Abstract
(1) Background: Transcranial magnetic stimulation (TMS) approaches are widely used to study cortical and corticospinal function. However, responses to TMS are subject to significant intra-and inter-individual variability. Acute and chronic exposure to recreational substances alters the excitability of the sensorimotor system and may contribute to the variability in TMS outcome measures. The increasing prevalence of recreational substance use poses a significant challenge for executing TMS studies, but there is a lack of clarity regarding the influence of these substances on sensorimotor function. (2) Methods: The literature investigating the influence of alcohol, nicotine, caffeine and cannabis on TMS outcome measures of corticospinal, intracortical and interhemispheric excitability was reviewed. (3) Results: Both acute and chronic use of recreational substances modulates TMS measures of excitability. Despite the abundance of research in this field, we identify knowledge gaps that should be addressed in future studies to better understand the influence of these substances on TMS outcomes. (4) Conclusions: This review highlights the need for TMS studies to take into consideration the history of participant substance use and to control for acute substance use prior to testing.
Keywords: transcranial magnetic stimulation, caffeine, alcohol, nicotine, cannabis
1. Introduction
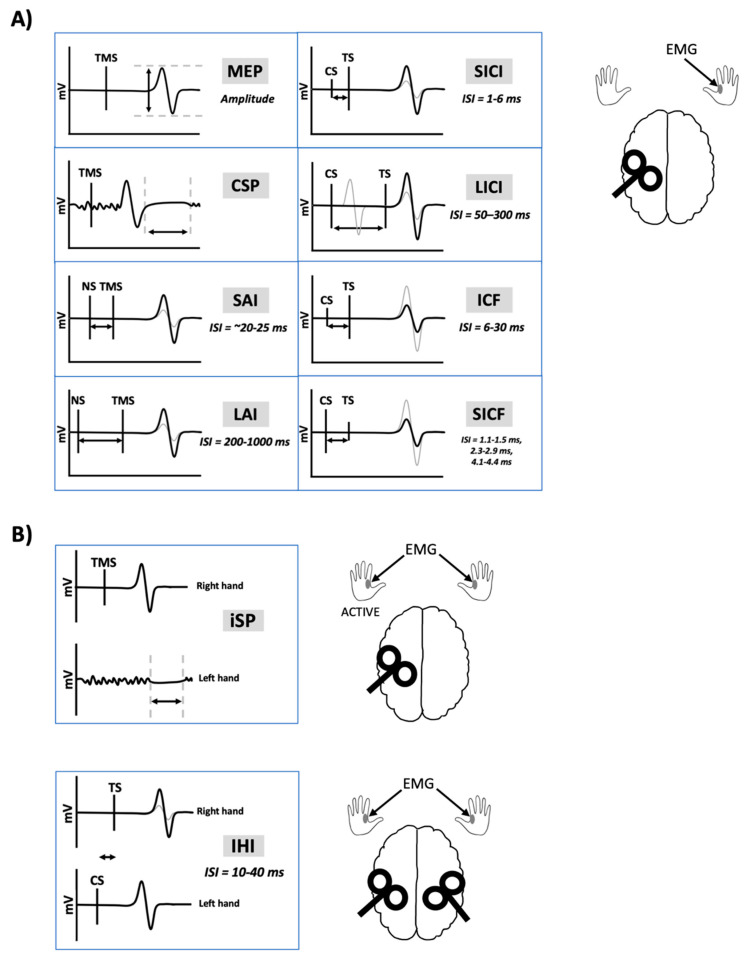
Transcranial magnetic stimulation (TMS) has been extensively used to non-invasively probe the motor system in healthy and clinical populations to study the neural mechanisms of human movement and evaluate neuroplasticity. TMS paradigms are used to acquire measurements of corticospinal, intracortical and transcallosal excitability (Figure 1). Importantly, exogenous substances are capable of influencing the physiological state at the time of testing and may change cortical and corticospinal excitability as measured by TMS-evoked responses. Exogenous substances include recreational drugs such as nicotine, alcohol, cannabis, caffeine and other controlled substances. It is common for TMS studies to enroll university students as participants out of convenience. However, consumption of recreational substances such as alcohol [1], nicotine [2], and cannabis [3] peaks in young adults between the ages of 18–29.
Figure 1.
Transcranial magnetic stimulation-electromyography (TMS-EMG) outcome measures. Black lines indicate traces following a single pulse of TMS. Grey lines indicate traces following nerve stimulation (NS) and TMS paired together or following a conditioning stimulus (CS) and test stimulus (TS) pair. (A) Unilateral measures. TMS delivered to the left motor cortex results in a motor-evoked potential (MEP) recorded from a muscle in the right-hand using EMG. Delivering TMS during isometric contraction of the right-hand muscle leads to an interruption of voluntary contraction known as the cortical silent period (CSP). Short-latency afferent inhibition (SAI) and long-latency afferent inhibition (LAI) occurs when electrical peripheral NS is delivered prior to the TMS pulse, at interstimulus intervals (ISIs) of 20–25 ms or 200–1000 ms, respectively. Short-interval intracortical inhibition (SICI) is measured when a subthreshold CS is delivered 1–6 ms prior to a suprathreshold TS. The resulting MEP is inhibited, compared to the MEP obtained following the TS alone. Long-interval intracortical inhibition (LICI) is measured when a suprathreshold CS is delivered 50–300 ms prior to a suprathreshold TS, leading to inhibition of the MEP. Intracortical facilitation (ICF) is measured when a subthreshold CS is delivered 6–30 ms prior to a suprathreshold TS, leading to facilitation of the MEP. Short-interval intracortical facilitation (SICF) is measured when a suprathreshold CS is delivered 1.1–1.5 ms, 2.3–2.9 ms, or 4.1–4.4 ms prior to a subthreshold TS, leading to facilitation of the MEP. (B) Transcallosal measures. Delivering TMS to the left motor cortex during isometric contraction of the left-hand muscle leads to an interruption of voluntary contraction known as the ipsilateral silent period (iSP). Interhemispheric inhibition (IHI) is measured when a suprathreshold CS is delivered to the right motor cortex prior to a suprathreshold TS delivered to the left motor cortex, leading to inhibition of the MEP. Short-latency IHI (SIHI) occurs at ISIs of ~10 ms and long-latency IHI (LIHI) occurs at ISIs of ~40 ms.
Safety guidelines for TMS delivery suggest screening participants for consumption of alcohol and drugs that lower seizure thresholds [4]. However, there are no official guidelines regarding the screening of other common, recreational substances including cannabis, nicotine and caffeine. At present, it is unclear whether or how short-and long-term use of these substances are capable of inducing changes in neuronal excitability that is reflected in TMS measures or their variability. In this review, we consider research investigating acute and chronic use of recreational substances on TMS outcome measures for the purpose of identifying knowledge gaps that, if filled, may lead to new guidelines to improve the reliability and safety of TMS research. In this review, our use of the term “chronic use” refers to those with current or previous addiction or substance abuse. The review focuses on recreational substance use of alcohol, cannabis, caffeine and nicotine, and their impact on measures of corticospinal and cortical excitability.
2. Methods
A literature search was conducted using PubMed and EMBASE electronic databases from 1980 through August 2020. Separate searches were conducted for each substance of alcohol, cannabis, nicotine, and caffeine. The following search terms were used:
(transcranial magnetic stimulation) AND ((alcohol) or (alcoholism) or (ethanol));
(transcranial magnetic stimulation) AND ((nicotine) or (tobacco));
(transcranial magnetic stimulation) AND (caffeine);
(transcranial magnetic stimulation) AND ((cannabis) or (THC) or (marijuana)).
Inclusion/Exclusion Criteria
Selected studies were required to meet the following inclusion criteria:
Studies assessed one or more of the following TMS measures: motor-evoked potential (MEP), resting motor threshold (RMT), active motor threshold (AMT), short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), short-interval intracortical facilitation (SICF), long-interval intracortical inhibition (LICI), interhemispheric inhibition (IHI), short-latency afferent inhibition (SAI), long-latency afferent inhibition (LAI), cortical silent period (CSP), ipsilateral silent period (iSP), or TMS-evoked electroencephalography (EEG) potentials.
Primary research articles (i.e., original research) only.
Article was written in English.
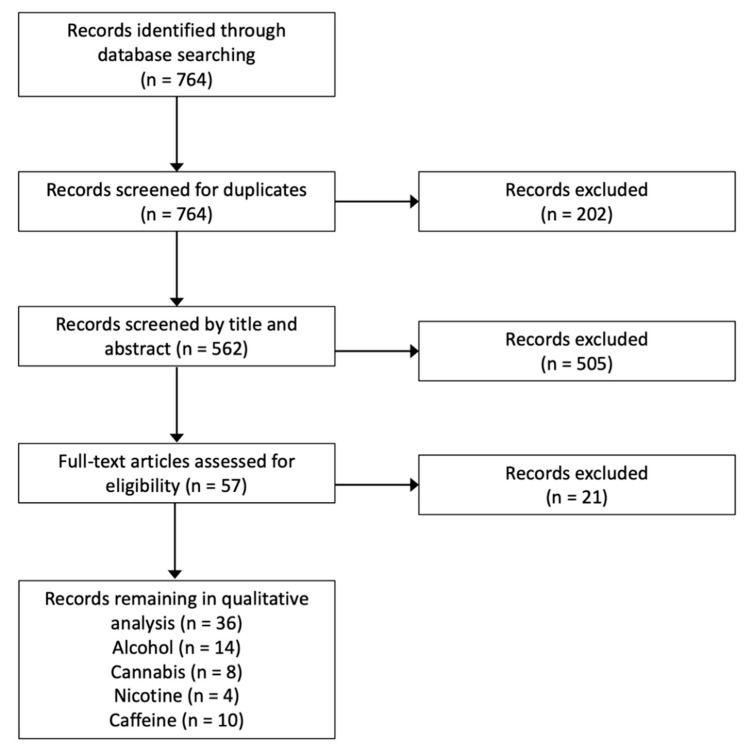
Studies assessing the influence of neuroplasticity-inducing protocols (e.g., repetitive transcranial magnetic stimulation, rTMS; paired associative stimulation, PAS; transcranial direct current stimulation, tDCS) on cravings or symptoms relating to chronic substance use were excluded as this was outside the scope of this review. For reviews on this topic, refer to Gorelick et al. [5], Hanlon et al. [6], Hauer et al. [7], or Mostafavi et al. [8]. Further, studies assessing the influence of substance use on the magnitude of corticospinal change following neuroplasticity-inducing protocols were excluded. Refer to Figure 2 for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. Details of all studies considered in this review are found in Table S1.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram.
3. Alcohol
3.1. Acute Effects of Alcohol
Table 1 shows the effects of alcohol on TMS measures. There is a general consensus that acute alcohol intake facilitates gamma aminobutyric acid (GABA) neurotransmission and reduces glutamatergic neurotransmission [9,10]. Acute ethanol exposure increases GABA-mediated chloride-ion currents [11,12,13], increases cortical GABA-mediated inhibition [14], and facilitates the effects of GABAA receptor (GABAAR) agonists [15]. In contrast, ethanol inhibits N-methyl-D-aspartate (NMDA) receptor activity [16,17] and reduces glutamate release [18,19,20].
Table 1.
