Abstract
At a recent symposium on aging biology, a debate was held as to whether or not we know what biological aging is. Most of the participants were struck not only by the lack of consensus on this core question, but also on many basic tenets of the field. Accordingly, we undertook a systematic survey of our 71 participants on key questions that were raised during the debate and symposium, eliciting 37 responses. The results confirmed the impression from the symposium: there is marked disagreement on the most fundamental questions in the field, and little consensus on anything other than the heterogeneous nature of aging processes. Areas of major disagreement included what participants viewed as the essence of aging, when it begins, whether aging is programmed or not, whether we currently have a good understanding of aging mechanisms, whether aging is or will be quantifiable, whether aging will be treatable, and whether many non-aging species exist. These disagreements lay bare the urgent need for a more unified and cross-disciplinary paradigm in the biology of aging that will clarify both areas of agreement and disagreement, allowing research to proceed more efficiently. We suggest directions to encourage the emergence of such a paradigm.
Keywords: Aged, Aging, Aging paradigm, biology of aging, aging mechanisms, aging interventions, epidemiology of aging, evolution of aging, philosophy of science
Introduction
The authors were all participants at the Biology of Aging Symposium: Understanding Aging to Better Intervene, held November 9–11, 2019 in Montreal, Quebec. The symposium featured 44 speakers with a diversity of expertise related to aging, including basic aging biology, translational geroscience, geriatric medicine, nutrition, immunosenescence, evolutionary ecology, demography, statistics, systems biology, epidemiology, and complex systems theory. During the course of the symposium, a debate was held on the question, “Do we know what aging is?” with Brian Kennedy ostensibly arguing the “Yes” side and Alan Cohen ostensibly arguing the “No” side. There was dynamic audience participation. Most participants agreed that the debate and the subsequent extensive discussion involving many participants were striking in how it highlighted the lack of a clear consensus paradigm (Kuhn, 1970) in the field, and collectively we agreed it would be important to describe this for the research community in our field.
Accordingly, we designed a survey that was sent to participants of the symposium, both invited speakers and students/other participants. The survey was meant to capture the opinions on both the key points of disagreement and basic features of aging in general. All participants who responded to the survey are co-authors. We use the term “aging” to refer to “aging biology,” though, as will be shown, some but not all participants felt that aging biology cannot be understood in isolation from psychological, social, and cultural factors.
Philosophers of science generally believe that at least some aspects of a shared paradigm or worldview are often critical in helping a field advance, though the precise nature and role of such paradigms is debated (Kuhn, 1970; Lakatos, 2014). Beyond the biology of aging that is our focus here, it has been argued that there is a broad gerontological paradigm spanning from biology to the social sciences (Ferraro, 2018), with six key features: causality, life course analysis, multifaceted change, heterogeneity, accumulation processes, and ageism. Our discussions at the symposium showed no consensus on questions relating to ageism and causality, but generally supported the other four proposed features. Nonetheless, the broad areas of disagreement shown below will pose challenges for the field, and the nature of a paradigm is likely to be quite different for aging biology than in gerontology more broadly. If we cannot agree on what aging is (definitions and mechanisms), how can we identify it, measure it, or know if we are measuring it (Belsky et al., 2015; Calimport et al., 2019; Horvath, 2013; Levine, 2013)? How can we evaluate potential anti-aging interventions (Justice et al., 2018)? How relevant are findings from other species in terms of understanding human aging (Austad, 2010; Jones et al., 2014)? We do not believe it is possible at this point to propose a paradigm that would be broadly accepted in the field; accordingly, the best we can do is to note the important differences and try to propose a roadmap for what would be needed to achieve consensus on key questions.
Methods
Survey
We used the tool Google Forms to distribute a survey to all participants at the symposium (44 invited speakers, 2 organizers, 14 students, and the 11 others). The survey collected the following information: (1) name; (2) demographic data (sex, country of origin, career stage) (3) domains of expertise (multiple responses permitted); (4) Likert-scale and other limited response questions (see below); and (5) a single open-ended question, “In no more than three sentences or 1000 characters, please describe your understanding of what causes aging and what it is, or is not, at a mechanistic level.”
The Likert scale used was, “For each of the following statements, do you (1) Strongly disagree; (2) Moderately disagree; (3) Slightly disagree; (4) Neutral; (5) Slightly agree; (6) Moderately agree; or (7) Strongly agree?” The statements were:
We have a relatively good understanding of the basic biological mechanisms of aging.
Some combination of the nine hallmarks (Lopez-Otin et al. 2013) or the seven pillars (Kennedy et al. 2014) does a relatively comprehensive job of describing the mechanisms of aging.
Aging proceeds uniformly across tissues.
It is or will soon be possible to have relatively reliable metrics of the overall aging process
Aging cannot and should not be measured by a single metric because it is multi-dimensional and heterogeneous
It should be possible to quantify aging well even in the absence of a clear consensus or mechanistic understanding of what aging is.
It should be possible to intervene in aging, and evaluate interventions, even in the absence of a clear consensus or mechanistic understanding of what aging is.
There are many species across the tree of life that do not age at a rate that is biologically relevant or appreciable
Broadly speaking, aging mechanisms are similar in most species
Mortality rates or survival curves are generally a reasonable proxy for aging at the organismal level
It is important for the field to have a consensus definition of aging
Aging is an undesirable process that should be treated, cured, or minimized
Aging biology can be understood largely as a cellular and molecular process
Aging is genetically programmed and not just a by-product of imperfections or evolutionary constraints.
Aging will be reversible with the right technologies.
Anti-aging interventions have the potential to dramatically extend human lifespan (beyond, say, 150 years).
