In this systematic review and meta‐analysis of 86 studies and 526,641 individuals without viral hepatitis and hepatic steatosis, the ALT upper threshold was 39 in overweight patients versus 28 in normal‐weight individuals, and 36 in diabetics versus 33 in nondiabetics. These findings were validated in a multicenter cohort of 6,058 patients. Diabetic and overweight patients with ALT values within these thresholds can potentially avoid extensive and possibly harmful invasive investigations after excluding viral hepatitis and nonalcoholic fatty liver disease. However, close monitoring and management of metabolic factors are required.

Abstract
The current alanine aminotransferase (ALT) upper limit of normal was defined using selected healthy Caucasian blood donors. Given the global rise in obesity and different body habitus in Asians, we aimed to perform a systematic review and meta‐analysis combined with bootstrap modeling and individual patient data validation to estimate the ALT upper threshold for Asians, including the overweight and diabetics. We included studies from PubMed, Embase, and Cochrane database searches that identified individuals without known liver diseases (i.e., viral hepatitis, alcohol, and ultrasound‐detected nonalcoholic fatty liver disease). The mean ALT (U/L) was estimated using a random‐effects mixed model and upper threshold (95th‐percentile value, U/L) via a bootstrap model with 10,000 resamples. We screened 4,995 studies and identified 86 studies that reported ALT values for 526,641 individuals without excessive alcohol intake or known liver diseases, yielding a mean ALT of 19 and ALT upper threshold of 32. The ALT upper threshold was 37 in males versus 31 in females, 39 in overweight versus 28 in normal‐weight individuals, and 36 for diabetics versus 33 for nondiabetics. We validated our study level data with individual patient level data in 6,058 individuals from five study centers in Japan. Consistent with our study‐level data, we found that the ALT upper threshold in our individual patient data analysis was indeed higher in overweight versus normal‐weight individuals (39 vs. 32) and in diabetics versus nondiabetics (42 vs. 33). Conclusion: We provide validated reference ranges for ALT upper threshold derived from Asians without known liver disease, including individuals with ultrasound‐detected nonalcoholic fatty liver disease who are normal weight, overweight, nondiabetic, and diabetic, to inform practice.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine aminotransferase
- BMI
body mass index
- CHB
chronic hepatitis B
- CI
confidence interval
- DM
diabetes mellitus
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IQR
interquartile range
- MetS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- ULN
upper limit of normal
Serum alanine aminotransferase (ALT) levels are markers of hepatocyte injury and are the most commonly used tests in routine practice to assess liver disease.( 1 , 2 ) However, there is no universally accepted upper limit of normal (ULN) threshold. A conventional value of 40 U/L has generally been used,( 3 , 4 ) and this threshold was established in the 1980s before routine hepatitis C virus (HCV) testing.( 5 , 6 ) In 2002, the ULN of 40 U/L without distinction between males and females was challenged by Prati et al., who, using data from healthy Italian blood donors, proposed an updated ULN level for ALT with separate thresholds for males and females (30 U/L and 19 U/L, respectively).( 7 ) In addition to the exclusion of donors with positive anti‐HCV antibody, this study comprehensively excluded patients with increased risk for liver disease, such as those with significant alcohol drinking as well as those with higher risk for nonalcoholic fatty liver disease (NAFLD), such as those with elevated body mass index (BMI), cholesterol, and glucose levels. Thus, this study included only very healthy individuals and not just individuals free of liver disease. The low ALT thresholds have also been validated in the Asian settings, with a Korean study describing a ULN of 33 IU/L for men and 25 IU/L for women, based on biopsy‐proven normal livers as well as a Taiwanese study of a healthy population proposing even lower ULN of 21 IU/L and 17 IU/L for men and women respectively.( 8 , 9 ) However, it remains unclear what the “normal” or “expected” ALT range for individuals with metabolic disorders, but without identifiable liver disease, would be.
We hypothesize that even without ultrasound‐detectable hepatic steatosis, the presence of diabetes mellitus (DM) and obesity results in elevated ALT levels. Nonalcoholic steatohepatitis (NASH) has traditionally been thought to be a result of an oxidative stress reaction to triglyceride accumulation in the liver, but an increasing body of evidence points toward the metabolites of fatty acids as the true culprits of hepatocellular injury, rather than steatosis, which may be a bystander.( 10 , 11 , 12 ) Defining the “expected” upper threshold of ALT for the metabolically active populations is important for several reasons, particularly in areas endemic for both NAFLD and chronic hepatitis B (CHB), such as Asia.( 13 ) First, the decision for antiviral therapy often rests on thresholds for the ULN of ALT, which the American Association for the Study of Liver Diseases (AASLD) has proposed as 35 U/L for males and 25 U/L for females, adapted from the 30 U/L and 19 U/L thresholds from Prati et al.( 14 ) Therefore, data on expected ULNs for individuals with metabolic disease may challenge the current thresholds for institution of antivirals for patients with concomitant CHB and stimulate further studies. Second, the large population with metabolic diseases continues to grow rapidly, and data on expected ULN may help to streamline evaluation in search of liver disease. In 2000, according to the WHO Global Health Observatory, 12.1% of Indians, 20.8% of Chinese nationals, and 36.8% of Malaysians were overweight.( 15 ) In 2016, those figures have risen to 19.7%, 32.3%, and 42.5%, respectively.( 15 ) As a result, there is now an epidemic of metabolic syndrome (MetS) in Asia, with an overall estimate of 37.1% of individuals in Asia afflicted, and up to 49% in certain countries.( 16 ) Third, Asians tend to develop NAFLD at a lower BMI,( 17 ) and there is a wide variation for the prevalence of NAFLD genes across ethnicities( 18 ) hence, there is an unmet need to evaluate the effect of the metabolic syndrome on ALT levels within Asia.
