Abstract
Background:
Medication review with follow-up (MRF) is a service where community pharmacists undertake a medication review with monthly follow-up to provide continuing care. The ConSIGUE Program assessed the impact and implementation of MRF for aged polypharmacy patients in Spanish Community Pharmacies. The present paper reports on the clinical impact evaluation phase of ConSIGUE.
Objective:
The main objective of the study was to measure the effect of MRF on the primary outcome of the number of uncontrolled health problems. Secondary objectives were to analyze the drug-related problems (DRPs) identified as potential causes of ineffective or unsafe medications and the pharmacists’ interventions implemented during MRF provision.
Methods:
An open-label multi-centered cluster randomized study with comparison group (CG) was carried out in community pharmacies from 4 provinces in Spain during 6 months. The main inclusion criteria were patients over 64 years old, using 5 or more medicines. The intervention group (IG) received the MRF service (advanced medication review-type 3 MR) whereas patients in the CG received usual care.
Results:
178 pharmacies recruited 1403 patients (IG= 688 patients; CG= 715 patients). During the 6 months of the study 72 patients were lost to follow up. The adjusted multi-level random effects models showed a significant reduction in the number of uncontrolled health problems over the periods in the IG (-0.72, 95% CI: -0.80, -0.65) and no change in the CG (-0.03, 95% CI: -0.10, 0.04). Main DRPs identified as potential causes of failures of uncontrolled health problems’ treatment were undertreated condition (559 DRPs; 35.81%), lack of treatment adherence (261 DRP; 16.67%) and risk of adverse effects (207 DRPs; 13.53%). Interventions performed by pharmacist to solve DRP mainly included the addition (246 interventions; 14.67%) and change (330 interventions; 19.68%) of a medicine and educational interventions on medicine adherence (231 interventions; 13.78%) and non-pharmacological interventions (369 interventions; 22.01%).
Conclusions:
This study provides evidence of the impact of community pharmacist on clinical outcomes for aged patients. It suggests that the provision of an MRF in collaboration with general medical practitioners and patients contributes to the improvement of aged polypharmacy patients’ health status and reduces their problems related with the use of medicines.
Keywords: Medication Therapy Management, Community Pharmacy Services, Pharmacies, Pharmacists, Polypharmacy, Treatment Adherence and Compliance, Randomized Controlled Trials as Topic, Spain
INTRODUCTION
A high percentage of over 64-year-old patients have co-morbidities and have multiple medications prescribed.1,2 The ageing process involves both psychosocial and physiological changes that can have an influence on clinical outcomes.3 This population is reported to have a high prevalence of medicines’ overuse, administration errors and poor medication adherence.4 Age-related changes in pharmacokinetics and pharmacodynamics and inter-individual variability in drug response increase the probability of interactions, contraindications and adverse effects.5 Aged patients are at a high risk of not achieving their pharmacotherapy goals and consequently, of presenting uncontrolled health problems despite being prescribed a high number of medications.6
There is obvious need for systematic approaches to be adopted by health professionals to optimize medicines´ use and clinical outcomes in aged polypharmacy patients. The World Health Organization recommends implementing pharmacy services to monitor and individualize pharmacotherapy.7 A professional pharmacy service (PPS) is “a set of actions undertaken in or organized by a pharmacy, delivered by a pharmacist or other health practitioner, who applies their specialized health knowledge personally or via an intermediary, with a patient/client, population or other health professional, to optimize the process of care, with the aim to improve health outcomes and the value of healthcare”.8 There are a wide range of professional pharmacy services, from the simple provision of medicines information to more clinically complex such us medication review, disease state management and prescribing.9 A Medication Review with Follow up (MRF) is a professional service where community pharmacists collaborate with other members of the health care team and the patient to prevent and solve drug related problems (DRP) for the improvement of clinical outcomes.10 MRF healthcare plans include tailored interventions and a monitoring process to improve the level of control of health problems. The important philosophical and operational difference to the usual type 3 or advanced medication review is that although not having access to medical records MRF has a protocol that includes a follow up over a period of time and is focused on clinical outcomes particularly control of the disease.11
There is a continuing controversy around the effectiveness of PPS including medication reviews. Two recent overviews of the scientific literature found high and moderate strength of evidence for the positive effect of certain PPSs on reducing the number and improving the appropriateness of medicines, but limited evidence on clinical outcome indicators.12,13 The authors concluded that, since PPS are complex interventions, there was a need of carrying out studies with high internal validity that measure their short-term impact by means of surrogate clinical outcome variables, such as the control of health problems.12 On the other hand, studies with high external validity are also required to ensure positive outcomes in the later stage of service implementation in real practice. The Medical Research Council guidelines for the development and evaluation of complex interventions, recommends the use of cluster randomized designs, with both process and outcome assessment, to achieve not only effective but also reproducible services and interventions.14
A national program was launched in Spain, called conSIGUE, to measure the clinical, economic and humanistic effectiveness of the MRF service on aged polypharmacy patients. This paper presents the results associated with the clinical impact of the service.