Additional questions:
Does aging begin approximately at (1) parental gamete formation; (2) conception; (3) birth; (4) sexual maturity; or (5) later in life?
Which of the following proposed anti-aging interventions are likely to slow aging in humans with tolerable levels of side-effects (check all that apply)? (1) senolytics/targeting senescent cells; (2) pharmacological or gene editing control of known aging pathways (metformin, rapamycin, NAD+, etc.); (3) enhancing cell replication (e.g. telomerase activation such as via TA65); (4) direct attempts to reduce macromolecular damage (e.g. proteasome activators such as 18aplha-GA, quercetin); (5) interventions in the immune system (e.g. growth hormone to reverse thymic involution); (6) lifestyle interventions; (7) other (list all that apply).
- What percent of what we observe as aging do you think can be attributed to the following types of mechanisms (understanding that they may coexist and interact with each other): [100 points that should be distributed among the five options below]
- Damage accumulation (DNA damage, protein aggregates, structural damage, etc.)
- Maladaptation/antagonistic pleiotropy: mechanisms that are useful early in life (e.g. for cancer prevention) become harmful later in life.
- Adaptation: Adjustments the organism makes to either pathological aspects of aging or to changing needs (e.g. immune repertoire) at different ages.
- Homeodynamic dysregulation: breakdown in the capacity of complex regulatory networks to maintain homeodynamics
- Other [Up to 100 words to specify]
We obtained a waiver from the ethics committee at the CIUSSS-ECHUS and post-hoc approval to conduct the survey (Project #2021–3728).
Statistics
We provide only descriptive statistics and do not calculate standard errors, confidence intervals, or p-values, which would only be relevant if we could suppose that our sample was representative of a larger population. We do not claim this to be the case, and prefer to present only raw data. We did assess pairwise Pearson correlations among the 16 Likert-scale variables by transforming them to a 7-point scale, and we also conducted exploratory principal components analysis (PCA) on the covariance matrix of this dataset, though we present only the broad strokes of those results due to fear that a 16-variable PCA with only 37 observations could be highly unstable.
Limitations
Even though the questions were subject to substantial discussion before the actual survey, with rewording performed throughout the rounds of debate if necessary, some questions could be viewed as ambiguous or be understood differently by some participants. In part, this was due to different understanding of phrases such as “we understand”, “damage”, etc. We acknowledge these limitations, which we think do not affect the core findings of this study.
Results
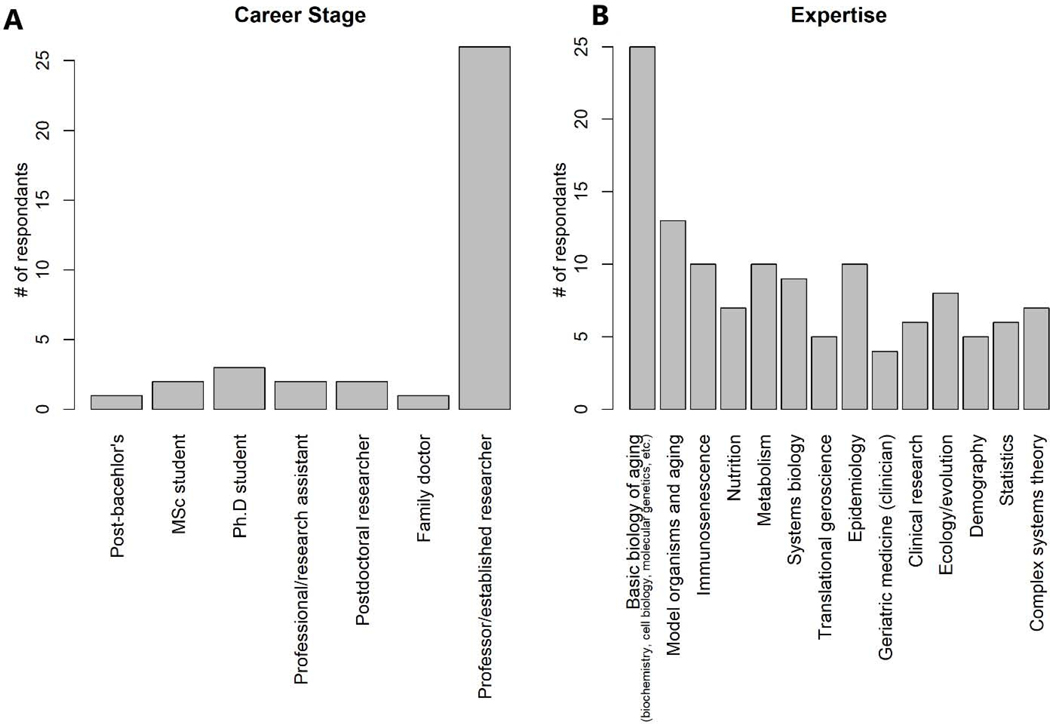
Thirty-seven researchers/students responded to the survey (the authors of this paper). Demographically, 27 of 37 respondents were male, with origins in 18 different countries. No country had more than 3 respondents except Canada, which had 14, though it is worth noting that some respondents marked their current country of residence, some marked their country of origin, some marked both, and some left the field blank. Respondents were at a variety of career stages as early as post-bachelor’s, but most (26) were established researchers/professors (Fig. 1A). A variety of expertise was represented, with the great majority having at least some expertise in the basic biology of aging, and many others with expertise in model organisms, metabolism, complex systems theory, evolutionary ecology, etc. (Fig. 1B). Respondents were also invited to volunteer additional expertise; these responses included: “Neuroscience and computer modelling,” “Neurodegenerative disease,” “Biodemography of aging, health, and longevity,” “Bioinformatics,” and “Cell signalling, cell death, degenerative diseases, microenvironment.” While our respondents do not represent a random sample of aging researchers, they do represent a wide variety of expertise in the field, and most are far enough advanced in their studies to have well-informed opinions on aging biology. Sample size was insufficient to formally compare responses by subgroups, but an informal analysis by sex suggests there are no particularly marked differences in opinions between male and female researchers.
