Abstract
Objectives
The SARS‐CoV‐2 global pandemic has subjected healthcare workers (HCWs) to high risk of infection through direct workplace exposure, coupled with increased workload and psychological stress. This review aims to determine the impact of SARS‐CoV‐2 on mental health outcomes of hospital‐based HCWs and formulate recommendations for future action.
Methods
A systematic review was performed between 31st December 2019 and 17th June 2020 through Ovid Medline and Embase databases (PROSPERO ID CRD42020181204). Studies were included for review if they investigated the impact of SARS‐CoV‐2 on mental health outcomes of hospital‐based HCWs and used validated psychiatric scoring tools. Prevalence of ICD‐10 classified psychiatric disorders was the primary outcome measure.
Results
The initial search returned 436 articles. Forty‐four studies were included in final analysis, with a total of 69,499 subjects. Prevalence ranges of six mental health outcomes were identified: depression 13.5%‐44.7%; anxiety 12.3%‐35.6%; acute stress reaction 5.2%‐32.9%; post‐traumatic stress disorder 7.4%‐37.4%; insomnia 33.8%‐36.1%; and occupational burnout 3.1%‐43.0%. Direct exposure to SARS‐CoV‐2 patients was the most common risk factor identified for all mental health outcomes except occupational burnout. Nurses, frontline HCWs, and HCWs with low social support and fewer years of working experience reported the worst outcomes.
Conclusion
The SARS‐CoV‐2 pandemic has significantly impacted the mental health of HCWs. Frontline staff demonstrate worse mental health outcomes. Hospitals should be staffed to meet service provision requirements and to mitigate the impact onmental health. This can be improved with access to rapid‐response psychiatric teams and should be continually monitored throughout the pandemic and beyond its conclusion.
Keywords: anxiety, burnout, depression, insomnia, SARS‐CoV‐2, stress
1. INTRODUCTION
The Severe Acute Respiratory Syndrome Coronavirus (SARS‐CoV‐2) pandemic originated from Wuhan city, Hubei Province, China, in December 2019. It continues to challenge healthcare services globally and has resulted in significant morbidity and mortality. 1 The World Health Organisation declared the outbreak an international public health emergency on January 30th 2020 and by June 24th 2020, the death toll worldwide stood at 479,496, with 9,343,448 confirmed cases. 2 , 3 Due to its high reproductive number (R 0), cases spread beyond Wuhan within 2 months to more than 25 countries. 4 The number of cases continue to rise with significant impact on healthcare workers (HCWs) and healthcare systems. Limited availability of personal protective equipment and increased workload increases the risk of both contracting and transmitting the disease. 5 Hence, HCWs are at risk of significant psychological distress.
SARS‐CoV, the predecessor to SARS‐CoV‐2, originated in Guangdong province, China, in November 2002. It became an epidemic that affected more than 8000 people across 26 countries, of which 20% of positive cases were HCWs. 6 There was evidence of considerable psychological burden on HCWs, including post‐traumatic stress disorder (PTSD) and depression. Chong et al reported that 75% of its HCWs in Taiwan experienced negative psychological effects. 7 Reconciling the fear of contagion, infecting patients, co‐workers, and family members, was commonplace, with associated stigmatization. 8 Given the deleterious effects of SARS‐CoV‐2 on HCWs currently, robust and rapidly available research on mental health outcomes is urgently required.
An understanding of SARS‐CoV‐2 impact on HCW mental health will inform an appropriate response as the pandemic continues. Support can be targeted to those at greatest risk of psychiatric decline. A previous review by Brooks et al identified key risk factors affecting HCW mental health outcomes during the SAR‐CoV pandemic: direct contact with infected patients, inadequate psychological support from employers, impacted social/family life, and working in the nursing profession (specifically for acute stress and PTSD). 9 Greater family support and strong belief in infection control procedures were protective for mental health outcomes. A meta‐analysis by Pappa et al on the prevalence of depression, anxiety, and insomnia in HCWs during the pandemic was recently published in May 2020. 10 This meta‐analysis limited its investigation to pooled prevalences of three mental health conditions from 13 studies, of which 12 were based in China. Included studies had substantial heterogeneity with varying disease‐specific outcome scales and diagnostic cut‐off scores. This systematic review investigates the prevalence of mental health conditions in HCWs during SARS‐CoV2. In addition it investigates the impact of mental health outcomes in hospital HCWs during SARS‐CoV‐2 and aims to identify outcomes and risk factors to inform future interventions.
2. METHODS
The protocol for this review (CRD42020181204) was guided by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.
2.1. Eligibility criteria
Inclusion:
Studies which examined the impact of SARS‐CoV‐2 on healthcare professionals.
Studies which investigated at least one International Classification of Diseases‐10th Revision (ICD‐10) defined psychiatric condition.
Use of at least one validated quantitative scoring scale to measure mental health outcomes, or a self‐designed one based on a pre‐existing, validated scale
Available in English Language
Hospital based
Conducted from 31st December 2019 (when China reported the first case of SARS‐CoV‐2 in Wuhan) to 17th June 2020
Exclusion:
Studies investigating non‐hospital–based HCWs exclusively
Written in non‐English language
Studies with fewer than 20 participants
2.2. Information sources and search
MEDLINE and Embase electronic databases were interrogated for studies published between 31st December 2019 and 17th June 2020. Due to the rapid production of publications during the current pandemic, pre‐print papers were also searched for using Medrix. Reference lists of selected studies were searched to identify further papers. The full list of search terms used is provided in Figure S1.
2.3. Study selection
Two authors (YH and JS) screened the total list of identified records to determine eligibility in a blinded, standardized manner. Screening was initially via title and then abstract. Disagreements between the two reviewers were resolved by a third author (NP). NP also searched the full reference lists of selected studies and identified pre‐print papers on Medrix. Correspondences with primary data and preprint studies were included in this review.
2.4. Data collection process
NP and JS completed an outcome extraction database for the review (Microsoft Excel, 2018). NP extracted the following data from the included studies: participant uptake; participant demographics (including age, occupation, exposure to SARS‐CoV‐2, and others); date of study; location of study; and outcome measure(s) used. JS checked the extracted data and any disagreements were reviewed by the third author YH. The authors of selected studies were contacted for clarification of data sets.
2.5. Data items
The primary outcome measure was the prevalence of ICD‐10 defined psychiatric conditions. The secondary outcome measures were the use of validated psychiatric scoring tools, differences in prevalence between sub‐groups of HCWs and independent risk factors associated with these conditions. Studies which used the same scoring tool but different diagnostic ‘cut‐off’ scores to calculate prevalence were highlighted. In these studies, the raw data were used to calculate standardized prevalence using the following cut‐off scores: DASS‐21 > 14, PHQ‐9 ≥ 10, GAD‐7 ≥ 10, IES‐R > 24/25, ISI ≥ 8, and MBI EE > 27 & DP > 10 using pre‐defined standards. 11 , 12 , 13 , 14 , 15 , 16
2.6. Risk of bias in individual studies
The methodology of each study was individually analyzed. With substantial study heterogeneity and large variation in study methodology, the use of a single bias scoring tool was inappropriate. Instead, differences between sampling methods in studies were identified (Table 1), compared, and limitations highlighted in the discussion.
TABLE 1.
