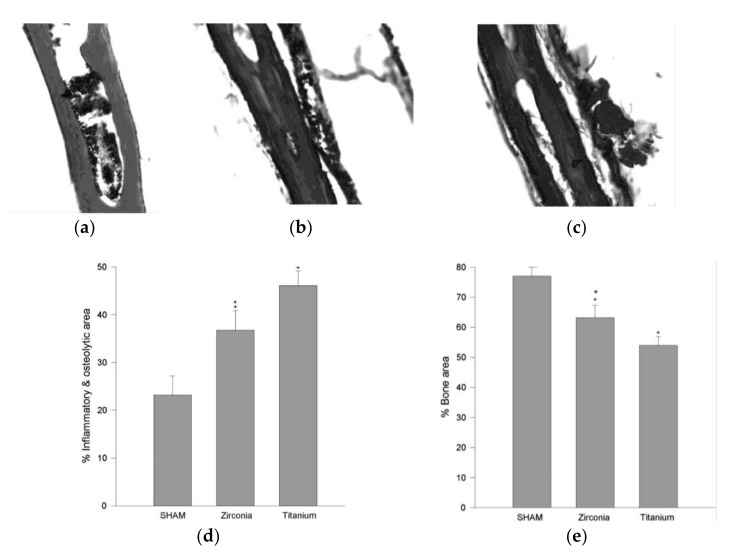
It should be remembered that the toxic symptoms due to titanium are not only allergic reactions, but also disorders in a whole body. Fretwurst et al., (2016) have proposed that a release of Ti ions could participate in peri-implant bone loss [83]. Additionally, acidic conditions in an oral cavity might increase the corrosion of titanium [84]. Moreover, the induction of osteoclastogenesis and the inhibition of osteoblastogenesis can lead to bone resorption around joint replacements [79]. As shown in Figure 3, mice treated with zirconium and titanium have expressed an inflammatory reaction and the reduction of bone surfaces in comparison to a sham group (PBS treated).
Figure 3.
(a–c) Histological microscopic images of murine calvaria stained with hematoxylin and eosin (magnification: ×200): (a) In sections obtained from PBS-treated animals (sham group); (b) In sections obtained from zirconia treated animals; (c) In sections obtained from titanium treated animals; The quantitative evaluation of tissue reactions, expressed as percentage of (d) osteolytic and (e) bone area; + indicates significant difference to sham group and * indicates significant difference to titanium treated group; One way analysis of variance (ANOVA) and Tukey’s test, p < 0.05. Adapted from [79] with permission from Elsevier, 2014.
Despite the abundant content of titanium in the Earth’s crust, water contamination by abnormal content of titanium might also affect human health. Titanium has been found in river water, and thus accumulated in aquatic insects [85]. Therefore, its possible impacts on the food chain and the agricultural damage must be considered. Moreover, a statistical study in Mexico has suggested that titanium in the blood, ingested by insufficiently treated water, might be related to low haemoglobin content, and thus anaemia in children [86].
The effect of titanium on bacteria cells has also been investigated, but contradictory results have been reported, i.e., (i) no significant bactericidal effect on oral bacterial species [87,88], and (ii) bactericidal activity of titanium [89]. Recently, Stolzoff et al. [84] have revealed the effect of surface topography of titanium on bacteria. It has been found that a high density of uniformly sized nanofeatures prevents bacterial adhesion and proliferation [90]. Considering that bacteria might cause implant failure, therefore, the development of bacteria-resistant titania implants would be highly valuable for patients.
Summarizing, titanium is one of the safest metals, as it has been widely used for clinical implants. However, it is necessary to consider and evaluate carefully all possible negative impacts on human body. Moreover, clinicians should pay attention when titanium-based implants are installed in patients.
3. Toxicity of Titanium(IV) Oxide
Titania (titanium(IV) oxide, titanium dioxide, TiO2) is the most widely used titanium compound, and thus its toxicity should be carefully examined. Similar to titanium, titania has been reported as inert, and thus safe for humans and the environment for many years. Non-toxicity of titania has been listed as one of the main advantages of titania photocatalysts among high activity, chemical and thermal stability, broad availability and low costs. However, considering the nanoparticulate nature of titania photocatalysts, the nanosize-governed toxicity of titania has been postulated [91,92]. Accordingly, various studies on titania toxicity have been performed, as shown in Figure 4. More than 6000 papers have been published on “titania (titanium dioxide, TiO2) toxicity” (searched in Web of Science), and about 60% of them (3674 results) in the last five years. Accordingly, an evaluation of cytotoxic, ecotoxic, genotoxic and carcinogenic potential of TiO2 has been represented in large number of scientific papers (Figure 4). However, it should be pointed out that the possible toxicity of titania has been intensively studied only in the last few years, and some potential effects are still unknown, e.g., carcinogenicity (only ca. 10 papers/year), which might suggest the low toxic effect of titania. Accordingly, the possible hazardous impact of TiO2 has been reviewed and discussed in the following sections, including carcinogenicity.
Figure 4.
Number of papers published annually on titanium toxicity searched in Web of Science (May 25, 2020) using: (a) “titanium dioxide toxicity” or “TiO2 toxicity” or “titania toxicity” (black) and “titanium dioxide cytotoxicity” or “TiO2 cytotoxicity” or “titania cytotoxicity” (red); (b) “titanium dioxide ecotoxicity” or “TiO2 ecotoxicity” or “titania ecotoxicity” (blue), “titanium dioxide genotoxicity” or “TiO2 genotoxicity” or “titania genotoxicity” (black) and “titanium dioxide carcinogenicity” or “TiO2 carcinogenicity” or “titania carcinogenicity” (red).
3.1. Can Inhalation of Titania Cause Cancer?
Pure titania pigment is a fine white powder, which looks like icing sugar. Typical TiO2 is characterized by a primary particle size between 0.2 and 0.5 µm (nanostructured titania has nanometre size, i.e., about 5–40 nm). However, a dusty form of titania could become airborne, and thus be easily inhaled. For this reason, titania (similar to other insoluble dusts, e.g., carbon, diesel black exhaust) has been considered as a potential health hazard [93].
A carcinogen is any substance, radiation or radionuclide, that promotes the formation of cancer (Carcinogenesis, oncogenesis or tumorigenesis). In the majority of cases, carcinogens do not cause acute toxicity (are not immediately toxic). Although titania has been considered as non-toxic, titania NPs of sizes up to 20 nm have been classified as a possible carcinogen to humans (Group 2B carcinogen) by the World Health Organization’s International Agency for Research on Cancer (IARC) since 2006. Accordingly, in 2015, the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) requested recognition of TiO2 as a carcinogen by inhalation to the Committee for Risk Assessment (RAC), which has prepared the opinions for the European Chemicals Agency (ECHA). The first time that the issue was discussed by RAC was at the meeting in March 2017. However, the ECHA committee stated (Helsinki, 9 June 2017) that the potential of titania as a carcinogen was less restrictive than presumed carcinogens (Group 1B carcinogen), and more research and requirements in classification, labelling and packaging (CLP) have been requested. The important aspect of this dispute was that the Titanium Dioxide Manufacturers Association (TDMA) pointed out that only rodent studies indicated the carcinogenic potential of TiO2 [94,95]. Finally, during the meeting of the CARACAL group (Competent Authorities Meeting for REACH and CLP regulations) on 18 September 2019, TiO2 was classified as a category 2 carcinogen, due to its inhalation hazard: “The classification is reported to apply to liquids as well as powders containing 1% or more of titanium dioxide in the form of or incorporated in particles with aerodynamic diameter ≤10 µm”. Accordingly, the appropriate act was submitted to the European Parliament and Council [96].
Similarly, the National Institute for Occupational Safety and Health (NIOSH), the United States federal agency responsible for the prevention of work-related injury and illness, has recommended the exposure limits of 2.4 mg/m3 for fine TiO2 and 0.3 mg/m3 for ultrafine (including NPs) TiO2 as the time-weighted average (TWA) concentrations (TWA is a method of calculating a worker’s daily exposure to a hazardous substance or agent, averaged to an 8-hour workday, taking into account the average levels of the substance or agent and the time spent in the area. A time-weighted average is equal to the sum of the portion of each time period (as a decimal) multiplied by the levels of the substance or agent during the time period divided by the hours in the workday) for up to 10 h per day during a 40-h work week. This means that over a working lifetime the risk of exposure is less than one person in 1000. The Occupational Safety and Health Administration (OSHA) has recommended an exposure limit (PEL) at 15 mg/m3 for TiO2 as a total dust and 5 mg/m3 as a respirable dust. Much lower limits have been applied in Germany, where the MAK (Maximum Concentration Values in the Workplace) values are 4 and 1.5 mg/m3, respectively. Since any exposure might involve some degree of risk, the general recommendations must result in the exposure reduction to the lowest achievable levels. As pointed by Hex et al. [93], the average concentration of respirable TiO2 dust depends on many factors, and varies in time (calendar year) and place (TiO2 factory). The median cumulative exposure of workers has been estimated at 1.98 mg/m3 × years, which is much less than the recommended exposure limits [93].
To better understand how titania affects human health, animal models, in particular rats or other rodents (mice and hamsters), have been used as exemplary for commercial titania, as presented by Relier et al. [97]. Accordingly, obtained data allow us to compare various conditions of TiO2 exposure and multiple exposure routes, and help to assess the model of action, even when the animal model of the organ injury does not correspond to the human organ injury or identification of conserved gene expression across organisms.
The importance of titania routes of exposure has been considered as follows: inhalation > oral exposure > dermal exposure (skin). Gas exchange, which involves an inhalation of oxygen and a release of carbon dioxide, is conducted by a respiratory system, which is a primary target structure. The sub-chronic and chronic effects have revealed that titania can be deposited in the lungs, and thus can be responsible for their injury, leading even to cancer development [98].
According to the National Library of Medicine (NLM)—Toxnet, there is only limited adequate evidence for the carcinogenicity of titania to animals’ respiratory systems [99]. Some human cases of lung injury, caused by the exposure to titania in various forms, are listed chronologically in Table 1. The mortality rate for these 13 cases (Table 1) is unknown.
Table 1.
The reported cases of human health effects by titania.
| Case | Contact | Exposed Persons | Results | References |
|---|---|---|---|---|
| 1 | 13 years | 53-year old man employed to pack titania | pneumoconiosis accompanied by right lung cancer | [100] |
| 2 | 9 years | Man employed to pack titania into cans | slight fibrosis of interstitial lung tissue surrounding bronchioles and alveolar spaces after 5 years | [101] |
| 3 | - | 55-year old man, heavily exposed to titania dust (rutile) | extensive pulmonary deposition of white pigment and absence of inflammatory and fibrotic changes | [101] |
| 4 | - | Four workers exposed to the inhalation of titania dust (not pure) | epithelioid granuloma, confirmed the inflammatory route of exposure | [102] |
| 5 | - | Four men and two women, between the ages of 22 and 65 years, unknown source of exposure | fibrosis and numerous macrophages with abundant deposition of a black pigment, confirmed presence of large quantity of titania in the pigment granule | [103] |
The vast majority (85%) of cases of lung cancer are caused by smoking or exposure to the smoke (secondhand smokers), whereas about 10–15% cases are due to a combination of genetic factors, confirmed by family history of lung cancer, or exposure to radon, arsenic, chromium and nickel or other forms of air pollutants. In practice, it is difficult to obtain a clear correlation between the mortality and the occupational exposure to titania, because in several cases, the smoking habits of study subjects have not been reported. The statistically valid epidemiologic studies of the mortality caused by titania were conducted by Chen and Fayerweather [104], Boffetta et al. [105] and Fryzek et al. [106]. In the first case, 1576 employees exposed to TiO2 were observed from 1956 to 1985 for cancer and chronic respiratory disease incidences, and from 1935 to 1983 for mortality. No cases of pulmonary fibrosis were found among TiO2-exposed employees. In the second case, 15,017 workers (14,331 men) employed in 11 factories producing TiO2 in Europe were examined. It was assumed that among men, the mortality from lung cancer did not increase with an increase in an employment duration or an estimated cumulative exposure to TiO2 dust. In the last study, 4241 workers, who were employed for at least 6 months in four TiO2 plants in the United States, were analyzed. It was found that employees with a higher exposure to titania had similar mortality rates to those with lower exposure. Therefore, in all these studies the same conclusion has been drawn, i.e., titania dust does not present a significant carcinogenic effect on human lungs.
The influence of titania properties, including the size, the surface area, the surface chemistry and charges, the crystallinity, the shape, the solubility and the agglomeration/aggregation state, on lung injury has been summarized in the review paper by Wang and Fan [107]. The most important conclusions from this work are: (i) titania NPs with the smaller sizes than 20 nm and small titania agglomerates (<100 nm) present the higher carcinogenic potential than fine TiO2 particles (ca. 200–250 nm), due to the induction of oxidative stress and DNA single and double-strand breaks; (ii) morphologically-ordered titania, including zero-, one-, two- and three-dimensional (0-D, 1-D and 3-D) assemblies as nanospheres, nanorods, nanotubes and nanobelts present varied effects in their deposition in the lungs (time- and shape-dependent); (iii) both anatase and rutile (two main polymorphs of titania) show the induction of inflammatory responses, cytotoxicity and ROS formation that lead to cell necrosis or initiate apoptosis; (iv) the ability of anatase to decrease the lung cell viability is slightly larger than that of rutile, (v) the surface modification (or surface coatings with inorganic or organic compounds) is an important factor influencing the toxicity of titania in the respiratory system; (vi) the main factors inducing the cytotoxicity (or oxidative stress induction) and genotoxicity are ROS, generated when TiO2 is light-activated; (vii) the crystalline forms of titania, the size, the specific surface area, the number of defect sites, etc. influence ROS formation. The toxicity of titania in dependent on the size of titania particles and the place of impact is exemplary as shown in Figure 5. Considering the surface charge of particles, Fröhlich [108] has postulated that there is no rigid rule of charge-dependent particle uptake, but it seems that cationic surfaces of NPs (not only TiO2) result in larger cytotoxicity.
Figure 5.
Comparison of titanium dioxide impact on cell and organismal level.
In summary, it should be pointed out that there is very little evidence that might link the increased mortality from lung cancer with the increased exposure to TiO2 dust. Additionally, a cumulative effect from airborne pollutants, e.g., nitrogen oxides, sulphur oxides, ozone, smoke, might cause (together or separately) severe sickness and premature death. For these reasons, although caution is advised, titania dust cannot be unambiguously defined as a human lung carcinogen.
3.2. Dermal and Oral Exposure to Titania
One hundred and fifty items of daily cosmetic products containing titania, such as toothpaste, sunscreens, food and beverage colorants, drugs, vaccines and nutritional supplements with long-term contact with human organs (e.g., skin, digestive track), have been examined by Shi et al. [109]. It should be pointed out that direct contact is also possible during manufacturing of titania (e.g., bagging, handling, labelling, sampling, overlaying). Accordingly, many studies involving skin lines have been conducted [110,111,112,113], and in vivo studies provide accurate and reliable data, due to the possibility to follow the course of active penetration during long-term exposure. In contrast, in vitro studies have a few limitations, e.g., (i) only passive distribution through skin layers can be verified; (ii) they are only limited to one cell type (e.g., keratocytes); and (iii) only short-term experiments can be conducted. Based on the results obtained from both types of experiments, it is thought that titania particles could not access intact skin through intercellular channels of stratum corneum, sweat glands and hair follicles [114,115]. Moreover, only the accumulation of titania NPs on the skin surface has been observed, which is caused by titania tendency to form aggregates, and additionally confirming the great stability of titania [110,115]. However, it could be basically imagined that scratching, small wounds, bites, burns and other slight damage might break the skin and facilitate titania penetration. However, in vitro and in vivo results, obtained by Xie et al. [113], have shown that 20-nm titania NPs could not penetrate skin even when its layers are damaged, Senzui et al. [111] have found that the concentration of titanium in skin increases when it has been applied on hair-removed skin. Moreover, TiO2 from cosmetics could pass through hair follicles and pores (greater than 1 mm) but has not been detected in dermis and viable epidermis layers [110,111]. Therefore, it has been proposed that the form of titania is crucial for the penetration rate. According to Bennat and Muller-Goymann [110], titania NPs applied as an oil emulsion become deeper inside the skin than that in the form of an aqueous colloid.
