Abstract
Background
Racial/ethnic disparities in prognosis have been reported in patients with hepatocellular carcinoma (HCC); however, few studies have evaluated racial/ethnic disparities in the context of insurance status.
Aims
Characterize racial/ethnic and insurance status in early tumor detection, receipt of curative therapy and overall survival in a multicenter diverse cohort of HCC patients from the USA.
Study
We included patients with HCC diagnosed between June 2012 and May 2013 at four centers in the USA. Generalized linear mixed effects models were used to compare early tumor detection (defined using Milan Criteria) and curative treatment receipt (liver transplantation, surgical resection, or local ablation) as a function of patient race/ethnicity and insurance status. A multivariable frailty survival model was used to compare risk of death between patient groups.
Results
Of 379 HCC patients (52.8% non-Hispanic White, 19.5% Hispanic White, 19.8% Black), 46.4% and 48.0% were found at an early stage and underwent curative therapy, respectively, and median overall survival of the cohort was 25.7 months. Early detection of HCC was associated with gastroenterology subspecialty care and receipt of HCC surveillance but not race/ethnicity or insurance status in adjusted models. However, commercial insurance was significantly associated with higher odds of curative treatment receipt, which in turn was the strongest correlate for overall survival. After adjusting for health system and insurance status, race/ethnicity was not associated with curative treatment receipt or overall survival.
Conclusions
Insurance status and access to gastroenterology subspecialty care may be important drivers of racial/ethnic disparities in prognosis among HCC patients.
Keywords: Disparities, Liver cancer, Survival, Racial, Insurance
Introduction
In the USA, the incidence of HCC has more than doubled over the past two decades and is projected to grow for the foreseeable future [1, 2]. HCC is also highly morbid, primarily due to low rates of early detection and curative treatment receipt [3, 4].
Significant health disparities exist in HCC incidence and prognosis [5–7]. Among Hispanic Whites and non-Hispanic Blacks, HCC incidence is rapidly rising [2]. Observed differences in incidence are thought to be multifactorial-related differences in epidemiology, risk factors, and access [8]. Similarly lower crude survival observed in racial/ethnic minorities, as compared to non-Hispanic Whites, may be attributed to differences in HCC surveillance and treatment receipt [9]. However, differences in care delivery may not fully explain racial/ethnic disparities, as Black patients with HCC continue to have lower survival than non-Hispanic Whites in adjusted models [10].
However, factors associated with socioeconomic status, like health insurance, and its potential intersectionality with race/ethnicity has had limited evaluation in most studies examining racial/ethnic disparities in HCC. Race/ethnicity is highly correlated with factors associated with SES, which has been also shown to be associated with lower rates of HCC surveillance and more advanced tumor stage [11, 12]. Failing to account for SES can falsely attribute socioeconomic disparities to race/ethnicity or vice versa. Prior analyses of HCC outcomes typically lack granularity to include any measure of SES [13–15], or there is limited variability in the SES across the populations studied [6, 16].
Exploring factors driving differential outcomes across the care continuum is crucial to inform interventions that can reduce HCC-related disparities [17]. Therefore, we aimed to characterize potential racial/ethnic and socioeconomic disparities in patients with HCC.
Materials and Methods
We conducted a retrospective cohort study of patients newly diagnosed with HCC at one of four health systems in the USA between June 1, 2012 and May 31, 2013. Each study site was associated with an academic medical center and included two tertiary care centers (University of Michigan and Loyola University) and two safety-net health systems (Parkland Health and Hospital System and Ben Taub Hospital—Harris Health System).
As previously described, patients were initially identified by ICD-9 codes for HCC (155.0–155.2), tumor conference presentation lists, and prospectively maintained databases of patients with HCC at each site [18, 19]. Authors adjudicated HCC cases to confirm they met diagnostic criteria as defined by the American Association for the Study of Liver Disease (AASLD) criteria [20]. For tumors larger than 1 cm, diagnosis was based on a typical vascular pattern on dynamic imaging (arterial enhancement and washout on delayed phase) or histology. We excluded patients without cross-sectional imaging given baseline tumor characteristics could not be accurately determined. Patients with HCC diagnosis prior to presentation in whom surveillance status was unknown and those with prior HCC-directed treatment were also excluded. This study was approved by the institutional review boards at each study site.
Data Collection
Data collection on patient demographics, clinical history, and laboratory data are available in the supplemental material. Four-phase CT and contrast-enhanced MRIs, as interpreted by radiologists at each site, were used to determine tumor characteristics including number of lesions, maximum tumor diameter, lymph node involvement, portal vein invasion, and presence of extrahepatic metastases. Early tumor detection was defined using Milan Criteria, the most common criteria for liver transplantation in the USA. Patient clinical course including dates of treatment, dates of follow-up imaging, and date of death or date of last follow-up were recorded for each patient. HCC treatments were categorized as liver transplantation, surgical resection, local ablative therapy (LAT), transarterial chemoembolization (TACE), transarterial radioembolization (TARE), stereotactic body radiation (SBRT), systemic therapy, or best supportive care. Receipt of curative treatment for HCC was determined whether it consisted of LAT, surgical resection, or liver transplantation. For patients who received multiple treatments, treatment was categorized as the most curative (i.e., liver transplantation > resection > LAT > TACE or TARE > systemic therapy > best supportive care).
