Abstract
Hepatocellular carcinoma (HCC) has a strong racial and ethnic association, with Hispanic patients having a higher incidence and mortality. However, there are limited data regarding clinical features and outcomes. This study includes Hispanic and non‐Hispanic White patients with HCC diagnosed between January 2000 and June 2014 from five United States academic medical centers. The chi‐square test for categorical variables and analysis of variance for continuous variables were used for statistical analysis, with two‐tailed P < 0.05 considered statistically significant. Of 5,327 patients, 4,217 met inclusion criteria, of whom 12.3% were Hispanic patients. Compared to their non‐Hispanic White counterparts, Hispanic patients were older at age of diagnosis (mean ± SD, 64.2 ± 10.9 vs. 61.9 ± 10.5 years; P < 0.0001), with higher body mass index (29.6 ± 6.5 vs. 28.8 ± 5.9 kg/m2; P = 0.01), and were more likely to have diabetes and hypertension. Hispanic patients had significantly more nonalcoholic fatty liver disease and alcohol‐related liver disease (both P < 0.0001). Hispanic patients presented with larger tumors, more advanced stage disease, and increased rates of macrovascular invasion and extrahepatic spread. HCCs in Hispanic patients were less likely to be within Milan criteria (26% vs. 38%; P < 0.0001) and were less likely to be treated with resection (9% vs. 13%; P = 0.03) or transplantation (8% vs. 19%; P < 0.0001). Hispanic patients had a median overall survival of 1.4 years (95% confidence interval [CI], 1.22‐1.56), which was similar to that of non‐Hispanic White patients (1.3 years; 95% CI, 1.26‐1.41; P = 0.07). Conclusion: Hispanic patients with HCC were more likely to have metabolic risk factors for chronic liver disease, including obesity. Despite diagnosis at more advanced stages with less curative intervention than non‐Hispanic White patients, median overall survival was similar between groups.
Hispanic patients are more likely to have metabolic risk factors leading to their chronic liver disease risk for hepatocellular carcinoma in comparison to non‐Hispanic whites. Hispanic patients have similar overall survival despite being diagnosed with larger, more advanced HCC despite fewer curative interventions.

Abbreviations
- AFP
alpha fetoprotein
- ALT
alanine aminotransferase
- APRI
aspartate aminotransferase to platelet ratio index
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- MELD
Model for End‐Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- SEER
Surveillance, Epidemiology, and End Results
Hepatocellular carcinoma (HCC) remains one of the leading causes of cancer mortality worldwide.( 1 , 2 ) It is the most common liver malignancy, with over half a million new cases diagnosed annually.( 3 ) The incidence and mortality of HCC has continued to rise, with mortality rates increasing faster than from any other cancer in the United States.( 4 , 5 , 6 , 7 ) Epidemiologic data for HCC, mainly using the Surveillance, Epidemiology, and End Results (SEER) database, attributes the majority of HCC cases to chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection.( 4 , 5 , 6 , 7 , 8 , 9 , 10 ) However, in the United States, the incidence and mortality of HCC has continued to increase despite advances in treatment of HBV and HCV.( 4 , 5 , 6 , 7 )
HCC has strong racial and ethnic associations, with a longstanding discrepancy in the incidence of HCC between the Hispanic and non‐Hispanic White populations.( 9 , 11 ) Hispanic individuals have the highest age‐adjusted incidence rates of HCC, surpassing that of Asian individuals, the previously most affected ethnicity.( 6 , 9 , 10 ) The yearly age‐adjusted incidence rates of HCC for Hispanic individuals are at least 2.5 times higher than their non‐Hispanic White counterparts.( 4 , 9 , 12 ) The Hispanic population is currently the second largest ethnic population in the United States, and the rising HCC mortality may be driven by increasing trends among Hispanic people.( 8 , 9 , 13 )
There are racial and ethnic disparities in cancer diagnosis and treatment due to unique social determinants, support mechanisms, and access to health care.( 14 ) These disparities can lead to increased cancer mortality.( 15 ) Early detection of HCC, especially through screening for asymptomatic disease at appropriate intervals, allows patients to benefit from potentially curative interventions, such as tumor resection and liver transplantation.( 16 , 17 , 18 ) Hispanic individuals tend to be diagnosed at later stages, and those that do present with early stage HCC have the lowest rates of undergoing curative treatments.( 18 , 19 , 20 , 21 ) Ha et al.( 21 ) reported that up to 70% of Hispanic patients with localized HCC received no HCC treatment.
