Abstract
Background
The first months postpartum can be challenging for parents, leading to elevated symptoms of parenting stress, depression and anxiety. In turn, distressed parents are at higher risk for providing suboptimal quality of caregiving. As psychoeducational interventions can be effective in reducing psychological distress, the goal of this randomized controlled trial was to examine the effectiveness of low-intensity universal psychoeducational program to prevent postpartum parenting stress, and to enhance parental well-being and caregiving quality.
Method
Between 26 and 34 weeks of pregnancy, 138 pregnant women and 96 partners were randomized to the intervention or a waitlist control group. The intervention consisted of a booklet, a video, a home visit, and a telephone call. Information was provided on (1) sensitive responsiveness, adapting to the parental role, and attending to own needs; (2) crying patterns; (3) feeding (arrangements); and (4) sleeping (arrangements). The primary outcome was parenting stress postpartum. Secondary outcomes were additional measures of distress (depression and anxiety), parental well-being, and caregiving quality.
Results
Both groups showed a rise in distress after birth. No between-group differences were observed on parenting stress, nor on the secondary outcomes. The intervention was rated as useful and of added value by the parents.
Conclusion
This study offered no evidence that our universal prevention program was effective in decreasing parental distress or in increasing caregiving quality. However, parents found aspects of the intervention useful. More research is needed, including a longer period of follow-up as well as observational measures of parents’ responsiveness.
Trial registration
This trial has been registered on 15 September 2016 in the Netherlands National Trial Register, ID: NTR6065, https://www.trialregister.nl/trial/5782.
Keywords: Psycho-education, Universal prevention program, Randomized controlled trial, Parenting stress, Pregnancy
The transition to parenthood is an important and challenging life event that can be accompanied by significant distress. Parents of a newborn report doubts about their own parenting skills [1] and feeling overwhelmed by the seemingly unlimited demands that come with the parenting role [2]. This results in lowered self-efficacy [3] and reduced self-esteem [4]. Moreover, these types of parenting distress seem to be associated with more general symptoms of depression, anxiety and stress in the first months after birth [3, 5]. Prevalence rates for maternal postpartum depression symptomatology range between 8 and 40% [6–9], and for postpartum anxiety symptomatology between 13 and 40% [10, 11]. Importantly, about 10% of fathers report symptoms of depression, anxiety and stress after the birth of their child as well [12, 13]. Moreover, next to compromising parents’ own health, parental postpartum psychological distress forecasts more problems in the child’s emotional, behavioural, and cognitive development [11, 14–16]. Clearly, there is a need to decrease symptoms of parenting stress and enhance parental well-being in the postpartum period.
Meta-analytic evidence indicates that brief psychoeducational interventions aimed at providing information can be effective in reducing symptoms of psychological distress [17]. Because these interventions are easy to implement and low-cost, they provide a fruitful option for universal prevention research. The primary goal of the present randomized controlled trial (RCT) was to examine the effectiveness of a brief universal prevention program to prevent postpartum symptoms of parenting stress. Parenting stress is the result of an experienced discrepancy between the demands associated with the parenting role and the available resources to fulfil these demands [18]. As parents report a need for reliable and non-judgmental information about parenting a newborn [1], we expect our psychoeducational intervention to be such a resource and to decrease parenting stress. Secondary aims of this study were to examine if the intervention was also effective in preventing general symptoms of depression, anxiety and stress and in enhancing the quality of parental caregiving.
Parental distress and quality of care
The proposed mechanism behind the associations between parental parenting distress and child outcomes is that parental distress negatively affects the quality of parenting [19–21]. For example, distress can prevent parents from focusing their attention on, and responding in a timely and sensitive manner to their infant’s needs [21]. Parental sensitivity is important for a range of child outcomes, including the formation of a secure attachment relationship between infants and their parents, social competence, regulatory capacities, and lower stress levels (e.g. [22–28]).
Next to sensitivity, parental distress can compromise the formation of the strong affective tie from parent to infant [29], commonly referred to as the maternal or paternal bond. This bond has been defined as the tie from parent to infant that facilitates parent-infant proximity and caregiving behavior, such as warmth and sensitivity [30, 31]. Lower quality of the parent-infant bond has been related to problems in children’s socio-emotional development [29, 32]. Bonding has also been related, through parenting stress, to child executive functioning at 24 months postpartum [33]. This study found that, for both mothers and fathers, feelings of bonding negatively predicted experienced parenting stress over time. In addition, for both parents, a negative indirect effect of bonding on child executive functioning problems was found via experienced parenting stress. As parenting stress is suggested to provide the child with a more negative, less predictive, and chaotic environment, these environmental circumstances can negatively affect the child’s own stress levels and subsequent neurocognitive development, but also prevent the child from a stimulating environment necessary for executive functioning skills to develop [19].
Parental distress might also affect caregiving practices, including breastfeeding and room-sharing arrangements, which are important for infant development. For example, maternal depression and anxiety have been linked to a shorter duration of breastfeeding [34, 35]. The World Health Organization recommends exclusive breastfeeding during the first 6 months after birth [36]. Breastfeeding has important and well-established beneficial effects on the child’s physical and mental health, for example protection against of infections and diabetes, and more favorable cognitive development [37, 38]. With regard to room-sharing, the American Academy of Pediatrics (AAP) recommends that children should sleep within the same room as the parents (in a separate cot) during the first 6 months after birth [39], as parent–infant room sharing is associated with reduced rates of Sudden Infant Death Syndrome (SIDS e.g., [40, 41]). Also, the availability of the parents seems to help buffering the infant’s distress [42, 43], and facilitates the breastfeeding process [44–46]. This RCT will thus also examine the effectiveness of the universal prevention program to increase parental caregiving quality (i.e. increased parental bonding, longer breastfeeding duration, and longer room-sharing duration).