Statistical Analysis
Univariable linear mixed effects models were used to compare the distributions of age, body mass index (BMI), and MELD score among those identifying as White, Black, Hispanic, and any other race. In these models, random intercepts were allowed for each hospital in order to account for the clustering of patients within their treatment facility type. Similarly, univariable generalized linear mixed effects models were used to make comparisons among these race groups on their sex, insurance status (used as a proxy of SES), use of tobacco and alcohol, HCC etiology, presence of cirrhosis, access to a GI specialist, Child-Turcotte-Pugh status, diagnosis mode, ECOG status, multidisciplinary review, BCLC stage, tumor burden, and receipt of a primary curative treatment. In addition to allowing random intercepts to account for patients’ within-hospital correlation, these models also specified a multinomial distribution with generalized logit link when the response variable comprised more than two nominal levels and a cumulative logit link when the response variable comprised more than two ordinal levels; otherwise, a binomial distribution with logit link was specified for the outcome. When expected cell frequencies were sparse, a Kenward-Roger correction was used to adjust the denominator degrees of freedom for small sample bias and conclusions were confirmed using Fisher’s exact test [21].
Univariable generalized linear mixed effects models were also used to estimate the odds of early tumor detection, defined using the Milan Criteria, as a function of patient characteristics and comorbidities. In these models, a binomial distribution with logit link was specified for early tumor detection and random intercepts were again allowed for each treatment facility. A follow-up multivariable model was used to further estimate the adjusted odds of early tumor detection as a function of patients’ race/ethnicity while controlling for their age, sex, insurance status, BMI, smoking status, presence of cirrhosis, access to a GI specialist, and mode of HCC diagnosis. These covariates were selected a priori or were entered because of their significance or nominal significance (p < .10) on univariable analysis. A similar approach was used to estimate the odds of receiving a curative treatment.
Finally, univariable and multivariable frailty survival models that allow for the clustering of patients within their treatment facility type were used to estimate the risk of mortality as function of patients’ race while controlling for their receipt of a curative treatment and insurance status. In this model, elapsed time was measured in months from the time of HCC diagnosis to date of death and living cases were censored at their last follow-up date. In these models, a log-normal distribution was specified for the frailty effect and the proportional hazards assumption for each covariate was assessed graphically using Martingale residuals as described by Lin et al. [22]. For all multivariable models, multicollinearity diagnostics were within an acceptable range as measured by variance inflation factors, shared variance values, and tolerance statistics. All analyses were conducted using SAS version 9.4 (Cary, NC).
Results
Patient Characteristics
We identified a total of 379 patients who met our inclusion criteria, ranging between 68 and 149 at each site. Patient characteristics are detailed in Tables 1 and 2. The median age was 59.9 (IGQ 55.4–66.7), and the majority were male (n = 284 or 74.9%). Our population was racially diverse, with approximately half identifying as White (52.7%), and the remaining identifying as Black (19.7%), Hispanic (19.5%), or another race (7.9%). Twenty-two percent had commercial insurance, while approximately a third had Medicare (32.5%) or a Medicaid/subsidiary plan (33.9%). Among racial groups, Whites had a higher proportion of commercial insurance and Medicare, Blacks had higher proportions of Medicaid, and Hispanics had a higher proportion of subsidy or no insurance. Almost all patients (91.8%) had underlying cirrhosis, with the most common etiologies being HCV infection (57.8%), NASH/cryptogenic (21.7%), or alcohol induced (15.6%). Most patients were exclusively followed by primary care providers (PCPs) prior to their HCC diagnosis, with only 144 (39.6%) patients receiving gastroenterology (GI) subspecialty care. There were no meaningful racial differences in Child-Pugh-Turcotte class as most patients had preserved liver function, with 47.4% Child-Pugh A cirrhosis, 34.6% Child-Pugh B, and 18% Child-Pugh C cirrhosis (overall p = 0.06).
Table 1.