The objectives were (1) to describe the effectiveness of MRF provision for aged polypharmacy patients on the number of uncontrolled health problems, (2) to identify DRPs potentially related to uncontrolled health problems along with pharmacists’ interventions carried out during MRF provision.
METHODS
Design and setting
An open-label multi-centered cluster randomized controlled study was carried out in 178 volunteer community pharmacies from 4 provinces in Spain selected by the General Council of Spanish Pharmacist (Gipuzkoa, Granada, Las Palmas and Santa Cruz de Tenerife) during eight months (May 2012-Jan 2013). Pharmacies in the intervention group (IG) provided the MRF service whereas comparison group (CG) pharmacies provided usual care and received no support on the provision of MRF. The Consort statement to improve reporting of cluster randomized controlled trials and minimize the risk of bias was applied.15
Patients
Design of this study was previously tested in a one-month pilot study carried out in one Spanish province (Cádiz). Sample size was calculated based on the pilot data to detect a hypothesized reduction of 10% in the proportion of uncontrolled health problems in the IG compared to the CG, considering an alpha error of 0.05 with 80% power in a two-way bilateral contrast. According to this calculation, 393 patients in each group would be required. Applying the standard criteria for cluster randomized trials, this initial sample size was multiplied by a design effect (DE) of 1.1854 to have into account the clustering effect of randomization at pharmacy level. The DE was calculated as follows:
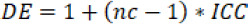
The ICC in the present work (0.0206) was calculated from the pilot study, and the mean cluster size was assumed to be 10 patients.16 A 12% inflation was applied to prevent the possibility of unequal cluster sizes.17 In addition, a potential loss at follow-up of 20% was estimated. Therefore, a minimum of 1,230 aged polypharmacy patients were required. Local pharmacist associations offered all pharmacies within their area the voluntary participation on the program and those pharmacies that accepted were randomized into intervention and control group. Within each pharmacy, patients were selected by convenience sampling to facilitate the access to patients and their physicians, according to the following criteria: patients over 64 years old, using five or more medicines for a period longer than six months and giving their informed consent to participate in the study.
Intervention
Pharmacies in the intervention group (IG) provided MRF during 6 months using the Dader method.10,11 The Dader method for MRF includes three stages: (1) Analysis of patients’ medication therapy: face to face patients’ interview to obtain information on their health problems and medicines, assessment of the pharmacotherapy to identify uncontrolled health problems as well as DRPs potentially related to pharmacotherapy failures. (2) Care plan: Interventions directed to the physician or to the patient to prevent or solve drug related problems and improve the level of control of health problems. Pharmacists-physician communication was agreed with the patient and it could have been both oral (by phone or face-to-face) and written (a letter given to patients). (3) Follow up: Visits with the patient on a monthly basis for the assessment of interventions’ results and continuance with patients’ care plan. Further details of the intervention elements are provided in TiDier Checklist (available at the
).18 Pharmacists within the IG were trained both for data collection and for MRF provision. Before the beginning of the study, they received a 5-day off-site training program on service provision, pharmacotherapy issues in treating patients, disease state management, evidence-based practice, communication with the patient and with other health professionals, data collection and clinical cases. During service provision, they received on-site support by a practice change facilitator (monthly visits as well as weekly telephone and email contact). Pharmacists within the CG attended a course on data collection and did not receive any information on MRF service.
Study outcomes
Assessment of control of health problems: Documentation on clinical history, including disease and medication history, were provided by the patient. The number of uncontrolled health problems, classified by means of International Classification of Primary Care, was assessed based on the achievement or not of the desired therapeutic outcomes for each individual patient, in 6 different points in time (on monthly basis).19 A combination of three different assessment criteria were used by the pharmacist, depending on the nature of the health problem, under the supervision of the facilitator of practice change: (1) achievement of desired values of the clinical parameters of control based on clinical guidelines recommendations, (2) improvement or resolution of signs or symptoms based on clinical information provided by the patient (3) achievement of the desired prevention by means of the patients’ self-reported adherence to the treatment. Several covariables were collected: age, gender, education level, civil status, number of health problems, and the number of medicines at each study period. DRP identified by the pharmacist were recorded according to Foro agreement (a Spanish national committee of professional and university representative).10
Statistical analysis
Data analysis was performed using PASW v-18.0. P-values less than 0.05 (p<0.05) were interpreted as statistically significant. Clustered data were analyzed using a multilevel model that included repeated measures in 6 different points and accounted for the clustering effect of the randomization at the pharmacy level.15 The initial multi-level random effects model for the outcome included all four nested levels (province, pharmacy, pharmacist and patient) and random effect for data collection point with an independent covariance structure. There were other independent variables collected on patients at 6 time-points that were included in the multi-level random effects linear model to account for other factors that might explain the change in the outcome over time and its variability at any given time, using a stepped approach. An interaction term of intervention/comparison group and time-point was included to estimate the outcome at each time-point. To show the number of uncontrolled health problems (by study group) at each time-point, marginal means were estimated to explore if there were changes in the outcome over time and if these changes were different in the study groups accounting for other factors.