Therefore, the primary purpose of this study was to provide the distribution and expected upper threshold for ALT in Asians without known liver disease (viral hepatitis, alcohol‐related liver disease, and ultrasound‐detectable NAFLD), but with and without various components of MetS. The secondary aim of this study was to describe the variation of ALT mean and upper threshold across Asian countries.
Patients and Methods
The protocol of this review was preregistered in PROSPERO (CRD42019135468). The study was carried out using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses standards.( 19 )
Search Strategy
We recently published a systematic review and meta‐analysis( 20 ) in which we searched PubMed, Embase, and the Cochrane Library databases up to January 17, 2019, for original research studies that defined NAFLD and provided data for NAFLD prevalence, incidence, and/or outcomes in Asia without other overlapping liver diseases or significant alcohol consumption. Details of this search strategy were previously published( 20 ) and are provided in the Supporting Information. From the results of this search, we identified studies that provided sufficiently detailed ALT data for the population without significant alcohol consumption and without known liver diseases including NAFLD. We included studies that (1) provided data for mean or median ALT levels, (2) demonstrated the absence of hepatic steatosis based on ultrasound, (3) excluded hepatitis B and C virus infection, and (4) excluded individuals with excessive alcohol consumption. We excluded studies that (1) demonstrated absence of hepatic steatosis by modalities other than ultrasound or (2) the cohort had any identifiable liver disease. The literature search did not have a language restriction, but all included articles were published in English; hence, translation was not needed.
Systematic Literature Review, Data Extraction, and Quality Assessment
We created a case report form specifically for this study for systematic study review/selection and structured data extraction. Two of the following three authors (D.H., E.T., or Y.H.Y.) independently reviewed, selected, and extracted relevant study data. ALT values for studies that included patients with various metabolic states (i.e., normal body weight, overweight, diabetic, and nondiabetic) were recorded. The individual study definition by BMI for overweight and obese varied from study to study, ranging from ≥23 kg/m2 to 25 kg/m2. For the purpose of this study, we defined categorized overweight as those with BMI ≥ 23 kg/m2 to 25 kg/m2, because all studies grouped overweight and obese individuals together, and the remainder as normal weight for meta‐analyzed data. For analysis of individual patient data, we categorized BMI into two groups, normal weight and overweight (<23 kg/m2 and ≥23 kg/m2), to match the meta‐analysis data. Discordance between the two reviewers and data extractors was resolved by discussion and/or by consultation with a third and senior investigator (M.H.N.).
We created a quality assessment tool based on the Newcastle‐Ottawa Scale (NOS) to grade the quality of the included studies.( 21 ) The NOS assesses quality in three domains, selection, comparability and outcome, with a maximum of four stars, two stars, and three stars per domain. Studies with seven or more stars have a low risk of bias, four to six stars have a moderate risk of bias, and three or less stars have a high risk of bias.
Individual Patient Data
To validate the findings of the meta‐analysis, we obtained individual patient data from five centers in Japan (Kumamoto University, Kumamoto; Ogaki Municipal Hospital, Ogaki; Eguchi Hospital Health Center, Saga; Kawamura Clinic Health Center, Hiroshima; and Kochi Medical School Hospital, Kochi). All included patients had an ultrasound that did not show steatosis, and all had negative serology for hepatitis B virus (HBV) and HCV. Patients with known liver disease and/or significant alcohol consumption were also excluded. The data from Kumamoto University was obtained from a health screening program performed by the Japanese Red Cross Kumamoto Health Care Center, Kumamoto (May 2003‐April 2012).( 22 ) The data from Ogaki Municipal Hospital was obtained from clinic records of consecutive patients who presented for either health screening or with a medical problem (March 2010‐September 2015). The data from the remaining three study centers were obtained from individuals who received a general health check‐up in 2009 to 2010 in one of three health centers: Eguchi Hospital Health Center in Saga Prefecture, Kawamura Clinic Health Center in Hiroshima Prefecture, and Kochi Medical School Hospital in Kochi Prefecture.( 23 , 24 ) Data on age, sex, ALT levels, BMI, DM, as well as cholesterol levels were collected. The study was performed in accordance with the Declaration of Helsinki( 25 ) and was approved by the institutional review board at each study center.
Statistical Analysis
Analysis of Study‐Level Data
All ALT means were estimated by pooling the data using a random intercepts reduced maximum likelihood model, in which the weight assigned to each study is the inverse of the within‐study variance plus the between‐study variance.( 26 ) For each model, random effects considered for individual study with the published ALT values served as the modeled response. For studies that reported median and interquartile range (IQR), we converted the median into mean based on the assumption that the distribution of data was symmetrical, as the sample size of the included studies were large and liver diseases were excluded. We considered this assumption by performing sensitivity analysis to estimate ALT upper threshold using only data from studies that provided mean ALT levels. We assessed heterogeneity using I 2 statistic. Estimates with I 2 ≥ 50% and P value of <0.05 in Q‐statistic were considered to have moderate to severe heterogeneity. We determined the pooled mean ALT in the following populations: overall (mixed, not focusing on a particular subgroup), males, females, overweight individuals, normal‐weight individuals, diabetics, and nondiabetics.
The upper threshold of ALT across the various populations was determined using an unrestricted bootstrap model with mean plus two SDs. Using the ALT data provided in the studies included in the meta‐analysis, the bootstrap model generated 10,000 resampled (sample rate = 1) data sets to estimate the mean ALT and the SD around the mean. Resampled percentile estimates were then pooled, from which the mean ALT 95th‐percentile value was derived. We refer to the ULN as the upper threshold for the purpose of this study, because the threshold may not necessarily be “normal” or “healthy” in general in the groups with metabolic disease. We performed sensitivity analyses to include only studies that also excluded other etiologies of liver diseases (besides viral hepatitis, ultrasound‐detected NALFD, and significant alcohol use). Sensitivity analyses were also performed to exclude studies in which the mean ALT and SD were not reported in the primary studies but derived from reported median and IQR, and finally by the risk of bias in the study.
