Abstract
Fatigue and pruritus are common in patients with chronic liver diseases of all etiologies, but clinical awareness is mostly restricted to those with cholestatic liver diseases. We assessed the impact of fatigue and pruritus on patient‐reported outcomes (PROs) of patients with advanced nonalcoholic steatohepatitis (NASH). Specifically, PROs (Short Form–36, Chronic Liver Disease Questionnaire–NASH, Euro‐Qol 5 Dimension, and Work Productivity and Activity Impairment instruments) were assessed at baseline in patients with histologically confirmed bridging fibrosis (F3) or compensated cirrhosis (F4) due to NASH enrolled in STELLAR 3 and 4. Presence of fatigue and pruritus were indicated by a score of 4 or less on the respective items of the Chronic Liver Disease Questionnaire–NASH (scale range, 1‐7). Among the included 1,669 patients with advanced NASH (mean age = 58 ± 9 years, 48% F3, 42% with psychiatric comorbidities), 33% and 27% had fatigue and pruritus, respectively. Patients with NASH with fatigue were younger, more likely to be female, cirrhotic, and diabetic, and had higher body mass index and more comorbidities (all P < 0.05). All PRO scores of patients with fatigue were significantly impaired (mean up to −31% of a PRO range size in comparison to patients without fatigue). In multivariate analysis, predictors of fatigue included diabetes, history of depression or nervous system comorbidities, and lower serum albumin (P < 0.05). Patients with pruritus had demographic characteristics similar to those with fatigue, but a higher prevalence of dermatologic comorbidities. All PROs were impaired (by up to −19% of a range size, all P < 0.01) in patients with NASH with pruritus. Female gender, lower serum albumin, and a history of depression, nervous system, and dermatologic comorbidities were associated with increased risk of pruritus (P < 0.05). Conclusion: Clinically significant fatigue and pruritus are common in patients with advanced NASH, and these symptoms negatively affect PROs.
Abbreviations
- ALP
alkaline phosphatase
- APRI
AST‐to‐platelet ratio index
- AST
aspartate aminotransferase
- BMI
body mass index
- CLDQ‐NASH
Chronic Liver Disease Questionnaire–NASH
- CRP
C‐reactive protein
- ELF
enhanced liver fibrosis
- EQ‐5D
Euro‐Qol 5 Dimension
- FIB‐4
Fibrosis‐4
- GGT
gamma‐glutamyl transferase
- HbA1C
hemoglobin A1c
- HCC
hepatocellular carcinoma
- HOMA‐IR
homeostasis model assessment of insulin resistance
- IQR
interquartile range
- medDRA
Medical Dictionary for Regulatory Activities
- MELD
Model of End‐Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- NFS
NAFLD fibrosis score
- NIT
noninvasive test
- OR
odds ratio
- PRO
patient‐reported outcome
- SF‐36
Short Form–36
- TIMP‐1
tissue inhibitor of metalloproteinase 1
- VCTE
vibration‐controlled transient elastography
- WPAI:SHP
Work Productivity and Activity Impairment: Specific Health Problem
- α‐SMA
alpha smooth muscle actin
The global prevalence of nonalcoholic fatty liver disease (NAFLD) is estimated at approximately 25%, and the prevalence of nonalcoholic steatohepatitis (NASH) ranges from 1.5% to 6.5%.( 1 , 2 , 3 , 4 ) In addition, as shown in a meta‐analysis, the latter could be as high as 37% among individuals with type 2 diabetes.( 2 ) Other reports have suggested that those prevalence rates could be even higher, both in the general population and in subpopulations with metabolic syndrome components such as obesity and diabetes.( 5 , 6 ) The prevalence of NASH also varies across demographic groups. In fact, in the United States, a substantially higher prevalence among Hispanic Americans in comparison to African Americans and Caucasians has been reported in multiple studies.( 5 , 6 ) Worldwide, the highest prevalence of NAFLD and NASH is believed to be in the Middle East and South America.( 1 )
This high prevalence of NASH has already led to significant clinical burden, as documented by increased rates of cirrhosis, hepatocellular carcinoma (HCC), and mortality. In fact, in one study that analyzed U.S. mortality data from 2007‐2016, investigators reported that 82% of liver‐related deaths were from cirrhosis, while 17% were from HCC, and that NAFLD/NASH was responsible for 35% of HCC deaths and for almost 50% of cirrhosis deaths.( 7 , 8 ) Using an age‐specific death rate (ASDR) metric, researchers reported that the ASDR for NAFLD in the United States increased by 15% over the decade.( 7 ) Consistent with these observations, other reports confirmed that NASH is currently the second leading indication for liver transplantation and is the most common indication in some demographic groups such as women in the United States.( 9 , 10 , 11 )
Despite the significant clinical burden, NASH has generally been considered to be asymptomatic and is often diagnosed inadvertently after a finding of elevated liver enzymes or evidence of fatty liver on ultrasound performed for other reasons. This paradigm is currently shifting with more providers becoming aware of NAFLD, NASH, and their associated risks. However, there remain substantial gaps in knowledge about these liver diseases, which is especially alarming for medical specialties who likely see most patients with NAFLD such as primary care specialists, endocrinologists, and cardiologists.( 12 )
Although early stages of NASH may not be associated with severe symptoms, it is increasingly appreciated that NASH is not an asymptomatic disease. In fact, systematic assessments of patients with NASH using validated health‐related quality‐of‐life instruments suggest significant impairment of patient‐reported outcomes (PROs).( 13 , 14 , 15 , 16 ) Physical health–related domains of PROs and those that reflect fatigue appear to be the most negatively affected, particularly in patients with NASH and advanced fibrosis.( 17 , 18 ) In addition to fatigue, other previously underappreciated symptoms of liver disease are being increasingly reported in patients with NASH. In fact, among patients with NASH enrolled in recent clinical trials, between 32% and 35% and 21% and 27% have reported clinically significant fatigue and pruritus at baseline, respectively.( 19 , 20 , 21 ) Therefore, the aim of this study was to assess fatigue and pruritus and their impact on PROs of patients with advanced fibrosis due to NASH.
Patients and Methods
Study Population
The data for this study were collected in the STELLAR phase 3 clinical trials of selonsertib (#NCT03053050 and #NCT03053063). The trials were conducted in 27 countries (North America, South America, Europe, Asia, Australia, and New Zealand) in 2017‐2019, and were terminated due to the lack of efficacy of the study drug.( 22 ) As previously described, enrolled subjects were required to have a liver biopsy consistent with NASH and bridging fibrosis or cirrhosis. Subjects with a prior history of decompensated liver disease, Child‐Pugh score greater than 6, Model for End‐Stage Liver Disease (MELD) score greater than 12, other causes of liver disease, liver transplant, HCC, human immunodeficiency virus infection, recent excessive alcohol or illicit drug use, any major or unstable comorbidities other than NASH and metabolic syndrome as determined by the investigators, concomitant use of certain medications, or those participating in other clinical trials were excluded.( 22 )
Assessments
Medical history was coded using the Medical Dictionary for Regulatory Activities (medDRA).( 23 ) Laboratory parameters were collected at baseline and during treatment, and were used to calculate commonly used noninvasive tests of fibrosis (NITs) at baseline and treatment week 48, including the Enhanced Liver Fibrosis score (ELF), aspartate aminotransferase (AST)–to‐platelet ratio index (APRI), Fibrosis‐4 index (FIB‐4), FibroTest, and the NAFLD fibrosis score (NFS).( 24 , 25 , 26 , 27 , 28 ) In addition, most enrolled patients underwent liver stiffness measurement by vibration‐controlled transient elastography (VCTE; FibroScan; Echosens, Paris, France) using a prespecified protocol.( 29 ) Liver biopsies, which were obtained at screening and week 48, were evaluated by a single central pathologist (Z.G.) using the NASH Clinical Research Network classification for hepatic fibrosis( 30 , 31 , 32 ) and the NAFLD activity score (NAS) for steatosis, hepatocellular ballooning, and lobular inflammation.( 32 ) The proportionate areas of hepatic collagen, alpha‐smooth muscle actin (α‐SMA) expression, and fat in biopsy specimens were assessed using computer‐assisted morphometry, as previously described.( 33 )
PRO Measures Including Pruritus and Fatigue Scores
In both studies, patients self‐administered four validated PRO instruments: Short Form–36 (SF‐36), the Euro‐Qol 5 Dimension (EQ‐5D), the Chronic Liver Disease Questionnaire–NASH (CLDQ‐NASH), and the Work Productivity and Activity Impairment: Specific Health Problem (WPAI:SHP)( 34 , 35 , 36 , 37 , 38 , 39 , 40 ) in their native languages. For the purpose of this study, pruritus and fatigue were quantified using the respective scores of the disease‐specific CLDQ‐NASH instrument. Scores of 4 or less on this semi‐quantitative scale, which ranges from 1 to 7, were considered indicative of clinically significant pruritus or fatigue.