Ethical considerations
The study was approved by ‘Virgen de las Nieves Hospital Clinical Research Ethics Committee’. Patients gave informed consent to participate in the study and for publication.
RESULTS
178 pharmacies (250 pharmacists) initially recruited 1,403 patients (IG=688 patients and CG=715 patients). Mean number of patients/pharmacies were 7.88 (SD=2.40). A total of 1,331 patients (94.9%) completed the study; 72 patients were lost to follow up and excluded from the analysis (Figure 1).
Figure 1. Flow diagram of patients.
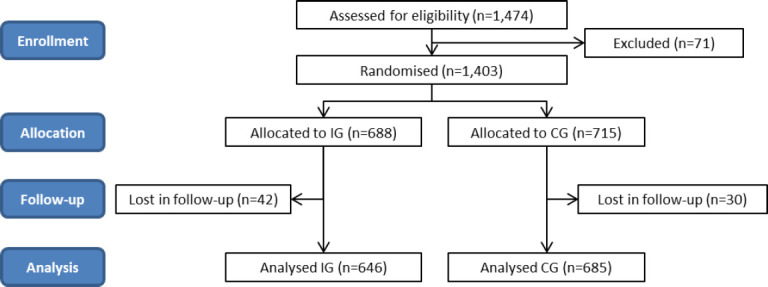
IG: intervention group; CG: comparison group
Socio-demographic characteristics of patients and baseline data are described in Table 1. Study groups presented statistically significant differences for the level of education, mean number of medicines, health problems and uncontrolled health problems. At baseline, a mean number of 4.65 (SD=1.66) conditions per patient were assessed by community pharmacists as controlled or uncontrolled using the mentioned assessment criteria and a mean number of 7.74 (SD=2.50) medicines per patient were studied by pharmacists in the IG to identify DRPs. Patients in the IG presented a significantly higher number of conditions (4.35 vs 4.96: p=0.001), uncontrolled health problems (1.46 vs 0.73: p<0.001) and medicines (7.39 vs 7.74: p=0.009) compared to patients in the CG.
Table 1. Baseline patient characteristics.
| Characteristic | valid (n=1403) | Summary Statistic | p-value | |
|---|---|---|---|---|
| Control Group (n=715) | Intervention Group (n=688) | |||
| Age; mean (SD) | 1403 | 74.92 (6.59) | 75.34 (6.46) | 0.243 |
| Gender: Female; n (%) | 1396 | 441 (61.7) | 409 (60.1) | 0.535 |
| Partner Status:with partner; n (%) | 1242 | 384 (59.3) | 355 (59.8) | 0.856 |
| Education; n (%) | 1174 | 0.004 | ||
| No formal education | 116 (18.7) | 149 (27) | ||
| Completed primary education | 313 (50.3) | 239 (43.3) | ||
| Completed secondary education | 135 (21.7) | 106 (19.2) | ||
| Completed university education | 58 (9.3) | 58 (10.5) | ||
| Number of medicines; mean (SD) | 1403 | 7.39 (2.37) | 7.74 (2.50) | 0.009 |
| Number of health problems | 1403 | 4.35 (1.49) | 4.96 (1.76) | <0.001 |
| Number of uncontrolled health problems;median (IQR) | 1403 | 0.73 (0.66-0.8) | 1.46 (1.36-1.56) | <0.001 |
Effect of MRF on the number of uncontrolled health problems
Over the 6 months of the study, there was a statistically significant reduction in the number of uncontrolled health problems in the IG (-0.81, 95%CI: -0.89, -0.73) while there was no significant change in the CG (-0.05, 95%CI: -0.1, 0.0002).
Figure 2 shows the prevalence of each type of uncontrolled health problems (International Classification of Primary Care) at baseline.19 The most frequent uncontrolled health conditions identified by pharmacists were, psychological problems (17.08% in the IG and 27.19% in the CG), musculoskeletal problems (16.09% in the IG and 14.83% in the CG) and general and unspecified conditions (10.33% in the IG and 16.92% in the CG). There were no statistically significant differences among the groups before the interventions for any of the categories of health problems, except for cardiovascular (p=0.007) and endocrine (p=0.001) conditions which were more prevalent in the IG.
Figure 2. Type of baseline uncontrolled health problems in the IG and CG.