From the late 1990s, TiO2 and ZnO NPs from sunscreens have been postulated to be involved in the generation of free radicals’ (including singlet oxygen) in skin cells [98,116,117,118,119], and thus resulting in possible protein dysfunction, mutations, direct DNA damage, and the further tumorigenesis and the cancer development. This is a kind of perversity since sunscreens are used for skin protection to prevent sunburn and to reduce skin cancers. However, it should be noticed that an evaluation of ROS influence on direct tumour formation is rather difficult, due to their short lifetime [120]. Interestingly, titania has been proposed as an efficient agent for possible local treatment of cancer, e.g., an increase in cells’ mortality and significant morphological alterations in living cells for MCF7 (breast adenocarcinoma) have been observed with an increase in titania dose (from 10 to 50 mg/μL) and under ultraviolet (UV-A) irradiation (as compared with experiments in the dark) [121].
The average dietary exposure to TiO2 (E 171) from its use as a food additive is between 0.4 and 10.4 mg of TiO2 per kg of body weight per day throughout life. Obviously, children are the most exposed group of studied individuals [122]. Growing concerns, related to acute and sub-chronic toxicity in rodents by their exposure to TiO2 through food, have already been noted [123,124,125,126,127,128]. Fortunately, no acute toxicity has been reported. For a sub-chronic effect, 28- and 90-day tests have been performed for male and female rats, dosed with rutile-type pigment of particles’ size from 73 nm to 145 nm, for no-adverse-effect level (NOAEL) from 1000 mg/kg bw/day to 24,000 mg/kg bw/day. Generally, negligible toxicological effects have been observed. However, LD50 (rat) value has been given for oral exposure to titania, reaching >5000 mg/kg bw [129]. Indeed, it has been found that an oral exposure of nano-TiO2 to pregnant mice clearly induces the dysplasia of skeleton and suppresses the development of mice embryos, as shown in Figure 6. Interestingly, the transport to other tissues outside the digestive system (e.g., lung tissues, spleen, kidney, brain and lymph) has also been confirmed [129]. Geraets et al. [124] have revealed another warning, i.e., slow elimination and potential accumulation of titania in tissues. The significant changes in body parameters (e.g., decrease in body weight gain) and the factors affecting fertility (e.g., an increase in sperm abnormalities, a decrease in a number and size of the epithelial lining of the tubuloalveolar gland and hyperplastic glandular epithelium of the seminal vesicle, and a decrease of sperm cell concentration and serum testosterone level) have also been reported after continuous feeding of the male albino rats with food containing 1–2% titania for 65 days [130]. An increase in the levels of dopamine and norepinephrine in the brain cerebral cortex appears to be the the most alarming symptom of possible neurotoxicity [125]. The results obtained by Mohammadipour et al. [131] have proven this hypothesis to a certain extent, i.e., rat offspring being forced to consume 100 mg/kg titania for 21 days impaired memory and learning abilities. Moreover, Bideskan et al. [132] have found that exposure to TiO2-NPs during rat pregnancy and lactation periods induces apoptosis and decreases neurogenesis in hippocampus. In this regard, it has been proposed that pregnant and lactating women should avoid food additives containing titania, such as E171 [133].
Figure 6.
Stereo microscopic images after treatment by Menegola’s method of mice embryos that were maternally exposed to nano-TiO2 at gestational day 18. Blue, cartilage. Purplish red, ossification. Red, incomplete ossification. Adapted from reference [135] with permission from Dove Medical Press, 2017.
Additionally, it should be pointed out that these aspects should be considered when titania is proposed as an antimicrobial agent (bare and modified titania, e.g., with NPs of noble metals) for applications connected with food protection against spoilage, e.g., for food storage containers [134].
The physicochemical characteristics of titania NPs, which might influence gastric toxicity, have been studied by many teams [136,137,138,139]. For example, it has been found that the properties of titania influence its entry pathway and further body penetration. The main titania absorption in the small and large intestines takes place through: (i) villi and microvilli of the epithelium and (ii) the lumen of the intestinal tract via aggregation of intestinal lymphatic tissue and the normal intestinal enterocytes. Moreover, the impact of a rutile form on gut microbiota is more pronounced than that of an anatase form. However, the reason for this phenomenon is unclear. It has also been reported that toxicity, induced by titania, might be attributed to mainly high dosing rather than the particle size and distribution [124]. However, Wang has found that for rats the 80 nm NPs of titania are more harmful than 25 nm ones [107].
An oral exposure to titania might induce an oxidative stress and alternation of enzymes activity. The changes in enzymes activity have been recognized for lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate transaminase (AST), superoxide dismutase (SOD) and glutathione reductase (GR), glutathione peroxidase (GP), glutathione-S-tranferase (GST) and catalase (CAT) [123,125]. It should be pointed out that the normal cellular metabolism is the main source of endogenous ROS [140]. Accordingly, due to a lack of light-activation inside a digestive track, it should be assumed that an increase in ROS content is caused by a decrease in the level of enzymatic scavengers of antioxidant defense (in particular CAT, SOD, GST) and non-enzymatic scavengers (e.g., vitamin A, vitamin E, vitamin C). The overproduction of ROS could break the balance down between the oxidative and antioxidative systems, resulting in DNA damage and apoptosis. Besides, free radicals might exacerbate the lipid peroxidation, calculated on the basis of thiobarbituric acid reactive substances (TBARS) that are formed as by-products [125,130]. Fadda et al. [128] have indicated that after an oral exposure to titania an increase in production of inflammatory mediators (e.g., tumor necrosis factor-α, interleukin-6, C-reactive protein and immunoglobin G) and angiogenic factors (e.g., vascular endothelial growth factor VEGF) might induce both hepatic injury and distant organ damage.
Additionally, it should be pointed that consumption of titania has an impact on gut microbes. According to Pinget et al. [141] titania consumption from 2 to 50 mg TiO2/kg body weight per day does not influence significantly the microbiota composition in the small intestine and colon. In contrast, in vitro studies have shown the opposite results, i.e., titania impairs haemostasis and affects the spatial distribution of commensal bacteria. Moreover, it has been found that rutile decreases a number of probiotic genera, such as Bifidobacterium sp. and increases opportunistic pathogens genera, such as Escherichia sp. and Shigella sp. [138]. In animal studies, a potential autoimmune disease and cancer development have been also reported [133,138,142,143,144]. Additionally, it has been presented that titania NPs-injected mice, which possess implanted tumours, have exhibited the promotion of tumour metastasis and the presence of cancer cells in the blood vessels [145].
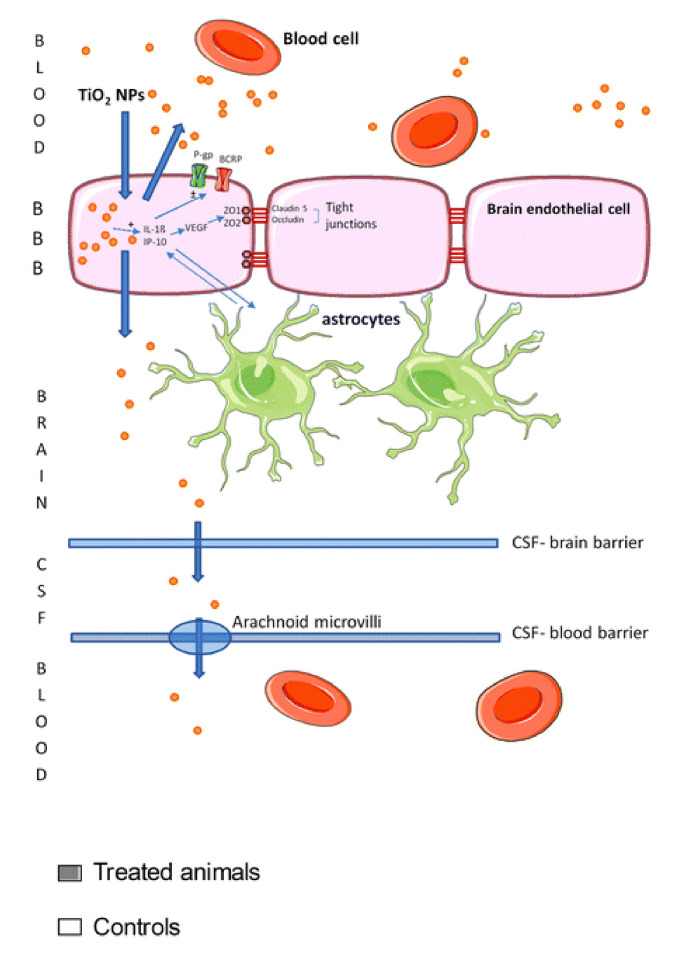
In toxicity studies, the intraperitoneal injection (IP) involves the injection of probable toxin (here: titania suspension) into the peritoneum. The IP method has been used in few animal investigations [146,147,148,149,150]. Accordingly, it has been found that the injection of TiO2/NaCl suspension influences only slightly the rat lifespan, i.e., from 8% prolongation to 15% shortening [146]. However, the emotional behaviour of rats has been alerted, and the anxious index has increased [149]. For example, as shown by Disdier et al. [148] (Figure 7), the accumulated titania NPs in liver, lungs and spleen might be transported to the brain by blood, and thus titania-containing organs show an increase in the levels of tight junction proteins (claudin-5 and occludin), interleukin 1β (IL-1β), chemokine ligand 1 and γ inducible protein-10 in brain endothelial cells (which consists of the brain blood barrier (BBB)) and also IL-1β in the brain. One of the most important studies has been performed by Kreyling et al. [150] for quantitative analysis of NPs’ presence in all organs, tissues, carcass and excretion using radiolabeled [48V] TiO2 NPs. Consequently, it has been shown that regions of titania accumulation might be put in the following order: liver > spleen > carcass > skeleton and the blood. Interestingly, the titania content in the blood decreases rapidly after 24 h, whereas its distribution in other organs and tissues remains rather constant during the whole experiment [150]. Based on histopathological examinations, Chen et al. [147] have concluded that titania particles cause the lesion of the mouse spleen. Moreover, hepatocellular necrosis or apoptosis, hepatic fibrosis, renal glomerulus swelling, and interstitial pneumonia, associated with alveolar septal thickening, have also been noted. In contrast, completely different results have been found for rainbow trout (Oncorhynchus mykiss), where high doses of intravenously injected titania NPs (mixed-phase: rutile and anatase, with crystallite size of 23.2 nm) have caused a very limited (if any) overt impairment of renal function and an oxidative stress in the blood, despite the evidence of significant uptake and retention in those tissues [151].
Figure 7.
Schematic image of the events and potential effects on brain blood barrier (BBB) physiology when titania NPs are accumulated in organs. Adapted from reference [148].
3.3. Titania Ecotoxicity
It should be pointed out that broad application of titania NPs might have serious consequences for ecosystems. Titania as other oxides’ NPs might be spread in the environment by various ways, e.g., wastewater treatment streams, accidental release during production or daily applications (e.g., rinsing creams from the skin during swimming, paint leaching from an exterior facade of buildings/walls) [98,152]. It is expected that the behavior of titania NPs in the environment depends not only on its properties, but also on the features of the environment. Therefore, various titania–environment interactions, e.g., different environmental components (e.g., reducing/oxidizing agents, surfactants, organic matter and humid acids) and further titania transformations (e.g., aggregation/agglomeration, adsorption, deposition, dissolution, redox reactions, and interaction with macromolecules) are almost impossible to evaluate and predict reliably. Moreover, the bioavailability of titania depends also on its characteristics, environmental parameters and route of exposure [153]. For example, Kiser et al. [154] have shown that concentrations of titanium in effluents from wastewater treatment plants range from 0.005 to 0.015 g/dm3, whereas that accumulated in settled solids reaches concentrations from 1000 to 6000 µg/g. Therefore, it has been proposed that toxicity should be considered for various external conditions (background) before the assessment of the risk across the world [155]. The modelled data for titania NPs released into environmental compartments for different continents have revealed that in Europe the predicted environmental concentration of TiO2 in water, soil, sediments and effluents from sewage treatment plants is a few times higher than that in the U.S. [152]. Recently, it has been found that titania (similar to other chemical compounds, e.g., benzophenone-3 (BP-3)) tends to release from sunscreens into the sea and might be life-threatening for aquatic fauna and flora [156]. According to calculations made by the Labille et al., (2018) for one small beach visited by 3000 people daily, 68 kg of creams could be deposited per day, and thus 2.2 tons over the summer. Assuming that half of used creams contain 5% of titania, it should be expected that ca. 1.7 kg of titania might release to the sea per day, and ca. 54 kg during the two months of high summer [157]. It is well known that titania and other metal oxides’ NPs agglomerate, forming the micrometer particles, which might cause other hazardous effects to marine ecosystem (e.g., direct interactions with sedimented animals as annelids, worms and bivalves) [158,159]. However, nano-sized titania at a concentration of 10 mg/dm3 has shown much higher accumulation than bulk titania (> six fold higher), particularly in digestive gland of marine bivalves Mytilus galloprovincialis [160]. Additionally, NPs possess different dissolution/dispersion properties, depending on water properties (e.g., freshwater and seawater). For example, Canesei et al. [161] observed the formation of agglomerates of different sizes for different types of NP in artificial sea water. The most attention has been on photocatalytic reactions leading to ROS generation, when titania in seawater is exposed to UV radiation. Interestingly, it has been found that ROS, produced under these conditions, have a long lifetime and high steady-state concentrations [162].
Studies on the toxicity of sunscreens and personal care products on coral species started at the beginning of the 21st century [163,164,165]. Many authors have argued that titania from sunscreens might be related to: (i) a decrease in biodiversity of marine ecosystems, (ii) worsening of the conditions of the coral reef, and (iii) affecting marine animals [158,159,160,162,166,167,168,169,170,171,172]. It has been found that under daylight illumination, nano-TiO2 is practically non-toxic for saline crustacea Artemia salina, and the EC50(24h) and EC50(48h) values have exceeded of 1.0 g/dm3 [172]. Nonetheless, an exposure to anatase and anatase/rutile nano-TiO2 under visible and UV light has increased the toxicity, showing values of EC50(48h) from 4.03 to 4.05 mg/dm3 and from 284.81 to 408.67 mg/dm3, respectively [169]. Interestingly, much lower values, e.g., EC50 = 38.56 mg/dm3 for the dark exposure conditions and EC50 = 16.39 mg/dm3 for the light/dark exposure, have been presented for marine bivalve Mytilus galloprovincialis [159]. On the other hand, a short-term pilot study in situ conducted by the Coral Restoration Foundation has shown no toxicity when Caribbean scleractinian staghorn coral Acropora cervicornis has been exposed to 10 different brands of sunscreens [171].