Effects of alcohol on TMS measures.
| Study | AMT | RMT | MEP | CSP | iSP | SAI | LAI | SICI | ICF | SICF | LICI | SIHI | LIHI | N100 | N45 | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ziemann et al. [21] | ○ | ○ | ○ | ▲ | – | – | – | ▲ | ▼ | – | – | – | – | – | – | Acute intake |
| Ziemann et al. [22] | – | ○ | ○ | – | – | – | – | – | – | ▼ | – | – | – | – | – | Acute intake |
| Conte et al. [23] | – | ○ | ○ | ▲ | – | – | – | – | – | – | – | – | – | – | – | Acute intake in controls |
| – | ○ | – | ○ | – | – | – | ○ | ○ | – | – | – | – | – | – | Alcoholics vs. controls | |
| Hoppenbrouwers et al. [24] | – | – | ▼ | – | – | – | – | – | – | – | – | ▼* | – | – | – | Acute intake; * significant in females only |
| Loheswaran et al. [25] | – | – | ○ | – | – | – | – | – | – | – | – | – | – | ▼* | – | Acute intake in alcoholics; * acquired in DLPFC |
| Kähkönen et al. [26] | – | – | – | – | – | – | – | – | – | – | – | – | – | ▼* | – | Acute intake; * acquired in M1 |
| Muralidharan et al. [27] | – | ○ | – | ▼ | ▼ | – | – | – | – | – | – | – | – | – | – | High-vs. low-risk for alcohol dependence |
| Nardone et al. [28] | ○ | ○ | – | ○ | – | – | – | ○ | ▲ | – | – | – | – | – | – | AWS vs. alcoholics and controls |
| Muralidharan et al. [29] | – | ○ | ○ | – | – | – | – | – | – | – | – | – | – | – | – | High-vs. low-risk for alcohol dependence |
| Naim-Feil et al. [30] | ▼ | ▼ | ▼ | ○ | – | – | – | ○ | ○ | – | ▼* | – | – | – | – | Alcoholics vs. controls; * LICI acquired in DLPFC |
| Kaarre et al. [31] | – | ○ | – | – | – | – | – | – | – | – | – | – | – | – | ▲ | Heavy alcohol use in adolescence vs. controls |
| Quoilin et al. [32] | – | – | ▼ | – | – | – | – | – | – | – | – | – | – | – | – | Alcoholics vs. controls |
▲ increase; ▼decrease; ○ indicates no change; − indicates did not assess; * refers to stipulations outlined in the right-hand column; AMT: active motor threshold; AWS: Alcohol Withdrawal Syndrome; CSP: cortical silent period; DLPFC: dorsolateral prefrontal cortex; ICF: intracortical facilitation; iSP: ipsilateral silent period; LAI: long-latency afferent inhibition; LICI: long-interval intracortical inhibition; LIHI: long-latency interhemispheric inhibition; M1: primary motor cortex; MEP: motor-evoked potential; N100: TMS-evoked electroencephalography potential at 100 ms latency; N45: TMS-evoked electroencephalography potential at 45 ms latency; RMT: resting motor threshold; SAI: short-latency afferent inhibition; SICF: short-interval intracortical facilitation; SICI: short-interval intracortical inhibition; SIHI: short-latency interhemispheric inhibition.
Acute ethanol intake does not induce changes in RMT [21,22] or AMT [21], suggesting that ethanol does not modulate the excitability of the lowest threshold neurons in the primary motor cortex (M1). Furthermore, several studies demonstrate no effect of ethanol intake on MEPs [21,22,25]. However, it is not known if ethanol impacts the variability in MEPs.
The TMS findings from Ziemann et al. [21] reflect the consensus that acute alcohol intake facilitates GABAergic neurotransmission and reduces glutamatergic neurotransmission. A single dose of ethanol administered to healthy individuals increased SICI [21], suggesting an upregulation of GABAAR activity [33], and reduced ICF [21], suggesting a downregulation of NMDA receptor activity [33]. Further, ethanol increased the CSP [21,23], which may reflect an increase in GABAB receptor (GABABR) [33] and/or GABAAR activity [34]. Acute ethanol intake reduced the TMS-evoked N100 potential following stimulation of the dorsolateral prefrontal cortex (DLPFC) [25] and M1 [26]. The N100 potential is modulated by both baclofen (a GABABR agonist) and benzodiazepines (positive allosteric modulators of the GABAAR) [35]. Several studies suggest that upregulation of GABABR activity may serve as a homeostatic mechanism to regulate the sensitivity of GABAARs to ethanol [36,37,38]. Therefore, modulation of the N100 potential likely reflects the complex mechanisms through which ethanol exerts its pre-and postsynaptic effects via GABABRs and GABAARs, respectively. To our knowledge, no study has assessed the influence of alcohol intake on the amplitude of the N45 potential. However, given that the N45 potential is reflective of GABAAR activity similar to SICI [35], this may suggest that alcohol intake would reduce the N45 potential. Finally, SICF is reduced following ethanol consumption [22]. This suggests that ethanol exerts its effects at the level of I-wave generating interneurons, which are thought to be regulated by GABAergic mechanisms [39].
Alcohol reduced short-latency interhemispheric inhibition (SIHI) in healthy females, but not males [24]. This aligns with evidence that alcohol has a larger physiological impact in females. Females are more susceptible to alcohol-related cognitive impairments than males [40,41,42,43], likely because females reach higher peak blood alcohol levels than males even when adjusting for difference in weight [44]. Circulating levels of sex steroid hormones also interact with ethanol. Specifically, testosterone injection inhibits ethanol-induced impairments in spatial memory [45] and estradiol increases the sensitivity of dopaminergic neurons in the ventral tegmental area (VTA) to ethanol [46]. Therefore, future research investigating the effect of alcohol on TMS should consider the influence of biological sex.
Summary
In summary, findings from the TMS literature appear to indicate that acute intake of alcohol increases SICI and CSP, while reducing ICF, SICF, SIHI, and the TMS-evoked N100 potential (Table 1). However, the acute administration of alcohol does not modulate MEP amplitude, and so does not appear to influence the corticospinal excitability.
3.2. Chronic Effects of Alcohol
Chronic alcohol exposure has the opposite effect of acute exposure characterized by a reduction in GABAergic neurotransmission and an increase in glutamatergic neurotransmission [9,10,47]. Chronic alcohol exposure reduces the sensitivity of GABAARs [48,49,50], the density of cortical GABAARs carrying α1-and α5-subunits [51,52] and blunts the effect of GABAAR agonists [37,53]. Furthermore, chronic alcohol exposure increases NMDA receptor-mediated excitatory post-synaptic potentials and glutamate release [19]. The dichotomy between the acute and chronic effects of alcohol may be due to physiological changes in the dopaminergic reward pathway that occur during the transition to chronic alcohol consumption [54]. Acutely, ethanol stimulates dopamine release in a dose-dependent fashion within the nucleus accumbens (NAc) [55,56,57,58,59]. Chronically, ethanol lowers circulating dopamine levels by increasing the rate of dopamine re-uptake in the NAc and the sensitivity of the D2 autoreceptor, which acts to inhibit dopamine release [60].
Alcohol-dependents exhibit reduced RMT, AMT, and MEPs compared to healthy controls [30,32]. In contrast, other studies report no chronic-related effects on RMT, AMT or MEPs [23,28,31]. Importantly, Naim-Feil et al. [30] recruited participants on the basis that they had successfully completed a detoxification program within the past two years. This indicates that while thresholds are not altered in chronic alcoholics, the physiological changes resulting from detoxification induced changes in thresholds. However, it is unknown if and for how long these physiological changes persist beyond two years.
In young adults who exhibited alcohol-dependence in adolescence, TMS-evoked N45 potentials were larger compared to controls [31]. This suggests an upregulation of GABAAR activity [35], which does not follow the expected chronic effects of alcohol [9,10,47]. Alcohol-dependence during a stage of development may have led to different physiological changes compared to the effect of alcohol-dependence in adulthood.
Several studies have reported no difference in SICI between alcohol-dependents and controls, suggesting that chronic alcohol intake does not modulate GABAAR activity within M1 [23,28,30]. The underlying effect of alcoholism on different subunits of the GABAARs may explain why alcohol dependence leads to an increase in the N45 potential but no change in SICI, which are both modulated by GABAAR activity. The N45 potential is increased by agonists to the α1-subunit [35], while SICI likely reflects GABAARs with α2/3-subunits [33,61,62]. This may suggest that alcohol-dependence has a physiological effect only on specific subunits of the GABAAR (i.e., the α1 subunit). LICI is reduced within the DLPFC of alcohol-dependents compared to healthy controls, which suggests hyperexcitability of the prefrontal cortex as a result of reduced GABABR activity [30].
The chronic effects of alcohol on SAI and LAI have yet to be investigated. Unlike SICI, SAI is likely modulated by GABAARs containing the α1-subunit [62]. Similar to SAI, LAI is also modulated by the GABAAR agonist lorazepam [63]. If chronic alcohol intake specifically alters functioning of GABAARs with the α1-subunit, it can be hypothesized that it would lead to an alteration in SAI and LAI.
Chronic ethanol exposure does not have an effect on CSP [23,28,30]. However, these studies may have been underpowered to expose an effect due to the relatively small sample sizes used [23,28,30]. Individuals at high-risk for alcohol dependence exhibit shorter CSP and iSP [27], but no difference in RMT or the % maximum stimulator output (%MSO) to evoke a 1 mV amplitude MEP in comparison to low-risk individuals [29]. This suggests that those predisposed to alcohol dependence may have inherited an imbalance of excitation/inhibition. Future studies should investigate if these predisposed changes in physiology also lead to differential effects of acute alcohol intake on cortical function. Evidence for a potential effect comes from in vitro studies, where rodent strains bred for high ethanol sensitivity exhibit greater effects of ethanol on GABA-receptor chloride conductance compared to low-alcohol sensitive rodents [9,64,65].
One important consideration is the dependency on alcohol at the time of testing. Compared to both controls and chronic alcoholics, individuals with alcohol-withdrawal syndrome exhibit increased ICF [28], which may reflect hyperexcitability as a result of chronic exposure. However, there is no difference in SICI between controls and alcoholics when individuals are not tested during withdrawal [23,30]. Overall, the effects of chronic alcohol consumption appear to be dependent upon an individual’s physiological state at the time of testing.
Summary
Based on the findings reviewed herein, chronic alcohol consumption appears to modulate intracortical circuitry underlying SICI, ICF, SICF and CSP (Table 1). Overall, the effects of chronic alcohol consumption appear to be dependent upon an individual’s physiological state at the time of testing—whether individuals are alcohol-dependents with versus without withdrawal symptoms in adulthood, were previous dependents in adolescence, recently completed detoxification, or have a familial history of alcohol dependence. This widespread effect clearly demonstrates the considerable impact that long-term consumption of alcohol has on neurophysiology.
4. Cannabis
According to the World Health Organization, ~2.5% of the global population consumes cannabis annually. Accompanying its legalization, the prevalence of cannabis use has risen by 15% in Canada [66] and 25% in the United States [67]. Long-term cannabis use is a risk factor for the development of schizophrenia [68] and neurocognitive impairments [69], and leads to structural changes such as increased cortical thickness [70,71,72] and cerebellar volume [73,74], and reduced hippocampal [75,76,77] and prefrontal cortex volume [77]. As such, it is essential to further our understanding of the impact that cannabis has on TMS measures.
In the brain, cannabis exerts its effect on cannabinoid type 1 receptors (CB1Rs), which presynaptically modulate GABA, glutamate, dopamine and acetylcholine levels. Animal studies show that CB1R agonists enhance dopaminergic neurotransmission in the basal ganglia [78,79] and mesolimbic pathway [80], leading to a reduction in prefrontal cortical activity [80]. CB1R agonists inhibit the release of GABA from pyramidal neurons in the prefrontal cortex [81], hippocampus and the VTA [80,82,83,84], and reduce glutamatergic neurotransmission in the prefrontal cortex [85], hippocampus [86,87], and NAc [88]. Finally, cannabinoids reduce acetylcholine release in the prefrontal cortex and hippocampus [89,90].
In humans, acute administration of delta-9-tetrahydrocannabinol (∆-9-THC) increases striatal dopamine release [91,92,93]. Cannabis addiction follows the neurobiological model of addiction proposed by Koob and Volkow [94], marked by dysregulation of neural circuitry within the mesocorticolimbic dopamine system, amygdala and prefrontal cortex [95]. Chronic cannabis exposure leads to a reduction in dopamine synthesis capacity in the striatum [96,97]. GABABR agonists and GABA reuptake inhibitors enhance the symptoms from acute ∆-9-THC intake [98,99], and long-term cannabis use reduces GABAergic function in the anterior cingulate cortex (ACC) [100,101]. Cannabis users also exhibit reduced glutamate levels in the basal ganglia [102], prefrontal cortex [103] and ACC [100,101]. Overall, evidence from animal models and humans clearly demonstrates diverse neurobiological effects of cannabis across brain regions.