Fig. 1.
Profile of the survey respondents in terms of self-declared career stage (A) and expertise (B). For career stage, respondents chose one category. Two respondents (post-bachelor’s and family doctor) volunteered responses not in the list. For expertise, each respondent checked as many boxes as they felt applied.
Most of the key questions were asked on a 7-level Likert scale, and responses are summarized in Fig. 2. Of 16 questions, 11 had at least one respondent in each of the most extreme categories (“Strongly agree” and “Strongly disagree”), and all 16 had responses ranging from Strongly on one end to Moderately on the other. For three questions, there was nonetheless a clear preponderance of respondents taking one opinion. For whether aging proceeds uniformly across tissues, 86% disagreed (strongly, moderately, or slightly). Regarding the multi-dimensionality of aging, 86% agreed strongly, moderately, or slightly that it is heterogeneous and cannot be measured with a single metric. Most respondents (81%) agreed strongly, moderately, or slightly that it is important for the field to have a consensus definition of aging. For four other questions, there was a clear tendency in one direction even if there was still substantial disagreement: more respondents than not concurred that aging is largely a cellular/molecular process (75%), that the hallmarks/pillars (Kennedy et al., 2014; López-Otín et al., 2013) do a decent job of summarizing the aging process (72%), that mortality rates are a reasonable proxy for organismal aging (72%), and that aging is undesirable (69%). In other words, for 9 out of 16 questions, there is no clear trend for a consensus of opinion (<65% on one side or the other), and diametrically opposed views were held by comparable numbers of researchers among our relatively small sample. Even for the two questions with the most agreement, it is worth noting that among the three researchers in the opposing camp in each case, two were among the best-known experts in the field (h-index > 100).
Fig. 2.
Responses to Likert-scale questions on aging, ordered from top to bottom by decreasing consensus.
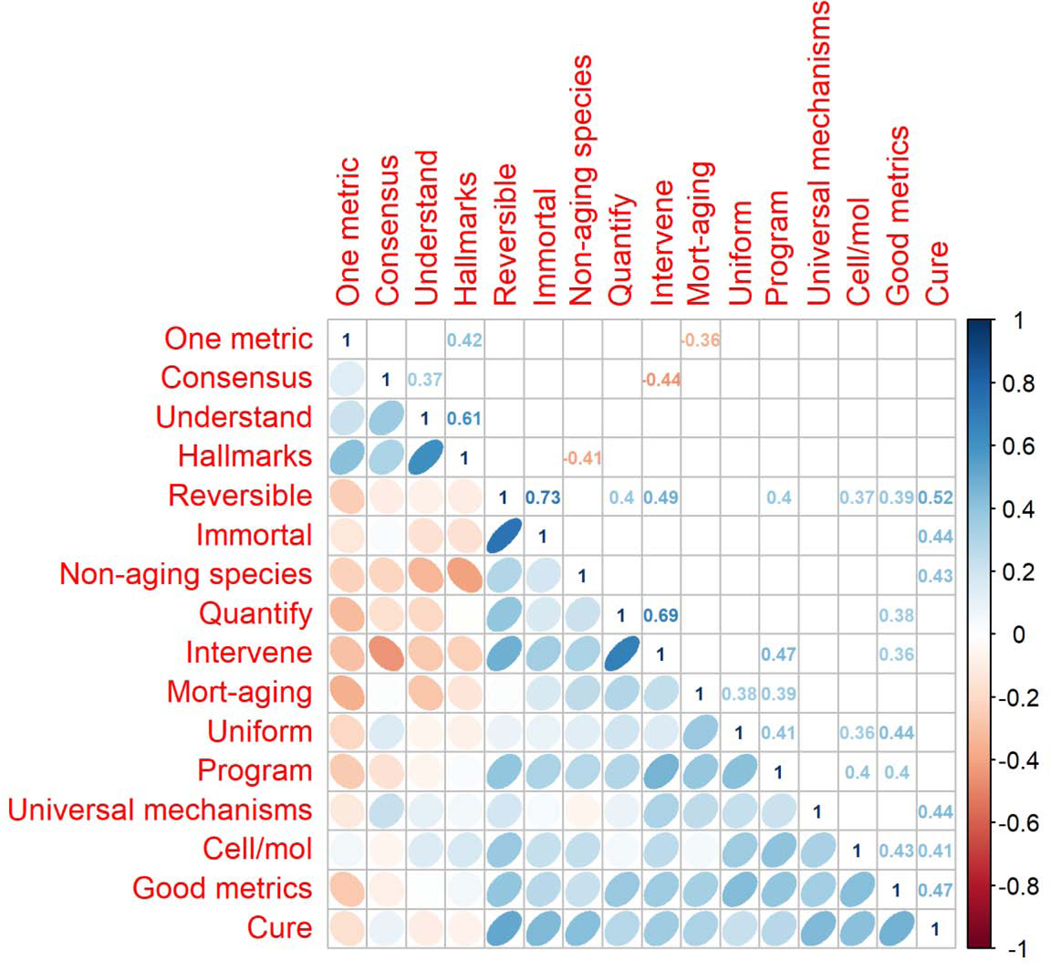
Unsurprisingly, there appeared to be a clear correlation structure among the Likert-scale questions (Fig. 3), though in many cases the limited sample size makes precise estimation of the coefficients doubtful. Broadly, those who thought aging should be cured/treated also tended to think it was more likely for this to be possible, and that aging was readily quantifiable, programmed, and universal. PCA confirmed this: 34% of the variance was explained by the first axis, and 15% by the second, with the loadings of the first axis qualitatively reflecting the summary of the correlation matrix. While the correlation structure is clear, it is not overwhelmingly strong, indicating that there are not simply two opposing camps with a range of opposing views.