We performed a subanalysis to evaluate the pooled mean ALT levels and bootstrapped estimates of upper threshold by sex and by metabolic subgroups (males, females, overweight individuals, normal‐weight individuals, diabetics, and nondiabetics). To evaluate the hypothesis that ALT levels may have risen over time, we performed subanalyses to estimate the pooled mean ALT levels as well as bootstrapped estimates of upper threshold for studies with a median study year before 2010 and compared them against studies with a median study year from 2010 onward.
To evaluate the effect of BMI and cholesterol levels on ALT, we performed meta‐regression using study‐level data. In addition, we used Egger’s test to assess for publication bias. All meta‐analyses were conducted using the meta packages in R statistical software (version 3.5.1) and bootstrap modeling using SAS (Ver. 9.4; SAS Institute, Cary, NC).
Analysis of Individual Patient‐Level Data
We used descriptive statistics to calculate the mean ALT with SD and median for the total data set and for subgroups by sex and by presence of metabolic disease. Based on the mean and SD, the 95th‐percentile ALT (mean + two SDs) value was then calculated. All analyses of individual patient‐level data were performed by Stata 15.1 (StataCorp, College Station, TX).
Results
Study Selection
From full‐text review of the total of 237 articles in the previous systematic review and meta‐analysis, we selected 86 articles (526,641 individuals) that met the inclusion and exclusion criteria for the current study (Fig. 1). Of these 86 articles, 59 articles provided data for the overall ALT mean and 95th‐percentile values, while some provided data for ALT means and upper thresholds for the various subgroups. Some studies provided data for more than one of these analyses. The quality assessment of each article can be found in Supporting Tables S1 and S2.
FIG. 1.
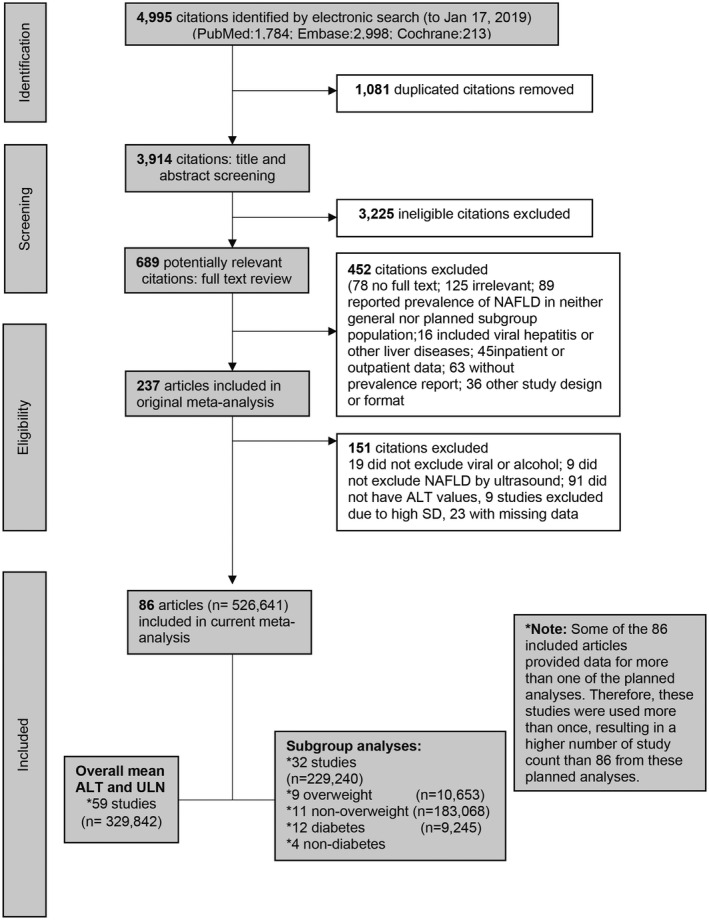
Flow chart of systematic literature search and screening for analysis of ALT values in patients without known liver disease.
Overall Cohort ALT Mean and Upper Threshold
In total, we included 59 studies (329,842 individuals) for the overall ALT mean and upper threshold analysis, of which 51 (86.4%) were of good quality, 8 (13.6%) were of moderate quality, and none were of poor quality. Most of the individuals came from Korea (n = 157,232, 47.7%), followed by mainland China (n = 138,059, 41.9%). The characteristics of the included studies are found in Supporting Table S3. The pooled mean ALT of the overall study population was 19 U/L (95% confidence interval [CI]: 19‐20) (Table 1). The bootstrapped estimate of ALT upper threshold in the overall study population was 32 U/L (95% CI: 29‐34) (Table 1).
TABLE 1.
Pooled ALT Means and Estimated Upper Thresholds in the Overall Population and by Sex
| Population | Number of Studies | Number of Participants | Pooled ALT Mean (U/L) ‡ | 95% CI | ALT Upper Threshold (95th Percentile) (U/L) |
|---|---|---|---|---|---|
| Overall | 59 | 329,842 | 19 | 19‐20 | 32 |
| Sensitivity analysis | |||||
| Only studies that also excluded additional liver diseases* | 26 | 167,656 | 19 | 18‐20 | 31 |
| Only studies with reported mean ALT † | 43 | 255,855 | 21 | 20‐21 | 34 |
| Only studies with low risk of bias | 51 | 326,224 | 19 | 19‐20 | 31 |
| Male | 11 | 25,914 | 22 | 20‐25 | 37 |
| Sensitivity analysis | |||||
| Only studies that also excluded additional liver diseases* | 5 | 10,764 | 19 | 16‐ 22 | 36 |
| Only studies with reported mean ALT † | 6 | 6,944 | 23 | 20‐26 | 37 |
| Only studies with low risk of bias | 10 | 25,763 | 23 | 19‐24. | 35 |
| Female | 13 | 51,357 | 18 | 17‐19 | 31 |
| Sensitivity analysis | |||||
| Only studies that also excluded additional liver diseases* | 7 | 20,034 | 17 | 16‐18 | 27 |
| Only studies with reported mean ALT † | 13 | 51,357 | 18 | 17‐19 | 32 |
| Only studies with low risk of bias | 13 | 51,357 | 18 | 17‐19 | 32 |
In addition to exclusion of HBV and HCV via serology, alcohol by defined criteria (30 g/day for males and 20 g/day for females), and NAFLD by ultrasound, these studies excluded additional liver diseases such as hepatotoxic drugs, autoimmune liver disease, metabolic liver disease, and cirrhosis.