Universal, selected and indicated prevention of parental distress
There are interventions that focus on the prevention of the development of clinical disorders (such as depressive or anxiety disorders) after birth. However, these interventions are mostly aimed at mothers with symptoms of clinical disorders (indicated prevention; e.g. [47, 48] or on mothers who belong to certain risk groups for developing a disorder (selected prevention; e.g. [49–51]. Examples of risk factors are a history of psychopathology, pregnancy complications or an infant born prematurely, adverse life events, low SES, or low social support [49–52].
Both indicated as well as selective prevention interventions have been proven to be effective in preventing depression [53–57]. However, much is unknown about the effectiveness of preventive interventions on other forms of distress beyond depression, such as anxiety and general stress [56, 58]. Furthermore, much less is known about universally applicable interventions that target all pregnant women, without pre-existing symptomatology or risk factors [56, 58]. This is important because research showed a high prevalence of parental symptomatology after birth, which extends in a more chronic level of sub-clinical symptomatology for about 20–30% of parents during the first postpartum years [52, 59]. In the absence of clear risk factors, this group of parents would not be targeted by existing (indicated and selected) preventive approaches. Since chronic sub-clinical symptomatology has also been linked to more negative child development [15], this finding suggests the importance of a universal preventive approach.
Existing interventions focus almost exclusively on the mother, instead of also including the partner. However, fathers also experience a significant degree of distress in the postpartum period [12, 13, 60] and a growing body of evidence indicates that paternal distress is also associated with problems in children’s emotional and behavioural development [61, 62]. Furthermore, including partners in interventions has been positively associated with higher breastfeeding rates at 6 weeks postpartum [63] and longer duration of the breastfeeding period [64]. Including partners seems thus to be important, not only for their own health and well-being, but also to prevent negative effects of paternal distress on infant development, as well as to enhance the quality of both parents’ caregiving.
The current study
In summary, there is a high prevalence of both maternal [6–11] as well as paternal postpartum distress symptomatology [12, 13]. Given the associations between parental symptomatology and the quality of parenting and, subsequently, child development [16, 61, 62, 65], there is a need for preventive interventions that are applicable to all expecting parents, both mothers and fathers, independent of pre-existing risk factors or symptomatology. Moreover, while existing interventions mainly focused on depression as an outcome measure, our main focus was on parenting stress. To be able to reach a broad range of parents and to foster real-world implementation, we developed an easy accessible and low-intensity intervention that can be implemented during pregnancy. The intervention consists of an information booklet, an educational video, and a prenatal home visit during pregnancy and a phone call during the first postpartum weeks. The intervention is targeted at both mothers and fathers. We will examine gender differences in distress outcomes, as well as differences in intervention effectiveness depending on prenatal levels of distress (i.e. between mothers and fathers with relatively high versus relatively low levels of distress during pregnancy). Lastly, this intervention is implemented in a Dutch sample. While there is a lack of data on parental postpartum distress in Dutch samples specifically, no indications are found that the Dutch context is different compared to other western countries.
We expected that the intervention would reduce parenting stress, as well as symptoms of depression and anxiety. Furthermore, by psycho-educating both parents already during pregnancy, we expected parents to experience more self-efficacy and satisfaction in fulfilling their roles. By preventing symptoms of parenting stress, depression, and anxiety and stimulating parental well-being, mothers as well as fathers might perceive less problems with infant crying, feeding, and sleeping. Moreover, by psycho-educating parents about typical infant crying, feeding and sleeping patterns, we expected that parents would perceive less problems with these infant behaviors. Specifically, by psycho-educating parents about typical infant sleep patterns, including frequent night-wakings, we aimed to prepare parents for broken nights and a possible lack of sleep postpartum, and expected that parental perception of the quality and quantity of their own sleep would be enhanced. Furthermore, by preventing distress symptomatology, the quality of parents’ caregiving (including bonding, breastfeeding and room-sharing) should be enhanced.
Materials and methods
Trial design
We used a randomized controlled trial with two parallel groups: an intervention and a waitlist control group. We used block randomization (blocks 6–8) stratified by birth order (first child/ no first child) and participation of the partner (yes/no). An independent researcher generated random number sequences with a 1:1 ratio and allocated each participant to either the control or the intervention group. Blinding of the researchers or the participants was not possible. The control group was offered access to the intervention after the final assessment, which was scheduled 10 weeks after birth. The design of the study was described in full in [66].
Participants and recruitment
Pregnant women and their partners were recruited between November 2016 and February 2018 through online media and midwifery practices in the Netherlands. Information about the study was provided digitally through 1) online newsletters providing information about the 26th week of pregnancy; and 2) advertisements on websites where pregnant women could find general information about being pregnant and where they could get in touch with other pregnant women. Furthermore, midwifery practices handed pregnant women a flyer with information about the study. In the online newsletters and advertisements, as well as in the flyer, a link to the website of the study was provided, through which potentially interested pregnant women could register for the study. Inclusion criteria were a) gestational age < 34th weeks; b) no severe pregnancy complications (i.e. gestational diabetes or pre-eclampsia); c) sufficient Dutch language proficiency; and d) access to the internet. Exclusion criteria were: a) current psychological treatment for psychopathology or psychological treatment in the 6 months before registration and b) the development of severe pregnancy complications during the course of the study. There were no requirements with respect to baseline distress level. All women were eligible. Pregnant women could participate with or without their partner.
Sample size calculation
Hiscock et al. [67] reported an odds ratio of .57 with regard to the prevention of maternal symptoms of depression in the intervention group. We converted this ratio into an effect size of d = .48. Using a power of 80% and setting alpha at .05, this resulted in a sample size of 64 participants per condition (total n = 128).