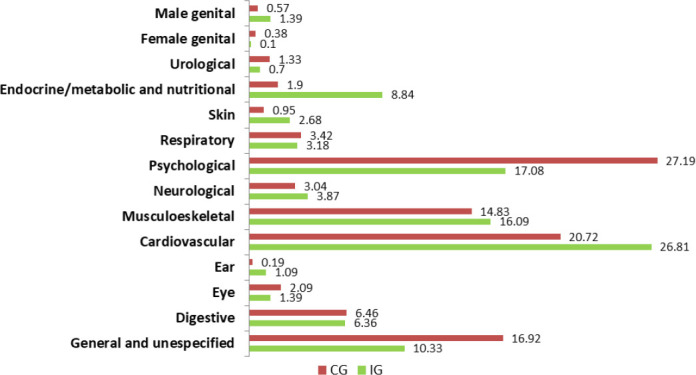
On accounting for the nested data collection in the multi-level random effects model (Table 2), the patients in the IG had statistically significantly more uncontrolled health problems (mean difference 0.66, 95%CI: 0.51, 0.82) than the CG at baseline, which remained on adjustment for other factors (gender, partner status, education, number of patient drugs, quality of life). Marginal mean effects calculated by means of this model show the change in the difference in number of uncontrolled health problems between the two groups over the periods (Table 3). In the unadjusted model, there is no significant difference in the number of uncontrolled health problems between IC and CG by the end of the timeframe (mean difference at 6th month -0.06, 95%CI: -0.20, 0.09). There is a significant mean reduction over the 6 months in the IG of -0.76 health problems (95%CI: -0.83, -0.69) compared to a non-significant reduction over the 6 months in the CG (-0.04, 95%CI: -0.10, 0.03). This change was also obtained in the multilevel analysis performed to control for baseline differences between groups, age, gender, partner status, education, number of patient´s drugs, quality of life measured by validated tool EuroQol, as well as the number of emergency room visits and hospital admissions in the prior 6 months (Table 2). On accounting for these factors, a significant reduction over the period in the IG (-0.72, 95%CI: -0.80, -0.65) no change in the CG (-0.03, 95%CI: -0.10, 0.04) and a mean difference between groups at 6th month of -0.04, (95% CI: -0.19, 0.11) were obtained.
Table 2. Associations with Number of uncontrolled health problems: Multilevel mixed-effects linear regression models*.
| Variable; mean (95%CI) | Number of uncontrolled health problems | |
|---|---|---|
| Univariate | Multivariable | |
| Intervention vs Control group | ||
| Difference between intervention and control groups at baseline | 0.66 (0.51,0.82) | 0.66 (0.5,0.81) |
| Age | ||
| Change per 10-year increase of age of patient at any given time-point | -0.03 (-0.09,0.04) | - |
| Gender | ||
| Difference in Men compared to Women at any given time-point | -0.36 (-0.45,-0.28) | -0.24 (-0.34,-0.14) |
| Partner status | ||
| Difference in those Without Partner compared to With Partner at any time-point | 0.11 (0.01,0.2) | -0.02 (-0.12,0.08) |
| Education | ||
| Difference in those with Primary Studies compared toNo studies at any time-point | -0.09 (-0.22,0.05) | -0.01 (-0.14,0.11) |
| Difference in those with Secondary Studies compared to No studies at any time-point | -0.28 (-0.45,-0.12) | -0.09 (-0.24,0.07) |
| Difference in those with University Studies compared to No studies at any time-point | -0.42 (-0.62,-0.23) | -0.18 (-0.37,0.01) |
| Number of patient’s drugs | ||
| Change per increase of number of drugs at any given time-point | 0.11 (0.095,0.124) | 0.09 (0.08,0.11) |
| EQ5D Index Value | ||
| Change per increase of 0.1 in EQ5D Index Value at any given time-point | -0.06 (-0.067,-0.052) | -0.04 (-0.05,-0.03) |
| EQ5D VAS Scale | ||
| Change per increase of 10 in EQ5D VAS scale at any given time-point | -0.07 (-0.08,-0.06) | -0.03 (-0.05,-0.02) |
| Number of ER visits in the six months prior to baseline | ||
| Change per increase in ER visit in 6 months prior to baseline, at any given time-point | 0.01 (-0.02,0.05) | -0.02 (-0.06,0.01) |
| Number of hospital admissions in the six months prior to baseline | ||
| Change per increase in Hospital Admissions in 6 months prior tobaseline, at any given time-point | -0.05 (-0.17,0.06) | -0.08 (-0.21,0.06) |
Table 3. Number of uncontrolled health problems over time and difference between Intervention and Control Groups: Marginal Means predicted from multilevel mixed-effects linear regression models*.