It is worth noting that marine organisms are very often more sensitive to nano-TiO2 than freshwater ones. For example, the brine shrimp A. salina is more susceptible to titania than freshwater Daphnia similis [169]. The well-documented responses to NPs’ exposure include: (i) inhibition of growth rate, (ii) significant coral bleaching, (iii) change in feeding behaviors of marine animals, (iv) inhibition of larva development, and (v) increased pre-apoptotic processes in animal cells [159,164,166,168,169]. Galloway et al. [166] have shown that sublethal effects of titania to lugworm (Arenicola marina) correlate with damage by free radicals that can react with most DNA components (e.g., purine and pyrimidine bases and the deoxyribose backbone) and bind directly to DNA or DNA repair enzymes, leading to the formation of strand breaks. The genotoxicity of photoactivated titania at concentrations above 2 mg/g, and direct and indirect toxicities of nano-TiO2 aggregates at higher concentrations (>10 mg/dm3) have been shown for embryos of marine snail Haliotis diversicolor (by a coherent anti-stokes Raman scattering (CARS) microscopy technique) [158]. In the same study, it was also found that the toxicity is enhanced by tributyltin TBT—an antifouling compound, widely introduced into marine environments as antifouling paints. Because of that, it has been proposed that research on the impacts of titania NPs on corals should be carried out not only for sole NPs, but also for co-mixed components, e.g., titania with cosmetics and other chemical ingredients to simulate real commercial products, such as sunscreens and personal care products (PPCPs). Many sunscreen producers claim that their sunscreens contain UV filters, which are “reef safe”, but in fact, the majority of them have not been tested under in situ conditions. Unfortunately, there are no current regulations that enforce companies to undertake long-term toxicological studies in real conditions [173]. It is known that marine toxicology is challenging, and thus most aquatic toxicology studies are performed in the laboratory settings that do not mirror the environment, in which the organisms reside [171]. Moreover, there is a need to develop non-lethal monitoring methods, and improvements of universal practices and standards to determine the effects on various marine organisms. A comprehensive database to cumulate information on the effects on marine organisms and ecotoxicological endpoints should be also developed [174]. Additionally, it has been proposed that cosmetics and products consisting of titania NPs, e.g., sunscreens, could be labelled with the health hazards’ information for consumers, as well as pictograms (as proposed by us in Figure 8), presenting an environmental risk and precautionary statements for coastal waters [173].
Figure 8.
Proposed pictogram for commercial products containing inorganic NPs (e.g., titania), which might release when exposed to costal water.
Small planktonic invertebrates, such as Daphnia magna and Thamnocephalus platyurus, have been used in the majority of the studies for freshwater organisms [175,176]. Based on the lifecycle of crustaceans, the ecotoxicity chronic studies require a 21-day exposure period to establish survival and reproductive endpoints. Additionally, suspension-feeding organisms could incorporate NPs into their body very easily by endocytosis and phagocytosis. Unfortunately, the reported data are very confusing. For example, Heinlaan et al. [177] have shown that suspension of nano and bulk titania at a concentration of 20.0 g/dm3 are not toxic for D. magna, whereas Liu et al. [176] have estimated medial lethal concentration (LC50) of 0.5 g/dm3. Accordingly, Menard et al. [152] have proposed that the physicochemical properties of titania (crystal phases, specific surface area, average particle size) and the presence/absence of light (titania activation) determine the overall toxicity effect. The median effective concentration (EC50) for invertebrates (including Mollusca), defined as concentration of a substance in an environmental medium expected to produce a certain effect in 50% of test organisms, has been higher than 0.1 g TiO2/dm3 [152,161,169]. According to the results of acute toxicity experiments (short-term), titania NPs are non-lethal to daphnia, even up to EC50 concertation. The opposite results have been obtained from long-term studies, where titania at concentrations lower than 0.1 g/dm3 has shown toxic effects to crustacea, leading to increased mortality, growth decline and a lower number of offspring. Therefore, it is clear that exposure duration might be one of the most important factors of titania toxicity [178]. It has also been reported that 20 nm-sized TiO2 induces higher toxicity than 30 and 50 nm ones, because smaller NPs are more likely to enter inside the cells or/and accumulate under the carapace, as well as on the external body surface [176]. Moreover, the crystalline form of titania might be also important. For example, Clemente et al. [169] have shown that under the UV light exposure, the EC50 (48 h) for Daphnia similis is 12 times lower for anatase/rutile mixture than that for pure anatase.
One of the biggest problems is the transfer of titania particles in the trophic chain. Aquatic algae, phytoplankton and aquatic vascular plants, as well as higher plants in a non-aquatic environment, are the first trophic level in the food chain, and thus being a source for heterotrophic organisms. It has been proposed that sediments could be the main available sources of titania NPs in water. The model freshwater and marine algae, used in toxicology research, are microalgae species that have an ubiquitous distribution, e.g., Pseudokirchneriella subcapitata, Desmodesmus subspicatusand, Chlamydomonas reinhardtii, Phaeodactylum tricornutum, Anabaena, Microcystis and Melosira [152,170,179,180,181]. Once again, significantly variable values of median effective concentration EC50 for P. subcapitata have been reported for titania NPs, i.e., from 5.83 to 241.0 mg/dm3. Similar to invertebrates, the smaller titania particles (380 nm) are much more toxic than larger ones (>416 nm) also for algae. It is worth mentioning that tested species of microalgae are quite sensitive to the presence of toxic substances (including metals) and are broadly used in toxicology studies. Wang et al. [170] have reported that nano-TiO2 exerts most severe inhibition effects on P. tricornutum during the first day of exposure with a low EC50 value of 12.65mg/dm3. Additionally, more than 50% of the chlorophyll-a concentration in cyanobacteria (Anabaena, Microcystis and Melosira) has been reduced by TiO2-coated Pyrex hollow glass beads under illumination with UV-A light [179].
Few works have been performed on titania toxicity towards higher water organisms, such as fishes, including Japanese rice fish or medaka (Oryzias latipes), zebrafish (Danio rerio) rainbow trout (Oncorhyncus mykiss) and carp (Cyprinus carpio) [151,176,182,183,184]. For example, ROS have been detected in zebrafish embryos in vivo after their treatment with titania NPs, and smaller titania NPs causes higher ROS generation (Figure 9) [185]. Moreover, Asztemborska et al. [186] have found that titania NPs could be transferred from D. magna to D. rerio by dietary exposure. The estimated LC50 of titania (particle size of 25 nm) for Japanese medaka is the same as that for D. magna, reaching 0.155 g/dm3. Moreover, it has been found that titania NPs have caused: reduced growth rate, decrease in the organ weight (e.g., liver), inhibition of antioxidant enzymes (e.g., CAT and SOD), destroyed gonadal tissue, reduced number of eggs, oxidative damage (e.g., lipid peroxidation) in cells and DNA, and histopathological changes of embryos and adults (e.g., thickening of edema and the gill lamellae) [151,176,183,187]. Some of these effects (e.g., pathological changes in the gill and liver) could cause fish suffering [188]. It has been shown that fish cells are generally more sensitive to oxidative injury than mammalian cells. Worrying toxicological data have been presented by Sun et al. [182] for nanocrystalline titania. It has been shown that nano-TiO2, due to its large specific surface area and the presence of surface hydroxyl groups, has a great adsorption capacity for highly toxic (to humans) arsenic ions (As(V)). The direct results of this adsorption might cause high accumulation of As/TiO2 in the stomach, intestine and gills of fishes. For example, after only 25 days of exposure to both titania and arsenate, arsenic concentration in carps has increased by 132% [182]). Moreover, arsenic might release from the titania surface, and then be uptaken by other organs, such as the liver and muscles. Similarly, it has been shown that titania NPs might increase an accumulation of other environmental toxins, e.g., cadmium (Cd) [189].
Figure 9.
Fluorescence (reactive oxygen species (ROS) indicator, CMH2DCFDA staining) and bright field images of ROS generation in the zebrafish embryos after a treatment with titania NPs of different sizes, i.e., 6, 12 and 15 nm. White arrowheads, non-specific fluorescence. Black arrows, titania NP-specific signal. Adapted from reference [185] with permission from Royal Society of Chemistry, 2014.
Agricultural contamination by titania is another aspect that should be considered. For example, contamination occurring in agricultural soil has caused an increase in Ti concentration up to 302% in sand. Fortunately, only low to negligible transfer to the soil leachate and the plant shoot has been noted [190]. Moreover, titania influences terrestrial ecosystems and causes changes in the microbial diversity and N cycle by decreasing of the denitrification directly and indirectly, and declining of the nitrification [191]. Drobne et al. [192] have found that the dietary exposure (3 and 14 days) of terrestrial isopod Porcellio scabe to titania NPs of 25 and 75 nm at concentrations of 10–1000 mg TiO2/g, i.e., much higher than that predicted for soil (0.0048 mg/g), enhances: (i) the feeding rate, (ii) the food absorption efficiency, and (iii) the activity of antioxidant enzymes CAT (catalase) and glutathione S-transferase (SOD). Fortunately, no adverse effects, such as mortality, body weight changes and reduced feeding, have been noticed. Quite opposite results have been found by Roh et al. [193] i.e., the toxic effect of 20-nm TiO2 particles to Caenorhabditis elegans, a free-living nematode [193]. Additionally, it has been confirmed that small NPs (7 nm) induce the ecotoxicity and affect: (i) cyp35a gene expression, (ii) fertility, and (iii) the survival of C. elegants. Hu et al. [194] have shown toxicological effects, induced by TiO2 NPs in the soil, on redworn Eisenia fetida. Moreover, it has been found that rutile (particle sizes of 10–20 nm) at concentrations above 1 g/kg bioaccumulates in the earthworms’ body and induces harmful effects, including oxidative stress, enzyme inhibition (cellulase), DNA damage and mitochondria damage [194].
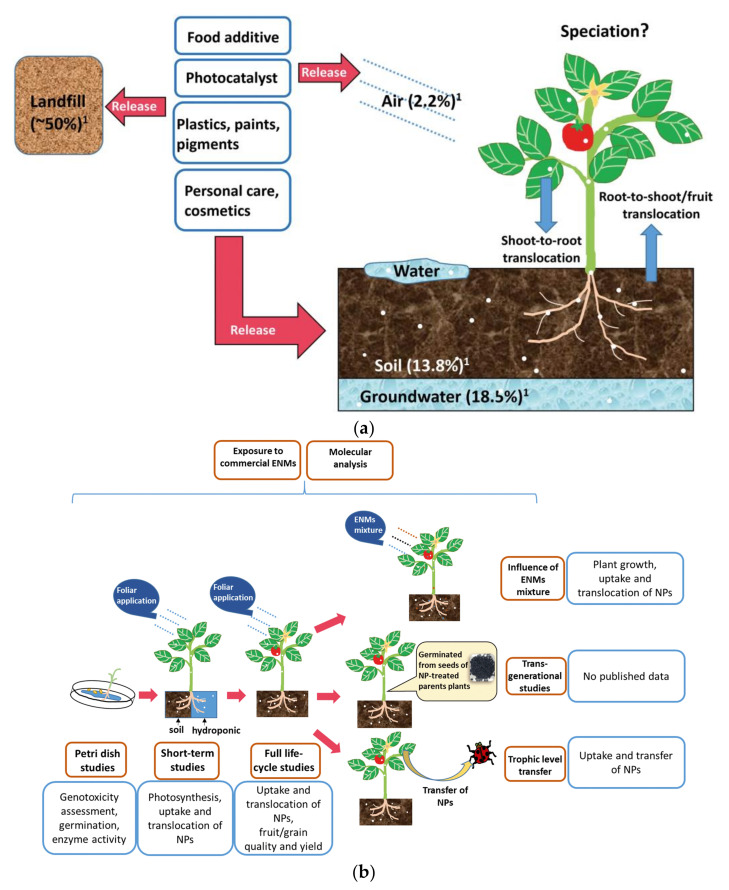
Although there are a limited number of papers concerning titania toxicity in higher plants [195,196]. Tan et al. [197] have summarized the interaction between nano-TiO2 and plants, including detection techniques, and possible effects of titania properties, such as the particle size, crystal phase, and surface coatings, as shown in Figure 10. Studies on the model plant Aribidopsis thaliana, crops and horticultural plants, such as Allium cepa, Avena sativa, Cucumis sativus, Nicotiana tabacum, Spinacia oleracea, Zea mays, Oryza sativa, Brassica campestris ssp. napusvar, Nippo-oleifera Makina, Phaseolus vulgaris var. humili, Solanum lycopersicum Glycine max, Daucus carota aquatic plants (e.g., Lemna minor), medical plants and willow tree Salix sp., have also been performed [152,195,196]. As summarized by Cox et al. [195], plants present various sensitivity to titania NPs. For example, for green algae Desmodesmus subspicatus, LC50 is 0.5g TiO2/dm3, whereas for the majority of other species, the value is larger than 1.0 g TiO2/dm3 [195,198]. Generally, plant basal metabolism and germination have not been affected by titania at a concentration of 2.0 g/dm3 [199]. It might be assumed that in some cases, titania might have even beneficial outcomes for some plants. For example, nano-anatase TiO2, which entered spinach cells easily, improves the whole chain of electron transport during photosynthesis, spinach growth and chlorophyll biosynthesis, and promotes the vigour of aged seeds [152,200]. Interestingly, improved root elongation and germination after exposition to titania at concentration of 20–40 mg/dm3 has also been found that could be associated with an increase in water uptake [54]. Similar results obtained by Andersen et al. [196] have shown that 1 g/dm3 titania (P25) does not cause widespread acute toxicity during germination and at early growth for 10 agronomic species. Although it has been known that NPs are uptaken by the seed coat, developing roots and leaves, the effects at later stages of the life cycle are still unknown. The toxic effects of small TiO2 particles (<100 nm) for A. cepa and N. tabacum have been caused by cellular oxidative stress, increased lipid peroxidation, antioxidative responses (enzyme activity) and induced DNA damage, e.g., chromosomal breaks, micronuclei formation, nuclear blebbing chromosome clumping and mitotic abnormalities [195].
Figure 10.
(a) Schematic representation of the titania interaction with plants; (b) trend of studies on the interaction between nano-TiO2 and plant systems. Blue box summarized the major findings in the study of each growth stage (orange box). Adapted from reference [197] with permission from Royal Society of Chemistry, 2018.
Although an increase in ROS generation correlates well with TiO2-NPs uptake and titania properties (i.e., NPs’ size, surface morphology and physical properties), the toxicity effect does not depend on the exposition time. Interestingly, it has been shown that agglomeration of NPs (generation of larger particles than 100 nm) could be a limiting factor for the titania uptake by plants. Additionally, even after NPs’ uptake into plant metabolic systems, titanium does not exhibit a toxic potential [195]. Moreover, Klancnik et al. [201] have found that 15-nm TiO2 does not have genotoxic potential under dark conditions (without UV light).
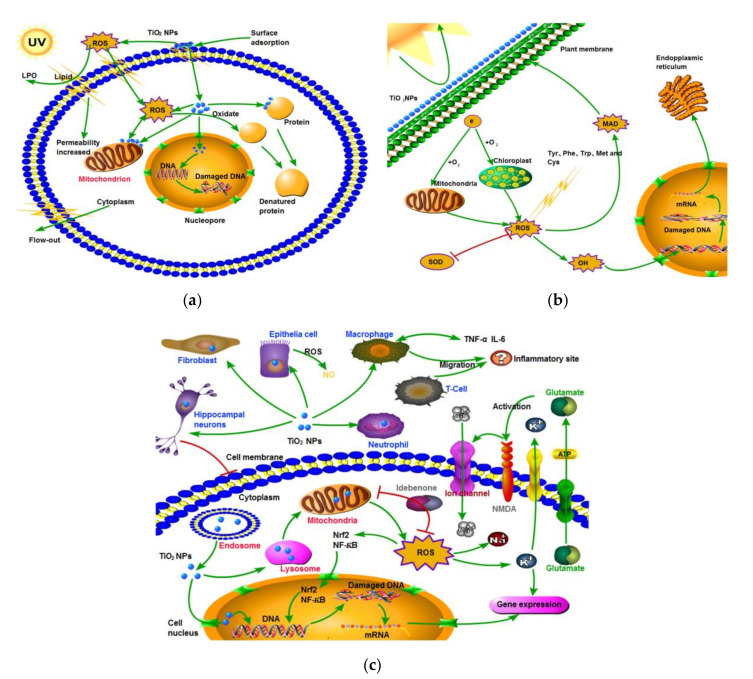
Titania NPs have been considered as safety antibacterial and antifungal agents for decades, as clearly reflected in a huge number of review papers concerning the application of titanium compounds as alternatives to antibiotic and antimicrobe agents [202,203,204,205,206,207,208,209,210,211]. This statement results from titania toxicity to prions, viruses, pathogenic Gram-positive and Gram-negative bacteria and microscopic fungi [212,213,214,215,216,217,218,219,220]. Most titania materials exhibit photocatalytic mechanism (by generation of ROS) of germ cells’ destruction. Titania likely interacts with cell walls and creates mechanical and chemical disruptions. Moreover, it has been found that the crystallite phase (anatase (A) or rutile (R) or mixture of them (AR)) and particle size influence the activity, as exemplary shown in Figure 11 [216].