The effects of cannabis on TMS measures can be seen in Table 2. In a case study with one participant comorbid for Tourette’s syndrome and attention deficit hyperactive disorder (ADHD), ∆-9-THC increased CSP length and SICI but did not affect RMT or the stimulation intensity to evoke a MEP of 1 mV [104]. This suggests that ∆-9-THC modulates intracortical inhibitory circuits within M1. However, this was observed in a single participant and may not generalize to larger sample sizes. In addition, the patient studied was consuming additional medications, making it harder to interpret the results [104]. The acute effects of cannabis on TMS measures should be tested in future studies.
Table 2.
Effects of cannabis on TMS measures.
| Study. | AMT | RMT | MEP | CSP | iSP | SAI | LAI | SICI | ICF | SICF | LICI | SIHI | LIHI | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hasan et al. [104] | – | ○ | ○ | ▲ | – | – | – | ▲ | – | – | – | – | – | Acute intake |
| Fitzgerald et al. [105] | ○ | ○ | ○ | ○ | – | – | – | ▼ | ○ | – | ○ | – | – | Heavy and light cannabis users vs. non-users |
| Martin-Rodriguez et al. [106] | ○ | ○ | ○ | – | – | – | – | ▼ | – | – | – | – | – | CUD and daily cannabis users vs. non-users |
| Wobrock et al. [107] | – | ○ | – | – | – | – | – | ▼ | ▲ | – | – | – | – | Schizophrenia cannabis users vs. non-users |
| Flavel et al. [108] | – | ○ | ○ | ○ | – | – | – | – | – | ○ | ○ | – | – | Cannabis users vs. nonusers |
| Goodman et al. [109] | – | ○ | – | ○ | – | – | – | ▲ | ○ | – | ○ | – | – | Schizophrenia cannabis users vs. non-users |
| – | ○ | – | ○ | – | – | – | ▼ | ○ | – | ○ | – | – | Control cannabis users vs. nonusers | |
| Russo et al. [110] | ○ | ○ | ○ | ○ | – | ○ | ○ | ▲ | ▼ | – | – | – | – | MS patients on 1 month of Sativex |
| Leocani et al. [111] | – | ○ | ○ | – | – | – | – | ○ | ○ | – | – | – | – | MS patients on 1 month of Sativex |
| Calabrò et al. [112] | – | – | ▲ | – | – | – | – | ▼ | ▼ | – | – | – | – | MS patients on 6 weeks of Sativex + gait training |
▲ increase; ▼decrease; ○ indicates no change; – indicates did not assess; CUD: cannabis use disorder; MS: multiple sclerosis.
Fitzgerald et al. [105] reported that both heavy and light cannabis users show reduced SICI compared to controls, which has since been replicated [109]. Similarly, Martin-Rodriguez et al. [106] recently showed that SICI is reduced in daily cannabis users and participants with cannabis abuse disorder compared to healthy controls. This is in line with other research suggesting that cannabis inhibits GABAergic neurotransmission [80,81,82,100,101]. In contrast, Flavel et al. [108] reported no difference in SICI between cannabis users and non-users. Discrepancies between these findings may relate to methodological differences. Specifically, Flavel et al. [108] tested a sample of individuals who were comorbid for chronic use of alcohol in addition to cannabis, whereas the other studies controlled for comorbid alcohol abuse [105,106,109]. Furthermore, the sample size used in Flavel et al. [108] was likely underpowered to detect a group difference in SICI. Neither study reported a difference in RMT, MEPs, CSP, or ICF between users and non-users [105,106,108,109]. Furthermore, there were no reported group differences in AMT [105,106] or LICI [105,109]. These findings suggest that, within M1, long-term cannabis use modulates GABAAR, but not GABABR or NMDA receptor function.
It is notable that all TMS studies showing an effect of cannabis abuse on SICI also reported that the cannabis group had significantly fewer years of education compared to controls [105,107,109]. The detrimental effects of cannabis on education outcomes may be influenced by other factors such as childhood adversity, family structure, and socioeconomic status [113,114]. It is currently unknown if intracortical inhibition is related to years of education, intelligence, or other sociocultural factors like socioeconomic status. Further longitudinal research is required to determine if education is a significant modifier of SICI.
Other studies investigating the chronic effects of cannabis use have been conducted in participants diagnosed with schizophrenia, as one third of schizophrenia patients report using cannabis daily [115]. These individuals experience more severe psychotic symptoms in response to ∆-9-THC [116], whereas the antipsychotic properties of cannabidiol (CBD) improves symptoms of schizophrenia [117,118]. Schizophrenia patients comorbid for cannabis abuse demonstrate increased ICF and reduced SICI compared to schizophrenia patients that do not use cannabis [107]. In contrast, Goodman et al. [109] reported that schizophrenia patients dependent on cannabis show increased SICI compared to non-users. This may be attributable to the inclusion of a sample of schizophrenia patients with a longer disease duration and a stricter criterion for defining cannabis abuse.
Sativex, an oromucosal spray containing ∆-9-THC and CBD, is commonly administered to multiple sclerosis (MS) patients for pain relief. MS patients treated with Sativex show no change in AMT, RMT or MEPs [110,111], or sensorimotor excitability as shown by no change in SAI or LAI [110]. Russo et al. [110] showed that Sativex reduces SICI and increases ICF, while Leocani et al. [111] showed no change in SICI or ICF after Sativex treatment.
In a recent study, Calabrò et al. [112] assessed the influence of cannabis on motor function following robot-aided gait training. Specifically, two groups of MS patients underwent 6 weeks of gait training along with administration of a THC:CBD oromucosal spray added onto ongoing oral antispastic therapy or oral antispastic therapy only. Those treated with THC:CBD demonstrated greater increases in MEP amplitude and greater decreases in SICI and ICF within the APB muscle following gait training. However, when obtained from the tibialis anterior (TA) muscle, a similar magnitude of MEP increase, SICI and ICF decrease were observed in both groups following gait training. These changes in cortical and corticospinal excitability were also accompanied by changes in function. Specifically, those treated with THC:CBD showed greater improvements in muscle stiffness, functional independence, ambulation, quality of life, and global disability following gait training compared to the group not treated with THC:CBD. Therefore, these results show that cannabis administration can potentiate rehabilitative outcomes following motor training.
Summary
Overall, chronic cannabis use appears to have no effect on corticospinal excitability but does impact SICI. Specifically, chronic exposure to cannabis consistently modulates SICI in healthy individuals and those diagnosed with schizophrenia. However, the effects of cannabis on other TMS measures including SAI, LAI, SICF, IHI and iSP have yet to be investigated. In addition, there is a gap in the knowledge regarding the acute effects of cannabis, within both long-term users and non-users.
5. Nicotine
Nicotine induces an inward depolarizing current by acting on nicotinic acetylcholine receptors (nAChRs) on one of two binding sites: α4β2 and α7-subunits [119]. Low doses of nicotine act on both binding sites, while higher doses act predominantly on α7-containing nAChRs due to the rapid desensitization of the α4β2-containing nAChRs [120,121]. Acutely, nicotine-induced activation of nAChRs leads to depolarization of glutamatergic neurons expressing the α7-nAChRs and GABAergic neurons expressing non-α7-nAChRs [122]. However, repeated nicotine exposure leads to the desensitization of non-α7-nAChRs on GABAergic neurons with no significant desensitizing effect on α7-nAChRs on glutamatergic neurons [120,122]. This translates to net excitation of mesolimbic dopaminergic neurons [120,122]. However, the net result of nicotine on excitation/inhibition balance varies across brain regions [123], leading to inhibition or disinhibition of pyramidal neurons [124]. Chronic exposure also leads to reduced cortical perfusion [125], reduced microstructural integrity of cerebral white matter [126], and increased dendritic arborization within M1 [127].
TMS can be used to gauge the effects of nicotine on motor function, although the reported results are mixed (Table 3). In healthy non-smokers, acute nicotine intake had either not changed SICI and SAI [128] or increased SICI and SAI [129]. SAI is reduced by muscarinic antagonists [130] and increased by acetylcholinesterase inhibitors [131,132], demonstrating its involvement in the cholinergic system. Therefore, increased SAI following nicotine intake likely reflects the upregulation of nAChR activity, whereas increased SICI may reflect the nAChR-modulation of GABAergic neurotransmission [62]. Orth et al. [128] may not have shown a significant effect of nicotine on SICI or SAI because of the low dose of 2 mg nicotine that was administered, whereas Grundey et al. [129] administered a higher dose of 16 mg. Furthermore, nicotine was administered in the form of gum [128] compared to a nicotine patch [129]. Approximately half of the nicotine administered in gum form is absorbed, reducing its effectiveness as an intervention compared to a nicotine patch [133,134,135]. Regardless, these studies showed no effect of nicotine on thresholds, MEP, CSP, SICF or ICF in non-smokers [128,129].
Table 3.
Effects of nicotine on TMS measures.
| Study | AMT | RMT | MEP | CSP | iSP | SAI | LAI | SICI | ICF | SICF | LICI | SIHI | LIHI | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grundey et al. [129] | ○ | ○ | ○ | – | – | ▲ | – | ▲ | ○ | ○ | – | – | – | Non-smokers after acute intake |
| ○ | ○ | ○ | – | – | ○ | – | ○ | ▲ | ○ | – | – | – | Smokers after acute intake | |
| ○ | ○ | ○ | – | – | ○ | – | ○ | ▼ | ○ | – | – | – | Smokers vs. non-smokers | |
| Orth et al. [128] | ○ | ○ | – | ○ | – | ○ | – | ○ | ○ | – | – | – | – | Non-smokers after acute intake |
| ○ | ○ | – | ○ | – | ○ | – | ○ | ○ | – | – | – | – | Tourette’s after acute intake | |
| Lang et al. [136] | ○ | ○ | ▼ | ▲ | – | ▲ | ○ | ○ | ▼ | – | ○ | – | – | Smokers vs. non-smokers |
| Khedr et al. [137] | ○ | ○ | ▲ | ○ | ○ | – | – | – | – | – | – | – | – | Smokers vs. non-smokers |
▲ increase; ▼decrease; ○ indicates no change; – indicates did not assess.
In smokers, acute nicotine intake also had no effect on MEPs [138,139]. Alternatively, acute nicotine intake increased ICF in smokers and did not modulate SAI or SICI [129]. These findings may be explained by the evidence mentioned above, where chronic exposure to nicotine may lead to the desensitization of non-α7 nAChRs that modulated GABAergic neurotransmission, while glutamatergic neurotransmission remains elevated [120].
Relative to non-smokers, smokers exhibited no difference in thresholds [129,136,137], iSP [137], SICF [129], SICI [129,136], LAI or LICI [136], but did show increased SAI [129] and reduced ICF [129,136]. Studies have reported that MEPs in smokers are either elevated [129,137], reduced [136], or not different to controls [136,140]. Lavendar et al. [140] likely did not see an effect of chronic nicotine exposure on MEPs as they only tested the %MSO required to evoke a 1 mV MEP while Grundey et al. [129] showed that, across a range of stimulation intensities, smokers only exhibit elevated MEPs at high intensities of 150% RMT only. Similarly, Lang et al. [136] showed that resting MEPs obtained at 110%, 120% or 140% RMT were not different between groups, while only MEPs obtained during active contraction were reduced in smokers compared to non-smokers. Additionally, CSP is either increased [136] or not different from non-smokers [137,140]. Discrepancies across studies may be a result of the different contraction levels maintained during CSP acquisition, which varied from 30–50% maximum voluntary contraction (MVC) [136], 50% MVC [137] or 10% maximum voluntary force (MVF) [140].
Summary
Acute nicotine intake appears to modulate SAI and SICI in non-smokers with no effects on corticospinal excitability. In smokers, chronic nicotine exposure modulates corticospinal excitability, CSP, SAI and ICF compared to non-smokers. However, SICI, LICI, SICF, iSP and LAI appear to be similar between smokers and non-smokers. As such, screening for both recent and chronic nicotine intake is important to consider in future TMS research.
6. Caffeine
Health Canada recommends 400 mg/day as an upper limit for caffeine intake in adults, as moderate caffeine intake of 400 mg/day is not associated with toxicity or adverse health effects [141]. Nevertheless, caffeine is considered to be a psychostimulant, and is known to affect brain function even when consumed at levels below the recommended upper limits. At low doses (20–200 mg), caffeine enhances attention, reaction time, and motor coordination [142,143,144,145]. At high doses (250–500 mg), caffeine increases unpleasant feelings of tension, irritability, and anxiousness, and reduces the amplitude of alpha and beta waves recorded with EEG [146].