Patient demographics and clinical characteristics
| Race | p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| White (n = 200) | Black (n = 75) | Hispanic (n = 74) | Other (n = 30) | Total (N = 379) | |||||||
| Mean age (SE) | 60.86 | 2.10 | 59.00 | 2.26 | 60.80 | 2.25 | 62.50 | 2.57 | 60.60 | 2.06 | .34 |
| Mean BMI(SE) N = 368 | 29.97 | 0.94 | 25.57 | 1.20 | 29.35 | 1.17 | 25.89 | 1.63 | 29.01 | 0.94 | .03 |
| Mean MELD (SE) N = 368 | 11.31 | 0.40 | 11.47 | 0.64 | 12.34 | 0.64 | 9.61 | 1.04 | 11.42 | 0.29 | .16 |
| Gender | |||||||||||
| Male | 157 | 78.5% | 54 | 72.0% | 53 | 71.6% | 20 | 66.7% | 284 | 74.9% | .37 |
| Female | 43 | 21.5% | 21 | 28.0% | 21 | 28.4% | 10 | 33.3% | 95 | 25.1% | |
| Insurance N = 372 | |||||||||||
| Uninsured | 9 | 4.6% | 10 | 13.7% | 16 | 22.2% | 7 | 24.1% | 42 | 11.3% | <.001 |
| Commercial | 61 | 30.8% | 8 | 11.0% | 8 | 11.1% | 6 | 20.7% | 83 | 22.3% | |
| Medicare | 82 | 41.4% | 21 | 28.8% | 13 | 18.1% | 5 | 17.2% | 121 | 32.5% | |
| Medicaid | 32 | 16.2% | 22 | 30.1% | 6 | 8.3% | 5 | 17.2% | 65 | 17.5% | |
| Subsidy | 14 | 7.1% | 12 | 16.4% | 29 | 40.3% | 6 | 20.7% | 61 | 16.4% | |
| Smoking N = 356 | |||||||||||
| Never | 42 | 23.1% | 16 | 22.2% | 37 | 50.0% | 14 | 50.0% | 109 | 30.6% | <.001 |
| Current | 61 | 33.5% | 36 | 50.0% | 13 | 17.6% | 5 | 17.9% | 115 | 32.3% | |
| Quit | 79 | 43.4% | 20 | 27.8% | 24 | 32.4% | 9 | 32.1% | 132 | 37.1% | |
| Alcohol N = 363 | |||||||||||
| Never | 85 | 45.0% | 23 | 31.5% | 26 | 35.6% | 17 | 60.7% | 151 | 41.6% | .31 |
| Current | 35 | 18.5% | 19 | 26.0% | 18 | 24.7% | 1 | 3.6% | 73 | 20.1% | |
| Quit | 69 | 36.5% | 31 | 42.5% | 29 | 39.7% | 10 | 35.7% | 139 | 38.3% | |
| Etiology N = 360 | |||||||||||
| NASH | 56 | 29.5% | 2 | 2.8% | 17 | 23.6% | 3 | 11.1% | 78 | 21.7% | <.001 |
| HBV | 3 | 1.6% | 5 | 7.0% | 3 | 4.2% | 7 | 25.9% | 18 | 5.0% | |
| ETOH | 32 | 16.8% | 3 | 4.2% | 20 | 27.8% | 1 | 3.7% | 56 | 15.6% | |
| HCV | 99 | 52.1% | 61 | 85.9% | 32 | 44.4% | 16 | 59.3% | 208 | 57.8% | |
| Cirrhosis N = 364 | |||||||||||
| No | 16 | 8.5% | 6 | 8.0% | 5 | 6.9% | 3 | 10.3% | 30 | 8.2% | .97 |
| Yes | 172 | 91.5% | 69 | 92.0% | 67 | 93.1% | 26 | 89.7% | 334 | 91.8% | |
| Ascites N = 373 | |||||||||||
| No | 107 | 54.6% | 59 | 79.7% | 38 | 51.4% | 18 | 62.1% | 222 | 59.5% | .002 |
| Yes | 27 | 13.8% | 6 | 8.1% | 5 | 6.8% | 5 | 17.2% | 43 | 11.5% | |
| Controlled | 62 | 31.6% | 9 | 12.2% | 31 | 41.9% | 6 | 20.7% | 108 | 29.0% | |
| GI specialist N = 364 | |||||||||||
| No | 94 | 49.5% | 54 | 74.0% | 54 | 75.0% | 18 | 62.1% | 220 | 60.4% | .15 |
| Yes | 96 | 50.5% | 19 | 26.0% | 18 | 25.0% | 11 | 37.9% | 144 | 39.6% | |
| Child-Turcotte-Pugh N = 361 | |||||||||||
| A | 90 | 47.9% | 40 | 56.3% | 26 | 35.1% | 15 | 53.6% | 171 | 47.4% | .063 |
| B | 69 | 36.7% | 22 | 31.0% | 25 | 33.8% | 9 | 32.1% | 125 | 34.6% | |
| C | 29 | 15.4% | 9 | 12.7% | 23 | 31.1% | 4 | 14.3% | 65 | 18.0% | |
The sample size for each comparison is N = 379 unless otherwise noted
Table 2.