The disparities in clinical features, incidence, tumor staging at diagnosis, and treatment for HCC of Hispanic compared to non‐Hispanic White individuals needs to be further investigated. Previous studies have been limited to population‐based data sets, particularly the SEER database. Although SEER is intended to capture a broad representative sample, generalizability for HCC trends may be limited. SEER data exclude a sizable portion of the U.S. population, including the state of Texas, which accounts for over one fifth of the U.S. Hispanic population.( 22 ) Additionally, SEER lacks information on HCC, such as etiology of underlying liver disease, comorbidities, risk factors, laboratory data, tumor burden at presentation, and some liver‐specific treatments.( 19 , 20 ) Therefore, the aim of our study was to address the current gaps in knowledge regarding HCC between Hispanic and non‐Hispanic patients in a large multicenter cohort to better understand unmet needs in caring for this significant U.S. patient population.
Participants and Methods
Study Population
This was a multicenter retrospective study of adults 18 years of age or older who were diagnosed with HCC between January 2000 and June 2014. Centers included Atrium Health, Columbia University Irving Medical Center, Indiana University Health Hospital, MD Anderson Cancer Center, and Vanderbilt University Medical Center. A further in‐depth description of ascertainment and characterization of this cohort can be reviewed at Gawrieh et al.( 23 ) Eligible patients were identified from institutional tumor registries (Fig. 1); afterwards, a comprehensive electronic medical chart review was conducted to extract data. We excluded patients with uncertain HCC diagnosis, diagnosis of recurrent HCC, fibrolamellar subtype HCC, cholangiocarcinoma, or insufficient data (less than 50% of the data available). Periodic intra‐institutional reviews were undertaken by the site principal investigator and followed by external harmonization by the data coordinating team at Indiana University.
Fig. 1.
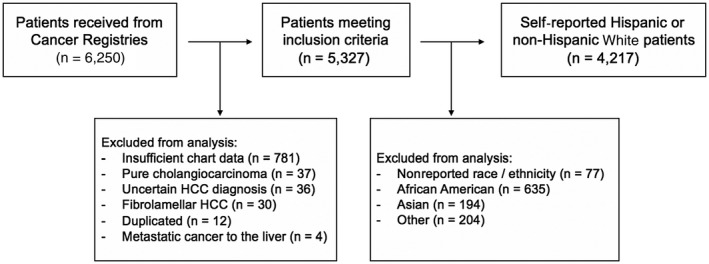
Flow diagram demonstrating patient inclusion and exclusion criteria in our large multicenter study.
Clinical Information
Collected data included demographics, concomitant morbidities, baseline investigational laboratory results, HCC‐related variables, and survival information. Demographics consisted of age at time of HCC diagnosis, sex, body mass index (BMI) within 3 months of HCC diagnosis, and self‐reported race (e.g., White, Black) or ethnicity (Hispanic vs. non‐Hispanic). Recorded comorbidities included diabetes mellitus, hypertension, coronary artery diseases, peripheral vascular diseases, any alcohol use, and human immunodeficiency virus (HIV). Performance status within 3 months of HCC diagnosis was reported using criteria of the Eastern Cooperative Oncology Group.( 24 )
Baseline laboratory tests were recorded within 3 months of HCC diagnosis and consisted of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, alkaline phosphatase, albumin, platelet count, creatinine, international normalized ratio, and alpha fetoprotein (AFP). The Model for End‐Stage Liver Disease (MELD) score and AST to platelet ratio index (APRI) were then calculated using these values.
