Abstract
Malaria risk and endemicity is often associated with the nature of human habitation and living environment. The disappearance of malaria from regions where it had been endemic for centuries, such as coastal areas of southern England, has been attributed, at least in part, to improvement in the quality of housing. Moreover, indigenous malaria transmission ceased throughout England without the necessity to eliminate the vector mosquitoes. The principles of malaria transmission, as formulated following the thinking of the pioneers of malaria epidemiology, Ronald Ross and George Macdonald, show how this may happen. Malaria ceases to be sustainable where its reproduction number, R0, the number of new cases generated on average for each existing case of malaria, falls below 1. In the terms of a Ross/Macdonald analysis the reduced contact between humans and blood-feeding mosquitoes that is achieved through housing that is secure against mosquito entry can have a powerful effect in reducing malaria R0. The island of Sri Lanka, where malaria had been endemic probably for centuries previously, has reported no indigenous cases of malaria since 2012. The disappearance of malaria from Sri Lanka followed an effective attack upon malaria transmission by the Sri Lanka Anti Malaria Campaign. The targeted and enhanced efforts of this campaign launched in 1999, drove the malaria R0 below 1 for most of the period up to 2012, leading to a nearly continuous decline in malaria cases until their extinction. The decades leading up to the launch of these efforts were ones of general improvement of living environment and notably in the quality of housing stock. Studies in the late 1980s had shown that quality of housing in a highly malarious district of Sri Lanka was a strong determinant of malaria risk. Through its effects on malaria R0, improved housing is likely to have facilitated the malaria control and cessation of indigenous malaria transmission in Sri Lanka and that it will help reduce the risk of the re-introduction of malaria to the island.
Keywords: Malaria transmission, Malaria control, Malaria elimination, Housing, Ross/Macdonald equations, Reproduction number, Sri Lanka, Living environment, Socio-economic development
Background
For the period of written history, and probably long before it, the nature of human habitation and the man-made environment has influenced the presence or absence of malaria transmission [1, 2]. In recent decades there has been renewed interest in this association [3–6] driven by awareness that better general standards of living and of housing tend to mitigate against malaria transmission. Here, the relationship between housing and living environment and the disappearance of malaria in a historical example in England and a recent example in Sri Lanka is discussed.
The disappearance of malaria from England
English wetlands, much of them southern coastal salt marsh, which had been highly malarious since at least late medieval times [7], became effectively malaria-free in the first decades of the 20th Century [8]. Between 1917 and 1926, as malaria-infected soldiers returned from the First World War, malarial infections re-appeared among local inhabitants across these same areas of England [8]. However, no further transmission took place. Even though the malaria vector mosquitoes (e.g.,. Anopheles atroparvus and Anopheles plumbeus [9]) were clearly still present and competent to generate new cases from introduced ones, the previously malarious regions of England had apparently become incapable of sustained malaria transmission.
How could this be? The answer, James [8] argued, lay to a large extent in two transformations. One was that by the early 20th Century the anti-malarial drug, quinine, had become widely affordable and available in England. The other lay in the quality of the human living environment, and above all of human dwellings. James describes it thus. In contrast to dwellings of “straw or stones or mud bricks, without windows or means of introducing light and ventilation….invariably infested with anopheles mosquitoes…In England… “civilising” social influences…particularly during the last seventy years (i.e. since about 1860) … (have resulted in) houses (that) are better lighted and ventilated; they have windows and are less damp; they have floors and are provided with ceilings shutting off the bedrooms from the rafters of the roof, they are more open and less crowded and are more frequently painted and whitewashed on the inside than they used to be. These changes, as well as more cleanly conditions in the home generally, have made the houses much less liable to harbour anopheles mosquitoes and have broken, to a considerable extent, the close association between those mosquitoes and man which existed when living conditions were primitive. Undoubtedly this disassociation has contributed materially towards the reduction of malaria.” The idea that James espoused as a major component to the disappearance of malaria from England was not the total elimination of the vector mosquitoes (important as their numerical reduction would have been through drainage of wetland [10]) but the sufficient reduction in contact between these mosquitoes and their human hosts through decent housing. Reduction in human and Anopheles contact had also occurred through increase in the cattle population as diversionary hosts to the mosquitoes [10].