Tumor and treatment characteristics
| Race | p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| White (n = 200) | Black (n = 75) | Hispanic (n = 74) | Other (n = 30) | Total (N = 379) | |||||||
| Median nodules (range) N = 318 | 1 | 1–8 | 1 | 1–6 | 1 | 1–13 | 1 | 1–6 | 1 | 1–13 | .76 |
| Mean tumor DIA (SE) N = 360 | 5.19 | 0.30 | 5.65 | 0.49 | 5.08 | 0.49 | 6.76 | 0.77 | 5.38 | 0.22 | .23 |
| Dx mode | |||||||||||
| N = 373 | |||||||||||
| Surveillance | 85 | 43.1% | 32 | 43.2% | 26 | 36.1% | 14 | 46.7% | 157 | 42.1% | .81 |
| Incidental | 112 | 56.9% | 42 | 56.8% | 46 | 63.9% | 16 | 53.3% | 216 | 57.9% | |
| PVT N = 369 | |||||||||||
| None | 158 | 82.3% | 46 | 62.2% | 59 | 79.7% | 21 | 72.4% | 284 | 77.0% | .046 |
| Bland | 3 | 1.6% | 1 | 1.4% | 1 | 1.4% | 0 | 0.0% | 5 | 1.4% | |
| Malignant | 31 | 16.1% | 27 | 36.5% | 14 | 18.9% | 8 | 27.6% | 80 | 21.7% | |
| HVT N = 367 | |||||||||||
| No | 180 | 94.7% | 69 | 93.2% | 71 | 95.9% | 27 | 93.1% | 347 | 94.6% | .91 |
| Yes | 10 | 5.3% | 5 | 6.8% | 3 | 4.1% | 2 | 6.9% | 20 | 5.4% | |
| Metastasis N = 368 | |||||||||||
| No | 177 | 92.7% | 63 | 85.1% | 67 | 90.5% | 28 | 96.6% | 335 | 91.0% | .65 |
| Yes | 14 | 7.3% | 11 | 14.9% | 7 | 9.5% | 1 | 3.4% | 33 | 9.0% | |
| ECOG | |||||||||||
| 0 | 104 | 52.0% | 34 | 45.3% | 24 | 32.4% | 15 | 50.0% | 177 | 46.7% | .68 |
| 1 | 58 | 29.0% | 29 | 38.7% | 38 | 51.4% | 10 | 33.3% | 135 | 35.6% | |
| 2 | 23 | 11.5% | 7 | 9.3% | 10 | 13.5% | 3 | 10.0% | 43 | 11.3% | |
| 3 | 12 | 6.0% | 5 | 6.7% | 9 | 2.7% | 2 | 6.7% | 21 | 5.5% | |
| 4 | 3 | 1.5% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 3 | 0.8% | |
| MDC | |||||||||||
| No | 74 | 37.0% | 22 | 29.3% | 28 | 37.8% | 12 | 40.0% | 136 | 35.9% | .94 |
| Yes | 126 | 63.0% | 53 | 70.7% | 46 | 62.2% | 18 | 60.0% | 243 | 64.1% | |
| BCLC stage N = 362 | |||||||||||
| Stage 0 | 13 | 7.0% | 3 | 4.1% | 3 | 4.1% | 5 | 17.2% | 24 | 6.6% | .22 |
| Stage 1 | 49 | 26.5% | 19 | 25.7% | 15 | 20.3% | 5 | 17.2% | 88 | 24.3% | |
| Stage 2 | 14 | 7.6% | 5 | 6.8% | 2 | 2.7% | 3 | 10.3% | 24 | 6.6% | |
| Stage 3 | 73 | 39.5% | 36 | 48.6% | 30 | 40.5% | 11 | 37.9% | 150 | 41.4% | |
| Stage 4 | 36 | 19.5% | 11 | 14.9% | 24 | 32.4% | 5 | 17.2% | 76 | 21.0% | |
| Milan Criteria N = 373 | |||||||||||
| No | 99 | 50.8% | 44 | 59.5% | 37 | 50.0% | 20 | 66.7% | 200 | 53.6% | .29 |
| Yes | 96 | 49.2% | 30 | 40.5% | 37 | 50.0% | 10 | 33.3% | 173 | 46.4% | |
| Treatment | |||||||||||
| None | 51 | 25.5% | 23 | 30.7% | 29 | 39.2% | 11 | 36.7% | 114 | 30.1% | .46 |
| OLT, SR, or LAT | 64 | 32.0% | 14 | 18.7% | 15 | 20.3% | 9 | 30.0% | 102 | 26.9% | |
| Other | 85 | 42.5% | 38 | 50.7% | 30 | 40.5% | 10 | 33.3% | 163 | 43.0% | |
| Treatment by Milan N = 373 | |||||||||||
| Milan no n = 200 | |||||||||||
| None | 34 | 34.3% | 15 | 34.1% | 22 | 59.5% | 10 | 50.0% | 81 | 40.5% | .81 |
| OLT, SR, or LAT | 11 | 11.1% | 2 | 4.6% | 3 | 8.1% | 10.0% | 18 | 9.0% | ||
| Other | 54 | 54.6% | 27 | 61.4% | 12 | 32.4% | 8 | 40.0% | 101 | 50.5% | |
| Milan yes n = 173 | |||||||||||
| None | 14 | 14.6% | 8 | 26.7% | 7 | 18.9% | 1 | 10.0% | 30 | 17.3% | .26 |
| OLT, SR, or LAT | 52 | 54.2% | 12 | 40.0% | 12 | 32.4% | 7 | 70.0% | 83 | 48.0% | |
| Other | 30 | 31.3% | 10 | 33.3% | 18 | 48.7% | 2 | 20.0% | 60 | 34.7% | |
The sample size for each comparison is N = 379 unless otherwise noted
OLT solid organ liver transplant, LAT local ablative therapy, Dx diagnosis, DIA diameter, PVT portal vein thrombosis, HVT hepatic venous invasion, ECOG Eastern Cooperative Oncology Group, MDC multidisciplinary conference, BCLC Barcelona Clinic Liver Cancer, SR surgical resection
Early Tumor Detection
HCC was diagnosed incidentally in 57.9% of cases, and less than half (46.4%) were within Milan Criteria at time of diagnosis. Among racial/ethnic groups, 49.2% of Whites, 40.5% of Blacks, and 50.0% of Hispanics were detected within Milan Criteria; however, differences between groups did not reach statistical significance (p = 0.29). Similarly, when compared to patients with commercial insurance, having Medicaid (p = 0.95), Medicare (p = 0.76), subsidy insurance (p = 0.62), or being uninsured (0.15) were not associated with early tumor detection. On univariable analysis, early tumor detection was associated with the presence of cirrhosis (p = 0.04), a gastroenterology clinic visit in the year prior to HCC diagnosis (p < 0.001), and detection by HCC surveillance (p < 0.001) (Table 3). On multivariable analysis, gastroenterology clinic visit in the year prior to HCC diagnosis (OR 5.38, 95% CI 2.60–11.12) and receipt of surveillance (OR 2.08, 95% CI 1.05–4.17) were independently associated with early tumor detection. Conversely, after controlling for all other variables in the model, race/ethnicity (p = 0.14) and insurance status (p = 0.90) were not associated with early tumor detection.
