Abstract
Cardiotoxicity as an off-target effect of doxorubicin therapy is a major limiting factor for its clinical use as a choice cytotoxic agent. Seeds of Irvingia gabonensis have been reported to possess both nutritional and medicinal values which include antidiabetic, weight losing, antihyperlipidemic, and antioxidative effects. Protective effects of Irvingia gabonensis ethanol seed extract (IGESE) was investigated in doxorubicin (DOX)-mediated cardiotoxicity induced with single intraperitoneal injection of 15 mg/kg of DOX following the oral pretreatments of Wistar rats with 100-400 mg/kg/day of IGESE for 10 days, using serum cardiac enzyme markers (cardiac troponin I (cTI) and lactate dehydrogenase (LDH)), cardiac tissue oxidative stress markers (catalase (CAT), malonyldialdehyde (MDA), superoxide dismutase (SOD), glutathione-S-transferase (GST), glutathione peroxidase (GSH-Px), and reduced glutathione (GSH)), and cardiac histopathology endpoints. In addition, both qualitative and quantitative analyses to determine IGESE's secondary metabolites profile and its in vitro antioxidant activities were also conducted. Results revealed that serum cTnI and LDH were significantly elevated by the DOX treatment. Similarly, activities of tissue SOD, CAT, GST, and GSH levels were profoundly reduced, while GPx activity and MDA levels were profoundly increased by DOX treatment. These biochemical changes were associated with microthrombi formation in the DOX-treated cardiac tissues on histological examination. However, oral pretreatments with 100-400 mg/kg/day of IGESE dissolved in 5% DMSO in distilled water significantly attenuated increases in the serum cTnI and LDH, prevented significant alterations in the serum lipid profile and the tissue activities and levels of oxidative stress markers while improving cardiovascular disease risk indices and DOX-induced histopathological lesions. The in vitro antioxidant studies showed IGESE to have good antioxidant profile and contained 56 major secondary metabolites prominent among which are γ-sitosterol, Phytol, neophytadiene, stigmasterol, vitamin E, hexadecanoic acid and its ethyl ester, Phytyl palmitate, campesterol, lupeol, and squalene. Overall, both the in vitro and in vivo findings indicate that IGESE may be a promising prophylactic cardioprotective agent against DOX-induced cardiotoxicity, at least in part mediated via IGESE's antioxidant and free radical scavenging and antithrombotic mechanisms.
1. Introduction
Doxorubicin (otherwise known as Adriamycin) is one of the antibiotic cytotoxic agent belonging to the anthracycline class of anticancer agents [1]. Doxorubicin is known to bind to and intercalate with DNA, thereby inhibiting the resealing action of topoisomerase II during normal DNA replication needed for cancer cell division and growth [2–5]. Doxorubicin is often used in clinical setting in combination with other classes of anticancer agents as “chemo cocktail” in the management of various types of solid and blood cancers such as breast and ovarian, leukemia (acute myelogenous leukemia (AML) and acute lymphoblastic leukemia), Hodgkin lymphoma, non-Hodgkin lymphoma, Wilm's tumor, neuroblastoma, and sarcoma [6–8]. For example, for breast cancer management, doxorubicin is typically combined and given with cyclophosphamide; for lymphomas and leukemias, it is combined with other cytotoxic agents to make regimens like CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone), R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone), and ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) [9–12]. However, the clinical use of doxorubicin have been reported to be associated with major common side effects such as pain at the injection site, anorexia, fever, nausea and vomiting, stomatitis, dyspnea, nose bleeding, alopecia, immunosuppression, weight gain, hepatic and renal injuries, and severe cardiotoxicity [3, 13], while its occasional side effects include hyperuricemia, heart failure, pericardial effusion, cardiomyopathy, conjunctivitis, and skin rashes [14, 15]. Of these side effects, cumulative and dose-related cardiomyopathy and heart failure are of grave concerns to cancer patients and managing physicians alike, thus, limiting its clinical use [16–18]. Although the pathogenesis of doxorubicin-induced cardiotoxicity has been reported to be complex and fuzzy, the pivotal role of iron-mediated formation of reactive oxygen species (ROS) cannot be underscored [19].
In preventing the development of doxorubicin-induced cardiotoxicity, chemocurative and chemopreventive strategies involving the use of flavonoids, especially monoHER, have been advocated [20, 21]. MonoHER has been reported to elicit potent antioxidant, iron chelating, and carbonyl reductase inhibiting effects while still protecting the antitumor activity of anthracycline anticancer agents [22]. Similarly, the effectiveness of dexrazoxane (an iron chelating agent) [5, 23], dextromethionine [24, 25], and angiotensin-converting enzyme inhibitors—zofenopril and lisinopril [26, 27]—in ameliorating doxorubicin-related cardiotoxicity have also been reported. These agents, especially dexrazoxane, are known to mitigate oxidative stress by chelating iron and catalytically inhibiting topoisomerase II, thus preventing doxorubicin-induced double strand DNA breaks [28, 29]. However, these chemopreventive agents are expensive and not readily accessible to patients, therefore, necessitating the need for the discovery and development of more effective but cheaper and more readily accessible alternatives especially ones of medicinal plant origin. One of these is the Irvingia gabonensis seed extract.
Irvingia gabonensis (Aubry-Le Comte ex O'Rorke) Bail belonging to the family, Irvingiaceae, is known as African Mango (in English). Its other common names include bread tree, African wild mango, wild mango, and bush mango [30, 31], and its local names include Apon (in Yoruba, Southwest Nigeria), Ogbono (in Igbo, Southeast Nigeria), and Goron or biri (in Hausa, Northern Nigeria) [32, 33]. Irvingia gabonensis is widely cultivated in West African countries including southwest and southeast Nigeria, southern Cameroon, Côte d'Ivoire, Ghana, Togo, and Benin, to produce its edible fruit whose seed is used in the preparation of local delicious viscous soup for swallowing yam and cassava puddings [34]. Fat extracted from its seeds is commonly known as dika fat and majorly consists of C12 and C14 fatty acids, alongside with smaller quantities of C10, C16, and C18, glycerides and proteins [34]. Irvingia gabonensis seeds are also a good source of nutrients including a variety of vitamins and minerals such as sodium, calcium, magnesium, phosphorus, and iron. It is also a rich source of flavonoids (quercetin and kaempferol), ellagic acid, mono-, di-, and tri-O-methyl-ellagic acids, and their glycosides which are potent antioxidants [35, 36].
Phytochemical analysis of its seeds showed that it contains tannins, alkaloids, flavonoids, cardiac glycosides, steroids, carbohydrate, volatile oils, and terpenoids [33, 37, 38] and its proximate composition of moisture 1.4 ± 0.11%, ash 6.8 ± 0.12%, crude lipid 7.9 ± 0.01%, crude fiber 21.6 ± 0.45%, and crude protein 5.6 ± 0.20% [33]. Pure compounds already isolated from the seed extract of include: methyl 2-[2-formyl-5-(hydroxymethyl)-1 H-pyrrol1yl]-propanoate, kaempferol-3-0-β-D-6″ (p-coumaroyl) glucopyranoside and lupeol (3β-lup-20(29)-en-3-ol). Erstwhile, the antioxidant property of Irvingia gabonensis seed extract has been largely attributed to its high lupeol content [39].
In view of the above, the current study was designed at evaluating the possible protective effect of the crude non-defatted ethanol seed extract of Irvingia gabonensis against doxorubicin-mediated cardiotoxicity in rats using cardiac injury markers, oxidative stress markers, and histopathology results as endpoint outcomes.
2. Materials and Methods
2.1. Extraction Process and Calculation of Percentage Yield
For Irvingia gabonensis seed extraction, 3 kg of pulverized Irvingia gabonensis dried seeds was macerated in 12 L of absolute ethanol for 72 hours after which it was continuously stirred for 1 hour before it was filtered using 180 mm of filter paper. The filtrate was then concentrated at 40°C to complete dryness using rotary evaporator. The dark-colored, oily paste-like residue left behind was weighed, stored in air- and water-proof container which was kept in a refrigerator at 4°C. This extraction process was repeated for two more times. From the stock, fresh solutions were made whenever required.
% yield was calculated as {weight of crude extract obtained (g) ÷ weight of pulverized dry seed extracted (g)} × 100.
2.2. Preliminary Qualitative Phytochemical Analysis of IGESE
The presence of saponins, tannins, alkaloids, flavonoids, anthraquinones, glycosides, and reducing sugars in IGESE was detected by the simple and standard qualitative methods described by Trease and Evans [40] and Sofowora [41].
2.3. Preliminary Quantitative Determination of Secondary Metabolites in and Phytoscan of IGESE
Preliminary quantitative analysis of the secondary metabolites (including phenol, flavonoids, tannin, terpenoids, steroids, reducing sugars, saponin, and phlobatannin) in IGESE was done using methods earlier described by Olorundare et al. [42]. Similarly, using gas chromatography-mass spectrophotometer (GC-MS) for phytoscan, the relative abundance of the secondary metabolites in IGESE was done using the procedures earlier described by Olorundare et al. [42].
2.4. In Vitro Antioxidant Studies of IGESE
DPPH scavenging activity, FRAP, and nitric oxide scavenging activities of IGESE were determined using the procedures earlier described by Olorundare et al. [42].
2.5. Experimental Animals
Young adult male Wistar Albino rats (aged 8-10 weeks old and body weight: 140-160 g) used in this study were obtained from the Animal House of the Lagos State University College of Medicine, Ikeja, Lagos State, Nigeria, after an ethical approval (UERC Approval number: UERC/ASN/2020/2022) was obtained from the University of Ilorin Ethical Review Committee for Postgraduate Research. The rats were handled in accordance with international principles guiding the Use and Handling of Experimental Animals [43]. The rats were maintained on standard rat feed (Ladokun Feeds, Ibadan, Oyo State, Nigeria) and potable water which were made available ad libitum. The rats were maintained at an ambient temperature between 28 and 30°C, humidity of 55 ± 5%, and standard (natural) photoperiod of approximately 12/12 hours of alternating light and dark periodicity.