HCC Diagnosis
We documented the date HCC diagnosis was confirmed by imaging or biopsy, whichever occurred first. Methods of establishing HCC diagnosis were noted as tissue assessment (biopsy sampling or surgical resection), diagnostic imaging, or a combination of both. Tumor/node/metastasis (TNM) classification from the American Joint Committee on Cancer was determined at the time of HCC diagnosis by findings on liver imaging and resection, if available. Cancer differentiation was documented from histopathologic reports. Tumor size was reported from imaging modalities or gross examination of the excised tumor if within 3 months of initial diagnosis and if the patient had not yet received other treatment modalities. To determine vascular invasion according to Milan criteria, only evidence of macroscopic involvement of great vessels (portal vein invasion) was considered.
Etiology of Liver Disease
Clinical notes, diagnostic imaging, and histopathologic reports were used to determine the etiology of the liver disease. The underlying etiology of the liver disease was classified as “idiopathic” if there was sufficient information to make a diagnosis but no underlying etiology was found. If there was not sufficient evidence to make a diagnosis, patients were classified as having “unknown” etiology of liver disease. Patients with evidence of steatosis on biopsy or imaging or patients that possessed multiple clinical risk factors for nonalcoholic fatty liver disease (NAFLD) were identified as having fatty liver disease as the underlying etiology of their disease.
Treatment and Recurrence
HCC treatment modalities received between the date of HCC diagnosis and time of death or last clinical encounter were recorded. These modalities included surgery, interventional radiology, liver transplantation, medical treatment, and other miscellaneous treatments. Recurrence of HCC after remission of the initial tumor was recorded; also, the dates and number of recurrences were documented.
Survival Outcome
Date of death or last follow‐up clinical encounter was recorded to calculate overall survival duration, which was defined as time between HCC diagnosis and last follow‐up or date of death, whichever occurred first.
Statistical Analyses
Categorical variables were summarized by frequency and percentage and compared using the chi‐square test, as published in other analyses of this cohort.( 23 ) Continuous variables were summarized by means and SD and compared using t tests. Overall survival time was defined from the date of HCC diagnosis to the date of death, censored at the date of last contact. The overall survival probability was estimated by the Kaplan‐Meier method and compared between groups using the log‐rank test. A Cox proportional hazards regression model was used to evaluate the association between risk factors and overall survival. The following risk factors were considered for the Cox model: patient demographics (age, sex, and ethnicity), tumor characteristics (tumor within Milan criteria and anatomic stage), and treatment modalities (resection and liver transplantation). Variables that were significant at P < 0.20 in the univariable analysis were included in a stepwise selection procedure to select which variables would be included in the final Cox multivariable regression model. Variables of clinical significance were retained in the model. P < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Demographics
We identified 5,327 eligible patients with HCC between January 2000 and June 2014, with 4,217 who were either Hispanic or non‐Hispanic White patients; 12% (n = 521) of these were confirmed to be Hispanic and 87.6% were non‐Hispanic patients (Table 1). The majority of Hispanic patients were from either Columbia University Medical Center in New York or MD Anderson Cancer Center in Houston, Texas (22% and 69%, respectively). Both Hispanic and non‐Hispanic patients were more likely to be men (75.2% vs. 78.2%; P = 0.1252). Additionally, Hispanic and non‐Hispanic White patients had similar rates of cirrhosis at the time of HCC diagnosis (86.8% vs. 85.2%; P = 0.1183). Compared to their non‐Hispanic White counterparts, Hispanic patients were older at age of diagnosis (mean ± SD, 64.2 ± 10.9 vs. 61.9 ± 10.5 years; P < 0.0001) with higher BMIs (mean ± SD, 29.6 ± 6.5 vs. 28.8 ± 5.9 kg/m2; P = 0.0113). Hispanic patients were more likely than non‐Hispanic patients to have diabetes (48.6% vs. 38%; P < 0.0001) and hypertension (68.3% vs. 60.6%; P = 0.0008) at the time of diagnosis. There were no statistically significant differences between sex, dyslipidemia, coronary artery disease, peripheral vascular disease, or HIV status in Hispanic versus non‐Hispanic patients. Hispanic patients had higher alkaline phosphatase (mean ± SD, 210.9 ± 176.5 vs. 172.5 ± 152.1 U/L; P < 0.0001) but similar AST (129.7 ± 311.5 vs. 114.5 ± 175.9 U/L; P = 0.1195) and ALT (66.1 ± 73.8 vs. 75.9 ± 117.2 U/L; P = 0.0787). Hispanic patients had higher albumin (mean ± SD, 3.5 ± 0.7 vs. 3.4 ± 0.7 g/dL; P = 0.0007) and platelets (178.1 ± 117.7 vs. 160.0 ± 111.0 K/µL; P = 0.0009) but similar creatinine, MELD, and APRI scores (Table 1).