Procedure
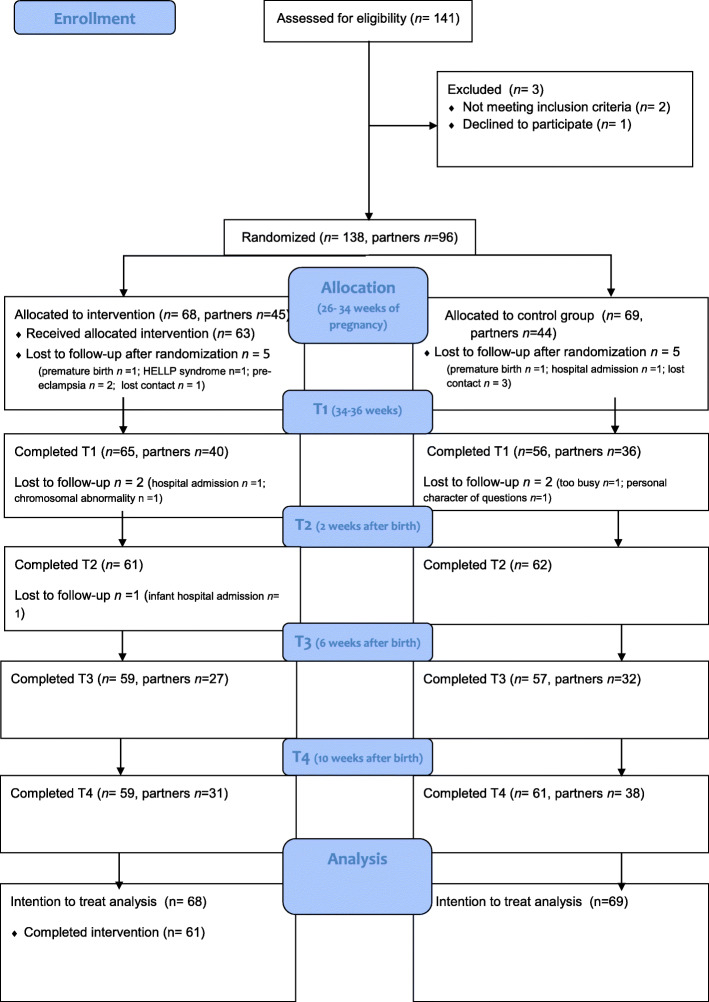
Upon registering through the study website, interested parents received a digital information brochure of the study and an informed consent form. After providing informed consent, parents received a link to the baseline questionnaire. Upon completion, couples (or pregnant women participating without their partner) were randomly assigned to either the intervention or the control group. Online assessments took place at baseline (T0, between 26 and 34 weeks of pregnancy), 34–36 weeks of pregnancy (T1), 2 weeks after giving birth (T2), 6 weeks after birth (T3) and 10 weeks after birth (T4). At each point in time, parents received an e-mail with a link to the questionnaire (Fig. 1). All parents were also offered the possibility to fill in the questionnaires by paper-and-pencil. None of them made use of this possibility. The study was registered at the Netherlands Trial Register (NTR6065). The study was approved by the Medical Ethical Committee Brabant (NL58528.028.16/P1620).
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram
Intervention
The intervention consisted of (1) an information booklet; (2) an online video, (3) a prenatal home visit; and (4) a postnatal phone call. The booklet and video were received during pregnancy, after the baseline assessment (T0). Given that parenting stress and the quality of parenting can affect child development from birth onwards [68, 69], we aimed to intervene already during pregnancy. Therefore, the main part of the intervention (booklet, video, home visit) was implemented before birth. This way, there was sufficient time for parents to digest and use the information in preparing for the birth of their child.
Booklet and video
The booklet consisted of four chapters on (1) interpreting of, and sensitive responding to, the infant’s needs and signals of distress; as well as adapting to the parental role and attending to own needs, taking a sufficient amount of rest, and seeking support; (2) patterns of crying and different soothing techniques; (3) the infant’s hunger signals and feeding arrangements (e.g. breastfeeding, availability of professional breastfeeding courses and support services, pumping and the use of a breast pump, formula feeding); and (4) sleeping patterns and sleeping arrangements (e.g. infant sleep development and consolidation, room-sharing, bed-sharing, and solitary sleeping). We based the booklet on the work of [66], which we further developed and extended based on recent empirical research and in collaboration with academic and clinical experts [66]. The video provided illustrations of the topics described in the booklet. In the video, the experiences of upcoming parents were shown. An expert on infant development commented on the fragments, and parents were actively engaged in the video by stimulating them to think about and discuss with each other how they could implement the information within their own lives. The video was developed by psychologists with extensive knowledge on infant development and with experience in translating scientific knowledge into tools for practice (Stichting Babywerk, The Netherlands). Watching the video and responding to the questions took about 15–20 min. More details on the content of the booklet and/or the video can be found in [66].
Prenatal home visit and postnatal phone call
Parents were asked to read and watch the materials before the prenatal home visit. The primary aim of the home visit was to discuss the information in the materials and to respond to the parent’s questions. A secondary aim of the home visit was to explain the parents that no part of the provided information was meant to be prescriptive. Rather, we asked parents to think about how the information would fit their own and their child’s needs, and to discuss how the information could be implemented in their lives. Thus, with the home visits, we aimed to offer both education and support. Another major reason for visiting parents in their homes is to facilitate their participation in the study and in the intervention (as parents did not have to travel to a clinic or parenting class). Furthermore, by visiting parents in their homes, we expected that especially for fathers there would be less of a barrier for participating in the study [70]. All parents were visited at their homes by the first author, who has a background in clinical psychology and infant developmental psychology. About 4 weeks after the birth of their child, a phone call was scheduled. The aim of this phone call was to ask how parents and their child were doing, and to discuss possible problems in the implementation of the provided information in their lives. All phone calls were performed by the first author. Parents were given the opportunity to ask questions with regard to sensitive responsiveness and contact-seeking of the infant; and any issues in relation to the infant’s crying, feeding, and sleeping behaviors. Parents were also given the opportunity to discuss their own well-being, such as feelings of depression, anxiety or stress. Both during the home visit and the postnatal phone call, as well as in the information booklet, we explicitly described that the birth of a child is a major life change that can evoke different emotions and that adapting to the new situation takes time. We also explained that many parents can feel tired, sad or frustrated during the first months after birth. We encouraged parents to discuss their emotions and needs with their partner, and to seek additional support from their social network or support services when needed.