2.6. Measurement of Body Weight
The rat body weights were taken at the beginning and last of the experiment using a digital rodent weighing scale (®Virgo Electronic Compact Scale, New Delhi, India). The obtained values were expressed in grams (g).
2.7. Induction of DOX-Induced Cardiotoxicity and Treatment of Rats
Prior to commencement of the experiment, rats were randomly allotted into 7 groups of 7 rats per group such that the weight difference between and within groups was not more than ±20% of the average weight of the sample population of rats used for the study. However, the choice of the therapeutic dose range of 100, 200, and 400 mg/kg/day of IGESE was made based on the result of the orientation studies conducted.
Treatments of rats with distilled water, 100-400 mg/kg/day of IGESE in 5% DMSO distilled water, 20 mg/kg/day of vitamin C (standard antioxidant drug) for 10 days, and subsequent treatment with single intraperitoneal dose (15 mg/kg) doxorubicin in 0.9% normal saline on day 11 are as indicated in Table 1.
Table 1.
Group treatment of rats.
| Groups | Treatments |
|---|---|
| Group I | 10 ml/kg of distilled water given p.o. for 10 days +1 ml/kg of 0.9% normal saline given i.p. on day 11 |
| Group II | 200 mg/kg/day of IGESE in 5% DMSO-distilled water given p.o. for 10 days +1 ml/kg of 0.9% normal saline given i.p. on day 11 |
| Group III | 10 ml/kg/day of distilled water given p.o. for 10 days +15 mg/kg of doxorubicin hydrochloride in 0.9% normal saline given i.p. on day 11 |
| Group IV | 20 mg/kg/day of Vit. C dissolved in 5% DMSO-distilled water given p.o. for 10 days +15 mg/kg of doxorubicin hydrochloride in 0.9% normal saline given i.p. on day 11 |
| Group V | 100 mg/kg/day of IGESE dissolved in 5% DMSO-distilled water given p.o. for 10 days +15 mg/kg of doxorubicin hydrochloride in 0.9% normal saline given i.p. on day 11 |
| Group VI | 200 mg/kg/day of IGESE dissolved in 5% DMSO-distilled water given p.o. for 10 days +15 mg/kg of doxorubicin hydrochloride in 0.9% normal saline given i.p. on day 11 |
| Group VII | 400 mg/kg/day of IGESE dissolved in 5% DMSO-distilled water given p.o. for 10 days +15 mg/kg of doxorubicin hydrochloride in 0.9% normal saline given i.p. on day 11 |
2.8. Collection of Blood Samples
72 hours postdoxorubicin injection, overnight fasted rats were humanely sacrificed under light inhaled diethyl ether anesthesia, and whole blood samples were collected directly from the heart with fine 21G injectable needle and 5 ml syringe without causing damage to the heart tissues. The rat heart, liver, kidneys, and testes were carefully identified, harvested, and weighed.
2.9. Bioassays
Blood samples collected into 10 ml plain sample bottles were allowed to clot at room temperature for 6 hours and then centrifuged at 5000 rpm to separate clear sera from the clotted blood samples. The clear samples were obtained for assays of the following biochemical parameters: serum cardiac troponin I, LDH, TG, TC, and cholesterol fractions (HDL-c, LDL-c) using estimated standard bioassay procedures and commercial kits.
2.10. AI and CRI Calculation
AI was calculated as LDL − c (mg/dl) ÷ HDL − c (mg/dl) [44], while CRI was calculated as TC (mg/dl) ÷ HDL − c (mg/dl) [45].
2.11. Determination of Cardiac Tissue Antioxidant Profile
After the rats were sacrificed humanely under inhaled diethyl ether, the heart was harvested en bloc. The heart was gently and carefully divided into two halves (each consisting of the atrium and ventricle) using a new surgical blade. The left half of the heart was briskly rinsed in ice-cold 1.15% KCl solution in order to preserve the oxidative enzyme activities of the heart before being placed in a clean sample bottle which itself was in an ice-pack filled cooler. This is to prevent the breakdown of the oxidative stress enzymes in these organs.
Activities of cardiac tissue oxidative stress markers such as SOD, CAT, MAD, GSH, GPx, and GST were assays using methods earlier described by Olorundare et al. [42].
2.12. Histopathological Studies
The right halves of the seven randomly selected rats from each treatment and control groups were subjected to histopathological examinations; the choice of the right ventricle was based on its reported most susceptibility to doxorubicin toxicity of the four heart chambers. The dissected right heart half was briskly rinsed in normal saline and then preserved in 10% formo-saline. It was then completely dehydrated in 100% ethanol before it was embedded in routine paraffin blocks. 4-5 μm thick sections of the cardiac tissue were prepared from these paraffin blocks and stained with hematoxylin-eosin. These were examined under a photomicroscope connected to a host computer for any associated histopathological lesions.
2.13. Statistical Analysis
Data were presented as mean ± S.E.M. of four observations for the in vitro studies and mean ± S.D. of seven observations for the in vivo studies, respectively. Statistical analysis was done using a two-way analysis of variance followed by the Student-Newman-Keuls test on GraphPad Prism Version 5. Statistical significance was considered at p < 0.05, p < 0.001, and p < 0.0001.
3. Results
3.1. % Yield
Complete extraction of Irvingia gabonensis ethanol seed extract in absolute ethanol resulted in an average yield of 4.31%, which was a very dark brown, oily, and sweet-smelling paste-like residue that was soluble in methanol and ethanol but not in water.
3.2. Preliminary Qualitative Phytochemical Analysis of IGESE
This shows the presence of phenol, flavonoids, tannin, terpenoids, steroids, and reducing sugars, while saponin and phlobatannin were absent.
3.3. Preliminary Quantification of the Secondary Metabolites in IGESE
Preliminary quantitative analysis of IGESE showing the relative abundance and quantification of secondary metabolites (expressed in mg/100 g of dry IGESE) shows the presence of phenol (57.18 ± 0.05), flavonoids (18.19 ± 0.07), alkaloids (50.51 ± 0.17), steroids (47.47 ± 0.03), tannin (41.60 ± 0.03), and reducing sugars (65.64 ± 0.23) (Table 2).
Table 2.
Quantitative analysis of the secondary metabolites in IGESE (mg/100 g of dry extract sample).
| Secondary metabolite | Quantity (mg/100 g of dry extract) |
|---|---|
| Flavonoids | 18.19 ± 0.07 |
| Alkaloids | 50.51 ± 0.17 |
| Reducing sugar | 65.64 ± 0.23 |
| Phenols | 57.18 ± 0.05 |
| Steroids | 47.47 ± 0.03 |
| Tannin | 41.60 ± 0.03 |
3.4. Phytoscan for Secondary Metabolites in IGESE Using Gas Chromatography-Mass Spectrometry
The presence and relative abundance of fifty-six (56) major secondary metabolites in IGESE obtained through gas chromatography-mass spectrometry and phytoscan based on CAS Library search included 4,6-di-O-methyl-alpha-d-galactose (27.08%), n-hexadecanoic acid (5.51%), undecanoic acid (5.08%), 9,12,15-octadecatrienoic acid, (Z,Z,Z) (4.84%), γ-sitosterol (4.18%), Phytol (3.84%), neophytadiene (3.77%), ethyl 9,12,15-octadecatrienoate (3.65%), stigmasterol (3.03%), vitamin E (2.91%), hexadecanoic acid, ethyl ester (2.51%), Phytyl palmitate (1.92%), campesterol (1.34%), lupeol (1.22%), 9,12-octadecadienoic acid (Z,Z) (0.96%), octadecanoic acid, ethyl ester (0.91%), lup-20(29)-en-3-one (0.84%), β-amyrone (0.82%), phenol (0.82%), 1-hexacosanol (0.77%), pyrrolidine, 1-(1-cyclohexen-1-yl)-(0.71%), triacontyl acetate (0.66%), octadecanoic acid, 2,3-dihydroxypropyl ester (0.59%), γ-tocopherol (0.35%), 1,2-bis(trimethylsilyl) benzene (0.34%), and squalene (0.26%) (Table 3 and Figure 1).
Table 3.
