Abstract
In autoimmune liver disease (AILD), including autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), and overlap syndrome of AIH and PSC (ASC), the presence of biliary injury portends a worse prognosis. We studied serum matrix metalloproteinase 7 (sMMP7) as a biomarker for pediatric sclerosing cholangitis (SC). We prospectively enrolled 54 children (median age, 16 years) with AILD (AIH, n = 26; ASC, n = 16; and PSC, n = 12) at our center. The sMMP7 concentrations were higher in patients with SC compared to those without cholangiopathy (P < 0.001). An sMMP7 concentration >23.7 ng/mL had a sensitivity and specificity of 79% and 96%, respectively, and outperformed alkaline phosphatase (ALP) and gamma‐glutamyltransferase (GGT) in segregating patients with SC. Serum concentrations correlated with liver gene expression levels for MMP7 (r = 0.70; P < 0.001). Using immunofluorescence, MMP7 was localized primarily to the cholangiocytes of patients with SC. In 46 subjects with liver biopsy available for blinded review, elevation in sMMP7 concentrations segregated with the presence of lymphocytic and neutrophilic cholangitis and periductal fibrosis and correlated with Ishak, Ludwig, and Nakanuma scoring systems. Liver stiffness measured by magnetic resonance elastography also correlated with sMMP7 concentrations (r = 0.56; P < 0.01). Using magnetic resonance cholangiopancreatography plus (MRCP+), sMMP7 in 34 patients correlated with the number of biliary dilatations (r = 0.54; P < 0.01) and strictures (r = 0.56; P < 0.01). MMP7 as a marker of biliary injury was validated in an independent cohort of children with ulcerative colitis. Higher sMMP7 concentrations also correlated with a history of SC‐related complication. Conclusion: MMP7 is a promising biomarker for pediatric SC that diagnostically outperforms ALP and GGT. sMMP7 may directly reflect biliary injury and fibrosis, the main drivers of disease progression in SC.
Abbreviations
- 3D
three dimensional
- AIH
autoimmune hepatitis
- AILD
autoimmune liver disease
- ALP
alkaline phosphatase
- ANOVA
analysis of variance
- ASC
autoimmune sclerosing cholangitis
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristic
- BA
biliary atresia
- BEC
biliary epithelial cell
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CI
confidence interval
- ECM
extracellular matrix
- ELE
elevated liver enzyme
- FFPE
formalin‐fixed paraffin embedded
- GGT
gamma‐glutamyltransferase
- HC
healthy control
- IBD
inflammatory bowel disease
- IBDc
inflammatory bowel disease, normal liver biochemistry
- IQR
interquartile range
- IRB
institutional review board
- mHAI
modified histologic activity index
- MMP7
matrix metalloproteinase 7
- MRCP
magnetic resonance cholangiopancreatography
- MRE
magnetic resonance elastography
- MRI
magnetic resonance imaging
- mRNA
messenger RNA
- NABA
nonautoimmune‐mediated biliary abnormality
- pMMP7
plasma matrix metalloproteinase 7
- PROTECT
Predicting Response to Standardized Pediatric Colitis Therapy
- PSC
primary sclerosing cholangitis
- PUCAI
pediatric ulcerative colitis activity index
- rMRCP
research magnetic resonance cholangiopancreatography
- ROC
receiver operating characteristic
- SC
sclerosing cholangitis
- sMMP7
serum matrix metalloproteinase 7
- TPM
transcripts per million
- UC
ulcerative colitis
Autoimmune liver diseases (AILDs), including autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), and autoimmune sclerosing cholangitis (ASC), which is an overlap of AIH and PSC, are well‐recognized causes of chronic liver disease in children.( 1 ) PSC is a progressive fibrosing cholangiopathy that is associated with concomitant inflammatory bowel disease (IBD) and characterized by cholestasis, inflammation and fibrosis of the biliary tree.( 2 ) AIH is characterized by necroinflammatory infiltrate and interface hepatitis on liver biopsy in the setting of hypergammaglobulinemia and autoantibodies.( 3 ) ASC, which has overlapping features of PSC and AIH, may be a more common disease entity in children than adults.( 4 )
In children with AILD, patients with sclerosing cholangitis (SC), whether PSC or ASC, are more likely to develop complications of chronic liver disease by 5 years from the time of diagnosis compared to patients with AIH.( 5 ) Thus, it is imperative to have highly sensitive and specific biomarkers to distinguish these two patient groups. Alkaline phosphatase (ALP) and gamma‐glutamyltransferase (GGT) are commonly used biomarkers to screen for biliary injury. In adult studies, ALP has been linked to prognosis and is used as an endpoint in clinical trials.( 6 ) In contrast, ALP is neither sensitive nor specific in pediatric PSC as ALP concentrations in children are confounded by rapid bone turnover during periods of growth.( 7 ) In children, GGT is more sensitive and specific than ALP for biliary injury. Multicenter retrospective studies have shown that normalization or greater than 75% reduction of GGT concentrations at 1 year compared to concentrations at the time of PSC diagnosis is predictive of event‐free survival in children.( 8 ) Despite these links to prognosis, neither GGT nor ALP has been linked to the degree of large duct disease or progression of fibrosis in PSC. Furthermore, GGT is a microsomal enzyme expressed in both hepatocytes and biliary epithelium and as such is also elevated in nonalcoholic fatty liver disease or following exposure to alcohol or medications.( 9 , 10 , 11 ) There is an unmet need for biomarkers that are directly related to progression of biliary duct injury and fibrosis in children with SC and that can be used to monitor disease progression.
Matrix metalloproteinase 7 (MMP7; also known as matrilysin) is an enzyme important for extracellular matrix (ECM) remodeling and the recruitment of inflammatory cells in response to cell injury.( 12 ) Recently, MMP7 has been shown to be a sensitive and specific marker of biliary atresia (BA), a rapidly progressive fibrosing cholangiopathy affecting the extrahepatic bile ducts in infants.( 13 ) Studies have shown MMP7 to be highly expressed in the extrahepatic bile ducts and to be sensitive and specific for distinguishing infants with BA from those with non‐BA causes of neonatal cholestasis. Liver MMP7 expression levels have also been shown to correlate with the stage of hepatic fibrosis in patients with BA following successful Kasai portoenterostomy.( 14 ) Given the biological possibility that pathways of ductal injury and biliary fibrosis are shared between BA and SC,( 15 ) we hypothesize that MMP7 may be a diagnostic biomarker of biliary injury and fibrosis in pediatric AILD.
In this study we evaluated the performance of serum MMP7 (sMMP7) as a biomarker for SC in patients with pediatric AILD; correlated serum and liver MMP7 gene expression; and determined the relationship between sMMP7 concentrations and bile duct injury and hepatic fibrosis as assessed by liver histopathology and prospective research magnetic resonance imaging (MRI) examinations.