Study characteristics (n = 44)
| Source | Participant no a | Dates of study | Location | Methodology | Primary outcomes |
|---|---|---|---|---|---|
| Apisarnt‐hanarak et al 27 (Research brief) | 160 | Mar 1‐31 2020 | Bangkok and Pratum, Thailand, 2 private and 2 university hospitals | Participants invited to survey using a ‘standardized data collection tool’ | GAD‐7 |
| Badahdah et al 67 | 194 | Early Apr 2020 | Oman | Web based survey link sent to participants |
GAD‐7 PSS‐10 |
| Barello et al 58 | 376 | Not disclosed | Italy | HCWs invited to complete an online questionnaire and those who reported direct exposure to SARS‐CoV‐2 patients were included | MBI |
| Cao et al 19 (Letter to editor) | 37 | Jan 20‐ Feb 10 2020 | Peking Union Medical College Hospital, Beijing, China |
Self‐report questionnaire administered to medical HCWs at end of rotation Qualitative interviews delivered through a 24‐hour hotline service |
PHQ‐9 MBI |
| Chatterjee et al 30 | 152 | Mar 28‐ Apr 6 2020 | West Bengal | Link to online questionnaire distributed through email, WhatsApp, social media groups (with only doctors), and to contacts of the investigators | DASS‐21 |
| Chen et al 71 (Letter to editor) | 105 | Not disclosed | Guiyang China | Anonymous questionnaire distributed |
SDS SAS |
| Chew et al 29 | 906 | Feb 19‐ Apr 17 2020 | Tertiary institutions in Singapore and India | Questionnaire distributed by hand‡ |
DASS‐21 IES‐R |
| Choudhury et al 20 | 106 | Apr 1‐7 2020 |
UK Lancashire Cardiac Centre, Blackpool Victoria Hospital |
Two anonymous questionnaires distributed to participants (one consisting of PHQ‐9 and PSS‐4, and the other GAD‐7) |
PHQ‐9 PSS‐4 GAD‐7 |
| Chung et al 21 (Letter to editor) | 69 | Feb 14‐24 2020 | 6 Hospitals in Hong Kong East Cluster | Online questionnaire distributed through COVID‐19 newsletter on email and hospital phone calls | PHQ‐9 |
| Dimitriu et al 34 | 100 | Apr 27‐ May 8 2020 | Romania | Survey distributed to participants in front line (Emergency department, radiology, ICU) and other departments (surgery, orthopedics) | MBI |
| Du et al 66 | 310 | Feb 13‐17 2020 | Wuhan, China | Smartphone‐based survey |
PSS BAI BDI‐II |
| Elbay et al 32 | 442 | Mar 10‐15 2020 | Istanbul, Turkey | Online survey shared on social network groups of different specialities (convenience sampling) | DASS‐21 |
| Garcia‐Fernandez et al 60 (Letter to editor) | 1787 | Mar 29‐ Apr 5 2020 | Spain |
National self‐reported online questionnaire distributed via social networks (exponential non‐discriminative snowball sampling) Patients with current/past psychological disorders excluded |
HARS BDI |
| Guo et al 61 | 11,118 | Feb 18‐ 20 2020 | China | Online survey distributed through WeChat |
SDS SAS |
| Huang et al 28 | 7236 | Feb 3‐17 2020 | China | Web‐based cross‐sectional voluntary survey distributed through WeChat and mainstream media |
GAD‐7 CES‐D PSQI |
| Jalili et al 59 (preprint) | 645 | Not disclosed |
Tehran, Iran 8 university‐affiliated hospitals |
Self‐reported questionnaire using a survey platform (EPoll), distributed through professional and informal networks | MBI |
|
Kang et al 98 |
994 | Jan 29‐ Feb 4, 2020 | Wuhan, China | Data collected through Wenjuanxing with anonymous self‐report questionnaire |
PHQ‐9 IES‐R GAD‐7 ISI |
| Lai et al 22 | 1257 | Jan 29 ‐ Feb 3 2020 | 34 hospitals in China: Wuhan (n = 20), other regions in Hubei (n = 7), other provinces (n = 7) | Random selection of one department from each hospital, and everyone in this department asked to participate |
PHQ‐9 IES‐R GAD‐7 ISI |
| Liang et al 72 (Letter to editor) | 59 | Feb 3‐21 2020 |
Guangdong Province, China |
Not disclosed |
SDS SAS |
| Li X et al 99 (Letter to editor) | 948 | Feb 15‐22 2020 |
Zhejiang province, China Ningbo Kangning Hospital |
Self‐reported questionnaire No further information provided |
AIS SRQ‐20 |
| Li Z et al 65 | 740 |
Feb 17‐21 2020 |
China | Anonymous questionnaire built using SoJump and distributed via WeChat | Chinese vicarious traumatization questionnaire (based on IES‐R) |
| Liu C et al 73 | 512 | Feb 10‐22 2020 | China | Self‐reported questionnaire distributed via WeChat | SAS |
| Liu Z et al 62 (preprint) | 4679 | Feb 17‐24 2020 |
China 348 hospitals in 31 provinces |
Anonymous voluntary self‐reported survey using Questionnaire Star program, distributed via WeChat |
SDS SAS |
| Lu et al 42 | 2299 | Feb 25‐ 26 2020 |
Fujian Province, China |
Online questionnaire, participants with psychological disorders excluded |
HAMA HAMD |
| Mo et al 68 | 180 | Feb 2020 | Wuhan, China | Online anonymous questionnaires sent to senior nurses and asked to forward onto colleagues |
SOS SAS |
| Naser et al 23 (pre‐print) | 4216 | Mar 22‐28 2020 | Jordan | Self‐reported questionnaire, participants invited through social media (Facebook and WhatsApp) |
PHQ‐9 GAD‐7 |
| Qi et al 74 (pre‐print) | 1306 | Feb 2020 | Multiple hospitals in Hubei Province |
Online self‐report survey distributed via WeChat b Participants with psychiatric disorders excluded |
PSQI AIS |
| Rossi et al 33 (Research Letter) | 1379 | Mar 27‐31 2020 | Italy | Online questionnaire spread via social networks (snowball sampling) and sponsored social network advertisements |
PHQ‐9 GPS PSS‐10 GAD‐7 ISI |
| Salman et al 24 (pre‐print) | 398 | Apr 15‐ May 20 2020 | Punjab province, Pakistan | Web‐based survey distributed via Google Forms (snowball sampling) |
PHQ‐9 GAD‐7 |
| Song et al 63 | 14,285 | Feb 28‐ Mar 18 2020 | China | Electronic self‐reported questionnaire distributed via snowball and convenience sampling |
PCL‐5 CES‐D |
| Sun et al 100 | 442 | Jan 31‐Feb 4 2020 | China | Online survey created using ‘Questionnaire Star’ platform | IES |
| Tan et al 31 | 470 | Feb 9 ‐ Mar 13 2020 | Two tertiary hospitals in Singapore | HCWs directly invited in person to participate in a self‐administered questionnaire |
DASS‐21 IES‐R |
| Thomaier et al 69 (pre‐print) | 374 | Mar 27‐Apr 10 2020 | America | Cross‐sectional anonymous online survey, distributed through social media (Twitter, Facebook, LinkedIn) | PHQ‐4 |
| Wang et al 64 | 123 | Jan 30‐Feb 7 2020 | Children's Healthcare Centre of Renmin Hospital, Wuhan, China | Self‐reported, anonymised questionnaire distributed to participants |
PSQI SAS SDS |
| Wu K et al 101 | 120 | Not disclosed |
China 2 undisclosed hospitals |
Online self‐reported questionnaires completed voluntarily |
PSQI SDS SAS PCL‐C |
| Wu Y et al 102 | 190 | Mar 13‐17 2020 | Wuhan | Questionnaire distributed to frontline and non‐frontline HCWs in 1:1 ratio | MBI |
| Xiao H et al 103 | 180 | Jan‐Feb 2020 | Wuhan, China | Self‐reported, anonymous questionnaires |
SAS PSQI GSE SSRS SASR |
| Xiao X et al 70 | 958 | Jan 28‐ Not disclosed | China | Online survey created using ‘Questionnaire Star’ program and distributed to medical personnel in multiple centers across the country via social media (WeChat and Tencent) |
PSS HAD |
| Yin et al 75 | 371 | Feb 1‐5 2020 | China |
Online survey conducted distributed via e‐mail, WeChat and online websites to the contacts of the investigators. Participants with psychiatric disorders excluded |
PSQI PCL‐5 |
| Zhang. C et al 25 | 1563 | Jan 29 ‐ Feb 3 2020 | China, including Wuhan workers | Questionnaire distributed through WeChat to groups of medical HCWs |
ISI IES‐R PHQ‐9 GAD‐7 |
| Zhang S et al 104 (Letter to editor) | 304 | Apr 5‐20 2020 |
Iran Public and private hospitals |
Not disclosed |
SF‐12 PHQ‐4 K6 |
| Zhang. W et al 47 | 2182 | Feb 9‐ Mar 6 2020 | Chinese provinces: Beijing (n = 888), Hebei (n = 546), Inner Mongolia (n = 126), Hubei (n = 28) |
Online survey distributed via Wenjuanxing Chinese citizens over 16 years old were invited if eligible |
ISI SCL‐90‐R PHQ‐4 |
| Zhu J et al 105 | 165 | Feb 1‐29 2020 |
Gansu province, China SARS‐CoV2 designated hospitals and fever clinics |
Online survey created using ‘Questionnaire Star’ program and distributed via WeChat |
SDS SAS SCSQ |
|
Zhu Z et al 26 (pre‐print) |
5062 | Feb 8‐10 2020 |
Wuhan, China Tongji Hospital |
Anonymous online questionnaires distributed via WeChat |
PHQ‐9 IES‐R GAD‐7 |
‘Participant No’ refers to total number of participants within the study (six included members of the general public).