| Time period | Univariate model | Multivariable model | ||||
|---|---|---|---|---|---|---|
| Mean (95% CI) number of uncontrolled health problems | Mean difference (95%CI) between IG and CG | Mean (95%CI) number of uncontrolled health problems | Mean difference (95% CI) between IG and CG | |||
| Intervention | Control | Intervention | Control | |||
| Month 1 | 1.42 (1.16,1.68) | 0.75 (0.49,1.01) | 0.66 (0.51,0.82) | 1.38 (1.15,1.6) | 0.72 (0.5,0.94) | 0.66 (0.5,0.81) |
| Month 2 | 1.26 (1.01,1.52) | 0.4 (0.49,1.00) | 0.52 (0.38,0.66) | 1.23 (1.01,1.45) | 0.71 (0.5,0.93) | 0.52 (0.37,0.67) |
| Month 3 | 1.11 (0.86,1.37) | 0.74 (0.48,0.99) | 0.38 (0.23,0.52) | 1.09 (0.87,1.31) | 0.71 (0.49,0.92) | 0.38 (0.24,0.52) |
| Month 4 | 0.96 (0.7,1.22) | 0.73 (0.47,0.98) | 0.23 (0.09,0.37) | 0.94 (0.73,1.16) | 0.7 (0.49,0.92) | 0.24 (0.1,0.38) |
| Month 5 | 0.81 (0.55,1.06) | 0.72 (0.47,0.98) | 0.09 (-0.06,0.23) | 0.8 (0.58,1.02) | 0.7 (0.48,0.91) | 0.1 (-0.04,0.25) |
| Month 6 | 0.66 (0.4,0.91) | 0.71 (0.46,0.97) | -0.06 (-0.2,0.09) | 0.65 (0.43,0.88) | 0.69 (0.47,0.91) | -0.04 (-0.19,0.11) |
considering the variation over time and between Provinces, Pharmacy’s and Pharmacists.
IG: intervention group; CG: control group; 95%CI: 95% confidence interval
DRPs and pharmacists’ interventions performed during the provision of MRF
During the six months of the study, pharmacists providing the MRF identified a total of 1,561 DRPs which were considered as potential causes of ineffective or unsafe medications of a total of 1,529 uncontrolled health problems’ treatment (Table 4). The most prevalent DRPs identified were, undertreated condition (559 DRPs; 35.81%), lack of treatment adherence (261 DRP; 16.7%) and risk of adverse effects (207 DRPs; 13.26%).
Table 4. Types of DRPs identified as potential causes of ineffectiveness or unsafety of uncontrolled health problems’ treatment.
| Month 1 (n=688) | Month 2 (n=669) | Month 3 (n=662) | Month 4 (n=661) | Month 5 (n=654) | Month 6 (n=646) | |
|---|---|---|---|---|---|---|
| Total number of uncontrolled health problems with a DRP | 349 (65.1%) | 391 (83.0%) | 274 (78.1%) | 213 (74.7%) | 197 (73.5%) | 105 (58.7%) |
| Wrong administration | 21 (6%) | 13 (3.3%) | 10 (3.6%) | 6 (2.8%) | 3 (1.5%) | 1 (1.0%) |
| Individual characteristics | 28 (8%) | 44 (11.3%) | 30 (10.9%) | 24 (11.3%) | 27 (13.7%) | 12 (11.4%) |
| Contraindication | 2 (0.6%) | 6 (1.5%) | 5 (1.8%) | 1 (0.5%) | 1 (0.5%) | - |
| Wrong dose/ posology/lenght | 40 (11.5%) | 46 (11.8%) | 29 (10.6%) | 22 (10.3%) | 17 (10.6%) | 15 (14.3%) |
| Duplicity | 8 (2.3%) | 8 (2.0%) | 4 (1.5%) | 3 (1.4%) | 3(1.5%) | 2 (1.9%) |
| Wrong prescription | 9 (2.6%) | 9 (2.3%) | 5 (1.8 %) | 3 (1.4%) | 4 (2.0%) | 1 (1.0%) |
| Non-adherence | 77 (22.1%) | 67 (17.1%) | 37 (13.5%) | 32 (15.0%) | 29 (14.7%) | 19 (18.1%) |
| Interactions | 9 (2.6%) | 9 (2.3%) | 8 (2.9%) | 3 (1.4%) | 3 (1.5%) | 1 (1.0%) |
| Not necessary drug | 5 (1.4%) | 3 (0.8%) | 3 (1.1%) | 1 (0.5%) | 2 (1.0%) | 2 (1.9%) |
| Other conditions that affect the treatment | 6 (1.7%) | 11 (2.8%) | 6 (2.2%) | 4 (1.9%) | 4 (2.0%) | 4 (3.8%) |
| Risk of adverse effects | 45 (12.9%) | 57 (14.6%) | 34 (12.4%) | 27 (12.7%) | 30 (15.2%) | 14 (13.3%) |
| Undertreated condition | 102 (29.2%) | 130 (33.2%) | 106 (38.7%) | 94 (44.1%) | 86 (43.7%) | 41 (39.0%) |
| Other DRPs | 13 (3.7%) | 10 (2.6%) | 11 (4.0%) | 9 (4.2%) | 6 (3.0%) | 3 (2.9%) |
DRP: drug related problem
Pharmacists performed a total of 1,676 interventions in an attempt to solve the problems related to the use of medicines, a mean of 1.1 interventions per DRP (Table 5). The most frequent were the suggestion of adding a medicine (246 interventions; 14.67%) and medication substitution (330 interventions; 19.68%), as well as educational interventions on medicine adherence (231 interventions; 13.78%) and non-pharmacological advices (369 interventions; 22.01%). Pharmacists interventions were directed to the patient (790 interventions; 47.13%), to the physician (750 interventions; 44.73%), and both the patient and the physician (136 interventions; 8.12%) and they were accepted in 58.1% of the cases (974 interventions).