Statistical Analysis
Clinico‐demographic parameters as well as PRO scores were compared between NASH patients with and without clinically significant pruritus or fatigue using Pearson’s chi‐square test or Wilcoxon rank sum test, as appropriate. Independent predictors of clinically significant pruritus and fatigue at baseline were evaluated using logistic regression models with stepwise selection of predictors out of the complete list of clinical, demographic, laboratory, and histologic parameters. Comparison of postbaseline scores to patients’ own baseline levels was performed using the sign‐rank test for matched pairs in patients treated with placebo during the trials. In those patients, independent predictors of changes in pruritus and fatigue from baseline to week 48 (observed cases only) were studied using generalized linear regression models with stepwise selection of parameters and changes in these parameters from baseline.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). The trials were approved by each site’s institutional review board, and all participants provided informed consent.
Results
Prevalence of Pruritus and Fatigue at Baseline
A total of 1,669 patients with advanced fibrosis due to NASH were included in this study. The mean age was 58 ± 9 years, 40% were male, 42% had psychiatric comorbidities, and 52% had cirrhosis (F4). Based on the respective scores of the CLDQ‐NASH, clinically significant pruritus and fatigue were present in 27% and 33% of patients, respectively. The median pruritus score was 6 (interquartile range [IQR] 4‐7) and did not differ between patients with bridging fibrosis and compensated cirrhosis (P = 0.55). The median fatigue score was 4.8 (IQR 3.7‐5.7). Although patients with bridging fibrosis had higher median fatigue scores (indicative of less fatigue) than those with cirrhosis (5.0 [IQR 4.0‐5.8] vs. 4.7 [3.5‐5.7]; P = 0.0028), the difference does not meet the threshold of a minimal clinically important difference for domain scores of the CLDQ‐NASH (approximately 0.5 points).
Predictors of Clinically Significant Pruritus
Clinico‐demographic, laboratory, and histologic parameters of patients with clinically significant pruritus at baseline are given in Table 1. Compared to patients without pruritus, those with pruritus were more likely to be female and white, less likely to be Asian, had a lower employment rate, and a higher body mass index (BMI). Clinically significant fatigue, as well as dermatologic and nondermatologic comorbidities (e.g., psychiatric, gastrointestinal, fatigue, immune, infections, musculoskeletal, nervous, respiratory, vascular, vision) were more common in patients with pruritus, but an association with diabetes was not observed. Patients with pruritus had lower hemoglobin and serum albumin, and higher serum alkaline phosphatase (ALP), gamma‐glutamyl transferase (GGT), bile acids, fasting glucose, homeostasis model assessment of insulin resistance (HOMA‐IR), hemoglobin A1c (HbA1c), and C‐reactive protein (CRP). Although patients with pruritus had greater liver stiffness by VCTE and higher ELF and NFS (all P < 0.05), there was no association between pruritus and the presence of cirrhosis or other histologic parameters (all P > 0.05) (Table 1).
Table 1.
Baseline Clinico‐demographic Parameters of Patients With NASH With Advanced Fibrosis by the Presence of Clinically Significant Itch
| Clinically Significant Itch (Score ≤ 4) | No Clinically Significant Itch | P | |
|---|---|---|---|
| N | 447 | 1,222 | |
| Age, years | 58.0 ± 8.3 | 57.8 ± 9.0 | 0.87 |
| Male gender | 144 (32.2%) | 529 (43.3%) | <0.0001 |
| White race | 353 (79.0%) | 873 (71.4%) | 0.0020 |
| Black race | 10 (2.2%) | 15 (1.2%) | 0.13 |
| Asian race | 71 (15.9%) | 306 (25.0%) | 0.0001 |
| Current smoker | 50 (11.2%) | 116 (9.5%) | 0.30 |
| Employed | 179 (40.0%) | 634 (52.1%) | <0.0001 |
| Enrolled in the United States | 268 (60.0%) | 659 (53.9%) | 0.0282 |
| Cirrhosis | 233 (52.1%) | 635 (52.0%) | 0.95 |
| BMI, kg/m2 | 34.5 ± 6.4 | 33.1 ± 6.7 | <0.0001 |
| Comorbidities from medical history (by MedDRA): | |||
| Diabetes mellitus | 341 (76.3%) | 890 (72.8%) | 0.16 |
| Anxiety | 122 (27.3%) | 213 (17.4%) | <0.0001 |
| Depression | 159 (35.6%) | 272 (22.3%) | <0.0001 |
| Clinically overt fatigue | 66 (14.8%) | 107 (8.8%) | 0.0004 |
| Gastrointestinal disorders | 342 (76.5%) | 846 (69.2%) | 0.0036 |
| Immune system disorders | 207 (46.3%) | 478 (39.1%) | 0.0082 |
| Infections or infestations | 190 (42.5%) | 435 (35.6%) | 0.0098 |
| Sleep disorders | 94 (21.0%) | 177 (14.5%) | 0.0013 |
| Musculo‐skeletal disorders | 299 (66.9%) | 688 (56.3%) | 0.0001 |
| Nervous system disorders | 227 (50.8%) | 449 (36.7%) | <0.0001 |
| Respiratory disorders | 206 (46.1%) | 447 (36.6%) | 0.0004 |
| Skin disorders | 176 (39.4%) | 321 (26.3%) | <0.0001 |
| Vascular disorders | 336 (75.2%) | 849 (69.5%) | 0.0233 |
| Eye disorders | 126 (28.2%) | 282 (23.1%) | 0.0314 |
| Laboratory parameters and derived scores | |||
| ALT, U/L | 55.7 ± 37.6 | 57.2 ± 35.4 | 0.13 |
| AST, U/L | 54.0 ± 30.1 | 52.1 ± 30.1 | 0.22 |
| Platelets, x109/L | 188.7 ± 65.9 | 188.2 ± 67.2 | 0.63 |
| Hemoglobin, g/dL | 13.7 ± 1.5 | 14.0 ± 1.5 | 0.0007 |