Authors contacted directly to obtain this information.
2.7. Synthesis of results
A narrative synthesis approach was used to summarize the diverse range of selected studies in a structured manner, following the European Social Research Council Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. 17 Outcome measures on each condition were collected from all studies and prevalences calculated. The studies were screened for independent risk factors associated with each condition, provided they were derived through regression analyses and were significant (P ≤ .05) (Tables S1‐S6). Comparisons between different sub‐groups were analyzed: frontline vs non‐frontline, doctors/nurses vs other HCWs, HCWs vs general public, doctors vs nurses, and further participant characteristics (eg, years of working experience and social support).
3. RESULTS
This systematic review demonstrates the prevalence of six mental health conditions in HCWs during SARS‐CoV‐2 with associated factors, incorporating 44 studies across 15 countries (Figure 1). The databases MEDLINE and Embase initially yielded 418 articles. A further 18 were identified through Google Scholar, Medrix, and reference lists of included studies. Following the removal of duplicates, 378 results remained and were compiled into a database for screening. A total of 305 articles were excluded in‐line with pre‐defined inclusion and exclusion criteria, leaving 73 for full‐text assessment. A further 29 were removed following assessment, leaving a total of 44 primary studies.
FIGURE 1.
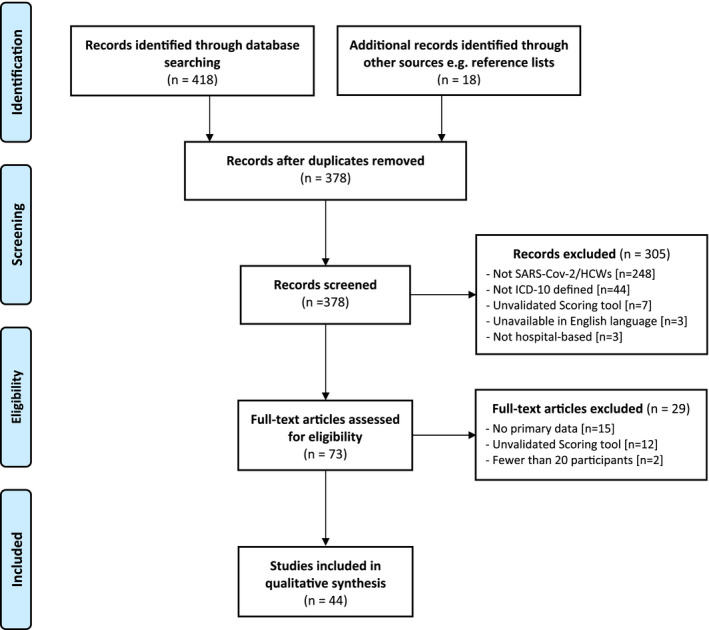
PRISMA flow diagram
3.1. Study characteristics
Of the 44 identified studies, 38 included nurses and 42 included doctors. Six studies also included members of the general population. 27 studies were conducted in China. The remaining were conducted in Thailand, Oman, Italy, India, Singapore, UK, Romania, Turkey, Spain, Iran, Jordan, Italy, Pakistan, and America. Seven of the China‐based studies recruited HCWs from Wuhan alone. The remaining China‐based studies had a varied participant demographic. The total number of participants across all studies was 69 499 (ranging from 37 to 14 285). All studies used a cross‐sectional design relying on self‐reported questionnaires. The characteristics of the studies are summarized in Table 1. A total of 23 studies investigated independent risk factors for mental health outcomes.
The healthcare professions included under the HCW term varied across studies and are detailed in Table S7. For ease in this review, when doctors and nurses are grouped together in sub‐group analysis, ‘other HCWs’ refers to all other professions within the hospital setting. Frontline HCWs are those working in departments that have direct contact with SARS‐CoV‐2 patients, eg, Intensive Care Unit (ICU) and respiratory wards.
All studies used psychiatric assessment tools as questionnaires to record outcomes, including: Patient Health Questionnaire (PHQ‐9), Generalised Anxiety Disorder score (GAD‐7), Insomnia Severity Index (ISI), Impact of Events Scale‐Revised (IES‐R), Pittsburgh Sleep Quality Index (PSQI), Zung's Self‐rating Depression Scale (SDS), Zung's Self‐rating Anxiety Scale (SAS), Depression Anxiety Stress Scales (DASS‐21), General Self‐Efficacy scale (GSE), Stanford Acute Stress Reaction Questionnaire (SASR), Social Support Rating Scale (SSRS), Stress Overload Scale (SOS) or Symptom Checklist‐90‐Revised (SCL‐90‐R), Maslach Burnout Inventory (MBI), Athens Insomnia Scale (AIS), Hamilton Anxiety Scale (HAMA), Hamilton Depression Scale (HAMD), Beck's Depression Inventory (BDI), Centre for Epidemiologic Studies Depression Scale (CES‐D), Hospital Anxiety and Depression Scale (HADS), Beck Anxiety Inventory (BAI), Perceived Stress Scale (PSS), Acute Stress Disorder Scale (ASDS), PTSD Checklist for DSM‐5 (PCL‐5), Global Psychotrauma Screen (GPS), and Kessler Screening Scale (K6). These are explained in Table S8. The scoring tool utilized in each study along with the key findings are summarized in Tables S9‐S14. The time periods during which the studies were conducted in relation to the first reported SARS‐CoV‐2 case are given in Table S15.
The final analysis identified six self‐reported mental health outcomes across 44 studies, with associated ICD‐10 codes: depression (F32), anxiety (F41), acute stress reaction (F43.0), PTSD (F43.1), insomnia (F51), and occupational burnout (Z73). 18 The prevalence ranges of the mental health outcomes were as follows: depression 13.5%‐44.7% 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 ; anxiety 12.3%‐35.6% 20 , 22 , 23 , 24 , 25 , 27 , 28 ; acute stress reaction 5.2%‐32.9% 29 , 30 , 31 , 32 ; PTSD 7.4%‐37.4% 22 , 25 , 29 , 31 ; insomnia 33.8%‐36.1% 22 , 25 , 33 ; and burnout 3.1%‐43%. 19 , 34
Direct exposure to SARS‐CoV‐2 patients was the most common risk factor identified across all mental health outcomes in this review, except occupational burnout (Tables S1‐S6). Nurses, frontline HCWs, HCWs with lack of social support, and HCWs with fewer years of work experience reported worse outcomes. Adequate social support was concluded as an important factor in reducing mental health morbidity.
3.2. Depression
Eight validated depression scores were used across 32 studies. 11 , 15 , 35 , 36 , 37 , 38 , 39 , 40 , 41 The most commonly adopted scoring tool was the Patient Health Questionnaire (PHQ‐9), used in 10 studies (Table S9). Using the cut‐off score ≥10, 12 eight studies showed depression prevalence ranging from 13.5% to 44.7%. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26
Independent risk factors for depression identified from the studies are shown in Table S1. Five studies identified direct exposure to SARS‐CoV‐2 patients as a risk factor. Of these, the largest study (Lu et al) reported the odds ratio for direct exposure as 2.016; 95% CI, 1.102‐3.685, P = .023. 42 Four studies reported lack of social support (including low peer/supervisor support, no family, unmarried or divorced/widowed) as a risk factor. Other risk factors corroborated by at least two studies included: having suspected/confirmed SARS‐CoV‐2, insufficient personal protection measures, younger age, eg, <35 years, and less working experience, eg, <10 years.
3.3. Anxiety
Seven validated assessment tools were used across 33 studies. 11 , 13 , 36 , 38 , 43 , 44 One of the most commonly adopted tools was GAD‐7, used in 11 studies (Table S10). Using the cut‐off score ≥10, 13 seven studies showed anxiety prevalence ranging from 12.3% to 35.6%. 20 , 22 , 23 , 24 , 25 , 27 , 28
Independent risk factors for anxiety identified from the studies are shown in Table S2. Five studies identified direct exposure to SARS‐CoV‐2 patients as a risk factor. Lu et al reported the odds ratio for direct exposure as OR 2.062; 95% CI, 1.349‐3.153, P = .001. 42 Risk factors corroborated by at least two studies included: having suspected/confirmed SARS‐CoV‐2, insufficient personal protection measures, intermediate job title/responsibility, lack of social support (low peer/supervisor support, no family, or divorced) and insufficient knowledge on SARS‐CoV‐2. Others included pre‐existing medical/psychiatric history and working experience <10 years.