Table 5. Types of pharmacists’ interventions performed during medication review with follow-up.
| Intervention | Month 1 (n=688) | Month 2 (n=669) | Month 3 (n=662) | Month 4 (n=661) | Month 5 (n=654) | Month 6 (n=646) |
|---|---|---|---|---|---|---|
| Proposal to general practitioner | ||||||
| Dose modification | 25 (7.1%) | 34 (8.6%) | 23 (8.3%) | 18 (8.4%) | 16 (8.1%) | 8 (7.6%) |
| Dosage modification | 18 (5.1%) | 21 (5.3%) | 11 (4.0%) | 8 (3.7%) | 9 (4.6%) | 4 (3.8%) |
| Schedule modification | 13 (3.7%) | 13 (3.3%) | 13 (4.7%) | 6 (2.8%) | 3 (1.5%) | 1 (1.0%) |
| Adding a medication | 44 (12.5%) | 52 (13.2%) | 43 (15.6%) | 46 (21.5%) | 42 (21.3%) | 19 (18.1%) |
| Medication withdrawal | 25 (7.1%) | 33 (8.4%) | 19 (6.9%) | 9 (4.2%) | 9 (4.6%) | 5 (4.8%) |
| Medication substitution | 64 (18.2%) | 77 (19.5%) | 71 (25.7%) | 47 (22%) | 47 (23.9%) | 24 (22.9%) |
| Referral with no specific proposal | 22 (6.3%) | 20 (5.1%) | 10 (3.6%) | 16 (7.5%) | 19 (9.6%) | 14 (13.3%) |
| Patient health education | ||||||
| Use and medicine administration | 29 (8.2%) | 23 (5.8%) | 16 (5.8 %) | 9 (4.2%) | 7 (3.6%) | 7 (6.7%) |
| Medication adherence | 71 (20.2%) | 57 (14.5%) | 33 (12%) | 28 (13.1%) | 25 (12.7%) | 17 (16.2%) |
| Non-pharmacological advice | 75 (21.3%) | 103 (26.1%) | 70 (25.4%) | 53 (24.8%) | 43 (21.8%) | 25 (23.8%) |
| Total number of interventions | 365 | 413 | 339 | 229 | 215 | 115 |
DISCUSSION
This study reports important evidence that may clarify some key aspects around the existing controversy on the effectiveness of MRF in aged polypharmacy patients’ clinical outcomes.12,13 To our knowledge, this is the first study using the control of health problems as a surrogate outcome indicator of PPS’s impact in the aged population. Its methodology combines several essential recommendations for the assessment of complex interventions.14 The detailed description of the intervention (TIDieR checklist) as well as the use of process indicators (DRPs) provides a reproducible intervention for other researchers and may contribute to a better understanding of the intervention. The use of a cluster-randomized controlled design allowed us to avoid contamination bias whereas the presence of a facilitator of practice change providing on-site support to pharmacists increased the quality of data collection, the fidelity of the intervention and decreased the assessment bias typical of non-blinded trials.
Interestingly, our population had approximately 5 health problems and was using more than 7 medications on average, which suggests a high health care expenditure. However, they presented more than one uncontrolled health problem at baseline. These data reinforce the need of having a health care professional such as a pharmacist ensuring that medications are used in an effective and safe manner. MRF has as a key feature of the continuous assessment of patient clinical outcomes, which allows the pharmacist to propose interventions directed to the control of health problems.11 The main direct effect expected from MRF on patients´ clinical outcomes was a reduction of the number of these uncontrolled health problems. A systematic review had highlighted the paucity of studies which actually report on this clinical outcome.20
Our results suggest that MRF has a beneficial impact in this clinical outcome indicator (significant reduction of 0.72 uncontrolled health problems within 6 months). Baseline differences between groups (more complicated patients with a lower level of education in the IG) are commonly reported in the intervention groups of these types of studies and may be due to potential practice biases. It appears that since selection criteria were broad and pharmacist providing MRF had the flexibility to choose those patients, they chose more complicated patients. In future studies, there may be a need not only to cluster the pharmacies but to have a more systematic method of patient sampling. Nevertheless, the multilevel and multivariate analysis controls the potential effect of these confounding variables. Interestingly the change observed in the decline in number of uncontrolled health problems was progressive throughout the study periods; in period 6 the prevalence of uncontrolled health problems, initially higher in the IG than in the CG, was reversed. This time lag of the effect could be related to the chronic conditions of aged patients, which need longer periods to be controlled and therefore logically need a follow up. Our results are supported by Sorensen et al. who evaluated the effect of a 6 month-medication review that included, as MRF does, physician–pharmacist collaboration and the monitoring of patients’ outcomes.21 They found a positive effect on the severity of illness as well as a reduction in frequency of adverse events.