| Albumin, g/dL | 4.39 ± 0.35 | 4.48 ± 0.33 | 0.0001 |
| ALP, U/L | 95.5 ± 32.7 | 92.0 ± 35.7 | 0.0055 |
| Bilirubin, mg/dL | 0.641 ± 0.350 | 0.690 ± 0.373 | 0.0025 |
| Direct bilirubin, mg/dL | 0.187 ± 0.105 | 0.189 ± 0.092 | 0.14 |
| Bile acids, umol/L | 13.5 ± 15.2 | 12.1 ± 13.5 | 0.0160 |
| Fasting glucose, mg/dL | 134.8 ± 49.9 | 127.5 ± 40.8 | 0.0195 |
| Fasting HOMA‐IR score | 11.7 ± 20.9 | 9.22 ± 15.74 | <0.0001 |
| HbA1c, % | 6.74 ± 1.18 | 6.57 ± 1.19 | 0.0034 |
| CRP, mg/dL | 0.580 ± 0.655 | 0.504 ± 0.741 | <0.0001 |
| GGT, U/L | 112.8 ± 127.4 | 109.1 ± 137.6 | 0.0250 |
| Alpha‐2 macroglobulin, mg/dL | 271.9 ± 77.8 | 275.6 ± 81.5 | 0.43 |
| Apolipoprotein A1, mg/dL | 142.2 ± 25.6 | 142.4 ± 26.0 | 0.67 |
| Apolipoprotein B, mg/dL | 91.2 ± 27.0 | 89.0 ± 24.6 | 0.20 |
| Haptoglobin, mg/dL | 125.6 ± 61.3 | 118.4 ± 66.2 | 0.0133 |
| Hyaluronic acid, ng/mL | 177.8 ± 217.8 | 160.0 ± 211.4 | 0.0123 |
| PIIINP, ng/mL | 14.2 ± 6.7 | 13.2 ± 6.5 | 0.0009 |
| TIMP‐1, ng/mL | 317.9 ± 99.6 | 300.3 ± 94.2 | 0.0002 |
| APRI score | 0.926 ± 0.640 | 0.914 ± 0.719 | 0.23 |
| FIB‐4 score | 2.60 ± 1.63 | 2.50 ± 1.60 | 0.15 |
| FibroTest score | 0.489 ± 0.225 | 0.518 ± 0.237 | 0.0156 |
| ELF score | 10.5 ± 1.0 | 10.3 ± 1.0 | 0.0007 |
| MELD score | 7.03 ± 1.57 | 7.13 ± 1.66 | 0.28 |
| NFS | 0.443 ± 1.381 | 0.168 ± 1.381 | 0.0024 |
| Liver imaging: | |||
| Liver stiffness by transient elastography, kPa | 20.7 ± 13.2 | 19.2 ± 12.3 | 0.0180 |
| Controlled attenuation parameter, dB/m | 325.8 ± 51.9 | 319.2 ± 55.4 | 0.17 |
| Liver histology: | |||
| NAFLD activity: steatosis grade 1 | 424 (94.9%) | 1,152 (94.3%) | 0.65 |
| NAFLD activity: steatosis grade 2 | 23 (5.1%) | 68 (5.6%) | 0.74 |
| NAFLD activity: lobular inflammation grade 1 | 37 (8.3%) | 112 (9.2%) | 0.57 |
| NAFLD activity: lobular inflammation grade 2 | 159 (35.6%) | 476 (39.0%) | 0.21 |
| NAFLD activity: lobular inflammation grade 3 | 251 (56.2%) | 634 (51.9%) | 0.12 |
| NAFLD activity: hepatocyte ballooning grade 1 | 72 (16.1%) | 247 (20.2%) | 0.06 |
| NAFLD activity: hepatocyte ballooning grade 2 | 375 (83.9%) | 973 (79.6%) | 0.05 |
| Total NAS | 5.37 ± 0.89 | 5.28 ± 0.92 | 0.06 |
| Hepatic collagen content, % | 8.32 ± 6.45 | 8.27 ± 6.10 | 0.91 |
| ‐SMA, % | 11.1 ± 8.3 | 10.5 ± 8.0 | 0.22 |
| Morphometric fat content, % | 11.2 ± 6.4 | 11.3 ± 6.6 | 0.89 |
Abbreviations: ALT, alanine aminotransferase; PIIINP, procollagen III amino terminal propeptide; TIMP‐1, tissue inhibitor of metalloproteinase 1.
In multivariate analysis, female gender, a history of depression, nervous system disorders, skin‐related comorbidities, and lower serum albumin were associated with an increased risk of pruritus in patients with advanced fibrosis due to NASH (all P < 0.05) (Table 2).
Table 2.
Multivariate Analysis of Clinico‐demographic Factors Independently Associated With Baseline Pruritus and Fatigue in Patients With Advanced NASH (Logistic Regression, P < 0.05 After Stepwise Selection of Clinico‐demographic and Laboratory Predictors From Table 1)
| Predictor of Pruritus | Odds Ratio (95% CI) | P |
|---|---|---|
| Male gender (ref: female) | 0.75 (0.59‐0.95) | 0.0194 |
| Depression | 1.53 (1.19‐1.96) | 0.0009 |
| Nervous system disorder | 1.40 (1.11‐1.77) | 0.0042 |
| Skin disorder | 1.59 (1.25‐2.01) | 0.0001 |
| Albumin, per g/dL | 0.56 (0.40‐0.78) | 0.0006 |
| Predictor of fatigue | ||
| Age, per year | 0.96 (0.95‐0.98) | <.0001 |
| Male gender (ref: female) | 0.66 (0.51‐0.86) | 0.002 |
| Asian race (ref: white) | 0.55 (0.39‐0.77) | 0.0006 |
| Type 2 diabetes | 1.51 (1.14‐1.99) | 0.0037 |
| Depression | 2.18 (1.67‐2.83) | <.0001 |
| Clinically overt fatigue | 1.85 (1.28‐2.67) | 0.0011 |
| Gastrointestinal disorder | 1.33 (1.00‐1.78) | 0.0497 |
| Nervous system disorder | 1.29 (1.01‐1.66) | 0.0454 |
| Platelet, per 109/L | 0.998 (0.996‐1.000) | 0.0225 |
| Albumin, per g/dL | 0.45 (0.31‐0.66) | <.0001 |
| Alpha‐2 macroglobulin, per mg/dL | 0.998 (0.996‐0.999) | 0.0094 |
| Apolipoprotein A1, per mg/dL | 0.994 (0.989‐0.999) | 0.0126 |
| Apolipoprotein B, per mg/dL | 1.006 (1.002‐1.011) | 0.0096 |
| TIMP‐1, per ng/mL | 1.002 (1.000‐1.003) | 0.0179 |
| Clinically significant pruritus | 3.30 (2.57‐4.24) | <.0001 |
Abbreviation: CI, confidence interval.
Predictors of Clinically Significant Fatigue
Parameters of patients with clinically significant fatigue are provided in Table 3. Compared to patients without fatigue, those with fatigue were younger (mean age: 56 vs. 59 years old), more likely to be female (70% vs. 55%) and white, and less likely to be employed. Patients with fatigue also had a higher BMI and more comorbidities including diabetes (78% vs. 72%) and smoking, and a higher prevalence of clinically significant pruritus (45% vs. 18%) (all P < 0.05). Patients with fatigue also had lower serum albumin and higher serum ALP, GGT, bile acids, fasting glucose, HbA1c, and CRP compared to patients without fatigue. In addition to a higher prevalence of cirrhosis (58% vs. 49%), patients with fatigue also had higher serum ELF scores, FibroTest and NFS, greater liver stiffness by VCTE, and higher hepatic collagen content and α‐SMA expression by morphometry (Table 3).
Table 3.
