Abstract
During the coronavirus disease-2019 (COVID-19) pandemic there were several barriers to treatment access and medication adherence in rheumatoid arthritis (RA) patients. There is no information regarding the RA patient health status in Egypt during the COVID-19. Thus,the aim of this work was to study the impact of the pandemic on RA patients through a patient-reported questionnaire and to determine the influence of gender, geographic regions. This multi-centre study initiated by the Egyptian College of Rheumatology (ECR) was conducted on 1037 RA patients attending rheumatology clinics from 10 governorates. The questionnaire provided covered socio-demographic data, health/disease status, information/knowledge about COVID-19 and medical/family history of the infection. Patients mean age was 44.2 ± 12.3 years;855 females and 182 males; 539(52%) from rural and 497(48%) from urban areas. 41.8% reported a striking difficulty to obtain hydroxychloroquine during the pandemic. The majority (70%) considered maintaining a regular visit to the rheumatologist in addition to remote contact mainly by phone (44.4%) or via WhatsApp (33.1%), in particular among male and urban patients. Urban patients were more likely to be infected by COVID-19 (12.9% vs 6.2%; p < 0.0001) than rural. Northern cities had more patients with suspected COVID-19 (13.9% vs 6.1%; p < 0.0001); was significantly associated with more disease flares (30.8% vs 5.8%) with subsequent change in the RA treatment (20.9% vs 6.4%; p < 0.0001). Patients with RA faced remarkable difficulty to obtain their medications with subsequent change in their disease status. The challenges of the pandemic have hastened changes in the way we deliver health care.
Electronic supplementary material
The online version of this article (10.1007/s00296-020-04736-9) contains supplementary material, which is available to authorized users.
Keywords: Coronavirus Disease 2019 (COVID-19), Rheumatoid arthritis, Multi-centre, Patient-reported questionnaire, Infection, North-south gradient
Introduction
Rheumatoid arthritis (RA) is the most common inflammatory arthritis [1] that can cause damage to the joints. It can also present with extra-articular manifestations, affecting other major organs [2]. Infections are the most important comorbidities associated with increased morbidity and mortality [1]. Despite the tremendous improvement that brought to its management, RA patients still confronting many challenges. Methotrexate (MTX) and hydroxychloroquine (HCQ) are key player medications in the management of RA with a high safety profile and a relatively low rate of discontinuation [3]. In Egyptian patients, the adherence rate was 62.5% and was higher (80%) in those receiving the medications within the first 6 months [4].
The rheumatology community in Egypt is now facing the most significant global public health care lifetime challenge, coronavirus disease 2019 (COVID-19) pandemic, that rapidly changed personal and professional outlooks [5]. Egyptian rheumatologists agreed to their key emerging frontline role in treating COVID-19. In a recent study conducted by the Egyptian College of Rheumatology (ECR), potential changes in rheumatology outpatient practice by staff members evolved since the COVID-19 pandemic [6]. Although HCQ may have favorable anti-viral and anti-inflammatory properties, it should be carefully used in the context of COVID-19 infection [7]. None of the university rheumatology physicians have prescribed HCQ to prevent or treat COVID-19 in a non-hospitalized patient who was not previously on it. COVID-19 pandemic is certainly conditioning the treatment strategy of RA, whose infectious risk is increased because of an overall impairment of the immune system combined with the effect of using steroids and immunosuppressives [8].
The impact of the COVID-19 infection was negative on the quality of life of RA patients in an African country and stress from being infected or having a flare contributed to this deterioration; the economic effect was significantly associated with temporary unemployment, decrease in monthly income, and drug discontinuation [9].COVID-19 infection worsens the outcome of RA patients and its severity is associated with pre-existing diseases, especially cardiac and renal, which may cause failure or even death [10]. A subset of patients, with severe COVID-19, develops profound inflammation and multi-organ dysfunction consistent with a “Cytokine Storm Syndrome” (CSS) [11].
During COVID-19, there are several barriers to treatment access and medication adherence in RA patients. Cultural and illness beliefs were noted to play an important role in how RA patients in various communities’ approach health care and are challenging [12]. The COVID-19 pandemic has triggered the unexpected implementation of telemedicine in the management of rheumatic diseases (RDs). Now patient-reported questionnaires may help ensure an optimum disease control and address the concerns of RA patients [13]. To date, no previous work evaluated the effect of COVID-19 pandemic on RA patients’ health status from the patient perspective. The aim of the present work was to study the impact of the COVID-19 pandemic on RA from a multi-centre patient-reported questionnaire and to determine the influence of gender and geographic distribution on the infection.