Figure 3.
Correlation matrix representing Pearson correlation coefficients between the 16 Likert-scale questions. Correlations that were not significant at α=0.05 are left blank above the diagonal.
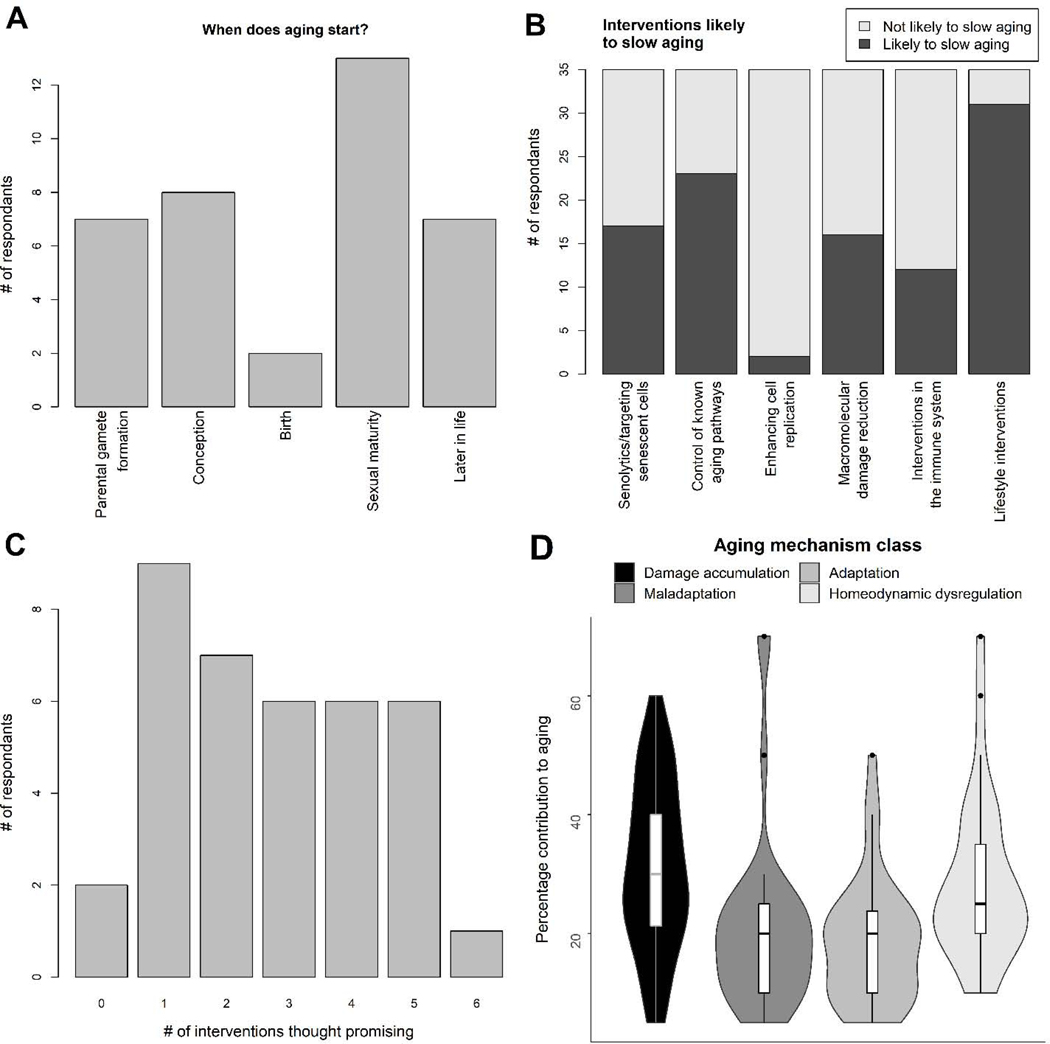
As for when aging begins, there was also marked disagreement, with a relatively uniform distribution of responses ranging from parental gamete formation to later in life (Fig. 4A). One participant has argued recently that the answer is none of the above: that aging begins very early in life (during embryogenesis), but the exact moment is not defined (Kinzina et al., 2019). For interventions likely to slow aging, there was near-consensus on some potential interventions but not others (Fig. 4B). Only 2 of 35 respondents thought enhancing cell replication was promising, and 91% (32/35) thought lifestyle interventions were promising, but for the other four interventions, between 29% and 63% thought them promising, showing substantial divergence in opinions of researchers. Furthermore, this was not due to a small set that found all interventions promising and a larger set that found none or only lifestyle promising; there was a relatively uniform distribution of how many interventions were thought promising (Fig. 4C). Some respondents noted additional interventions they thought promising: “Healthy, diversified nutrition, in particular digital tool aided personalized nutrition,” “Minimize somatotroph dysfunction,” “Combination of pharmacological (including those affecting immune function) and lifestyle,” and “Prevention of early changes in the organism (disease-dependent etc).”
Fig. 4.
Responses to non-Likert questions. (A) Counts (absolute number) of answers to when aging starts. (B) Counts (absolute number) of respondents endorsing intervention classes thought likely to slow aging. (C) Counts (absolute number) of respondents endorsing intervention classes thought likely to slow aging (D) Relative contribution (%) of different classes of mechanisms to the aging process.