Waitlist control group
Parents randomized to the waitlist control group received the psychoeducational materials after they had completed the last assessment, about 10 weeks after the birth of their child. The information was still applicable for them at that time. Also, they were given the opportunity to schedule a phone call to discuss the materials. None of the parents from the control group made use of the possibility for a phone call. All parents in the study, both in the intervention and control group, had access to care-as-usual (e.g. visits to the well-baby clinic, general practitioner) during the pre- and postpartum period.
Measures
Online questionnaires were sent to both the mother and the father. The questionnaires assessed the primary outcome (parenting stress), as well as the other indicators of distress (symptoms of depression and anxiety) and parental well-being (satisfaction with the parenting role, parenting self-efficacy, and sleep quality and quantity). Furthermore, we measured quality of caregiving, including parent-infant bonding, duration of exclusive breastfeeding, duration of room-sharing (the infant sleeping in the parents’ room on a separate surface) and parental perception of problems with infant crying, feeding, and sleeping. We also assessed potential confounders, namely: characteristics of the delivery and birth, attachment styles of the parents, and marital satisfaction. Finally, we measured the uptake of the intervention.
Parental distress
Parenting stress
Parenting stress was assessed with 10 items of the Parenting Stress Index (PSI [71, 72];). Since the PSI has been originally developed for parents of children up to 14 years [72], we selected the 10 items that are most relevant for parents of a newborn child and added one item because it was considered central to the parenting stress construct (especially for parents of a newborn): “The responsibility I have for my children weighs on me” (see also [73]). An example of the other items is: “I feel restricted by my responsibilities as a parent.” Response options varied from 1 (totally disagree) to 6 (totally agree). A total score is derived by summing the individual item scores ranging from 11 (no stress) to 66 (very high stress). For mothers, Cronbach’s alpha in the current study was .63 (T0), .67 (T1), .73 (T3), and .75 (T4). For partners, Cronbach’s alpha was .75 (T0), .73 (T1), .75 (T3), and .84 (T4).
Depressive symptoms
Depressive symptoms were assessed with the Edinburgh Postnatal Depression Scale [74, 75]. This scale consists of 10 items. Participants could indicate the experienced frequency of each depression-related statement on a 4-point scale. Items are scored from 0 to 3 and the total score ranges from 0 (no depressed feelings) to 30 (severely depressed feelings). The scale shows good psychometric properties: Pop et al. [75] reported a Cronbach’s alpha of .82 and sufficient concurrent validity. Cronbach’s alpha in the current study ranged from .77 (T1) to .85 (T3) for mothers, and from .71 (T0) to .83 (T4) for partners. EPDS cut-off scores were subsequently used for descriptive purposes. To screen for (minor) depression and describe depression symptomatology in the general population, a cut-off score of 10 or more was used, as previously recommended [74, 76]. As it has been suggested that men are emotionally less expressive compared to women [77], the procedure was followed as reported by Leung, Letourneau, Giesbrecht, Ntanda, and Heart [78], and a slightly lower cut-off score was used for fathers (EPDS score of 9 or more). To be consistent and for sake of clarity, the same cut-off scores for both mothers and fathers were used in the prenatal and the postnatal period (i.e. 10 or more for mothers and 9 or more for fathers).
Anxiety symptoms
Symptoms of anxiety were measured with the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS [79]; Dutch translation [80]). This subscale consists of seven items. For each item, participants could indicate their level of experienced anxiety on a 4-point scale. Total scores range from 0 (no anxiety) to 21 (severe anxiety). Spinhoven et al. [80] reported good psychometric properties for the Dutch version. In the current study, Cronbach’s alpha ranged from .64 (T1) to .71 (T4) for mothers, and from .73 (T0) to .76 (T1) for partners. A cut-off score of 8 or more was used to identify anxiety symptomatology within the clinical range [81].
Parental well-being
Satisfaction with the parental role
Satisfaction with the parental role was measured with three items of the Dutch translation of the Parenting Stress Index (PSI [72, 82]). As has been done before, four items were added to the scale (see [66, 73]). The addition of these items was necessary because to our knowledge, no measure of parents’ satisfaction with their new role currently exists. An example item is: “I enjoy spending time with my child.” Cronbach’s alpha in this study ranged from .64 (t0) to .76 (t3) for mothers, and from .77 (t4) to .89 (t1) for partners.
Self-efficacy
Self-efficacy was assessed with 1 item, through which parents could indicate their (expected) efficacy as a parent on a 5-point scale, ranging from 1 (not very good) to 5 (a very good parent [66, 82].
Perception of squality and quantity
Perception of sleep quality and quantity were measured with two items of the Pittsburgh Sleep Quality Index (PSQI [83]; see also [84]). The items were phrased as follows: ‘Over the last 2 weeks, how would you rate your own sleep quality/sleep quantity?’. Parents could indicate their experienced sleep quality on a 4-point scale, varying from ‘Not nearly good enough’ to ‘More than good enough’. Because of high correlations between quality and quantity at T3 (r = .61, p < .05), and at T4 (r = .67, p < .05), and our desire to reduce the number of analyses, sleep quality and quantity scores were standardized and subsequently averaged across the two time points.