Patients and methods
The study included RA cases aged 18 years and older diagnosed according to the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria [14] and were followed up by Academic staff members at various locations: University teaching hospitals, medical Insurance hospitals and private practice clinics during the first wave of the pandemic (June through August 2020) as an initiative of the Egyptian College of Rheumatology. All participants were provided with detailed information about the study. An informed consent from the patients was provided. The work conforms to the ethical standards of the 1995 Helsinki declaration and was approved by the local institutional ethical committees of the corresponding universities.
The idea of the questionnaire (Online Appendix I) was proposed by the first and last authors. The form was constructed by the first author and was then revised, refined and adapted by the rest of the authors. It was provided to the patients in Arabic language after being translated. To validate the survey, a pilot test was run and a revision of the accuracy of the translation, survey design, content, terms, comprehension and easiness to fill in was performed by 5 Rheumatology staff members. Areas covered by the questionnaire include patients’ sociodemographic data, health/disease status, information and knowledge about COVID-19 pandemic and medical and family history of the COVID-19 infection. A “yes or no” response was provided and all questions were close-ended (only 2 questions in the ‘History of the COVID-19 infection’ section were open-ended) and the response was according to the mentioned instructions. Patients were contacted (face-to-face) and the questionnaire was explained to each patient and trade name examples of the generic names of medications were provided. Patients of low education were guided to fill in the form. It was designed so as to be easily filled in by the patient and takes an average of 5 − 10 min. Answers were recorded and transferred into an excel sheet.
Statistical analysis: Data were analyzed using SPSS version 25.0. Continuous data were expressed as mean ± standard deviation or frequency and percentages. Normality of distribution was assessed using the Shapiro–Wilk test. Student’s t and Chi square tests were considered for comparison of the normal distributed variables of two groups, Mann–Whitney test for comparison of the non-normal distributed variables, and ANOVA for more than two groups. Regression analysis was conducted to determine the independent risk factors predicting COVID-19 infection and the coefficient with 95% confidence interval (CI) was determined. p < 0.05 was considered significant.
Results
The work initially included 1108 cases, and after excluding incomplete or invalid patients’ data as well as juvenile cases, 1037 adult RA patients remained. The mean age of 44.2 ± 12.3 years (17–85 years) and 855 was females and 182 males (F:M 4.7:1). They were from 10 governorates: 11.9% from Cairo, 5.6% Kalyoubia, 13.7% the Delta region (Mansoura and Tanta), 11.5% from Alexandria and 57.4% were from Upper Egypt (Fayoum, Beni-Suef, Minia, Assiut and Sohag). The patients were 539 (52%) from rural and 497 (48%) from urban areas. The health status of the patients involving the medications received, availability and their contact with the rheumatologist are presented in Table 1. The mean disease duration was 8.3 ± 6.9 years. In addition to the presented findings, several cases reported associated thyroid dysfunction, secondary Sjögren, and fibromyalgia syndrome. There are 182 (17.5%) patients who reported receiving other medications for the associated chronic diseases and comorbidities especially hypertension and diabetes.
Table 1.
Sociodemographic and health status of the patients involving the medications received, availability and their contact with the rheumatologist
| Parameters | RA patients (n = 1037) N (%) |
|
|---|---|---|
| Sociodemographic parameters | ||
| Age (mean ± SD) | 44.2 ± 12.3 | |
| Sex (Female) | 855 (82.4) | |
| Education level | ||
| Low | 278 (26.8) | |
| Moderate | 466 (44.9) | |
| High | 293 (28.3) | |
| Special habits | ||
| Tea/Coffee | 771 (74.3) | |
| Smoking | 81 (7.8) | |
| Alcohol | 0 (0) | |
| Addiction | 13 (1.25) | |
| Health Status during COVID-19 pandemic parameters | ||
| Medications used | ||
| NSAIDs | 880 | (84.9) |
| Steroids | 466 | (44.9) |
| Methotrexate | 664 | (64) |
| CQ/HCQ | 661 | (63.7) |
| Sulfasalazine | 180 | (17.4) |