3.4. Insomnia
Three validated assessment tools were used across 12 studies. 45 , 46 The most commonly used tool was ISI, used in five studies (Table S11). Using the cut‐off score ≥8, 14 three studies showed insomnia prevalence ranging from 33.8% to 36.1%. 22 , 25 , 33
Independent risk factors for insomnia identified from the studies are shown in Table S4. Three studies identified direct exposure to SARS‐CoV‐2 patients as a risk factor for insomnia. Of these, the largest study (Zhang W et al) reported the odds ratio for direct exposure as 2.53; 95% CI, 1.74‐3.68, P < .01. 47 Other risk factors included: fear of self‐infection, working in isolation units, uncertainty regarding effective disease control, and lack of faith in psychological support from news/social media.
3.5. Acute stress reaction
Five validated assessment tools were used across 11 studies. 11 , 48 , 49 , 50 , 51 The most commonly used tool was DASS‐21, used in four studies (Table S12). Using the cut‐off score >14 for the stress subscale, 11 these studies showed acute stress reaction prevalence ranging from 5.2% to 32.9%. 29 , 30 , 31 , 32
Independent risk factors for acute stress reaction identified from the studies are shown in Table S5. These included direct exposure to SARS‐CoV‐2 patients, longer working hours, being a single child, having colleagues affected by SARS‐CoV‐2, and pre‐existing psychiatric history.
3.6. PTSD
Four validated assessment tools were used across 13 studies. 52 , 53 , 54 , 55 The most commonly used tool was IES‐R, used in seven studies (Table S13). Using the cut‐off score >24/25, 56 four studies showed PTSD prevalence ranging from 7.4% to 37.4%. 22 , 25 , 29 , 31
Independent risk factors for PTSD identified from the studies are shown in Table S3. Three studies identified direct exposure to SARS‐CoV‐2 patients as a risk factor. Of these, the largest study (Rossi et al) reported the odds ratio for direct exposure as 1.37; 95% CI 1.05‐1.80, P = .03. 33 Two studies identified the nursing profession as a risk factor. Others included working in isolation wards, being quarantined, working >12 hours/day, having family/friends affected by SARS‐CoV‐2, and poor sleep quality. Interestingly, Zhu et al identified longer working experience >10 years as a risk factor for PTSD. 26
3.7. Occupational burnout
The validated MBI assessment tool was used in five studies to evaluate HCW burnout. 57 This scale is divided into three categories: emotional exhaustion (EE), depersonalization (DP), and personal accomplishment (PA). 16 EE refers to the feelings of overextension and loss of motivation in one's work, which can be suspected among HCWs burdened with the SARS‐CoV‐2 pandemic. Using the cut‐off score >27 for EE specifically, 14 two studies showed burnout prevalence ranging from 3.1% to 43.0%. 19 , 34
Two studies derived independent risk factors for occupational burnout. Both Barello et al and Jalili et al associated the nursing profession with burnout. 58 , 59 Jalili et al also found that physicians with intermediate responsibility, ie, residents (OR 6.64; 95% CI, 2.16‐20.41, P = .001), and being of younger age were risk factors. 59
4. SUBGROUP ANALYSIS
4.1. Doctors versus nurses
Eight studies investigated differences in mental health outcomes between medical professions. 22 , 58 , 59 , 60 , 61 , 62 , 63 , 64 Six studies show greater prevalence of disorders among nurses. 22 , 58 , 60 , 61 , 62 , 63 For example nurses in studies by Garcia‐Fernandez et al, Guo et al, and Lai et al scored significantly higher on depression and anxiety scales. 22 , 60 , 61 Liu Z et al also corroborated this finding, reporting greater prevalences among nurses compared to physicians. 62 Garcia‐Fernandez et al and Lai et al reported higher scores for acute stress and PTSD among nurses, respectively. 22 , 60 Lai et al also outlined a significantly higher prevalence of insomnia in nurses. 22 Zhang C et al defined being a doctor as a protective factor again insomnia (OR = 0.44; 95%CI 0.24‐0.80, P = .007). 25 Barello et al demonstrated higher burnout prevalence in nurses. 58
Contrary to this, Wang et al reported a significantly greater percentage of doctors with insomnia than nurses. 64 Jalili et al concluded that resident doctors were at a greater risk of burnout than nurses or specialist doctors. 59
4.2. Doctors/nurses versus other HCWs
The literature comparing doctors/nurses against other HCWs varies. Lu et al reported greater anxiety prevalence in other HCWs (eg, administration staff) which was statistically significant, but not for depression. 42 Tan et al directly compared depression, anxiety, and acute stress in these subgroups, using the DASS‐21 scale. 31 Other HCWs (eg, technicians, maintenance workers, and clerical staff) reported more anxiety (adjusted prevalence ratio 1.85; 95%CI, 1.15‐2.99, P = .011). There were no significant differences for depression and acute stress.
4.3. HCWs versus general public
Six studies included the general public in their samples, in order to compare the impact of SARS‐CoV‐2 on HCWs to people in other professions. 23 , 28 , 47 , 60 , 61 , 65 Zhang W et al reported higher prevalences of depression, anxiety, and insomnia in HCWs, compared to the general public, eg, teachers (P = .04, P < .01, and P < .01, respectively). 47 Acute stress scores were also higher in HCWs in the study by Garcia‐Fernandez et al 60 HCWs in the study by Huang et al reported the highest rate of poor sleep quality compared to other occupational groups (teachers, students, and enterprise workers). 28 Naser et al found that depression and anxiety was most prevalent in undergraduate students, followed by HCWs (61.4% vs 44.8% and 45.9% vs 32.8%, respectively). 23
4.4. Social support
Social support was often described in the literature as being provided by peers or family. 63 If HCWs lived alone or were unmarried/divorced/widowed, this was deemed as a lack of social support. Du et al reported that depressive and anxiety symptoms were more common among those lacking family support (P < .05, P < .01). 66 Using a specific scoring scale, Song et al found strong association between low‐moderate social support and symptoms of depression and PTSD. 63 Unmarried HCWs were more likely to have depression, but less likely to have PTSD. Badahdah et al found that married doctors reported lower levels of stress than non‐married, however, there was no difference in anxiety. 67 Elbay et al reported higher depression, anxiety, and acute stress scores in HCWs living alone. 32 With regards to burnout, Jalili et al showed high emotional exhaustion prevalence in those without children, but no significant difference between married and single participants. 59
4.5. Working experience
Across eight studies, there was a consensus that fewer years of working experience corresponded to worse mental health outcomes. 30 , 32 , 59 , 60 , 63 , 68 , 69 , 70 Chatterjee et al and Elbay et al showed that HCWs reporting depression and anxiety had less experience in their roles. 30 , 32 Jalili et al reported higher levels of burnout in those with ≤5 years than >5 years of working experience. 59 Song et al and Thomaier et al attained similar findings regarding depression and PTSD, and anxiety, respectively. 63 , 69
4.6. Frontline versus non‐frontline
A total of 15 studies compared the mental health outcomes of frontline and non‐frontline HCWs. 22 , 67 , 71 , 72 , 73 , 74 , 75 Lai et al demonstrated that median GAD‐7, PHQ‐9, ISI, and IES‐R scores were significantly higher in frontline HCWs (P < .001). 22 Guo et al, Lu et al, and Liu Z et al all reported higher anxiety and depression scores in frontline HCWs. 42 , 61 , 62 However, Liang et al found no significant differences in these disorders despite using the same scoring scales as Guo et al 61 , 72 Dimitriu et al identified burnout as more frequent in HCWs in normal wards, as opposed to frontline departments (86% vs 66% P < .05). 34 Qi et al reported significantly higher scores of PSQI and AIS insomnia scales in frontline HCWs. 74 This is corroborated by Lai et al 22
Elbay et al derived the following factors which are detrimental to the mental health of frontline HCWs: increased weekly working hours, increased number of SARS‐CoV‐2 patients, and lack of peer support. 32 Naser et al found that pulmonologists and ENT specialists, who often work on the frontline during this pandemic, had higher depression and anxiety median scores than HCWs. 23
5. DISCUSSION
This analysis of HCW mental health across 15 countries shows two key findings. Firstly, all six mental health outcomes were prevalent across the studies which investigated them. Secondly, direct exposure to SARS‐CoV‐2 patients was the most common risk factor identified for all mental health outcomes except burnout, followed by lack of social support and pre‐existing medical and/or psychiatric disorders.