Patients and Methods
Study Design and Patients
Two pediatric cohorts recruited under prospective research studies were evaluated to address our research questions: (1) a prospective single‐center cross‐sectional cohort of patients with AILD from Cincinnati Children’s Hospital Medical Center (CCHMC) and (2) a multicenter inception cohort of patients with IBD enrolled into the Predicting Response to Standardized Pediatric Colitis Therapy (PROTECT) study.( 16 ) Human study protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki and were approved by the CCHMC Institutional Review Board (IRB).
Prospective AILD Cohort
Patients receiving care at CCHMC were enrolled into the prospective observational study of pediatric patients with AILD (NCT03175471) between February 2017 and November 2018. Following consent to the CCHMC IRB‐approved study (IRB#2016‐7388), serum was collected from patients with an established or suspected diagnosis of AIH, PSC, or ASC. The clinical diagnosis of PSC was assigned based on established guidelines.( 2 ) Patients were assigned the diagnosis of AIH if they met the international autoimmune hepatitis study group simplified criteria( 3 ) and did not have radiologic or histologic evidence of cholangiopathy. Patients with ASC had histopathologic or radiographic evidence of biliary injury consistent with PSC and serologic evidence of AIH with a liver histopathology compatible with AIH.( 5 ) A retrospective chart review of this cohort was performed to identify clinical endpoints of AILD, as previously described, including ascites, hepatic encephalopathy, endoscopic evidence of esophageal varices, cholangitis, biliary strictures requiring intervention, cholangiocarcinoma, liver transplantation, and death from liver disease.( 5 , 17 ) Excess liver tissue was stored from clinically indicated liver biopsies, and study participants underwent a research MRI examination at the time of blood collection.
We report results from the first 60 consecutively enrolled patients from this cohort. Three patients withdrew from the study before any study‐related investigations. Of the remaining 57 patients, 3 patients had nonautoimmune‐mediated biliary abnormalities (NABA) on subsequent investigations (choledochal cyst, n = 2; pancreatic head mass, n = 1). An additional 8 children (median, 11 years; interquartile range [IQR], 10‐17 years) undergoing minor surgeries (upper endoscopy, 4 children; dental surgery, 1; pectus excavatum repair, 1; plastic surgery lesion excision, 1; normal visit, 1) at CCHMC without known history of liver disease or IBD were recruited as healthy controls (HCs) for collection of serum samples.
PROTECT Cohort
The PROTECT cohort is a multicenter prospective study of pediatric patients with newly diagnosed ulcerative colitis (UC); 431 total patients are enrolled (NCT01536535) and described in detail elsewhere.( 16 ) Informed consent/assent had been obtained from all patients, and the study had been approved by the local investigational review board at all investigative sites. Of the 431 patients at the time of enrollment, 8 had been diagnosed with PSC or ASC, 29 were found to have elevated liver enzymes (ELEs) without a diagnosis of chronic liver disease, and the remaining 394 had had IBD with normal liver biochemistries (IBDc). Plasma samples from the patients in this cohort with SC (n = 8) were matched by age, sex, and severity of colitis, using the pediatric ulcerative colitis activity index (PUCAI) at enrollment to samples from patients in this cohort with ELEs (n = 8) and IBDc (n = 16).
MRI
Research MRI examinations were performed on the AILD cohort at a field strength of 1.5 tesla (Ingenia; Philips Healthcare, Best, the Netherlands) and included coronal T2‐weighted single‐shot fast spin‐echo, axial T2‐weighted fast spin‐echo fat‐suppressed, and coronal three‐dimensional (3D) T2‐weighted fast spin‐echo magnetic resonance cholangiopancreatography (MRCP) pulse sequences. Axial 2D spin‐echo echo‐planar magnetic resonance elastography (MRE) was performed, as described.( 18 ) Research MRCP (rMRCP) images were reviewed by a board‐certified pediatric radiologist (J.R.D.) blinded to all clinical, biochemical, and histologic information. A radiologic diagnosis of SC was made according to established guidelines.( 2 )
Perspectum MRCP+
Using MRCP+, a proprietary MRI postprocessing software (Perspectum Diagnostics, Oxford, United Kingdom), 3D T2‐weighted MRCP images from the research MRI examinations were processed to generate a quantitative model of the biliary ducts and cross‐sectional diameters identifying candidate strictures and dilatations, as described.( 19 )
Histology
All patients with suspected AIH undergo liver biopsy for diagnosis and patients with AIH in remission for >3 years undergo repeat liver biopsy before withdrawal of immunosuppression. Given the high prevalence of overlap with AIH, the majority of patients with suspected PSC undergo liver biopsy at diagnosis. Patients who experience flares in their serum aminotransferases on treatment with immunosuppression often undergo repeat liver biopsy. Archived liver tissue sections from clinically indicated needle core biopsies for patients in the AILD cohort stained for hematoxylin and eosin and Masson’s trichrome were reviewed by an experienced pathologist (D.S.) blinded to clinical, biochemical, and radiographic data. Orcein staining for the Nakanuma score was performed on unstained slides when available.( 20 ) Tissues were scored based on the presence of biliary injury (lymphocytic cholangitis, acute cholangitis, pericholangitis, periductal fibrosis [“onion skinning”], bile duct proliferation, and bile ductular reaction), Ishak grade using the modified histologic activity index (mHAI) and stage, Ludwig score, and the Nakanuma scoring system( 21 , 22 , 23 ) (Supporting Information).
RNA Isolation
RNA was isolated from liver biopsy samples from patients in the AILD cohort with the MiRNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. Sequencing was performed by the University of Cincinnati DNA Core Center using 101 base pair, pair end reads at a depth of 50 million reads. Following removal of primers and barcodes, raw reads were aligned to the Hg19 genome and quantified using Kallisto v0.45.0 (Pachter Lab( 24 )) to accurately quantify transcript abundances using pseudoalignment. Further transcriptomic analyses were performed in GeneSpring 14.9 GX (Agilent Technologies), where transcripts per million (TPM) were log2 transformed and baselined to the median of all samples. Transcripts were filtered based on expression, requiring more than three reads in at least 20% of samples, resulting in 13,706 reasonably expressed transcripts available for differential and statistical analyses. Statistical analyses involving tissue‐based transcript expression and clinical and serologic variables were performed in R. Transcripts were filtered based on expression, requiring more than three reads in at least 20% of samples (n = 13,706 transcripts). Statistical analyses were performed in GeneSpring 14.9 GX.
MMP7 Quantification
MMP7 concentrations in serum and plasma obtained from both cohorts were determined by using Milliplex Multiplex kits (MilliporeSigma, Darmstadt, Germany) according to the manufacturer’s protocol, as described( 13 ) (Supporting Information).