Lastly, there was substantial heterogeneity in how respondents saw the mechanisms of aging (Fig. 4D). Damage accumulation was perhaps seen as slightly more important than the others on average, contributing from 5% to 60% with a median of 30%. Next was homeodynamic dysregulation, contributing from 0% to 70% with a median of 25%. Maladaptation and adaptation both had means of 20%, though maladaptation had a larger range (0% to 70%) than adaptation (5% to 50%). Some respondents indicated additional classes of mechanisms, with the following texts and percentages: “This will be different for each individual depending on genetic and environmental exposition. Personalized medicine will help.”; “The intrinsic (causal) interactions between the abovementioned mechanisms makes it artificial to rate them separately”; “Pre-programmed cellular death and aging orchestrated by genetics/epigenetics (40%)”; and “reserve depletion (20%); slowdown in metabolism and information processing (20%).”
In addition to this summary of the survey results, we provide the results on a website that will allow other members of the field to fill out the survey, and allows users to visualize and download data by different subsets of respondents (established researchers, domains of expertise, etc.). If many of our colleagues and readers fill out the same survey, we may increase both the sample size and potentially the representativity.
Definitions of aging
Responses to the question “In no more than three sentences or 1000 characters, please describe your understanding of what causes aging and what it is, or is not, at a mechanistic level” were highly diverse. Some representative examples of this diversity are shown in Table 1, and the full list of definitions can be found in Supplement 1. Note that some definitions refer explicitly to programs, and others are explicitly non-programmed. Some are primarily focused on damage, others on system dynamics. Some refer to cellular mechanisms, other to systemic or organism-level mechanisms. Some definitions suggest that aging rate is modified by the environment, others that it is not. Some situate aging explicitly in an evolutionary context, others focus exclusively on cellular/biochemical mechanisms. While not all these contrasts are mutually exclusive, they represent a remarkable diversity of perspectives.
Table 1:
Examples of the diverse mechanistic definitions of aging
| “We do not know what causes aging. I think the trigger is endogenous but not necessarily programmed.” (G. Ferbeyre) |
| “Aging is mainly accumulation of macromolecular damage and failure of adaptation to a continuous changing environment. It is also an epiphenomenon of the outcome of the protective conditions we humans have achieved thanks to civilization over time and to this end somehow it doesnť comply fully with typical Darwinian Biology.” (E.S. Gonos) |
| “As an evolutionary biologist, I see ‘aging’ as the decrease in the age-specific contribution to fitness. […] At a mechanistic level, aging (or I should say ‘senescence’) corresponds to any deterioration of cellular/physiological traits that will ultimately impact fitness.” (J.F. Lemaître) |
| “Mechanistically, aging is a disruption of the homeostasis established between cellular processes. Age-related-diseases are the external symptoms of this disruption. […] Aging is the result of [an] imperfect optimization to maintain a balance between evolutionary constraints, cellular dynamical equilibrium and environmental constraints. […] Aging is not a programmed process but a consequence of this search for an equilibrium.” (Q. Vanhaelen) |
| “Time-dependent degradation of interactions or their (quasi)adaptative changes; sluggish and/or ineffective responses to challenges; when more than one such response is biologically relevant, aging would manifest as preference for a response different than one preferred at youth. The latter response may still be effective, but at higher cost for an aging organism (e.g. increased innate, inflammatory responses to pathogens in the old).” (J.M. Witkowski) |
| “Aging is the progressive decline of function with increasing chronological age due to internal factors that are not dependent on environment, which leads to an increased probability of death. Aging is caused by a genetically programmed switch that downregulates cellular pathways involved in homeostasis, stress response, repair etc. that evolved to limit competition for resources between offspring and parent.” (J. Van Raamsdonk) |
| “I believe that some mechanism that clears damage from the germline (which is immortal) is toxic to the rest of the organism. This toxicity interferes with transcription at the DNA or protein level, leading to imbalanced proteostasis. Chromatin condensation therefore becomes abnormal (as measured by epigenetic clocks) which disrupts function of the organism as a whole and likely exponentially through a negative feedback loop.” (T. Liontis) |
| “Ageing is caused by a breakdown in repair mechanisms due to a shift of resource allocation after reproduction, modulated according to the environmental niche of the organism.” (G. Pawelec) |
| “Aging is not programmed. Aging is the result of complex interactions between the genome and physiological and environmental changes, with feedback loops that lead to physical and mental impairment. Organisms are not adapted to naturally live indefinitely.” (F. Dufour) |
| “Repair machineries fail to keep up with internal and external damage; systems become dysregulated and eventually collapse.” (V. Gorbunova) |
| “The gradual break-down of cellular components, leading to the eventually death of the organism, associated with time. This is caused by DNA damage, mutations, genetic/epigenetic pre-programmed senescence, protein aggregates and environmental stress.” (U. Anglas) |
Discussion
The results of our survey undeniably confirm the impression at the symposium: there is no clear consensus in the field of aging biology, even on the most fundamental questions. There was a near-consensus (but not complete) that aging is heterogeneous, reflected in a clear preponderance of respondents considering that aging does not proceed uniformly across tissues and that aging cannot be measured with a single, unidimensional metric. The only other question with such a clear preponderance of support was on the need for the consensus, which we have demonstrated here does not currently exist.
Some degree of disagreement in a scientific discipline is expected – it would be surprising to find complete consensus, particularly on questions under active investigation. Nonetheless, we argue that the disagreement is striking for the types of questions we posed, at least for many of them. For example, there was major disagreement as to whether we have a good understanding of the basic biological mechanisms of aging, whether it will soon be possible to reliably measure aging, whether there are many species that do not age appreciably, whether aging mechanisms are similar across species, whether aging is largely a cellular and molecular process, and whether aging is genetically programmed, among others. Given our relatively detailed knowledge of molecular pathways related to aging (Pan and Finkel, 2017), this level of disagreement among well renowned, established researchers/professors on such larger questions should perhaps be of major concern to the field.