Caffeine exerts its psychomotor effects by inhibiting adenosine receptors, specifically the A1 and A2A receptor subtypes [147,148,149]. A1 receptors are expressed in the hypothalamus, hippocampus, basal ganglia, and cortex; A2A receptors are localized in the striatum, NAc and olfactory bulb [150]. Caffeine also induces a presynaptic increase in glutamate release and reduces miniature excitatory post-synaptic currents via the blockade of α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) ionotropic glutamate receptors [151]. However, it is unlikely that regular caffeine intake in humans would have a significant effect on glutamate ionotropic receptors, as this effect was found under extreme conditions of caffeine toxicity [151]. Research suggests dopamine’s involvement in eliciting the psychostimulant effects that follow caffeine intake. The motor and discriminative stimulus effects of caffeine are diminished when dopamine is depleted or when dopamine receptors are blocked [152,153]. Moreover, the presence of caffeine increases dopaminergic transmission, which is linked to an increase in arousal and motivation, coupled with enhanced serotonin release and increased post-synaptic serotonergic input [150].
The effect of caffeine on TMS measures are shown in Table 4. First, acute caffeine intake has no effect on threshold [154,155,156,157,158]. Multiple studies have reported no change in resting MEPs following caffeine intake, at intensities ranging from 100–150% RMT [154,155,156,157,158,159,160,161]. Bowtell et al. [162] also found no change in resting MEPs following caffeine intake, while MEPs obtained during maximal contraction were potentiated by caffeine. However, Specterman et al. [163] found that 68 mg of caffeine increased resting MEPs from 30–90 min after intake. This effect was potentiated when participants were administered Lucozade, an energy drink containing 68 g of caffeine and 46 g of glucose. Notably, these finding were obtained from a small sample of four and six participants, respectively. Alternatively, caffeine has been demonstrated to potentiate post-exercise facilitation of MEPs on multiple occasions [158,159,161].
Table 4.
Effects of caffeine on TMS measures.
| Study | AMT | RMT | MEP | CSP | iSP | SAI | LAI | SICI | ICF | SICF | LICI | SIHI | LIHI | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kalmar et al. [159] | – | – | ○ | – | – | – | – | – | – | – | – | – | – | Acute intake |
| Orth et al. [154] | ○ | ○ | ○ | ○ | – | – | – | ○ | ○ | – | – | – | – | Acute intake |
| Specterman et al. [163] | – | – | ▲ | – | – | – | – | – | – | – | – | – | – | Acute intake |
| Cerqueira et al. [156] | – | ○ | ○ | ▼ | – | – | – | – | – | – | – | – | – | Acute intake |
| de Carvahlo et al. [157] | – | ○ | ○ | ▼ | – | – | – | ○ | ○ | – | ○ | – | – | Acute intake |
| Concerto et al. [158] | – | ○ | ○ | ○ | – | ○ | ○ | ○ | ▼ | – | – | – | – | Acute intake |
| Hanajima et al. [160] | – | – | ○ * | – | – | – | – | – | – | – | – | – | – | Acute intake; * between-subject comparison |
| Kalmar et al. [161] | – | – | ○ * | – | – | – | – | – | – | – | – | – | – | Acute intake; * trending increase after caffeine intake, but not significant |
| Bowtell et al. [162] | – | – | ▲ * | ○ | – | – | – | – | – | – | – | – | – | Acute intake; * caffeine only potentiated MEPs obtained during maximal contraction |
| Mesquita et al. [155] | ○ | – | ○ | ▼ | – | – | – | ○ * | ○ | – | – | – | – | Acute intake; * SICI reduced in caffeine and placebo condition |
▲ increase; ▼decrease; ○ indicates no change; – indicates did not assess; * refers to stipulations outlined in the right-hand column.
Acute caffeine intake has no reported effect on SAI and LAI [158], or SICI [154,157,158]. One study reported a reduction in SICI following caffeine intake, although a similar finding was also observed in the placebo condition [155]. This suggests that the change in SICI was not due exclusively to the effects of caffeine.
Most studies have reported no effect of caffeine on ICF [154,155,157]. However, Concerto et al. [158] found a decrease in ICF after administration of a sugar-free energy drink, which contained caffeine as the main active ingredient. The authors attributed this effect to taurine, which is present within energy drinks in significant concentrations. Taurine is a free amino acid that modulates GABAAR activity [164,165] and glutamatergic neurotransmission [165,166,167]. In a follow-up study, Infortuna et al. [168] found that taurine alone was not capable of modulating ICF. The reduction in ICF reported by Concerto et al. [158] may be attributed to time of testing, as TMS measures were acquired 45 min following ingestion of the energy drink. Studies reporting no effect of caffeine on ICF performed testing at least 1 h following caffeine administration [154,155,157].
CSP is either reduced [155,156,157] or not affected by caffeine [154,158,162]. Studies reporting a decrease following caffeine intake obtained CSP at low TMS intensities of 110% RMT [156,157]. Alternatively, studies reporting no change in CSP used higher intensities of 120–175% AMT [154,162] or 150% RMT [158]. Indeed, Cerqueira et al. [156] showed that only CSP obtained at 110% RMT and not 150% RMT was reduced following caffeine intake. Mesquita et al. [155] revealed a reduction in CSP following caffeine intake at a higher intensity of 130% RMT, although this was acquired in the soleus muscle. CSP length increases with increasing TMS intensity [169]. It is possible that the neuromodulatory effects of caffeine are not capable of inducing a change in CSP at higher TMS intensities.
Summary
Based on these findings from the literature, caffeine does not appear to have a significant modulatory effect on motor thresholds, MEPs, SAI, LAI, SICI or LICI. However, studies acquiring CSP or ICF should consider restricting caffeine prior to testing. At present, it is unknown if caffeine leads to a change in interhemispheric measures of IHI or iSP, and it is currently unknown whether cortical excitability is different between habitual versus non-habitual coffee consumers.
7. Current Gaps in Knowledge
There are several notable gaps in the research reviewed. Notably, as seen in Table 1 through Table 4, the acute and chronic effects of recreational substances on several measures are unknown. For example, the acute and chronic effects of alcohol on measures such as LICI, LAI, SAI, IHI and iSP are unknown. Furthermore, the effects of chronic cannabis use on SAI, LAI, SICF, IHI and iSP have yet to be tested. Therefore, until further investigation can be undertaken, studies employing all of these TMS measures should screen participants for recent recreational substance use.
The time-course of substance effects on TMS measures has not been thoroughly investigated. Future studies examining the effect of acute substance intake on TMS measures should include multiple post-intake measurements to determine how long the substance exerts an observable effect of motor physiology. Furthermore, it would also be useful to have information about blood plasma levels of substances over time, and how this related to TMS measures. For drugs consumed orally, the variability in gastric emptying due to food constituents in the stomach may contribute to variability in the time course of effects.
In extension, the time course of variation in TMS measures within chronic substance users, users undergoing withdrawal, and those in recovery is unknown. Studies examining the effect of chronic exposure on TMS measures should include samples at different points of withdrawal or recovery to determine if and when motor function normalizes. For example, the studies reviewed in Table 4 instructed participants to refrain from caffeine intake for some time before TMS testing in order to better identify the effects of the administered caffeine dose. A side effect of prolonged abstinence from caffeine is the emergence of withdrawal symptoms [170]. It is unknown whether corticospinal or cortical excitability is altered during periods of caffeine withdrawal in habitual coffee consumers. Concrete testing is needed to fully elucidate whether the physiological state of withdrawal or recovery has a differential impact on TMS measures compared to periods of chronic substance use.
There has been limited investigation into the interaction between biological sex and recreational substance use on TMS measures. However, biological sex appears to be a determinant of the brain’s response to cannabis and nicotine. Female cannabis users have lower glutamate and glutamine levels in the dorsal striatum [171] and impaired frontal dopaminergic neurotransmission [172] compared to controls. Male cannabis users do not show this effect [171,172]. It is unknown whether differences in neurotransmitter profiles between male and female cannabis users are reflected within TMS outcome measures. Furthermore, females are less sensitive to nicotine [173] and are less responsive to nicotine replacement therapies [174]. This suggests that nicotine induces differential changes in physiological state between biological sexes. Future TMS studies should determine whether there is an interaction between acute or chronic nicotine or cannabis exposure and biological sex on outcome measures.
Fitzgerald et al. [105] divided cannabis consumers into groups of light and heavy consumers, and showed that both groups experienced reduced SICI. This indicates that infrequent consumption can still lead to persisting changes in cortical excitability. However, the cross-sectional design of this study prevented the quantification of the average dose consumed by participants. Importantly, no studies have assessed the dose-dependent effects of substance use on TMS measures, except for Cerquiera et al. [156] who found that both 200 mg and 400 mg of caffeine reduced CSP. Therefore, future studies should examine the dose-response relationship between recreational substances and TMS metrics, which would more suitably tested with an intervention study.
We can attribute 60–70% of the variance in nicotine dependence to genetic factors [175]. There are a number of molecular variants that have been linked to nicotine dependence [176]. For example, individuals expressing variations in CHRNA4, CHRNA5, and CHRNB4, which code for nAChRs subunits, exhibit greater nicotine dependence [177,178,179], greater number of cigarettes smoked per day [180,181], and increased vulnerability to smoking [182]. Studies should investigate if individuals expressing these variants, or variants related to dependence on other recreational substances, show altered cortical excitability relative to individuals expressing the wildtype alleles.
Specifically related to cannabis research, an important consideration for future studies is the effect that different strains of cannabis have on neurophysiological function. The two main ingredients of cannabis are ∆-9-THC, the psychoactive component, and CBD, the non-psychoactive component. These components may have differential effects on brain function [183,184]. Specifically, ∆-9-THC intake reduced striatal activation while CBD increased striatal activity during a word retrieval task [183]. ∆-9-THC and CBD had similar opposing effects on task-related amygdala, hippocampal, temporal and occipital activation [183]. Future studies should account for the differential effects of CBD and ∆-9-THC in TMS research.
Substance abuse is often comorbid with other psychiatric conditions, as exemplified by the comorbidity of schizophrenia and cannabis use. Further, those with alcoholism may present comorbidities for depression [185], bipolar disorder [186], or Wernicke–Korsakoff syndrome [187]. Due to the prevalence of comorbidities in those with substance use disorders, it is important that future TMS studies assessing the chronic effects of recreational substances take care to screen participants for existing comorbidities. Furthermore, studies should also continue to assess the interactions between substance use and comorbid psychiatric disorders, and how this changes neurophysiological function.
It is unknown whether the reliability of TMS measures is impacted by recreational substances. While intake of recreational substances did not significantly change TMS outcome measures in some cases, we cannot conclude that they had no effect on the variability of these measures. Finally, eight of the 21 intervention studies reviewed did not include a placebo control (see Table S1). As such, the results of these studies should be interpreted with caution. Future studies should continue to employ placebo-controlled and blinded study designs to increase outcome validity.
8. Conclusions
According to the studies reviewed herein, both acute and chronic use of recreational substances appear to modulate the excitability of the motor system, reflected by a change in TMS outcome measures. Overall, we note that most studies suffered from a small sample size and did not report power or sample size calculations. Heterogeneity stemming from participant demographics and parameters used to acquire TMS outcome measures also limits our interpretation of these studies. However, the results do suggest that there is a need for future studies to take into consideration the history of substance use and to control for acute substance use at the time of testing.
Although there is not enough information to provide definitive screening guidelines, our assessment of the literature suggests that this information can still be implemented in TMS screening tools. However, preliminary screening guidelines may include excluding participants with a history of chronic recreational substance use and acute use within the 24 h prior to testing, even though the influence of abstinence duration following intake still needs to be determined. Finally, the information provided in this review allows for a retrospective assessment of datasets demonstrating a causal effect of substance use on TMS measures.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3425/10/10/751/s1: Table S1: TMS studies investigating the effects of recreational substance use.