Multiparameter IF
Archived 4‐µm formalin‐fixed paraffin‐embedded (FFPE) liver sections from patients with AILD in the AILD cohort were obtained from the CCHMC Biobank. Sequential heat‐induced epitope retrieval, incubation with primary and with horseradish peroxidase‐conjugated secondary antibodies, and tyramide signal amplification were performed using the PerkinElmer Tyramide Signal Amplification Kit (OP7TL2001KT; PerkinElmer) according to the manufacturer’s recommendations. Ethylene diamine tetraacetic acid (pH 9) was used for antigen retrieval before incubation with antibodies against cytokeratin (clone PAN‐CK; 1:100 dilution; Thermo Fisher Scientific), while citrate buffer (pH 6) was used before staining with antibodies against MMP7 (PAA102Hu01; 1:100 dilution; Cloud Corporation). Images were captured with an inverted Nikon Eclipse Ti2 widefield microscope (Nikon Instruments Inc., Tokyo, Japan). Image analysis was performed using Nikon Elements Advanced Research.
Statistics
Differences of continuous variables and categorical variables between groups were assessed for statistical significance (P < 0.05) using the Student t test (two sided) and Fisher’s exact test. Associations between continuous variables and ordinal variables were examined using a proportional odds model. Analysis of variance (ANOVA) and Tukey’s multiple comparison tests were used where appropriate. Graphs were generated using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA; www.graphpad.com), The area under the receiver operating characteristic (AUROC) was calculated for MMP7, ALP, and GGT to predict clinical diagnosis and abnormal rMRCP findings of cholangiopathy. The DeLong test was applied to compare AUROCs.
Results
Serum MMP7 as a Diagnostic Biomarker for Autoimmune Cholangiopathy
In order to examine whether sMMP7 concentrations could serve as a diagnostic biomarker to distinguish immune‐mediated bile duct injury of SC from AIH, NABA, and HCs, sMMP7 concentrations were measured in 54 patients with the diagnosis of SC or AIH from the AILD cohort, 3 children from the cohort with suspected AILD subsequently diagnosed with NABA, and 8 HCs. Baseline characteristics of the 54 patients with AILD are summarized in Table 1. Age and disease duration were similar between AIH and SC groups. In the patients with AIH and ASC, liver‐directed immunosuppression was started at the time of diagnosis of AILD. Concomitant IBD was more prevalent in the SC group, and platelets were lower in the AIH group. Liver biochemistries were similar in patients with AIH and SC, except ALP and GGT concentrations were higher in patients with SC. As expected, the Autoimmune Hepatitis Study Group‐simplified score at diagnosis was higher in patients with AIH and ASC compared to PSC.
Table 1.
Baseline Characteristics of 54 Enrolled Patients With AILD
| Characteristic | AIH (n = 26) | SC (ASC = 16 and PSC = 12) | P Value |
|---|---|---|---|
| Age, (years) | 16 (13‐18) | 16 (11‐19) | 0.70 |
| Male, n (%) | 13 (50) | 16 (57) | 0.79 |
| Disease duration, (years) | 1.9 (0.2‐4.0) | 1.6 (0.3‐4.2) | 0.80 |
| IBD, n (%) | 0 (0) | 13 (46) | <0.001 |
| ALT, U/L | 47 (23‐84) | 59 (34‐109) | 0.28 |
| AST, U/L | 34 (19‐66) | 36 (23‐92) | 0.32 |
| ALP, U/L | 129 (88‐200) | 196 (125‐326) | 0.02 |
| GGT, U/L | 34 (15‐109) | 85 (30‐232) | 0.02 |
| Total bilirubin, U/L | 0.8 (0.4‐1.1) | 0.5 (0.4‐1.1) | 0.52 |
| Direct bilirubin, U/L | 0.2 (0.1‐0.3) | 0.2 (0.1‐0.2) | 0.47 |
| Platelets (×103/μL) | 217 (74‐275) | 289 (127‐367) | 0.04 |
| APRI | 0.62 (0.27‐2.57) | 0.47 (0.19‐2.51) | 0.49 |
| Ishak stage | 2 (1‐5) | 4 (2‐6) | 0.07 |
| Ursodiol, n (%) | 0 (0) | 15 (54) | 0.001 |
| Oral vancomycin, n (%) | 0 (0) | 5 (18) | 0.05 |
| AIHSG simplified score* | 6 (5‐7) | 5 (3‐6)* | 0.08 |
Ranges are median (IQR). P < 0.05 indicates statistically significant differences between groups.
ASC 6 (5‐7) and PSC 3 (2‐5).
Abbreviations: AIHSG, Autoimmune Hepatitis Study Group; ALT, alanine aminotransferase; APRI, aspartate aminotransferase‐to‐platelet ratio index.
Serum samples for MMP7 concentrations were collected at a median of 1 day from laboratory investigations. sMMP7 concentrations were significantly higher in patients with SC compared to patients with AIH, NABA, and HCs (median, 34.6; IQR, 22.1‐70.9 vs. median, 14.0; IQR, 11.5‐16.7 vs. median, 18.7; IQR, 2.0‐19.1 vs. median, 7.2; IQR, 5.6‐9.4 ng/mL in SC vs. AIH vs. NABA vs. HCs, respectively; Fig. 1A). The optimal sMMP7 cutoff based on ROC analysis to distinguish SC from AIH was 23.7 ng/mL with sensitivity 79% (95% confidence interval [CI], 60%‐90%) and specificity 96% (95% CI, 81%‐100%). All patients with NABA had sMMP7 concentrations less than 23.7 ng/mL. sMMP7 concentrations correlated with serum aspartate aminotransferase (AST), ALP, and GGT concentrations (Supporting Fig. S1). ALP and GGT concentrations were normal in 41% and 25%, respectively, of SC patients with sMMP7 above 23.7 ng/mL. For the diagnosis of SC, the AUROC for sMMP7 (AUROC, 0.87; 95% CI, 0.77‐0.91) was significantly higher than that for ALP and GGT (AUROC, 0.66; 95% CI, 0.51‐0.81 and AUROC, 0.70; 95% CI, 0.51‐0.81, respectively) (Fig. 1B). Combining sMMP7 with either ALP or GGT did not improve the AUROC over sMMP7 alone.
Fig. 1.
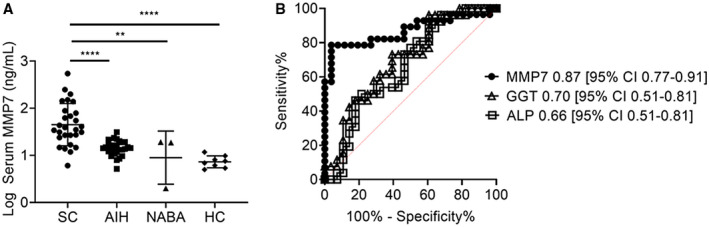
Serum MMP7 is a diagnostic biomarker of PSC/ASC. (A) sMMP7 concentrations were measured in 65 children (AIH, 24; ASC, 18; PSC, 12; NABA, 3; and HCs, 8) by Luminex. Differences between groups were tested for statistical significance using a one‐way ANOVA and Tukey’s test **P < 0.001, ****P < 0.0001. (B) ROC curves were generated for serum MMP7, ALP, and GGT in distinguishing SC from AIH. Cut‐off values for MMP7, ALP, and GGT were 23.7 ng/mL, 123 U/L, and 181 U/L, respectively, as determined by the maximal Youden’s index. ROCs of the three biomarkers were compared by applying the DeLong test with **P < 0.001, ****P < 0.0001.