Baseline Clinico‐demographic Parameters of Patients With NASH With Advanced Fibrosis by the Presence of Clinically Significant Fatigue
| Clinically Significant Fatigue (Score ≤ 4) | No Clinically Significant Fatigue | P | |
|---|---|---|---|
| N | 549 | 1,121 | |
| Age, years | 56.2 ± 8.9 | 58.7 ± 8.6 | <0.0001 |
| Male gender | 167 (30.4%) | 507 (45.2%) | <0.0001 |
| White race | 459 (83.6%) | 768 (68.5%) | <0.0001 |
| Black race | 12 (2.2%) | 13 (1.2%) | 0.10 |
| Asian race | 63 (11.5%) | 314 (28.0%) | <0.0001 |
| Current smoker | 69 (12.6%) | 97 (8.7%) | 0.0122 |
| Employed | 232 (42.3%) | 582 (52.1%) | 0.0002 |
| Enrolled in the United States | 357 (65.0%) | 571 (50.9%) | <0.0001 |
| Cirrhosis | 319 (58.1%) | 550 (49.1%) | 0.0005 |
| BMI, kg/m2 | 35.3 ± 6.8 | 32.6 ± 6.3 | <0.0001 |
| Comorbidities from medical history (MedDRA): | |||
| Diabetes mellitus | 428 (78.0%) | 803 (71.6%) | 0.0058 |
| Anxiety | 171 (31.1%) | 165 (14.7%) | <0.0001 |
| Blood and lymphatic system disorders | 143 (26.0%) | 219 (19.5%) | 0.0024 |
| Depression | 233 (42.4%) | 199 (17.8%) | <0.0001 |
| Clinically overt fatigue | 93 (16.9%) | 80 (7.1%) | <0.0001 |
| Gastrointestinal disorders | 442 (80.5%) | 747 (66.6%) | <0.0001 |
| Immune system disorders | 272 (49.5%) | 413 (36.8%) | <0.0001 |
| Infections or infestations | 227 (41.3%) | 399 (35.6%) | 0.0225 |
| Sleep disorders | 128 (23.3%) | 143 (12.8%) | <0.0001 |
| Musculo‐skeletal disorders | 369 (67.2%) | 618 (55.1%) | <0.0001 |
| Nervous system disorders | 292 (53.2%) | 384 (34.3%) | <0.0001 |
| Respiratory disorders | 262 (47.7%) | 392 (35.0%) | <0.0001 |
| Skin disorders | 175 (31.9%) | 322 (28.7%) | 0.19 |
| Vascular disorders | 397 (72.3%) | 789 (70.4%) | 0.41 |
| Eye disorders | 129 (23.5%) | 280 (25.0%) | 0.51 |
| Clinically significant itch (score ≤ 4) | 249 (45.4%) | 198 (17.7%) | <0.0001 |
| Laboratory parameters and derived scores: | |||
| ALT, U/L | 55.9 ± 37.7 | 57.2 ± 35.2 | 0.06 |
| AST, U/L | 54.2 ± 35.3 | 51.9 ± 27.2 | 0.73 |
| Platelets, 109/L | 188.9 ± 70.2 | 188.0 ± 65.1 | 0.84 |
| Hemoglobin, g/dL | 13.7 ± 1.5 | 14.1 ± 1.5 | <0.0001 |
| Albumin, g/dL | 4.36 ± 0.35 | 4.50 ± 0.32 | <0.0001 |
| ALP, U/L | 98.5 ± 36.5 | 90.2 ± 33.9 | <0.0001 |
| Bilirubin, mg/dL | 0.635 ± 0.361 | 0.697 ± 0.369 | <0.0001 |
| Direct bilirubin, mg/dL | 0.185 ± 0.105 | 0.190 ± 0.090 | 0.0196 |
| Bile acids, umol/L | 14.5 ± 15.8 | 11.5 ± 12.9 | 0.0001 |
| Fasting glucose, mg/dL | 136.4 ± 51.2 | 126.0 ± 38.9 | 0.0003 |
| Fasting HOMA‐IR score | 12.8 ± 21.9 | 8.45 ± 14.34 | <0.0001 |
| HbA1c, % | 6.82 ± 1.25 | 6.51 ± 1.15 | <0.0001 |
| CRP, mg/dL | 0.675 ± 0.812 | 0.450 ± 0.658 | <0.0001 |
| GGT, U/L | 112.8 ± 131.2 | 108.7 ± 136.7 | 0.0149 |
| Alpha‐2 macroglobulin, mg/dL | 264.4 ± 76.0 | 279.6 ± 82.2 | 0.0002 |
| Apolipoprotein A1, mg/dL | 139.3 ± 25.3 | 143.9 ± 26.1 | 0.0003 |
| Apolipoprotein B, mg/dL | 92.7 ± 28.4 | 88.0 ± 23.4 | 0.0098 |
| Haptoglobin, mg/dL | 129.0 ± 67.0 | 116.1 ± 63.6 | 0.0001 |
| Hyaluronic acid, ng/mL | 181.5 ± 217.9 | 156.4 ± 210.4 | 0.0449 |
| PIIINP, ng/mL | 14.5 ± 7.3 | 13.0 ± 6.1 | <0.0001 |
| TIMP‐1, ng/mL | 327.1 ± 111.5 | 294.1 ± 85.3 | <0.0001 |
| APRI score | 0.961 ± 0.801 | 0.896 ± 0.643 | 0.25 |
| FIB‐4 score | 2.60 ± 1.83 | 2.49 ± 1.48 | 0.96 |
| FibroTest score | 0.474 ± 0.234 | 0.528 ± 0.232 | <0.0001 |
| ELF score | 10.5 ± 1.1 | 10.3 ± 1.0 | 0.0007 |
| MELD score | 7.10 ± 1.72 | 7.10 ± 1.59 | 0.38 |
| NFS | 0.464 ± 1.488 | 0.132 ± 1.319 | <0.0001 |
| Liver imaging: | |||
| Liver stiffness by transient elastography, kPa | 21.7 ± 14.6 | 18.5 ± 11.2 | 0.0008 |
| Controlled attenuation parameter, dB/m | 326.9 ± 57.0 | 317.8 ± 52.9 | 0.0029 |
| Liver histology: | |||
| NAFLD activity: steatosis grade 1 | 521 (94.9%) | 1056 (94.2%) | 0.56 |
| NAFLD activity: steatosis grade 2 | 27 (4.9%) | 64 (5.7%) | 0.50 |
| NAFLD activity: lobular inflammation grade 1 | 46 (8.4%) | 103 (9.2%) | 0.59 |
| NAFLD activity: lobular inflammation grade 2 | 208 (37.9%) | 428 (38.2%) | 0.91 |
| NAFLD activity: lobular inflammation grade 3 | 295 (53.7%) | 590 (52.6%) | 0.67 |
| NAFLD activity: hepatocyte ballooning grade 1 | 93 (16.9%) | 226 (20.2%) | 0.12 |
| NAFLD activity: hepatocyte ballooning grade 2 | 455 (82.9%) | 894 (79.8%) | 0.13 |
| Total NAS | 5.33 ± 0.90 | 5.29 ± 0.92 | 0.32 |
| Hepatic collagen content, % | 9.03 ± 7.03 | 7.94 ± 5.72 | 0.0373 |
| ‐SMA, % | 11.8 ± 9.1 | 10.2 ± 7.6 | 0.0081 |
| Morphometric fat content, % | 11.6 ± 6.6 | 11.1 ± 6.5 | 0.19 |
Abbreviations: ALT, alanine aminotransferase; PIIINP, procollagen III amino terminal propeptide.
To eliminate the potential impact of biopsy sampling error on classification of fibrosis, the association between fatigue at baseline with cirrhosis was also assessed in a subgroup of patients in whom the fibrosis stage was unchanged between baseline and week 48 (n = 1,161, including n = 638 with stable bridging fibrosis and n = 523 with stable cirrhosis). In this sensitivity analysis, clinically significant fatigue remained more common in patients with cirrhotic NASH versus bridging fibrosis (60% vs. 52%; P = 0.02).