In the majority of studies comparing HCWs to the general public during this pandemic, HCWs suffered worse mental health outcomes. Furthermore, the ‘China Mental Health Survey’ prior to SARS‐CoV‐2 found that in the general public, the weighted 12‐month prevalences of anxiety and depression were 5.0% and 3.6%, respectively. 76 In this review, the prevalence ranges of anxiety and depression in HCWs are substantially higher at 12.3%‐35.6% and 13.5%‐44.7%, respectively.
During the SARS‐CoV 2003 pandemic, Chong et al demonstrated that fear and anxiety amongst HCWs appeared early, but depression and PTSD symptoms arose later in the ‘repair phase’ as the virus was brought under control. 7 As the included studies were conducted in different countries and at different time points in relation to their case trajectory curves (Table S15), similar conclusions cannot be made. However, the high insomnia prevalences in this review may suggest its importance as a precursor of HCW mental health deterioration. This is supported by a recent meta‐analysis, which identified chronic insomnia as a risk factor for depression. 77 However, this relationship is likely bidirectional. 78
HCW mental health is clearly multifaceted. A range of interlinking factors should be addressed when devising psychological interventions, including external factors such as social support and demographic risk factors. 79 Maunder et al demonstrated that high levels of HCW post‐traumatic stress and burnout were sustained up to 12‐26 months after the SARS‐CoV pandemic. 80 As SARS‐CoV‐2 has arguably impacted healthcare services on a greater scale, it is likely that HCW mental health will be affected in a similar way. Hence, longitudinal follow‐up studies are required to investigate and inform treatment of these disorders.
5.1. Frontline versus non‐frontline HCWs
Frontline HCWs worked in high‐risk departments such as ICU, respiratory wards, and 24‐hour fever clinics. Direct exposure to SARS‐CoV‐2 patients was the most consistent risk factor identified. The reasons for this may be three‐fold. Firstly, frontline workers have the most exposure risk and having seen first‐hand the effects of SARS‐CoV‐2 on patients, fear being infected themselves and transmitting to others. This includes their colleagues, other patients, friends, and family. Much like SARS‐CoV‐2, during the SARS‐CoV pandemic, a greater disease exposure was associated with higher stress levels. 81 Secondly, Personal Protective Equipment (PPE) is often double layered and uncomfortable. They must be worn by staff for several hours on end without eating, drinking, or using the toilet. Many become dehydrated from excessive sweating and develop skin conditions from excessive hand cleaning. 82 In the case of PPE shortages, their risk of infection increases dramatically. 83 Thirdly, due to the high morbidity and mortality associated with the disease, in addition to the reported unpredictable nature of deterioration, medical workers experience feelings of helplessness. 84 , 85
5.2. HCW medical/psychiatric health
Due the added strain of SARS‐CoV‐2 on HCWs with pre‐existing medical and/or psychiatric comorbidity, their symptoms may become exacerbated with a decline in overall function. HCWs with chronic medical conditions may experience anxiety over self‐infection and the potential increase in mortality risk. Added stress and reduced sleep may worsen existing depression or trigger mood episodes in bipolar disorder. 86
The presence of respiratory‐related symptoms such as sore throat, breathlessness, or cough, as well as other systemic symptoms of myalgia and lethargy may raise HCWs’ fear of SARS‐CoV‐2 infection. HCWs may feel conflicted between self‐isolating for further testing or continuing to work alongside their colleagues, especially if they are under‐staffed. This dilemma could result in anxiety, distress, and burnout. 87
5.3. Working hours
In order to provide 24‐hour care, hospitals organize staff rotas in shifts and HCWs must often work during unsociable hours. Before the pandemic, shift work had been independently associated with burnout among nurses. 88 Dyrbye et al reported 3%‐9% increased odds of physician burnout for each additional night or weekend on call. 89 SARS‐CoV‐2 has brought a surplus of patients with complex management needs, placing an even greater strain on healthcare systems and HCW workload. 90 A recent survey by Zhang et al identified that frontline HCWs were working longer hours than preferred during the SARS‐CoV‐2 pandemic. 91 This was particularly the case in fever clinics and isolation wards. Longer working hours on the frontline not only increases exposure to SARS‐CoV‐2 patients but also their risk of acute stress reaction/PTSD. 63 , 68
5.4. Variability in HCWs
The SARS‐CoV pandemic has demonstrated similarly higher rates of mental health outcomes in nurses, compared to doctors. 9 , 92 The reasoning behind this may be multifactorial. Nursing staff may have increased contact with infected patients compared to other HCWs. 93 For example they are responsible for monitoring vital signs, administering oxygen, and attending to patient needs. Given the morbidity associated with the disease, all HCWs are likely to experience increased ‘Emotional Labour’. This refers to the mental effort of suppressing emotions such as fear and concern, while displaying optimism and empathy. 93 There has been no research comparing this phenomenon between different HCWs. However, this has been linked to occupational burnout in the nursing profession. 93 The observed increased strain on all healthcare sectors may exacerbate inadequate staffing. 1 Cao et al reported nursing staff caring for up to 200 SARS‐CoV‐2 patients a day due to understaffing. 19
5.5. Social support
A lack of social support (including from peers, supervisors, family, spouse, and/or children) was shown to have a negative impact on HCWs’ mental health. Social support can provide HCWs with an outlet for managing work‐related stress and improving self‐confidence in their abilities. Wang et al demonstrated that support from peers and supervisors was associated with reduced job strain. 94 Conversely, living with family was identified as a risk factor in this review. 26 This is likely due to fear of transmitting the virus to loved ones, which brings a significant psychosocial burden.
5.6. Working experience
HCWs with more clinical experience are more likely to have developed individual coping mechanisms for increased workload. This is in‐line with the study by Chong et al during the SARS‐CoV pandemic, in which IES scores were significantly higher in HCWs with less than 2 years of experience. 7 In addition, according to Xiao X et al, junior doctors and nurses had more contact with SARS‐CoV‐2 patients (both with confirmed and suspected diagnoses) compared to their seniors. 70
5.7. Limitations of studies
Within the challenging environment of the SARS‐CoV‐2 pandemic, this review has highlighted varying methods used to measure the prevalence of mental health outcomes in HCWs. These methods largely utilized self‐reported data and were mostly cross‐sectional. Due to this study design, no definitive causal relationships can be made. Although psychiatric assessment tools such as PHQ‐9 and GAD‐7 have shown efficacy for screening and monitoring purposes, their use in quantifying prevalence is inherently subjective. 95 In addition, crucial ‘effect modifiers’ such as pre‐existing psychiatric disease and low socioeconomic status were not considered in the majority of studies. Following the onset of SARS‐CoV‐2, HCWs in these subgroups may have displayed exacerbated symptoms which may have confounded the study findings. Some studies used social media platforms such as WeChat to maximize questionnaire distribution through convenience sampling. As it cannot be ascertained how many HCWs received them, it is impossible to quantify response rates. There is also high selection bias as HCWs not using these platforms were unable to participate. ‘Wenjuanxing’, a Chinese survey website used by two studies, financially rewards participants on survey completion. This raises the possibility of data falsification, rushed answers, and multiple entries using different accounts. Due to the novelty of SARS‐CoV‐2 and the rapid publication of relevant work, preprint studies were included in this review. Preprint studies have not undergone rigorous academic peer review and the conclusions drawn from their datasets may not be as robust. Medrix was the sole search platform for preprint studies. 96 The long‐term mental health consequences cannot be concluded from currently available literature and there is a need for future follow‐up studies to quantify the long‐term implications.
5.8. Recommendations
HCWs should follow working patterns which balance service provision and staff safety, with designated rest periods to prevent burnout and insomnia.
All HCWs should have access to Psychiatric Rapid Response teams.
Healthcare employers should systematically screen HCWs for mental health illness using risk factors identified and use this to implement mental health programs locally.
Social support is crucial and can be provided through hospital support groups. Staff should be actively encouraged to remain in contact with their friends and family.