Quantitative analysis of the secondary metabolites (PhytoScan) of Irvingia gabonensis ethanol seed extract (IGESE) using gas chromatography-mass spectrometry.
| Pk# | RT | Area (%) | Library/IDRef# | CAS# | Quality (%) |
|---|---|---|---|---|---|
| 1. | 4.069 | 0.1378 | Ethanol, 2-(ethylamino)- | 000110-73-6 | 80 |
| 2. | 4.906 | 0.0411 | Oxime-, methoxy-phenyl- | 1000222-86-6 | 91 |
| 3. | 5.137 | 0.1764 | 1,2-Cyclopentanedione | 003008-40-0 | 78 |
| 4. | 5.455 | 0.0811 | Cyclotetrasiloxane, octamethyl- | 000556-67-2 | 83 |
| 5. | 5.721 | 0.8170 | Phenol | 000108-95-2 | 90 |
| 6. | 5.905 | 0.1070 | Phenol | 000108-95-2 | 60 |
| 7. | 8.291 | 0.1399 | Z,Z-7,11-Hexadecadien-1-ol | 1000131-01-4 | 50 |
| 8. | 8.458 | 0.0616 | Cyclotetrasiloxane, octamethyl- | 000556-67-2 | 64 |
| 9. | 10.387 | 0.0843 | Naphthalen-4a,8a-imine, octahydro- | 005735-21-7 | 50 |
| 10. | 10.503 | 0.7119 | Pyrrolidine, 1-(1-cyclohexen-1-yl)- | 001125-99-1 | 50 |
| 11. | 11.288 | 0.1380 | Cycloheptasiloxane, tetradecamethyl- | 000107-50-6 | 60 |
| 12. | 12.137 | 0.4489 | 4-Methyl-2,5-dimethoxybenzaldehyde | 004925-88-6 | 60 |
| 13. | 13.125 | 5.0814 | Undecanoic acid | 000112-37-8 | 53 |
| 14. | 14.516 | 3.7713 | Neophytadiene | 000504-96-1 | 89 |
| 15. | 15.088 | 27.0790 | 4,6-di-O-methyl-alpha-d-galactose | 024462-98-4 | 52 |
| 16. | 15.695 | 5.5072 | n-Hexadecanoic acid | 000057-10-3 | 99 |
| 17. | 15.816 | 2.5123 | Hexadecanoic acid, ethyl ester | 000628-97-7 | 98 |
| 18. | 16.116 | 0.0474 | Heptadecanoic acid | 000506-12-7 | 55 |
| 19. | 16.595 | 0.1190 | Heptadecanoic acid, ethyl ester | 014010-23-2 | 60 |
| 20. | 16.774 | 3.8358 | Phytol | 000150-86-7 | 91 |
| 21. | 17.063 | 4.8375 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | 000463-40-1 | 99 |
| 22. | 17.185 | 3.6541 | Ethyl 9,12,15-octadecatrienoate | 1000336-77-4 | 99 |
| 23. | 17.352 | 0.9067 | Octadecanoic acid, ethyl ester | 000111-61-5 | 98 |
| 24. | 17.508 | 0.3478 | 14-Pentadecenoic acid | 017351-34-7 | 86 |
| 25. | 18.420 | 0.1330 | Bicyclo[3.1.1]heptan-2-one, 6,6-dimethyl- | 024903-95-5 | 55 |
| 26. | 18.605 | 0.0633 | Cis-vaccenic acid | 000506-17-2 | 91 |
| 27. | 18.761 | 0.1262 | Heptadecanoic acid, ethyl ester | 014010-23-2 | 70 |
| 28. | 19.264 | 0.0555 | Cyclopentadecanone, 2-hydroxy- | 004727-18-8 | 90 |
| 29. | 19.425 | 0.173 | Ethyl 9-hexadecenoate | 054546-22-4 | 58 |
| 30. | 19.599 | 0.594 | Octadecanoic acid, 2,3-dihydroxypropyl ester | 000123-94-4 | 87 |
| 31. | 19.818 | 0.0961 | 1,4-benzenedicarboxylic acid, mono(1-methylethyl) ester | 1000400-56-6 | 52 |
| 32. | 19.934 | 0.0379 | Cis-9-tetradecenoic acid, heptyl ester | 1000405-20-8 | 70 |
| 33. | 20.078 | 0.1537 | Docosanoic acid, ethyl ester | 005908-87-2 | 93 |
| 34. | 20.251 | 0.0452 | 18-nonadecenoic acid | 076998-87-3 | 64 |
| 35. | 20.742 | 0.3606 | 1,3,12-nonadecatriene | 1000131-11-1 | 64 |
| 36. | 20.887 | 0.1046 | 2-methyl-Z,Z-3,13-octadecadienol | 1000130-90-5 | 55 |
| 37. | 21.510 | 0.2565 | Squalene | 000111-02-4 | 90 |
| 38. | 22.844 | 0.3462 | γ-Tocopherol | 007616-22-0 | 98 |
| 39. | 23.052 | 0.6599 | Triacontyl acetate | 041755-58-2 | 95 |
| 40. | 23.341 | 2.9085 | Vitamin E | 000059-02-9 | 99 |
| 41. | 24.040 | 1.3362 | Campesterol | 000474-62-4 | 99 |
| 42. | 24.277 | 3.0258 | Stigmasterol | 000083-48-7 | 99 |
| 43. | 24.427 | 0.7673 | 1-hexacosanol | 000506-52-5 | 91 |
| 44. | 24.542 | 0.1545 | Hexadecanoic acid, 2-hydroxy-,methyl ester | 016742-51-1 | 59 |
| 45. | 24.750 | 4.1775 | γ-Sitosterol | 000083-47-6 | 99 |
| 46. | 24.843 | 0.8204 | β-Amyrone | 000638-97-1 | 94 |
| 47. | 25.241 | 0.8408 | Lup-20(29)-en-3-one | 001617-70-5 | 97 |
| 48. | 25.443 | 1.2194 | Lupeol | 000545-47-1 | 58 |
| 49. | 25.559 | 0.0751 | Benz[b]-1,4-oxazepine-4(5H)-thione, 2,3-dihydro-2,8-dimethyl- | 1000258-63-4 | 50 |
| 50. | 25.969 | 0.3833 | Stigmast-4-en-3-one | 001058-61-3 | 87 |
| 51. | 26.431 | 0.0624 | 2,4-Cyclohexadien-1-one, 3,5-bis(1,1-dimethylethyl)-4-hydroxy- | 054965-43-4 | 50 |
| 52. | 26.829 | 1.9166 | Phytyl palmitate | 1000413-67-8 | 96 |
| 53. | 27.170 | 0.1216 | 1,4-Bis(trimethylsilyl)benzene | 013183-70-5 | 78 |
| 54. | 27.592 | 0.0250 | 2,4-Cyclohexadien-1-one, 3,5-bis(1,1-dimethylethyl)-4-hydroxy- | 054965-43-4 | 50 |
| 55. | 28.463 | 0.3430 | 1,2-Bis(trimethylsilyl)benzene | 017151-09-6 | 76 |
| 56. | 29.376 | 0.9577 | 9,12-Octadecadienoic acid (Z,Z)- | 000060-33-3 | 50 |
Pk#: peak number; RT: retention time; Area%: percentage area covered; Library/ID Ref#: library/identification number; CAS#: chemical abstract scheme number.
Figure 1.
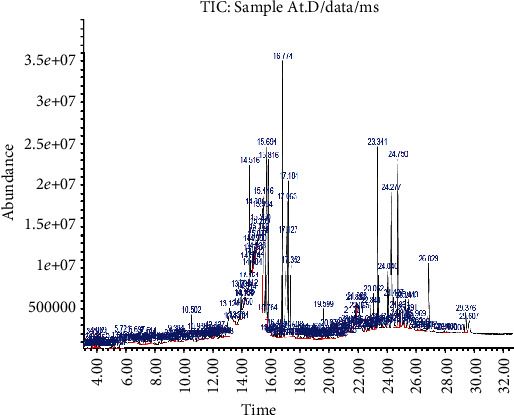
GC-MS analysis showing the relative abundance of the secondary metabolites in IGESE.
3.5. In Vitro Antioxidant Profiling of IGESE
3.5.1. Determination of DPPH Scavenging Activity of IGESE
Table 4 shows the in vitro DPPH scavenging activities of 25 μg/ml, 50 μg/ml, 75 μg/ml, and 100 μg/ml of IGESE in comparison with those of corresponding doses of the standard antioxidant drug (Vit. C) used. IGESE's DPPH scavenging activities were significantly (p < 0.001 and p < 0.0001) dose related at 75 μg/ml and 100 μg/ml, and these were comparable to that of Vit. C (Table 4).
Table 4.
In vitro DPPH scavenging activity (% inhibition) of 25-100 μg/ml of IGESE and Vit. C.
| Drug | Graded doses | |||
|---|---|---|---|---|
| 25 μg/ml | 50 μg/ml | 75 μg/ml | 100 μg/ml | |
| IGESE | 14.59 ± 0.31 | 43.53 ± 0.19 | 67.98 ± 0.38b | 75.44 ± 0.51c |
| Vit. C | 45.06 ± 0.28 | 56.55 ± 0.55a | 76.92 ± 0.31c | 89.83 ± 0.21c |
a, b, and c represent significant increases at p < 0.05, p < 0.001, and p < 0.0001, respectively, when compared to the baseline value at 25 μg/ml.
3.5.2. Determination of NO Scavenging Activity of IGESE
Table 5 shows the in vitro NO scavenging activities of 25 μg/ml, 50 μg/ml, 75 μg/ml, and 100 μg/ml of IGESE in comparison with those of corresponding doses of the standard antioxidant drug (Vit. C). IGESE's NO scavenging activities of the extract were significantly (p < 0.001, p < 0.0001) dose related and comparable to that of Vit. C at 75 μg/ml and 100 μg/ml of IGESE (Table 5).
Table 5.
In vitro nitric oxide (NO) scavenging activity of 25-100 μg/ml of IGESE and Vit. C.
| Drug | Graded doses | |||
|---|---|---|---|---|
| 25 μg/ml | 50 μg/ml | 75 μg/ml | 100 μg/ml | |
| IGESE | 13.55 ± 0.70 | 39.98 ± 0.70 | 68.39 ± 0.32b | 77.09 ± 0.13c |
| Vit. C | 47.89 ± 0.14 | 63.09 ± 0.24b | 76.07 ± 0.47c | 84.91 ± 0.31c |
a, b, and c represent significant increases at p < 0.05, p < 0.001, and p < 0.0001, when compared to the baseline value at 25 μg/ml.
3.5.3. Determination of FRAP of IGESE
Table 6 shows IGESE's in vitro ferric reducing activity power of 25 μg/ml, 50 μg/ml, 75 μg/ml, and 100 μg/ml in comparison with those of corresponding doses of the standard antioxidant drug. Again, IGESE's FRAP activities were significantly (p < 0.05, p < 0.001, p < 0.0001) dose dependent and comparable to that of Vit. C especially at 50 μg/ml, 75 μg/ml, and 100 μg/ml of IGESE (Table 6).
Table 6.