In multivariate analysis, independent predictors of clinically significant fatigue included clinically significant pruritus (odds ratio [OR] 3.30; 95% confidence interval 2.57‐4.24; P < 0.0001), female gender, younger age, non‐Asian race, history of depression, diabetes and other comorbidities, and some laboratory tests including lower serum albumin and platelets (all P < 0.05; Table 2). After adjustment for laboratory tests including platelet count, serum albumin, and several serum NITs, the association between fatigue and cirrhosis was not statistically significant (P = 0.21).
Comorbid Fatigue and Pruritus in Patients With NASH
Due to the strong association between clinically significant pruritus and fatigue shown previously, we aimed to specifically study patients who have both pruritus and fatigue. In this context, 15% of enrolled patients with NASH had both fatigue and pruritus, 18% had fatigue without pruritus, 12% had pruritus without fatigue, and 55% had neither. Patients who had both fatigue and pruritus were predominantly female and white, had more cirrhosis (59.4% vs. 50.7%), higher BMI (35.8 ± 6.6 vs. 33.1 ± 6.6), and significantly more comorbidities of all types (with the exception of only diabetes) (all P < 0.05) (Supporting Table S1). Consistent with the higher rate of cirrhosis, those patients also had higher NIT fibrosis scores, greater liver stiffness by imaging, lower serum albumin, and higher ‐SMA percentage (P < 0.05) (Supporting Table S1).
In multivariate analysis, independent predictors of having both pruritus and fatigue included female gender (OR = 1.58 [1.15‐2.16], P = 0.0043), higher BMI (OR = 1.034 [1.012‐1.057] per kg/m2, P = 0.0021), history of depression (OR = 2.04 [1.52‐2.75], P < 0.0001), clinically overt fatigue (1.92 [1.30‐2.83], P = 0.0011), nervous system disorders (1.47 [1.10‐1.97], P = 0.0104), and lower serum albumin (0.52 [0.33‐0.81] per g/dL, P = 0.0037). It is important to note that these predictors are similar to those of having either of these conditions.
Impact of Pruritus and Fatigue on Other PROs
Patients with NASH with advanced fibrosis who reported clinically significant pruritus also had significant impairment in all concurrently measured PROs (Table 4). The greatest impairments were observed in Role Physical of the SF‐36 as well as Abdominal Symptoms and Fatigue of the CLDQ‐NASH (mean up to −19.5% of a PRO range size; all P < 0.01). Furthermore, correlations of the CLDQ‐NASH pruritus score with other PRO scores were all statistically significant with Spearman correlation (all P < 0.001). The strongest correlation with pruritus among the scales of the SF‐36 was with Vitality (rs= 0.34) and the Systemic domain among the domains of the CLDQ‐NASH (rs = 0.61).
Table 4.
Baseline PRO Scores of Patients With NASH with Advanced Fibrosis by the Presence of Clinically Significant Itch and Fatigue (All P < 0.001 Between the Groups)
| PRO Score | Clinically Significant Itch (Score ≤ 4) | No Clinically Significant Itch | Clinically Significant Fatigue (Score ≤ 4) | No Clinically Significant Fatigue |
|---|---|---|---|---|
| SF‐36 (range 0‐100) | ||||
| Physical Functioning | 61.0 ± 26.7 | 75.9 ± 24.1 | 55.6 ± 26.5 | 79.9 ± 21.1 |
| Role physical | 59.9 ± 29.8 | 78.3 ± 26.1 | 52.3 ± 28.9 | 83.7 ± 21.4 |
| Bodily pain | 54.1 ± 25.4 | 71.3 ± 24.7 | 48.9 ± 24.4 | 75.4 ± 22.0 |
| General health | 44.4 ± 20.5 | 55.9 ± 21.0 | 38.8 ± 18.2 | 59.7 ± 19.5 |
| Vitality | 44.7 ± 22.2 | 59.8 ± 22.7 | 34.7 ± 18.3 | 66.1 ± 18.4 |
| Social functioning | 68.9 ± 27.1 | 83.9 ± 22.7 | 62.0 ± 26.9 | 88.6 ± 18.3 |
| Role emotional | 70.9 ± 29.1 | 85.0 ± 22.9 | 64.3 ± 29.9 | 89.4 ± 17.9 |
| Mental health | 66.0 ± 20.5 | 76.7 ± 18.6 | 61.5 ± 20.0 | 79.8 ± 16.4 |
| Physical summary | 42.1 ± 9.5 | 48.0 ± 8.9 | 40.0 ± 9.6 | 49.5 ± 7.6 |
| Mental summary | 45.4 ± 11.4 | 51.0 ± 9.6 | 42.3 ± 11.1 | 53.1 ± 7.9 |
| Health utility scores (range 0‐1) | ||||
| SF‐6D | 0.613 ± 0.120 | 0.706 ± 0.135 | 0.576 ± 0.099 | 0.732 ± 0.124 |
| EQ‐5D | 0.759 ± 0.156 | 0.853 ± 0.130 | 0.725 ± 0.155 | 0.877 ± 0.106 |
| CLDQ‐NASH (range 1‐7) | ||||
| Abdominal | 4.52 ± 1.56 | 5.69 ± 1.34 | 4.24 ± 1.50 | 5.94 ± 1.13 |
| Activity | 4.66 ± 1.38 | 5.71 ± 1.20 | 4.31 ± 1.29 | 5.97 ± 0.95 |
| Emotional | 4.58 ± 1.26 | 5.59 ± 1.07 | 4.29 ± 1.11 | 5.82 ± 0.89 |
| Fatigue | 3.85 ± 1.32 | 4.96 ± 1.31 | 3.02 ± 0.79 | 5.47 ± 0.81 |
| Systemic | 3.89 ± 1.12 | 5.45 ± 1.03 | 3.96 ± 1.13 | 5.56 ± 0.95 |
| Worry | 4.43 ± 1.62 | 5.37 ± 1.39 | 4.23 ± 1.58 | 5.56 ± 1.27 |
| Total CLDQ‐NASH score | 4.32 ± 1.13 | 5.46 ± 0.98 | 4.01 ± 0.91 | 5.72 ± 0.75 |
| WPAI:SHP (range 1‐0) | ||||
| Work productivity impairment | 0.188 ± 0.248 | 0.102 ± 0.200 | 0.271 ± 0.280 | 0.059 ± 0.140 |
| Absenteeism | 0.036 ± 0.127 | 0.020 ± 0.104 | 0.062 ± 0.176 | 0.007 ± 0.058 |
| Presenteeism | 0.154 ± 0.201 | 0.082 ± 0.163 | 0.212 ± 0.220 | 0.051 ± ± 0.125 |
| Activity impairment | 0.298 ± 0.293 | 0.130 ± 0.219 | 0.341 ± 0.292 | 0.093 ± 0.181 |
Similarly, patients with clinically significant fatigue had significant impairment in all the measured PROs. The greatest impairments were observed in Vitality of the SF‐36 and Systemic and Fatigue among the CLDQ‐NASH (mean impairment up to −31.4% of a PRO range size) (Table 4). The correlations among those with fatigue and the measured PROs were noticeably stronger than for pruritus, especially for the Vitality scale of the SF‐36 (rs = 0.75) and the Emotional domain score (rs = 0.74) and total score of the CLDQ‐NASH (rs = 0.86).
Finally, in patients who had both fatigue and pruritus, PRO scores were the most profoundly impaired; the mean impairment in comparison to patients who had neither fatigue nor pruritus was between −5% and −37.5% of a PRO range size (Fig. 1).