| Azathioprine | 11 | (1.1) |
| Leflunomide | 382 | (36.8) |
| Biologics | 156 | (15) |
| Mode to obtain medications | ||
| Self-payment | 457 | (44.1) |
| Medical Insurance | 298 | (28.7) |
| Governmental supply | 331 | (31.9) |
| Regularity of intake | 798 | (76.9) |
| Difficulty to obtain the drug | 608 | (58.6) |
| Methotrexate | 64 | (6.2) |
| CQ/HCQ | 433 | (41.8) |
| Leflunomide | 11 | (1.1) |
| Biologics | 22 | (2.1) |
| All medications | 75 | (7.2) |
| Disease affected by drug shortage | 422 | (40.7) |
| Regular contact with the rheumatologist | 726 | (70) |
| Modes of Remote Contact | ||
| Phone | 460 | (44.4) |
| 343 | (33.1) | |
| Messenger | 42 | (4.1) |
| Telegram | 18 | (1.7) |
| Physician’s website/page | 36 | (3) |
| Tele-video meeting | 18 | (1.7) |
| Associated Diseases/Comorbidities | ||
| Diabetes mellitus | 105 | (10.1) |
| Hypertension | 185 | (17.8) |
| Cardiovascular | 27 | (2.5) |
| Chest problems | 33 | (3.2) |
| Renal problems | 12 | (1.2) |
| Hepatic problems | 27 | (2.6) |
| Allergy problems | 51 | (4.9) |
NSAIDs non-steroidal anti-inflammatory drugs, CQ chloroquine, HCQ hydroxychloroquine, RA rheumatoid arthritis
Protective measures taken for COVID-19, source of information, influence of the pandemic and history of infection are presented in Table 2. The job was affected in 470 (45.3%) patients. Only 11 travelled abroad during the preceding 60 days and 181 were in contact with individuals just arriving from out of the country. Most patients reported more frequent hand washing when possible. Sporadic cases reported receiving HCQ tablets as further protective measure while others considered frequent hot drinks. The complementary therapeutic agents and natural herbs were received by some patients and involved mainly vitamin C, nutritional supplements. Among 70 (6.8%) patients who required hospitalization, 30 (42.9%) were mainly due to disease activity and 26 (37.1%) due to COVID-19 infection. Preventive measures followed by the patients when they had a relative with COVID-19 infection included considering the governmental lockdown measures, staying at home, self-isolation, social distancing, wearing the mask, using disinfectants, regular hand wash.
Table 2.
Protective measures taken for COVID-19, source of information and influence of the pandemic
| During COVID-19 pandemic parameters |
RA patients (n = 1037) n (%) |
|
|---|---|---|
| Work affected | 470 | (45.3) |
| Mild | 207 | (20) |
| Moderate | 88 | (8.5) |
| Severe | 129 | (12.4) |
| Lost | 46 | (4.4) |
| Protective measures taken | ||
| Staying at home | 612 | (59) |
| Wearing a mask | 812 | (79.3) |
| Keeping social distancing | 597 | (57.6) |
| Using disinfectants/alcohol | 492 | (47.4) |
| Source of information on COVID-19 | ||
| Physicians | 428 | (41.3) |
| Friends | 424 | (40.9) |
| Media | 775 | (74.7) |
| Social media | 370 | (35.7) |
| Websites | 153 | (14.8) |
| Others (relatives/MOH/at work) | 21 | (2) |
| Consider their knowledge sufficient | 408 | (39.3) |
| Stopped or reduced taking NSAIDs | 171 | (16.5) |
| Considered complementary therapy | 228 | (22) |
| Received HCQ for prevention | 127 | (12.2) |
| Needed hospitalization | 70 | (6.8) |
| Activity | 30 | (42.9) |
| COVID-19 | 26 | (37.1) |
| Others* | 14 | (20) |
| Found Difficulty in Hospitalization | 48 | (4.6) |
| History of COVID-19 Infection | ||
| Close relatives affected | 320 | (30.9) |
| Protective measures considered | 310 | (29.9) |
| Patient with suspected COVID-19 | 97 | (9.4) |
| Free after treatment | 38 | (39.2) |
| Carrier | 29 | (29.9) |
| Still infected | 30 | (30.9) |
| The disease was affected (flare) | 123 | (11.9) |
| Change in the RA medications | 85 | (8.2) |
NSAIDs: non-steroidal anti-inflammatory drugs, HCQ: hydroxychloroquine, RA: rheumatoid arthritis, and MOH: Ministry of health. *Others: include respiratory distress and exacerbated asthma in 3, diabetic coma in 2, renal colic/stone in 2, hematologic dyscrasias in 2, uncontrolled hypertension, myocardial infarction, very low potassium, meniscal tear and abortion in one patient each
Patients who had suspected COVID-19 considered measures mostly in harmony with the protocols updated by the Egyptian ministry of health (MOH) whether at hospital or at home and would include paracetamol, azithromycin antibiotic, HCQ, vitamin C and zinc supplements as well as oseltamivir by few. Corticosteroids were considered in the management plan of one case. Sporadic cases were also provided acetyl cysteine and rivaroxaban.