Principles of malaria transmission and the human living environment
James’ ideas are well supported by the theoretical principles of malaria transmission. Pioneered by Ronald Ross [11] they were formulated by George Macdonald [12–14] in terms that, although subject to ongoing analysis and modification, are still broadly accepted. A central concept presented by Macdonald is that of the “basic reproduction number for malaria”—the number of new cases resulting from each existing case of malaria—now designated R0, is given in what is widely known as a Ross/Macdonald equation [14]. In such an equation (e.g., Box 1) R0 is, among other factors, a function of ‘M’, the number of adult female malaria vector mosquitoes in a defined locality, and of ‘a’, their daily biting rate upon humans. Reducing either or both M and a reduces R0. Because R0 is proportional to a2 (Box 1), anything that reduces a, the daily rate at which vector mosquitoes take a human blood meal, is particularly powerful in reducing the value of R0. Improved house-type construction that is secure against mosquito entry reduces a. It is likely that there are other malaria transmission-reducing effects that result from those types of housing that resist entry by mosquitoes. These include their impact upon mosquito egg-laying rates due to the lower frequency of blood meals. Recent analysis indicates that such effects on M (Box 1) could also significantly reduce R0 [15]. Improvements in housing are, therefore, as James proposed, likely to have contributed greatly to the reduction leading to disappearance of indigenous malaria transmission in England.
Box 1 A Ross/Macdonald equation.
The following formulation is given of a Ross/Macdonald equation:
R0, as defined by Macdonald (see below) is “The number of infections distributed in a community as a direct result of a single primary non-immune case”. In the case of malaria he proposed the following relationship between R0 and its determining factors:
R0 = (M/H)a2.c.b.pv/(−lnp.r)
M is the density (e.g., numbers per sq km) of adult female vector Anopheles mosquitoes in a (defined) locality;
H is the density of humans (i.e., numbers per sq km) in this locality;
a is the proportion of female mosquitoes that feed on humans each day;
c is the proportion of blood meals on an infected human that will yield an infection in the mosquito;
b is the proportion of bites by an infectious mosquito that infect a human;
p is the daily probability of survival of a mosquito;
v is the time in days from a human blood meal until a mosquito becomes potentially infectious to a human;
r is the daily rate at which each infected human completely clears infection.
As pointed out in the text, the probability that an adult female mosquito takes a human blood meal, ‘a’, has a powerful effect upon R0 because it is squared in the equation for R0. For example reducing a by a half reduces R0 by a quarter; reducing a by 90% reduces R0 to 1% and so on. Compared to many traditional forms of rural housing in Sri Lanka, modern housing with sealed doors, windows, eaves, etc. and interiors with fewer resting places for mosquitoes, reduces the human biting rate by mosquitoes, a, many fold. In addition, reducing the frequency of mosquito blood meals proportionately reduces their egg-laying rate and hence the numbers of adult female mosquitoes ‘M’.
The Ross/Macdonald equation, presented here, derives from that given by George Macdonald in 1952 [12] when he put forward the concept of a reproduction number for malaria. Indeed, this appears to have been the first statement of the idea of a reproduction number, R0 (which he called Z0 at the time), for any infectious disease. As he would point out [13] “Should this (number, i.e. R0) fall below one, successive generations of cases would be smaller than their predecessors and the disease would disappear; should it be greater than one, successive generations would increase and the disease would mount in the population.”