Impact of Disease Activity of IBD on sMMP7 Concentrations in SC
Colonic epithelium and inflammatory cells have previously been found to up‐regulate MMP7 messenger RNA (mRNA) expression in UC.( 25 , 26 ) Given the association between SC and IBD, we investigated whether IBD confounded sMMP7 concentrations. We compared sMMP7 concentrations in patients with SC from the AILD cohort with (n = 13) and without (n = 15) concomitant IBD and found no difference (IBD, 31.0 ng/mL vs. non‐IBD, 42.4 ng/mL; P = 0.13). To further validate our findings in an independent cohort, we assayed plasma samples from the matched cohort of patients in the PROTECT study (Table 2). In this cohort, plasma MMP7 (pMMP7) concentrations in the patients with SC were higher than in the patients with ELEs and IBDc (median, 9.6; IQR, 6.4‐24.7 vs. median, 2.0; IQR, 1.5‐2.6 vs. median, 3.0; IQR, 2.1‐4.0 ng/mL in SC vs. ELEs vs. IBDc, respectively) (Fig. 2A). There was no difference in pMMP7 concentrations between patients with ELEs and IBDc (P = 0.81). Based on ROC analysis, pMMP7 concentrations >5.7 ng/mL distinguished patients with SC from those with ELEs and IBDc with an AUROC of 0.88 (95% CI, 0.71‐1.0; P = 0.002) (Fig. 2B). IBD activity as assessed by PUCAI did not correlate with pMMP7 concentrations (P = 0.72). The cut‐off levels for predicting SC were lower in plasma compared to serum samples. In summary, our findings suggest that circulating MMP7 concentrations (serum or plasma) do not appear to be confounded by IBD severity.
Table 2.
PROTECT Cohort Patient Characteristics
| Characteristic | Liver Disease (ASC, n = 2, PSC, n = 6) | Elevated Liver Enzymes Without Established Liver Disease (n = 8) | IBD Controls (n = 16) | LD vs. (ELE+IBDc) |
|---|---|---|---|---|
| Age (years), mean ± SD | 13.8 ± 1.8 | 12.8 ± 2 .5 | 13.6 ± 2.7 | 0.69 |
| Female n (%) | 2 (25%) | 4 (50%) | 6 (38%) | 0.68 |
| PUCAI score (range, 0‐85), mean ± SD | 37 ± 14 | 35 ± 18 | 37 ± 18 | 0.98 |
| ALT (U/L), median (IQR) | 97.5 (43‐130) | 76 (39‐175) | 17.0 (13‐24) | 0.006 |
| AST (U/L), median (IQR) | 88 (37‐100) | 61 (32‐146) | 22 (17‐25) | 0.21 |
| Total bilirubin, median (IQR) | 0.6 (0.4‐0.7) | 0.3 (0.2‐0.5) | 0.3 (0.3‐0.4) | <0.001 |
| Direct bilirubin, median (IQR) | 0.2 (0.2‐0.3) | 0.2 (0.1‐0.2) | 0.1 (0.1‐0.2) | <0.001 |
| ALP (U/L), median (IQR) | 543 (346‐705) | 242 (134‐428) | 149 (106‐195) | <0.001 |
| GGT (U/L), median (IQR) | 260.0 (178‐474) | 86 (25‐124) | 11 (9‐14) | <0.001 |
Abbreviation: ALT, alanine aminotransferase.
Fig. 2.
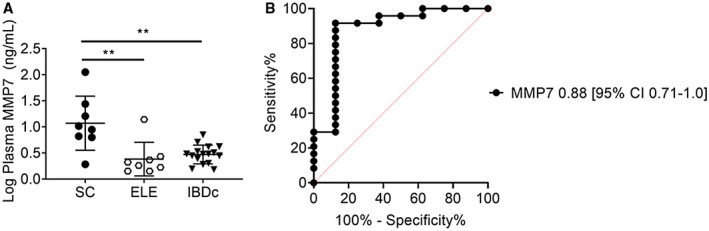
Validation of plasma MMP7 as a diagnostic biomarker for concomitant PSC/ASC in an inception cohort of pediatric patients with UC. (A) MMP7 concentrations were measured by Luminex in archived plasma samples from patients with UC at the time of diagnosis. Results were grouped according to presence of ASC/PSC (n = 8), ELEs (n = 8), or IBDc (n = 16). Differences among groups were tested for statistical significance using a one‐way ANOVA and Tukey’s test; **P < 0.005. (B) An ROC curve for pMMP7 concentrations in distinguishing ASC/PSC from ELEs and IBDc was constructed.
Tissue and Cellular Origin of sMMP7 in AILD
MMP7 expression has previously been reported in gallbladder, kidney, lung, and colonic epithelium.( 13 , 27 , 28 , 29 ) To examine whether elevation of sMMP7 concentration originated from the liver, we examined the correlation between sMMP7 and liver MMP7 mRNA expression. RNA was isolated from 20 liver biopsy samples (SC, n = 10 and AIH, n = 10) from patients in the AILD cohort that were obtained at a median of 1.5 (IQR, 0.25‐119) days after serum collection and subjected to RNA sequencing. Expression of MMP7 mRNA in whole‐liver tissue was higher in patients with SC than in patients with AIH (15 TPM vs. 3 TPM; P = 0.002) (Fig. 3A). There was a strong correlation between liver tissue MMP7 mRNA expression and sMMP7 concentration (r = 0.70; 95% CI, 0.39‐0.88; P < 0.001) (Fig. 3B). In contrast, the liver tissue expression of ALP and GGT mRNA did not correlate with serum concentrations of ALP and GGT (Supporting Fig. S2A,B).
Fig. 3.