Table 3.
Unadjusted and adjusted odds of early tumor detection
| Characteristic | Unadjusted odds ratio (95% CI) | p | Adjusted odds ratio (95% CI) | p |
|---|---|---|---|---|
| Age (per 1-year increase) | 1.02 (0.99–1.04) | .28 | ||
| BMI (per 1 kg/m2 increase) | 0.99 (0.96–1.02) | .51 | 0.98 (0.94–1.02) | .23 |
| Sex female versus male | 1.44 (0.90–2.32) | .13 | 0.99 (0.52–1.91) | .98 |
| Insurance (vs commercial) | .50 | .90 | ||
| Medicaid | 0.98 (0.46–2.06) | .95 | 1.67 (0.51–5.49) | .39 |
| Medicare | 1.10 (0.61–1.97) | .76 | 1.41 (0.59–3.37) | .43 |
| Subsidy | 0.81 (0.32–2.03) | .62 | 1.49 (0.28–7.89) | .60 |
| Uninsured | 0.49 (0.18–1.32) | .15 | 1.11 (0.15–7.95) | .91 |
| Smoking (vs never) | .08 | .25 | ||
| Quit | 1.06 (0.63–1.78) | .82 | 0.86 (0.41–1.79) | .68 |
| Current | 0.61 (0.36–1.05) | .08 | 0.54 (0.25–1.18) | .12 |
| Alcohol (vs never) | .50 | |||
| Quit | 0.75 (0.45–1.24) | .26 | ||
| Current | 0.95 (0.52–1.72) | .86 | ||
| Cirrhosis yes versus no | 2.35 (1.04–5.32) | .04 | 2.12 (0.71–6.29) | .18 |
| HIV yes versus no | 1.04 (0.28–3.83) | .96 | ||
| GI specialist yes versus no | 7.01 (4.19–11.73) | <.001 | 5.38 (2.60–11.12) | <.001 |
| Dx mode incidental versus surveillance | 0.20 (0.13–0.32) | <.001 | 0.48 (0.24–0.95) | .03 |
| Race (vs White) | .29 | .14 | ||
| Black | 0.71 (0.37–1.37) | .30 | 0.73 (0.20–2.68) | .62 |
| Hispanic | 1.08 (0.55–2.10) | .82 | 1.31 (0.50–3.46) | .58 |
| Other | 0.53 (0.23–1.21) | .13 | 0.35 (0.11–1.09) | .07 |
Receipt of Curative Treatment
Among patients with early stage tumors, just under half (48%) received potentially curative therapy including OLT, resection or ablation, while only 9% of those outside of Milan Criteria received potentially curative therapy (Table 2). Overall, only 32% Whites, 18.7% of Blacks, 20.3% of Hispanics, and 30% of another race received curative treatment (p = 0.26 within Milan; p = 0.83 outside of Milan) (Table 2). Receipt of curative treatment occurred in 9.7% of those who had commercial insurance, 9.2% with Medicare, 3.9% with Medicaid, and 3.2% of those with a subsidy plan. On univariable analysis, receipt of curative therapy was positively associated with early tumor detection (OR 9.38, 95% CI 5.24–16.78), seeing a GI specialist in year prior to HCC diagnosis (OR 3.36, 95% CI 2.01–5.59), and detection by HCC surveillance (OR 3.29, 95% CI 2.01–5.38; p < .001). Medicare (OR 0.50, 95% CI 0.26–0.96), Medicaid (0.37, 95% CI 0.15–0.88), and being uninsured or on a subsidy plan (OR 0.17, 95% CI 0.04–0.66) were negatively associated with receipt of curative therapy when compared to commercial insurance. On multivariable analysis that controlled for race/ethnicity and insurance status, early tumor detection remained associated with curative therapy (OR 9.08, 95% CI 4.52–18.24). In this model, patients with Medicaid (OR 0.26, 95% CI 0.10–0.66), Medicare (OR 0.37, 95% CI 0.18–0.79), or being uninsured or on a subsidy plan (OR 0.17, 95% CI 0.05–0.57) remained less likely than those with commercial insurance to receive curative therapy (Fig. 1). In order to eliminate selection bias associated with liver transplantation, we performed a sub-analysis of those receiving surgical resection and LAT and found that associations remained unchanged (data not shown). Conversely, controlling for all other variables in the model, race/ethnicity was not meaningfully associated with receiving curative therapy (p = 0.28).
Fig. 1.