Author Contributions
Conceptualization, C.V.T., S.O.A., A.J.N.; Methodology, C.V.T.; Writing–Original Draft Preparation, C.V.T.; Writing–Reviewing and Editing, C.V.T., S.O.A., A.J.N.; Funding Acquisition, A.J.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Sciences and Engineering Research Council, grant number RGPIN-2020-06757, to A.J.N.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Delker E., Brown Q., Hasin D.S. Alcohol consumption in demographic subpopulations: An epidemiologic overview. Alcohol Res. Curr. Rev. 2016;38:7–15. [PMC free article] [PubMed] [Google Scholar]
- 2.SAMHSA . Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. SAMHSA; Rockville, MD, USA: 2013. p. 222. NSDUH Ser. H-46, HHS Publ. No. 13-4795. [Google Scholar]
- 3.Haug N.A., Padula C.B., Sottile J.E., Vandrey R., Heinz A.J., Bonn-Miller M.O. Cannabis use patterns and motives: A comparison of younger, middle-aged, and older medical cannabis dispensary patients. Addict. Behav. 2017;72:14–20. doi: 10.1016/j.addbeh.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi S., Hallett M., Rossini P.M., Pascual-Leone A., Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinial practice and research. Clinial Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelick D.A., Zangen A., George M.S. Transcranial magnetic stimulation in the treatment of substance addiction. Ann. N. Y. Acad. Sci. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanlon C.A., Dowdle L.T., Scott Henderson J. Modulating neural circuits with transcranial magnetic stimulation: Implications for addiction treatment development. Pharmacol. Rev. 2018;70:661–683. doi: 10.1124/pr.116.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauer L., Scarano G.I., Brigo F., Golaszewski S., Lochner P., Trinka E., Sellner J., Nardone R. Effects of repetitive transcranial magnetic stimulation on nicotine consumption and craving: A systematic review. Psychiatry Res. 2019;281:112562. doi: 10.1016/j.psychres.2019.112562. [DOI] [PubMed] [Google Scholar]
- 8.Mostafavi S.A., Khaleghi A., Mohammadi M.R. Noninvasive brain stimulation in alcohol craving: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;101:109938. doi: 10.1016/j.pnpbp.2020.109938. [DOI] [PubMed] [Google Scholar]
- 9.Nevo I., Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem. Int. 1995;26:305–336. doi: 10.1016/0197-0186(94)00139-L. [DOI] [PubMed] [Google Scholar]
- 10.Roberto M., Varodayan F.P. Synaptic targets: Chronic alcohol actions. Neuropharmacology. 2017;122:85–99. doi: 10.1016/j.neuropharm.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair L.A.C., Levitan E.S., Marshall J., Dionne V.E., Barnard E.A. Single subunits of the GABAA receptor form ion channels with properties of the native receptor. Science (80-.) 1988;242:577–579. doi: 10.1126/science.2845583. [DOI] [PubMed] [Google Scholar]
- 12.Tatebayashi H., Motomura H., Narahashi T. Alcohol modulation of single GABA(A) receptor-channel kinetics. Neuroreport. 1998;9:1769–1775. doi: 10.1097/00001756-199806010-00018. [DOI] [PubMed] [Google Scholar]
- 13.Aguayo L.G. Ethanol potentiates the GABAA-activated Cl- current in mouse hippocampal and cortical neurons. Eur. J. Pharmacol. 1990;187:127–130. doi: 10.1016/0014-2999(90)90349-B. [DOI] [PubMed] [Google Scholar]
- 14.Nestoros J.N. Ethanol specifically potentiates GABA-mediated neurotransmission in feline cerebral cortex. Science (80-.) 1980;209:708–710. doi: 10.1126/science.7394531. [DOI] [PubMed] [Google Scholar]
- 15.Suzdak P.D., Schwartz R.D., Skolnick P., Paul S.M. Ethanol stimulates γ-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc. Natl. Acad. Sci. USA. 1986;83:4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrisett R.A., Martin D., Oetting T.A., Lewis D.V., Wllson A., Swartzwelder H.S. Ethanol and magnesium ions inhibit N-methyl-d-aspartate-mediated synaptic potentials in an interactive manner. Neuropharmacology. 1991;30:1173–1178. doi: 10.1016/0028-3908(91)90162-5. [DOI] [PubMed] [Google Scholar]
- 17.Steffensen S.C., Nie Z., Criado J.R., Siggins G.R. Ethanol inhibition of N-methyl-D-aspartate responses involves presynaptic γ-aminobutyric acid(B) receptors. J. Pharmacol. Exp. Ther. 2000;294:637–647. [PubMed] [Google Scholar]
- 18.Carboni S., Isola R., Gessa G.L., Rossetti Z.L. Ethanol prevents the glutamate release induced by N-methyl-d-aspartate in the rat striatum. Neurosci. Lett. 1993;152:133–136. doi: 10.1016/0304-3940(93)90501-B. [DOI] [PubMed] [Google Scholar]
- 19.Roberto M., Schweitzer P., Madamba S.G., Stouffer D.G., Parsons L.H., Siggins G.R. Acute and Chronic Ethanol Alter Glutamatergic Transmission in Rat Central Amygdala: An In Vitro and In Vivo Analysis. J. Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nie Z., Madamba S.G., Siggins G.R. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J. Pharmacol. Exp. Ther. 1994;271:1566–1573. [PubMed] [Google Scholar]
- 21.Ziemann U., Lönnecker S., Paulus W. Inhibition of human motor cortex by ethanol a transcranial magnetic stimulation study. Brain. 1995;118:1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- 22.Ziemann U., Tergau F., Wischer S., Hildebrandt J., Paulus W. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr. Clin. Neurophysiol.-Electromyogr. Mot. Control. 1998;109:321–330. doi: 10.1016/S0924-980X(98)00023-X. [DOI] [PubMed] [Google Scholar]
- 23.Conte A., Attilia M.L., Gilio F., Iacovelli E., Frasca V., Bettolo C.M., Gabriele M., Giacomelli E., Prencipe M., Berardelli A., et al. Acute and chronic effects of ethanol on cortical excitability. Clin. Neurophysiol. 2008;119:667–674. doi: 10.1016/j.clinph.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Hoppenbrouwers S.S., Hofman D., Schutter D.J.L.G. Alcohol breaks down interhemispheric inhibition in females but not in males: AAlcohol and frontal connectivity. Psychopharmacology. 2010;208:469–474. doi: 10.1007/s00213-009-1747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loheswaran G., Barr M.S., Zomorrodi R., Rajji T.K., Blumberger D.M., Le Foll B., Daskalakis Z.J. Alcohol Impairs N100 Response to Dorsolateral Prefrontal Cortex Stimulation. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-21457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kähkönen S., Wilenius J. Effects of alcohol on TMS-evoked N100 responses. J. Neurosci. Methods. 2007;166:104–108. doi: 10.1016/j.jneumeth.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Muralidharan K., Venkatasubramanian G., Pal P.K., Benegal V. Abnormalities in cortical and transcallosal inhibitory mechanisms in subjects at high risk for alcohol dependence: A TMS study. Addict. Biol. 2008;13:373–379. doi: 10.1111/j.1369-1600.2007.00093.x. [DOI] [PubMed] [Google Scholar]
- 28.Nardone R., Bergmann J., Kronbichler M., Caleri F., Lochner P., Tezzon F., Ladurner G., Golaszewski S. Altered motor cortex excitability to magnetic stimulation in alcohol withdrawal syndrome. Alcohol. Clin. Exp. Res. 2010;34:628–632. doi: 10.1111/j.1530-0277.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- 29.Muralidharan K., Venkatasubramanian G., Pal P.K., Benegal V. Relationship between motor threshold and externalizing symptoms in subjects at high risk for alcohol dependence: A TMS study. Am. J. Addict. 2013;22:84–85. doi: 10.1111/j.1521-0391.2013.00274.x. [DOI] [PubMed] [Google Scholar]
- 30.Naim-Feil J., Bradshaw J.L., Rogasch N.C., Daskalakis Z.J., Sheppard D.M., Lubman D.I., Fitzgerald P.B. Cortical inhibition within motor and frontal regions in alcohol dependence post-detoxification: A pilot TMS-EEG study. World J. Biol. Psychiatry. 2016;17:547–556. doi: 10.3109/15622975.2015.1066512. [DOI] [PubMed] [Google Scholar]
- 31.Kaarre O., Kallioniemi E., Könönen M., Tolmunen T., Kekkonen V., Kivimäki P., Heikkinen N., Ferreri F., Laukkanen E., Määttä S. Heavy alcohol use in adolescence is associated with altered cortical activity: A combined TMS–EEG study. Addict. Biol. 2018;23:268–280. doi: 10.1111/adb.12486. [DOI] [PubMed] [Google Scholar]
- 32.Quoilin C., Wilhelm E., Maurage P., De Timary P., Duque J. Deficient inhibition in alcohol-dependence: Let’s consider the role of the motor system! Neuropsychopharmacology. 2018;43:1851–1858. doi: 10.1038/s41386-018-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziemann U., Reis J., Schwenkreis P., Rosanova M., Strafella A., Badawy R., Müller-Dahlhaus F. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Mohammadi B., Krampfl K., Petri S., Bogdanova D., Kossev A., Bufler J., Dengler R. Selective and nonselective benzodiazepine agonists have different effects on motor cortex excitability. Muscle Nerve. 2006;33:778–784. doi: 10.1002/mus.20531. [DOI] [PubMed] [Google Scholar]
- 35.Premoli I., Castellanos N., Rivolta D., Belardinelli P., Bajo R., Zipser C., Espenhahn S., Heidegger T., Müller-Dahlhaus F., Ziemann U. TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J. Neurosci. 2014;34:5603–5612. doi: 10.1523/JNEUROSCI.5089-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan F.J., Berton F., Madamba S.G., Francesconi W., Siggins G.R. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc. Natl. Acad. Sci. USA. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang M.H., Spigelman I., Olsen R.W. Alteration in the sensitivity of GABA(A) receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol. Clin. Exp. Res. 1998;22:2165–2173. doi: 10.1111/j.1530-0277.1998.tb05930.x. [DOI] [PubMed] [Google Scholar]
- 38.Ariwodola O.J., Weiner J.L. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: Role of presynaptic GABAB receptors. J. Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Lazzaro V., Ziemann U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front. Neural Circuits. 2013;7:18. doi: 10.3389/fncir.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mumenthaler M.S., Taylor J.L., O’Hara R., Yesavage J.A. Gender differences in moderate drinking effects. Alcohol Res. Health. 1999;23:55–64. [PMC free article] [PubMed] [Google Scholar]
- 41.Jones B.M., Jones M. Alcohol and memory impairment in male and female social drinkers. In: Birnbaum I.M., Parker E.S., editors. Alcohol and Human Memory. Lawrence Erlbaum; Hillsdale, NJ, USA: 1977. pp. 127–138. [Google Scholar]
- 42.Haut J.S., Beckwith B.E., Petros T.V., Russell S. Gender differences in retrieval from long-term memory following acute intoxication with ethanol. Physiol. Behav. 1989;45:1161–1165. doi: 10.1016/0031-9384(89)90103-0. [DOI] [PubMed] [Google Scholar]
- 43.Miller M.A., Weafer J., Fillmore M.T. Gender differences in alcohol impairment of simulated driving performance and driving-related skills. Alcohol Alcohol. 2009;44:586–593. doi: 10.1093/alcalc/agp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frezza M., di Padova C., Pozzato G., Terpin M., Baraona E., Lieber C.S. High Blood Alcohol Levels in Women: The Role of Decreased Gastric Alcohol Dehydrogenase Activity and First-Pass Metabolism. N. Engl. J. Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 45.Khalil R., King M.A., Soliman M.R.I. Testosterone reverses ethanol-induced deficit in spatial reference memory in castrated rats. Pharmacology. 2005;75:87–92. doi: 10.1159/000087188. [DOI] [PubMed] [Google Scholar]