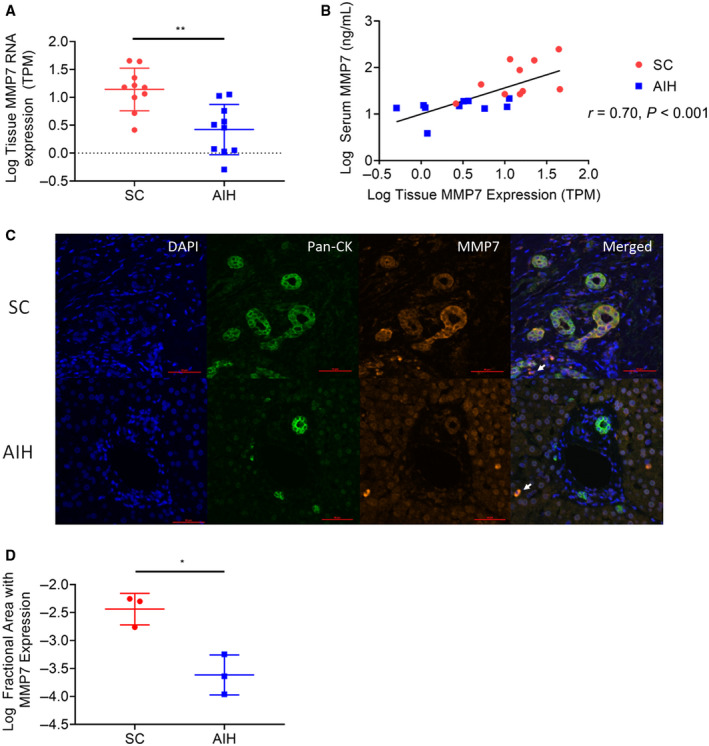
Relationship between concentration of MMP7 in serum and its expression in the liver. (A) RNA sequencing was performed on excess liver tissue from clinically indicated liver biopsies in 20 patients with AILD (AIH, 10; ASC/PSC, 10), and tissue mRNA concentrations were compared between both groups by applying the unpaired t test with **P < 0.01. (B) Liver tissue mRNA expression was correlated with sMMP7 concentrations, as determined by Luminex. The P value represents Pearson’s correlation coefficient. (C) Archived FFPE liver sections from 3 patients with ASC/PSC and 3 patients with AIH were subjected to multiparameter IF with antibodies against Pan‐CK and MMP7. MMP7 expression decorating cholangiocytes in patients with ASC/PSC is shown in representative photomicrographs. Minimal MMP7 expression on nonparenchymal cells found in patients with ASC/PSC and AIH is denoted with a white arrow. Magnification 200x. (D) Automated image analysis on digitalized IF images was performed to determine the MMP7+ area in livers from patients with ASC/PSC and AIH. Statistical significance was determined using an unpaired t test with *P < 0.05. Abbreviations: DAPI, 4′,6‐diamidino‐2‐phenylindole; Pan‐CK, pan cytokeratin.
To identify the cellular sources of MMP7 within the liver, archived FFPE liver tissue of 6 patients from the AILD cohort (SC, 3 and AIH, 3) were subjected to immunofluorescence (IF). MMP7 localized primarily to biliary epithelial cells (BECs) in patients with SC (Fig. 3C), while it was not prominently expressed by BECs in patients with AIH. A few nonparenchymal cells also expressed MMP7 in patients with AILD. Image analysis revealed that liver tissue from patients with SC had more area of MMP7‐expressing cells compared to patients with AIH (P = 0.03) (Fig. 3D).
Our liver gene expression and IF studies suggest that biliary injury is associated with up‐regulation of MMP7 expression in intrahepatic BECs in SC, which correspondingly results in increased sMMP7 concentration.
Association of sMMP7 Concentrations With Histologic Findings of AILD
Archived liver tissue slides were available for 46 patients from the AILD cohort (AIH , 25; SC, 21). The median duration between serum collection for sMMP7 quantification and liver biopsy was 43 days (IQR, 1‐298 days). Pericholangitis and periductal fibrosis were more prevalent in patients with SC compared to patients with AIH (Supporting Fig. S3). Importantly, sMMP7 concentration correlated with the typical features of immune‐mediated biliary injury of SC (Table 3). Periductal fibrosis, a histologic feature closely linked to PSC, also highly correlated with sMMP7 concentration. ALP and GGT also correlated with a number of histologic features of immune‐mediated biliary injury, but sMMP7 was the only biomarker that correlated with bile duct proliferation.
Table 3.
Correlation of MMP7, ALP, and GGT to Histologic Parameters of Biliary Injury and Fibrosis
| Histologic Parameter | MMP7 | ALP | GGT |
|---|---|---|---|
| All patients (n = 46) | Chi‐square P value | ||
| Lymphocytic infiltrate | <0.01 | 0.03 | <0.01 |
| Acute cholangitis | 0.02 | 0.08 | 0.04 |
| Acute pericholangitis | <0.01 | <0.01 | 0.01 |
| Periductal fibrosis | 0.01 | 0.03 | 0.09 |
| Bile duct proliferation | 0.04 | 0.05 | 0.09 |
| Bile duct atrophy | <0.01 | 0.06 | 0.11 |
| Bile ductular reaction | 0.25 | 0.49 | 0.36 |
| ASC/PSC (n = 21 patients) | Proportional odds model: odds ratio (95% CI) | ||
| Ishak stage | 53.5 (3.3‐880) | 3.5 (0.3‐37.0) | 1.6 (0.3‐7.8) |
| Ludwig’s score | 43.8 (2.2‐871) | 4.7 (0.3‐62.2) | 0.7 (0.2‐5.3) |
| Nakanuma stage | 38.5 (1.4‐1085) | 7.3 (0.3‐186.8) | 3.4 (0.4‐32.1) |
Ishak stage, Ludwig score, and the Nakanuma staging system, which includes features of chronic biliary injury, have been shown to predict the prognosis of adult patients with PSC.( 20 , 23 ) Therefore, we examined the correlation between these histologic scoring systems and sMMP7 concentrations. While sMMP7 concentrations correlated with all three liver scores in patients with SC (n = 21), ALP and GGT did not (Table 3). Notably, there was no correlation between the Ishak stage and sMMP7 concentrations (P = 0.80) in patients with AIH (n = 25). Furthermore, there was no relationship between the mHAI score (as a marker of hepatic inflammation) and sMMP7 concentrations (P = 0.99), suggesting that MMP7 is specific to biliary injury and biliary fibrosis.
In summary, histologic features of bile duct injury and biliary fibrosis were associated with increased sMMP7 concentrations in AILD. Furthermore, sMMP7 concentrations strongly correlated with validated histologic prognostic scores of PSC.
Correlation of sMMP7 Concentrations With Imaging Biomarkers of Hepatobiliary Injury in AILD
Cholangiogram
Fifty‐four patients from the AILD cohort had sMMP7 concentrations correlated with rMRCP images to determine whether elevations in sMMP7 were associated with radiographic evidence of biliary injury. Twenty‐nine of 54 patients had abnormal cholangiograms by rMRCP, 24 from the SC group and 5 from the AIH group. Among serum biomarkers obtained within a median of 2 days of the rMRCP, sMMP7 and GGT predicted an abnormal cholangiogram with an AUROC of 0.73 (95% CI, 0.59‐0.87) and 0.68 (95% CI, 0.53‐0.82), respectively, while ALP did not (AUROC, 0.56; 95% CI, 0.41‐0.72) (Fig. 4A). Of the 24 abnormal rMRCPs in patients with SC, 19 had intrahepatic and extrahepatic biliary disease while 5 had isolated intrahepatic biliary disease. There was no difference in sMMP7 concentrations based on location of disease (intrahepatic vs. extrahepatic; P = 0.95). In patients with SC, there was no difference in sMMP7 concentrations between those with large (n = 24) and small duct (n = 4) disease defined by the rMRCP (median, 39.2; IQR, 20.3‐83.7 vs. median, 39.2; IQR, 28.9‐160 ng/mL; P = 0.75) (Supporting Fig. S4A).
Fig. 4.