Table 1.
Baseline Characteristics
| Variable | Non‐Hispanic Patients (n = 3,696) | Hispanic Patients (n = 521) | P Value |
|---|---|---|---|
| Age at HCC (years) Diagnosis | 61.9 ± 10.5 | 64.2 ± 10.9 | <0.0001 |
| Male sex | 2,891 (78.2%) | 392 (75.2%) | 0.1252 |
| BMI (kg/m2) | 28.8 ± 5.9 | 29.6 ± 6.5 | 0.0113 |
| Diabetes | 1,393 (38.0%) | 252 (48.6%) | <0.0001 |
| Hypertension | 2,223 (60.6%) | 355 (68.3%) | 0.0008 |
| Dyslipidemia | 925 (25.3%) | 130 (25.0%) | 0.8879 |
| Coronary artery disease | 706 (19.3%) | 83 (16%) | 0.0700 |
| Peripheral vascular disease | 307 (8.4%) | 40 (7.7%) | 0.5823 |
| ALT (U/L) | 75.9 ± 117.2 | 66.1 ± 73.8 | 0.0787 |
| AST (U/L) | 114.5 ± 175.9 | 129.7 ± 311.5 | 0.1195 |
| Alkaline phosphatase (U/L) | 172.5 ± 152.1 | 210.9 ± 176.5 | <0.0001 |
| Albumin (g/dL) | 3.4 ± 0.7 | 3.5 ± 0.7 | 0.0007 |
| Platelets (K/µL) | 160.0 ± 111.0 | 178.1 ± 117.7 | 0.0009 |
| Creatinine (mg/dL) | 1.0 ± 0.7 | 1.0 ± 0.6 | 0.0908 |
| AFP (ng/mL) | 13,060.2 ± 79,738.9 | 18,816.8 ± 94,682.5 | 0.1604 |
| MELD score | 11.8 ± 5.4 | 11.3 ± 5.2 | 0.107 |
| APRI score | 2.8 ± 6.3 | 2.9 ± 9.3 | 0.9178 |
Data shown as mean ± SD or number (%).
There were significant differences in the etiology of liver disease between Hispanic and non‐Hispanic patients (P < 0.0001). HCC in Hispanic patients was more likely to be secondary to alcohol‐related liver disease or NAFLD than in non‐Hispanic patients (Table 2).
Table 2.
Underlying Etiologies of Liver Disease
| Etiologies of Underlying Liver Disease | Non‐Hispanic Patients (n = 3,696) | Hispanic Patients (n = 521) | P Value |
|---|---|---|---|
| AIH/PBC/PSC | 68 (1.8%) | 2 (0.4%) | <0.0001 |
| Alcohol alone | 492 (13.3%) | 108 (20.7%) | |
| HBV/alcohol | 38 (1.0%) | 5 (1.0%) | |
| HBV | 97 (2.6%) | 17 (3.3%) | |
| HCV/A1ATD | 77 (2.1%) | 3 (0.6%) | |
| HCV/alcohol | 774 (20.9%) | 73 (14.0%) | |
| HCV/HBV (+/– alcohol) | 103 (2.8%) | 13 (2.5%) | |
| HCV | 865 (23.4%) | 113 (21.7%) | |
| NAFLD | 635 (17.2%) | 104 (20.0%) | |
| Rare etiologies | 18 (0.5%) | 0 (0.0%) | |
| Unclear/Unknown | 529 (14.3%) | 83 (15.9%) |
Data shown as number (%).