There is a need for a validated, standardized HCW mental health scoring tool for specific use during a contagion outbreak. One such scale for work‐related stress and anxiety during SARS‐CoV‐2 (‘SAVE‐9’) has been recently devised. 97
Future research should assess the international incidence of long‐term psychiatric disorders in HCWs as a result of the SARS‐CoV‐2 pandemic to inform mitigation and future prevention strategies.
6. CONCLUSION
This systematic review aimed to investigate the prevalence of mental health issues in hospital‐based HCWs during the SARS‐CoV‐2 pandemic, and identify associated risk factors for psychological interventions. Disturbances in mental health such as insomnia were commonplace and may be predicted by risk factors such as direct exposure to SARS‐CoV‐2 patients and pre‐existing medical comorbidity. Hospitals should screen their staff and provide early supportive psychological intervention. Future global research should be focused on the long‐term psychological impact of SARS‐CoV‐2 and formulating a standardized questionnaire for use in future pandemics. The repercussions of SARS‐CoV‐2 have far surpassed its predecessors such as SARS‐CoV. It has brought new challenges to mental health, and the impact on HCWs is likely be present far after the conclusion of the pandemic.
7. TRANSPARENCY DECLARATION
NP, JS, and JB confirm that the manuscript is honest, accurate, and that a transparent review of studies included is reported and that no important aspects of the study have been omitted.
DISCLOSURE STATEMENT
Ethical Approval of Research Protocol: N/A; Informed Consent: N/A; Registration No. (PROSPERO): CRD42020181204; Animal Studies: N/A; Conflict of Interest: N/A.
AUTHOR CONTRIBUTIONS
JS and NP share joint first authorship and contributed to this study equally. JB conceived the research question and is the study guarantor. JS and YH drafted the protocol. JS, NP, and YH carried out the literature search and screening of studies. YH drafted the methodology, JS and NP extracted and analyzed the data, and then drafted the manuscript. JB, KV, MC, AB, and YH critically revised the draft manuscript providing text and intellectual corrections. All authors reviewed the final manuscript and approved it for submission. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supporting information
Fig S1
Table S1‐S15
ACKNOWLEDGMENTS
All authors receive no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. JB holds a Royal College of Surgeons of England clinical research fellowship supported by Bowel Research UK. The views expressed in this publication are those of the authors and not necessarily those of the NHS, RCS England, Bowel Research UK, Health Education England, or the Department of Health.
Sanghera J, Pattani N, Hashmi Y, et al. The impact of SARS‐CoV‐2 on the mental health of healthcare workers in a hospital setting—A Systematic Review. J Occup Health. 2020;62:e12175 10.1002/1348-9585.12175
Guarantor: Mr Joshua R. Burke.
Jaspinder Sanghera and Nikhil Pattani Joint 1st Authorship.
REFERENCES
- 1. Ruan S. Likelihood of survival of coronavirus disease 2019. Lancet Infect Dis. 2020;20(6):630‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019‐nCoV). Available from https://bit.ly/2Y4v5Z0. Published 2020.
- 3. Johns Hopkins Coronavirus Resource Center . COVID‐19 Map. Available from https://coronavirus.jhu.edu/map.html. Published 2020.
- 4. Liu Y, Gayle AA, Wilder‐Smith A, Rocklöv J. The reproductive number of COVID‐19 is higher compared to SARS coronavirus. J Travel Med. 202027(2):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Who.int . Shortage of personal protective equipment endangering health workers worldwide. Available from https://www.who.int/news‐room/detail/03‐03‐2020‐shortage‐of‐personal‐protective‐equipment‐endangering‐health‐workers‐worldwide. Published 2020.
- 6. WHO . Summary table of SARS cases by country, 1 November 2002–7 August 2003. Available from https://www.who.int/csr/sars/country/2003_08_15/en/. Accessed 29 April, 2020
- 7. Chong M‐Y, Wang W‐C, Hsieh W‐C, et al. Psychological impact of severe acute respiratory syndrome on health workers in a tertiary hospital. Br J Psychiatry. 2004;185(2):127‐133. [DOI] [PubMed] [Google Scholar]
- 8. Maunder R, Hunter J, Vincent L, et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003;168(10):1245‐1251. [PMC free article] [PubMed] [Google Scholar]
- 9. Brooks SK, Dunn R, Amlôt R, Rubin GJ, Greenberg N. A systematic, thematic review of social and occupational factors associated with psychological outcomes in healthcare employees during an infectious disease outbreak. J Occup Environ Med. 2018;60(3):248‐257. [DOI] [PubMed] [Google Scholar]
- 10. Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID‐19 pandemic: a systematic review and meta‐analysis. Brain Behav immun. 2020;88:901‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee J, Lee E‐H, Moon SH. Systematic review of the measurement properties of the depression anxiety stress Scales–21 by applying updated COSMIN methodology. Qual Life Res. 2019;28(9):2325‐2339. [DOI] [PubMed] [Google Scholar]
- 12. Levis B, Benedetti A, Thombs BD. Accuracy of Patient Health Questionnaire‐9 (PHQ‐9) for screening to detect major depression: individual participant data meta‐analysis. Br Med J. 2019;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Arch Intern Med. 2006;166(10):1092‐1097. [DOI] [PubMed] [Google Scholar]
- 14. Smith MT, Wegener ST. Measures of sleep: the insomnia severity index, medical outcomes study (MOS) sleep scale, Pittsburgh sleep diary (PSD), and Pittsburgh sleep quality index (PSQI). Arthritis Rheum. 2003;49(S5):S184‐S196. [Google Scholar]
- 15. Arroll B, Goodyear‐Smith F, Crengle S, et al. Validation of PHQ‐2 and PHQ‐9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doulougeri K, Georganta K, Montgomery A. “Diagnosing” burnout among healthcare professionals: can we find consensus? Cogent Med. 2016;3(1):1237605. [Google Scholar]
- 17. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version. Comput Sci. 2006;1:b92. [Google Scholar]
- 18. WHO . ICD‐10 Version: 2016. Available from https://icd.who.int/browse10/2016/en?fbclid=IwAR20kpRpc4bVxahHC_CwIe4iQYPyFh4819EOJO83u8WyXZ20m43GdReyvSQ. Published 2020. Accessed 29 April, 2020.