We note an important caveat on the apparent disagreement we show: it was nearly impossible to formulate questions to be uniformly understood so as to elicit perfectly clear responses, and the wording of the questions was subject to substantial discussion before the final survey was circulated. For example, a participant’s definition/conception of aging likely influenced responses to downstream questions. Perhaps the clearest example of this is when aging begins: if aging is largely damage accumulation, it likely begins before birth (Kinzina et al., 2019), but if it is based on mortality/reproduction patterns or maladaptation/antagonistic pleiotropy (mechanisms that are useful early in life become harmful later in life), it begins closer to sexual maturity or later in life (Gaillard and Lemaître, 2017; Williams, 1957). Another example would be a statement like “We have a good understanding of X.” Who is “we,” the researcher or the field? How good is good? What do we mean by understanding? In this sense, the apparent disagreements in responses to our questions may thus be somewhat exaggerated. Nonetheless, our questions were formulated in ways that are typical of discussions in the field, and at the very least the disagreement we show indicates that we have major communication issues to overcome. For example, it is common to read that aging is reversible (López-León and Goya, 2017) (and 42% of our respondents felt so to differing degrees), but what do we mean by aging? Mortality rates, or biomarker signatures? Which biomarkers? What exactly is reversible, and to what extent? Likewise, if we ask whether aging proceeds uniformly across tissues (86% of us felt it doesn’t), the answer might be different if we are considering indicators of conserved genetic pathways versus epigenetic signals versus tissue functionality.
Despite this caveat, many disagreements are clearly about facts/reality rather than definitions. For example, disagreement as to whether aging is programmed or not might be slightly attributable to communication, but, based on the definitions, also clearly reflects real differences of opinion. Our objective here is not to settle these differences or to take a side, but simply to point them out.
The definitions of aging are particularly enlightening and often explicitly showed the differences in opinions reflected in the guided questions. Answers ranged from mechanistic to evolutionary, with answers sometimes referring explicitly to either a presence or absence of programming (Blagosklonny, 2013; Goldsmith, 2012; Kowald and Kirkwood, 2016). Some respondents thought the basis is fundamentally cellular and molecular, whereas others saw important roles for higher-order processes, or defined aging around functionality and phenotype rather than underlying processes. While these ideas are not necessarily mutually exclusive, the combination of the survey questions and the definitions indicate substantial disagreement as to whether an understanding of aging biology should include mechanisms beyond cellular/molecular biology. Certain notions were repeatedly referenced, notably damage accumulation (e.g. via imperfect repair mechanisms) (Gladyshev, 2014) and loss of homeostasis (Cohen, 2016), reflecting areas of active interest among many researchers. This highlights that while there is immense disagreement at some levels, certain subsets of researchers are also actively converging on similar ideas, even if these ideas may not be shared by all. The lack of consensus is thus not a complete absence of convergence or coherence.
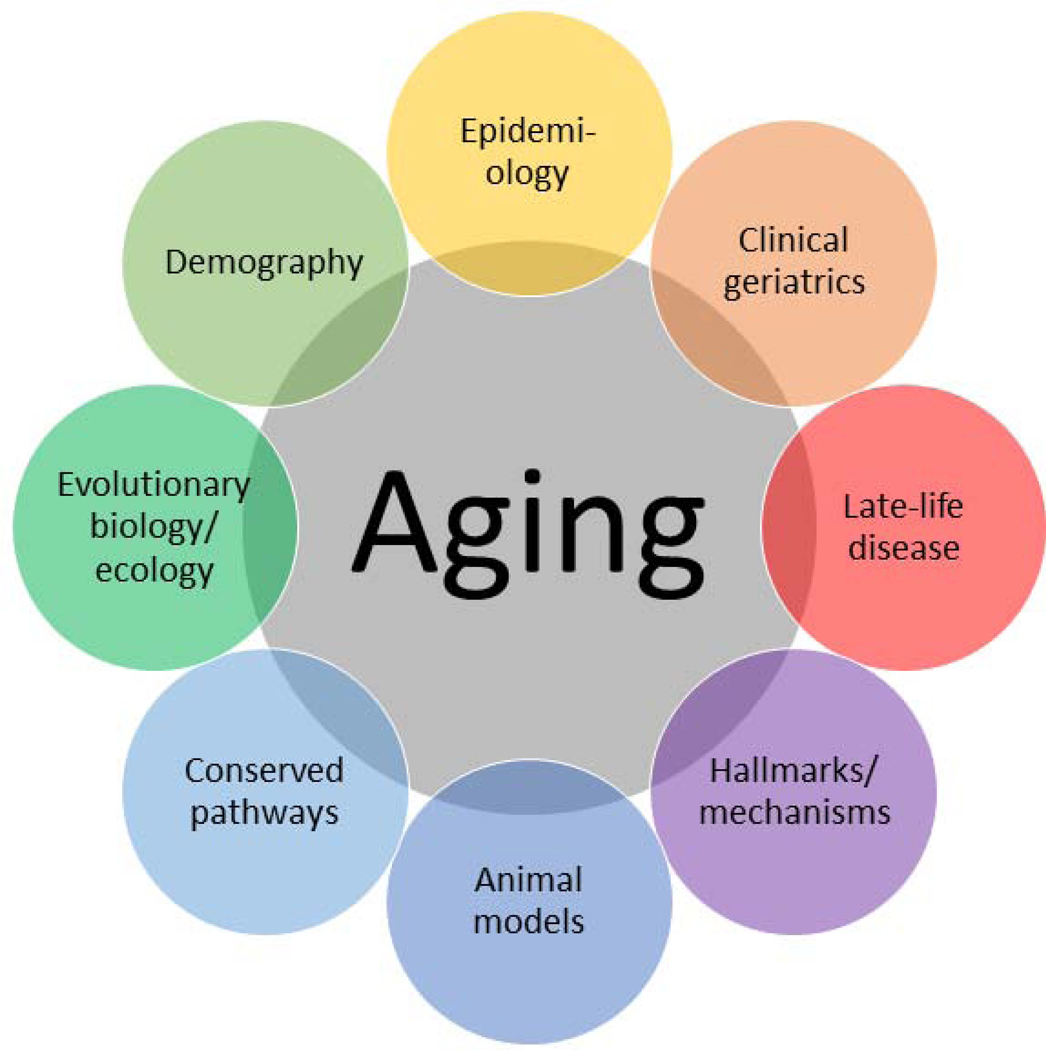
What should we do as a research community, in the face of this disagreement? We hope that simply becoming aware of the problem may stimulate researchers to consider aging in new ways, and to interact with colleagues that may have a different perspective. Beyond that, we suggest three complementary approaches that may help. First, though basic biologists tend to avoid theory, we think it is important for the field to develop an explicit theoretical paradigm. Of course there will be some disagreement, but this should largely occur within areas of the paradigm that are still unclear. This paradigm should include an attempt to link a variety of sub-disciplines, encompassing mechanistic, evolutionary, and demographic approaches to aging (e.g. 22). It should also link the conserved signaling pathways and the downstream mechanisms, and should integrate comparative, epidemiological, and clinical perspectives. Any understanding of aging cannot be considered complete if it fails to explain or disagrees with a major subdiscipline. For example, our understanding of the mechanistic basis of aging should complement and clarify our understanding of how lifespan evolves across evolutionary time in response to selection pressure, and vice-versa. It should thus be possible to construct an interdisciplinary understanding of aging progressively merging mechanistic and evolutionary aspects (Cui et al., 2019; MacRae et al., 2015). This may require the application of approaches to clearly define the questions, disagreements, and terminological confusion. Much as the Hallmarks/Pillars of Aging unified what was before a relatively fragmented landscape of mechanistic research of aging (Kennedy et al., 2014; López-Otín et al., 2013), a similar effort is probably required to unify into a single paradigm mechanistic approaches, research on conserved regulatory pathways, epidemiology, evolutionary biology, clinical geriatrics, late-life diseases, and so forth (Fig. 5).
Fig. 5:
A conceptual model of how our understanding of aging biology will need to integrate perspectives from diverse disciplines. The precise definition of disciplines is somewhat arbitrary.
Accordingly, the second approach is to bridge some of the gaps between our sub-disciplines, not just with theory, but with concrete collaborations. While there is certainly some movement of information across the sub-disciplines, there are major questions that are not being sufficiently tackled. How might a mechanistic understanding of aging impact clinical geriatrics? What can species differences in aging mechanisms tell us about the evolution of life history strategies? Beyond the construction of a paradigm, there is a need to conduct research at the interface of sub-disciplines of aging. This would present an opportunity to benefit from the productivity and effectiveness of what has been called the multidisciplinary edge effect (Varpio and MacLeod, 2019): In ecology, the edge effect refers to characteristics observed when the boundaries of two different habitats meet (e.g., when forests meet rocky outcrops). When the edges of ecosystems intersect, a greater biodiversity exists. Likewise, in research on aging biology, we might expect the most productive research questions to exist at the frontiers between our subdisciplines. These two efforts, theoretical and empirical, should nourish each other. Third and complementary, training of young researchers in aging biology should explicitly involve exposure to this multidisciplinary context, with courses and discussions designed to ensure that students are exposed to a wide range of mechanistic, genetic, evolutionary, ecological, clinical, and epidemiological perspectives.
What might an emerging paradigm look like? Ideally, it would provide a framework that largely integrates the basic knowledge from these varied domains, identifying key areas of consensus, as well as areas of continued disagreement. It would provide a coherent structure for terminology, and indeed perhaps a more precise vocabulary for words that are used in different ways in different sub-disciplines – including the word “aging” itself (Cohen et al., n.d.; Gladyshev, 2016) – might go a long way toward creating this framework. As needed, it would include a mathematical framework to ensure appropriate rigor.
We are not under any illusion that this will be an easy task. Disagreement on, say, the programmed vs. non-programmed nature of aging is unlikely to be resolved soon, and without consensus on such points it becomes difficult to also have consensus on broader questions such as how to integrate an evolutionary and mechanistic understanding of aging. However, we also argue that cross-talk between sub-disciplines will gradually clarify the points of disagreement and eventually lead to a more effective and valuable paradigm on aging biology. If such a paradigm does not emerge organically over the next several years, it may be worth considering organizing a more formal consultation process with key experts from various subdisciplines in order to generate a consensus framework.
Highlights.