- 46.Vandegrift B.J., You C., Satta R., Brodie M.S., Lasek A.W. Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0187698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabakoff B., Hoffman P.L. The neurobiology of alcohol consumption and alcoholism: An integrative history. Pharmacol. Biochem. Behav. 2013;113:20–37. doi: 10.1016/j.pbb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrow A.L., Suzdak P.D., Karanian J.W., Paul S.M. Chronic ethanol administration alters γ-aminobutyric acid, pentobarbital and ethanol-mediated 36Cl- uptake in cerebral cortical synaptoneurosomes. J. Pharmacol. Exp. Ther. 1988;246:158–164. [PubMed] [Google Scholar]
- 49.Allan A.M., Harris R.A. Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol. Biochem. Behav. 1987;27:665–670. doi: 10.1016/0091-3057(87)90192-4. [DOI] [PubMed] [Google Scholar]
- 50.Sanna E., Serra M., Cossu A., Colombo G., Follesa P., Cuccheddu T., Concas A., Biggio G. Chronic Ethanol Intoxication Induces Differential Effects on GABAA and NMDA Receptor Function in the Rat Brain. Alcohol. Clin. Exp. Res. 1993;17:115–123. doi: 10.1111/j.1530-0277.1993.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 51.Devaud L.L., Fritschy J.-M., Sieghart W., Morrow A.L. Bidirectional Alterations of GABAA Receptor Subunit Peptide Levels in Rat Cortex During Chronic Ethanol Consumption and Withdrawal. J. Neurochem. 2002;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- 52.Matthews D.B., Devaud L.L., Fritschy J.M., Sieghart W., Morrow A.L. Differential Regulation of GABAA Receptor Gene Expression by Ethanol in the Rat Hippocampus Versus Cerebral Cortex. J. Neurochem. 2002;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- 53.Buck K.J., Harris R.A. Benzodiazepine agonist and inverse agonist actions on GABA(A) receptor-operated chloride channels. I. Acute effects of ethanol. J. Pharmacol. Exp. Ther. 1990;253:706–712. [PubMed] [Google Scholar]
- 54.Diana M., Brodie M., Muntoni A., Puddu M.C., Pillolla G., Steffensen S., Spiga S., Little H.J. Enduring effects of chronic ethanol in the CNS: Basis for alcoholism. Alcohol. Clin. Exp. Res. 2003;27:354–361. doi: 10.1097/01.ALC.0000057121.36127.19. [DOI] [PubMed] [Google Scholar]
- 55.Brodie M.S., Shefner S.A., Dunwiddie T.V. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-Z. [DOI] [PubMed] [Google Scholar]
- 56.Aalto S., Ingman K., Alakurtti K., Kaasinen V., Virkkala J., Någren K., Rinne J.O., Scheinin H. Intravenous ethanol increases dopamine release in the ventral striatum in humans: PET study using bolus-plus-infusion administration of [11 C]raclopride. J. Perinatol. 2015;35:424–431. doi: 10.1038/jcbfm.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boileau I., Assaad J.M., Pihl R.O., Benkelfat C., Leyton M., Diksic M., Tremblay R.E., Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 58.Yan Q.S. Extracellular dopamine and serotonin after ethanol monitored with 5- minute microdialysis. Alcohol. 1999;19:1–7. doi: 10.1016/S0741-8329(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimoto K., McBride W.J., Lumeng L., Li T.K. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-T. [DOI] [PubMed] [Google Scholar]
- 60.Karkhanis A.N., Rose J.H., Huggins K.N., Konstantopoulos J.K., Jones S.R. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 2015;150:24–30. doi: 10.1016/j.drugalcdep.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teo J.T.H., Terranova C., Swayne O., Greenwood R.J., Rothwell J.C. Differing effects of intracortical circuits on plasticity. Exp. Brain Res. 2009;193:555–563. doi: 10.1007/s00221-008-1658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Lazzaro V., Pilato F., Dileone M., Profice P., Ranieri F., Ricci V., Bria P., Tonali P., Ziemann U. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: A TMS study. Clin. Neurophysiol. 2007;118:2207–2214. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Turco C.V., El-Sayes J., Locke M.B., Chen R., Baker S., Nelson A.J. Effects of lorazepam and baclofen on short- and long-latency afferent inhibition. J. Physiol. 2018;596 doi: 10.1113/JP276710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allan A.M., Spuhler K.P., Harris R.A. γ-Aminobutyric acid-activated chloride channels: Relationship to genetic differences in ethanol sensitivity. J. Pharmacol. Exp. Ther. 1988;244:866–870. [PubMed] [Google Scholar]
- 65.Harris R.A., Allan A.M. Alcohol intoxication: Ion channels and genetics. FASEB J. 1989;3:1689–1695. doi: 10.1096/fasebj.3.6.2467834. [DOI] [PubMed] [Google Scholar]
- 66.Rotermann M. What has changed since cannabis was legalized? Health Rep. 2020;31:11–20. doi: 10.25318/82-003-x202000200002-eng. [DOI] [PubMed] [Google Scholar]
- 67.Cerdá M., Wall M., Feng T., Keyes K.M., Sarvet A., Schulenberg J., O’Malley P.M., Pacula R.L., Galea S., Hasin D.S. Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatr. 2017;171:142–149. doi: 10.1001/jamapediatrics.2016.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Semple D.M., McIntosh A.M., Lawrie S.M. Cannabis as a risk factor for psychosis: Systematic review. J. Psychopharmacol. 2005;19:187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- 69.Karila L., Roux P., Rolland B., Benyamina A., Reynaud M., Aubin H.-J., Lancon C. Acute and Long-Term Effects of Cannabis Use: A Review. Curr. Pharm. Des. 2014;20:4112–4118. doi: 10.2174/13816128113199990620. [DOI] [PubMed] [Google Scholar]
- 70.Jacobus J., Castro N., Squeglia L.M., Meloy M.J., Brumback T., Huestis M.A., Tapert S.F. Adolescent cortical thickness pre- and post marijuana and alcohol initiation. Neurotoxicol. Teratol. 2016;57:20–29. doi: 10.1016/j.ntt.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobus J., Squeglia L.M., Meruelo A.D., Castro N., Brumback T., Giedd J.N., Tapert S.F. Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev. Cogn. Neurosci. 2015;16:101–109. doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alejandra Infante M., Courtney K.E., Castro N., Squeglia L.M., Jacobus J. Adolescent brain surface area pre-and post-cannabis and alcohol initiation. J. Stud. Alcohol Drugs. 2018;79:835–843. doi: 10.15288/jsad.2018.79.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Batalla A., Bhattacharyya S., Yücel M., Fusar-Poli P., Crippa J.A., Nogué S., Torrens M., Pujol J., Farré M., Martin-Santos R. Structural and Functional Imaging Studies in Chronic Cannabis Users: A Systematic Review of Adolescent and Adult Findings. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blithikioti C., Miquel L., Batalla A., Rubio B., Maffei G., Herreros I., Gual A., Verschure P., Balcells-Oliveró M. Cerebellar alterations in cannabis users: A systematic review. Addict. Biol. 2019;24:1121–1137. doi: 10.1111/adb.12714. [DOI] [PubMed] [Google Scholar]
- 75.Rocchetti M., Crescini A., Borgwardt S., Caverzasi E., Politi P., Atakan Z., Fusar-Poli P. Is cannabis neurotoxic for the healthy brain? A meta-analytical review of structural brain alterations in non-psychotic users. Psychiatry Clin. Neurosci. 2013;67:483–492. doi: 10.1111/pcn.12085. [DOI] [PubMed] [Google Scholar]
- 76.Lorenzetti V., Chye Y., Silva P., Solowij N., Roberts C.A. Does regular cannabis use affect neuroanatomy? An updated systematic review and meta-analysis of structural neuroimaging studies. Eur. Arch. Psychiatry Clin. Neurosci. 2019;269:59–71. doi: 10.1007/s00406-019-00979-1. [DOI] [PubMed] [Google Scholar]
- 77.Bloomfield M.A.P., Hindocha C., Green S.F., Wall M.B., Lees R., Petrilli K., Costello H., Ogunbiyi M.O., Bossong M.G., Freeman T.P. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol. Ther. 2019;195:132–161. doi: 10.1016/j.pharmthera.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J., Paredes W., Lowinson J.H., Gardner E.L. Strain-specific facilitation of dopamine efflux by Δ9-tetrahydrocannabinol in the nucleus accumbens of rat: An in vivo microdialysis study. Neurosci. Lett. 1991;129:136–140. doi: 10.1016/0304-3940(91)90739-G. [DOI] [PubMed] [Google Scholar]
- 79.Malone D.T., Taylor D.A. Modulation by fluoxetine of striatal dopamine release following Δ9-tetrahydrocannabinol: A microdialysis study in conscious rats. Br. J. Pharmacol. 1999;128:21–26. doi: 10.1038/sj.bjp.0702753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pistis M., Porcu G., Melis M., Diana M., Gessa G.L. Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur. J. Neurosci. 2001;14:96–102. doi: 10.1046/j.0953-816x.2001.01612.x. [DOI] [PubMed] [Google Scholar]
- 81.Pistis M., Ferraro L., Pira L., Flore G., Tanganelli S., Gessa G.L., Devoto P. Δ9-Tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: An in vivo microdialysis study. Brain Res. 2002;948:155–158. doi: 10.1016/S0006-8993(02)03055-X. [DOI] [PubMed] [Google Scholar]
- 82.Hoffman A.F., Lupica C.R. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J. Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katona I., Sperlágh B., Sík A., Käfalvi A., Vizi E.S., Mackie K., Freund T.F. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katona I., Sperlágh B., Maglóczky Z., Sántha E., Köfalvi A., Czirják S., Mackie K., Vizi E.S., Freund T.F. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/S0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- 85.Auclair N., Otani S., Soubrie P., Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J. Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- 86.Misner D.L., Sullivan J.M. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J. Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Derkinderen P., Valjent E., Toutant M., Corvol J.C., Enslen H., Ledent C., Trzaskos J., Caboche J., Girault J.A. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J. Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robbe D., Alonso G., Duchamp F., Bockaert J., Manzoni O.J. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J. Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gessa G.L., Casu M.A., Carta G., Mascia M.S. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. Eur. J. Pharmacol. 1998;355:119–124. doi: 10.1016/S0014-2999(98)00486-5. [DOI] [PubMed] [Google Scholar]
- 90.Mishima K., Egashira N., Matsumoto Y., Iwasaki K., Fujiwara M. Involvement of reduced acetylcholine release in Δ9-tetrahydrocannabinol-induced impairment of spatial memory in the 8-arm radial maze. Life Sci. 2002;72:397–407. doi: 10.1016/S0024-3205(02)02274-9. [DOI] [PubMed] [Google Scholar]
- 91.D’Souza D.C., Ranganathan M., Braley G., Gueorguieva R., Zimolo Z., Cooper T., Perry E., Krystal J. Blunted psychotomimetic and amnestic effects of Δ-9- tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhattacharyya S., Crippa J.A., Allen P., Martin-Santos R., Borgwardt S., Fusar-Poli P., Rubia K., Kambeitz J., O’Carroll C., Seal M.L., et al. Induction of psychosis by Δ9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch. Gen. Psychiatry. 2012;69:27–36. doi: 10.1001/archgenpsychiatry.2011.161. [DOI] [PubMed] [Google Scholar]
- 93.Martin-Santos R.A., Crippa J., Batalla A., Bhattacharyya S., Atakan Z., Borgwardt S., Allen P., Seal M., Langohr K., Farre M., et al. Acute Effects of a Single, Oral dose of d9-tetrahydrocannabinol (THC) and Cannabidiol (CBD) Administration in Healthy Volunteers. Curr. Pharm. Des. 2012;18:4966–4979. doi: 10.2174/138161212802884780. [DOI] [PubMed] [Google Scholar]