Figure 11.
Transmission electron microscopy (TEM) images of E. coli bacteria unsliced (A–F) and sliced (G–L) without (A,G) and with the treatments of TiO2-NP 10A (B,H), TiO2-NP 25A (C,I), TiO2-NP 25AR (D,J), TiO2-NP 50A (E,K) and TiO2-NP 50R (F,L). Blue arrows, cells and red arrows, aggregated TiO2 NPs. Adapted from reference [216].
Significant antibacterial activity of titania materials occurs only for suitable (determined experimentally for given conditions) concentrations of microorganisms, types of titania and methods of application (e.g., dissolution or immobilization). However, it has been reported that electrostatic interactions (determined by Zeta potential) between bacteria membranes and NPs are probably the most important for toxicity [216,221]. Additionally, such factors as nature and intensity of light illumination and characteristics of titania dopants/modifiers should be also considered [212,216,217,222,223]. It is thought that the form, size and morphology of particles are also very important factors [214,224,225,226,227,228,229]. In recent studies, Baysal et al. [230] have shown that the physicochemical characteristics of titania (e.g., agglomeration properties) are related to the environment type. Moreover, the content/composition of matrix influences titania’s fate and behavior in aquatic and soil environments. The mechanism of toxicity is similar to that described for other groups of organisms, including ROS interactions with cell-wall composites, lipid peroxidation of cell membranes, an inhibition of crucial antioxidant enzymes and DNA damage (Figure 12) [222,226,231,232]. However, the mechanism for different titania compositions has not been clarified yet [231,232].
Figure 12.
The mechanism of toxicity of titania NPs to: (a) microorganisms; (b) algae and plants; and (c) invertebrates and vertebrates. Adapted from reference [231] with permission from Elsevier, 2019.
4. Summary and Conclusions
The correct evaluation of the risk of titanium and its compounds requires understanding of all the factors involved in their behavior, as well as the generation of toxicity. As shown in this review, the effects of titanium and its compounds depend on the physicochemical properties, tested organisms, exposure methodology (e.g., in vivo or in vitro, ex situ or in situ), exposure time, illumination conditions, and thus full characterization of all factors must be considered when toxicity is discussed.
It might be concluded that there are no fully convincing studies proving that titanium implants and titania photocatalysts cause serious health and environmental problems. The potential carcinogenicity of titania powders to humans has been considered by authorities in some European Union (EU) countries and appropriate procedures are in progress. However, broad applications of titanium compounds result in their accumulation in various organisms and environments, thus disturbing environmental sustainability. Moreover, excessive accumulation of titanium (as well as any other element) poses a threat and thus cannot be ignored. Therefore, it should be carefully considered if the use of titanium and its compounds is necessary, reasonable, and causes more pros than cons.
Studies indicating titania toxicity (or the contact allergy) to human and animals cannot be ignored, and all people having direct contact with titania need to be aware of the sporadic problem of its noxiousness. Efforts should be made to obtain scientifically sound toxicity data from toxicity tests in the nearest future. It is believed that those data would result in avoidance of an unnecessary protection burden for industry (e.g., a need for personal protective equipment, PPE) and confusion for customers.
Abbreviations
| ALT | Alanine aminotransferase |
| ANOVA | Analysis of variance |
| ANSES | French Agency for Food, Environmental and Occupational Health and Safety |
| AST | Aspartate transaminase |
| BBB | Brain Blood Barrier |
| BMSCs | Bone Marrow Stem Cells |
| BP-3 | Benzophenone-3 |
| CARS | Coherent Anti-stokes Raman Scattering and Coherent Anti-stokes Raman Scattering spectroscopy |
| CAT | Catalase |
| CLP | Classification, Labelling and Packaging |
| CMH2DCFDA | General Oxidative Stress Indicator, i.e., 5-(and-6)-chlomethyl-2′7′-dichlorodihydrofluorescein diacetate acetyl ester |
| DNA | Deoxyribonucleic acid |
| EC | European Council |
| EC50 | Half maximal Effective Concentration |
| EU | European Union |
| ECHA | European Chemicals Agency |
| GP | Glutathione Peroxidase |
| GR | Glutathione Reductase |
| GST | Glutathione-S-Transferase |
| IARC | International Agency for Research on Cancer |
| IL | Interleukin |
| IP | Intraperitoneal injection |
| LD50 | Median Lethal Dose |
| LDH | Lactate Dehydrogenase |
| MAK | Maximum Concentration values in the Workplace |
| MCF7 | Breast cancer cells (Michigan Cancer Foundation-7 cell line) |
| MC3T3-E1 | Mouse osteoblastic cells |
| mRNA | Messenger RNA |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of activated B cells |
| NLM | National Library of Medicine |
| NIOSH | National Institute for Occupational Safety and Health |
| NOAEL | No-Observed-Adverse-Effect Level |
| NPs | Nanoparticles |
| OSHA | The Occupational Safety and Health Administration |
| P25 | Evonik (Degussa) titanium dioxide (type P25) |
| PEL | Permissible Exposure Limit |
| PBS | Phosphate-Buffered Saline |
| PDL-hTERT | Periodontal Ligament human Telomerase Reverse Transcriptase |
| PPCPs | Pharmaceuticals and Personal Care Products |
| PPE | Personal Protective Equipment |
| RAC | Risk Assessment Committee |
| RANK-RANKL | Receptor Activator of Nuclear factor Kappa-Β–Receptor Activator of Nuclear factor Kappa-Β Ligand |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TBT | Tributyltin |
| TDMA | Titanium Dioxide Manufacturers Association |
| TNF-α | Tumor Necrosis Factor α |
| TPS | Titanium Plasma-Sprayed |
| TWA | Time-Weighted Average |
| UV-A | Ultraviolet A |
| VEGF | Vascular Endothelial Growth Factor |
| YAP | Yes-Associated Protein |
| YNS | Yellow Nail Syndrome |
Funding
This research was funded by from a Grand Challenges Explorations Grant (GCE RB, OPP1060234) for antimicrobial study on titania and “Yugo-Sohatsu Kenkyu” for an Integrated Research Consortium on Chemical Sciences (IRCCS) project from the Ministry of Education and Culture, Sport, Science and Technology-Japan (MEXT). The APC was funded A. M-S.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schwartz M. Encyclopedia and Handbook of Materials, Parts and Finishes. 3rd ed. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 2.Hock C.W. How TiCl3 Catalysts Control Texture of as-polymerized Polypropylene. Polym. Sci. 1966;4:3055–3065. doi: 10.1002/pol.1966.150041212. [DOI] [Google Scholar]
- 3.Niinomi M. Mechanical Properties of Biomedical Titanium Alloys. Mat. Sci. Eng. A Struct. 1998;243:231–236. doi: 10.1016/S0921-5093(97)00806-X. [DOI] [Google Scholar]
- 4.Kawamura Y., Hayashi K., Inoue A., Masumoto T. Rapidly Solidified Powder Metallurgy Mg97Zn1Y2 Alloys with Excellent Tensile Yield Strength above 600 MPa. Mater. Trans. 2001;42:1172–1176. doi: 10.2320/matertrans.42.1172. [DOI] [Google Scholar]
- 5.Ezugwu E.O. Key Improvements in the Machining of Difficult-to-cut Aerospace Superalloys. Int. J. Mach. Tools Manu. 2005;45:1353–1367. doi: 10.1016/j.ijmachtools.2005.02.003. [DOI] [Google Scholar]
- 6.Ouyang S.H., Huang Q.L., Liu Y., Ouyang Z.X., Liang L.X. Powder Metallurgical Ti-Mg Metal-metal Composites Facilitate Osteoconduction and Osseointegration for Orthopedic Application. Bioact. Mater. 2019;4:37–42. doi: 10.1016/j.bioactmat.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W., Tian J., Liu Z., Lu X., Hayat M.D., Yan Y., Li Z., Qu X., Wen C. Novel porous Ti35Zr28Nb scaffolds fabricated by powder metallurgy with excellent osteointegration ability for bone-tissue engineering applications. Mat. Sci. Eng. C Mater. 2019;105:110015. doi: 10.1016/j.msec.2019.110015. [DOI] [PubMed] [Google Scholar]
- 8.Maharubin S., Hu Y., Sooriyaarachchi D., Cong W., Tan G.Z. Laser Engineered Net Shaping of Antimicrobial and Biocompatible Titanium-silver Alloys. Mat. Sci. Eng. C. 2019;105:110059. doi: 10.1016/j.msec.2019.110059. [DOI] [PubMed] [Google Scholar]
- 9.Wu H., Xie J., Mao L. One-pot assembly Tannic Acid-titanium Dual Network Coating for Low-pressure Nanofiltration Membranes. Sep. Purif. Technol. 2020;233:116051. doi: 10.1016/j.seppur.2019.116051. [DOI] [Google Scholar]
- 10.Schkroeder H.A., Balassa J.J., Tipton I.H. Abnormal Trace Metals in Man: Titanium. J. Chronic Dis. 1963;16:55–69. doi: 10.1016/0021-9681(63)90019-5. [DOI] [PubMed] [Google Scholar]
- 11.Branemark P. Osseointegration and Its Experimental Background. J. Prosthet. Dent. 1983;50:399–410. doi: 10.1016/S0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 12.Pouilleau J., Devilliers D., Garrido F., Durand-Vidal S., Mahé E. Structure and Composition of Passive Titanium Oxide Films. Mat. Sci. Eng. B. 1997;47:235–243. doi: 10.1016/S0921-5107(97)00043-3. [DOI] [Google Scholar]
- 13.Hanawa T. Metal Ion Release from Metal Implants. Mat. Sci. Eng. C. 2004;24:745–752. doi: 10.1016/j.msec.2004.08.018. [DOI] [Google Scholar]
- 14.Hanawa T. Surface Treatment and Modification of Metals to Add Biofunction. Dent. Mater. J. 2017;36:533–538. doi: 10.4012/dmj.2017-154. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi T.P. An Overview of the Corrosion Aspect of Dental Implants (Titanium and its Alloys) Indian J. Dent. Res. 2009;20:91–98. doi: 10.4103/0970-9290.49068. [DOI] [PubMed] [Google Scholar]
- 16.Fage S.W., Muris J., Jakobsen S.S., Thyssen J.P. Titanium: A Review on Exposure, Release, Penetration, Allergy, Epidemiology, and Clinical Reactivity. Contact Dermat. 2016;74:323–345. doi: 10.1111/cod.12565. [DOI] [PubMed] [Google Scholar]
- 17.Zabrzyński J., Jaworski Ł. Myths and Facts about Combining Metals in Orthopedic surgery. Chir. Narz. Ruchu Ortop. Pol. 2017;82:58–62. [Google Scholar]
- 18.Spriano S., Yamaguchi S., Baino F., Ferraris S. A Critical Review of Multifunctional Titanium Surfaces: New Frontiers for Improving Osseointegration and Host Response, Avoiding Bacteria Contamination. Acta Biomater. 2018;79:1–22. doi: 10.1016/j.actbio.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Kim K.T., Eo M.Y., Nguyen T.T.H., Kim S.M. General Review of Titanium Toxicity. Int. J. Implant. Dent. 2019;5:10. doi: 10.1186/s40729-019-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strietzel R., Hösch A., Kalbfleisch H., Buch D. In Vitro Corrosion of Titanium. Biomaterials. 1998;19:1495–1499. doi: 10.1016/S0142-9612(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 21.Sicilia A., Cuesta S., Coma G., Arregui I., Guisasola C., Ruiz E., Maestro A. Titanium Allergy in Dental Implant Patients: A Clinical Study on 1500 Consecutive Patients. Clin. Oral Implant. Res. 2008;19:823–835. doi: 10.1111/j.1600-0501.2008.01544.x. [DOI] [PubMed] [Google Scholar]
- 22.Bhola R., Bhola S.M., Mishra B., Olson D.L. Corrosion in Titanium Dental Implants/prostheses—A Review. Trends Biomater. Artif. Organs. 2011;25:34–46. [Google Scholar]
- 23.Evrard L. Titanium: A new allergen. In: Turkyilmaz I., editor. Implant Dentistry—A Rapidly Evolving Practice. IntechOpen; London, UK: 2011. [Google Scholar]
- 24.Jacobs J.J., Skipor A.K., Patterson L.M., Hallab N.J., Paprosky W.G., Black J., Galante J.O. Metal Release in Patients Who Have Had a Primary Total Hip Arthroplasty: A Prospective, Controlled, Longitudinal Study. J. Bone Jt. Surg. 1998;80:1447–1458. doi: 10.2106/00004623-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kasai Y., Iida R., Uchida A. Metal Concentrations in the Serum and Hair of Patients with Titanium Alloy Spinal Implants. Spine. 2003;28:1320–1326. doi: 10.1097/01.BRS.0000065482.41115.B4. [DOI] [PubMed] [Google Scholar]
- 26.Soto-Alvaredo J., Blanco E., Bettmer J., Hevia D., Sainz R.M., López Cháves C., Sánchez C., Llopis J., Sanz-Medel A., Montes-Bayón M. Evaluation of the Biological Effect of Ti Generated Debris from Metal Implants: Ions and Nanoparticles. Metallomics. 2014;6:1702–1708. doi: 10.1039/C4MT00133H. [DOI] [PubMed] [Google Scholar]
- 27.Delgado-Ruiz R., Romanos G. Potential Causes of Titanium Particle and Ion Release in Implant Dentistry: A Systematic Review. Int. J. Mol. Sci. 2018;19:3585. doi: 10.3390/ijms19113585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiff N., Grosgogeat B., Lissac M., Dalard F. Influence of Fluoride Content and pH on the Corrosion Resistance of Titanium and its Alloys. Biomaterials. 2002;23:1995–2002. doi: 10.1016/S0142-9612(01)00328-3. [DOI] [PubMed] [Google Scholar]
- 29.Yu F., Addison O., Davenport A.J. A Synergistic Effect of Albumin and H2O2 Accelerates Corrosion of Ti6Al4V. Acta Biomater. 2015;26:355–365. doi: 10.1016/j.actbio.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 30.Penarrieta-Juanito G., Sordi M.B., Henriques B., Dotto M.E.R., Teughels W., Silva F.S., Magini R.S., Souza J.C.M. Surface Damage of Dental Implant Systems and Ions Release After Exposure to Fluoride and Hydrogen Peroxide. J. Periodontal. Res. 2019;54:46–52. doi: 10.1111/jre.12603. [DOI] [PubMed] [Google Scholar]
- 31.Haynes D.R., Rogers S.D., Hay S., Pearcy M.J., Howie D.W. The Differences in Toxicity and Release of Bone-resorbing Mediators Induced by Titanium and Cobalt-chromium-alloy Wear Particles. J. Bone Jt. Surg. 1993;75:825–834. doi: 10.2106/00004623-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Shettlemore M.G., Bundy K.J. Toxicity Measurement of Orthopedic Implant Alloy Degradation Products Using a Bioluminescent Bacterial Assay. J. Biomed. Mater. Res. 1999;45:395–403. doi: 10.1002/(SICI)1097-4636(19990615)45:4<395::AID-JBM15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q., Thouas G.A. Metallic Implant Biomaterials. Mat. Sci. Eng. R. 2015;87:1–57. doi: 10.1016/j.mser.2014.10.001. [DOI] [Google Scholar]
- 34.Khadija G., Saleem A., Akhtara Z., Naqvi Z., Gull M., Masood M., Mukhtar S., Batool M., Saleem N., Rasheed T., et al. Short Exposure to Titanium, Aluminum and Vanadium (Ti 6Al 4V) Alloy Powder Drastically Affects Behavior and Antioxidant Metabolites in Vital Organs of Male Albino Mice. Toxicol. Rep. 2018;5:765–770. doi: 10.1016/j.toxrep.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovan V., Tugce T., Topal E.S. The Effect of Molybdenum on Titanium’s Castability in Dental Prosthesis Applications: A Numerical Analysis. Proc. Inst. Mech. Eng. 2019;L 233:1966–1971. doi: 10.1177/1464420718802464. [DOI] [Google Scholar]
- 36.McMahon R.E., Ma J., Verkhoturov S.V., Munoz-Pinto D., Karaman I., Rubitschek F., Maier H.J., Hahn M.S. A Comparative Study of the Cytotoxicity and Corrosion Nickel-Titanium and Titanium-niobium Shape Memory Alloys. Acta Biomater. 2012;8:2863–2870. doi: 10.1016/j.actbio.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Kirmanidou T., Sidira M., Drosou M.E., Bennani V., Bakopoulou A., Tsouknidas A., Michailidis N., Michalakis K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016;2:1–21. doi: 10.1155/2016/2908570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikarashi Y., Toyoda K., Kobayashi E., Doi H., Yoneyama T., Hamanaka H., Tsuchiya T. Improved Biocompatibility of Titanium-zirconium (Ti–Zr) Alloy: Tissue Reaction and Sensitization to Ti–Zr Alloy Compared with Pure Ti and Zr in Rat Implantation Study. Mater. Trans. 2005;46:2260–2267. doi: 10.2320/matertrans.46.2260. [DOI] [Google Scholar]