FIG. 1.
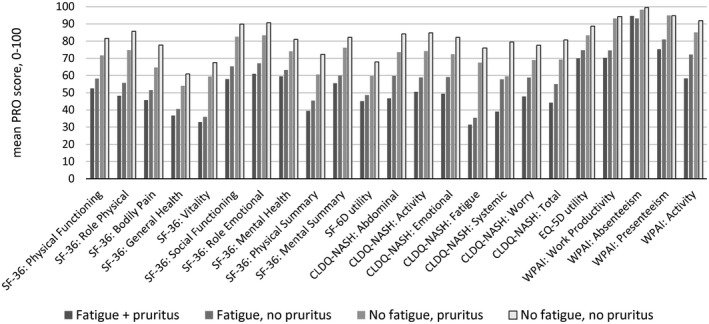
Mean PRO scores (normalized to 0‐100 score) in patients with NASH by the presence of clinically significant fatigue and pruritus.
Changes in Pruritus and Fatigue During Follow‐up
Postbaseline data were available in 331 placebo‐treated patients, including 86 patients (26%) with clinically significant pruritus and 103 patients (31%) with fatigue at baseline. In patients with baseline pruritus, mean pruritus scores increased (indicative of improvement) such that, by week 48 of placebo treatment, only 38 (44%) still had clinically significant pruritus (Fig. 2A). However, only 32 patients (13%) without clinically significant pruritus at baseline developed it by week 48. Multivariate analysis showed that greater spontaneous improvement in pruritus in patients with clinically significant pruritus at baseline, after adjustment for the baseline value, was independently associated with white race (β = +1.09 ± 0.41; P = 0.0094), lower baseline serum ALP (−0.017 ± 0.005 per U/L; P = 0.0017), and lack of immune system disorders (−1.34 ± 0.35; P = 0.0002).
FIG. 2.
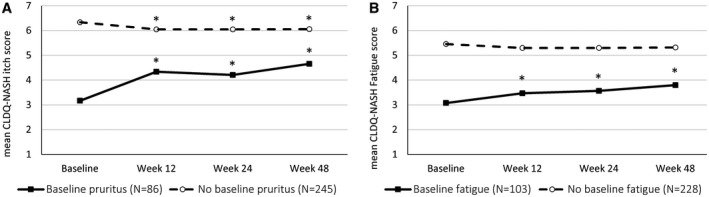
Changes in pruritus (A) and fatigue (B) scores in patients with NASH treated with placebo. *P < 0.01 when compared with patients’ own baseline score levels.
Similar trends were observed regarding temporal changes in fatigue scores. Specifically, by week 48 of treatment with placebo, only 57 patients (55%) with fatigue at baseline still had clinically significant fatigue, whereas 31 patients (14%) without clinically significant fatigue at baseline developed it later (Fig. 2B). In multivariate analysis, the only independent predictor of spontaneous improvement in fatigue among patients with clinically significant fatigue at baseline was the absence of current smoking (β = −0.61 ± 0.30; P = 0.047).
Discussion
In this study, we assessed the prevalence, predictors, and impact of clinically significant fatigue and pruritus among patients with advanced fibrosis due to NASH who participated in two large phase 3 clinical trials. Because these symptoms are sometimes not recognized as common or considered as important in NASH compared with cholestatic liver disorders, the aim was to relate their presence to health‐related quality of life and other PROs in patients with NASH.
Our data suggest that about one‐third of patients with advanced fibrosis due to NASH enrolled in these studies may have clinically significant fatigue, as indicated by a CLDQ‐NASH fatigue score at or below the middle of the range of this PRO. Patients with fatigue were more frequently younger, female, Caucasian, unemployed, and obese. As expected, fatigue was also associated with a number of comorbidities including diabetes mellitus, smoking, as well as gastrointestinal, sleep, and psychiatric disorders. In addition, fatigue was more common among patients with cirrhosis, and those with fatigue had significantly higher NITs of fibrosis including the ELF score, NFS, and liver stiffness by VCTE. Furthermore, the PROs of patients with NASH with fatigue were significantly lower across all domains when compared to those without fatigue, supporting the profound impact of this symptom on health‐related quality of life. Domains related to general health, vitality, and the ability to go to and perform work, as well as activities of daily living, were the domains that were most substantially affected. Multivariate analysis determined that type 2 diabetes mellitus, depression, and having a nervous system disorder were independently associated with the presence of fatigue among patients with NASH with advanced fibrosis. In contrast, younger age, male sex, Asian ethnicity, having higher platelets and serum albumin levels, and lower NITs were associated with a reduced likelihood of fatigue. Collinearity between the latter biochemical parameters and cirrhosis likely explain the lack of association between fatigue and histologic cirrhosis in this analysis.
In addition to fatigue, clinically significant pruritus was commonly observed in these patients with NASH with advanced fibrosis, affecting approximately 27% of individuals. Although fewer clinico‐demographic factors were found to be associated with pruritus than observed for fatigue, the PRO scores across all measured domains were significantly lower among patients with versus without pruritus. As noted regarding fatigue, patients with depression and/or a nervous system disorder were more likely to report clinically significant pruritus. Not surprisingly, the presence of comorbid dermatologic disorders was also independently associated with pruritus. In contrast, being male and having a higher serum albumin level were associated with a lower likelihood of pruritus. An interesting observation is the lack of difference in the prevalence of pruritus between patients with bridging fibrosis and those with cirrhosis. These findings support the relative homogeneity of these patient populations, at least from a pruritus perspective. However, sampling error of liver biopsy may have affected these findings, because patients with pruritus had higher NITs (e.g., liver stiffness by VCTE, ELF score, NFS) than those without pruritus.
Interestingly, we also found a strong association between the presence of fatigue and pruritus in this patient population with NASH, in both univariate and multivariate analyses. Although the exact reasons are unclear, it is plausible that nocturnal pruritus can cause sleep disturbance, which contributes to fatigue. Indeed, sleep disorders were more common in patients with fatigue and pruritus versus those without these symptoms. It is also possible that both symptoms are driven, at least in part, by a common cause in patients with advanced NASH. Indeed, the pathophysiology behind these symptoms may be linked to hepatic injury and hepatic and/or chronic systemic inflammation as well as disturbed bile acid metabolism or cholestasis associated with chronic liver disease.( 41 , 42 ) In fact, the analysis of bile acid composition in patients with NASH who experience pruritus will be of great interest not only for development of potential treatment options to manage pruritus, but also to understand drug‐induced pruritus that is seen among side effects of some of the drugs tested for treatment of NASH. Considering fatigue in NASH, it is possible that it is not only associated with metabolic abnormalities such as diabetes or insulin resistance, but can also be exacerbated by reduced muscle mass or performance in patients with advanced liver disease.( 43 ) In this context, a systematic assessment for sarcopenia in NASH and its management with a targeted nutrition regimen may be required. However, fatigue in NASH may also be associated with sleep disturbances or neuropsychiatric disorders.( 42 , 43 , 44 ) In light of this, a targeted treatment approach that would address the underlying fatigue drivers in patients with NASH should be considered.( 44 )
These findings suggest that patients with advanced NASH are not asymptomatic. Indeed, clinically significant fatigue and/or pruritus are quite common and negatively affect almost all aspects of patients’ daily functioning, perception of their health, and overall wellbeing. In this context, we found that both fatigue and pruritus appear to adversely affect patients’ ability to work, as only approximately 40% of patients with either of these conditions reported being employed, despite most being in the employment age range. As such, the economic burden of NASH may be greater than previously reported, especially now that the impact of NASH‐related fatigue and pruritus has been quantified.( 45 ) However, further work is necessary to better appreciate the true economic burden of NASH, especially as NAFLD and NASH are forecasted to substantially increase in the coming years due to the unmitigated increase in the rates of obesity and type 2 diabetes mellitus.( 46 , 47 )
Given these findings, the development of therapies with potential histologic and clinical benefits is paramount, as improvement in patients’ PRO burden may ensue. At the same time, these trials must also account for the effect of interventions on pruritus and fatigue. In particular, because some currently investigated therapies (e.g., farnesoid X receptor agonists) may exacerbate pruritus,( 48 ) it is critical to optimize management of treatment‐emergent pruritus in these trials. In this context, our data demonstrate that a notable proportion of patients experience spontaneous improvement in both fatigue and pruritus after placebo treatment. This is not unexpected, given both regression to the mean and the placebo effect, which has been reported to be as high as 72% in some studies.( 49 ) Owing to the limited sample size in the current study, we were unable to quantify the contribution of spontaneous improvement in laboratory or histologic parameters to the improvements in pruritus or fatigue that we observed.