- 19. Cao J, Wei J, Zhu H, et al. A study of basic needs and psychological wellbeing of medical workers in the fever clinic of a tertiary general hospital in Beijing during the COVID‐19 outbreak. Psychother Psychosom. 2020;89(4):252‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choudhury T, Debski M, Wiper A, et al. Covid‐19 pandemic: looking after the mental health of our healthcare workers. J Occup Environ Med. 2020;62(7):e373‐e376. [DOI] [PubMed] [Google Scholar]
- 21. Chung JP, Yeung W‐S. Staff mental health self‐assessment during the COVID‐19 outbreak. East Asian Arch of. Psychiatry. 2020;30(1):34. [DOI] [PubMed] [Google Scholar]
- 22. Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naser AY, Dahmash EZ, Al‐Rousan R, et al. Mental health status of the general population, healthcare professionals, and university students during 2019 coronavirus disease outbreak in Jordan: a cross‐sectional study. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salman M, Raza MH, Mustafa ZU, et al. The psychological effects of COVID‐19 on frontline healthcare workers and how they are coping: a web‐based, cross‐sectional study from Pakistan. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang C, Yang L, Liu S, et al. Survey of insomnia and related social psychological factors among medical staff involved in the 2019 novel coronavirus disease outbreak. Front Psychiatry. 2020;11:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu Z, Xu S, Wang H, et al. COVID‐19 in Wuhan: immediate psychological impact on 5062 health workers. medRxiv. 2020. [Google Scholar]
- 27. Apisarnthanarak A, Apisarnthanarak P, Siripraparat C, Saengaram P, Leeprechanon N, Weber DJ. Impact of anxiety and fear for COVID‐19 toward infection control practices among thai healthcare workers. Infect Control Hosp Epidemiol. 2020:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID‐19 outbreak in China: a web‐based cross‐sectional survey. Psychiatry Res. 2020;112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chew NW, Lee GK, Tan BY, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID‐19 outbreak. Behav Immun Brain. 2020;88:559‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chatterjee SS, Bhattacharyya R, Bhattacharyya S, Gupta S, Das S, Banerjee BB. Attitude, practice, behavior, and mental health impact of COVID‐19 on doctors. Indian J Psychiatry. 2020;62(3):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan BY, Chew NW, Lee GK, et al. Psychological impact of the COVID‐19 pandemic on health care workers in Singapore. Ann Intern Med. 2020;173(4):317‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elbay RY, Kurtulmuş A, Arpacıoğlu S, Karadere E. Depression, anxiety, stress levels of physicians and associated factors in covid‐19 pandemics. Psychiatry Res. 2020;290:113‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rossi R, Socci V, Pacitti F, et al. Mental health outcomes among frontline and second‐line health care workers during the coronavirus disease 2019 (COVID‐19) pandemic in Italy. JAMA Netw Open. 2020;3(5):e2010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dimitriu MC, Pantea‐Stoian A, Smaranda AC, et al. Burnout syndrome in Romanian medical residents in time of the COVID‐19 pandemic. Med Hypotheses. 2020:109972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fava GA, Kellner R, Munari F, Pavan L. The Hamilton depression rating scale in normals and depressives. Acta Psychiatr Scand. 1982;66(1):26‐32. [DOI] [PubMed] [Google Scholar]
- 36. Dunstan DA, Scott N, Todd AK. Screening for anxiety and depression: reassessing the utility of the Zung scales. BMC Psychiatry. 2017;17(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Breedvelt JJ, Zamperoni V, South E, et al. A systematic review of mental health measurement scales for evaluating the effects of mental health prevention interventions. Eur J Public Health. 2020;30(3):539‐545. [DOI] [PubMed] [Google Scholar]
- 38. Löwe B, Wahl I, Rose M, et al. A 4‐item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire‐4 (PHQ‐4) in the general population. J Affect Disord. 2010;122(1–2):86‐95. [DOI] [PubMed] [Google Scholar]
- 39. Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the beck depression inventory. Psychopathology. 1998;31(3):160‐168. [DOI] [PubMed] [Google Scholar]
- 40. Cosco TD, Prina M, Stubbs B, Wu Y‐T. Reliability and validity of the Center for Epidemiologic Studies Depression Scale in a population‐based cohort of middle‐aged US adults. J Nurs Meas. 2017;25(3):476‐485. [DOI] [PubMed] [Google Scholar]
- 41. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69‐77. [DOI] [PubMed] [Google Scholar]
- 42. Lu W, Wang H, Lin Y, Li L. Psychological status of medical workforce during the COVID‐19 pandemic: a cross‐sectional study. Psychiatry Res. 2020;288:112936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maier W, Buller R, Philipp M, Heuser I. The Hamilton anxiety scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14(1):61‐68. [DOI] [PubMed] [Google Scholar]
- 44. Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the Beck Anxiety Inventory. J Anxiety Disord. 1992;6(1):55‐61. [Google Scholar]
- 45. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non‐clinical samples: a systematic review and meta‐analysis. Sleep Med Rev. 2016;25:52‐73. [DOI] [PubMed] [Google Scholar]
- 46. Chiu H‐Y, Chang L‐Y, Hsieh Y‐J, Tsai P‐S. A meta‐analysis of diagnostic accuracy of three screening tools for insomnia. J Psychosom Res. 2016;87:85‐92. [DOI] [PubMed] [Google Scholar]
- 47. Zhang W‐R, Wang K, Yin LU, et al. Mental health and psychosocial problems of medical health workers during the COVID‐19 epidemic in China. Psychother Psychosom. 2020;89(4):242‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee E‐H. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res. 2012;6(4):121‐127. [DOI] [PubMed] [Google Scholar]
- 49. Bryant RA, Moulds ML, Guthrie RM. Acute stress disorder scale: a self‐report measure of acute stress disorder. Psychol Assess. 2000;12(1):61. [PubMed] [Google Scholar]
- 50. Amirkhan JH, Urizar GG Jr, Clark S. Criterion validation of a stress measure: the stress overload scale. Psychol Assess. 2015;27(3):985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cardena E, Koopman C, Classen C, Waelde LC, Spiegel D. Psychometric properties of the Stanford Acute Stress Reaction Questionnaire (SASRQ): a valid and reliable measure of acute stress. J Trauma Stress. 2000;13(4):719‐734. [DOI] [PubMed] [Google Scholar]
- 52. Wu K, Chan S. Psychometric properties of the Chinese version of the Impact of Event Scale‐Revised. Hong Kong J Psychiatry. 2004;14(4):2‐9. [Google Scholar]
- 53. Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The posttraumatic stress disorder checklist for DSM‐5 (PCL‐5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489‐498. [DOI] [PubMed] [Google Scholar]
- 54. Olff M, Bakker A, Frewen P, et al. Screening for consequences of trauma – an update on the global collaboration on traumatic stress. Eur J Psychotraumatol. 2020;11(1):1752504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kessler RC, Green JG, Gruber MJ, et al. Screening for serious mental illness in the general population with the K6 screening scale: results from the WHO World Mental Health (WMH) survey initiative. Int J Methods Psychiatr Res. 2010;19(S1):4‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Asukai N, Kato H, Kawamura N, et al. Reliabiligy and validity of the Japanese‐language version of the impact of event scale‐revised (Ies‐RJ): four studies of different traumatic events. J Nerv Ment Dis. 2002;190(3):175‐182. [DOI] [PubMed] [Google Scholar]
- 57. Coker A, Omoluabi P. Validation of maslach burnout inventory. IFE PsychologIA. 2009;17(1):231‐242. [Google Scholar]
- 58. Barello S, Palamenghi L, Graffigna G. Burnout and somatic symptoms among frontline healthcare professionals at the peak of the Italian COVID‐19 pandemic. Psychiatry Res. 2020;290:113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jalili M, Niroomand M, Hadavand F, Zeinali K, Fotouhi A. Burnout among healthcare professionals during COVID‐19 pandemic: a cross‐sectional study. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. García‐Fernández L, Romero‐Ferreiro V, López‐Roldán PD, et al. Mental health impact of COVID‐19 pandemic on Spanish healthcare workers. Psychol Med. 2020;1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guo J, Liao L, Wang B, et al. Psychological effects of COVID‐19 on Hospital Staff: a national cross‐sectional survey of China Mainland. Available at SSRN 3550050; 2020.
- 62. Liu Z, Han B, Jiang R, et al. Mental health status of doctors and nurses during COVID‐19 epidemic in China. Available at SSRN 3551329; 2020.