- 39.Rehbock C., Jakobi J., Gamrad L., Van Der Meer S., Tiedemann D., Taylor U., Kues W., Rath D., Barcikowski S. Current State of Laser Synthesis of Metal and Alloy Nanoparticles as Ligand-free Reference Materials for Nano-toxicological Assays. Beilstein J. Nanotechnol. 2014;5:1523–1541. doi: 10.3762/bjnano.5.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee D.W., Yun Y.P., Park K., Kim S.E. Gentamicin and Bone Morphogenic Protein-2 (BMP-2)-delivering heparinized-titanium Implant with Enhanced Antibacterial Activity and Osteointegration. Bone. 2012;50:974–982. doi: 10.1016/j.bone.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Grischke J., Eberhard J., Stiesch M. Antimicrobial Dental Implant Functionalization Strategies—A Systematic Review. Dent. Mat. J. 2016;35:545–558. doi: 10.4012/dmj.2015-314. [DOI] [PubMed] [Google Scholar]
- 42.Mandell J.B., Deslouches B., Montelaro R.C., Shanks R.M.Q., Doi Y., Urish K.L. Elimination of Antibiotic Resistant Surgical Implant Biofilms Using an Engineered Cationic Amphipathic Peptide WLBU2. Sci. Rep. 2017;7:18098. doi: 10.1038/s41598-017-17780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rae T. The Toxicity of Metals Used in Orthopaedic Prostheses. An Experimental Study Using Cultured Human Synovial Fibroblasts. J. Bone Jt. Surg. 1981;63-B:435–440. doi: 10.1302/0301-620X.63B3.7263760. [DOI] [PubMed] [Google Scholar]
- 44.Olmedo D.G., Tasat D., Guglielmotti M.B., Cabrini R.L. Titanium Transport Through the Blood Stream. An Experimental Study on Rats. J. Mater. Sci. Mater. Med. 2013;14:1099–1103. doi: 10.1023/B:JMSM.0000004007.26938.67. [DOI] [PubMed] [Google Scholar]
- 45.He X., Reichl F.X., Wang Y., Michalke B., Milz S., Yang Y., Stolper P., Lindemaier G., Graw M., Hickel R., et al. Analysis of Titanium and Other Metals in Human Jawbones with Dental Implants—A Case Series Study. Dent. Mater. 2016;32:1042–1051. doi: 10.1016/j.dental.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Lukina E., Laka A., Kollerov M., Sampiev M., Mason P., Wagstaff P., Noordeen H., Yoon W.W., Blunn G. Metal Concentrations in the Blood and Tissues After Implantation of Titanium Growth Guidance Sliding Instrumentation. Spine J. 2016;16:380–388. doi: 10.1016/j.spinee.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 47.Schliephake H., Reiss G., Urban R., Neukam F.W., Guckel S. Metal Release from Titanium Fixtures During Placement in the Mandible: An Experimental Study. Int. J. Oral Maxillofac. Implant. 1993;8:502–511. [PubMed] [Google Scholar]
- 48.Ipach I., Schäfer R., Mittag F., Leichtle C., Wolf P., Kluba T. The Development of Whole Blood Titanium Levels After Instrumented Spinal Fusion—Is There a Correlation Between the Number of Fused Segments and Titanium Levels? BMC Musculoskelet. Disord. 2012;13:159. doi: 10.1186/1471-2474-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Temiz M., Dayi E., Saruhan N. Evaluation of Blood Titanium Levels and Total Bone Contact Area of Dental Implants. BioMed Res. Int. 2018:4121639. doi: 10.1155/2018/4121639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warme B.A., Epstein N.J., Trindade M.C., Miyanishi K., Ma T., Saket R.R., Regula D., Goodman S.B., Smith R.L. Proinflammatory Mediator Expression in a Novel Murine Model of Titanium-particle-induced Intramedullary Inflammation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004;71:360–366. doi: 10.1002/jbm.b.30120. [DOI] [PubMed] [Google Scholar]
- 51.Dalal A., Pawar V., McAllister K., Weaver C., Hallab N.J. Orthopedic Implant Cobalt-alloy Particles Produce Greater Toxicity and Inflammatory Cytokines than Titanium Alloy and Zirconium Alloy-based Particles in vitro, in Human Osteoblasts, Fibroblasts, and Macrophages. J. Biomed. Mater. Res. Part A. 2012;100:2147–2158. doi: 10.1002/jbm.a.34122. [DOI] [PubMed] [Google Scholar]
- 52.Wachi T., Shuto T., Shinohara Y., Matono Y., Makihira S. Release of Titanium Ions from an Implant Surface and their Effect on Cytokine Production Related to Alveolar Bone Resorption. Toxicology. 2015;327:1–9. doi: 10.1016/j.tox.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Safioti L.M., Kotsakis G.A., Pozhitkov A.E., Chung W.O., Daubert D.M. Increased Levels of Dissolved Titanium are Associated with Peri-implantitis—A Cross-sectional Study. J. Periodontol. 2017;88:436–442. doi: 10.1902/jop.2016.160524. [DOI] [PubMed] [Google Scholar]
- 54.Hatami M., Ghorbanpour M., Salehiarjomand H. Nano-anatase TiO2 Modulates the Germination Behavior and Seedling Vigority of Some Commercially Important Medicinal and Aromatic Plants. J. Biol. Environ. Sci. 2014;8:53–59. [Google Scholar]
- 55.Penmetsa S.L.D., Shah R., Thomas R., Kumar A.B.T., Gayatri P.S.D., Mehta D.S. Titanium particles in tissues from peri-implant mucositis: An exfoliative cytology-based pilot study. J. Indian Soc. Periodontol. 2017;21:192–194. doi: 10.4103/jisp.jisp_184_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suárez-López del Amo F., Garaicoa-Pazmiño C., Fretwurst T., Castilho R.M., Squarize C.H. Dental Implants—Associated release of Titanium Particles: A A systematic Review. Clin. Oral Implant. Res. 2018;29:1085–1100. doi: 10.1111/clr.13372. [DOI] [PubMed] [Google Scholar]
- 57.Goutam M., Giriyapura C., Mishra S.K., Gupta S. Titanium Allergy: A Literature Review. Indian J. Dermatol. 2014;59:630. doi: 10.4103/0019-5154.143526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bircher A.J., Stern W.B. Allergic Contact Dermatitis from “Titanium” Spectacle Frames. Contact Dermat. 2001;45:244–245. doi: 10.1034/j.1600-0536.2001.450417.x. [DOI] [PubMed] [Google Scholar]
- 59.Egusa H., Ko N., Shimazu T., Yatani H. Suspected Association of An Allergic Reaction with Titanium Dental Implants: A Clinical Report. J. Prosthet. Dent. 2008;100:344–347. doi: 10.1016/S0022-3913(08)60233-4. [DOI] [PubMed] [Google Scholar]
- 60.Thomas P., Bandl W.D., Maier S., Summer B., Przybilla B. Hypersensitivity to Titanium Osteosynthesis with Impaired Fracture Healing, Eczema, and T-cell Hyperresponsiveness In Vitro: Case Report and Review of the Literature. Contact Dermat. 2006;55:199–202. doi: 10.1111/j.1600-0536.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- 61.Wood W., Vermilyea S.G. A Review of Selected Dental Literature on Evidence-based Treatment Planning for Dental Implants: Reports of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. J. Prosthet. Dent. 2004;92:447–462. doi: 10.1016/j.prosdent.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Berglund F. Titanium and Yellow Nail Syndrome. In: Vannelli A., editor. Novel Strategies in Lymphedema. IntechOpen; London, UK: 2011. EPublishing. [Google Scholar]
- 63.Decker A., Daly D., Scher R.K. Role of Titanium in the Development of Yellow Nail Syndrome. Skin Appendage Disord. 2015;1:28–30. doi: 10.1159/000375171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ataya A., Kline K.P., Cope J., Alnuaimat H. Titanium Exposure and Yellow Nail Syndrome. Respir. Med. Case Rep. 2015;16:146–147. doi: 10.1016/j.rmcr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bianco P.D., Ducheyne P., Cuckler J.M. Local Accumulation of Titanium Released from a Titanium Implant in the Absence of Wear. J. Biomed. Mater. Res. 1996;31:227–234. doi: 10.1002/(SICI)1097-4636(199606)31:2<227::AID-JBM9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 66.Pettersson M., Pettersson J., Thoren M.M., Johansson A. Release of Titanium After Insertion of Dental Implants with Different Surface Characteristics—An ex vivo Animal Study. Acta Biomater. Odontol. Scand. 2018;3:63–73. doi: 10.1080/23337931.2017.1399270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martini D., Fini M., Franchi M., Pasquale V.D., Bacchelli B., Gamberini M., Tinti A., Taddei P., Giavaresi G., Ottani V., et al. Detachment of Titanium and Fluorohydroxyapatite Particles in Unloaded Endosseous Implants. Biomaterials. 2003;24:1309–1316. doi: 10.1016/S0142-9612(02)00508-2. [DOI] [PubMed] [Google Scholar]
- 68.Meng B., Chen J., Guo D., Ye Q., Liang X. The Effect of Titanium Particles on Rat Bone Marrow Stem Cells In Vitro. Toxicol. Mech. Method. 2009;19:552–558. doi: 10.3109/15376510903401716. [DOI] [PubMed] [Google Scholar]
- 69.Gomes C.C., Moreira L.M., Santos V.J.S.V., Ramos A.S., Lyon J.P., Soares C.P., Santos F.V. Assessment of the Genetic Risks of a Metallic Alloy Used in Medical Implants. Genet. Mol. Biol. 2011;34:116–121. doi: 10.1590/S1415-47572010005000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anastassopoulou J. Metal-DNA Interactions. J. Mol. Struct. 2003;651–653:19–23. doi: 10.1016/S0022-2860(02)00625-7. [DOI] [Google Scholar]
- 71.Shamsi M.H., Kraatz H.M. Interactions of Metal Ions with DNA and Some Applications. J. Inorg. Organomet. Polym. 2012;23:4–23. doi: 10.1007/s10904-012-9694-8. [DOI] [Google Scholar]
- 72.Egorova K.S., Ananikov V.P. Toxicity of Metal Compounds: Knowledge and Myths. Organometallics. 2017;36:4071–4090. doi: 10.1021/acs.organomet.7b00605. [DOI] [Google Scholar]
- 73.He X., Hartlieb E., Rothmund L., Waschke J., Wu X., Van Landuyt K.L., Milz S., Michalke B., Hickel R., Reichl F.X., et al. Intracellular Uptake and Toxicity of Three Different Titanium Particle. Dental Mater. 2015;31:734–744. doi: 10.1016/j.dental.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 74.Kumazawa R., Watari F., Takashi N., Tanimura Y., Uo M., Totsuka Y. Effects of Ti Ions and Particles on Neutrophil Function and Morphology. Biomaterials. 2002;23:3757–3764. doi: 10.1016/S0142-9612(02)00115-1. [DOI] [PubMed] [Google Scholar]
- 75.Evans E.J. Cell Damage In Vitro Following Direct Contact with Fine Particles of Titanium, Titanium Alloy and Cobalt-chrome-molybdenum Alloy. Biomaterials. 1994;15:713–717. doi: 10.1016/0142-9612(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 76.Tamura K., Takashi N., Kumazawa R., Watari F., Totsuka Y. Effects of Particle Size on Cell Function and Morphology in Titanium and Nickel. Mater. Trans. 2012;43:3052–3057. doi: 10.2320/matertrans.43.3052. [DOI] [Google Scholar]
- 77.Zhu W., Ming P., Qiu J., Shao S., Yu Y., Chen J., Yang J., Xu L., Zhang S., Tang C. Effect of Titanium Ions on the Hippo/YAP Signaling Pathway in Regulating Biological Behaviors of MC3T3-E1 Osteoblasts. J. Appl. Toxicol. 2018;38:824–833. doi: 10.1002/jat.3590. [DOI] [PubMed] [Google Scholar]
- 78.Suska F., Gretzer C., Esposito M., Emanuelsson L., Wennerberg A., Tengvall P., Thomsen P. In Vivo Cytokine Secretion and NF-κB Activation Around Titanium and Copper Implants. Biomaterials. 2005;26:519–527. doi: 10.1016/j.biomaterials.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 79.Obando-Pereda G.A., Fischer L., Stach-Machado D.R. Titanium and Zirconia Particle-induced Pro-inflammatory Gene Expression in Cultured Macrophages and Osteolysis, Inflammatory Hyperalgesia and Edema In Vivo. Life Sci. 2014;97:96–106. doi: 10.1016/j.lfs.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 80.Schuster G.S., Caughman G.B. Alterations of Cell Lipids by Metal Salts. J. Biomed. Mater. Res. A. 2004;70:347–353. doi: 10.1002/jbm.a.30091. [DOI] [PubMed] [Google Scholar]
- 81.López-Jornet P., Perrez F.P., Calvo-Guirado J.L., Lor-Ros I.L., Ramírez-Fernández P. Metallic Ion Content and Damage to the DNA in Oral Mucosa Cells Patients Treated Dental Implants. J. Mater. Sci. Mater. Med. 2014;25:1819–1824. doi: 10.1007/s10856-014-5203-7. [DOI] [PubMed] [Google Scholar]
- 82.Liao H., Wurtz T., Li J. Influence of Titanium Ion on Mineral Formation and Properties of Osteoid Nodules in Rat Calvaria Cultures. J. Biomed. Mater. Res. 1999;47:220–227. doi: 10.1002/(SICI)1097-4636(199911)47:2<220::AID-JBM12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 83.Fretwurst T., Buzanich G., Nahles S., Woelber J.P., Riesemeier H., Nelson K. Metal Elements in Tissue with Dental Peri-implantitis: A Pilot Study. Clin. Oral Implants Res. 2016;27:1178–1186. doi: 10.1111/clr.12718. [DOI] [PubMed] [Google Scholar]
- 84.Fretwurst T., Nelson K., Tarnow D.P., Wang H.L., Giannobile W.V. Is Metal Particle Release Associated with Peri-implant Bone Destruction? An Emerging Concept. J. Dent. Res. 2018;97:259–265. doi: 10.1177/0022034517740560. [DOI] [PubMed] [Google Scholar]
- 85.Aydoğan Z., Sisman T., Icekara U., Gurol A. Heavy Metal Accumulation in Some Aquatic Insects (Coleoptera: Hydrophilidae) and Tissue of Chondrostoma regium (Heckel, 1843) Relevant to Their Concentration in Water and Sediments from Karasu River, Erzurum, Turkey. Environ. Sci. Pollut. Res. 2017;24:9566–9574. doi: 10.1007/s11356-017-8629-x. [DOI] [PubMed] [Google Scholar]
- 86.López-Rodríguez G., Galván M., González-Unzaga M., Ávila J.H., Pérez-Labra M. Blood Toxic Metals and Hemoglobin Levels in Mexican Children. Environ. Monit. Assess. 2017;189:179. doi: 10.1007/s10661-017-5886-6. [DOI] [PubMed] [Google Scholar]
- 87.Elagli K., Neut C., Romond C., Hildebrand H.F. In Vitro Effects of Titanium Powder on Oral Bacteria. Biomaterials. 1992;13:25–27. doi: 10.1016/0142-9612(92)90090-B. [DOI] [PubMed] [Google Scholar]
- 88.Leonhardt A., Dahlén G. Effect of Titanium on Selected Oral Bacterial Species in Vitro. Eur. J. Oral Sci. 1995;103:382–387. doi: 10.1111/j.1600-0722.1995.tb01861.x. [DOI] [PubMed] [Google Scholar]
- 89.Berry C.W., Moore T.J., Safar J.A., Henry C.A., Wagner M.J. Antibacterial Activity of Dental Implant Metals. Implant Dent. 1992;1:59–65. doi: 10.1097/00008505-199200110-00006. [DOI] [PubMed] [Google Scholar]
- 90.Stolzoff M., Burns J.E., Aslani A., Tobin E.J., Nguyen C., De La Torre N., Golshan N.H., Ziemer K.S., Webster T.J. Decreased Bacterial Growth on Titanium Nanoscale Topographies Created by Ion Beam Assisted Evaporation. Int. J. Nanomed. 2017;12:1161–1169. doi: 10.2147/IJN.S119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pichat P. A Brief Survey of the Potential Health Risks of TiO2 Particles and TiO2-Containing Photocatalytic and Non-photocatalytic Materials. J. Adv. Oxid. Technol. 2010;13:238–246. doi: 10.1515/jaots-2010-0302. [DOI] [Google Scholar]
- 92.Friehs E., Al Salka Y., Jonczyk R., Lavrentieva A., Jochums A., Walter J.G., Stahl F., Scheper T., Bahnemann D. Toxicity, Phototoxicity and Biocidal Activity of Nanoparticles Employed in Photocatalysis. J. Photochem. Photobiol. C. 2016;29:1–28. doi: 10.1016/j.jphotochemrev.2016.09.001. [DOI] [Google Scholar]
- 93.Hex P.M., Tomenson J.A., Thompson P. Titanium Dioxide: Inhalation Toxicology and Epidemiology. Ann. Occup. Hyg. 2005;49:461–472. doi: 10.1093/annhyg/mei012. [DOI] [PubMed] [Google Scholar]
- 94.Bomgardner M.M. Europe May Call TiO2 a Carcinogen. Inhalation Studies in Rats Spark Push for Classification. Chem. Eng. News. 2017;95:13. [Google Scholar]
- 95.Boucher J. EU Moves Forward with TiO2 Carcinogen Classification. Food Packing Forum; Zurich, Switzerland: 2019. [(accessed on 25 August 2020)]. Available online: https://www.foodpackagingforum.org/news/eu-moves-forward-with-tio2-carcinogen-classification. [Google Scholar]
- 96.EC, 2019 Commission Delegated Regulation (EU)…/…of XXX Amending, for the Purposes of its Adoption to Technical and Scientific Progress, Regulation (EC) 1272/2008 of the European Parliament and of the Council on Classification, Labelling and Packaging of Substances and Mixtures and Correcting that Regulation. [(accessed on 12 December 2019)]; Available online: https://ec.europa.eu/transparency/regdoc/rep/3/2019/EN/C-2019-7227-1-EN-MAIN-PART-1.PDF.