Quality of caregiving
Bonding
Bonding between parent and child was measured with the Maternal Postnatal Attachment Scale (MPAS [85, 86]). This scale consists of 19 statements, for example: “When I am with the baby, I feel tense and fearful.” Parents could indicate how much they agreed with each statement on a 2-point; 4-point; or 5-point scale. By summing up all items, a total score between 19 and 95 can be reached. Lower scores are an indication of bonding problems between parent and child. When administered between 2 and 3 months after birth, Van Bussel et al. [86] reported a Cronbach’s alpha of .75. In the current study, Cronbach’s alpha was .81 for mothers (T4) and .86 (T4) for partners.
Breastfeeding
The duration of exclusive breastfeeding was assessed by asking mothers to indicate the number of weeks their child received exclusive breastfeeding, and thus no formula.
Room-sharing
Mothers were asked to indicate the number of weeks of room-sharing, defined as the infant sleeping in the room of the parents at night (in a separate cot or in the bed of the parents).
Perception of problems with infant sleeping, crying, feeding
Additionally, both mothers and fathers were asked in the online questionnaire (t3 and t4) whether they had experienced a problem with infant sleeping, crying, or feeding (at day and/or at night). If they responded affirmatively, they were asked to indicate the severity of this problem on a 7-point Likert scale. Response options varied from 1 (hardly any problem) to 7 (a severe problem) (see also [67]).
Confounding variables
We included the following confounding variables and tested for between-group differences on these variables. In case of non-significant results, we did not include the variable in our main analyses.
Birth characteristics
Characteristics of the delivery and birth were assessed by parents’ self-report and included birth weight, Apgar score at 5 min, and spontaneous delivery versus caesarean section.
Attachment style of the parents
To control for possible insecure attachment styles of the parents (which can impact on their caregiving quality [87, 88];, parental attachment style was measured with the short form of the Experiences in Close Relationships Questionnaire (ECR- short form [89]; Dutch translation [90]). The 12 items of this instrument are derived from the avoidance and anxious attachment subscales of the ECR-R (six items of each subscale [91]). Response options vary from 1 (strongly disagree) to 7 (strongly agree). The avoidance subscale measures the need to stay independent from others and to avoid intimacy [89], see also [92]. The anxiety subscale measures the degree to which the subject worries about rejection and abandonment [89, 92]. Following recoding of items 15, 25, 27, 29, and 31; for each subscale (anxiety and avoidance) an average score of between 1 and 7 can be computed. Higher scores reflect more attachment anxiety and avoidance. The ECR-short form showed good psychometric properties in different samples: Lafontaine et al. [89] reported Cronbach’s alpha’s of .78 to .87 for the anxiety subscale and .74 to .83 for the avoidance subscale. In the current study, for mothers, Cronbach’s alpha was .76 for the anxiety subscale and .85 for the avoidance subscale. For partners, these values were .76 (anxiety) and .85 (avoidance).
Marital satisfaction
Participants’ satisfaction with their relationship was measured with the global satisfaction items of The Investment Model Scale (IMS [93]; Dutch translation: [94]). This scale consists of five items, with answering options varying from 1 (totally disagree) to 9 (totally agree). An example item is: “My relationships fulfills my needs for intimacy.” The total score ranges from 5 (not satisfied) to 45 (very satisfied). Montgomery et al. [94], reported a Cronbach’s alpha of .93.
Intervention uptake and satisfaction
The uptake of the intervention was measured by asking parents whether they had read and watched the materials before the birth of their child. We also asked them whether they looked into the materials again after the birth of their child. Furthermore, we asked them to rate the frequency of using the information in their daily lives, with the item: “How many times did you use the information from the booklet, video, or the home visit during the daily care for your baby?” Response options were: “Daily”; “Several times a week”; “About once a week”; “About once every 2 weeks”; “About once a month”; and “Never.” We also asked them to rate the usefulness of the booklet, video, and the home visit on a 5-point scale ranging from 1 “Not very useful” to 5 “Very useful.”
Statistical analyses
Preliminary analyses
We investigated the effect of the intervention on all different outcomes. First, we inspected distress scores over time for men and women separately (Tables 2 and 3). We analyzed whether there were between-group differences at the follow-up (T3) and final assessments (T4) for all distress (parenting stress, depression, anxiety), well-being (self-efficacy, satisfaction with the parenting role) and quality of caregiving outcomes (bonding, duration of breastfeeding, duration of room-sharing, perception of infant problems).
Table 2.