- 63. Song X, Fu W, Liu X, et al. Mental health status of medical staff in emergency departments during the Coronavirus disease 2019 epidemic in China. Brain Behav Immun. 2020;88:60‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang S, Xie L, Xu Y, Yu S, Yao B, Xiang D. Sleep disturbances among medical workers during the outbreak of COVID‐2019. Occup Med (Lond). 2020;70(5):364‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li Z, Ge J, Yang M, et al. Vicarious traumatization in the general public, members, and non‐members of medical teams aiding in COVID‐19 control. Brain Behav Immun. 2020;88:916‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Du J, Dong L, Wang T, et al. Psychological symptoms among frontline healthcare workers during COVID‐19 outbreak in Wuhan. Gen Hosp Psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Badahdah AM, Khamis F, Al MN. The psychological well‐being of physicians during COVID‐19 outbreak in Oman. Psychiatry Res. 2020;289:113053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mo Y, Deng L, Zhang L, et al. Work stress among Chinese nurses to support Wuhan in fighting against COVID‐19 epidemic. J Nurs Manag. 2020;28(5):1002‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thomaier L, Teoh D, Jewett P, et al. Emotional health concerns of oncology physicians in the United States: fallout during the COVID‐19 pandemic. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xiao X, Zhu X, Fu S, Hu Y, Li X, Xiao J. Psychological impact of healthcare workers in China during COVID‐19 pneumonia epidemic: a multi‐center cross‐sectional survey investigation. J Affect Disord. 2020;274:405‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen Y, Zhou H, Zhou Y, Zhou F. Prevalence of self‐reported depression and anxiety among pediatric medical staff members during the COVID‐19 outbreak in Guiyang, China. Psychiatry Res. 2020;288:113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liang Y, Chen M, Zheng X, Liu J. Screening for Chinese medical staff mental health by SDS and SAS during the outbreak of COVID‐19. J Psychosom Res. 2020;133:110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu C‐Y, Yang Y‐Z, Zhang X‐M, et al. The prevalence and influencing factors in anxiety in medical workers fighting COVID‐19 in China: a cross‐sectional survey. Epidemiol Infect. 2020;148(e98):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Qi J, Xu J, Li B‐Z, et al. The evaluation of sleep disturbances for Chinese frontline medical workers under the outbreak of COVID‐19. Sleep Med. 2020;72:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yin Q, Sun Z, Liu T, et al. Posttraumatic Stress Symptoms of Health Care Workers during the Corona Virus Disease 2019 (COVID‐19). Clin Psychol & Psychother. 2020;27(3):384‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xiang Y‐T, Zhang Q, Wang G, Zeng L‐N, Ungvari GS. Prevalence of mental disorders in China. Lancet Psychiat. 2019;6(6):467‐468. [DOI] [PubMed] [Google Scholar]
- 77. Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the risk of depression: a meta‐analysis of prospective cohort studies. BMC Psychiatry. 2016;16(1):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sivertsen B, Salo P, Mykletun A, et al. The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med. 2012;74(7):758‐765. [DOI] [PubMed] [Google Scholar]
- 79. Jacob K. Recovery model of mental illness: a complementary approach to psychiatric care. Indian J Psychol Med. 2015;37(2):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maunder R, Lancee W, Balderson K, et al. Long‐term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerg Infect Dis. 2006;12(12):1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Maunder R. The experience of the 2003 SARS outbreak as a traumatic stress among frontline healthcare workers in Toronto: lessons learned. Philos Trans R Soc Lond B Biol Sc. 2004;359(1447):1117‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hu K, Fan J, Li X, Gou X, Li X, Zhou X. The adverse skin reactions of health care workers using personal protective equipment for COVID‐19. Medicine. 2020;99(24):e20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lancet T. COVID‐19: protecting health‐care workers. Lancet. 2020;395(10228):922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. European Medicines Agency . Update on treatments and vaccines against COVID‐19 under development. Available from https://bit.ly/2SeZArl. Accessed 25 April, 2020
- 85. Shira F. Coronavirus symptoms might get better before they get worse, and the downturn can happen very quickly, doctors say. Available from https://bit.ly/35h9uOs. Accessed 28 April, 2020
- 86. Lewis KS, Gordon‐Smith K, Forty L, et al. Sleep loss as a trigger of mood episodes in bipolar disorder: individual differences based on diagnostic subtype and gender. BJPsych. 2017;211(3):169‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Menon V, Padhy SK. Ethical dilemmas faced by health care workers during COVID‐19 pandemic: issues, implications and suggestions. Asian J Psychiatr. 2020;51:102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wisetborisut A, Angkurawaranon C, Jiraporncharoen W, Uaphanthasath R, Wiwatanadate P. Shift work and burnout among health care workers. Occup Med. 2014;64(4):279‐286. [DOI] [PubMed] [Google Scholar]
- 89. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. [DOI] [PubMed] [Google Scholar]
- 90. Lucchini A, Iozzo P, Bambi S. Nursing workload in the COVID‐19 ERA. Intensive Crit Care Nurs. 2020;102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang X, Jiang Z, Yuan X, et al. (COVID‐19) epidemic: a cross‐sectional survey. Int J Nurs Stud. 2019;2020:103635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Maunder RG, Lancee WJ, Rourke S, et al. Factors associated with the psychological impact of severe acute respiratory syndrome on nurses and other hospital workers in Toronto. Psychosom Med. 2004;66(6):938‐942. [DOI] [PubMed] [Google Scholar]
- 93. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang J‐N, Sun W, Chi T‐S, Wu H, Wang L. Prevalence and associated factors of depressive symptoms among Chinese doctors: a cross‐sectional survey. Int Arch Occup Environ Health. 2010;83(8):905‐911. [DOI] [PubMed] [Google Scholar]
- 95. Richardson T, Wrightman M, Yeebo M, Lisicka A. Reliability and score ranges of the PHQ‐9 and GAD‐7 in a primary and secondary care mental health service. J Psychosoc Rehabil Ment Health. 2017;4(2):237‐240. [Google Scholar]
- 96. medRxiv . medRxiv. Available from https://www.medrxiv.org/. Published 2020. Accessed 30 June, 2020.
- 97. Chung S. Development of the Stress and Anxiety to Viral Epidemics‐9 (SAVE‐9) scale for assessing work‐related stress and anxiety in healthcare workers in response to viral epidemics. PsyArXiv Preprints. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kang L, Ma S, Chen M, et al. Impact on mental health and perceptions of psychological care among medical and nursing staff in Wuhan during the 2019 novel coronavirus disease outbreak: a cross‐sectional study. Brain Behav Immun. 2020;87:11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li X, Yu H, Bian G, et al. Prevalence, risk factors, and clinical correlates of insomnia in volunteer and at home medical staff during the COVID‐19. Brain Behav Immun. 2020;87:140‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sun D, Yang D, Li Y, et al. Novel Coronavirus (2019‐nCoV) outbreak on health workers in China. Epidemiol Infect. 2019;2020:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wu K, Wei X. Analysis of psychological and sleep status and exercise rehabilitation of front‐line clinical staff in the fight against COVID‐19 in China. Med Sci Monit Basic Res. 2020;26:e924081‐e924085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wu Y, Wang J, Luo C, et al. A comparison of burnout frequency among oncology physicians and nurses working on the front lines and usual wards during the COVID‐19 epidemic in Wuhan, China. J Pain Symptom Manag. 2020;60(1):e60‐e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xiao H, Zhang Y, Kong D, Li S, Yang N. The effects of social support on sleep quality of medical staff treating patients with coronavirus disease 2019 (COVID‐19) in January and February 2020 in China. Med Sci Monit. 2020;26:e923541‐e923549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhang SX, Liu J, Jahanshahi AA, et al. At the height of the storm: healthcare staff’s health conditions and job satisfaction and their associated predictors during the epidemic peak of COVID‐19. Brain Behav Immun. 2020;87:144‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhu J, Sun L, Zhang L, et al. Prevalence and influencing factors of anxiety and depression symptoms in the first‐line medical staff fighting against covid‐19 in Gansu. Front Psychiatry. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Beck JG, Grant DM, Read JP, et al. The impact of event scale‐revised: psychometric properties in a sample of motor vehicle accident survivors. J Anxiety Disord. 2008;22(2):187‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193‐213. [DOI] [PubMed] [Google Scholar]
- 110. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371‐379. [DOI] [PubMed] [Google Scholar]
- 111. Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. Sydney: Psychology Foundation of Australia; 1996. [Google Scholar]
- 112. Ke X, Liu C, Li N. Social support and Quality of Life: a cross‐sectional study on survivors eight months after the 2008 Wenchuan earthquake. BMC Public Health. 2010;10(1):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Amirkhan JH. A brief stress diagnostic tool: the short Stress Overload Scale. Assessment. 2018;25(8):1001‐1013. [DOI] [PubMed] [Google Scholar]
- 114. Lee H‐F, Kuo H‐T, Chang C‐L, Hsu C‐C, Chien T‐W. Determining cutting points of the Maslach Burnout Inventory for nurses to measure their level of burnout online. History Research. 2017;5(1):1. [Google Scholar]
- 115. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD‐10 criteria. J Psychosom Res. 2000;48(6):555‐560. [DOI] [PubMed] [Google Scholar]
- 116. Sharp R. The Hamilton rating scale for depression. Occup Medicine. 2015;65(4):340. [DOI] [PubMed] [Google Scholar]
- 117. Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory‐II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 118. Carleton RN, Thibodeau MA, Teale MJ, et al. The center for epidemiologic studies depression scale: a review with a theoretical and empirical examination of item content and factor structure. PLoS One. 2013;8(3):e58067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Carney CE, Moss TG, Harris AL, Edinger JD, Krystal AD. Should we be anxious when assessing anxiety using the Beck Anxiety Inventory in clinical insomnia patients? J Psychiatr Res. 2011;45(9):1243‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cohen S, Kamarck T, Mermelstein R. Perceived stress scale Measuring Stress: A Guide for Health and Social Scientists. 1994;10:1‐2. [Google Scholar]
- 122. Sveen J, Bondjers K, Willebrand M. Psychometric properties of the PTSD Checklist for DSM‐5: a pilot study. Eur J Psychotraumatol. 2016;7(1):30165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Prochaska JJ, Sung HY, Max W, Shi Y, Ong M. Validity study of the K6 scale as a measure of moderate mental distress based on mental health treatment need and utilization. Int J Methods Psychiatr Res. 2012;21(2):88‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S15