The termination of autochthonous malaria transmission in Sri Lanka
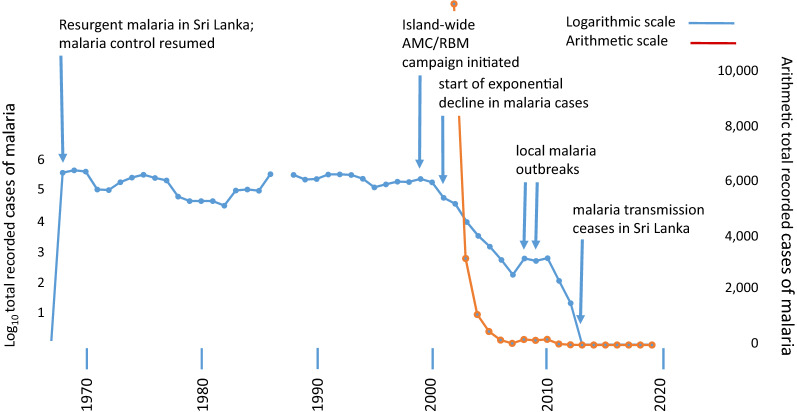
When the malaria R0 is reduced to a stable value below 1, irrespective of the cause, then malaria incidence can be expected to decline continuously and exponentially. These expectations are well met by the recorded cases of malaria in Sri Lanka for most of the period from 2001 to 2012 (Fig. 1) [16, 17] (Fig. 2). In 2013 no indigenously acquired case of malaria was recorded in Sri Lanka. There have been none since [17–19] except for a recent case acquired by infection from a foreign migrant [20].
Fig. 1.
Recorded cases of indigenously transmitted malaria in Sri Lanka, 1967 to 2020 (Source: Anti Malaria Campaign, Ministry of Health, Sri Lanka) (AMC: Anti-malaria Campaign; RBM: Roll Back Malaria). logarithmic scale blue lines; arithmetic scale red lines
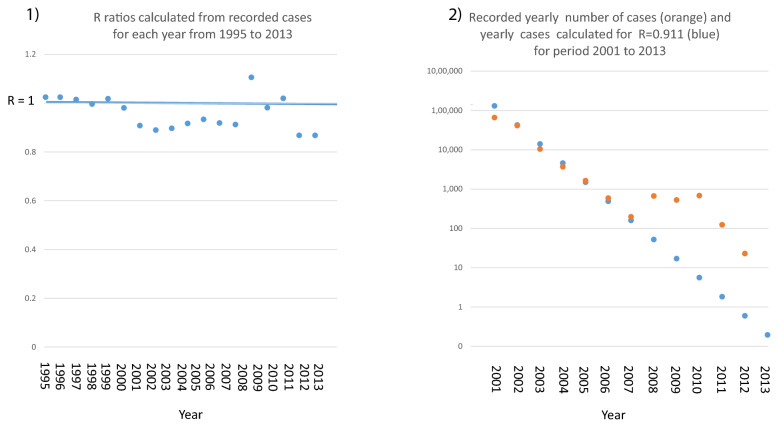
Fig. 2.
Panel 1 R ratios calculated from recorded cases for each year from 1995 to 2013. R ratio is below 1 for the years 2001 to 2007 and again for the years 2011 and 2012. In the years 2008 and 2009 there were two local outbreaks of malaria leading to the island wide R rising to 1 or above for the years 2008 to 2010. Panel 2 Recorded yearly number of cases (orange) and yearly cases calculated for R = 0.911 (blue) for period 2001 to 2013
Fig. 2 R and the decline of malaria transmission in Sri Lanka.
Let ‘R’ = (number of new cases after one round of malaria transmission)/(number of cases at the start of this round of transmission).
Let ‘n’ be the number of cases at the start of this round of transmission.
After one round of transmission, the number of new cases = n.R, after two rounds of transmission = n.R.R = n.R2 after three = n.R3 and so on. Where R is fewer than 1, this progression is a simple exponential decline.
The rate of decline of malaria cases in Sri Lanka from 2001 through 2007 was about two-thirds per year. Based on the above progression and assuming there are 12 rounds of malaria transmission per year, then from 2001 to 2007 this corresponds to an almost stable R = 0.91 per round of transmission (panel 1) leading to an exponential decline that matches very closely with the recorded decline in malaria cases during this period (panel 2). With the exception of local malaria outbreaks in 2008 and 2009 and their subsequent suppression, it continued to the termination of indigenous malaria transmission on the island of Sri Lanka in 2013 (Fig. 2).
It is pointed out that the evidence quoted here on house type and malaria [22, 23] concerns malaria risk. This is not a measure of malaria transmission rate. However, the factors that influence malaria risk, up or down, closely overlap those (e.g., human biting rate) that influence the case reproduction number, R.