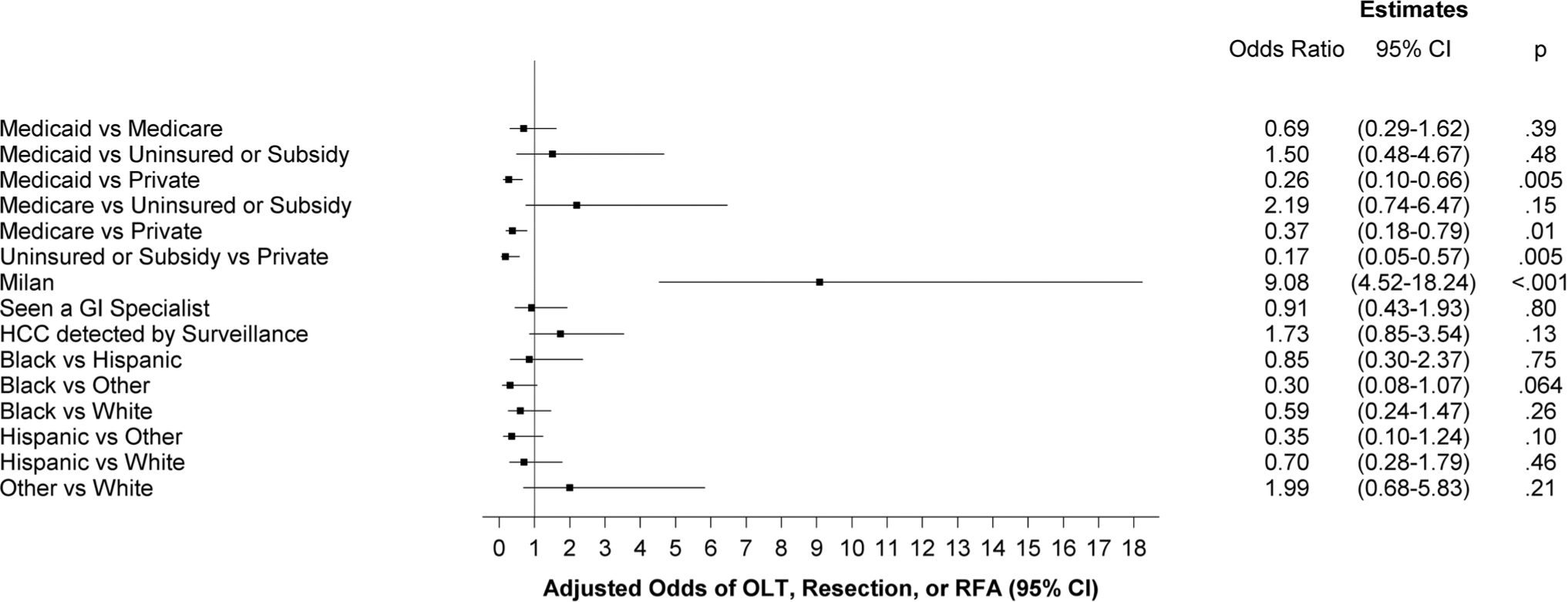
Adjusted odds of receiving curative treatment
Overall Survival
Finally, the median survival of the cohort was 25.7 months and 1-year probability of survival was 62.4%. At the end of the study period, 48% of Blacks, 39% of Whites, 37.4% of Hispanics, and 26.6% of those identified as other race were deceased. Over half (55%) of those with Medicaid, 42.6% of those with subsidy insurance, 40.5% of uninsured were deceased at the end of the study period, while the lowest mortality was observed among those with Medicare (34.7%) and commercial insurance (30.1%). On multivariable analysis, patients receiving a curative therapy had lower mortality when compared to those receiving any other HCC treatment (HR 0.33, 95% CI 0.19–0.56) after controlling for race/ethnicity and insurance status. However, race/ethnicity (p = .31) and insurance status (p = .20) were not associated with overall survival (Table 4).
Table 4.
Adjusted risk of mortality
| Valid N (events) | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Receipt of curative therapy | 260 (87) | 0.30 (0.18–0.50) | <.001 | 0.33 (0.19–0.56) | <.001 |
| Race (vs White) | 260 (87) | .17 | .31 | ||
| Black | 0.98 (0.54–1.77) | .94 | 0.80 (0.43–1.50) | .49 | |
| Hispanic | 0.52 (0.24–1.11) | .09 | 0.60 (0.28–1.30) | .19 | |
| Other | 1.57 (0.65–3.82) | .32 | 1.55 (0.62–3.89) | .35 | |
| Insurance (vs private) | 259 (87) | .01 | .20 | ||
| Medicaid | 2.65 (1.35–5.24) | .005 | 1.85 (0.91–3.76) | .09 | |
| Medicare | 1.74 (0.92–3.30) | .09 | 1.27 (0.66–2.45) | .47 | |
| Subsidy | 1.20 (0.45–3.20) | .72 | 0.93 (0.34–2.57) | .89 | |
| Uninsured | 4.22 (1.38–12.88) | .01 | 2.27 (0.71–7.27) | .17 | |
Valid N (events) = The number of patients and mortality events used for the unadjusted estimates. For the adjusted estimates, valid N = 259 with 87 mortality events. HR = hazard ratio. Recipient of curative therapy is OLT, Resection, or RFA versus other primary treatment
Discussion
In our multicenter study of two academic and two safety-net hospitals, we characterized racial/ethnic an economic disparities in HCC prognosis. We found no differences in early tumor detection, curative treatment receipt, or overall survival by race/ethnicity. Although insurance status was not associated with early HCC detection, it was significantly associated with curative treatment receipt, with significantly higher treatments in patients with commercial insurance than their counterparts. In turn, receipt of curative treatment was associated with improved survival. Overall, these results underscore insurance status as an important prognostic factor and potential driver of prognostic disparities.