In vitro FRAP activities of 25-100 μg/ml of IGESE and Vit. C.
| Drug | Graded doses | |||
|---|---|---|---|---|
| 25 μg/ml | 50 μg/ml | 75 μg/ml | 100 μg/ml | |
| IGESE | 0.08 ± 0.00 | 0.13 ± 0.04a | 0.28 ± 0.00b | 0.48 ± 0.00c |
| Vit. C | 0.24 ± 0.00 | 0.38 ± 0.00b | 0.48 ± 0.00c | 0.63 ± 0.00c |
a, b, and c represent significant increases at p < 0.05, p < 0.001, and p < 0.0001, respectively, when compared to the baseline value at 25 μg/ml.
3.6. Effect of IGESE on the Cardiac Tissue Oxidative Stress Markers (GSH, GST, GPx, SOD, CAT, and MDA) of DOX-Treated Rats
Intraperitoneal injection of DOX to rats resulted in significant (p < 0.05, p < 0.001, and p < 0.0001) decreased activities of SOD, CAT, GPx, GST, and GSH levels while significantly increasing (p < 0.001) MDA activities (Table 7). However, oral pretreatment with IGESE significantly (p < 0.05, p < 0.001, and p < 0.0001) attenuated the alterations in the activities of these cardiac tissue enzyme markers. Similarly, IGESE pretreatment significantly (p < 0.001 and p < 0.0001) and dose dependently reduced MDA levels (Table 7).
Table 7.
Antioxidant enzyme activities of 100-400 mg/kg/day of IGESE in DOX-treated rat cardiac tissue.
| Groups | Antioxidant parameters | |||||
|---|---|---|---|---|---|---|
| GSH | GST | GPx | SOD | CAT | MDA | |
| I | 18.8 ± 1.6 | 1.6 ± 0.2 | 1.2 ± 0.1 | 9.5 ± 1.8 | 43.6 ± 4.7 | 4.3 ± 0.5 |
| II | 20.4 ± 0.6 | 2.7 ± 0.2b+ | 2.0 ± 0.2 | 10.4 ± 1.2 | 62.7 ± 4.4 | 6.0 ± 0.5 |
| III | 14.8 ± 0.8b− | 1.1 ± 0.2a− | 1.0 ± 0.1a− | 6.9 ± 0.6b− | 16.7 ± 2.3c− | 12.8 ± 1.0c+ |
| IV | 21.3 ± 1.3e+,e | 2.6 ± 0.3e+,e | 2.4 ± 0.2e+,e | 11.5 ± 1.5e+,e | 51.4 ± 5.2d+,d | 5.2 ± 0.5e− |
| V | 17.0 ± 1.4d | 2.0 ± 0.2d | 1.3 ± 0.1 | 11.1 ± 1.5d+,d | 54.2 ± 6.5d+,d | 5.3 ± 0.6e− |
| VI | 19.6 ± 1.8d+,d | 2.4 ± 0.1e+,e | 2.3 ± 0.3e+,e | 12.8 ± 1.4e+,e | 56.6 ± 4.3d+,d | 3.9 ± 0.4f− |
| VII | 24.7 ± 1.3e+,e | 3.0 ± 0.4f+,f | 3.3 ± 0.4f+,f | 15.4 ± 1.6e+,e | 78.7 ± 6.9f+,f | 3.5 ± 0.4f− |
b+ represents a significant increase at p < 0.001 when compared to untreated negative (normal) control (Group I) values; c+ represents a significant increase at p < 0.0001 when compared to IGESE-only treated (Group II) values; a-,b-, andc- represent significant decreases at p < 0.05, p < 0.001, and p < 0.0001, respectively, when compared to Groups I values; d+ and e+ represent significant increases at p < 0.001 and p < 0.0001, respectively, when compared to untreated positive control (DOX-only treated, Group III) values; while e- and f- represent significant decreases at p < 0.001 and p < 0.0001, respectively, when compared to untreated positive control (DOX-treated only, Group III) values, respectively. d, e, and f represent significant increases at p < 0.05, p < 0.001, and p < 0.0001, respectively, when compared to untreated positive control (DOX-treated only, Group III).
3.7. Effect of IGESE on Cardiac Marker Enzymes (cTnI and LDH) of DOX-Treated Rats
Single intraperitoneal injection of DOX resulted in significant (p < 0.0001) increases in the serum LDH and cTnI levels when compared to that of untreated negative control (Group I) values (Table 8). However, with oral pretreatments with 100-400 mg/kg/day of IGESE significantly attenuated (p < 0.05, p < 0.001, and p < 0.0001) increases in the serum cTnI and LDH levels dose dependently (Table 8), and these attenuations were comparable to that induced by oral pretreatment with 20 mg/kg/day of Vit. C (Table 8).
Table 8.
Effect of 100-400 mg/kg/day of IGESE on serum LDH and cardiac troponin I (cTnI) levels in DOX-intoxicated rats.
| Treatment groups | LDH (U/L) | cTnI (ng/ml) |
|---|---|---|
| I | 4347 ± 596.4 | 3.4 ± 1.1 |
| II | 4338 ± 238.1 | 3.7 ± 1.1 |
| III | 8151 ± 441.0c+ | 40.5 ± 3.5c+ |
| IV | 4887 ± 217.5a− | 11.4 ± 3.5c− |
| V | 4737 ± 260.2a− | 25.5 ± 3.3a− |
| VI | 4188 ± 229.2b− | 19.8 ± 2.4b− |
| VII | 3679 ± 346.1c− | 14.8 ± 1.1c− |
c+ represents a significant increase at p < 0.0001 when compared to untreated normal control (Group I) and IGESE only treated (Group II) values, while a-, b- and c- represent significant decreases at p < 0.05, p < 0.001, and p < 0.0001, respectively, when compared to DOX-only treated (Group III) values, respectively.
3.8. Effect of IGESE on the Serum Lipids (TG, TC, HDL-c, LDL-c) Level of DOX-Treated Rats
Acute intraperitoneal DOX injection resulted in significant (p < 0.05) decreases in the serum TG, significantly (p < 0.001) increased serum TC and LDL-c while inducing insignificant (p > 0.05) alterations in the serum HDL-c level (Table 9). However, with 100-400 mg/kg/day of IGESE oral pretreatment, there were significant (p < 0.05 and p<0.0001) dose-related increases in the serum TG and HDL-c concentrations, while there were significant (p > 0.05 and p < 0.001) decreases in the serum TC and LDL-c concentrations when compared to DOX-only treated rats (Table 9). Oral pretreatment with 20 mg/kg/day of vitamin C elicited similar effects on the measured serum lipids parameters (Table 9).
Table 9.
Effect of 100-400 mg/kg/day of IGESE on complete serum lipid profile.
| Groups | Serum lipids | |||
|---|---|---|---|---|
| TG(mmol/l) | TC(mmol/l) | HDL-c(mmol/l) | LDL-c(mmol/l) | |
| I | 1.2 ± 0.1 | 2.0 ± 0.1 | 0.7 ± 0.0 | 0.7 ± 0.0 |
| II | 1.1 ± 0.1 | 1.8 ± 0.1 | 0.6 ± 0.0 | 0.6 ± 0.1 |
| III | 0.9 ± 0.1a− | 2.7 ± 0.3c+ | 0.7 ± 0.0 | 1.6 ± 0.2c+ |
| IV | 1.2 ± 0.1d+ | 1.4 ± 0.2f− | 0.8 ± 0.1a+ | 0.4 ± 0.2f− |
| V | 1.0 ± 0.2 | 2.4 ± 0.2d− | 0.8 ± 0.1a+ | 1.2 ± 0.1d− |
| VI | 1.3 ± 0.2d+ | 2.3 ± 0.2d− | 0.9 ± 0.1b+ | 1.1 ± 0.2d− |
| VII | 1.5 ± 0.0e+ | 1.6 ± 0.1f− | 0.6 ± 0.0 | 0.6 ± 0.1f− |
a- represents a significant decrease at p < 0.05 when compared to (Groups I and II) values, while c+ represents a significant increase at p < 0.0001 when compared to Groups I and II values; d- and f- represent significant decreases at p < 0.05 and p < 0.0001, respectively, when compared to DOX-only treated (Group III) values; d+ and e+ represent significant increases at p < 0.05 and p < 0.001, respectively, when compared to DOX-only treated (Group III) values.
3.9. Effect of Oral IGESE Pretreatment on Cardiovascular Risk Indices (AI and CRI) of DOX-Treated Rats
Acute intraperitoneal injections with DOX resulted in significant (p < 0.001) increases in the AI and CRI values when compared to Groups I and II values (Table 10). However, with oral pretreatment with 100-400 mg/kg/day of IGESE, there were significant (p < 0.05, p < 0.001, and p < 0.0001) dose-related decreases in the AI and CRI values with similar effect induced by oral pretreatments with 20 mg/kg/day of Vit. C (Table 10).
Table 10.
Effect of 100-400 mg/kg/day of IGESE on cardiovascular risk indices (atherogenic index (AI) and coronary risk index (CRI)) values in DOX-intoxicated rats.
| Treatment groups | TC ÷ HDL − c (AI) | LDL − c ÷ HDL − c (CRI) |
|---|---|---|
| I | 2.83 ± 0.05 | 1.05 ± 0.07 |
| II | 2.86 ± 0.12 | 1.11 ± 0.15 |
| III | 3.85 ± 0.19b+ | 2.28 ± 0.20c+ |
| IV | 1.88 ± 0.39a− | 0.64 ± 0.33c− |
| V | 3.10 ± 0.23a− | 1.51 ± 0.16a− |
| VI | 2.56 ± 0.27b− | 1.30 ± 0.27b− |
| VII | 2.62 ± 0.18b− | 0.98 ± 0.16c− |
b+ and c+ represent significant increases at p < 0.001 and p < 0.0001, respectively, when compared to Groups I and II values, respectively, while a-, b-, and c- represent significant decreases at p < 0.05, p < 0.001, and p < 0.0001, respectively, when compared to untreated positive control (DOX-only treated) (Group III) values, respectively.