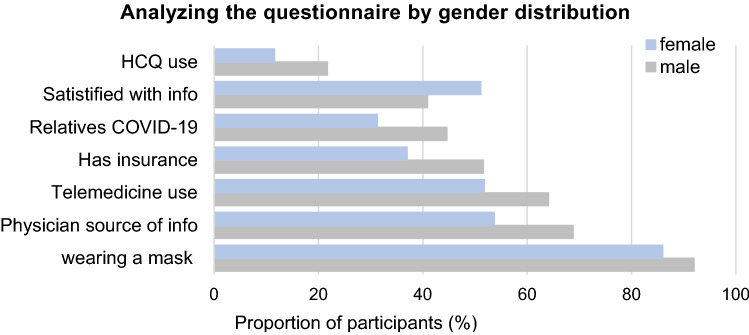
Figure 1 shows the differences between males and females in response to the questionnaire. While, males would use phone and messenger for remote contact with the rheumatologist (64.1% vs 51.8%; p = 0.006 and 13.4% vs 5.5%; p = 0.032), wearing a mask (92% vs 86%; p = 0.016), consider HCQ as a preventive measure (21.7% vs 11.6%; p = 0.003), females would feel more satisfied with the information known (51.1% vs 40.9%; p = 0.028).
Fig. 1.
The frequency of the response to questionnaire in Egyptian rheumatoid arthritis patients by gender
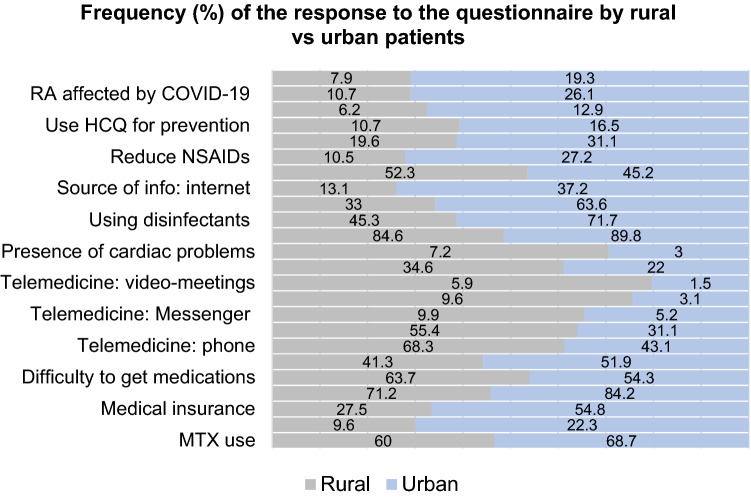
Regarding the disparities between patients from rural and urban areas, it was found that rural patients were of older age (45.8 ± 12.6 vs 42.4 ± 11.8 years; p < 0.0001) and of lower education and economic status (p < 0.0001). More urban patients had their work affected (p < 0.0001) and came in contact with individuals recently arrived from abroad (25.9% vs 14.4%, p < 0.0001). Detailed descriptions of differences in the response to the questionnaire between rural and urban are presented in Fig. 2.
Fig. 2.
The frequency of the response to questionnaire in Egyptian rheumatoid arthritis patients by residence
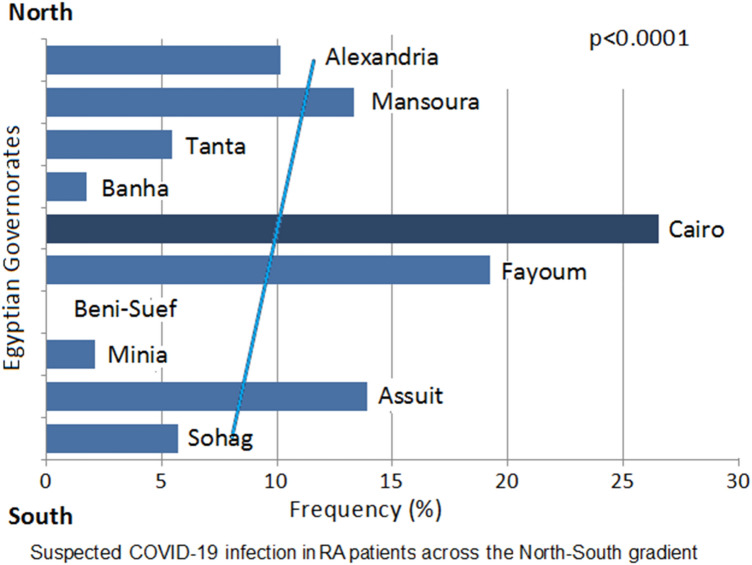
On considering the patients from Upper Egypt vs Lower Egypt (from Cairo across the Delta region till Alexandria), the age and gender were comparable (p = 0.82 and p = 0.79, respectively). Upper Egypt patients were significantly of lower education and had a shorter disease duration (7.5 ± 5.5 years vs 9.4 ± 8.2 years) (p < 0.0001). Patients from Upper Egypt were more in regular contact with their rheumatologist (77.8% vs 66.3%; p < 0.0001). The proportion of questionnaire results by geographic regions (Upper vs. Lower Egypt) is presented in Fig. 3. Northern cities had more patients were infected by COVID-19 (13.9% vs 6.1%; p < 0.0001). The COVID-19 pandemic was significantly associated with more disease flares (30.8% vs 5.8%) with subsequent change in the RA treatment regimen (20.9% vs 6.4%; p < 0.0001). The distribution of suspected COVID-19 patients according to the North–South gradient is presented in Fig. 4.