Thus, an island-wide tipping point for malaria transmission (R0 < 1) appears to have arisen at around the year 2000 sending malaria case incidence into its exponential decline (Fig. 1, Box 2). It coincides with the enhanced malaria control in endemic areas begun in 1999 by the Sri Lanka Anti Malaria Campaign in line with the Roll Back Malaria initiative of the World Health Organization [19]. The effectiveness of this campaign, which involved targeted and coordinated interventions against multiple components of malaria transmission in the human host and in the mosquito vectors, can be understood, in part, in terms of the Ross/Macdonald equation (Box 1).
Its success would also have been facilitated by the steadily improving human–environment, and specifically better housing conditions across the island in the preceding decades [21] (Figs. 3 and 4). A strong association between better type of house construction and reduced malaria risk had indeed, been demonstrated in studies conducted in the late 1980s and early 1990s in Moneragala [22, 23], a district of Sri Lanka that was at the time highly malarious. The results of these studies are a direct demonstration of the relationship between good housing and reduced malaria as proposed by James [8]. The trend towards better housing in Sri Lanka since the 1980s would undoubtedly have created an ever-more malaria transmission-resistant environment within which to conduct active malaria control.
Fig. 3.
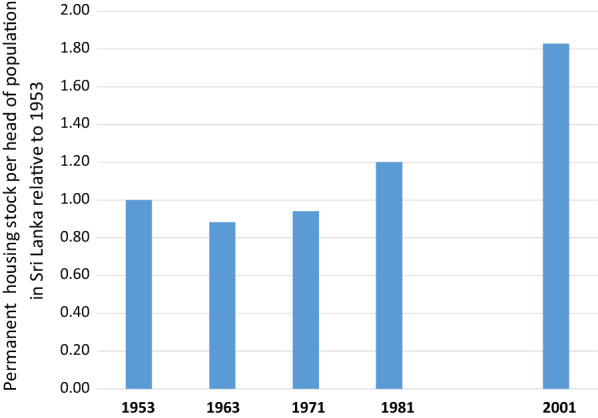
Permanent housing stock per head of population in Sri Lanka relative to values in 1953. Calculated from data in Table 3.5 in Housing and Sustainable Urban Development in Sri Lanka. National Report for the Third United Nations Conference on Human Settlements Habitat III. 2015. http://habitat3.org/wp-content/uploads/Sri-Lanka-%EF%BC%88Final-in-English%EF%BC%89.pdf. No census data available for 1991 due to the armed conflict that prevailed during that period
Fig. 4.

Housing in Kataragama, Moneragala District, Sri Lanka, from around 1990 and in 2018. a, b Kataragama, Moneragala District (circa 1990). c Kataragama, Moneragala District (2018)
While caution and vigilance should be maintained against the possible return to endemic malaria in Sri Lanka [24], continuing socio-economic improvements in malaria-prone areas, including in housing and life style, will help mitigate against its resurgence. Such improvements represent national economic and social development as significant forces in assisting malaria control and prevention.
Acknowledgements
We thank Kamini Mendis, David L. Smith and Geoffrey Pasvol for their insightful comments and advice.
Abbreviations
- R0
Reproduction number
- M
Number of adult female malaria vector mosquitoes in a defined locality
- a
Daily mosquito biting rate upon humans
Authors’ contributions
RC was the major contributor in synthesis of the theories behind the article. Both authors contributed to researching the context of the article and to writing the manuscript. Both authors read and approved the final manuscript.