Measurement of the control of chronic health problems in primary care settings has many potential benefits for the management of polypharmacy aged patients however this concept is not well integrated in primary care setting routine activities for pharmacy. This study demonstrated the feasibility of measuring it in the daily clinical practice of a community pharmacy with our data indicating that there were 1,529 uncontrolled health problems. However, reliability of this clinical outcome variable constitutes an important challenge for community pharmacists. To enhance this key activity of the pharmacists, future studies should analyze this assessment procedure by using consensus techniques that include other professional views and further efforts should be done to enable pharmacists to monitor the most prevalent uncontrolled health problems, especially endocrine metabolic and nutritional problems, cardiovascular conditions and musculoskeletal problems.
Another difficulty of complex interventions assessment is to understand which part of the intervention contributed to the effects observed. The description of DRPs identified and interventions implemented across the six months provides important evidence about the potential to link them with the clinical impact. The more we understand the causes of lack of control of health problems, the more effective our interventions will be. In a recent study with community-dwelling aged patients a mean of 4.8 DRPs per patient were identified, a higher prevalence compared to our results, despite being a slightly younger population taking less medicines.22 The main reason for this difference is that pharmacists in our study have reported only those DRPs potentially related to uncontrolled health problems whereas most of the studies on DRPs do not distinguish between potential and real causes of medication failures. With this new approach pharmacists can address specifically those process indicators that may have a higher impact on patient´s clinical outcomes. These intervention and acceptance ratios are also like those obtained in the study of Rhalimi et al.23
CONCLUSIONS
This study provides evidence on the role of the pharmacist for aged patients’ clinical outcomes. It suggests that the addition of a skilled community pharmacist to the primary care team could contribute to the improvement of aged polypharmacy patients’ health status and to reduce their problems related with the use of medicines. MRF conducted by community pharmacists during 6 months significantly reduced the number of uncontrolled health problems of aged polypharmacy patients by means of identifying DRPs, performing educational interventions with patients, and proposing medication changes to general practitioners. Decision makers could use this evidence when designing the management strategies for polypharmacy patients, considering the inclusion of the pharmacist in the primary health care team to improve the efficiency of the health care system.
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no competing interests.
FUNDING
This work was supported by an unrestricted grant from Cinfa Laboratories to the Spanish Council of Pharmacy Colleges which in turn funded the University of Granada.
Contributor Information
Raquel Varas-Doval, M.Pharm, BPharm, Pharmaceutical Services, Department of Innovation and Education, Spanish General Council of Official Pharmacists Associations. Madrid (Spain). raquelvaras@redfarma.org.
Miguel A. Gastelurrutia, PhD, MSc, BPharm. Pharmaceutical Research Group of the University of Granada, Faculty of Pharmacy, University of Granada. Granada (Spain). magastelu@farmanorte.org
Shalom I. Benrimoj, PhD, BPharm (hons). Pharmaceutical Research Group of the University of Granada, Faculty of Pharmacy, University of Granada. Granada (Spain). shalom.benrimoj@gmail.com
Victoria García-Cárdenas, PhD, MPharm, BPharm. Senior Lecturer, Graduate School of Health, Discipline of Pharmacy University of Technology Sydney. Sydney, NSW (Australia). victoria.garciacardenas@uts.edu.au.
Loreto Sáez-Benito, PhD, MPharm. Teaching and Research Academic Staff, Faculty of Health Sciences, San Jorge University. Zaragoza (Spain). lsaezbenito@usj.es.
Fernando Martinez-Martínez, PhD, BPharm. Professor, Pharmaceutical Research Group of the University of Granada, Faculty of Pharmacy, University of Granada. Granada (Spain). femartin@ugr.es.