Fig. 3.
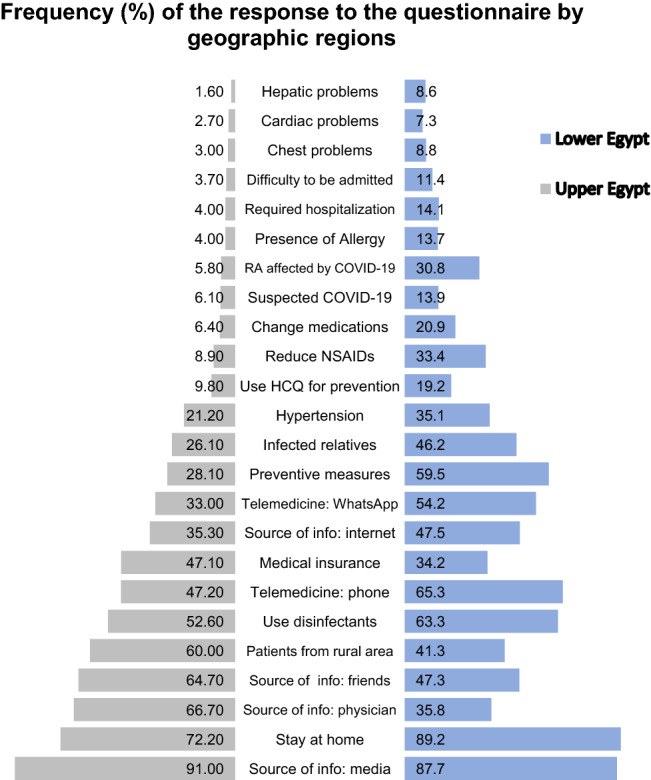
The frequency of the response to the questionnaire in Egyptian rheumatoid arthritis patients according to the geographic regions
Fig. 4.
The frequency of suspected coronavirus disease 2019 (COVID-19) infection in Egyptian rheumatoid arthritis patients along the north = south gradient of the country governorates
To further analyze the independent factors that may influence or predict suspected COVID-19 infection in Egyptian RA patients, a regression analysis was performed considering the following independent factors: demographic features, disease duration, medications received, mode of payment, adherence and regularity of visits to the rheumatologist, in contact with persons arriving from abroad, recently travelled, presence of co-morbidities and applying protective measures. Significant risk factors included age (95%CI: 0 to 0.004; p = 0.03), being from upper Egypt (95%CI: 0.029 to 0.128; p = 0.002), urban residency (95%CI: -0.085to -0.002; p = 0.042), intake of leflunomide (95%: 0.004 to 0.088; p = 0.031), receiving biologics (95% CI 0.008 to 0.153; p = 0.02), irregular intake of medications (95% CI − 0.096 to − 0.001; p = 0.045), presence of renal (95% CI 0.065 to 0.675; p = 0.017) and chest (95% CI 0.001 to 0.263; p = 0.037) co-morbidities and travelling over the last 60 days (95% CI 0.225 to 0.61; p < 0.0001).
Discussion
Rheumatoid arthritis is one of the leading causes of chronic morbidity in developing nations [15]. Developing countries have fewer resources for managing the medical, surgical and social consequences of RA, more often seen in patients presenting with established disease. To address the current critical public health issue, we assessed the impact of the COVID-19 pandemic on patients with RA from a multi-centre patient-reported questionnaire. To the best of our knowledge, no equivalent questionnaire has been conducted in other Middle East countries and till present, there are no other questionnaires incorporating the patient’s perspective in the assessment of the impact of COVID-19 on the health status.
This work included 1037 adult RA patients from 10 governorates that were alike from rural and urban areas and the majority was of moderate education level and economic class. 7.8% were smoker and 1.3% declared consuming alcoholics or addictive drugs. Smoking was not increased as most of the patients were females, however, passive smoking, is a main risk factor for the development, activity and severity of RA [16]; yet, details on the habits were lacking.