- 97.Relier C., Dubreuil M., Lozano Garcia O., Cordelli E., Mejia J., Eleuteri P., Robidel F., Loret T., Pacchierotti F., Lucas S., et al. Study of TiO2 P25 Nanoparticles Genotoxicity on Lung, Blood, and Liver Cells in Lung Overload and Non-overload Conditions After Repeated Respiratory Exposure in Rats. Toxol. Sci. 2017;156:527–537. doi: 10.1093/toxsci/kfx006. [DOI] [PubMed] [Google Scholar]
- 98.Skocaj M., Filipic M., Petkovic J., Novak S. Titanium Dioxide in Our Everyday Life; Is It Safe? Radiol. Oncol. 2011;45:227–247. doi: 10.2478/v10019-011-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toxnet Toxicological Data Network. [(accessed on 7 December 2019)]; Available online: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+869.
- 100.Yamadori I., Ohsumi S., Taguchi K. Titanium Dioxide Deposition and Adenocarcinoma of the Lung. Acta Pathol. Jpn. 1986;36:783–790. doi: 10.1111/j.1440-1827.1986.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 101.IARC . Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans 1972–Present. World Health Organization, International Agency for Research on Cancer; Geneva, Switzerland: 1989. Multivolume Work. [Google Scholar]
- 102.Pimentel J.C. Systemic Granulomatous Disease, of the Sarcoid Type, Caused by Inhalation of Titanium Dioxide. Anatomo-clinical and Experimental Study. Acta Med. Port. 1992;5:307–313. [PubMed] [Google Scholar]
- 103.Moran C.A., Mullick F.G., Ishak K.G., Johnson F.B., Hummer W.B. Identification of Titanium in Human Tissues: Probable Role in Pathologic Processes. Hum. Pathol. 1991;22:450–454. doi: 10.1016/0046-8177(91)90130-H. [DOI] [PubMed] [Google Scholar]
- 104.Chen J.L., Fayerweather W.E. Epidemiologic study of workers exposed to titanium dioxide. J. Occup. Med. 1988;30:937–942. doi: 10.1097/00043764-198812000-00011. [DOI] [PubMed] [Google Scholar]
- 105.Boffetta P., Soutar A., Cherrie J.W., Granath F., Andersen A., Anttila A., Blettner M., Gaborieau V., Klug S.J., Langard S., et al. Mortality Among Workers Employed in the Titanium Dioxide Production Industry in Europe. Cancer Causes Control. 2004;15:697–706. doi: 10.1023/B:CACO.0000036188.23970.22. [DOI] [PubMed] [Google Scholar]
- 106.Fryzek J.P., Chadda B., Marano D., White K., Schweitzer S., McLaughlin J.K., Blot W.J. A Cohort Mortality Study Among Titanium Dioxide Manufacturing Workers in the United States. J. Occup. Environ. Med. 2003;45:400–409. doi: 10.1097/01.jom.0000058338.05741.45. [DOI] [PubMed] [Google Scholar]
- 107.Wang J., Fan Y. Lung Injury Induced by TiO2 Nanoparticles Depends on Their Structural Features: Size, Shape, Crystal Phases, and Surface Coating. Int. J. Mol. Sci. 2014;15:22258–22278. doi: 10.3390/ijms151222258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fröhlich E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Naomed. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi H., Magaye R., Castranova V., Zhao J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part. Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bennat C., Muller-Goymann C.C. Skin Penetration and Stabilization of Formulations Containing Microfine Titanium Dioxide as Physical UV Filter. Int. J. Cosmet. Sci. 2000;22:271–283. doi: 10.1046/j.1467-2494.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 111.Senzui M., Tamura T., Miura K., Ikarashi Y., Watanabe Y., Fujii M. Study on Penetration of Titanium Dioxide (TiO2) Nanoparticles into Intact and Damaged Skin in Vitro. J. Toxicol. Sci. 2010;35:107–113. doi: 10.2131/jts.35.107. [DOI] [PubMed] [Google Scholar]
- 112.Castanedo-Cázares J.P., Martínez-Rosales K., Hernández-Blanco D., Valdés-Rodríguez G., Torres-Álvarez B. In Vitro Assessment of Commercial Sunscreens Available in Latin America. Invest. Clin. 2014;55:142–154. [PubMed] [Google Scholar]
- 113.Xie G., Lu W., Lu D. Penetration of Titanium Dioxide Nanoparticles Through Slightly Damaged Skin in Vitro and in Vivo. J. Appl. Biomater. Funct. Mater. 2015;13:e356–e361. doi: 10.5301/jabfm.5000243. [DOI] [PubMed] [Google Scholar]
- 114.Monteiro-Riviere N.A., Wiench K., Landsiedel R., Schulte S., Inman A.O., Riviere J.E. Safety Evaluation of Sunscreen Formulations Containing Titanium Dioxide and Zinc Oxide Nanoparticles in UVB Sunburned Skin: An in Vitro and in Vivo Study. Toxicol. Sci. 2011;123:264–280. doi: 10.1093/toxsci/kfr148. [DOI] [PubMed] [Google Scholar]
- 115.Crosera M., Prodi A., Mauro M., Pelin M., Florio C., Bellomo F., Adami G., Apostoli P., De Palma G., Bovenzi M., et al. Titanium Dioxide Nanoparticle Penetration Into the Skin and Effects on HaCaT cells. Int. J. Environ. Res. Public Health. 2015;12:9282–9297. doi: 10.3390/ijerph120809282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dunford R., Salinaro A., Cai L., Serpone N., Horikoshi S., Hidaka H., Knowland J. Chemical Oxidation of DNA Damage Catalysed by Inorganic Sunscreen Ingredients. FEBS Lett. 1997;418:87–90. doi: 10.1016/S0014-5793(97)01356-2. [DOI] [PubMed] [Google Scholar]
- 117.McHugh P.J., Knowland J. Characterization of DNA Damage Inflicted by Free Radicals from a Mutagenic Sunscreen Ingredient and its Location Using an in vitro Genetic Reversion Assay. J. Photochem. Photobiol. 1997;66:276–281. doi: 10.1111/j.1751-1097.1997.tb08655.x. [DOI] [PubMed] [Google Scholar]
- 118.Hidaka H., Kobayashi H., Koike T., Sato T., Serpone N. DNA Damage Photoinduced by Cosmetic Pigments and Sunscreen Agents Under Solar Exposure and Artificial UV Illumination. J. Oleo Sci. 2006;55:249–261. doi: 10.5650/jos.55.249. [DOI] [Google Scholar]
- 119.Buchalska M., Kras G., Oszajca M., Lasocha W., Macyk W. Singlet Oxygen Generation in the Presence of Titanium Dioxide Materials Used as Sunscreens in Suntan Lotions. J. Photochem. Photobiol. A. 2010;213:158–163. doi: 10.1016/j.jphotochem.2010.05.019. [DOI] [Google Scholar]
- 120.Borza C., Muntean D., Dehelean C., Săvoiu G., Şerban C., Simu G., Andoni M., Butur M., Drăgan S. Oxidative Stress and Lipid Peroxidation—A Lipid Metabolism Dysfunction. In: Baez R.V., editor. Lipid Metabolism. IntechOpen; London, UK: 2013. [Google Scholar]
- 121.Markowska-Szczupak A., Wei Z., Kowalska E. The Influence of the Light-activated Titania P25 on Human Breast Cancer Cells. Catalysts. 2020;10:238. doi: 10.3390/catal10020238. [DOI] [Google Scholar]
- 122.Mortensen A., Aguilar F., Crebelli R., Di Domenico A., Dusemund B., Frutos M.J., Galtier P., Gott D., Gundert-Remy U., Lambre C., et al. Re-evaluation of Titanium Dioxide (E 171) as a Food Additive. Eur. Food Saf. Auth. J. 2016;14:e04545. [Google Scholar]
- 123.Wang J., Zhou G., Chen C., Yu H., Wang T., Ma Y., Jia G., Gao Y., Li B., Sun J., et al. Acute Toxicity and Biodistribution of Different Sized Titanium Dioxide Particles in Mice After Oral Administration. Toxicol. Lett. 2007;168:176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 124.Geraets L., Oomen A., Krystek P., Jacobsen N., Wallin H., Laurentie M., Verharen H., Brandon E., de Jong W.H. Tissue Distribution and Elimination After Oral and Intravenous Administration of Different Titanium Dioxide Nanoparticles in Rats. Part. Fibre Toxicol. 2014;11:30. doi: 10.1186/1743-8977-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shrivastava R., Raza S., Yadav A., Kushwaha P., Flora S.J. Effects of Sub-acute Exposure to TiO2, ZnO and Al2O3 Nanoparticles on Oxidative Stress and Histological Changes in Mouse Liver and Brain. Drug Chem. Toxicol. 2014;37:336–347. doi: 10.3109/01480545.2013.866134. [DOI] [PubMed] [Google Scholar]
- 126.Warheit D.B., Brown S.C., Donner E.M. Acute and Subchronic Oral Toxicity Studies in Rats with Nanoscale and Pigment Grade Titanium Dioxide Particles. Food Chem. Toxicol. 2015;84:208–224. doi: 10.1016/j.fct.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 127.Dekanski D., Spremo-Potparević B., Bajić V., Živković L., Topalović D., Sredojević D.N., Lazić V., Nedeljković J.M. Acute Toxicity Study in Mice of Orally Administrated TiO2 Nanoparticles Functionalized with Caffeic Acid. Food Chem. Toxicol. 2017;115:42–48. doi: 10.1016/j.fct.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 128.Fadda L.M., Hagar H., Mohamed A.M., Ali H.M. Quercetin and Idebenone Ameliorate Oxidative Stress, Inflammation, DNA Damage, and Apoptosis Induced by Titanium Dioxide Nanoparticles in Rat Liver. Dose Response. 2018;16 doi: 10.1177/1559325818812188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shakeel M., Jabeen F., Shabbir S., Asghar M.S., Khan M.S., Chaudhry A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Eexposure: A Review. Biol. Trace Elem. Res. 2016;172:1–36. doi: 10.1007/s12011-015-0550-x. [DOI] [PubMed] [Google Scholar]
- 130.El-Sharkawy N.I., Hamza S.M., Abou-Zeid E.H. Toxic Impact of Titanium Dioxide (TiO2) in Male Albino rats with Special Reference to Its Effect on Reproductive System. J. Am. Sci. 2010;6:865–872. [Google Scholar]
- 131.Mohammadipour A., Hosseini M., Fazel A., Haghir H., Rafatpanah H., Pourganji M., Bideskan A.E. The Effects of Exposure to Titanium Dioxide Nanoparticles During Lactation Period on Learning and Memory of Rat Offspring. Toxicol. Ind. Health. 2013;32:221–228. doi: 10.1177/0748233713498440. [DOI] [PubMed] [Google Scholar]
- 132.Bideskan A.E., Mohammadipour A., Fazel A., Haghir H., Rafatpanah H., Hosseini M., Rajabzadeh A. Maternal Exposure to Titanium Dioxide Nanoparticles During Pregnancy and Lactation Alters Offspring Hippocampal mRNA BAX and Bcl-2 levels, Induces Apoptosis and Decreases Neurogenesis. Exp. Toxicol. Pathol. 2017;69:329–337. doi: 10.1016/j.etp.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 133.Baranowska-Wójcik E., Szwajgier D., Oleszczuk P., Winiarska-Mieczan A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 1993:118–129. doi: 10.1007/s12011-019-01706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kowalska E. Materials for Food Storage with Antiseptic Properties. Global Grand Challenges: Bill and Melinda Gates Foundation (Grand Challenges Explorations Grant; GCE RB, OPP1060234) [(accessed on 10 September 2020)]; Available online: https://gcgh.grandchallenges.org/grant/materials-food-storage-antiseptic-properties.