Studies with ALT converted from median were not included.
All I 2 > 98.0% and all P values for I 2 < 0.05.
Subgroup ALT Mean and Upper Threshold
By Sex
A total of 11 studies provided ALT data specifically for 25,914 males, and 13 studies provided ALT data specifically for 51,357 females (Supporting Table S4A,B). The pooled ALT mean was higher in males compared with females: 22 U/L (95% CI: 20‐25) versus 18 (95% CI: 17‐19) (P = 0.0034) (Table 1). The corresponding bootstrapped upper threshold estimate for males was also higher than that of females: 37 U/L versus 31 U/L (Table 1).
By BMI
Nine studies provided ALT data specifically in 10,653 overweight individuals (Supporting Table S4C). Eleven studies provided ALT data in 183,068 normal‐weight individuals (Supporting Table S4D). The pooled mean ALT was significantly higher in overweight individuals compared with normal‐weight individuals: 25 U/L (95% CI: 20‐29) versus 16 U/L (95% CI: 14‐19) (P < 0.001) (Table 2). The ALT upper threshold was also higher in overweight individuals compared with normal‐weight individuals: 39 U/L versus 28 U/L (Table 2).
TABLE 2.
Pooled ALT Means and Estimated Upper Thresholds, by Metabolic Subgroups
| Population | Number of Studies | Number of Participants | Pooled ALT Mean (U/L) † | 95% CI | ALT Upper Threshold (95th Percentile) (U/L) |
|---|---|---|---|---|---|
| Normal weight | 11 | 183,068 | 16 | 14‐19 | 28 |
| Sensitivity analysis | |||||
| Only studies with reported mean ALT* | 9 | 175,930 | 18 | 15‐20 | 30 |
| Only studies with low risk of bias | 11 | 183,068 | 17 | 15‐ 19 | 29 |
| Overweight/obese | 9 | 10,653 | 25 | 20‐29 | 39 |
| Sensitivity analysis | |||||
| Only studies with reported mean ALT* | 8 | 7,415 | 25 | 20‐30 | 40 |
| Only studies with low risk of bias included | 8 | 10,581 | 21 | 17‐26 | 33 |
| No DM | 4 | 26,274 | 19 | 17‐22 | 33 |
| Sensitivity analysis | |||||
| Only studies with low risk of bias | 4 | 26,274 | 19 | 17‐22 | 34 |
| DM | 12 | 9,245 | 22 | 20‐25 | 36 |
| Sensitivity analysis | |||||
| Only studies with reported mean ALT* | 8 | 4,011 | 25 | 21‐29 | 39 |
| Only studies with low risk of bias | 5 | 4,371 | 24 | 20‐28 | 44 |
Studies with ALT converted from median were not included.
All I 2 > 98.0% and all P values for I 2 < 0.05.
By Presence of DM
Twelve studies provided data specifically for 9,245 diabetic individuals, and four studies provided data for 26,274 nondiabetic individuals (Table 2 and Supporting Table S4E,F). The bootstrapped estimate of ALT upper threshold was significantly higher in diabetics compared with nondiabetics: 36 U/L versus 33 U/L (Table 2).
By Country
Fifty‐nine studies from eight countries provided ALT data for patients without known liver disease (NAFLD and viral hepatitis): Mainland China (26 studies, n = 138,059), South Korea (20 studies, n = 157,232), Taiwan (5 studies, n = 28,909), Japan (4 studies, n = 4,863), Iran (1 study, n = 289), Israel (1 study, n = 228), India (1 study, n = 155), and Malaysia (1 study, n = 107). The pooled mean ALT estimates ranged from 19 U/L (95% CI: 18‐20) for China to 28 U/L (95% CI: 16‐30) for Malaysia (Table 3). The highest ALT upper threshold estimate was for Iran at 40 U/L, and the lowest was for Israel at 27 U/L; however, data for both these countries were limited with only one study for each country and fewer than 300 study participants in each. The upper threshold for ALT for China, South Korea, Taiwan, and Japan were 32 U/L (95% CI: 27‐37), 31 U/L (95% CI: 29‐33), 38 U/L (95% CI: 36‐61), and 37 U/L (95% CI: 24‐164), respectively. Figure 2 presents data for bootstrapped ALT upper threshold estimates and by country.
TABLE 3.
Pooled ALT Means, by Country
| Population | Number of Studies | Number of Participants | Pooled ALT Mean* (U/L) | 95% CI | P |
|---|---|---|---|---|---|
| By country | < 0.001 † | ||||
| 0.47 ‡ | |||||
| Mainland China | 26 | 138,059 | 19 | 18‐20 | |
| South Korea | 20 | 157,232 | 19 | 18‐20 | |
| Taiwan | 5 | 28,909 | 20 | 17‐24 | |
| Japan | 4 | 4,863 | 21 | 18‐24 | |
| Iran | 1 | 289 | 24 | 22‐26 | |
| Israel | 1 | 228 | 20 | 19‐21 | |
| India | 1 | 155 | 23 | 22‐23 | |
| Malaysia | 1 | 107 | 28 | 16‐30 |
I 2 > 98% for all country analyses with more than one study, and all P values for I 2 < 0.05.