A substantial number of patients (41.8%) reported a striking difficulty to obtain HCQ during the pandemic, and consequently, about 40.7% of patients reported uncontrolled/flare of their disease status. HCQ was ineffective for COVID-19 prophylaxis in RA [17] and in response to the growing shortage amid the pandemic, there has been an incessant search for alternatives in treatment of RA [18]. Moreover, the doubts raised about the likely side effects lead to the urgent situation arising from patients’ fear of taking antimalarials during these COVID-19 times [19]. Recurrence of COVID-19 was reported with long-term use of HCQ, leflunomide, and steroids and was paradoxically aggravated after use of tocilizumab [20]. Data for other drugs are conflicting or incomplete and the rationale for their use is based on the immune system runaway and the secretion of pro-inflammatory cytokines in severe forms of the disease [21]. Even though several national authorities have defined patients under immunosuppressive therapy as at risk for severe COVID-19, it is currently unknown whether immunosuppressive and/or immunomodulating agents, such as biologics, affect the rate and the outcome of COVID-19 infections in RDs patients [22]. Thus, a call for careful consideration and close monitoring in the administration of biologics in RDs patients with COVID-19 is warranted [20].
The existing COVID-19 pandemic has heightened the need to care for patients with RA in an increasingly virtual environment. Many rheumatology clinics were closed or had only limited face-to-face appointments due to social distancing restrictions and health system surge capacity planning [23]. During the pandemic, 70% of the patients considered maintaining a regular visit to the rheumatologist in addition to remote contact mainly by phone or via the WhatsApp. Using tele-video meetings was limited to 1.7%. The risk of patients with rheumatic diseases of having a more severe clinical course if they become infected with the COVID-19 infection is very high and mandates the establishment of telemedicine programs [24]. The workflow of rheumatology outpatient clinics worldwide has changed. The complexity of the care of RA patients implies challenges in the evaluation of new patients and follow-up [25]. How the rheumatology patients were managed during this period was similar to outlines considered in many areas of the world, as patients’ visits were spaced and were instructed to follow up remotely [26]. The ACR recently recommended that RA disease activity and functional status measures can be adapted for use in telehealth settings to support high-quality clinical care [23]. Social media platforms are becoming major players in the era of COVID-19 [27].
The present questionnaire revealed that 9.4% contracted suspected COVID-19 infection. 11.9% of the patients reported a flare due to the pandemic and 8.2% had a change in their RA medication regimen. Features of COVID-19 pneumonia could be challenging to differentiate from idiopathic or RA-related interstitial lung disease (ILD) [28]. The documented COVID-19 case in Egypt on August 31, 2020 is around 1 per 1000 of the population [29] and that is due to shortage in the screening the numbers could have been higher. In this work, estimation of likely or suspicious COVID-19 among RA patients could explain the possibly low officially announced numbers and at the same time confirms the extra fear these vulnerable patients are in or the true higher risk to infection that they face. As there is conflicting evidence of a higher incidence of COVID in patients with autoimmune rheumatic diseases, maintaining the ongoing treatment regimens was supported. However, once infected, there is a small but significant increased risk of mortality. Older age, urban residency, irregular intake of medications, renal and chest co-morbidities were significant risk factors for COVID-19 infection in RA patients. Moreover, intake of leflunomide and biologics increased the hazard.
In this work, only 6.8% of RA patients reported the need for hospitalization mainly due to a flare of the disease or COVID-19 infection and most of those cases found difficulty to be admitted. RA patients with severe COVID-19 and who warranted hospitalization were significantly more likely to be older and have comorbid hypertension, chronic obstructive pulmonary disease, diabetes, renal failure and end-stage renal disease [30, 31]. Data from the COVID-19 Global Rheumatology Alliance physician-reported registry including 600 rheumatic diseases cases from 40 countries revealed that 9% died and that 46% were hospitalized. Hospitalization was associated with daily intake of steroids, was reduced in those receiving anti-TNF and did not differ in those exposed to DMARDs or NSAIDs[32]. Current global proposals advocate that patients with rheumatic diseases on immunosuppressives should not stop glucocorticoids during COVID-19 infection, although minimum potential doses may be used. Disease-modifying drugs should be continued [33].
Data from previous outbreaks showed the importance of incorporating a gender analysis into response efforts to improve the effectiveness of health interventions and promote gender and health equity goals. There were overall gender differences in response to many of the patient-reported questions. Males significantly would use phone and messenger for remote contact with the rheumatologist, wearing a mask, would gain information through the physician, and consider complementary therapy and HCQ as a preventive measure. Females were more likely to be satisfied with the information known about the pandemic. RA women desire more information than men and placed higher importance on most topics [34]. Given their front-line interaction with communities, the WHO Executive Board recognizing the need to include women in decision-making for outbreak preparedness and response.
Rural patients were of lower education and fewer were receiving biologics with lower adherence to medications and faced more difficulty to get them; however, the disease was less affected by the shortage of the treatments. Unsurprisingly, urban patients were using more remote contact options. More urban cases were suspected to be infected by COVID-19 with consequent affection of the disease and change in their medications. In line with the present study, the frequency of adherent patients was comparable and rural residence was among the most important factors associated with non-adherence to medications in Egyptian RA cases [4]. Similarly, in Romania, urban RA patients had a significantly higher frequency of biologic access than those from rural areas [35]. Rural clinic location was associated with lower use of biologics suggesting a possible prescription bias or patient preferences due to convenience [36].