A recent symposium debate highlighted disagreements and confusion in aging biology
Symposium participants followed up by completing an online survey
Survey results show little common ground on most questions in aging biology
However, there is a near-consensus that aging is heterogeneous and multifactorial
Work is needed to achieve a common paradigm in aging biology
Acknowledgements
A.A.C. is supported by a CIHR New Investigator Salary Award and is a member of the FRQ-S funded Centre de recherche du CHUS and Centre de recherche sur le vieillissement, as well as by a CIHR project grant (153011). A.M.S. is supported by a DECRA fellowship from the Australian Research Council (DE180101520). VG and VNG are supported by grants from US National Institutes of Health. D.F. is supported by NIH AG059719 and AG023717. OTN is supported by the Brazilian Council for Scientific and Technological Development (CNPq) with a research productivity grant (3035402019–2). S.U. is supported by the NIA/NIH grants R01AG062623 and R01AG070487. A.I.Y is supported by the NIA/NIH grants RF1AG046860, R01AG070487, and U19AG063893. CF and MI are supported by a grant of the Ministry of Education and Science of the Russian Federation Agreement No. 074–02-2018–330.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Austad SN, 2010. Methusaleh’s Zoo: how nature provides us with clues for extending human health span. J. Comp. Pathol. 142, S10–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, Harrington H, Israel S, Levine ME, Schaefer JD, Sugden K, Williams B, Yashin AI, Poulton R, Moffitt TE, 2015. Quantification of biological aging in young adults. Proc. Natl. Acad. Sci 112, E4104–E4110. 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV, 2013. Aging is not programmed: Genetic pseudo-program is a shadow of developmental growth. Cell Cycle. 10.4161/cc.27188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calimport SRG, Bentley BL, Stewart CE, Pawelec G, Scuteri A, Vinciguerra M, Slack C, Chen D, Harries LW, Marchant G, Alexander Fleming G, Conboy M, Antebi A, Small GW, Gil J, Lakatta EG, Richardson A, Rosen C, Nikolich K, Wyss-Coray T, Steinman L, Montine T, de Magalhães JP, Campisi J, Church G, 2019. To help aging populations, classify organismal senescence. Science (80-. ). 10.1126/science.aay7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, 2016. Complex systems dynamics in aging: new evidence, continuing questions. Biogerontology 17 10.1007/s10522-015-9584-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Legault V, Fulop T, n.d. What if there’s no such thing as “aging”? Mech. Ageing Dev. [DOI] [PubMed] [Google Scholar]
- Cui R, Medeiros T, Willemsen D, Iasi LNM, Collier GE, Graef M, Reichard M, Valenzano DR, 2019. Relaxed Selection Limits Lifespan by Increasing Mutation Load. Cell. 10.1016/j.cell.2019.06.004 [DOI] [PubMed] [Google Scholar]
- Ferraro KF, 2018. The Gerontological Imagination: An Integrative Paradigm of Aging. Oxford University Press, New York, NY. [Google Scholar]
- Gaillard JM, Lemaître JF, 2017. The Williams’ legacy: A critical reappraisal of his nine predictions about the evolution of senescence. Evolution (N. Y). 10.1111/evo.13379 [DOI] [PubMed] [Google Scholar]
- Gladyshev VN, 2016. Aging: progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell. 10.1111/acel.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladyshev VN, 2014. The free radical theory of aging is dead. Long live the damage theory! Antioxidants Redox Signal. 10.1089/ars.2013.5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith TC, 2012On the programmed/non-programmed aging controversy. Biochem. 10.1134/S000629791207005X [DOI] [PubMed] [Google Scholar]
- Horvath S, 2013. DNA methylation age of human tissues and cell types. Genome Biol. 14, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones OR, Scheuerlein A, Salguero-Gomez R, Camarda CG, Schaible R, Casper BB, Dahlgren JP, Ehrlen J, Garcia MB, Menges ES, Quintana-Ascencio PF, Caswell H, Baudisch A, Vaupel JW, 2014. Diversity of ageing across the tree of life. Nature 505, 169–173. 10.1038/nature12789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, Espeland MA, Marcovina S, Pollak MN, Kritchevsky SB, Barzilai N, Kuchel GA, 2018. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. GeroScience. 10.1007/s11357-018-0042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, 2014. Geroscience: linking aging to chronic disease. Cell 159, 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzina ED, Podolskiy DI, Dmitriev SE, Gladyshev VN, 2019. Patterns of Aging Biomarkers, Mortality, and Damaging Mutations Illuminate the Beginning of Aging and Causes of Early-Life Mortality. Cell Rep. 10.1016/j.celrep.2019.11.091 [DOI] [PubMed] [Google Scholar]
- Kowald A, Kirkwood TBL, 2016. Can aging be programmed? A critical literature review. Aging Cell. 10.1111/acel.12510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn TS, 1970. The Structure of Scientific Revolutions Second Edition, Enlarged, International Encyclopedia of Unified Science. [Google Scholar]
- Lakatos I, 2014. Falsification and the methodology of scientific research programmes, in: Philosophy, Science, and History: A Guide and Reader. 10.4324/9780203802458 [DOI] [Google Scholar]
- Levine ME, 2013. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 68, 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-León M, Goya RG, 2017. The Emerging View of Aging as a Reversible Epigenetic Process. Gerontology. 10.1159/000477209 [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae SL, Croken MMK, Calder RB, Aliper A, Milholland B, White RR, Zhavoronkov A, Gladyshev VN, Seluanov A, Gorbunova V, Zhang ZD, Vijg J, 2015. DNA repair in species with extreme lifespan differences. Aging (Albany. NY). 10.18632/aging.100866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Finkel T, 2017. Key proteins and pathways that regulate lifespan. J. Biol. Chem. 10.1074/jbc.R116.771915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varpio L, MacLeod A, 2019. Philosophy of Science Series: Harnessing the Multidisciplinary Edge Effect by Exploring Paradigms, Ontologies, Epistemologies, Axiologies, and Methodologies. Acad. Med. 10.1097/ACM.0000000000003142 [DOI] [PubMed] [Google Scholar]
- Williams GC, 1957. Pleiotropy, natural selection and the evolution of senescence. Evolution (N. Y). 11, 398–411. [Google Scholar]