Stress, depression, anxiety, satisfaction, self-efficacy and bonding mean scores for mothers in intervention and control group over time (mean; standard deviation), and p-values for group differences at T3 and T4
| T0 (26–34 weeks pregnant) | T1 (34–36 weeks pregnant) | T3 (6 weeks after birth) | T4 (10 weeks after birth) |
p-value* T3 |
p- value* T4 |
|
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| Stress | ||||||
| Intervention | 32.97 (6.75) | 32.91 (7.15) | 39.03 (3.08) | 32.42 (8.43) | .72 | .22 |
| Control | 33.01 (6.25) | 32.73 (6.92) | 38.59 (3.48) | 30.59 (7.57) | ||
| Secondary outcomes | ||||||
| Depression | ||||||
| Intervention | 4.48 (3.08) | 4.44 (3.60) | 5.53 (4.02) | 4.82 (4.20) | .86 | .59 |
| Control | 4.86 (3.83) | 3.87 (2.60) | 5.67 (4.40) | 4.44 (3.34) | ||
| Anxiety | ||||||
| Intervention | 5.10 (2.10) | 5.59 (2.29) | 5.69 (2.53) | 5.64 (2.77) | .47 | .78 |
| Control | 5.35 (2.14) | 5.05 (1.93) | 6.05 (2.83) | 5.50 (2.56) | ||
| Satisfaction | ||||||
| Intervention | 38.39 (3.52) | 38.82 (3.67) | 32.84 (8.61) | 39.40 (2.41) | .46 | .71 |
| Control | 38.32 (3.41) | 38.93 (3.25) | 32.31 (7.33) | 39.21 (2.91) | ||
| Parental self-efficacy | ||||||
| Intervention | 3.94 (.52) | 4.05 (.45) | 3.88 (.65) | 3.98 (.53) | .68 | .99 |
| Control | 3.87 (.54) | 3.98 (.53) 3.83 (.70) | 3.98 (.53) | |||
| Sleep quality | ||||||
| Intervention | n/a | n/a | 2.41 (.70) | 2.58 (.74) | .15 | .68 |
| Control | n/a | n/a | 2.59 (.56) | 2.73 (.61) | ||
| Sleep quantity | ||||||
| Intervention | n/a | n/a | 2.41 (.70) | 2.58 (.74) | .70 | .23 |
| Control | n/a | n/a | 2.59 (.56) | 2.73 (.61) | ||
| Parent-infant bonding | ||||||
| Intervention | n/a | n/a | n/a | 66.15 (6.02) | n/a | .83 |
| Control | n/a | n/a | n/a | 66.55 (5.18) | ||
Table 3.
Stress, depression, anxiety, satisfaction, self-efficacy, and bonding mean scores for partners in intervention and control group over time (mean; standard deviation), and p-values for group differences at T3 and T4
| T0 (26–34 weeks pregnant) | T1 (34–36 weeks pregnant) | T3 (6 weeks after birth) | T4 (10 weeks after birth) |
p-value T3 |
p-value T4 |
|
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| Stress | ||||||
| Intervention | 25.38 (6.74) | 26.55 (6.58) | 38.82 (3.27) | 28.66 (9.66) | .61 | .59 |
| Control | 24.49 (7.08) | 25.67 (6.67) | 38.54 (4.35) | 27.36 (9.56) | ||
| Secondary outcomes | ||||||
| Depression | ||||||
| Intervention | 3.62 (3.20) | 2.70 (3.07) | 2.93 (2.62) | 3.21 (3.67) | .38 | .54 |
| Control | 3.93 (2.56) | 3.00 (2.29) | 3.66 (3.61) | 3.75 (3.40) | ||
| Anxiety | ||||||
| Intervention | 6.24 (3.26) | 5.48 (2.86) | 5.25 (2.07) | 5.55 (3.11) | .73 | .96 |
| Control | 5.79 (2.28) | 5.86 (2.60) | 5.49 (3.02) | 5.58 (2.35) | ||
| Satisfaction | ||||||
| Intervention | 36.76 (5.42) | 36.68 (6.11) | 28.61 (7.26) | 38.86 (3.29) | .78 | .58 |
| Control | 37.67 (3.22) | 37.72 (3.48) | 27.60 (7.99) | 38.36 (3.48) | ||
| Self-efficacy | ||||||
| Intervention | 4.11 (.65) | 4.22 (.62) | 4.11 (.57) | 4.00 (.71) | .27 | .60 |
| Control | 4.02 (.56) | 4.22 (.59) | 3.94 (.59) | 3.92 (.55) | ||
| Sleep quality | ||||||
| Intervention | n/a | n/a | 2.75 (.59) | 2.93 (.66) | .22 | .18 |
| Control | n/a | n/a | 2.54 (.70) | 2.69 (.71) | ||
| Sleep quantity | ||||||
| Intervention | n/a | n/a | 2.64 (.68) | 2.86 (.85) | .39 | .42 |
| Control | n/a | n/a | 2.49 (.74) | 2.69 (.75) | ||
| Parent-infant bonding | ||||||
| Intervention | n/a | n/a | n/a | 64.46 (6.42) | n/a | .73 |
| Control | n/a | n/a | n/a | 63.78 (6.73) | ||
Because of high intercorrelations, scores at T0 and T1 were averaged for parenting stress (r = .75; p < .01 and r = .77; p < .01 for partners), depression (r = .66 for mothers and .62 for partners; p < .01), anxiety (r = .58 for mothers and .70 for partners; p < .01), satisfaction with the parenting role (r = .45 for mothers and .38 for partners; p < .01), and self-efficacy (r = .61 for mothers and .58 for partners; p < .01).
Main analyses
To deal with the nested nature of the data (mothers and fathers in couples), we used multilevel linear modelling (MLM). MLM is robust for missing data and is unaffected by unequal number of data points per unit, in this case the mother-father dyad [95]. Therefore, there was no need to control for the fact that more mothers than fathers participated. We could run the analyses on the full data set, including data from participants with incomplete data. As we hypothesized that parenting stress and parental mental problems would be highest around 6 weeks of infant age (i.e. the infant crying peak [96]) analyses were focused on the outcomes at T3. To test the robustness of the results, we repeated all analyses for T4.
MLM is based on a set of regression equations. First, the intercepts-only model (a model without predictors) was ran to check whether a multilevel model was required, by means of the intraclass correlation. The intraclass correlations ranged between .20 and .40, thus MLM analyses were appropriate for all variables. Second, following [95], a build-up strategy was used. Variables were added one by one to the intercept-only model. After each addition, the − 2 log likelihood ratio scale after generalized least square estimation was examined. The − 2 log likelihood tracks model fit. If model fitness increases, the added variable is kept. If model fitness decreases, the added variable is cut from the model.