3.10. Histopathological Studies of the Effect of IGESE Oral Pretreatment on DOX-Intoxicated Treated Heart
Figure 2 is a photomicrograph of a cross-sectional representative of DOX-only treated heart showing myocyte congestion and antemortem coronary microthrombi when compared to untreated normal (Figure 3) and IGESE-only treated heart tissues with normal cardiac architecture (Figure 4). However, pretreatment with varying doses of IGESE resulted in dose-related improvements in the histological distortions induced by DOX especially at 200 mg/kg/day (Figure 5) and 400 mg/kg/day of IGESE (Figure 6); although, histological features of vascular congestion were still seen with 100 mg/kg/day of IGESE oral pretreatment (Figure 7). On the contrary, there were histological features of persistent coronary microthrombi in rat heart pretreated with 20 mg/kg/day of Vit. C, indicating the lingering DOX-induced histological lesions, even with the standard antioxidant drug (Figure 8).
Figure 2.
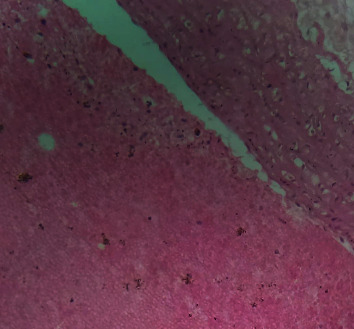
A cross-sectional representative of 15 mg/kg of DOX-only intoxicated rat cardiac tissue showing antemortem coronary artery microthrombi and congested cardiomyocytes suggestive of coronary intravascular thrombosis (×400 magnification, Hematoxylin and Eosin stain).
Figure 3.
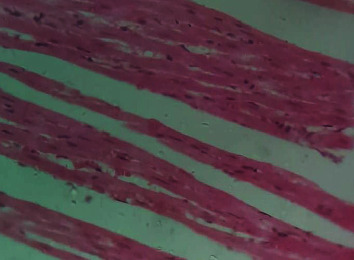
A cross-sectional representative of normal rat cardiac tissue showing normal cardiac histoarchitecture (×400 magnification, Hematoxylin and Eosin stain).
Figure 4.
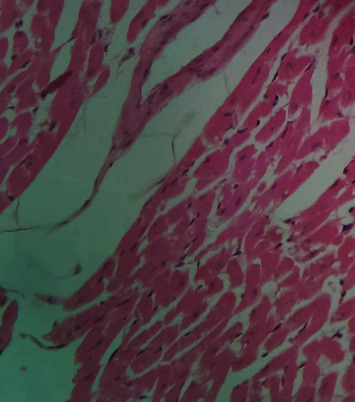
A cross-sectional representative of 200 mg/kg/day of IGESE-only pretreated rat cardiac tissue showing normal cardiac histoarchitecture (×400 magnification, Hematoxylin and Eosin stain).
Figure 5.
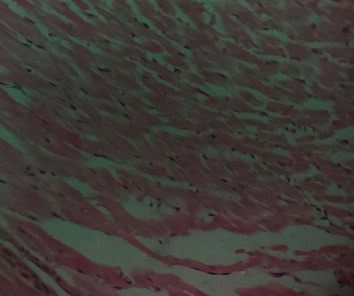
A cross-sectional representative of 200 mg/kg of IGESE pretreated, DOX intoxicated rat cardiac tissue showing mildly congested cardiomyocytes (×400 magnification, Hematoxylin and Eosin stain).
Figure 6.
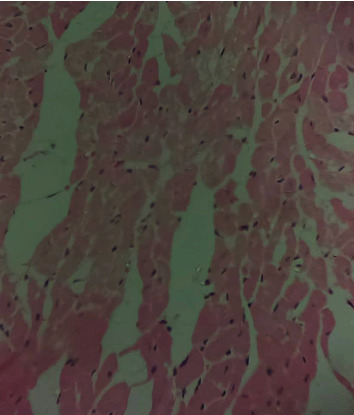
A cross-sectional representative of 400 mg/kg of IGESE pretreated, DOX intoxicated rat cardiac tissue showing normal cardiac histoarchitecture (×400 magnification, Hematoxylin and Eosin stain).
Figure 7.
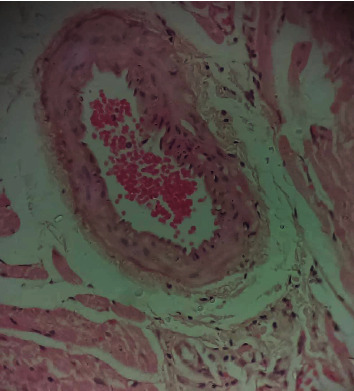
A cross-sectional representative of 100 mg/kg/day of IGESE-pretreated, DOX-intoxicated cardiac tissue showing mild-to-moderate vascular congestion but normal myocardiocytes (×400 magnification, Hematoxylin-Eosin stain).
Figure 8.
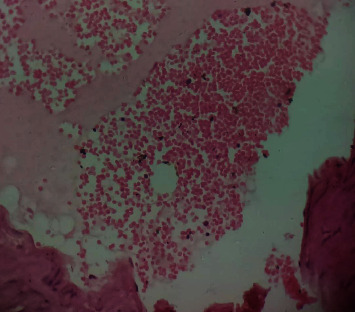
A cross-sectional representative of 20 mg/kg/day of vitamin C pretreated, DOX intoxicated rat cardiac tissue showing mild-to-moderate antemortem coronary artery thrombus and normal cardiomyocytes suggestive of coronary intravascular thrombosis (×400 magnification, Hematoxylin and Eosin stain).
4. Discussion
The clinical use of doxorubicin in the management of solid and hematological cancers has been widely limited by its off-target severe cardiotoxicity which manifests biochemically by elevation of serum enzyme markers of cardiotoxicity. The diagnostic serum marker enzymes of cardiotoxicity are AST, ALT, CK-MB, LDH, and cTnI which leak from cardiac tissue damage to the bloodstream due to their tissue specificity and serum catalytic activity [46]. DOX administration may result in the damage to the myocardial cell membrane or make myocytes more permeable, resulting in the leakage of the diagnostic cardiac enzyme markers cardiac AST, ALT, CK-MB, LDH, and cTnI into the bloodstream and their high circulating levels. In the present study, DOX-mediated cardiotoxicity was fully established as evidenced by the profound elevations in the serum cTnI and LDH levels which is in complete agreement with previous studies [47–52]. With oral IGESE pretreatments, the serum levels of cTnI and LDH were profoundly attenuated toward normal serum level indicating the ameliorative potential of IGESE in DOX-mediated cardiotoxicity. These effects were probably mediated through high antioxidant and/or free radical scavenging activities of IGESE on the myocardium, thus reducing the damaging effects of DOX to the cardiac muscle fibers, subsequently minimizing the leakage of such enzymes in the serum. Similarly, ROS-mediated mechanism is one of the proposed DOX-mediated cardiotoxicity mechanisms, leading to oxidative stress that causes cardiomyopathy [53]. Oxidative stress has been reported to increase lipid peroxidation as indicated by an increase in MDA levels and altered enzymatic and nonenzymatic antioxidant systems [54, 55]. In this study, MDA level was profoundly increased by DOX treatment, while DOX treatment also suppressed the cardiac tissue activities of SOD, CAT, GPx, GST, and GSH levels in the treated rats in agreement with other studies. These altered biochemical alterations were supported by histological lesions characterized by myocyte congestion and coronary intravascular microthrombi formation. DOX has been previously reported to profoundly reduce vascular blood flow, disintegrate vascular endothelium, and promote GPIIb/IIIa-mediated platelet adhesion and aggregation, all resulting in microthrombi formation [56–58]. The fact that IGESE prevented microthrombi formation in DOX-treated coronary vasculature as evidenced by histopathological results of this study highlighted the possible inherent antithrombotic potential of IGESE; although, further studies are still needed in this respect in order to validate this hypothesis. However, IGESE profoundly attenuated significant alterations in the cardiac tissue oxidative markers whose activities were significantly suppressed by DOX intoxication. IGESE has the tendency to neutralize ROS like superoxide radicals, singlet oxygen, nitric oxide, and peroxynitrite, thereby reducing the damage to lipid membranes [39]. Similarly, oral IGESE pretreatments profoundly improved and reversed the DOX-induced histological lesions especially at 200 mg/kg/day and 400 mg/kg/day of IGESE pretreatments.
The effects of DOX on serum lipids are also significant. DOX has been reported to cause hyperlipidemia (which include increased serum cholesterol, triglyceride, LDL-c, and FFAs) [59–64] and increases cardiovascular disease risk [65]. This hyperlipidemia is thought to be mediated via downregulation of PPAR-γ and subsequently affect GLUT4 and FAT/CD36 expression resulting in glucose and fatty acid transporters expression and causing hyperglycemia and hyperlipidemia [65]. Irvingia gabonensis seeds have been reported to induce weight loss, antihyperlipidemia, and reduced cardiovascular disease risk factors in both animal [59–64] and human studies [66–72] which were reportedly mediated via downregulation of the PPAR-γ and leptin genes and upregulation of the adiponectin gene mechanisms [67]. Thus, the results of this study are in tandem with those of earlier studies.