Abbreviations: A1AT, alpha 1 antitrypsin deficiency; AIH, autoimmune hepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
HCC Presentation
At the time of HCC diagnosis, Hispanic patients were more likely to present with larger tumors (mean ± SD, 6.4 ± 4.2 vs. 5.6 ± 4.2 cm; P < 0.0001). Hispanic patients also presented with higher AFP values compared to non‐Hispanic patients; 38.7% of AFP values in Hispanic patients were over 200 ng/mL compared to 32.6% in non‐Hispanic patients (P = 0.0046). Hispanic and non‐Hispanic patients differed significantly when classified by Milan criteria (P < 0.0001), with 26.3% of Hispanic patients and 37.7% non‐Hispanic patients being within the Milan criteria. HCC tumor characteristics were significantly different between the two groups (P = 0.0025); non‐Hispanic patients had increased rates of single tumors (35.6% vs. 31.3%) and three tumors <3 cm (10.1% vs. 6.7%), while Hispanic patients had increased rates of macrovascular invasion and extrahepatic spread (39% vs. 37.7%; Fig. 2). When classified by TNM staging, Hispanic patients were also more likely to present with more advanced staging (P < 0.0001), including anatomic stage III or IV (55.2% vs. 44.3%), and non‐Hispanic White patients were more likely to present with stage I or II (55.7% vs. 44.8%). Similarly, with Barcelona Clinic Liver Cancer staging, Hispanic patients were more likely to be stages C and D than A and B (P = 0.0135).
Fig. 2.
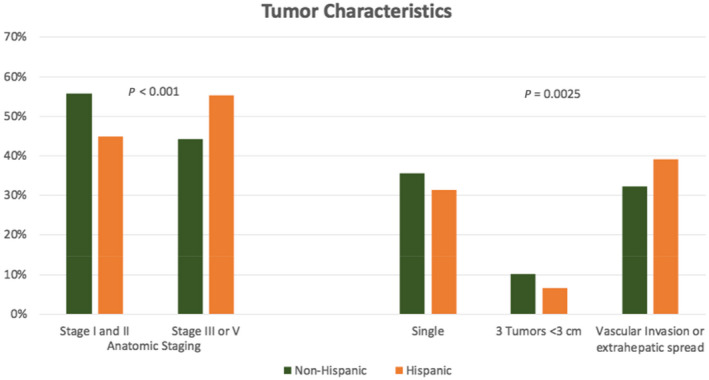
Tumor characteristics of anatomic staging and tumor number in Hispanic versus non‐Hispanic patients.
Hispanic patients diagnosed with anatomic stage III or IV disease were younger than those diagnosed with stage I and II disease (mean ± SD, 63.4 ± 11.3 vs. 65.9 ± 10.2 years; P = 0.0107). However, this age difference was not present in Non‐Hispanic White patients (mean ± SD, 61.7 ± 11.3 vs 61.7 ± 10.0 years; P = 0.96). Similarly, Hispanic patients with tumors within the Milan criteria were older than those with tumors not within the Milan criteria (mean ± SD, 66.0 ± 10.4 vs. 63.6 ± 11.0 years; P = 0.0290), whereas non‐Hispanic White patients with tumors within the Milan criteria were younger (60.9 ± 9.3 vs. 62.4 ± 11.1 years; P < 0.0001). Non‐Hispanic White patients with more advanced disease (stage III and IV and not within the Milan criteria) were more likely to be men; however, Hispanic patients had a similar sex distribution regardless of disease progression.
When compared to patients with earlier disease, Hispanic patients with stage III and IV disease had similar BMIs as well as rates of diabetes, hypertension, and dyslipidemia, whereas non‐Hispanic White patients with stage III and IV disease had lower BMIs (mean ± SD, 28.4 ± 5.9 vs. 29.1 ± 5.9 kg/m2 ; P = 0.0047), lower rates of diabetes (35.7% vs. 39.5%; P = 0.0302), and similar rates of hypertension and dyslipidemia.