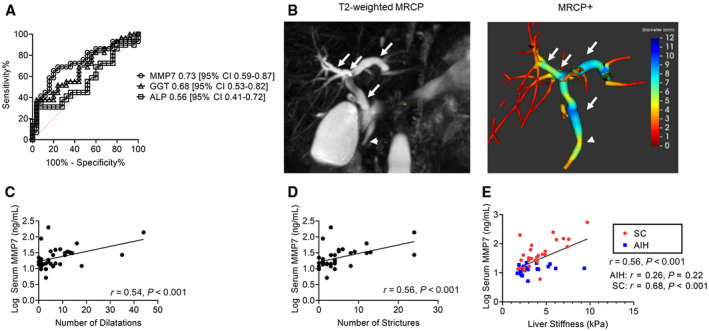
Correlation between serum MMP7 concentrations and MRI‐based determination of large bile duct damage and fibrosis. (A) Prediction of cholangiopathy on concomitant rMRCP by sMMP7, ALP, and GGT. (B) Biliary injury was quantitated on T2‐weighted 3D rMRCP using the proprietary Perspectum MRCP+ software. sMMP7 concentrations were correlated with the numbers of candidate (C) dilatations and (D) strictures. (E) sMMP7 concentrations were correlated with liver stiffness as measured by MRE. P values represent Pearson’s correlation coefficients.
MRCP+
To further explore the relationship between sMMP7 concentrations and imaging evidence of biliary injury, 34 rMRCPs were quantitatively analyzed using MRCP+ software (Fig. 4B). The number of candidate dilatations and strictures correlated with sMMP7 concentrations with r values of 0.54 (95% CI, 0.25‐0.75) and 0.56 (95% CI, 0.27‐0.75), respectively (Fig. 4C,D).
Taken together, sMMP7 appears to be a biomarker of all types of biliary injury, irrespective of extrahepatic or intrahepatic location and large or small duct disease.
MRE
Measured liver stiffness has been shown to be predictive of hepatic decompensation in adults with PSC.( 30 ) In our AILD cohort, sMMP7 concentrations correlated with liver stiffness (r = 0.56; 95% CI, 0.34‐0.73; P < 0.001) (Fig. 4E). Notably, in the subgroup of patients with SC, sMMP7 and liver stiffness measurements were strongly correlated (r = 0.68; 95% CI, 0.43‐0.84; P < 0.001), whereas, there was no significant correlation between sMMP7 and liver stiffness in the subgroup of patients with AIH (r = 0.26; (95% CI, −0.17 to 0.61; P = 0.22). This suggests that processes responsible for progression of biliary fibrosis may be associated with secretion of MMP7. Serum GGT also correlated (r = 0.50; 95% CI, 0.26‐0.68; P < 0.001) with liver stiffness, but the relationship was not restricted to one subgroup of patients (Supporting Fig. S4B).
Correlation of sMMP7 and Clinical Endpoints
Because sMMP7 concentrations correlated with biliary injury and fibrosis, we examined whether patients with SC and higher sMMP7 concentrations were more likely to have experienced a clinical endpoint of chronic liver disease (ascites, hepatic encephalopathy, endoscopic evidence of esophageal varices, cholangitis, biliary strictures requiring intervention, cholangiocarcinoma, liver transplantation, or death from liver disease). In the patients with SC in the AILD cohort (n = 28), sMMP7 concentrations were higher in patients with a history of complications related to liver disease (P = 0.03). Among patients with sMMP7 >69 ng/mL (n = 7), which represented the top quartile, 71% had a history of a complication related to liver disease. In contrast, only 1 patient with an sMMP7 <23 ng/mL, representing the bottom quartile, developed a complication of portal hypertension. Serum ALP and GGT did not correlate with a history of clinical endpoints. Thus, sMMP7 concentrations may reflect active biliary inflammation and fibrosis and may be useful to stratify patients at risk of complications related to their liver disease.
Discussion
We report the results of a single‐center cross‐sectional study to determine the performance of sMMP7 as a biomarker for biliary injury and liver fibrosis in patients with pediatric onset AILD. The analysis includes baseline characteristics of 54 patients with pediatric onset AIH, PSC, and ASC who were enrolled into an observational study involving the collection of clinical information, serum samples, a research 3D MRCP, MRE, and a review of liver histopathology. Our analysis shows that sMMP7 is a specific marker for biliary injury in SC. We have shown that in children with AILD, an sMMP7 concentration >23.7 ng/mL can distinguish patients with SC from those with AIH with an AUROC of 0.87. This level of diagnostic performance was significantly better than that for concomitantly obtained GGT and ALP. Importantly, because IBD may coexist with SC, circulating MMP7 was not confounded by IBD or IBD severity.
Our data support that sMMP7 is a marker of immune‐mediated biliary disease in patients with SC because all subjects with NABA had low sMMP7 concentrations. In patients with SC, liver MMP7 expression was up‐regulated on BECs, the site of immune‐mediated injury. sMMP7 concentrations correlated closely with hepatic MMP7 expression and was linked to histologic evidence of biliary injury. Furthermore, sMMP7 concentration was linked to histopathologic predictors of disease progression, including the Ishak stage and the Nakanuma and Ludwig scoring systems, in patients with SC. This linkage of sMMP7 to histopathologic disease staging was reinforced by the linkage we have shown between sMMP7 and imaging markers of disease, including liver stiffness by MRE and features of biliary disease on MRCP. Our findings suggest that elevation of sMMP7 concentrations are driven by both progressive biliary injury and fibrosis, which are the primary disease processes linked to clinical endpoints in PSC; this is a quality that sets sMMP7 apart from the current clinically available biomarkers GGT and ALP.
Elevated sMMP7 may directly reflect immune‐mediated biliary injury in children with AILD. The immunomodulatory role of MMP7 in activating local macrophages and amplifying the local inflammatory response has been reported in IBD and systemic lupus erythematosus.( 31 , 32 ) In biliary atresia, MMP7 was most strongly expressed in the extrahepatic bile ducts, reflecting the site of injury in this disease.( 13 ) In SC, immune‐mediated biliary injury can occur from the interlobular level to the level of the extrahepatic ducts. We found similar sMMP7 concentrations in patients with small and large duct disease, suggesting that any biliary injury can increase sMMP7 concentrations. Indeed, our IF from needle core biopsies localized MMP7 expression mainly to cholangiocytes, even at the interlobular level. Our findings suggest that pathomechanisms causing bile duct injury and fibrosis in SC are associated with secretion of MMP7. Given the up‐regulation of MMP7 on BECs in SC as the primary cellular source for MMP7, we propose that biliary injury is the primary process raising sMMP7 concentrations in SC. This is corroborated by a recent study showing higher concentrations of MMP7 in the bile aspirates of adult patients with PSC compared to healthy and IBD controls.( 33 ) Moreover, there was no correlation between sMMP7 concentration and mHAI, a measure of hepatic inflammation, reinforcing the specificity of MMP7 to biliary injury. Because MMP7 was not localized to the biliary epithelium of patients with AIH, we speculate that MMP7 is induced following biliary injury and released from damaged cholangiocytes into the surrounding tissue and systemic circulation (Supporting Fig. S5).