The variables were tested in a certain order. First, gender (mother or father) was included as a fixed factor, and then as a random factor. Thereafter, intervention condition was added. Finally, interaction terms between intervention X parental gender, and between intervention X prenatal state (including the main effect of prenatal state) were added. These interaction terms tested whether intervention effects differed between mothers and fathers, and between parents varying on prenatal levels of the outcome of interest (e.g. whether the intervention was effective in decreasing postpartum parenting stress symptoms depending on their levels of prenatal parenting stress). Also, the interaction between intervention X parity was of interest to investigate whether the intervention was effective for first-time parents versus experienced parents (i.e. parents with children). As the majority of our sample (91.2%) consisted of first-time parents, multilevel analyses were repeated in the sample with first-time parents only. The final models are presented in the results. All analyses were done using SPSS 25.0.0.
Results
Descriptives
We included 138 women (68 in the intervention group and 69 in the control group) and 96 partners (48 in the intervention and 48 in the control group). Figure 1 shows the CONSORT flow diagram. The response rate of the mothers was high: 88.3% (T1); 89.7% (T2), 84.7% (T3) and 87.6% (T4). Non-response was mainly caused by medical complications during pregnancy or birth (Fig. 1). For partners, the response rate was also high: 88.4% (T1), 100%1 (T2); 74.7% (T3) and 85.2% (T4).
The mothers were on average between 32 and 33 years of age (Table 1). Partners were slightly older, with a mean age of about 35 years. The majority of the mothers (94.8%) were either married or cohabitating. Most participants received higher vocational education and about one third (34.7%) of mothers worked fulltime at baseline (during pregnancy). More than half of the participants reported a net family income of more than 4000 euros per month. The majority (91.2%) of participating mothers was pregnant of their first child. No significant differences between the intervention and control group emerged on the demographic variables.
Table 1.
Demographics and confounder variables
| Mothers | Partners | |||||
|---|---|---|---|---|---|---|
| Intervention group (n = 68) | Control group (n = 69) | p-value | Intervention group (n = 45) | Control Group (n = 44) | p-value | |
| Age (mean, SD) | 32.69 (3.37) | 32.23 (3.54) | 0.44 | 35.03 (4.08) | 34.73 (5.67) | 0.79 |
| % married or cohabitating | 94.1 | 95.6 | 0.51 | 95.5 | 97.7 | 0.27 |
| % ≥ higher vocational education | 91.1 | 91.2 | 0.51 | 86.7 | 77.2 | 0.31 |
| Working hours (weekly) | 0.13 | 0.13 | ||||
| employed ≥37 h (%) | 28.3 | 39.8 | 57.8 | 72.8 | ||
| employed 21–36 h (%) | 65.5 | 54.4 | 35.5 | 22.7 | ||
| employed 0–20 h (%) | 1.5 | 4.4 | 4.4 | 1.5 | ||
| no paid employment | 4.5 | 1.5 | 2.2 | 2.3 | ||
| Family incomea (% ≥ 4000 euro) | 52.2 | 60.2 | 0.40 | 57.8 | 56.8 | 0.78 |
| Birth ordera (% 1e kind) | 91.2 | 91.3 | 0.96 | 88.9 | 84.1 | 0.45 |
| # weeks pregnant at inclusion | 28.24 (2.60) | 27.81 (4.18) | 0.45 | 28.20 (2.49) | 27.56 (4.61) | 0.39 |
| Attachment style: avoidance | 11.52 (5.82) | 10.52 (3.93) | 0.25 | 13.52 (6.54) | 12.78 (5.31) | 0.57 |
| Attachment style: anxiety | 16.64 (5.35) | 17.24 (5.79) | 0.54 | 17.49 (6.68) | 17.44 (5.73) | 0.97 |
| Marital satisfaction | 30.97 (4.39) | 31.63 (3.72) | 0.35 | 30.96 (3.87) | 30.93 (3.49) | 0.97 |
| % cesarean section | 9.9 | 11.3 | 0.69 | |||
| Birth weight | 3484.84 (472.94) | 3484.57 (424.02) | 0.99 | |||
| Apgar score 5 min (% ≥7) | 98.4 | 98.3 | 0.23 | |||
| Apgar score 5 min | 9.43 (1.3) | 9.66 (.81) | ||||
adifferences between mothers and partners because not all partners participated in the study
Treatment adherence and drop-out
Of the 68 mothers who were allocated to the intervention group, 63 received the intervention (booklet, video, and home visit). The other mothers (n = 5) were not able to receive the intervention because of pregnancy complications developed after inclusion (i.e. HELLP syndrome or premature birth). Two mothers dropped out of the study after the home visit because of hospital admission and chromosomal abnormality (Fig. 1). In the control group, 5 women dropped out right after randomization: two because of pregnancy complications and three because they could not be contacted anymore. Two more women dropped out after T1 because they indicated they felt they were too busy to continue participation (n = 1) or did not feel comfortable sharing personal information (n = 1).
Parental distress
Table 2 shows the distress scores over time for mothers. As can be seen from the mean scores, both the intervention and control group showed a rise in distress (parenting stress, depression, and anxiety) and a decrease in well-being (satisfaction with the parenting role and self-efficacy) from T0 to T3, after which distress levels returned to baseline at T4. There were no significant differences on the distress variables between the intervention and control group at 6 (T3) or 10 weeks after birth (T4).
At baseline, 5 mothers in the intervention group (3.3%) scored above the cut-off for depression (10 or more on the EPDS) and 8 mothers in the control group (12.1%). With regard to anxiety, in the intervention group, 11 mothers (16, 4%) scored 8 or more on the HADS at baseline and 14 mothers in the control group (20,6%). Ten weeks after birth (t4) 5 mothers in the intervention group (9.1%) scored above the threshold for depression, and 5 mothers in the control group (8.2%). A total of 7 mothers in the intervention group (12.7%) scored above the cut-off for anxiety (8 or more on the HADS) at t4, and 13 mothers in the control group (21.7%).