This study has several limitations. First, the patients included in this study were enrolled in clinical trials with highly specific enrollment criteria; hence, the generalizability of our findings to patients enrolled in real‐world settings and across a wider spectrum of NAFLD severity are unclear. In fact, data from a registry suggest that the prevalence of fatigue may be even higher in the real‐world setting.( 50 ) Second, the results may have been affected by the subjectivity of PROs and recall bias, as subjects were asked to think back over a certain time period in completing the PRO assessments. Third, the severity of pruritus was not assessed with an instrument that has been validated for this purpose. Therefore, the absence of an association between pruritus and histologic parameters should be interpreted with caution. Similarly, the definitions of clinically significant fatigue and pruritus used in this study, based on the midrange of the respective scales, were somewhat arbitrary. Nevertheless, the correlations of both continuous scores with other PROs were highly significant across the range evaluated, suggesting the presence of monotonous trends. Moreover, all PRO tools used in this study are valid and reliable and have been used extensively in PRO research, so that any bias introduced should be consistent over time.
In summary, the STELLAR trials demonstrate that among patients with advanced fibrosis due to NASH, up to 30% experience significant pruritus or fatigue. These symptoms are associated with extraordinarily low PRO scores across all measured domains. This still largely underappreciated symptom burden suggests that NASH is not an asymptomatic disease; indeed, symptoms such as fatigue and pruritus worsen patients’ experience with their disease. These data emphasize the need for continued evaluation of novel therapies for NASH that may lead to improved patient‐relevant outcomes including PROs.
Supporting information
Table S1
Supported by Gilead Sciences.
Potential conflict of interest: Dr. Lawitz received grants from 89Bio, Allergan, Akero, BMS, BI, Durect, Eli Lilly, Enanta, Gilead, Intercept, Madrigal, Metacrine, Viking, and Zydus. Dr. Trauner consults for, is on the speakers’ bureau, and received grants from Falk, Gilead, Intercept, and MSD. He consults for and received grants from Alibireo. He is on the speakers’ bureau for and received grants from Roche. He consults for Albireo, BiomX, Boehringer Ingelheim, Genfit, Novartis, Phenex, and Regulus. He received grants from Cymabay, Takeda, and AbbVie. Dr. Romero‐Gomez consults and received grants from Gilead and Intercept. Dr. Camargo owns stock in and is employed by Gilead. Dr. Younossi consults for and received grants from Gilead, Intercept, BMS, NovoNordisk, Viking, Terns, Siemens, Shionogi, AbbVie, Merck, and Novartis. Dr. Harrison consults for, advises, received grants from, and owns stock in Cirius, Galectin, Genfit, Madrigal, NGM Bio, and Northsea. He consults for, advises, and owns stock in Akero, Histoindex, and Metacrine. He consults for, advises, and received grants from Axcella, Civi Biopharma, Cymabay, Galmed, Gilead, Hepion, Hightide Bio, Intercept, Novartis, Novo Nordisk, Sagimet, and Viking. He consults for and advises Altimmune, Blade Therapeutics, CLDF, Echosens, Foresite Labs, Gelesis, Indalo, Innovate, Medpace, Merck, Perspectum, Poxel, Prometic, Ridgeline Therapeutics, and Terns. He consults for and received grants from Enyo. He advises Arrowhead. He consults for Fortress, Kowa, and Silverback. He received grants from BMS, Conatus, Genetech, Immuron, Pfzier, Second Genome, and Tobira/Allergan. Dr. Myers owns stock in and is employed by Gilead. Dr. Kersey owns stock in and is employed by Gilead. Dr. Wong advises, consults for, and received grants from Gilead. He advises and consults for 3V‐BIO, AbbVie, Allergan, Boehringer Ingelheim, Center for Outcomes Research in Liver Diseases, Echosens, Hanmi Pharmaceutical, Intercept, Merck, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, ProSciento, Sagimet Biosciences, TARGET PharmaSolutions, and Terns. Dr. Anstee consults for, is on the speakers’ bureau for, received grants from, and has active research collaborations with Allergan/Tobira. He consults for, received grants from, and has active research collaborations with AstraZeneca, Novartis, and Pfzier. He consults for, is on the speakers’ bureau for, and has active research collaborations with BMS and Genfit SA. He consults for and is on the speakers’ bureau for Abbott and Gilead. He consults for and has active research collaborations with Eli Lilly, HistoIndex, Intercept, and Novo Novartis. He received grants from and has active research collaborations with AbbVie, GlaxoSmithKline, and Glympse Bio. He consults for 89Bio, Acuitas Medical, Altimmune, Axcella, Blade, BNN Cardio, Celgene, Cirius, CymaBay, EcoR1, E3Bio, Galmed, Genentech, Grunthal, lndalo, Imperial Innovations, lnventiva, IQVIA, Janssen, Madrigal, MedImmune, Matacrine, NewGene, NGMBio, North Sea Therapeutics, Poxel, Prosciento, Raptor Pharma, Servier, Terns, and Viking Therapeutics. He is on the speakers’ bureau for Clinical Care Options, Falk, Fishawack, Integritas Communications, Kenes, and Medscape. He received grants from Vertex. He has active research collaborations with Antares Medical, Boehringer Ingelheim, Echosens, Ellegaard Gottingen Minipigs AS, Exalenz Bioscience, iXscient, Nordic Bioscience, OWL Genomics, Perspectum, Resound, Sanofi, Soma Logic, and Takeda. He receives royalties from Elsevier.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Golabi P, Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol 2019;71:793‐801. [DOI] [PubMed] [Google Scholar]
- 3. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El‐Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol 2016;14:301‐308.e1‐e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non‐alcoholic fatty liver disease in Asia, 1999‐2019: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol 2019;4:389‐398. [DOI] [PubMed] [Google Scholar]
- 5. Pedrosa M, Balp M, Janssens N, Lopez P, Mckenna S, Chatterjee S, et al. Global prevalence of nonalcoholic steatohepatitis (NASH): findings from a targeted literature review. Value Health 2018;21:S82. [Google Scholar]
- 6. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, et al. Prevalence of nonalcoholic steatohepatitis‐associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol 2017;112:581‐587. [DOI] [PubMed] [Google Scholar]
- 7. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of nonalcoholic fatty liver disease. Hepatology 2020. Feb 11. 10.1002/hep.31173. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8. Paik JM, Golabi P, Biswas R, Algahtani S, Venkatesam C, Younossi ZM. Nonalcoholic fatty liver disease and alcoholic liver disease are major drivers of liver mortality in the United States. Hepatol Commun 2020 April 4. 10.1002/hep4.1510. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 10. Golabi P, Bush H, Stepanova M, Locklear CT, Jacobson IM, Mishra A, et al. Liver transplantation (LT) for cryptogenic cirrhosis (CC) and nonalcoholic steatohepatitis (NASH) cirrhosis: data from the Scientific Registry of Transplant Recipients (SRTR): 1994 to 2016. Medicine (Baltimore) 2018;97:e11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Said A, Gagovic V, Malecki K, Givens M, Nieto F. Primary care practitioners’ survey of non‐alcoholic fatty liver disease. Ann Hepatol 2013;12:758‐765. [PubMed] [Google Scholar]