Screening and Diagnosis
HCC was diagnosed as part of screening in 29.1% of Hispanic and 25.9% of non‐Hispanic White patients (P = 0.1233). HCC was diagnosed secondary to symptoms more frequently in non‐Hispanic than in Hispanic patients (57.5% vs. 52.5%; P = 0.0358). Forty‐one percent of Hispanic patients had not undergone regular screening within 2 years before a diagnosis of HCC compared with only 29% of non‐Hispanic patients (P < 0.001). However, the screening status was unknown for 32.7% of Hispanic and 40.3% of non‐Hispanic White patients (P < 0.001). Non‐Hispanic White patients were also more likely to have AFP for screening than Hispanic patients (40.7% vs. 27.5%; P = 0.0037).
Treatment and Survival
Hispanic patients were less likely to undergo resection (9% vs. 13%; P = 0.0266) or liver transplantation (8% vs. 19%; P < 0.0001). Hispanic patients were more likely to be treated by palliative care or enter hospice (28.4% vs. 23.9%; P = 0.0257). Median overall survival in Hispanic patients was 1.4 years (95% confidence interval [CI], 1.22‐1.56), which was similar to that of non‐Hispanic White patients (1.3 years; 95% CI, 1.26‐1.41; P = 0.07) (Fig. 3). In multivariable analysis controlling for ethnicity, age, sex, anatomic stage, tumor within Milan criteria, and whether resection and/or liver transplant took place, non‐Hispanic patients had worse survival than Hispanic patients (hazard ratio, 1.15; 95% CI, 1.03‐1.28; P = 0.0120).
Fig. 3.
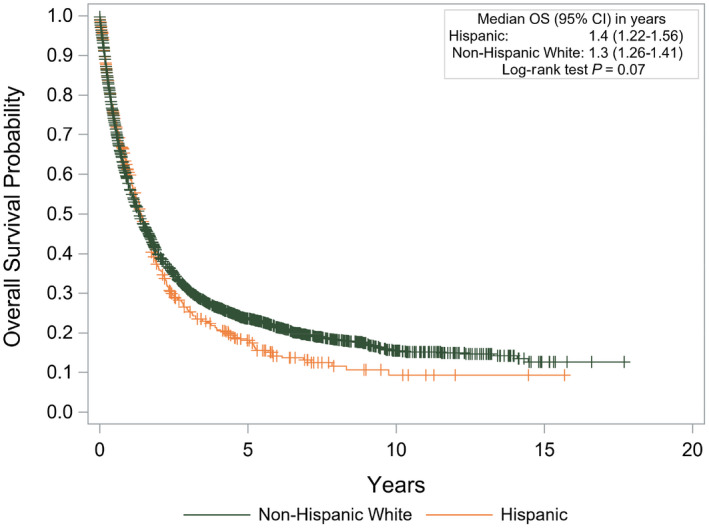
Kaplan‐Meier survival curve of outcomes between Hispanic and non‐Hispanic White patients. Abbreviation: OS, overall survival.
Discussion
This large, multicenter, cohort study in the United States provides primary data on the presentation, treatment, and outcomes of Hispanic patients in comparison to their non‐Hispanic White counterparts, demonstrating the significant differences and disparities between the two. These differences are likely contributing to the increased incidence and mortality of HCC throughout the country. This is one of the largest and most diverse cohorts for which the primary laboratory data, underlying etiology of liver disease, treatments, and outcomes were collected and classified through manual clinical review.
Our results are consistent with studies that have shown that Hispanic individuals tend to be diagnosed with HCC at later stages and with more advanced disease.( 18 , 19 , 20 , 21 ) Hispanic patients that present with early stage HCC have the lowest rates of undergoing curative treatment. Furthermore, Hispanic patients were less likely to receive liver transplants within 360 days compared with non‐Hispanic White patients.( 21 ) Interestingly, despite diagnosis at more advanced stages, we found no difference in overall survival and Hispanic patients had better survival than their non‐Hispanic White counterparts in multivariable analysis.