Patients from Upper Egypt were more from rural areas, and more in regular contact with their rheumatologist. Furthermore, fewer patients were suspected to be infected by COVID-19. It seems that there are unrevealed factors that could be considered accountable for the decreased frequency of COVID-19 infection in those from upper Egypt compared to those from the capital and northern cities, such as the hot weather, less vibrant lifestyle and moderate challenging circumstances. RA patients are more prone to have anxiety, depression and cognitive impairment [2].
This study represents the largest sample to date of national RA patient-reported disease outcomes during COVID-19 pandemic from rheumatology practices across Egypt. Despite the strengths of the current study, its limitations should be addressed. The small number of cases reported by various governorates which affected the accuracy of reported infected cases. The main limitation is that the self-assessment process often differs from the accreditation process. The duration of conducting the survey should have been more limited to avoid the dramatic changes in the characteristics of the COVID-19 pandemic and surrounding circumstances. On the other hand, being filled in by the patients, it was not possible to include essential variables, such as the disease activity. In addition, self-reported answers may be subjected to various biases may affect the results like, embarrassment.
In summary, using data from multi-centre patient-reported outcomes questionnaire, we reported the largest sample to date. We found a substantial number of patients faced a striking difficulty to obtain their medications with subsequent change in their disease status. The challenges of the pandemic have hastened changes in the way we deliver care and raised the need to introduce the tele-medicine in the health care service. It is important to consider social determinants variables, such as education, economic, environment and access to care, that influence patients’ differential response to infection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the colleagues who assisted in executing the questionnaire and work: Dr. NouraGhareeb and Dr. Samar Ali (assistant lecturers Internal Medicine and Rheumatology, Tanta University); Dr. Salwa Moussa (lecturer of Rheumatology, Ain Shams University); Dr. AsmaaKotb (assistant lecturer Rheumatology, Minia University), Dr. Aya Ahmed and Dr. Reem Gad (residents Rheumatology, Minia University); Dr. Ayman Eid (assistant lecturer Rheumatology, Beni-Suef University).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thomas K, Vassilopoulos D. Infections in patients with rheumatoid arthritis in the era of targeted synthetic therapies. Mediterr J Rheumatol. 2020;31(1):129–136. doi: 10.31138/mjr.31.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lwin MN, Serhal L, Holroyd C, Edwards CJ. Rheumatoid arthritis: the impact of mental health on disease: a narrative review. Rheumatol Ther. 2020;7:457–471. doi: 10.1007/s40744-020-00217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anvari B. Leading causes of methotrexate and antimalarial drugs discontinuation in Iranian patients with rheumatoid arthritis. Egypt Rheumatol. 2016;38:147–152. doi: 10.1016/j.ejr.2015.12.003. [DOI] [Google Scholar]
- 4.Ragab OM, Zayed HS, Abdelaleem EA, Girgis AE. Effect of early treatment with disease-modifying anti-rheumatic drugs and treatment adherence on disease outcome in rheumatoid arthritis patients. Egypt Rheumatol. 2017;39:69–74. doi: 10.1016/j.ejr.2016.11.004. [DOI] [Google Scholar]
- 5.McInnes IB. COVID-19 and rheumatology: first steps towards a different future? Ann Rheum Dis. 2020;79:551–552. doi: 10.1136/annrheumdis-2020-217494. [DOI] [PubMed] [Google Scholar]
- 6.Gheita TA, Salem MN, Eesa NN, et al. Rheumatologists’ practice during the Coronavirus disease 2019 (COVID-19) pandemic: a survey in Egypt. Rheumatol Int. 2020 doi: 10.1007/s00296-020-04655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahouati M, Mériglier E, Martin L, et al. COVID-19 infection also occurs in patients taking hydroxychloroquine. J Antimicrob Chemother. 2020;75:2014–2015. doi: 10.1093/jac/dkaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favalli EG, Ingegnoli F, De Lucia O, et al. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev. 2020;19:102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zomalheto Z, Assogba C, Dossou-yovo H. Impact of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection and disease-2019 (COVID-19) on the quality of life of rheumatoid arthritis patients in Benin. Egypt Rheumatol. 2020 doi: 10.1016/j.ejr.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C, Wang Z, Li J, et al. Implications of SARS-CoV-2 infection for patients with rheumatic disease. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218050. [DOI] [PubMed] [Google Scholar]