- 94.Koob G.F., Volkow N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zehra A., Burns J., Liu C.K., Manza P., Wiers C.E., Volkow N.D., Wang G.J. Cannabis Addiction and the Brain: A Review. J. Neuroimmune Pharmacol. 2018;13:438–452. doi: 10.1007/s11481-018-9782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bloomfield M.A.P., Morgan C.J.A., Egerton A., Kapur S., Curran H.V., Howes O.D. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol. Psychiatry. 2014;75:470–478. doi: 10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 97.Bloomfield M.A.P., Morgan C.J.A., Kapur S., Curran H.V., Howes O.D. The link between dopamine function and apathy in cannabis users: An [18F]-DOPA PET imaging study. Psychopharmacology. 2014;231:2251–2259. doi: 10.1007/s00213-014-3523-4. [DOI] [PubMed] [Google Scholar]
- 98.Lile J.A., Kelly T.H., Hays L.R. Separate and combined effects of the GABA reuptake inhibitor tiagabine and Δ 9-THC in humans discriminating Δ 9-THC. Drug Alcohol Depend. 2012;122:61–69. doi: 10.1016/j.drugalcdep.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lile J.A., Kelly T.H., Hays L.R. Separate and combined effects of the GABAB agonist baclofen and Δ9-THC in humans discriminating Δ9-THC. Drug Alcohol Depend. 2012;126:216–223. doi: 10.1016/j.drugalcdep.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prescot A.P., Locatelli A.E., Renshaw P.F., Yurgelun-Todd D.A. Neurochemical alterations in adolescent chronic marijuana smokers: A proton MRS study. Neuroimage. 2011;57:69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prescot A.P., Renshaw P.F., Yurgelun-Todd D.A. γ-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Depend. 2013;129:232–239. doi: 10.1016/j.drugalcdep.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang L., Cloak C., Yakupov R., Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J. Neuroimmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rigucci S., Xin L., Klauser P., Baumann P.S., Alameda L., Cleusix M., Jenni R., Ferrari C., Pompili M., Gruetter R., et al. Cannabis use in early psychosis is associated with reduced glutamate levels in the prefrontal cortex. Psychopharmacology. 2018;235:13–22. doi: 10.1007/s00213-017-4745-z. [DOI] [PubMed] [Google Scholar]
- 104.Hasan A., Rothenberger A., Münchau A., Wobrock T., Falkai P., Roessner V. Oral Δ9-tetrahydrocannabinol improved refractory Gilles de la Tourette syndrome in an adolescent by increasing intracortical inhibition: A case report. J. Clin. Psychopharmacol. 2010;30:190–192. doi: 10.1097/JCP.0b013e3181d236ec. [DOI] [PubMed] [Google Scholar]
- 105.Fitzgerald P.B., Williams S., Daskalakis Z.J. A transcranial magnetic stimulation study of the effects of cannabis use on motor cortical inhibition and excitability. Neuropsychopharmacology. 2009;34:2368–2375. doi: 10.1038/npp.2009.71. [DOI] [PubMed] [Google Scholar]
- 106.Martin-Rodriguez J.F., Ruiz-Veguilla M., Alvarez de Toledo P., Aizpurua-Olaizola O., Zarandona I., Canal-Rivero M., Rodriguez-Baena A., Mir P. Impaired motor cortical plasticity associated with cannabis use disorder in young adults. Addict. Biol. 2020:e12912. doi: 10.1111/adb.12912. [DOI] [PubMed] [Google Scholar]
- 107.Wobrock T., Hasan A., Malchow B., Wolff-Menzler C., Guse B., Lang N., Schneider-Axmann T., Ecker U.K.H., Falkai P. Increased cortical inhibition deficits in first-episode schizophrenia with comorbid cannabis abuse. Psychopharmacology. 2010;208:353–363. doi: 10.1007/s00213-009-1736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Flavel S.C., White J.M., Todd G. Motor cortex and corticospinal excitability in humans with a history of illicit stimulant use. J. Appl. Physiol. 2012;113:1486–1494. doi: 10.1152/japplphysiol.00718.2012. [DOI] [PubMed] [Google Scholar]
- 109.Goodman M.S., Bridgman A.C., Rabin R.A., Blumberger D.M., Rajji T.K., Daskalakis Z.J., George T.P., Barr M.S. Differential effects of cannabis dependence on cortical inhibition in patients with schizophrenia and non-psychiatric controls. Brain Stimul. 2017;10:275–282. doi: 10.1016/j.brs.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Russo M., Calabrò R.S., Naro A., Sessa E., Rifici C., D’Aleo G., Leo A., De Luca R., Quartarone A., Bramanti P. Sativex in the Management of Multiple Sclerosis-Related Spasticity: Role of the Corticospinal Modulation. Neural Plast. 2015;2015:656582. doi: 10.1155/2015/656582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leocani L., Nuara A., Houdayer E., Schiavetti I., Del Carro U., Amadio S., Straffi L., Rossi P., Martinelli V., Vila C., et al. Sativex® and clinical–neurophysiological measures of spasticity in progressive multiple sclerosis. J. Neurol. 2015;262:2520–2527. doi: 10.1007/s00415-015-7878-1. [DOI] [PubMed] [Google Scholar]
- 112.Calabrò R.S., Russo M., Naro A., Ciurleo R., D’Aleo G., Rifici C., Balletta T., La Via C., Destro M., Bramanti P., et al. Nabiximols plus robotic assisted gait training in improving motor performances in people with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2020;43:102177. doi: 10.1016/j.msard.2020.102177. [DOI] [PubMed] [Google Scholar]
- 113.Townsend L., Flisher A.J., King G. A systematic review of the relationship between high school dropout and substance use. Clin. Child Fam. Psychol. Rev. 2007;10:295–317. doi: 10.1007/s10567-007-0023-7. [DOI] [PubMed] [Google Scholar]
- 114.Macleod J., Oakes R., Copello A., Crome P.I., Egger P.M., Hickman M., Oppenkowski T., Stokes-Lampard H., Smith G.D. Psychological and social sequelae of cannabis and other illicit drug use by young people: A systematic review of longitudinal, general population studies. Lancet. 2004;363:1579–1588. doi: 10.1016/S0140-6736(04)16200-4. [DOI] [PubMed] [Google Scholar]
- 115.Jablensky A. Prevalence and Incidence of Schizophrenia Spectrum Disorders: Implications for Prevention. Aust. N. Z. J. Psychiatry. 2000;34:S26–S34. doi: 10.1177/000486740003401S05. [DOI] [PubMed] [Google Scholar]
- 116.D’Souza D.C., Abi-Saab W.M., Madonick S., Forselius-Bielen K., Doersch A., Braley G., Gueorguieva R., Cooper T.B., Krystal J.H. Delta-9-tetrahydrocannabinol effects in schizophrenia: Implications for cognition, psychosis, and addiction. Biol. Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 117.Batalla A., Janssen H., Gangadin S.S., Bossong M.G. The Potential of Cannabidiol as a Treatment for Psychosis and Addiction: Who Benefits Most? A Systematic Review. J. Clin. Med. 2019;8:1058. doi: 10.3390/jcm8071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zuardi A.W., Morais S.L., Guimaraes F.S., Mechoulam R. Antipsychotic effect of cannabidiol. J. Clin. Psychiatry. 1995;56:485–486. [PubMed] [Google Scholar]
- 119.Dajas-Bailador F., Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 120.Mansvelder H.D., McGehee D.S. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- 121.Wooltorton J.R.A., Pidoplichko V.I., Broide R.S., Dani J.A. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.D’Souza M.S., Markou A. The “Stop” and “Go” of nicotine dependence: Role of GABA and glutamate. Cold Spring Harb. Perspect. Med. 2013;3:a012146. doi: 10.1101/cshperspect.a012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Even N., Cardona A., Soudant M., Corringer P.J., Changeux J.P., Cloëz-Tayarani I. Regional differential effects of chronic nicotine on brain α4-containing and α6 -containing receptors. Neuroreport. 2008;19:1545–1550. doi: 10.1097/WNR.0b013e3283112703. [DOI] [PubMed] [Google Scholar]
- 124.Alkondon M., Pereira E.F.R., Almeida L.E.F., Randall W.R., Albuquerque E.X. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. doi: 10.1016/S0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 125.Durazzo T.C., Meyerhoff D.J., Murray D.E. Comparison of regional brain perfusion levels in chronically smoking and non-smoking adults. Int. J. Environ. Res. Public Health. 2015;12:8198–8213. doi: 10.3390/ijerph120708198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gons R.A.R., Van Norden A.G.W., De Laat K.F., Van Oudheusden L.J.B., Van Uden I.W.M., Zwiers M.P., Norris D.G., De Leeuw F.E. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain. 2011;134:2116–2124. doi: 10.1093/brain/awr145. [DOI] [PubMed] [Google Scholar]
- 127.Gonzalez C.L.R., Gharbawie O.A., Whishaw I.Q., Kolb B. Nicotine stimulates dendritic arborization in motor cortex and improves concurrent motor skill but impairs subsequent motor learning. Synapse. 2005;55:183–191. doi: 10.1002/syn.20106. [DOI] [PubMed] [Google Scholar]
- 128.Orth M., Amann B., Robertson M.M., Rothwell J.C. Excitability of motor cortex inhibitory circuits in Tourette syndrome before and after single dose nicotine. Brain. 2005;128:1292–1300. doi: 10.1093/brain/awh473. [DOI] [PubMed] [Google Scholar]
- 129.Grundey J., Freznosa S., Klinker F., Lang N., Paulus W., Nitsche M.A. Cortical excitability in smoking and not smoking individuals with and without nicotine. Psychopharmacology. 2013;229:653–664. doi: 10.1007/s00213-013-3125-6. [DOI] [PubMed] [Google Scholar]
- 130.Di Lazzaro V., Oliviero A., Profice P., Pennisi M., Di Giovanni S., Zito G., Tonali P., Rothwell J. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp. Brain Res. 2000;135:455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- 131.Fujiki M., Hikawa T., Abe T., Ishii K., Kobayashi H. Reduced short latency afferent inhibition in diffuse axonal injury patients with memory impairment. Neurosci. Lett. 2006;405:226–230. doi: 10.1016/j.neulet.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 132.Di Lazzaro V. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J. Neurol. Neurosurg. Psychiatry. 2005;76:1064–1069. doi: 10.1136/jnnp.2004.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Benowitz N.L. Pharmacokinetic considerations in understanding nicotine dependence. In: Bock G., Marsh J., editors. The Biology of Nicotine Dependence. Wiley; Chichester, UK: 1990. pp. 186–209. [DOI] [PubMed] [Google Scholar]
- 134.Shiffman S., Dresler C.M., Hajek P., Gilburt S.J.A., Targett D.A., Strahs K.R. Efficacy of a nicotine lozenge for smoking cessation. Arch. Intern. Med. 2002;162:1267–1276. doi: 10.1001/archinte.162.11.1267. [DOI] [PubMed] [Google Scholar]
- 135.Shiffman S., Dresler C.M., Rohay J.M. Successful treatment with a nicotine lozenge of smokers with prior failure in pharmacological therapy. Addiction. 2004;99:83–92. doi: 10.1111/j.1360-0443.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 136.Lang N., Hasan A., Sueske E., Paulus W., Nitsche M.A. Cortical Hypoexcitability in Chronic Smokers? A Transcranial Magnetic Stimulation Study. Neuropsychopharmacology. 2008;33:2517–2523. doi: 10.1038/sj.npp.1301645. [DOI] [PubMed] [Google Scholar]
- 137.Khedr E.M., Tony A.A., Abdelwarith A., Safwat M. Effect of chronic nicotine consumption on motor cortical excitability: A transcranial magnetic stimulation study. Neurophysiol. Clin. 2020;50:33–39. doi: 10.1016/j.neucli.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 138.Grundey J., Thirugnanasambandam N., Kaminsky K., Drees A., Skwirba A.C., Lang N., Paulus W., Nitsche M.A. Neuroplasticity in cigarette smokers is altered under withdrawal and partially restituted by nicotine exposition. J. Neurosci. 2012 doi: 10.1523/JNEUROSCI.3660-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Grundey J., Thirugnasambandam N., Amu R., Paulus W., Nitsche M.A. Nicotinic restoration of excitatory neuroplasticity is linked to improved implicit motor learning skills in deprived smokers. Front. Neurol. 2018;9:367. doi: 10.3389/fneur.2018.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lavender A.P., Obata H., Kawashima N., Nakazawa K. Effect of paired associative stimulation on corticomotor excitability in chronic smokers. Brain Sci. 2019;9 doi: 10.3390/brainsci9030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nawrot P., Jordan S., Eastwood J., Rotstein J., Hugenholtz A., Feeley M. Effects of caffeine on human health. Food Addit. Contam. 2003;20:1–30. doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- 142.Bolton S., Null G. Caffeine psychological effects, use and abuse. J. Orthomol. Psychiatry. 1981;10:202–211. [Google Scholar]