The GC-MS analysis and phytoscan of IGESE are also notably significant. IGESE is shown to contain high contents of 4,6-di-O-methyl-alpha-d-galactose, n-hexadecanoic acid, undecanoic acid, 9,12,15-octadecatrienoic acid, γ-sitosterol, phytol, neophytadiene, ethyl 9,12,15-octadecatrienoate, stigmasterol, vitamin E, hexadecanoic acid ethyl ester, Phytyl palmitate, campesterol, and lupeol. Phytosterols such as sitosterol, stigmasterol, campesterol, and phytols have been reported to effectively mitigate lipid peroxidation through antioxidant and free radical scavenging mechanisms and physically stabilize cell membrane [73] as well as effectively lowered cholesterol especially the LDL-c fraction [74–78]. Similarly, stigmasterol, γ-sitosterol, lupeol, lupeol acetate, and α-amyrin are known to exhibit other important pharmacological activities such as anticancer, anti-inflammatory, and antibacterial activities [79]. Lupeol in particular is known to mediate anti-inflammatory, antimicrobial, antiprotozoal, antiproliferative, anti-invasive, antiangiogenic, and cholesterol-lowering activities [79, 80]. Phytol is an important diterpene that possesses antimicrobial, antioxidant, and anticancer activities [81, 82]. Hexadecanoic acid is known to exhibit strong antimicrobial and anti-inflammatory activity [83]. Squalene, a triterpene, is a natural antioxidant [84], possessing various other pharmacological properties including antimicrobial property [85, 86]. Neophytadiene is a good analgesic, antipyretic, anti-inflammatory, antimicrobial, and antioxidant compound [87, 88]. Thus, the presence of stigmasterol, γ-sitosterol, lupeol, phytols, and neophytadiene in high amounts in IGESE could be responsible for the cholesterol-lowering, antioxidant, and antilipiperoxidation activities of IGESE in DOX-mediated cardiotoxic rats. Similarly, flavonoids, steroids, cardiac glycosides, tannin, and saponin have been reported to elicit antithrombotic activities [89–91], and more specifically, plant-derived sitosterol has been reported to have anticoagulant and thrombus-preventing activities in mice [78, 92, 93]. Thus, the presence of these phytochemicals especially steroids and tannin in high amounts in IGESE could be responsible for the observed antithrombotic action of IGESE in DOX-intoxicated rats.
5. Conclusion
Overall, results of this study showed that IGESE effectively attenuated DOX-mediated cardiotoxicity and its cardioprotective activities were mediated via antioxidant, free radical scavenging, antilipoperoxidation, and antithrombotic mechanisms.
Acknowledgments
The authors deeply appreciate the technical assistance provided by the Laboratory Manager, Dr. Sarah John-Olabode, and other staff of the Laboratory Services, AFRIGLOBAL MEDICARE, Mobolaji Bank Anthony Branch Office, Ikeja, Lagos, Nigeria, in assaying for the serum cardiac biomarkers and lipid profile. Similarly, the technical support of staff of LASUCOM Animal House, for the care of the Experimental Animals used for this study and Mr. Sunday Adenekan of BIOLIFE CONSULTS in the area of oxidative stress markers analysis are much appreciated. This research was funded by the Tertiary Education Trust Fund (TETFUND) Nigeria, through its National Research Fund (TETFUND/NRF/UIL/ILORIN/STI/VOL.1/B2.20.12) as a collaborative research award to Professors Olufunke Olorundare, Phillip Kolo, and Hasan Mukhtar.
Abbreviations
- AI:
Atherogenic index
- ALT:
Alanine transaminase
- AST:
Aspartate transaminase
- CAT:
Catalase
- CK-MB:
Creatine kinase-MB
- CRI:
Coronary artery index
- DMSO:
Dimethyl sulfoxide
- DNA:
Deoxyribonucleic acid
- DOX:
Doxorubicin
- DPPH:
1,1-diphenyl-2-picrylhydrazyl
- FAT/CD36:
Fatty acid translocase
- FFAs:
Free fatty acids
- FRAP:
Ferric reducing activity power
- GC-MS:
Gas chromatography mass spectrometer
- GLUT4:
Glucose transporter member 4
- GPIIb/IIIa:
Glycoprotein IIb/IIIa
- GPx:
Glutathione peroxidase
- GSH:
Reduced glutathione
- GST:
Glutathione S-transferase
- HDL-c:
High density lipoprotein cholesterol
- i.p.:
Intraperitoneal
- IGESE:
Irginvia gabonensis ethanol seed extract
- KCl:
Potassium chloride
- LDH:
Lactate dehydrogenase
- LDL-c:
Low density lipoprotein cholesterol
- MDA:
Malondialdehyde
- NO:
Nitric oxide
- p.o.:
Per os
- PPARγ:
Peroxisome proliferator-activator receptor gamma
- ROS:
Reactive oxygen species
- rpm:
Revolution per minute
- S.E.M.:
Standard error of the mean
- SOD:
Superoxidase dismutase
- TC:
Total cholesterol
- TG:
Triglyceride
- UERC:
UNILORIN ethics and research committee
- UNILORIN:
University of Ilorin
- Vit. C:
Vitamin C.
Data Availability
Answer: Yes. Comment
Conflicts of Interest
The authors have none to declare.
Authors' Contributions
Olufunke Olorundare designed the experimental protocol for this study and was involved in the manuscript writing; Adejuwon Adeneye supervised the research, analyzed data, and wrote the manuscript; Akinyele Akinsola and Olalekan Agede are postgraduate students in Olufunke Olorundare's laboratory that performed the laboratory research under supervision; Phillip Kolo was part of the protocol design and read through the manuscript; Ikechukwu Okoye prepared the cardiac tissue slides; Sunday Soyemi and Alban Mgbehoma independently read and interpreted the cardiac tissue slides; Ralph Albrecht and Hasan Mukhtar are our collaborators in the U.S.A. who read through the manuscript.
References
- 1.Henriksen P. A. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104(12):971–977. doi: 10.1136/heartjnl-2017-312103. [DOI] [PubMed] [Google Scholar]
- 2.Pommier Y., Leo E., Zhang H., Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chemistry & Biology. 2010;17(5):421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tacar O., Sriamornsak P., Dass C. R. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. The Journal of Pharmacy and Pharmacology. 2013;65(2):157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 4.Pang B., Qiao X., Janssen L., et al. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nature Communications. 2013;4(1, article 1908) doi: 10.1038/ncomms2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kropp J., Roti Roti E. C., Ringelstetter A., Khatib H., Abbott D. H., Salih S. M. Dexrazoxane diminishes doxorubicin-induced acute ovarian damage and preserves ovarian function and fecundity in mice. PLoS One. 2015;10(11, article e0142588) doi: 10.1371/journal.pone.0142588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith L. A., Cornelius V. R., Plummer C. J., et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10(1):p. 337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spallarossa P., Maurea N., Cadeddu C., et al. A recommended practical approach to the management of anthracycline-based chemotherapy cardiotoxicity: an opinion paper of the working group on drug cardiotoxicity and cardioprotection, Italian Society of Cardiology. Journal of Cardiovascular Medicine. 2016;17(Supplement 1):e84–e92. doi: 10.2459/JCM.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menna P., Salvatorelli E. Primary prevention strategies for anthracycline cardiotoxicity: a brief overview. Chemotherapy. 2017;62(3):159–168. doi: 10.1159/000455823. [DOI] [PubMed] [Google Scholar]
- 9.Engert A., Franklin J., Eich H. T., et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. Journal of Clinical Oncology. 2007;25(23):3495–3502. doi: 10.1200/JCO.2006.07.0482. [DOI] [PubMed] [Google Scholar]
- 10.Brayfield A. Doxorubicin in: Martindale: The Complete Drug Reference. London: Pharmaceutical Press; 2013. [Google Scholar]
- 11.Zhao M., Ding X.-F., Shen J.-Y., Zhang X. P., Ding X. W., Xu B. Use of liposomal doxorubicin for adjuvant chemotherapy of breast cancer in clinical practice. Journal of Zhejiang University. Science. B. 2017;18(1):15–26. doi: 10.1631/jzus.B1600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magni M., Biancon G., Rizzitano S., et al. Tyrosine kinase inhibition to improve anthracycline-based chemotherapy efficacy in T-cell lymphoma. British Journal of Cancer. 2019;121(7):567–577. doi: 10.1038/s41416-019-0557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaczmarek A., Brinkman B. M., Heyndrickx L., Vandenabeele P., Krysko D. V. Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways. The Journal of Pathology. 2012;226(4):598–608. doi: 10.1002/path.3009. [DOI] [PubMed] [Google Scholar]