References
- 1.World Population Ageing 1950-2050 [Internet] New York: United Nations; [accessed Feb 13 2014]. Available at: http://www.un.org/esa/population/publications/worldageing19502050/ [Google Scholar]
- 2.Turner G, Clegg A. British Geriatrics Society;Age UK;Royal College of General Practioners. Best practice guidelines for the management of frailty:a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014;43(6):744–747. doi: 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen L, Stokes JA, Purdie DM, Woodward M, Roberts MS. Medication management at home:medication-related risk factors associated with poor health outcomes. Age Ageing. 2005;34(6):626–632. doi: 10.1093/ageing/afi202. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall S, Sevick MA, Donohue J, Maher R, Hanlon JT. Medication errors in older adults:a review of recent publications. Am J Geriatr Pharmacother. 2007;5(1):75–84. doi: 10.1016/j.amjopharm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 5.ElDesoky ES. Pharmacokinetic-pharmacodynamic crisis in the elderly. Am J Ther. 2007;14(5):488–498. doi: 10.1097/01.mjt.0000183719.84390.4d. [DOI] [PubMed] [Google Scholar]
- 6.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 7.Wiedenmayer K, Summers RS, Mackie CA, Gous AG, Everard M. Developing Pharmacy Practice:A Focus on Patient Care. [accessed Aug 17 2020]. Available at: https://www.fip.org/files/fip/publications/DevelopingPharmacyPractice/DevelopingPharmacyPracticeEN.pdf .
- 8.Moullin JC, Sabater-Hernández D, Fernandez-Llimos F, Benrimoj SI. Defining professional pharmacy services in community pharmacy. Res Social Adm Pharm. 2013;9(6):989–995. doi: 10.1016/j.sapharm.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Benrimoj SI, Feletto E, Gastelurrutia MA, Martinez-Martinez F, Faus MJ. A holistic and integrated approach to implementing cognitive pharmaceutical services. Ars Pharm. 2010;51(2):69–87. [Google Scholar]
- 10.Grupo de Expertos de Foro de Atención Farmacéutica. [Consensus document] Madrid: CGCOF; 2008. [Google Scholar]
- 11.Pharmaceutical Care Research Group, University of Granada (Spain). Pharmacotherapy follow-up:The Dader method (3rd revision (2005) Pharm Pract (Granada) 2006;4(1):44–53. [Google Scholar]
- 12.Sáez-Benito L, Fernandez-Llimos F, Feletto E, Gastelurrutia MA, Martinez-Martinez F, Benrimoj SI. Evidence of the clinical effectiveness of cognitive pharmaceutical services for aged patients. Age Ageing. 2013;42(4):442–449. doi: 10.1093/ageing/aft045. [DOI] [PubMed] [Google Scholar]
- 13.Jokanovic N, Tan EC, Sudhakaran S, Kirkpatrick CM, Dooley MJ, Ryan-Atwood TE, Bell JS. Pharmacist-led medication review in community settings:An overview of systematic reviews. Res Social Adm Pharm. 2017;13(4):661–685. doi: 10.1016/j.sapharm.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions:new guidance [Internet] London: Medical Research Council; [accessed Aug 17 2020]. Available at: https://mrc.ukri.org/documents/pdf/complex-interventions-guidance/ [Google Scholar]
- 15.Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group. Consort 2010 statement:extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmjne5661. [DOI] [PubMed] [Google Scholar]
- 16.Cosby RH, Howard M, Kaczorowski J, Willan AR, Sellors JW. Randomizing patients by family practice:sample size estimation, intracluster correlation and data analysis. Fam Pract. 2003;20(1):77–82. doi: 10.1093/fampra/20.1.77. [DOI] [PubMed] [Google Scholar]
- 17.van Breukelen GJ, Candel MJ. Efficiency loss due to varying cluster sizes in cluster randomized trials and how to compensate for it:comment on You et al (2011) Clin Trials. 2012;9(1):125–127. doi: 10.1177/174077451142⇉. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S. Better reporting of interventions:template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 19.ComitéInternacional de Clasificación de la WONCA. International Classification of Primary Care second edition. CIAP - 2. Barcelona: Masson; 1999. [Google Scholar]
- 20.Viswanathan M, Kahwati LC, Golin CE, Blalock SJ, Coker-Schwimmer E, Posey R, Lohr KN. Medication therapy management interventions in outpatient settings:a systematic review and meta-analysis. JAMA Intern Med. 2015;175(1):76–87. doi: 10.1001/jamainternmed.2014.5841. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen L, Stokes JA, Purdie DM, Woodward M, Elliott R, Roberts MS. Medication reviews in the community:results of a randomized, controlled effectiveness trial [published correction appears in Br J Clin Pharmacol 2005. 2005 Mar;59(3):376] Br J Clin Pharmacol. 2004;58(6):648–664. doi: 10.1111/j.1365-2125.2004.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Meng L, Liu Y, Lv L, Sun S, Long R, Shan X, Song J, Qiu F. Drug-related problems among community-dwelling older adults in mainland China. Int J Clin Pharm. 2018;40(2):368–375. doi: 10.1007/s11096-017-0587-3. [DOI] [PubMed] [Google Scholar]
- 23.Rhalimi M, Rauss A, Housieaux E. Drug-related problems identified during geriatric medication review in the community pharmacy. Int J Clin Pharm. 2018;40(1):109–118. doi: 10.1007/s11096-017-0571-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