- 13. Younossi ZM, Stepanova M, Lawitz EJ, Reddy KR, Wai‐Sun Wong V, Mangia A, et al. Patients with nonalcoholic steatohepatitis experience severe impairment of health‐related quality of life. Am J Gastroenterol 2019;114:1636‐1641. [DOI] [PubMed] [Google Scholar]
- 14. Golabi P, Otgonsuren M, Cable R, Felix S, Koenig A, Sayiner M, et al. Non‐alcoholic fatty liver disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual Life Outcomes 2016;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinstein AA, Kallman Price J, Stepanova M, Poms LW, Fang Y, Moon J, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics 2011;52:127‐132. [DOI] [PubMed] [Google Scholar]
- 16. Huber Y, Boyle M, Hallsworth K, Tiniakos D, Straub BK, Labenz C, et al. Health‐related quality of life in nonalcoholic fatty liver disease associates with hepatic inflammation. Clin Gastroenterol Hepatol 2019;17:2085‐2092.e2081. [DOI] [PubMed] [Google Scholar]
- 17. Younossi ZM, Stepanova M, Younossi I, Racila A. Validation of chronic liver disease questionnaire for nonalcoholic steatohepatitis in patients with biopsy‐proven nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2019;17:2093‐2100.e3. [DOI] [PubMed] [Google Scholar]
- 18. Younossi ZM, Stepanova M, Lawitz EJ, Reddy KR, Wai‐Sun Wong V, Mangia A, et al. Patients with nonalcoholic steatohepatitis experience severe impairment of health‐related quality of life. Am J Gastroenterol 2019;114:1636‐1641. [DOI] [PubMed] [Google Scholar]
- 19. Younossi ZM, Stepanova M, Nader F, Loomba R, Anstee QM, Ratziu V, Harrison SA, et al. Assessment of patient‐reported outcomes (PROs) in patients with non‐alcoholic steatohepatitis (NASH) treated with obeticholic acid (OCA): results from REGENERATE phase 3 clinical trial In: Proceedings of the Liver Meeting of the American Society of the Study of Liver Diseases, Boston, MA, 2019. [Google Scholar]
- 20. Younossi ZM, Stepanova M, Anstee QM, Lawitz EJ, Wai‐Sun Wong V, Romero‐Gomez M, et al. Reduced patient‐reported outcome scores associate with level of fibrosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2019;17:2552‐2560.e10. [DOI] [PubMed] [Google Scholar]
- 21. Younossi ZM, Yu ML, Yilmaz Y, El Kassas M, Castellanos Fernandez M, et al. The impact of fatigue on patient‐reported outcomes in patients with chronic liver disease: data from the Global Liver Registry In: Proceedings of the Digestive Disease Week, San Diego, CA, 2019. [Google Scholar]
- 22. Harrison SA, Wai‐Sun Wong V, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized ph III STELLAR trials [published online ahead of print, 2020 Mar 5]. J Hepatol 2020;73:26‐39. [DOI] [PubMed] [Google Scholar]
- 23. Introductory Guide MedDRA version 13.1. https://www.meddra.org/sites/default/files/guidance/file/intguide_13_1_english.pdf. Accessed on March 18, 2020.
- 24. Neuschwander‐Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp‐Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European liver fibrosis panel and exploring simple markers. Hepatology 2008;47:455‐460. [DOI] [PubMed] [Google Scholar]
- 26. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 27. Vallet‐Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin‐Venier V, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology 2007;46:32‐36. [DOI] [PubMed] [Google Scholar]
- 28. Ratziu V, Massard J, Charlotte F, Messous D, Imbert‐Bismut F, Bonyhay L, et al. Diagnostic value of biochemical markers (FibroTest‐FibroSURE) for the prediction of liver fibrosis in patients with non‐alcoholic fatty liver disease. BMC Gastroenterol 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Juluri R, Vuppalanchi R, Olson J, Unalp A, Van Natta ML, Cummings OW, et al. Generalizability of the nonalcoholic steatohepatitis Clinical Research Network histologic scoring system for nonalcoholic fatty liver disease. J Clin Gastroenterol 2011;45:55‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Afdhal NH. Fibroscan (transient elastography) for the measurement of liver fibrosis. Gastroenterol Hepatol (N Y) 2012;8:605‐607. [PMC free article] [PubMed] [Google Scholar]
- 31. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 32. Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis 2001;21:3‐16. [DOI] [PubMed] [Google Scholar]
- 33. Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology 2018;155:1140‐1153. [DOI] [PubMed] [Google Scholar]
- 34. Ware JE, Kosinski M. Interpreting SF‐36 summary health measures: a response. Qual Life Res 2001;10:405‐413; discussion 415‐420. [DOI] [PubMed] [Google Scholar]
- 35. Younossi ZM, Stepanova M, Younossi I, Racila A. Validation of Chronic Liver Disease Questionnaire for Nonalcoholic Steatohepatitis in patients with biopsy‐proven nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2019;17 2093‐2100.e3. [DOI] [PubMed] [Google Scholar]
- 36. Webster K, Odom L, Peterman A, Lent L, Cella D. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res 1999;8:604. [Google Scholar]
- 37. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353‐365. [DOI] [PubMed] [Google Scholar]
- 38. Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5‐D itch scale: a new measure of pruritus. Br J Dermatol 2010;162:587‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012;92:497‐501. [DOI] [PubMed] [Google Scholar]
- 40. EuroQol Research Foundation . EQ‐5D. https://euroqol.org/. Accessed on March 23, 2020.
- 41. Jüngst C, Berg T, Cheng J, Green RM, Jia J, Mason AL, et al. Intrahepatic cholestasis in common chronic liver diseases. Eur J Clin Invest 2013;43:1069‐1083. [DOI] [PubMed] [Google Scholar]
- 42. Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 2017;65:350‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ekerfors U, Sunnerhagen KS, Westin J, Jakobsson Ung E, Marschall HU, Josefsson A, et al. Muscle performance and fatigue in compensated chronic liver disease. Scand J Gastroenterol 2019;54:925‐933. [DOI] [PubMed] [Google Scholar]
- 44. Swain MG, Jones DEJ. Fatigue in chronic liver disease: new insights and therapeutic approaches. Liver Int 2019;39:6‐19. [DOI] [PubMed] [Google Scholar]
- 45. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577‐1586. [DOI] [PubMed] [Google Scholar]
- 46. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 48. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non‐alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet 2019;394:2184‐2196. [DOI] [PubMed] [Google Scholar]
- 49. Enck P, Klosterhalfen S, Weimer K, Horing B, Zipfel S. The placebo response in clinical trials: more questions than answers. Philos Trans R Soc Lond B Biol Sci 2011;366:1889‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Younossi Z, Yu ML, Yilmaz Y, El Kassas M, Fernández MI, Wong V, et al. The impact of fatigue on patient‐reported outcomes (PRO) in patients with chronic liver disease (CLD): data from the Global Liver Registry (GLR) In: Proceedings from Digestive Disease Week, Chicago, IL; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