We suspect the association between sMMP7 concentration and liver fibrosis results from biliary fibrosis accompanying chronic cholangiopathy. MMP7 is a zinc‐ and calcium‐dependent endopeptidase that has a broad number of substrates, including components of the ECM and the basement membrane. In renal fibrosis, MMP7 plays an active profibrogenic role through transforming growth factor beta signaling and ECM deposition.( 28 ) Two earlier studies in infants with BA also implicated the role of MMP7 in hepatic fibrosis.( 14 , 34 ) Therefore, it is a biomarker that is directly involved in the disease mechanisms it is used to measure. In pediatric SC, we show a strong correlation between sMMP7 and histopathologic stage and liver stiffness. By providing information regarding both biliary inflammation and fibrosis, the two main drivers of disease progression in SC, patients with low MMP7 concentrations are less likely to have significant biliary injury and fibrosis and therefore less likely to have had complications related to chronic liver disease. In support of this, patients in our cohort with SC in the lowest sMMP7 quartile were less likely to have had a liver disease‐related complication compared to those in the highest quartile. While our study does not examine how sMMP7 predicts disease course, we do show a correlation between sMMP7 and the Nakanuma score, Ludwig score, Ishak stage, and liver stiffness, all of which have been shown to be outcomes in the adult PSC population.( 23 , 30 ) Therefore, MMP7 also has the potential to serve as a prognostic biomarker.
Radiologic biomarkers of biliary injury and fibrosis have been used as surrogate endpoints in the adult PSC population. MRCP+ is a novel technology that has received U.S. Food and Drug Administration (FDA) clearance to quantitatively characterize the biliary tree (see FDA letter to Perspectum Diagnostics). While MRCP+ separates children with SC from AIH,( 19 ) we are the first to correlate a circulating biomarker with the number of candidate strictures and dilatations reported by this technology. Recently, the change in liver stiffness measurement by MRE per year was independently associated with hepatic decompensation in adults with PSC.( 35 ) Given the strong correlation between liver stiffness measurement by MRE and sMMP7 concentration in our cohort, changes in sMMP7 concentrations over time may provide prognostic information.
sMMP7 can be incorporated into the initial workup of AILD in conjunction with liver enzymes, autoantibodies, immunoglobulin G, liver biopsy, and imaging studies. With a specificity of 96%, an elevated MMP7 level would suggest a diagnosis of SC. When used to monitor patients with AIH, an elevated sMMP7 level may identify those who may have developed ASC. Similarly, when added to routine laboratory investigations in patients with UC, an elevated MMP7 may herald the development of PSC and differentiates those patients with transient elevation of liver enzymes. A prompt diagnosis of SC is critical as there are implications regarding surveillance for hepatobiliary and colorectal malignancies. Up to 50% of cholangiocarcinomas, a major cause of mortality in patients with PSC, are diagnosed at or within 1 year of the diagnosis of PSC, which may reflect unrecognized chronic biliary inflammation.( 36 )
To our knowledge, we are the first to report MMP7 as a novel biomarker of biliary injury in pediatric AILD. Given the slow progressive nature of PSC, adverse clinical outcomes may not be adequately captured in the setting of a clinical trial. Therefore, surrogate markers are needed to demonstrate the efficacy of an intervention. In adult studies, normalization or reduction of ALP has been used as an endpoint in all clinical trials in the last 2 decades.( 37 ) While in pediatrics, a greater than 75% reduction or normalization of GGT by 1 year may be associated with a higher event‐free survival.( 8 ) However, most patients may have spontaneous reductions in ALP and GGT, and it is still unclear how ALP and GGT relate to the underlying mechanism of injury in the primary disease process. The advantage of MMP7 is that elevated concentrations may directly reflect ongoing biliary injury and fibrosis.
The strengths of our study include its prospective design, clear correlation between sMMP7 concentrations and tissue‐level MMP7 expression and localization of tissue MMP7 primarily to the BECs. Furthermore, we used multiple modalities, including histopathology and quantitative MRI, to correlate sMMP7 concentrations with biliary injury and fibrosis in a blinded fashion. We also validated MMP7 as a marker of biliary injury in an independent cohort of patients with IBD. Our cross‐sectional approach enabled us to study sMMP7 across a wide spectrum of disease, including patients from time of diagnosis to end‐stage liver disease. The main limitation to our study was our relatively small sample size. Pediatric AILDs are rare diseases, with the prevalence of PSC, ASC, and AIH at 1.5, 0.6, and 3 in 100,000 patient years, respectively.( 5 ) However, despite this small sample size, we show that sMMP7 outperformed ALP and GGT in segregating patients with SC from AIH and correlated with histopathologic and radiographic features linked to disease outcome. Another limitation was that MMP7 concentrations were not studied longitudinally or following interventions (i.e., stent placement in a dominant stricture). The role of sMMP7 as a dynamic biomarker remains to be seen. Future investigations include validation of MMP7 in an independent multicenter cohort of pediatric patients with AILD, examining sMMP7 in a longitudinal fashion, and prospectively evaluating sMMP7 as a prognostic biomarker. If our observations are validated in larger independent cohorts, sMMP7 may serve as a diagnostic, dynamic, and prognostic biomarker in pediatric AILD and has the potential to be a surrogate endpoint for clinical trials in pediatric SC.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Supplementary Material
Acknowledgment
The authors thank the investigators and coordinators of the PROTECT study.
Supported by the Center for Autoimmune Liver Disease, Center for Translational Fibrosis Research, American Association for the Study of Liver Disease Foundation‐Advanced Transplant Hepatology (S.L.), University of Calgary Helios (Scholarship to S.L.), Cincinnati Digestive Health Center, Connecticut Children’s Hospital Medical Center through the Predicting Response to Standardized Pediatric Colitis Therapy Study (number U01DK095745 to J.S.H.), Cincinnati Digestive Diseases Research Core Center (PHS Grant P30DK078392), and Perspectum Diagnostics (Oxford, United Kingdom; in‐kind image processing). The National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) grant DK095001 supported this study (to A.G.M.).