Between all available countries.
Between countries with four or more studies (Mainland China, South Korea, Taiwan, and Japan).
FIG. 2.
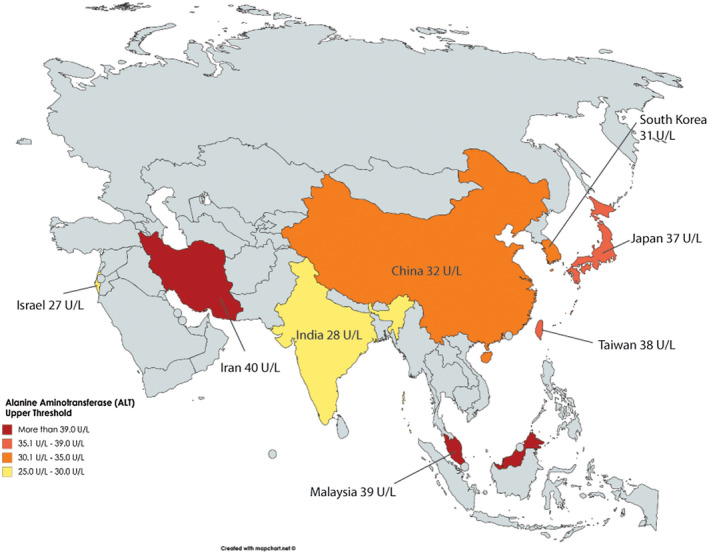
Bootstrapped estimates of ALT upper threshold (95th percentile), by country.
By Time Period
Twenty‐eight studies (179,868 individuals) had a median study year earlier than 2010, and 31 studies (149,974 individuals) had a median study year from 2010 onward. There were no statistically significant differences in the pooled mean ALT levels (20 U/L vs. 19 U/L, P = 0.283) or in the bootstrapped upper thresholds (33 U/L vs. 32 U/L) between studies before 2010 compared to those from 2010 and after (Supporting Table S5).
Meta‐regression: Relationship Between ALT With BMI and Other Metabolic Factors
Meta‐regression of ALT showed significant correlation with study‐level data average BMI readings. The coefficient was 1.20 (95% CI: 0.16‐2.25, P = 0.024), meaning that for each 1‐kg/m2 increase in BMI, the ALT was 1.2 U/L higher (Table 4).
TABLE 4.
Meta‐regression of ALT Against BMI and Other Metabolic Factors
| Coefficient | 95% CI | P | |
|---|---|---|---|
| BMI | 1.20* | 0.16‐2.25 | 0.024 |
| Fasting plasma glucose | 0.02 | −0.26‐0.30 | 0.87 |
| Total cholesterol | 0.034 | −0.06‐0.13 | 0.48 |
| Low‐density lipoprotein | 0.00 | −0.17‐0.17 | 0.98 |
| High‐density lipoprotein | −0.02 | −0.30‐0.26 | 0.88 |
| Triglyceride | 0.05 | −0.01‐0.11 | 0.10 |
For each 1‐kg/m2 increase in BMI, the ALT was 1.2 U/L higher.
Validation With Individual Patient Data
We validated our findings from meta‐analysis and bootstrap modeling with individual patient‐level data from 6,058 individuals who had negative serology for hepatitis B and C virus and did not have ultrasound‐detected NAFLD or significant alcohol history. Close to half (41.2%) of this cohort were male, and the mean age was 52.1 ± 12.8 years. Most (71.2%) had normal weight (BMI < 23), and about one‐quarter (28.8%) were overweight (BMI ≥ 23). Close to half had hyperlipidemia (43.4%), and 12.3% had DM. As indicated in Table 5, the ALT upper threshold for the total cohort (95th percentile) was 34 U/L overall, 39 U/L for males, and 30 U/L for females, closely approximating the overall meta‐analyzed data of 32, 37, and 31 U/L, respectively. Among the individuals with low metabolic risk (those with normal weight, no DM, and no hyperlipidemia), the upper threshold of ALT was 30 U/L overall, 35 U/L for males, and 28 U/L for females.
TABLE 5.
Individual Patient Data Analysis of ALT Upper Threshold (95th Percentile)
| Groups | Overall | Male | Female | |||
|---|---|---|---|---|---|---|
| Number of Individuals | ALT Upper Threshold (U/L) | Number of Individuals | ALT Upper Threshold (U/L) | Number of Individuals | ALT Upper Threshold (U/L) | |
| Overall | 6,058 | 34 | 2,495 | 39 | 3,563 | 30 |
| Low metabolic risk* | 2,521 | 30 | 857 | 35 | 1,664 | 28 |
| DM | 748 | 42 | 479 | 44 | 269 | 37 |
| No DM | 5,310 | 33 | 2,016 | 38 | 3,294 | 29 |
| Hyperlipidemia | 2,630 | 36 | 1,123 | 40 | 1,507 | 31 |
| No hyperlipidemia | 3,428 | 33 | 1,372 | 38 | 2,056 | 28 |
| BMI < 23 | 4,314 | 32 | 1,521 | 36 | 2,793 | 29 |
| BMI ≥ 23 | 1,744 | 39 | 974 | 44 | 770 | 32 |
| BMI < 23; DM | 408 | 39 | 242 | 40 | 166 | 36 |
| BMI ≥ 23; DM | 340 | 48 | 237 | 50 | 103 | 39 |
| BMI < 23; no DM | 3,906 | 31 | 1,279 | 35 | 2,627 | 29 |
| BMI ≥ 23; no DM | 1,404 | 38 | 737 | 41 | 667 | 32 |
Low‐risk: BMI < 23, no diabetes, no hyperlipidemia.