In our cohort, Hispanic patients were less likely to have undergone HCC screening within the 2 years before diagnosis compared with non‐Hispanic White patients, despite having equal rates of cirrhosis. Evidence exists that social determinants and access to health care may play a role.( 14 ) We propose that lower rates of screening might be one factor contributing to more advanced disease at the time of presentation in Hispanic patients in our cohort. Interestingly, overall rates of screening were low in the entire cohort not just among Hispanic patients, with the majority of HCC diagnosed secondary to symptoms.
One major contributor to differences between Hispanic and non‐Hispanic White patients is likely related to the differences in rates of metabolic syndrome and NAFLD, both of which are independently associated with increased risk of HCC.( 23 , 25 , 26 , 27 , 28 , 29 ) In our cohort, Hispanic patients had higher BMI and higher rates of diabetes and hypertension and were also more likely to have NAFLD as the underlying etiology of liver disease compared with their non‐Hispanic White counterparts. The correlation between metabolic syndrome and its components (e.g., diabetes, obesity, hypertension) and HCC has been described.( 26 , 27 , 28 , 30 ) Several different case and cohort studies have also demonstrated that HCC is almost twice as likely to develop in patients with type 2 diabetes compared to those without diabetes.( 27 , 28 ) Heiss et al.( 30 ) found that the metabolic syndrome was highly prevalent in Hispanic/Latino patients, with close to 50% of patients meeting diagnostic criteria at 45‐64 years of age. Given the prevalence of metabolic syndrome and its components in Hispanic patients as well as the increased risk these risk factors portend to developing HCC, both primary care physicians and specialists should work closely with this population to prevent the onset of metabolic syndrome as this could subsequently reduce the incidence of HCC.
NAFLD and nonalcoholic steatohepatitis are currently the most common liver diseases worldwide, and NAFLD is a known risk factor for the development of HCC.( 23 , 26 , 29 , 31 ) NAFLD is more common in the Hispanic population, likely due to the higher prevalence of obesity and insulin resistance in these patients.( 32 ) We found that Hispanic patients with HCC were more likely to have underlying NAFLD compared to their non‐Hispanic counterparts. Understanding trends in HCC among the Hispanic population in the United States may provide important insights on NAFLD–HCC trends.( 20 )
There are several limitations to this study. As a retrospective study, data were extracted from patient charts within 6 months of the date of HCC diagnosis; as patients were not followed longitudinally before their diagnosis of HCC, this has limitations in implications for screening data and recommendations. Although the entire study focuses on five different locations, 91% of the Hispanic patients were from Texas‐ and New York‐based institutions. Additionally, all patients were seen at large tertiary care centers, limiting the generalizability to the entire United States as well as patients seen at community‐based institutions. As with any retrospective study, some data points were missing; however, patient charts were manually reviewed to significantly reduce missing data points and also to allow for collection of meaningful data. Despite manual review, there were many patients for whom data on HCC screening within 2 years of diagnosis were not available. Ethnicity was self‐designated by patients, with a small number (77 patients) missing ethnicity data. The Hispanic population is a heterogeneous group that was unable to be further stratified given the retrospective nature of this study. However, the diversity in location of the multiple academic centers helped to capture a more diverse population.
Despite these limitations, this study has several strengths. Data extraction was performed by manual chart review, which allowed for extensive data collection, including laboratory data, patient and tumor information, and outcomes. Identification of the underlying etiology of liver disease, for example, was made through review of clinical and histopathologic reports, with reliance on detailed documentation in the medical record. This is one of the largest multicenter cohorts with primary information not included in the SEER database.
With the increasing prevalence of metabolic syndrome, diabetes, and NAFLD, especially within the Hispanic population, increased public health efforts with a focus on prevention should be undertaken. While this study demonstrates the risk factors associated with HCC in Hispanic patients, significant questions for future exploration arise regarding the unexpected survival despite later stage diagnosis and noncurative treatment.
Supporting information
Supplementary Material
Acknowledgment
We thank Ava Farrell for meeting coordination assistance.