- 135.Hong F., Zhou Y., Zhao X., Sheng L., Wang L. Maternal Exposure to Nanosized Titanium Dioxide Suppresses Embryonic Development in Mice. Int. J. Nanomed. 2017;12:6197–6204. doi: 10.2147/IJN.S143598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bannunah A.M., Vllasaliu D., Lord J., Stolnik S. Mechanisms of Nanoparticle Internalization and Transport Across an Intestinal Epithelial Cell Model: Effect of Size and Surface Charge. Mol. Pharm. 2014;11:4363–4373. doi: 10.1021/mp500439c. [DOI] [PubMed] [Google Scholar]
- 137.Gitrowski C., Al-Jubory A.R., Handy R.D. Uptake of Different Crystal Structures of TiO2 Nanoparticles by Caco-2 Intestinal Cells. Toxicol. Lett. 2014;226:264–276. doi: 10.1016/j.toxlet.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 138.Li J., Yang S., Lei R., Gu W., Qin Y., Ma S., Chen K., Chang Y., Bai X., Xia S., et al. Oral Administration of Rutile and Anatase TiO2 Nanoparticles Shifts Mouse Gut Microbiota Structure. Nanoscale. 2018;10:7736–7745. doi: 10.1039/C8NR00386F. [DOI] [PubMed] [Google Scholar]
- 139.Yong J.M., Mantaj J., Cheng Y., Vllasaliu D. Delivery of Nanoparticles Across the Intestinal Epithelium via the Transferrin Transport Pathway. Pharmaceutics. 2019;11:298. doi: 10.3390/pharmaceutics11070298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pinget G., Tan J., Janac B., Kaakoush N.O., Angelatos A.S., O’Sullivan J., Koay Y.C., Sierro F., Davis J., Divakarla S.K., et al. Impact of the Food Additive TiLitanium Dioxide (E171) on Gut Microbiota-host Interaction. Front. Nutr. 2019;6:57. doi: 10.3389/fnut.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Landy J., Ronde E., English N., Clark S.K., Hart A.L., Knight S.C., Ciclitira P.J., Al-Hassi H.O. Tight Junctions in Inflammatory Bowel Diseases and Inflammatory Bowel Disease Associated Colorectal Cancer. World J. Gastroenterol. 2016;22:3117–3126. doi: 10.3748/wjg.v22.i11.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bettini S., Boutet-Robinet E., Cartier C., Coméra C., Gaultier E., Dupuy J., Naud N., Taché S., Grysan P., Reguer S., et al. Food-grade TiO2 Impairs Intestinal and Systemic Immune Homeostasis, Initiates Preneoplastic Lesions and Promotes Aberrant Crypt Development in the Rat Colon. Sci. Rep. 2017;7:40373. doi: 10.1038/srep40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen H., Zhao R., Wang B., Cai C., Zheng L., Wang H., Wang M., Ouyang H., Zhou X., Chi Z., et al. The Effects of Orally Administered Ag, TiO2 and SiO2 Nanoparticles on Gut Microbiota Composition and Colitis Induction in Mice. NanoImpact. 2017;8:80–88. doi: 10.1016/j.impact.2017.07.005. [DOI] [Google Scholar]
- 145.Peng F., Setyawati M.I., Kai T.J., Ding I., Wang J., Nga M.E., Ho H.K., Leong D.T. Nanoparticles Promote in Vivo Breast Cancer Cell Intravasation and Extravasation by Inducing Endothelial Leakiness. Nat. Nanotech. 2019;14:279–286. doi: 10.1038/s41565-018-0356-z. [DOI] [PubMed] [Google Scholar]
- 146.Pott F., Roller M. Carcinogenicity Study with Nineteen Granular Dusts in Rats. Eur. J. Oncol. 2005;10:249–281. [Google Scholar]
- 147.Chen J., Dong X., Zhao J., Tang G. In Vivo Acute Toxicity of Titanium Dioxide Nanoparticles to Mice After Intraperitioneal Injection. J. Appl. Toxicol. 2009;29:330–337. doi: 10.1002/jat.1414. [DOI] [PubMed] [Google Scholar]
- 148.Disdier C., Devoy J., Cosnefroy A., Chalansonnet M., Herlin-Boime N., Brun E., Lund A., Mabondzo A. Tissue Biodistribution of Intravenously Administrated Titanium Dioxide Nanoparticles Revealed Blood-brain Barrier Clearance and Brain Inflammation in Rat. Part. Fibre Toxicol. 2015;12:27. doi: 10.1186/s12989-015-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Younes N.B.R., Amara S., Mrad I., Ben-Slama I., Jeljeli M., Omri K., El Ghoul K., El Mir L., Rhouma K.B., Abdelmelek H., et al. Subacute Toxicity of Titanium Dioxide (TiO2) Nanoparticles in Male Rats: Emotional Behavior and Pathophysiological Examination. Environ. Sci. Pollut. Res. Int. 2015;22:8728–8737. doi: 10.1007/s11356-014-4002-5. [DOI] [PubMed] [Google Scholar]
- 150.Kreyling W.G., Holzwarth U., Haberl N., Kozempel J., Hirn S., Wenk A., Schleh C., Schäffler M., Lipka J., Semmler-Behnke M., et al. Quantitative Biokinetics of Titanium Dioxide Nanoparticles After Intravenous Injection in Rats: Part 1. Nanotoxicology. 2017;11:434–442. doi: 10.1080/17435390.2017.1306892. [DOI] [PubMed] [Google Scholar]
- 151.Scown T.M., Van Aerle R., Johnston B.D., Cumberland S., Lead J.R., Owen R., Tyler C.R. High Doses of Intravenously Administered Titanium Dioxide Nanoparticles Accumulate in the Kidneys of Rainbow Trout but with no Observable Impairment of Renal Function. Toxicol. Sci. 2009;109:372–380. doi: 10.1093/toxsci/kfp064. [DOI] [PubMed] [Google Scholar]
- 152.Menard A., Drobne D., Jemec A. Ecotoxicity of Nanosized TiO2. Review of in Vivo Data. Environ. Poll. 2011;159:677–684. doi: 10.1016/j.envpol.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 153.Amde M., Liu J., Tan Z.Q., Bekana D. Transformation and Bioavailability of Metal Oxide Nanoparticles in Aquatic and Terrestrial Environments. A Review. Environ. Poll. 2017;230:250–267. doi: 10.1016/j.envpol.2017.06.064. [DOI] [PubMed] [Google Scholar]
- 154.Kiser M.A., Westerhoff P., Benn T., Wang Y., Pérez-Rivera J., Hristovski K. Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants. Environ. Sci. Technol. 2009;43:6757–6763. doi: 10.1021/es901102n. [DOI] [PubMed] [Google Scholar]
- 155.Bystrzejewska-Piotrowska G., Golimowski J., Urban P.L. Nanoparticles: Their Potential Toxicity, Waste and Environmental Management. Waste Manag. 2009;29:2587–2595. doi: 10.1016/j.wasman.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 156.Brausch J.M., Rand G.M. A Review of Personal Care Products in the Aquatic Environment: Environmental Concentrations and Toxicity. Chemosphere. 2011;82:1518–1532. doi: 10.1016/j.chemosphere.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 157.Labille J., Slomberg D., Catalano R., Robert S., Boudenne J.L., Apers-Tremelo M.L., Masion A., De Garidel C. Evaluation of the Environmental Exposure to Nanoparticulate UV-filters Used in Sunscreens; Proceedings of the Goldschmidt Conference; Boston, MA, USA. 12–17 August 2018. [Google Scholar]
- 158.Zhu X., Zhou J., Cai Z. TiO2 Nanoparticles in the Marine Environment: Impact on the Toxicity of Tributyltin to Abalone (Haliotis diversicolor supertexta) Embryos. Environ. Sci. Technol. 2011;45:3753–3758. doi: 10.1021/es103779h. [DOI] [PubMed] [Google Scholar]
- 159.Libralato G., Minetto D., Totaro S., Mičetić S., Pigozzo A., Sabbioni E., Marcomini A., Ghirardini A.V. Embryotoxicity of TiO2 Nanoparticles to Mytilus galloprovincialis (Lmk) Mar. Environ. Res. 2013;92:71–78. doi: 10.1016/j.marenvres.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 160.D’Agata A., Fasulo S., Dallas L.J., Fisher A.S., Maisano M., Readman J.W., Jha A.N. Enhanced Toxicity of ’Bulk’ Titanium Dioxide Compared to ’Fresh’ and ’Aged’ nano-TiO2 in Marine Mussels (Mytilus galloprovincialis) Nanotoxicology. 2014;8:549–558. doi: 10.3109/17435390.2013.807446. [DOI] [PubMed] [Google Scholar]
- 161.Canesi L., Ciacci C., Vallotto D., Gallo G., Marcomini A., Pojana G. In Vitro Effects of Suspensions of Selected Nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes. Aquat. Toxicol. 2010;96:151–158. doi: 10.1016/j.aquatox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 162.Miller R.J., Bennett S., Keller A.A., Pease S., Lenihan H.S. TiO2 Nanoparticles are Phototoxic to Marine Phytoplankton. PLoS ONE. 2012;7:e30321. doi: 10.1371/journal.pone.0030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Danovaro R., Corinaldesi C. Sunscreen Products Increase Virus Production Through Prophage Induction in Marine Bacterioplankton. Microb. Ecol. 2003;45:109–118. doi: 10.1007/s00248-002-1033-0. [DOI] [PubMed] [Google Scholar]
- 164.Danovaro R., Bongiorni L., Corinaldesi C., Giovannelli D., Damiani E., Astolfi P., Greci L., Pusceddu A. Sunscreens Cause Coral Bleaching by Promoting Viral Infections. Environ. Health Perspect. 2008;116:441–447. doi: 10.1289/ehp.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Giokas D.L., Salvador A., Chisvert A. UV Filters: From Sunscreens to Human Body and the Environment. TRAC Trend. Anal. Chem. 2007;26:360–374. doi: 10.1016/j.trac.2007.02.012. [DOI] [Google Scholar]
- 166.Galloway T., Lewis C., Dolciotti I., Johnston B.D., Moger J., Regoli F. Sublethal Toxicity of Nano-titanium Dioxide and Carbon Nanotubes in a Sediment Dwelling Marine Polychaete. Environ. Poll. 2010;158:1748–1755. doi: 10.1016/j.envpol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 167.Zhu X., Zhou J., Cai Z. The Toxicity and Oxidative Stress of TiO2 Nanoparticles in Marine Abalone (Haliotis diversicolor supertexta) Mar. Poll. Bull. 2011;63:334–338. doi: 10.1016/j.marpolbul.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 168.Barmo C., Ciacci C., Canonico B., Fabbri R., Cortese K., Balbi T., Marcomini A., Pojana G., Gallo G., Canesi L. In Vivo Effects of N-TiO2 on Digestive Gland and Immune Function of the Marine Bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2013;132–133:9–18. doi: 10.1016/j.aquatox.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 169.Clemente Z., Castro V.L., Jonsson C.M., Fraceto L.F. Minimal Levels of Ultraviolet Light Enhance the Toxicity of TiO2 Nanoparticles to Two Representative Organisms of Aquatic Systems. J. Nanoparticle Res. 2014;16:2559. doi: 10.1007/s11051-014-2559-z. [DOI] [Google Scholar]
- 170.Wang Y., Zhu X., Lao Y., Lv X., Tao Y., Huang B., Wang J., Zhou J., Cai Z. TiO2 Nanoparticles in the Marine Environment: Physical Effects Responsible for the Toxicity on Algae Phaeodactylum tricornutum. Sci. Total Environ. 2016;565:818–826. doi: 10.1016/j.scitotenv.2016.03.164. [DOI] [PubMed] [Google Scholar]
- 171.Johnsen E.C. Toxicological Effects of Commercial Sunscreens on Coral Reef Ecosystems: New Protocols for Coral Restoration. HCNSO Student Capstones, Nova Southeastern University NSU Works. [(accessed on 18 March 2020)];2018 Available online: https://nsuworks.nova.edu/cnso_stucap/335.