Also similar to the meta‐analyzed data, the ALT upper threshold for those with DM was higher than those without DM (42 U/L vs. 33 U/L) (Table 5). Within the DM and non‐DM subgroups, the ALT upper threshold was consistently higher in males compared with females (44 vs. 37 U/L and 38 vs. 29 U/L, respectively). Similar findings were observed for the hyperlipidemic and nonhyperlipidemic groups.
Similar to the meta‐analysis data, ALT upper threshold for overweight individuals was higher than normal‐weight individuals (39 U/L vs. 32 U/L) (Table 5). ALT upper thresholds were again higher in males compared with females within each of the BMI/weight subgroups. Even when stratified for the presence of DM, the ALT upper thresholds remained consistently higher in the overweight versus normal‐weight groups.
In addition, the mean ALT values in this individual patient cohort were consistent with the meta‐analyzed data (Supporting Table S6), with higher values in males compared with females overall (21 U/L vs. 16 U/L), in those with DM compared to those without DM (21 U/L vs. 18 U/L), in those with hyperlipidemia compared to those without hyperlipidemia (19 U/L vs. 17 U/L), and in the overweight group compared with normal‐weight group (21 U/L vs. 17 U/L) (all P < 0.001).
Sensitivity Analyses
In the multiple sensitivity analyses for pooled ALT, the means and bootstrapped estimates of the upper threshold that removed (1) studies that did not exclude other etiologies of liver diseases besides exclusion of viral hepatitis, NAFLD, and significant alcohol use, (2) studies in which the median/IQR values were converted to mean/SD, and (3) studies that were at moderate to high risk of bias (Tables 1 and 2), the upper threshold and pooled mean ALT remained higher in males compared with females, consistent with the data in the main analysis (Table 1).
For the comparison between normal‐weight to overweight individuals, our sensitivity analyses also demonstrated similar results to the main analysis for ALT upper threshold and mean ALT (Table 2). When only studies at low risk of bias were analyzed, the ALT upper threshold was also higher in those with DM compared to those without DM (44 U/L vs. 34 U/L) (Table 2).
Heterogeneity and Publication Bias
There was considerable heterogeneity among the studies for the overall and subgroup ALT results (all I 2 statistic ≥98.00). Egger’s test was not suggestive of significant publication bias in the overall and subgroup analysis (P = 0.18).
Discussion
Globalization, urbanization, and westernization of Asian diets have all led to a rise in obesity and MetS in Asia.( 27 , 28 , 29 ) In this meta‐analysis, from data involving 86 studies and 526,641 individuals without significant alcohol use, ultrasound‐detected NAFLD, and viral hepatitis, we found that the ALT upper threshold was higher in overweight and diabetic individuals at 39 U/L and 36 U/L, respectively (vs. 28 U/L and 33 U/L, respectively, in normal‐weight and nondiabetic individuals). This study complements the information provided by Prati et al. in 2002, when they proposed lower ALT ULN in patients with no known liver disease and no risk factors for metabolic disease, whereas we propose the “expected” ALT ULN or upper threshold in overweight individuals and in diabetics.( 7 ) However, this phenotypic‐oriented approach toward a higher ALT ULN does not mean that the liver is entirely healthy. Rather, it should alert care providers and patients that the metabolic disease present is likely having an effect on liver health, even though a specific liver‐disease diagnosis such as NAFLD cannot be made through liver ultrasound, the accepted diagnostic standard for NAFLD in most practice settings currently.
Despite the absence of NAFLD based on ultrasound findings, obesity and DM still led to higher ALT levels. Lipid droplets formed by accumulating triglycerides were previously thought to be the driver of NASH, but a growing body of evidence suggests that the formation of lipid droplets is a parallel process, and triglycerides may even be a protective mechanism against progressive liver disease.( 30 , 31 ) On the other hand, the true drivers of NASH may be the metabolites of free fatty acids, such as phosphatidic acid, ceramides, and diacylglycerol.( 32 , 33 ) Hence, while steatosis may not have been detected on ultrasound, the presence of DM and obesity may have already resulted in “nontriglyceride lipotoxic liver injury” and resulted in an elevation of ALT levels.( 11 ) A prospective trial that involved healthy patients in the intervention arm eating two meals of fast food a day resulted in a significant ALT elevation with minimal change in liver fat content.( 34 )
Another possible factor to explain the raised ALT values despite the absence of steatosis in these patients with metabolic disease is the fact that ultrasound is only sensitive for steatosis when more than 33% of hepatocytes are steatotic.( 35 ) NAFLD is most commonly diagnosed by abdominal ultrasound, and it will not be feasible to perform liver biopsy on all patients. A recent meta‐analysis found in patients with over 20%‐30% hepatic steatosis, using liver biopsy as the gold standard, ultrasound had a pooled sensitivity of 84.8% and a pooled specificity of 93.6%.( 36 ) Therefore, patients with MetS without known NAFLD by ultrasound evaluation may actually already have NAFLD, but under the ultrasound‐detection threshold they are at risk for future NAFLD development. It is not clear whether those with MetS, elevated ALT, but undetectable NAFLD would be at higher risk for full‐blown NAFLD and/or NASH development, but this warrants additional investigation.
A defined ALT upper threshold for diabetic and overweight patients can potentially help to streamline investigations for elevated ALT readings. In a large population‐based study consisting of 95,977 individuals from Scotland, only 3.9% of individuals with an abnormal ALT were found to have significant liver disease within 5 years of the test.( 37 ) On the other hand, while the expected upper thresholds for diabetic and obese individuals are higher, this does not mean that those with these higher ALT levels are “healthy,” and clinicians should still be wary of alternative causes for borderline raised ALT levels, such as drug‐induced liver injury, viral hepatitis, and alcohol. A recent long‐term cohort study shows that ALT above a “low cutoff” (30 U/L for men, 19 U/L for females) has good predictive power for future development of liver‐related mortality, HCC, and cirrhosis.( 38 ) In diabetic or overweight patients with “elevated ALT” but still below our proposed upper threshold, one could adopt a judicious approach toward investigating for less common etiologies for liver disease. However, there should be increased effort toward controlling the metabolic risk factors and follow‐up for the subsequent development of ultrasound‐detectable NAFLD, as these patients are at higher risk for developing NAFLD and cardiovascular disease. However, caution must still be exercised in screening these additional donors for infectious diseases such as HCV and HBV.