- 143.Nehlig A. Is caffeine a cognitive enhancer? J. Alzheimer’s Dis. 2010;20:S85–S94. doi: 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- 144.Smith A., Sturgess W., Gallagher J. Effects of a low dose of caffeine given in different drinks on mood and performance. Hum. Psychopharmacol. 1999;14:473–482. doi: 10.1002/(SICI)1099-1077(199910)14:7<473::AID-HUP129>3.0.CO;2-D. [DOI] [Google Scholar]
- 145.Griffiths R.R., Evans S.M., Heishman S.J., Preston K.L., Sannerud C.A., Wolf B., Woodson P.P. Low-dose caffeine discrimination in humans. J. Pharmacol. Exp. Ther. 1990;252:970–978. [PubMed] [Google Scholar]
- 146.Kaplan G.B., Greenblatt D.J., Ehrenberg B.L., Goddard J.E., Cotreau M.M., Harmatz J.S., Shader R.I. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J. Clin. Pharmacol. 1997;37:693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 147.Daly J.W., Fredholm B.B. Caffeine-An atypical drug of dependence. Drug Alcohol Depend. 1998;51:199–206. doi: 10.1016/S0376-8716(98)00077-5. [DOI] [PubMed] [Google Scholar]
- 148.Fredholm B.B., IJzerman A.P., Jacobson K.A., Linden J., Müller C.E. International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors-An update. Pharmacol. Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Snyder S.H., Katims J.J., Annau Z., Bruns R.F., Daly J.W. Adenosine receptors and behavioral actions of methylxanthines [caffeine/theophylline/N 6-cyclohexyladenosine/N 6-(phenylisopropyl)adenosine] Proc. Natl. Acad. Sci. USA. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Böttger A., Vothknecht U., Bolle C., Wolf A. Lessons on Caffeine, Cannabis & Co. Springer International Publishing; Cham, Switzerland: 2018. Adenosine Receptors: Caffeine; pp. 64–68. [Google Scholar]
- 151.Vyleta N.P., Smith S.M. Fast inhibition of glutamate-activated currents by caffeine. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ferré S., Fuxe K., von Euler G., Johansson B., Fredholm B.B. Adenosine-dopamine interactions in the brain. Neuroscience. 1992;51:501–512. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- 153.Garrett B.E., Griffiths R.R. The role of dopamine in the behavioral effects of caffeine in animals and humans. Pharmacol. Biochem. Behav. 1997;57:533–541. doi: 10.1016/S0091-3057(96)00435-2. [DOI] [PubMed] [Google Scholar]
- 154.Orth M., Amann B., Ratnaraj N., Patsalos P.N., Rothwell J.C. Caffeine has no effect on measures of cortical excitability. Clin. Neurophysiol. 2005;116:308–314. doi: 10.1016/j.clinph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 155.Mesquita R.N.O., Cronin N.J., Kyröläinen H., Hintikka J., Avela J. Effects of caffeine on neuromuscular function in a non-fatigued state and during fatiguing exercise. Exp. Physiol. 2020;105:690–706. doi: 10.1113/EP088265. [DOI] [PubMed] [Google Scholar]
- 156.Cerqueira V., de Mendonça A., Minez A., Dias A.R., de Carvalho M. Does caffeine modify corticomotor excitability? Neurophysiol. Clin. 2006;36:219–226. doi: 10.1016/j.neucli.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 157.De Carvalho M., Marcelino E., De Mendonça A. Electrophysiological studies in healthy subjects involving caffeine. J. Alzheimer’s Dis. 2010;20:S63–S69. doi: 10.3233/JAD-2010-1377. [DOI] [PubMed] [Google Scholar]
- 158.Concerto C., Infortuna C., Chusid E., Coira D., Babayev J., Metwaly R., Naenifard H., Aguglia E., Battaglia F. Caffeinated energy drink intake modulates motor circuits at rest, before and after a movement. Physiol. Behav. 2017;179:361–368. doi: 10.1016/j.physbeh.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 159.Kalmar J.M., Cafarelli E. Central fatigue and transcranial magnetic stimulation: Effect of caffeine and the confound of peripheral transmission failure. J. Neurosci. Methods. 2004;138:15–26. doi: 10.1016/j.jneumeth.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 160.Hanajima R., Tanaka N., Tsutsumi R., Shirota Y., Shimizu T., Terao Y., Ugawa Y. Effect of caffeine on long-term potentiation-like effects induced by quadripulse transcranial magnetic stimulation. Exp. Brain Res. 2019;237:647–651. doi: 10.1007/s00221-018-5450-9. [DOI] [PubMed] [Google Scholar]
- 161.Kalmar J.M., Cafarelli E. Central excitability does not limit postfatigue voluntary activation of quadriceps femoris. J. Appl. Physiol. 2006;100:1757–1764. doi: 10.1152/japplphysiol.01347.2005. [DOI] [PubMed] [Google Scholar]
- 162.Bowtell J.L., Mohr M., Fulford J., Jackman S.R., Ermidis G., Krustrup P., Mileva K.N. Improved Exercise Tolerance with Caffeine Is Associated with Modulation of both Peripheral and Central Neural Processes in Human Participants. Front. Nutr. 2018;5:6. doi: 10.3389/fnut.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Specterman M., Bhuiya A., Kuppuswamy A., Strutton P.H., Catley M., Davey N.J. The effect of an energy drink containing glucose and caffeine on human corticospinal excitability. Physiol. Behav. 2005;83:723–728. doi: 10.1016/j.physbeh.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 164.Jia F., Yue M., Chandra D., Keramidas A., Goldstein P.A., Homanics G.E., Harrison N.L. Taurine is a potent activator of extrasynaptic GABAA receptors in the thalamus. J. Neurosci. 2008;28:106–115. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.El Idrissi A., Trenkner E. Taurine as a Modulator of Excitatory and Inhibitory Neurotransmission. Neurochem. Res. 2004;29:189–197. doi: 10.1023/B:NERE.0000010448.17740.6e. [DOI] [PubMed] [Google Scholar]
- 166.Oja S.S., Saransaari P. Modulation of taurine release by glutamate receptors and nitric oxide. Prog. Neurobiol. 2000;62:407–425. doi: 10.1016/S0301-0082(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 167.El Idrissi A., Harris C., Trenkner E. Taurine modulates glutamate- and growth factors-mediated signaling mechanisms. Adv. Exp. Med. Biol. 1998;442:385–396. doi: 10.1007/978-1-4899-0117-0_48. [DOI] [PubMed] [Google Scholar]
- 168.Infortuna C., Milisits T.F., Shaju S., Desai J.K., Patel S.P., Sheikh A.M., Chusid E., Han Z., Battaglia F. Effects of taurine acute intake on cortical excitability and post-exercise facilitation: A TMS study. Behav. Brain Res. 2019;359:719–722. doi: 10.1016/j.bbr.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 169.Kojima S., Onishi H., Sugawara K., Kirimoto H., Suzuki M., Tamaki H. Modulation of the cortical silent period elicited by single- and paired-pulse transcranial magnetic stimulation. BMC Neurosci. 2013;14:43. doi: 10.1186/1471-2202-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.dos Santos M.K.F., Gavioli E.C., Rosa L.S., de Paula Soares-Rachetti V., Lobão-Soares B. Craving espresso: The dialetics in classifying caffeine as an abuse drug. Naunyn. Schmiedebergs. Arch. Pharmacol. 2018;391:1301–1318. doi: 10.1007/s00210-018-1570-9. [DOI] [PubMed] [Google Scholar]
- 171.Muetzel R.L., Marjańska M., Collins P.F., Becker M.P., Valabrègue R., Auerbach E.J., Lim K.O., Luciana M. In vivo 1H magnetic resonance spectroscopy in young-adult daily marijuana users. NeuroImage Clin. 2013;2:581–589. doi: 10.1016/j.nicl.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wiers C.E., Shokri-Kojori E., Wong C.T., Abi-Dargham A., Demiral Ş.B., Tomasi D., Wang G.J., Volkow N.D. Cannabis Abusers Show Hypofrontality and Blunted Brain Responses to a Stimulant Challenge in Females but not in Males. Neuropsychopharmacology. 2016;41:2596–2605. doi: 10.1038/npp.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Perkins K.A. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp. Clin. Psychopharmacol. 1996;4:166–177. doi: 10.1037/1064-1297.4.2.166. [DOI] [Google Scholar]
- 174.Hatsukami D., Skoog K., Allen S., Bliss R. Gender and the Effects of Different Doses of Nicotine Gum on Tobacco Withdrawal Symptoms. Exp. Clin. Psychopharmacol. 1995;3:163–173. doi: 10.1037/1064-1297.3.2.163. [DOI] [Google Scholar]
- 175.Li M.D. The genetics of nicotine dependence. Curr. Psychiatry Rep. 2006;8:158–164. doi: 10.1007/s11920-006-0016-0. [DOI] [PubMed] [Google Scholar]
- 176.MacKillop J., Obasi E.M., Amlung M.T., McGeary J.E., Knopik V.S. The Role of Genetics in Nicotine Dependence: Mapping the Pathways from Genome to Syndrome. Curr. Cardiovasc. Risk Rep. 2010;4:446–453. doi: 10.1007/s12170-010-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hutchison K.E., Allen D.L., Filbey F.M., Jepson C., Lerman C., Benowitz N.L., Stitzel J., Bryan A., McGeary J., Haughey H.M. CHRNA4 and tobacco dependence: From gene regulation to treatment outcome. Arch. Gen. Psychiatry. 2007;64:1078–1086. doi: 10.1001/archpsyc.64.9.1078. [DOI] [PubMed] [Google Scholar]
- 178.Broms U., Wedenoja J., Largeau M.R., Korhonen T., Pitkäniemi J., Keskitalo-Vuokko K., Häppölä A., Heikkilä K.H., Heikkilä K., Ripatti S., et al. Analysis of detailed phenotype profiles reveals CHRNA5-CHRNA3-CHRNB4 gene cluster association with several nicotine dependence traits. Nicotine Tob. Res. 2012;14:720–733. doi: 10.1093/ntr/ntr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Saccone N.L., Culverhouse R.C., Schwantes-An T.H., Cannon D.S., Chen X., Cichon S., Giegling I., Han S., Han Y., Keskitalo-Vuokko K., et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: A meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Furberg H., Kim Y., Dackor J., Boerwinkle E., Franceschini N., Ardissino D., Bernardinelli L., Mannucci P.M., Mauri F., Merlini P.A., et al. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Berrettini W., Yuan X., Tozzi F., Song K., Francks C., Chilcoat H., Waterworth D., Muglia P., Mooser V. A-5/A-3 Nicotinic Receptor Subunit Alleles Increase Risk for Heavy Smoking. Mol. Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hartz S.M., Short S.E., Saccone N.L., Culverhouse R., Chen L.S., Schwantes-An T.H., Coon H., Han Y., Stephens S.H., Sun J., et al. Increased genetic vulnerability to smoking at CHRNA5 in early-onset smokers. Arch. Gen. Psychiatry. 2012;69:854–861. doi: 10.1001/archgenpsychiatry.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bhattacharyya S., Morrison P.D., Fusar-Poli P., Martin-Santos R., Borgwardt S., Winton-Brown T., Nosarti C., O’Carroll C.M., Seal M., Allen P., et al. Opposite effects of δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pertwee R.G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ 9-tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Becker A., Ehret A.M., Kirsch P. From the neurobiological basis of comorbid alcohol dependence and depression to psychological treatment strategies: Study protocol of a randomized controlled trial. BMC Psychiatry. 2017;17:153. doi: 10.1186/s12888-017-1324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Frye M.A., Salloum I.M. Bipolar disorder and comorbid alcoholism: Prevalence rate and treatment considerations. Bipolar Disord. 2006;8:677–685. doi: 10.1111/j.1399-5618.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 187.McCormick L.M., Buchanan J.R., Onwuameze O.E., Pierson R.K., Paradiso S. Beyond alcoholism: Wernicke-korsakoff syndrome in patients with psychiatric disorders. Cogn. Behav. Neurol. 2011;24:209–216. doi: 10.1097/WNN.0b013e31823f90c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.