Previous studies have identified racial/ethnic-specific disparities across the HCC cancer continuum [14, 23, 24]. For example, Rich et al. [24] examined over 1000 patients at two large health organizations and found Blacks and Hispanics were less likely to be diagnosed with early stage HCC and less likely to undergo curative treatment compared to Whites. In Surveillance, Epidemiology, and End-Results Program (SEER) database, Whites and Blacks had higher odds of mortality compared with Asians [10]. Similar studies suggest Blacks consistently had more advanced stage at diagnosis and lower rates of receiving any treatment [23]. In analysis of the Veterans Administration, Hispanics had lower odds of all-cause mortality compared to Whites, Blacks, and Asians [16]. However, we did not find significant racial/ethnic disparities in surveillance receipt or overall survival. Our study may be discordant from previous literature for a few reasons. First, our study included granular, multicentered data containing variables such as degree of liver dysfunction, insurance status, and specialty referral, which were not present in all previous studies. The inclusion of insurance status as a measure of SES is particularly important as it possible that previously reported racial/ethnic differences were actually related to differences in SES. It is well known the two are highly correlated so attributed disparities to one construct or the other can be quite difficult. Second, we included patients seen at two academic centers and two safety-net health systems, and these results may differ from patients seen in other practice settings such as community practices or integrated health systems such as the Veterans Affairs. Finally, prior studies have suggested racial/ethnic differences in access and engagement in medical care, and our study was restricted to patients who presented for outpatient medical care so may not generalize to those without any medical coverage or not engaged in medical care. Reasons for the differences seen among races/ethnicities in HCC care are difficult to identify, but likely involve factors including, but not limited to, financial/SES, tumor biology, provider-and patient-related factors [24–26].
The care of the patient with HCC is a complex, multilevel process that includes identification of at-risk patients, primary prevention, early detection, diagnosis, treatment, and survivorship [27]. Disparities in HCC healthcare delivery can influence appropriate care along all aspects of the cancer care continuum. Our study found that early tumor detection was not significantly associated with age, race or insurance status. These data suggest that unidentified factors independent of demographics influence the rate of HCC surveillance and early tumor detection and further studies are needed to understand patient, provider, and health system factors.
In our study only 26.9% of the entire cohort and just under half (48.6%) of those diagnosed within Milan Criteria received curative treatment. This finding is unfortunately comparable to other studies reporting underutilization of curative therapy, ranging from 21 to 40% [24, 25]. Many factors affect the utilization of curative therapy including, but not limited to patient-related factors (patient choice, comorbid conditions, substance abuse, social support), provider-level factors (local expertise and availability of OLT or liver resection) and technical factors (tumor characteristics including size and location, organ availability, and changes to allocation system). Patient-related factors other than insurance status including education level, health literacy, social support, transportation, substance abuse can significantly affect decisions on curative therapy [17]. Unfortunately, we were unable to evaluate many of these additional factors given the retrospective nature of our study. In our study, factors independently associated with receipt of curative treatment were early tumor detection and having seen a GI specialist in the previous year. Lack of commercial insurance coverage was independently associated with lower odds of receiving curative therapy. Although the effect of having health insurance on health outcomes have mixed evidence on cancer stage [28–30], our study found that having commercial insurance may play a significant factor in receiving curative therapy in patients with HCC. Access to insurance coverage appears vital to closing the disparities gap.
In addition, our study reinforces that GI subspecialty care is significantly associated with early tumor detection and may be an important driver of improved outcomes. Several studies have highlighted the association between GI subspecialty care and HCC surveillance [31]. Prior studies suggested PCPs have substantial barriers to HCC surveillance including competing clinical concerns and deficits in knowledge about surveillance benefits [26, 32]. Racial/ethnic minorities and socioeconomically disadvantaged patients typically have differential access to medical care, including less clinic visits and higher likelihood of being seen in safety-net health systems that may have less treatment availability [33]. Improving access to subspecialty care and alternative care deliver models such population health programs or telehealth [34, 35], targeted toward racial/ethnic minorities and socioeconomically disadvantaged patient populations may be effective interventions to improve HCC outcomes.