Patients with concomitant HBV and DM or obesity may be prescribed unnecessary antiviral treatment when stringent ALT ULNs are used without considering the impact of metabolic risk factors on ALT. In 2018, based on the ALT ULN by Prati, the updated AASLD CHB guidance proposed the ALT ULNs of 35 U/L for men and 25 U/L for women.( 39 ) Our data help to support these higher ALT thresholds, but our data also suggest that different thresholds be considered for normal weight versus overweight and nondiabetic versus diabetic, in addition to the distinction between men and women. It is of interest that among all three major international liver societies’ guidelines on HBV treatment, only the AASLD guideline uses values that are gender‐specific and has the lowest ALT threshold for HBV antiviral therapy. None of the current guidelines take weight or DM into consideration for the ALT treatment threshold. Given the high prevalence of CHB infection and MetS in Asia, further trials are warranted to define the optimal ALT for antiviral therapies in patients with CHB with MetS. In addition, there may also be a “dose‐dependent” relationship that is dependent on how many components of MetS are present. For example, we found higher ALT levels in patients who were overweight and diabetic compared with those who were overweight but not diabetic.
This meta‐analysis provides the most comprehensive assessment to date of estimated upper threshold and mean ALT in Asians, including groups with metabolic disorders such as diabetic and overweight patients. A limitation of this study is a lack of data from large Asian countries such as Philippines and Thailand, limiting the generalizability to these areas. Countries such as Malaysia, India, Iran, and Israel only had one study each, limiting the robustness of the bootstrapped upper‐threshold estimate for these countries. There is high heterogeneity among studies, which is common in a meta‐analysis of this size. There could have been selection bias in some of the analyses due to the small number of published studies, such as in the nondiabetic subgroup, with only four studies. This may also explain why there was no statistically significant difference in the pooled mean ALT between diabetics and nondiabetics, although a significant difference was found in the validation cohort using individual patient data. Additionally, we were not able to subcategorize the results between overweight and obesity within the meta‐analysis data, as all included studies reported overweight and obese patients as a single group. The individual study definitions of overweight varied from ≥23 kg/m2 to 25 kg/m2, and a standard definition could not be applied across all studies. We were also unable to evaluate for the effect of hyperlipidemia on ALT levels, as there were insufficient studies segregating their cohorts by the presence of hyperlipidemia, but the data provided by the individual patient cohort did suggest an association between hyperlipidemia and ALT. A further limitation would be the variability of ALT readings among different laboratories, which is largely related to the different chemical analyzers used.( 40 ) Although we only included studies that excluded individuals with excessive alcohol intake, we recognize that alcoholic liver disease is frequently underestimated, and some of the included individuals may have undeclared excessive alcohol intake.
In summary, this large meta‐analysis with bootstrap modeling provides reference ranges for ALT upper threshold in Asian overweight and diabetic individuals, and its findings are validated by our individual patient‐level data (see Supporting Fig. S1). It should also be emphasized that these higher ALT ULN thresholds in metabolically active individuals do not necessarily mean “healthy,” Therefore, obese and/or diabetic Asian patients with ALT levels within this newly defined upper threshold should be monitored for subsequent development of detectable NAFLD as well as nonliver complications, and due diligence should still be exercised by clinicians to exclude common causes for mildly raised ALT such as alcohol and viral hepatitis. This study also challenges the concept of using the same ALT threshold for all patients with CHB infection using only categorization for sex, and additional studies are needed to correlate CHB histologic activities with ALT in the setting of metabolic diseases for patients of diverse race/ethnicities and geographic region/countries.
Introductory Statement
Overweight or diabetic Asian individuals without fatty liver on ultrasound have higher ALT levels compared with healthy individuals.
Supporting information
Supplementary Material
Acknowledgment
The map of Asia was created using mapchart.net.
Potential conflict of interest: Dr. Cheung received grants from Gilead. Dr. Huang received grants from Exxon Mobil. Dr. S.G. Lim advises, is on the speakers’ bureau, and received grants from Gilead and Roche. He advises and is on the speakers’ bureau for Abbott. He advises Springbank and Kaleido. He received grants from Sysmex, Fibronostics, and Merck. Dr. Nguyen advises, received grants from, received research support, and received honorarium from BMS and Gilead. She advises, received grants from, and received research support from Janssen. She advises and received honorarium from Intercept, Roche, Dynavax, and Alnylam. She advises and received research report from Lab for Advance Medicine and Exact Sciences. She advises Novartis, Eisai, Bayer, and Spring Brank. She received grants from NCI and B.K. Kee Foundation. Dr. Toyoda is on the speakers’ bureau of AbbVie, MSD, and Bayer. Dr. Wong consults for and received grants from Gilead. He consults for 3V‐BIO, AbbVie, Allergan, Boehringer Ingelheim, Center for Outcomes Research in Liver Diseases, Echosens Intercept, Janssen, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, TARGET‐NASH, and Terns.
References
Author names in bold designate shared co‐first authorship.