Supported in part by Indiana University School of Medicine, David W. Crabb Professorship Endowment (N.C.).
Potential conflict of interest: Dr. Gawrieh received grants from Galmed, Zydus, and Viking; he consults for Transmedics. Dr. Wattacheril advises Astra Zeneca; she received grants from Janssen, Intercept, Galectin, Genfit, Conatus and Zydus. The other authors have nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 3. Mittal S, El‐Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47(Suppl.):S2‐S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed F, Perz JF, Kwong S, Jamison PM, Friedman C, Bell BP. National trends and disparities in the incidence of hepatocellular carcinoma, 1998‐2003. Prev Chronic Dis 2008;5:A74. [PMC free article] [PubMed] [Google Scholar]
- 5. El‐Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology 2004;127(Suppl.1):S27‐S34. [DOI] [PubMed] [Google Scholar]
- 6. White DL, Thrift AP, Kanwal F, Davila J, El‐Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 2017;152:812‐820.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El‐Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med 2007;167:1983‐1989. [DOI] [PubMed] [Google Scholar]
- 10. Han SS, Kelly SP, Li Y, Yang B, Nguyen M, So S, et al. Changing landscape of liver cancer in California: a glimpse into the future of liver cancer in the United States. J Natl Cancer Inst December 2019;111:550‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu L, Kim Y, Spolverato G, Gani F, Pawlik TM. Racial disparities in treatment and survival of patients with hepatocellular carcinoma in the United States. Hepatobiliary Surg Nutr 2016;5:43‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Méndez‐Sánchez N, Zamora‐Valdés D, Vásquez‐Fernández F, Uribe M. Hepatocellular carcinoma in Hispanics. Ann Hepatol 2007;6:279‐280. [PubMed] [Google Scholar]
- 13. Endeshaw M, Hallowell BD, Razzaghi H, Senkomago V, McKenna MT, Saraiya M. Trends in liver cancer mortality in the United States: dual burden among foreign‐ and US‐born persons. Cancer 2019;125:726‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol 2018;36:25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 2002;94:334‐357. [DOI] [PubMed] [Google Scholar]
- 16. El‐Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008;134:1752‐1763. [DOI] [PubMed] [Google Scholar]
- 17. El‐Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118‐1127. [DOI] [PubMed] [Google Scholar]
- 18. Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992‐2008. Hepatology 2012;55:476‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2019;17:551‐559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robinson A, Ohri A, Liu B, Bhuket T, Wong RJ. One in five hepatocellular carcinoma patients in the United States are Hispanic while less than 40% were eligible for liver transplantation. World J Hepatol 2018;10:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ha J, Yan M, Aguilar M, Tana M, Liu B, Frenette CT, et al. Race/ethnicity‐specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol 2016;50:423‐430. [DOI] [PubMed] [Google Scholar]
- 22. Ramirez AG, Munoz E, Holden AEC, Adeigbe RT, Suarez L. Incidence of hepatocellular carcinoma in Texas Latinos, 1995‐2010: an update. PLoS One 2014;9:e99365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gawrieh S, Dakhoul L, Miller E, Scanga A, deLemos A, Kettler C, et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: a United States multicentre study. Aliment Pharmacol Ther 2019;50:809‐821. [DOI] [PubMed] [Google Scholar]
- 24. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649‐655. [PubMed] [Google Scholar]
- 25. El‐Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014;60:1767‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Welzel TM, Graubard BI, Zeuzem S, El‐Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER‐Medicare database. Hepatology 2011;54:463‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El‐Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006;4:369‐380. [DOI] [PubMed] [Google Scholar]
- 28. El‐Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004;126:460‐468. [DOI] [PubMed] [Google Scholar]
- 29. Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non‐alcoholic fatty liver disease. Gastroenterology 2018;155:1828‐1837.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heiss G, Snyder ML, Teng Y, Schneiderman N, Llabre MW, Cowie C, et al. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: the Hispanic Community Health Study/Study of Latinos. Diabetes Care 2014;37:2391‐2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol 2017;23:8263‐8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387‐1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