Funding
NDK is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01AI136033. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boyd MF. Malariology. Philadelphia: WB Saunders; 1949. [Google Scholar]
- 2.Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev. 2002;15:564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tusting LS, Willey B, Lines J. Building malaria out: improving health in the home. Malar J. 2016;15:320. doi: 10.1186/s12936-016-1349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatem AJ, Gething PW, Smith DI, Hay SI. Urbanization and the global malaria recession. Malar J. 2013;12:133. doi: 10.1186/1475-2875-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S-Q, Li Y-C, Zhang Z-M, Wang G-Z, Hu X-M, Qualls WA, et al. Prevention measures and socioeconomic development result in a decrease in malaria in Hainan, China. Malar J. 2014;13:362. doi: 10.1186/1475-2875-13-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rek JC, Alegana V, Arinaitwe E, Cameron E, Kamya MR, Katureebe A, et al. Rapid improvements to rural Ugandan housing and their association with malaria from intense to reduced: a cohort study. Lancet Planet Health. 2018;2:e83–e94. doi: 10.1016/S2542-5196(18)30010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson MJ. Malaria in England: a geographical and historical perspective. Parassitologia. 1994;36:35–60. [PubMed] [Google Scholar]
- 8.James SP. The disappearance of malaria from England. Proc R Soc Med. 1929;23:71–87. doi: 10.1177/003591572902300127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shute PG. Indigenous P. vivax malaria in London believed to have been transmitted by Anopheles plumbeus. Mon Bull Minist Health Public Health Lab Serv. 1954;13:48–51. [PubMed] [Google Scholar]
- 10.Kuhn KG, Campbell-Lendrum DH, Armstrong B, David CR. Malaria in Britain: past, present, and future. Proc Natl Acad Sci USA. 2003;100:9997–10001. doi: 10.1073/pnas.1233687100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross R. The prevention of malaria. London: John Murray; 1911. [Google Scholar]
- 12.Macdonald G. The analysis of equilibrium in malaria. Trop Dis Bull. 1952;49:813–1129. [PubMed] [Google Scholar]
- 13.Macdonald G. The epidemiology and control of malaria. London: Oxford University Press; 1957. [Google Scholar]
- 14.Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. Ross, Macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 2012;8:e1002588. doi: 10.1371/journal.ppat.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brady OJ, Godfray HCJ, Tatem AJ, Gething PW, Cohen JM, McKenzie FE, et al. Adult vector control, mosquito ecology and malaria transmission. Int Health. 2015;7:121–129. doi: 10.1093/inthealth/ihv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karunaweera ND, Galappaththy GNL, Wirth DF. On the road to eliminate malaria in Sri Lanka: lessons from history, challenges, gaps in knowledge and research needs. Malar J. 2014;13:59. doi: 10.1186/1475-2875-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijesundere DA, Ramasamy R. Analysis of historical trends and recent elimination of malaria from Sri Lanka and its applicability to malaria control in other countries. Front Public Health. 2017;5:212. doi: 10.3389/fpubh.2017.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sri Lanka free of malaria . Case study. New Delhi: World Health Organization, Regional Office for South-East Asia; 2017. [Google Scholar]
- 19.Premaratne R, Wickremasinghe R, Ranaweera D, Kumudu WM, Gunasekera AW, Hevawitharana M, et al. Technical and operational underpinnings of malaria elimination from Sri Lanka. Malar J. 2019;18:256. doi: 10.1186/s12936-019-2886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karunasena VM, Marasinghe M, Koo C, Amarasinghe S, Senaratne AS, Hasantha R, et al. The first introduced malaria case reported from Sri Lanka after elimination: implications for preventing re-introduction of malaria in recently eliminated countries. Malar J. 2019;18:210. doi: 10.1186/s12936-019-2843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Housing and Sustainable Urban Development in Sri Lanka. National Report for the Third United Nations Conference on Human Settlements Habitat III. 2015. http://habitat3.org/wp-content/uploads/Sri-Lanka-%EF%BC%88Final-in-English%EF%BC%89.pdf.
- 22.Gamage-Mendis AC, Carter R, Mendis C, De Zoysa APK, Herath PRJ, Mendis KN. Clustering of malaria infections within an endemic population: risk of malaria associated with the type of housing construction. Am J Trop Med Hyg. 1991;45:77–85. doi: 10.4269/ajtmh.1991.45.77. [DOI] [PubMed] [Google Scholar]
- 23.Gunawardena DM, Wickremasinghe AR, Muthuwatta L, Weerasingha S, Rajakaruna J, Senanayaka T, et al. Malaria risk factors in an endemic region of Sri Lanka, and the impact and cost implications of risk factor-based interventions. Am J Trop Med Hyg. 1998;58:533–542. doi: 10.4269/ajtmh.1998.58.533. [DOI] [PubMed] [Google Scholar]
- 24.Premaratne R, Ortega L, Jankan N, Mendis KN. Malaria elimination in Sri Lanka: what it would take to reach the goal. WHO South-East Asia J Public Health. 2014;3:85–89. doi: 10.4103/2224-3151.206892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.