- 1. Karmen A, Wróblewski F, LaDue JS. Transaminase activity in human blood. J Clin Invest 1955;34:126‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017;112:18‐35. [DOI] [PubMed] [Google Scholar]
- 3. Pratt DS, Kaplan MM. Evaluation of abnormal liver‐enzyme results in asymptomatic patients. N Engl J Med 2000;342:1266‐1271. [DOI] [PubMed] [Google Scholar]
- 4. Ruhl CE, Everhart JE. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology 2012;55:447‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alberti A, Morsica G, Chemello L, Cavalletto D, Noventa F, Pontisso P, et al. Hepatitis C viraemia and liver disease in symptom‐free individuals with anti‐HCV. Lancet 1992;340:697‐698. [DOI] [PubMed] [Google Scholar]
- 6. Puoti C, Magrini A, Stati T, Rigato P, Montagnese F, Rossi P, et al. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology 1997;26:1393‐1398. [DOI] [PubMed] [Google Scholar]
- 7. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1‐10. [DOI] [PubMed] [Google Scholar]
- 8. Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim Y‐S, et al. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology 2010;51:1577‐1583. [DOI] [PubMed] [Google Scholar]
- 9. Wu W‐C, Wu C‐Y, Wang Y‐J, Hung H‐H, Yang H‐I, Kao W‐Y, et al. Updated thresholds for serum alanine aminotransferase level in a large‐scale population study composed of 34 346 subjects. Aliment Pharmacol Ther 2012;36:560‐568. [DOI] [PubMed] [Google Scholar]
- 10. Thaler H. Relation of steatosis to cirrhosis. Clin Gastroenterol 1975;4:273‐280. [PubMed] [Google Scholar]
- 11. Neuschwander‐Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 2010;52:774‐788. [DOI] [PubMed] [Google Scholar]
- 12. Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998;114:842‐845. [DOI] [PubMed] [Google Scholar]
- 13. Polaris Observatory Collaborators . Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383‐403. [DOI] [PubMed] [Google Scholar]
- 14. Terrault NA, Lok ASF, McMahon BJ, Chang K‐M, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization . Overweight and obesity. http://www.who.int/gho/ncd/risk_factors/overweight/en/. Accessed Jun 3, 2019. [Google Scholar]
- 16. Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the asia‐pacific region: a systematic review. BMC Public Health 2017;17:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei JL, Leung JC‐F, Loong TC‐W, Wong GL‐H, Yeung DK‐W, Chan RS‐M, et al. Prevalence and severity of nonalcoholic fatty liver disease in non‐obese patients: a population study using proton‐magnetic resonance spectroscopy. Am J Gastroenterol 2015;110:1306‐1314, quiz 1315. [DOI] [PubMed] [Google Scholar]
- 18. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non‐alcoholic fatty liver disease in Asia, 1999‐2019: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol 2019;4:389‐398. [DOI] [PubMed] [Google Scholar]
- 21. Ottawa Hospital Research Institute . http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Aug 8, 2019.
- 22. Oniki K, Saruwatari J, Izuka T, Kajiwara A, Morita K, Sakata M, et al. Influence of the PNPLA3 rs738409 polymorphism on non‐alcoholic fatty liver disease and renal function among normal weight subjects. PLoS One 2015;10:e0132640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanaka K, Takahashi H, Hyogo H, Ono M, Oza N, Kitajima Y, et al. Epidemiological survey of hemoglobin A1c and liver fibrosis in a general population with non‐alcoholic fatty liver disease. Hepatol Res 2019;49:296‐303. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka K, Hyogo H, Ono M, Takahashi H, Kitajima Y, Ono N, et al. Upper limit of normal serum alanine aminotransferase levels in Japanese subjects. Hepatol Res 2014;44:1196‐1207. [DOI] [PubMed] [Google Scholar]
- 25. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 26. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synth Methods 2010;1:97‐111. [DOI] [PubMed] [Google Scholar]
- 27. Chang X, DeFries RS, Liu L, Davis K. Understanding dietary and staple food transitions in China from multiple scales. PLoS One 2018;13:e0195775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Du SF, Wang HJ, Zhang B, Zhai FY, Popkin BM. China in the period of transition from scarcity and extensive undernutrition to emerging nutrition‐related non‐communicable diseases, 1949‐1992. Obes Rev 2014;15(Suppl. 1):8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mu M, Xu L‐F, Hu D, Wu J, Bai M‐J. Dietary patterns and overweight/obesity: a review article. Iran J Public Health 2017;46:869‐876. [PMC free article] [PubMed] [Google Scholar]
- 30. Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007;45:1366‐1374. [DOI] [PubMed] [Google Scholar]
- 31. Cheung O, Sanyal AJ. Abnormalities of lipid metabolism in nonalcoholic fatty liver disease. Semin Liver Dis 2008;28:351‐359. [DOI] [PubMed] [Google Scholar]
- 32. Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 2003;144:5159‐5165. [DOI] [PubMed] [Google Scholar]
- 33. van Herpen NA, Schrauwen‐Hinderling VB. Lipid accumulation in non‐adipose tissue and lipotoxicity. Physiol Behav 2008;94:231‐241. [DOI] [PubMed] [Google Scholar]
- 34. Kechagias S, Ernersson A, Dahlqvist O, Lundberg P, Lindström T, Nystrom FH, et al. Fast‐food‐based hyper‐alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut 2008;57:649‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745‐750. [DOI] [PubMed] [Google Scholar]
- 36. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta‐analysis. Hepatology 2011;54:1082‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al. Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record‐linkage population cohort study and decision analysis (ALFIE). Health Technol Assess 2009;13:iii‐iv, ix‐xi, 1‐134. [DOI] [PubMed] [Google Scholar]
- 38. Park JH, Choi J, Jun DW, Han SW, Yeo YH, Nguyen MH. Low alanine aminotransferase cut‐off for predicting liver outcomes; a nationwide population‐based longitudinal cohort study. J Clin Med 2019;8:1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terrault NA, Bzowej NH, Chang K‐M, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dutta A, Saha C, Johnson CS, Chalasani N. Variability in the upper limit of normal for serum alanine aminotransferase levels: a statewide study. Hepatology 2009;50:1957‐1962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