- 14.Swain S. M., Whaley F. S., Ewer M. S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 15.Feijen E. A. M., Leisenring W. M., Stratton K. L., et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncology. 2019;5(6):864–871. doi: 10.1001/jamaoncol.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theodoulou M., Hudis C. Cardiac profiles of liposomal anthracyclines: greater cardiac safety versus conventional doxorubicin? Cancer. 2004;100(10):2052–2063. doi: 10.1002/cncr.20207. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee K., Zhang J., Honbo N., Karliner J. S. Doxorubicin cardiomyopathy. Cardiology. 2010;115(2):155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGowan J. V., Chung R., Maulik A., Piotrowska I., Walker J. M., Yellon D. M. Anthracycline chemotherapy and cardiotoxicity. Cardiovascular Drugs and Therapy. 2017;31(1):63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Štěrba M., Popelová O., Vávrová A., et al. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxidants & Redox Signaling. 2013;18(8):899–929. doi: 10.1089/ars.2012.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalay N., Basar E., Ozdogru I., et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. Journal of the American College of Cardiology. 2006;48(11):2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 21.Cai F., Luis M., Lin X., et al. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: preventive strategies and treatment (Review) Molecular and Clinical Oncology. 2019;11(1):15–23. doi: 10.3892/mco.2019.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiserová H., Šimůnek T., van der Vijgh W. J. F., Bast A., Kvasničková E. Flavonoids as protectors against doxorubicin cardiotoxicity: role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochimica et Biophysica Acta (BBA) - Molecular Basis of DiseaseBiochimica et Biophysica Acta. 2007;1772(9):1065–1074. doi: 10.1016/j.bbadis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Minotti G., Menna P., Salvatorelli E., Cairo G., Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 24.Che F. F., Liu Y., Xu C. G. Study on the effect and mechanism of dextromethionine on cardiotoxicity induced by doxorubicin. Journal of Sichuan University. 2010;41:24–28. [PubMed] [Google Scholar]
- 25.Gao X., Han Z., Du X. Observation of the effects of dextromethine on cardiotoxicity induced by epirubicin. Chinese Journal of Cancer Prevention and Treatment. 2010;17:296–298. [Google Scholar]
- 26.Sacco G., Bigioni M., Evangelista S., Goso C., Manzini S., Maggi C. A. Cardioprotective effects of zofenopril, a new angiotensin-converting enzyme inhibitor, on doxorubicin-induced cardiotoxicity in the rat. European Journal of Pharmacology. 2001;414(1):71–78. doi: 10.1016/S0014-2999(01)00782-8. [DOI] [PubMed] [Google Scholar]
- 27.Sacco G., Bigioni M., Lopez G., Evangelista S., Manzini S., Maggi C. A. ACE inhibition and protection from doxorubicin-induced cardiotoxicity in the rat. Vascular Pharmacology. 2009;50(5-6):166–170. doi: 10.1016/j.vph.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Vavrova A., Jansova H., Mackova E., et al. Catalytic inhibitors of topoisomerase II differently modulate the toxicity of anthracyclines in cardiac and cancer cells. PLoS One. 2013;8(10, article e76676) doi: 10.1371/journal.pone.0076676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng S., Yan T., Jendrny C., et al. Dexrazoxane may prevent doxorubicin-induced DNA damage via depleting both topoisomerase II isoforms. BMC Cancer. 2014;14(1):p. 842. doi: 10.1186/1471-2407-14-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burkill H. M. The Useful Plants of West Tropical Africa. Vol. 2. Kew, London: Royal Botanic Gardens; 1985. [Google Scholar]
- 31.Karalliedde L., Gawarammana I. Traditional Herbal Medicines - a Guide to the Safer Use of Herbal Medicines. London: Hammersmith Press; 2008. [Google Scholar]
- 32.Unaeze B. C., Ilo C. E., Egwuatu C., Orabueze I., Obi E. Anti-diarrhoeal effects of three Nigerian medicinal plant extracts on E.coli-induced diarrhea. International Journal of Biological and Chemical Sciences. 2017;11(1):414–419. doi: 10.4314/ijbcs.v11i1.33. [DOI] [Google Scholar]
- 33.Mahunu G. K., Quansa L., Tahir H. E., Mariod A. A. Irvingia gabonensis: phytochemical constituents, bioactive compounds, traditional and medicinal uses. In: Mariod A., editor. Wild Fruits: Composition, Nutritional Value and Products. New York, NY, USA: Springer Cham; 2019. [DOI] [Google Scholar]
- 34.Okogun J. I. Drug discovery through ethnobotany in Nigeria: some results. In: Iwu M. M., Wootton J. C., editors. Advances in Phytomedicine - Ethnomedicine and Drug Discovery. Vol. 1. London: Elsevier; 2002. pp. 145–154. [DOI] [Google Scholar]
- 35.Sun J., Chen P. Ultra high-performance liquid chromatography with high-resolution mass spectrometry analysis of African mango (Irvingia gabonensis) seeds, extract, and related dietary supplements. Journal of Agricultural and Food Chemistry. 2012;60(35):8703–8709. doi: 10.1021/jf302703u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awah F. M., Uzoegwu P. N., Ifeonu P., et al. Free radical scavenging activity, phenolic contents and cytotoxicity of selected Nigerian medicinal plants. Food Chemistry. 2012;131(4):1279–1286. doi: 10.1016/j.foodchem.2011.09.118. [DOI] [Google Scholar]
- 37.Wolfe O. A., Ijeoma U. F. Effects of aqueous extracts of Irvingia gabonensis seeds on the hormonal parameters of male Guinea pigs. Asian Pacific Journal of Tropical Medicine. 2010;3(3):200–204. doi: 10.1016/s1995-7645(10)60009-0. [DOI] [Google Scholar]
- 38.Don Lawson D. C. Proximate analysis and phytochemical screening of Irvingia gabonensis (Ogbono cotyledon) Biomedical Journal of Scientific & Technical Research. 2018;5(4):4643–4646. doi: 10.26717/bjstr.2018.05.001227. [DOI] [Google Scholar]
- 39.Ekpe O. O., Nwaehujor C. O., Ejiofor C. E., Arikpo Peace W., Woruji Eliezer E., Amor Emmanuel T. Irvingia gabonensis seeds extract fractionation, its antioxidant analyses and effects on red blood cell membrane stability. PhOL. 2019;1:337–353. [Google Scholar]
- 40.Trease G. E., Evans W. C. A Textbook of Pharmacognosy. London, UK: Bailliere Tindall Ltd; 1989. [Google Scholar]
- 41.Sofowora A. Medicinal Plants and Traditional Medicine in Africa. Ibadan, Nigeria: Spectrum Books Ltd; 1993. [Google Scholar]
- 42.Olorundare O. E., Adeneye A. A., Akinsola A. O., Sanni D. A., Koketsu M., Mukhtar H. Clerodendrum volubile ethanol leaf extract: a potential antidote to doxorubicin-induced cardiotoxicity in rats. Journal of Toxicology. 2020;2020:17. doi: 10.1155/2020/8859716.8859716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Washington, DC, USA: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 44.Abbott R. D., Wilson P. W., Kannel W. B., Castelli W. P. High density lipoprotein-cholesterol, total cholesterol screening and myocardial infarction: the Framingham study. Arteriosclerosis. 1988;8(3):207–211. doi: 10.1161/01.ATV.8.3.207. [DOI] [PubMed] [Google Scholar]
- 45.Alladi S., Shanmugasundaram K. R. Induction of hypercholesterolemia by supplementing soy protein with acetate generating amino acids. Nutrition Reports International. 1989;40:893–899. [Google Scholar]
- 46.Zheng J., Lee H. C. M., bin Sattar M. M., Huang Y., Bian J. S. Cardioprotective effects of epigallocatechin-3-gallate against doxorubicin-induced cardiomyocyte injury. European Journal of Pharmacology. 2011;652(1-3):82–88. doi: 10.1016/j.ejphar.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 47.Polena S., Shikara M., Naik S., et al. Troponin I as a marker of doxorubicin induced cardiotoxicity. Proceedings of the Western Pharmacology Society. 2005;48:142–144. [PubMed] [Google Scholar]
- 48.Shahzadi A., Sonmez I., Allahverdiyev O., et al. Cardiac troponin-I (cTnI) a biomarker of cardiac injuries induced by doxorubicin alone and in combination with ciprofloxacin, following acute and chronic dose protocol in Sprague Dawley rats. International Journal of Pharmacology. 2014;10(5):258–266. [Google Scholar]
- 49.Simões R., Silva L. M., Cruz A. L. V. M., Fraga V. G., de Paula Sabino A., Gomes K. B. Troponin as a cardiotoxicity marker in breast cancer patients receiving anthracycline-based chemotherapy: a narrative review. Biomedicine & Pharmacotherapy. 2018;107:989–996. doi: 10.1016/j.biopha.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 50.Zilinyi R., Czompa A., Czegledi A., et al. The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: the role of autophagy. Molecules. 2018;23(5, article 1184) doi: 10.3390/molecules23051184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alam M. F., Khan G., Safhi M. M., et al. Thymoquinone ameliorates doxorubicin-induced cardiotoxicity in Swiss albino mice by modulating oxidative damage and cellular inflammation. Cardiology Research and Practice. 2018;2018:6. doi: 10.1155/2018/1483041.1483041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adamcova M., Skarkova V., Seifertova J., Rudolf E. Cardiac troponins are among targets of doxorubicin-induced cardiotoxicity in hiPCS-CMs. International Journal of Molecular Sciences. 2019;20(11, article 2638) doi: 10.3390/ijms20112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong E. K. C., Yu S., Sanderson J. E., Chen K. B., Huang Y., Yu C. M. A novel anti-fibrotic agent, baicalein for the treatment of myocardial fibrosis in spontaneously hypertensive rats. European Journal of Pharmacology. 2011;658(2-3):175–181. doi: 10.1016/j.ejphar.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 54.Freeman B. A., Crapo J. D. Biology of disease free radicals and tissue injury. Laboratory Investigation. 1982;47(5):412–426. [PubMed] [Google Scholar]
- 55.Gaweł S., Wardas M., Niedwork E., Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci Lekarskie. 2004;57(9-10):453–455. [PubMed] [Google Scholar]
- 56.Kim S. H., Lim K. M., Noh J. Y., et al. Doxorubicin-induced platelet procoagulant activities: an important clue for chemotherapy-associated thrombosis. Toxicological Sciences. 2011;124(1):215–224. doi: 10.1093/toxsci/kfr222. [DOI] [PubMed] [Google Scholar]
- 57.Ben Aharon I., Bar Joseph H., Tzabari M., et al. Doxorubicin-induced vascular toxicity – targeting potential pathways may reduce procoagulant activity. PLoS One. 2013;8(9, article e75157) doi: 10.1371/journal.pone.0075157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lv H., Tan R., Liao J., et al. Doxorubicin contributes to thrombus formation and vascular injury by interfering with platelet function. American Journal of Physiology-Heart and Circulatory Physiology. 2020;319(1):H133–H143. doi: 10.1152/ajpheart.00456.2019. [DOI] [PubMed] [Google Scholar]