There are a few notable limitations in our study. First, the retrospective study design has inherent biases including the possibility of unmeasured confounders not being included in the analysis. For example, degree of social support, accurate alcohol and tobacco use, and ECOG status may affect HCC treatment decisions. Second, we attempted to adjust for SES utilizing insurance status as a proxy marker; however, insurance status is an imperfect surrogate, and further analyses including both individual, such as education and household income, and neighborhood SES are still needed [36]. Despite this, we feel that our study adds to the current literature and provides an impetus for prospective studies with more comprehensive SES assessments to evaluate the intersectionality between race/ethnicity and SES. Third, defining early tumor detection as within Milan Criteria could have had an impact on treatment decision that ultimately affected HCC morbidity and mortality. Fourth, the mode of HCC detection was not available for analysis; although insurance status may affect the type of imaging for surveillance, ultrasound-based surveillance is considered standard and used for most patients at each of the sites. Fifth, our study is limited by selection bias, as all patients in our study were seen at one of the four health systems and prior studies have demonstrated differential access to care between racial/ethnic groups and therefore may limit overall generalizability of our findings. It is possible we would find greater racial/ethnic and socioeconomic disparities in population-based samples of HCC patients. We did include two safety-net health systems, capturing socioeconomically disadvantaged patients that are not captured in studies isolated to tertiary care referral centers. We believe the strengths of our multicentered study design outweigh the limitations.
In summary, our multicenter study examining healthcare delivery among patients with HCC in the US failed to find any racial/ethnic disparities in early tumor detection, receipt of curative treatment, and overall survival; however, we instead found disparities by insurance status with regard to receipt of curative therapy. Further, we found access to GI subspecialty care was one of the most important factors associated with early tumor detection and receipt of curative treatment. These findings highlight the important role of insurance coverage, and access to specialty care when evaluating racial/ethnic disparities in HCC outcomes.
Supplementary Material
Funding
Dr. Singal was supported in part by NIH RO1 MD12565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10620-019-05890-2) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petrick JL, Kelly SP, Altekruse SF, et al. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White DL, Thrift AP, Kanwal F, et al. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology. 2017;152:812–820.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulahannan SV, Duffy AG, McNeel TS, et al. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology. 2014;60:1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;. 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shebl FM, Capo-Ramos DE, Graubard BI, et al. Socioeconomic status and hepatocellular carcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21:1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367–1377. [DOI] [PubMed] [Google Scholar]
- 7.Mathur AK, Osborne NH, Lynch RJ, et al. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145:1158–1163. [DOI] [PubMed] [Google Scholar]
- 8.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. [DOI] [PubMed] [Google Scholar]
- 9.Singal AG, Yopp A, Skinner CS, et al. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singal AG, Li X, Tiro J, et al. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med. 2015. 10.1016/j.amjmed.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaydfudim V, Whiteside MA, Griffin MR, et al. Health insurance status affects staging and influences treatment strategies in patients with hepatocellular carcinoma. Ann Surg Oncol. 2010;17:3104–3111. [DOI] [PubMed] [Google Scholar]
- 13.Siegel AB, McBride RB, El-Serag HB, et al. Racial disparities in utilization of liver transplantation for hepatocellular carcinoma in the United States, 1998–2002. Am J Gastroenterol. 2008;103:120–127. [DOI] [PubMed] [Google Scholar]
- 14.Xu L, Kim Y, Spolverato G, et al. Racial disparities in treatment and survival of patients with hepatocellular carcinoma in the United States. Hepatobiliary Surg Nutr. 2016;5:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonnenday CJ, Dimick JB, Schulick RD, et al. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. J Gastrointest Surg. 2007;11:1636–1646. (discussion 1646). [DOI] [PubMed] [Google Scholar]
- 16.Serper M, Taddei TH, Mehta R, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology. 2017;152:1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099–1106.e1. [DOI] [PubMed] [Google Scholar]
- 19.Parikh ND, Scaglione S, Li Y, et al. A comparison of staging systems for hepatocellular carcinoma in a multicenter US cohort. Clin Gastroenterol Hepatol. 2018;16:781–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. [DOI] [PubMed] [Google Scholar]
- 21.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 22.Lin DY, Wei L, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. BioMetrika. 1993;80:557–572. [Google Scholar]
- 23.Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50:423–430. [DOI] [PubMed] [Google Scholar]
- 24.Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2018;16:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan D, Yopp A, Beg MS, et al. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013;38:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalton-Fitzgerald E, Tiro J, Kandunoori P, et al. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:791–798.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol. 2015;13:2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommers BD, Gawande AA, Baicker K. Health insurance coverage and health—what the recent evidence tells us. N Engl J Med. 2017;377:586–893. [DOI] [PubMed] [Google Scholar]
- 29.Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry LR, Miller S. Early coverage, access, utilization, and health effects associated with the affordable care act medicaid expansions: a quasi-experimental study. Ann Intern Med. 2016;164:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davila JA, Morgan RO, Richardson PA, et al. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons OL, Feng Y, Parikh ND, et al. Primary care provider practice patterns and barriers to hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol. 2019;17:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mokdad AA, Murphy CC, Pruitt SL, et al. Effect of hospital safety net designation on treatment use and survival in hepatocellular carcinoma. Cancer. 2018;124:743–751. [DOI] [PubMed] [Google Scholar]
- 34.Su GL, Glass L, Tapper EB, et al. Virtual consultations through the veterans administration SCAN-ECHO project improves survival for veterans with liver disease. Hepatology. 2018;68:2317–2324. [DOI] [PubMed] [Google Scholar]
- 35.Singal AG, Tiro JA, Marrero JA, et al. Mailed outreach program increases ultrasound screening of patients with cirrhosis for hepatocellular carcinoma. Gastroenterology. 2017;152:608–615.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99:1013–1023. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