Potential conflict of interest: Dr. Goldfinger is employed by Perspectum. Dr. Dillman advises and has other interests in Perspectum. Dr. Haramija and Dr. Ridgway own stock in and are employed by Perspectum. Dr. Miethke consults for Mirum and Metacrine. Dr. Trout received grants from Perspectum. The other authors have nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Mieli‐Vergani G, Vergani D. Autoimmune liver diseases in children—what is different from adulthood? Best Pract Res Clin Gastroenterol 2011;25:783‐795. [DOI] [PubMed] [Google Scholar]
- 2. Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660‐678. [DOI] [PubMed] [Google Scholar]
- 3. Mileti E, Rosenthal P, Peters MG. Validation and modification of simplified diagnostic criteria for autoimmune hepatitis in children. Clin Gastroenterol Hepatol 2012;10:417‐421.e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D, et al. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16‐year prospective study. Hepatology 2001;33:544‐553. [DOI] [PubMed] [Google Scholar]
- 5. Deneau M, Jensen MK, Holmen J, Williams MS, Book LS, Guthery SL. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology 2013;58:1392‐1400. [DOI] [PubMed] [Google Scholar]
- 6. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis‐a comprehensive review. J Hepatol 2017;67:1298‐1323. [DOI] [PubMed] [Google Scholar]
- 7. Miloh T, Arnon R, Shneider B, Suchy F, Kerkar N. A retrospective single‐center review of primary sclerosing cholangitis in children. Clin Gastroenterol Hepatol 2009;7:239‐245. [DOI] [PubMed] [Google Scholar]
- 8. Deneau MR, Mack C, Abdou R, Amin M, Amir A, Auth M, et al. Gamma glutamyltransferase reduction is associated with favorable outcomes in pediatric primary sclerosing cholangitis. Hepatol Commun 2018;2:1369‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J 2003;79:307‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, et al. Guidelines on the management of abnormal liver blood tests. Gut 2018;67:6‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology 2009;50:1282‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parks WC, Wilson CL, Lopez‐Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617‐629. [DOI] [PubMed] [Google Scholar]
- 13. Lertudomphonwanit C, Mourya R, Fei L, Zhang Y, Gutta S, Yang L, et al. Large‐scale proteomics identifies MMP‐7 as a sentinel of epithelial injury and of biliary atresia. Sci Transl Med 2017;9:eaan8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerola A, Lampela H, Lohi J, Heikkila P, Mutanen A, Hagstrom J, et al. Increased MMP‐7 expression in biliary epithelium and serum underpins native liver fibrosis after successful portoenterostomy in biliary atresia. J Pathol Clin Res 2016;2:187‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo Z, Jegga AG, Bezerra JA. Gene‐disease associations identify a connectome with shared molecular pathways in human cholangiopathies. Hepatology 2018;67:676‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hyams JS, Davis S, Mack DR, Boyle B, Griffiths AM, LeLeiko NS, et al. Factors associated with early outcomes following standardised therapy in children with ulcerative colitis (PROTECT): a multicentre inception cohort study. Lancet Gastroenterol Hepatol 2017;2:855‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deneau MR, El‐Matary W, Valentino PL, Abdou R, Alqoaer K, Amin M, et al. The natural history of primary sclerosing cholangitis in 781 children: a multicenter, international collaboration. Hepatology 2017;66:518‐527. [DOI] [PubMed] [Google Scholar]
- 18. Trout AT, Serai S, Mahley AD, Wang H, Zhang Y, Zhang B, et al. Liver stiffness measurements with MR elastography: agreement and repeatability across imaging systems, field strengths, and pulse sequences. Radiology 2016;281:793‐804. [DOI] [PubMed] [Google Scholar]
- 19. Gilligan LA, Trout AT, Lam S, Singh R, Tkach JA, Serai SD, et al. Differentiating pediatric autoimmune liver diseases by quantitative magnetic resonance cholangiopancreatography. Abdom Radiol (NY) 2020;45:168‐176. [DOI] [PubMed] [Google Scholar]
- 20. de Vries EM, Verheij J, Hubscher SG, Leeflang MM, Boonstra K, Beuers U, et al. Applicability and prognostic value of histologic scoring systems in primary sclerosing cholangitis. J Hepatol 2015;63:1212‐1219. [DOI] [PubMed] [Google Scholar]
- 21. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696‐699. [DOI] [PubMed] [Google Scholar]
- 22. Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology 1989;10:430‐436. [DOI] [PubMed] [Google Scholar]
- 23. de Vries EM, de Krijger M, Farkkila M, Arola J, Schirmacher P, Gotthardt D, et al. Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: an international cohort study. Hepatology 2017;65:907‐919. [DOI] [PubMed] [Google Scholar]
- 24. Bray NL, Pimentel H, Melsted P, Pachter L. Near‐optimal probabilistic RNA‐seq quantification. Nat Biotechnol 2016;34:525‐527. [DOI] [PubMed] [Google Scholar]
- 25. Rath T, Roderfeld M, Halwe JM, Tschuschner A, Roeb E, Graf J. Cellular sources of MMP‐7, MMP‐13 and MMP‐28 in ulcerative colitis. Scand J Gastroenterol 2010;45:1186‐1196. [DOI] [PubMed] [Google Scholar]
- 26. Pedersen G, Saermark T, Kirkegaard T, Brynskov J. Spontaneous and cytokine induced expression and activity of matrix metalloproteinases in human colonic epithelium. Clin Exp Immunol 2009;155:257‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben‐Dor A, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99:6292‐6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ke B, Fan C, Yang L, Fang X. Matrix metalloproteinases‐7 and kidney fibrosis. Front Physiol 2017;8:21. Erratum in: Front Physiol 2017;8:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jakubowska K, Pryczynicz A, Iwanowicz P, Niewinski A, Maciorkowska E, Hapanowicz J, et al. Expressions of matrix metalloproteinases (MMP‐2, MMP‐7, and MMP‐9) and their inhibitors (TIMP‐1, TIMP‐2) in inflammatory bowel diseases. Gastroenterol Res Pract 2016;2016:2456179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eaton JE, Dzyubak B, Venkatesh SK, Smyrk TC, Gores GJ, Ehman RL, et al. Performance of magnetic resonance elastography in primary sclerosing cholangitis. J Gastroenterol Hepatol 2016;31:1184‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rath T, Roderfeld M, Graf J, Wagner S, Vehr AK, Dietrich C, et al. Enhanced expression of MMP‐7 and MMP‐13 in inflammatory bowel disease: a precancerous potential? Inflamm Bowel Dis 2006;12:1025‐1035. [DOI] [PubMed] [Google Scholar]
- 32. Vira H, Pradhan V, Umare V, Chaudhary A, Rajadhyksha A, Nadkar M, et al. Role of MMP‐7 in the pathogenesis of systemic lupus erythematosus (SLE). Lupus 2017;26:937‐943. [DOI] [PubMed] [Google Scholar]
- 33. Vesterhus M, Holm A, Hov JR, Nygard S, Schrumpf E, Melum E, et al. Novel serum and bile protein markers predict primary sclerosing cholangitis disease severity and prognosis. J Hepatol 2017;66:1214‐1222. [DOI] [PubMed] [Google Scholar]
- 34. Huang CC, Chuang JH, Chou MH, Wu CL, Chen CM, Wang CC, et al. Matrilysin (MMP‐7) is a major matrix metalloproteinase upregulated in biliary atresia‐associated liver fibrosis. Mod Pathol 2005;18:941‐950. [DOI] [PubMed] [Google Scholar]
- 35. Eaton JE, Sen A, Hoodeshenas S, Schleck CD, Harmsen WS, Gores GJ, et al. Changes in liver stiffness, measured by magnetic resonance elastography, associated with hepatic decompensation in patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2020;18:1576‐1583.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 2011;54:1842‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ponsioen CY, Chapman RW, Chazouilleres O, Hirschfield GM, Karlsen TH, Lohse AW, et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology 2016;63:1357‐1 367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Supplementary Material