- 59.Samelis G. F., Stathopoulos G. P., Kotsarelis D., Dontas I., Frangia C., Karayannacos P. E. Doxorubicin cardiotoxicity and serum lipid increase is prevented by dextrazoxane (ICRF-187) Anticancer Research. 1998;18(5A):3305–3309. [PubMed] [Google Scholar]
- 60.Hong Y. M., Kim H. S., Yoon H.-R. Serum lipid and fatty acid profiles in adriamycin-treated rats after administration of L-carnitine. Pediatric Research. 2002;51(2):249–255. doi: 10.1203/00006450-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 61.Lee J., Chung M., Fu Z., Choi J., Lee H. J. The effects of Irvingia gabonensis seed extract supplementation on anthropometric and cardiovascular outcomes: a systematic review and meta-analysis. Journal of the American College of Nutrition. 2020;39(5):388–396. doi: 10.1080/07315724.2019.1691956. [DOI] [PubMed] [Google Scholar]
- 62.Kuyooro S. E., Abam E. O., Agbede E. B. Hypolipidemic effects of Irvingia gabonensis - supplemented diets in male Albino rats. Biochemistry & Analytical Biochemistry. 2017;6(2, article 1000316) [Google Scholar]
- 63.Fatoki J. O., Adedosu O. T., Afolabi O. K., et al. Dyslipidemic effect of doxorubicin and etoposide: a predisposing factor for the antineoplastic drugs-induced cardiovascular diseases. Research & Reviews: Journal of Pharmacology and Toxicological Studies. 2018;6(1):34–42. [Google Scholar]
- 64.Mentoor I., Nell T., Emjedi Z., van Jaarsveld P. J., de Jager L., Engelbrecht A. M. Decreased efficacy of doxorubicin corresponds with modifications in lipid metabolism markers and fatty acid profiles in breast tumors from obese vs. lean mice. Frontiers in Oncology. 2020;10:p. 306. doi: 10.3389/fonc.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arunachalam S., Tirupathi Pichiah P. B., Achiraman S. Doxorubicin treatment inhibits PPARγ and may induce lipotoxicity by mimicking a type 2 diabetes-like condition in rodent models. FEBS Letters. 2013;587(2):105–110. doi: 10.1016/j.febslet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 66.Ngondi J. L., Oben J. E., Minka S. R. The effect of Irvingia gabonensis seeds on body weight and blood lipids of obese subjects in Cameroon. Lipids in Health and Disease. 2005;4(1):p. 12. doi: 10.1186/1476-511X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oben J. E., Ngondi J. L., Blum K. Inhibition of Irvingia gabonensis seed extract (OB131) on adipogenesis as mediated via down regulation of the PPARgamma and leptin genes and up-regulation of the adiponectin gene. Lipids in Health and Disease. 2008;7(1):p. 44. doi: 10.1186/1476-511X-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ngondi J. L., Etoundi B. C., Nyangono C. B., Mbofung C. M. F., Oben J. E. IGOB131, a novel seed extract of the West African plant Irvingia gabonensis, significantly reduces body weight and improves metabolic parameters in overweight humans in a randomized double-blind placebo controlled investigation. Lipids in Health and Disease. 2009;8(1):p. 7. doi: 10.1186/1476-511X-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Onakpoya I., Davies L., Posadzki P., Ernst E. The efficacy of Irvingia gabonensis supplementation in the management of overweight and obesity: a systematic review of randomized controlled trials. Journal of Dietary Supplements. 2013;10(1):29–38. doi: 10.3109/19390211.2012.760508. [DOI] [PubMed] [Google Scholar]
- 70.Lee M., Nam D. E., Kim O. K., Shim T. J., Kim J. H., Lee J. Anti-obesity effects of African mango (Irvingia gabonesis, IGOB 131TM) extract in leptin-deficient obese mice. Journal of the Korean Society of Food Science and Nutrition. 2014;43(10):1477–1483. doi: 10.3746/jkfn.2014.43.10.1477. [DOI] [Google Scholar]
- 71.Azantsa B., Kuate D., Chakokam R., Paka G., Bartholomew B., Nash R. The effect of extracts of Irvingia gabonensis (IGOB131) and Dichrostachys glomerata (Dyglomera™) on body weight and lipid parameters of healthy overweight participants. Functional Foods in Health and Disease. 2015;5(6):200–208. doi: 10.31989/ffhd.v5i6.184. [DOI] [Google Scholar]
- 72.Patra S., Nithya S., Srinithya B., Meenakshi S. M. Review of medicinal plants for anti-obesity activity. Translational Biomedicine. 2015;6(3) doi: 10.21767/2172-0479.100021. [DOI] [Google Scholar]
- 73.Yoshida Y., Niki E. Antioxidant effects of phytosterol and its components. Journal of Nutritional Science and Vitaminology. 2003;49(4):277–280. doi: 10.3177/jnsv.49.277. [DOI] [PubMed] [Google Scholar]
- 74.Meguro S., Higashi K., Hase T., et al. Solubilization of phytosterols in diacylglycerol versus triacylglycerol improves the serum cholesterol-lowering effect. European Journal of Clinical Nutrition. 2001;55(7):513–517. doi: 10.1038/sj.ejcn.1601173. [DOI] [PubMed] [Google Scholar]
- 75.Demonty I., Ras R. T., van der Knaap H. C. M., et al. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. The Journal of Nutrition. 2009;139(2):271–284. doi: 10.3945/jn.108.095125. [DOI] [PubMed] [Google Scholar]
- 76.Ras R. T., Geleijnse J. M., Trautwein E. A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies. The British Journal of Nutrition. 2014;112(2):214–219. doi: 10.1017/S0007114514000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trautwein E., Vermeer M., Hiemstra H., Ras R. LDL-cholesterol lowering of plant sterols and stanols - which factors influence their efficacy? Nutrients. 2018;10(9, article 1262) doi: 10.3390/nu10091262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan C., Zhang X., Long X., Jin J., Jin R. Effect of β-sitosterol self-microemulsion and β-sitosterol ester with linoleic acid on lipid-lowering in hyperlipidemic mice. Lipids in Health and Disease. 2019;18(1):p. 157. doi: 10.1186/s12944-019-1096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abu-Lafi S., Rayan M., Masalha M., et al. Phytochemical composition and biological activities of wild Scolymus maculatus L. Medicine. 2019;6(2):p. 53. doi: 10.3390/medicines6020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saleem M., Murtaza I., Tarapore R. S., et al. Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis. 2009;30(5):808–817. doi: 10.1093/carcin/bgp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei L. S., Wee W., Siong J. Y. F., Syamsumir D. F. Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Medica Iranica. 2011;49(10):670–674. [PubMed] [Google Scholar]
- 82.Song Y., Cho S. K. Phytol induces apoptosis and ROS mediated protective autophagy in human gastric adenocarcinoma AGS cells. Biochemistry and Analytical Biochemistry. 2015;4:p. 211. [Google Scholar]
- 83.Amarowicz R. Squalene: a natural antioxidant? European Journal of Lipid Science and Technology. 2009;111(5):411–412. doi: 10.1002/ejlt.200900102. [DOI] [Google Scholar]
- 84.Kim S.-K., Karadeniz F. Biological importance and applications of squalene and squalane. Advances in Food and Nutrition Research. 2012;65:223–233. doi: 10.1016/B978-0-12-416003-3.00014-7. [DOI] [PubMed] [Google Scholar]
- 85.Ben Arfa A., Combes S., Preziosi-Belloy L., Gontard N., Chalier P. Antimicrobial activity of carvacrol related to its chemical structure. Letters in Applied Microbiology. 2006;43(2):149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 86.Muzalevskaya E. N., Miroshnichenko L. A., Nikolaevskii V. A., et al. Squalene: physiological and pharmacological properties. Eksperimental'naia i Klinicheskaia Farmakologiia. 2015;78(6):30–36. [PubMed] [Google Scholar]
- 87.Venkata Raman B., Samuel L. A., Saradhi M. P., et al. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian Journal of Pharmaceutical and Clinical Research. 2012;5(2):99–106. [Google Scholar]
- 88.Swamy M. K., Arumugam G., Kaur R., Ghasemzadeh A., Yusoff M. M., Sinniah U. R. GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of MalaysianPlectranthus amboinicusLeaves. Evidence-Based Complementary and Alternative Medicine. 2017;2017:10. doi: 10.1155/2017/1517683.1517683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cordier W., Steenkamp V. Herbal remedies affecting coagulation: a review. Pharmaceutical Biology. 2012;50(4):443–452. doi: 10.3109/13880209.2011.611145. [DOI] [PubMed] [Google Scholar]
- 90.Mahmud S., Akhter S., Rahman M. A., et al. Antithrombotic effects of five organic extracts of Bangladeshi plants in vitro and mechanisms in in silico models. eCAM. 2015;2015, article 782742:1–8. doi: 10.1155/2015/782742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thom V. T., Tung N. H., van Diep D., et al. Antithrombotic activity and saponin composition of the roots of Panax bipinnatifidus Seem. growing in Vietnam. Pharmacognosy Research. 2018;10(4):333–338. doi: 10.4103/pr.pr_58_18. [DOI] [Google Scholar]
- 92.Gogoi D., Pal A., Chattopadhyay P., Paul S., Deka R. C., Mukherjee A. K. First report of plant-derived β-sitosterol with antithrombotic, in vivo anticoagulant, and thrombus-preventing activities in a mouse model. Journal of Natural Products. 2018;81(11):2521–2530. doi: 10.1021/acs.jnatprod.8b00574. [DOI] [PubMed] [Google Scholar]
- 93.Salunkhe P. S., Patil S. D., Dhande S. R. Anti-thrombotic activity of isolated β-sitosterol from roots of Hemidesmus indicus Linn. in rat model. Journal of Pharmacognosy and Phytochemistry. 2018;7(SP6):10–14. doi: 10.22271/phyto.2018.v7.isp6.1.03. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Answer: Yes. Comment