- 11.England JT, Abdulla A, Biggs CM, et al. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar K, Dubey S, Samanta A, et al. COVID-19 and ethnicity: challenges in rheumatology. Rheumatol Oxf Engl. 2020;59:1802–1803. doi: 10.1093/rheumatology/keaa329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor PC. Adopting PROs in virtual and outpatient management of RA. Nat Rev Rheumatol. 2020;16(9):477–478. doi: 10.1038/s41584-020-0449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 15.Rudan I, Sidhu S, Papana A, et al. Prevalence of rheumatoid arthritis in low- and middle-income countries: a systematic review and analysis. J Glob Health. 2015;5:010409. doi: 10.7189/jogh.05.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammam N, Gheita TA. Impact of secondhand smoking on disease activity in women with rheumatoid arthritis. Clin Rheumatol. 2017;36:2415–2420. doi: 10.1007/s10067-017-3795-2. [DOI] [PubMed] [Google Scholar]
- 17.Hydroxychloroquine ineffective as a preventive antiviral against COVID-19, study finds. In: ScienceDaily. https://www.sciencedaily.com/releases/2020/08/200817124914.htm
- 18.Husayn SS, Brown JD, Presley CL, et al. Hydroxychloroquine alternatives for chronic disease: response to a growing shortage amid the Global COVID-19 Pandemic. J Pharm Pract. 2020 doi: 10.1177/0897190020942658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos-Moreno P, Buitrago-Garcia D, Villarreal L, et al. Emergency arising from patients’ fear of taking antimalarials during these COVID-19 times: are antimalarials as unsafe for cardiovascular health as recent reports suggest? Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218259. [DOI] [PubMed] [Google Scholar]
- 20.Cai S, Sun W, Li M, Dong L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin Rheumatol. 2020;39:2797–2802. doi: 10.1007/s10067-020-05234-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grange L, Guilpain P, Truchetet M-E, et al. Challenges of autoimmune rheumatic disease treatment during the COVID-19 pandemic: a review. Therapie. 2020;75:335–342. doi: 10.1016/j.therap.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulze-Koops H, Krueger K, Inka Vallbracht I, et al. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218075. [DOI] [PubMed] [Google Scholar]
- 23.England BR, Barber CEH, Bergman M, et al. Brief Report: adaptation of American College of Rheumatology Rheumatoid Arthritis Disease Activity and functional status measures for telehealth visits. Arthritis Care Res. 2020 doi: 10.1002/acr.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos Moreno P, Chafez-Chafez J, Hernández-Zambrano SM, et al. Experience of telemedicine use in a big cohort of patients with rheumatoid arthritis during COVID-19 pandemic. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218165. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa-Parra G, Gamboa-Alonso CM, Galarza-Delgado DA, et al. Challenges and opportunities in telerheumatology in the COVID-19 era. Response to: “Online management of rheumatoid arthritis during COVID-19 pandemic” by Zhang. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217631. [DOI] [PubMed] [Google Scholar]
- 26.Gheita TA, Kenawy SA. Egypt’s groundwork blessing during the COVID-19 pandemic curse: rheumatologic experience. Eur J Rheumatol. 2020;7:S134–S136. doi: 10.5152/eurjrheum.2020.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed S, Zimba O, Gasparyan AY. Moving towards online rheumatology education in the era of COVID-19. Clin Rheumatol. 2020;17:1–8. doi: 10.1007/s10067-020-05405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manfredi A, Luppi F, Cassone G, et al. Pathogenesis and treatment of idiopathic and rheumatoid arthritis-related interstitial pneumonia. The possible lesson from COVID-19 pneumonia. Expert Rev Clin Immunol. 2020;16:751–770. doi: 10.1080/1744666X.2020.1803064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egypt Coronavirus: 104,516 Cases and 6,052 Deaths - Worldometer. https://www.worldometers.info/coronavirus/country/egypt/. Accessed 11 Oct 2020
- 30.Haberman RH, Castillo R, Chen A, et al. COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and DMARDs on clinical outcomes. Arthritis Rheumatol. 2020 doi: 10.1002/art.41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji W, Huh K, Kang M, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35(25):e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020;39:2055–2062. doi: 10.1007/s10067-020-05073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrie RA, Walker JR, Graff LA, et al. Gender differences in information needs and preferences regarding depression among individuals with multiple sclerosis, inflammatory bowel disease and rheumatoid arthritis. Patient Educ Couns. 2019;102:1722–1729. doi: 10.1016/j.pec.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Codreanu C, Popescu CC, Mogoşan C. Area of residence and socioeconomic factors reduce access to biologics for rheumatoid arthritis patients in Romania. Biomed Res Int. 2018;2018:7458361. doi: 10.1155/2018/7458361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Movahedi M, Joshi R, Rampakakis E, et al. Impact of residential area on the management of rheumatoid arthritis patients initiating their first biologic DMARD: results from the Ontario Best Practices Research Initiative (OBRI) Medicine (Baltimore) 2019;98:e15517. doi: 10.1097/MD.0000000000015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.