- 172.Anaya-Esparza L.M., González-Silva N., Yahia E.M., González-Vargas O.A., Montalvo-González E., Pérez-Larios A. Effect of TiO2-ZnO-MgO Mixed Oxide on Microbial Growth and Toxicity Against Artemia salina. Nanomaterials. 2019;9:992. doi: 10.3390/nano9070992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sobek A., Bejgarn S., Rudén C., Molander L., Breitholtz M. In the Shadow of the Cosmetic Directive—Inconsistencies in EU Environmental Hazard Classification Requirements for UV-Filters. Sci. Total Environ. 2013;461–462:706–711. doi: 10.1016/j.scitotenv.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 174.Prichard E., Granek E.F. Effects of Pharmaceuticals and Personal Care Products on Marine Organisms: From Single-species Studies to an Ecosystem-based Approach. Environ. Sci. Poll. Res. 2016;23:22365–22384. doi: 10.1007/s11356-016-7282-0. [DOI] [PubMed] [Google Scholar]
- 175.Baun A., Hartmann N.B., Grieger K., Kusk K.O. Ecotoxicity of Engineered Nanoparticles to Aquatic Invertebrates: A Brief Review and Recommendations for Future Toxicity Testing. Ecotoxicology. 2008;17:387–396. doi: 10.1007/s10646-008-0208-y. [DOI] [PubMed] [Google Scholar]
- 176.Liu J., Fan D., Wang L., Shi L., Ding J., Chen Y., Shen S. Effects of ZnO, CuO, Au, and TiO2 Nanoparticles on Daphnia magna and Early Life Stages of Zebrafish Danio Rerio. Environ. Prot. Eng. 2014;40:139–149. [Google Scholar]
- 177.Heinlaan M., Ivask A., Blinova I., Dubourguier H.C., Kahru A. Toxicity of Nanosized and Bulk ZnO, CuO and TiO2 to Bacteria Vibrio fischeri and Crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 178.Zhu X., Chang Y., Chen Y. Toxicity and Bioaccumulation of TiO2 Nanoparticle Aggregates in Daphnia magna. Chemosphere. 2010;78:209–215. doi: 10.1016/j.chemosphere.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 179.Kim S.C., Lee D.K. Preparation of TiO2-coated Hollow Glass Beads and Their Application to the Control of Algal Growth in Eutrophic Water. Microchem. J. 2005;80:227–322. doi: 10.1016/j.microc.2004.07.008. [DOI] [Google Scholar]
- 180.Kahru A., Dubourguier H.C. From Ecotoxicology to Nanoecotoxicology. Toxicology. 2010;269:105–119. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 181.Lead J.R., Batley G.E., Alvarez P.J.J., Croteau M.N., Handy R.D., McLaughlin M.J., Judy J.D., Schirmerh K. Nanomaterials in the Environment: Behavior, Fate, Bioavailability, and Effects—An Updated Review. Environ. Toxicol. Chem. 2018;37:2029–2063. doi: 10.1002/etc.4147. [DOI] [PubMed] [Google Scholar]
- 182.Sun H.W., Zhang X.Z., Niu Q., Chen Y.S., Crittenden J.C. Enhanced Accumulation of Arsenate in Carp in the Presence of Titanium Dioxide Nanoparticles. Water Air Soil Poll. 2007;178:245–254. doi: 10.1007/s11270-006-9194-y. [DOI] [Google Scholar]
- 183.Ramsden C.S., Smith T.J., Shaw B.J., Handy R.D. Dietary Exposure to Titanium Dioxide Nanoparticles in Rainbow Trout (Oncorhynchus mykiss): No Effect on Growth, but Subtle Biochemical Disturbances in the Brain. Ecotoxicology. 2009;18:939–995. doi: 10.1007/s10646-009-0357-7. [DOI] [PubMed] [Google Scholar]
- 184.Linhua H., Zhenyu W., Baoshan X. Effect of Sub-acute Exposure to TiO2 Nanoparticles on Oxidative Stress and Histopathological Changes in Juvenile Carp (Cyprinus carpio) J. Environ. Sci. 2009;21:1459–1466. doi: 10.1016/s1001-0742(08)62440-7. [DOI] [PubMed] [Google Scholar]
- 185.Kim M.S., Louis K.M., Pedersen J.A., Hamers R.J., Peterson R.E., Heideman W. Using Citrate-functionalized TiO2 Nanoparticles to Study the Effect of Particle Size on Zebrafish Embryo Toxicity. Analyst. 2014;139:964–972. doi: 10.1039/c3an01966g. [DOI] [PubMed] [Google Scholar]
- 186.Asztemborska M., Jakubiak M., Stęborowski R., Chajduk E., Bystrzejewska-Piotrowska G. Titanium Dioxide Nanoparticle Circulation in an Aquatic Ecosystem. Water Air Soil Poll. 2018;229:208. doi: 10.1007/s11270-018-3852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vevers W.F., Jha A.N. Genotoxic and Cytotoxic Potential of Titanium Dioxide (TiO2) Nanoparticles on Fish Cells in Vitro. Ecotoxicology. 2008;17:411–421. doi: 10.1007/s10646-008-0226-9. [DOI] [PubMed] [Google Scholar]
- 188.Reeves J.F., Davies S.J., Dodd N.J., Jha A.N. Hydroxyl Radicals (OH) are Associated with Titanium Dioxide (TiO2) Nanoparticle-induced Cytotoxicity and Oxidative DNA Damage in Fish Cells. Mut. Res. 2008;640:113122. doi: 10.1016/j.mrfmmm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 189.Zhang X.Z., Sun H.W., Zhang Z.Y., Niu Q., Chen Y.S., Crittenden J.C. Enhanced Bioaccumulation of Cadmium in Carp in the Presence of Titanium Dioxide Nanoparticles. Chemosphere. 2007;67:160–166. doi: 10.1016/j.chemosphere.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 190.Larue C.C., Baratange C., Vantelon D., Khodja H., Surblé S., Elger A., Carrière M. Influence of Soil Type on TiO2 Nanoparticle Fate in an Agro-ecosystem. Sci. Total Environ. 2018;630:609–617. doi: 10.1016/j.scitotenv.2018.02.264. [DOI] [PubMed] [Google Scholar]
- 191.Simonin M., Richaume A., Guyonnet J.P., Dubost A., Martins J.F.M., Pommier T. Titanium Dioxide Nanoparticles Strongly Impact Soil Microbial Function by Affecting Archaeal Nitrifiers. Sci. Rep. 2016;6:33643. doi: 10.1038/srep33643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Drobne D., Jemec A., Tkalec Z.P. In Vivo Screening to Determine Hazards of Nanoparticles: Nanosized TiO2. Environ. Poll. 2009;157:1157–1164. doi: 10.1016/j.envpol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 193.Roh J.Y., Park Y.K., Park K., Choi J. Ecotoxicological Investigation of CeO2 and TiO2 Nanoparticles on the Soil Nematode Caenorhabditis elegans Using Gene Expression, Growth, Fertility, and Survival as Endpoints. Environ. Toxicol. Pharmacol. 2010;29:167–172. doi: 10.1016/j.etap.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 194.Hu C.W., Li M., Cui Y.B., Li D.S., Chen J., Yang L.Y. Toxicological Effects of TiO2 and ZnO Nanoparticles in Soil on Earthworm Eisenia fetida. Soil Biol. Biochem. 2010;42:586–591. doi: 10.1016/j.soilbio.2009.12.007. [DOI] [Google Scholar]
- 195.Cox A., Venkatachalam P., Sahi S., Sharma N. Silver and Titanium Dioxide Nanoparticle Toxicity in Plants: A Review of Current Research. Plant Physiol. Biochem. 2016;107:147–163. doi: 10.1016/j.plaphy.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 196.Andersen C.P., King G., Plocher M., Storm M., Pokhrel L.R., Johnson M.G., Rygiewicz P.T. Germination and Early Plant Development of Ten Plant Species Exposed to Titanium Dioxide and Cerium Oxide Nanoparticles. Environ. Toxicol. Chem. 2016;35:2223–2229. doi: 10.1002/etc.3374. [DOI] [PubMed] [Google Scholar]
- 197.Tan W., Peralta-Videa J.R., Gardea-Torresdey J.L. Interaction of Titanium Dioxide Nanoparticles with Soil Components and Plants: Current Knowledge and Future Research Needs—A Critical Review. Environ. Sci. Nano. 2018;5:257–278. doi: 10.1039/C7EN00985B. [DOI] [Google Scholar]
- 198.Song U., Shin M., Lee G., Roh J., Kim J., Lee E.J. Functional Analysis of TiO2 Nanoparticle Toxicity in Three Plant Species. Biol. Trace Elem. Res. 2013;155:93–103. doi: 10.1007/s12011-013-9765-x. [DOI] [PubMed] [Google Scholar]
- 199.Yang Z.Z., Chen J., Dou R.Z., Gao X., Mao C.B., Wang L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.) Int. J. Environ. Res. Public Health. 2015;12:15100–15109. doi: 10.3390/ijerph121214963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lei Z., Mingyu S., Chao L., Liang C., Hao H., Xiao W., Xiaoqing L., Fan Y., Fengqing G., Fashui H. Effects of Nanoanatase TiO2 on Photosynthesis of Spinach Chloroplasts Under Different Light Iillumination. Biol. Trace Elem. Res. 2007;119:68–76. doi: 10.1007/s12011-007-0047-3. [DOI] [PubMed] [Google Scholar]
- 201.Klancnik K., Drobne D., Valant J., Koce J.D. Use of a Modified Allium Test with NanoTiO2. Ecotoxicol. Environ. Saf. 2011;74:85–92. doi: 10.1016/j.ecoenv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 202.Gumy D., Rincon A.G., Hajdu R., Pulgarin C. Solar Photocatalysis for Detoxification and Disinfection of Water: Different Types of Suspended and Fixed TiO2 Catalysts Study. Sol. Energy. 2006;80:1376–1381. doi: 10.1016/j.solener.2005.04.026. [DOI] [Google Scholar]
- 203.Malato S., Blanco J., Alarcón D.C., Maldonado M.I., Fernández-Ibáñez P., Gernjak W. Photocatalytic Decontamination and Disinfection of Water with Solar Collectors. Catal. Today. 2007;122:137–149. doi: 10.1016/j.cattod.2007.01.034. [DOI] [Google Scholar]
- 204.Foster H.A., Ditta I.B., Varghese S., Steele A. Photocatalytic Disinfection Using Titanium Dioxide: Spectrum and Mechanism of Antimicrobial Activity. Appl. Microbiol. Biotechnol. 2011;90:1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Markowska-Szczupak A., Ulfig K., Morawski A.W. The Application of Titanium Dioxide for Deactivation of Bioparticulates: An Overview. Catal. Today. 2011;169:249–257. doi: 10.1016/j.cattod.2010.11.055. [DOI] [Google Scholar]
- 206.Pigeot-Rémy S., Simonet F., Errazuriz-Cerda E., Lazzaroni J.C., Atlan D., Guillard C. Photocatalysis and Disinfection of Water: Identification of Potential Bacterial Targets. Appl. Cat. B. 2011;104:390–398. doi: 10.1016/j.apcatb.2011.03.001. [DOI] [Google Scholar]
- 207.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 208.Qu X., Alvarez P.J.J., Li Q. Applications of Nanotechnology in Water and Wastewater Treatment. Water Res. 2013;47:3931–3946. doi: 10.1016/j.watres.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 209.Hossain F., Perales-Perez O.J., Hwang S., Román F. Antimicrobial Nanomaterials as Water Disinfectant: Applications, Limitations and Future Perspectives. Sci. Total Environ. 2014;466–467:1047–1059. doi: 10.1016/j.scitotenv.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 210.Wang L., Hu C., Shao L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Abo-Zeid Y., Williams G.R. The Potential Anti-infective Applications of Metal Oxide Nanoparticles: A Systematic Review. Wiley Interdiscp. Nanomed. Nanobiotechnol. 2020;12:e1592. doi: 10.1002/wnan.1592. [DOI] [PubMed] [Google Scholar]
- 212.Gogniat G., Dukan S. TiO2 Photocatalysis Causes DNA Damage via Fenton Reaction-Generated Hydroxyl Radicals During the Recovery Period. Appl. Environ. Microbiol. 2007;73:7740–7743. doi: 10.1128/AEM.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Van Grieken R., Marugan J., Pablos C., Lopez A. Comparison Between the Photocatalytic Inactivation of Gram-positive E. faecalis and Gram-negative E. coli Faecal Contamination Indicator Microorganisms. Appl. Catal. B. 2010;100:212–220. doi: 10.1016/j.apcatb.2010.07.034. [DOI] [Google Scholar]
- 214.Markowska-Szczupak A., Wang K., Rokicka P., Endo M., Wei Z., Ohtani B., Morawski A.W., Kowalska E. The Effect of Anatase and Rutile Crystallites Isolated from Titania P25 Photocatalyst on Growth of Selected Mould fungi. J. Photch. Photobiol. B. 2015;151:54–62. doi: 10.1016/j.jphotobiol.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 215.Liou J.W., Chang H.H. Bactericidal Effects and Mechanisms of Visible Light-responsive Titanium Dioxide Photocatalysts on Pathogenic Bacteria. Arch. Immunol. Ther. Ex. 2012;60:267–275. doi: 10.1007/s00005-012-0178-x. [DOI] [PubMed] [Google Scholar]
- 216.Lin X., Li J., Ma S., Liu G., Yang K., Tong M., Lin D. Toxicity of TiO2 Nanoparticles to Escherichia coli: Effects of Particle Size, Crystal Phase and Water Chemistry. PLoS ONE. 2014;10:110247. doi: 10.1371/journal.pone.0110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kowalska E., Wei Z., Karabiyik B., Herissan A., Janczarek M., Endo M., Markowska-Szczupak A., Remita H., Ohtani B. Silver-modified Titania with Enhanced Photocatalytic and Antimicrobial Properties under UV and Visible Light Irradiation. Catal. Today. 2015;252:136–142. doi: 10.1016/j.cattod.2014.10.038. [DOI] [Google Scholar]
- 218.Leung Y.H., Xu X., Ma A.P., Liu F., Ng A.M., Shen Z., Gethings L.A., Guo M.Y., Djurišić A.B., Lee P.K., et al. Toxicity of ZnO and TiO2 to Escherichia coli Cells. Sci. Rep. 2016;6:35243. doi: 10.1038/srep35243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wei Z., Endo M., Wang K., Charbit E., Markowska-Szczupak A., Ohtani B., Kowalska E. Noble Metal-modified Octahedral Anatase Titania Particles with Enhanced activity for Decomposition of Chemical and Microbiological Pollutants. Chem. Eng. J. 2017;318:121–134. doi: 10.1016/j.cej.2016.05.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wanag A., Rokicka P., Kusiak-Nejman E., Kapica-Kozar J., Wróbel R.J., Markowska-Szczupak A., Morawski A.W. Antibacterial Properties of TiO2 Modified with Reduced Graphene Oxide. Ecotox. Environ. Saf. 2018;147:788–793. doi: 10.1016/j.ecoenv.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 221.Planchon M., Ferrari R., Guyot F., Gélabert A., Menguy N., Chanéac C., Thill A., Benedetti M.F., Spalla O. Interaction Between Escherichia coli and TiO2 Nanoparticles in Natural and Artificial Waters. Colloids Surf. B. 2013;102:158–164. doi: 10.1016/j.colsurfb.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 222.Marugan J., Van Grieken R., Sordo C., Cruz C. Kinetics of the Photocatalytic Disinfection of Escherichia coli Suspensions. Appl. Catal. B Environ. 2008;82:27–36. doi: 10.1016/j.apcatb.2008.01.002. [DOI] [Google Scholar]
- 223.Benabbou A.K., Derriche Z., Felix C., Lejeune P., Guillard C. Photocatalytic Inactivation of Escherischia coli: Effect of Concentration of TiO2 and Microorganism, Nature, and Intensity of UV Irradiation. Appl. Catal. B Environ. 2007;76:257–263. doi: 10.1016/j.apcatb.2007.05.026. [DOI] [Google Scholar]
- 224.Cho M., Chung H., Choi W., Yoon J. Linear Correlation Between Inactivation of E. coli and OH Radical Concentration in TiO2 Photocatalytic Disinfection. Water Res. 2004;38:1069–1077. doi: 10.1016/j.watres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 225.Kiwi J., Nadtochenko V. New Evidence for TiO2 Photocatalysis During Bilayer Lipid Peroxidation. J. Chem. Phys. Chem. B. 2004;108:17675–17684. doi: 10.1021/jp048281a. [DOI] [Google Scholar]
- 226.Dalrymple O.K., Stefanakos E., Trotz M.A., Goswami D.Y. A Review of the Mechanisms and Modeling of Photocatalytic Disinfection. Appl. Catal. B Environ. 2010;98:27–38. doi: 10.1016/j.apcatb.2010.05.001. [DOI] [Google Scholar]
- 227.Kim S., Ghafoor K., Lee J., Feng M., Hong J., Lee D.U., Park J. Bacterial Inactivation in Water, DNA Strand Breaking, and Membrane Damage Induced by Ultraviolet-assisted Titanium Dioxide Photocatalysis. Water Res. 2013;47:4403–4411. doi: 10.1016/j.watres.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 228.Endo M., Janczarek M., Wei Z., Wang K., Markowska-Szczupak A., Ohtani B., Kowalska E. Bactericidal Properties of Plasmonic Photocatalysts Composed of Noble-metal Nanoparticles on Faceted Anatase Titania. J. Nanosci. Nanotechnol. 2019;9:442–452. doi: 10.1166/jnn.2019.15780. [DOI] [PubMed] [Google Scholar]
- 229.Endo M., Wei Z., Wang K., Karabiyik B., Kenta Y., Rokicka P., Ohtani O., Markowska-Szczupak A., Kowalska E. Noble Metal-modified Titania with Visible-Light Activity for the Decomposition of Microorganisms. Beilstein J. Nanotech. 2018;9:829–841. doi: 10.3762/bjnano.9.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Baysal A., Saygin H., Ustabasi G.S. Physicochemical Transformation of ZnO and TiO2 Nanoparticles in Sea Water and its Impact on Bacterial Toxicity. Environ. Health Eng. Manag. 2019;6:73–80. doi: 10.15171/EHEM.2019.08. [DOI] [Google Scholar]
- 231.Hou J., Wang L., Wang C., Zhang S., Liu H., Li H., Wang X. Toxicity and Mechanisms of Action of Titanium Dioxide Nanoparticles in Living Organisms. J. Environ. Sci. 2019;75:40–53. doi: 10.1016/j.jes.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 232.Wang P., Li K., Qian J., Wang C., Lu B., Tian X., Jin W., He X. Differential Toxicity of Anatase and Rutile TiO2 Nanoparticles to the Antioxidant Enzyme System and Metabolic Activities of Freshwater Biofilms Based on Microelectrodes and Fluorescence in situ Hybridization. Environ. Sci. Nano. 2019;6:2626–2640. doi: 10.1039/C9EN00389D. [DOI] [Google Scholar]