Table 3 shows the different scores over time for fathers. Also here, both groups showed a rise in distress levels and a decrease in well-being from t0 to t3, after which distress levels returned to baseline. There were no significant differences in distress between the intervention and control groups at 6 (t3) or 10 weeks after birth (t4).
At baseline, 4 fathers in the intervention group (8.9%) scored above the cut-off score for depression (9 or more on the EPDS) and 2 fathers in the control group (4.65%). With regard to anxiety, 13 fathers in the intervention group (28.9%) scored 8 or more on the HADS at baseline and 11 fathers in the control group (25,6%). Ten weeks after birth (t4), 4 fathers in the intervention group (13.8%) scored above the threshold for depression, and 5 fathers in the control group (13.9%). A total of 7 fathers in the intervention group (24.1%) scored above the cut-off for anxiety (8 or more on the HADS) compared to 7 fathers in the control group (19.4%).
Multilevel analyses
The final multilevel models are presented in Table 4. For both maternal and paternal parenting stress at t3, the multilevel analyses indicated no effect of the intervention. Similarly, the interaction terms intervention X parental gender, and intervention X prenatal state did not significantly improve model fit. The intervention was thus not effective in decreasing parenting stress. Moreover, multilevel analyses indicated no effect of the intervention on parental depressive symptoms, anxiety symptoms nor on any other parental secondary outcome (satisfaction with the parenting role, self-efficacy, and perception of sleep quality and quantity). The interaction terms were also not significant, indicating that the intervention was not effective for both mothers and fathers, or for parents varying in their prenatal levels on the outcome of interest.
Table 4.
Estimates for the best fitting multilevel models
| Estimate | SE | p | |
|---|---|---|---|
| Primary outcome: parenting stress (T3) | |||
| Intercept | 14.22 | 3.18 | < 0.001 |
| Gender (1 = mother, 2 = father) | −.41 | .96 | .67 |
| Prenatal parenting stressa | .57 | .07 | < 0.001 |
| Deviance: 1165.48 | |||
| Secondary outcomes | |||
| Depressive symptoms (T3) | |||
| Intercept | 4.58 | .83 | < 0.001 |
| Partner (1 = mother, 2 = father) | −1.69 | .46 | < 0.001 |
| Prenatal depressive symptomsa | .63 | .09 | < 0.001 |
| Deviance: 926.42 | |||
| Anxiety symptoms (T3) | |||
| Intercept | 3.26 | .65 | < 0.001 |
| Partner (1 = mother, 2 = father) | −.81 | .36 | .03 |
| Prenatal anxiety symptomsa | .66 | .08 | < 0.001 |
| Deviance: 799.03 | |||
| Satisfaction with the parenting role (T3) | |||
| Intercept | 19.96 | 3.08 | < 0.001 |
| Partner (1 = mother, 2 = father) | .30 | .48 | .53 |
| Prenatal levels of satisfactiona | .48 | .08 | < 0.001 |
| Deviance: 911.98 | |||
| Self-efficacy (T3) | |||
| Intercept | 1.38 | .36 | < 0.001 |
| Partner (1 = mother, 2 = father) | .04 | .09 | .68 |
| Prenatal levels of self-efficacya | .61 | .09 | < 0.001 |
| Deviance: 305.34 | |||
| Perception of sleep (T3)b | |||
| Intercept | −.40 | .18 | .03 |
| Partner (1 = mother, 2 = father) | .29 | .13 | .02 |
| Deviance: 458.38 | |||
| Bonding (T4)b | |||
| Intercept | 67.92 | 1.94 | < 0.001 |
| Partner (1 = mother, 2 = father) | −.12 | .96 | .90 |
| Deviance: 1141.44 | |||
aprenatal state levels are averaged across T0 and T1 because of high intercorrelations (ranging between .38 and .77)
bno prenatal levels available
Additionally, the multilevel analyses indicated that mothers had higher levels of postpartum depressive and anxiety symptoms, and worse perceptions of their sleep quality and quantity, compared to fathers. Furthermore, for all outcomes of interest, prenatal levels were predictive of postpartum levels, indicating that symptoms were to some extent stable over the course of childbirth. Repeating the multilevel analyses with the outcomes measured at infant age 10 weeks (T4), and on the sample including only first-time parents yielded similar results.
Quality of caregiving
Bonding
The multilevel model for parent-infant bonding is presented in Table 5. For both maternal and paternal bonding with the infant at T3, the multilevel analyses indicated no effect of the intervention. Similarly, the interaction terms intervention X parental gender, and intervention X prenatal bonding did not significantly improve model fit. The intervention was thus not effective in improving parent-infant bonding.
Table 5.
Mean number of weeks of exclusive breastfeeding and the mean number of weeks the child slept in the same room as the parents (in own cot) at 10 weeks after birth (t4)a
| Intervention | Control | p-value | |
|---|---|---|---|
| # weeks exclusive breastfeeding | 7.66 (4.19) | 7.26 (4.52) | .78 |
| # weeks room-sharing | 8.64 (3.52) | 9.12 (3.28) | .77 |
aFor room-sharing, only parents that slept with their child in the same room, while the child was in his or her own cot were taken into account (control, n = 51/ intervention, n = 50)
Breastfeeding and room-sharing
Independent samples t-tests indicated that there were no differences between the intervention (M = 7.66, SD = 4.19) and the control group (M = 7.26, SD = 4.52) with regard to the mean duration (in weeks) of exclusive breastfeeding, p = .78 (Table 5). Also, no differences emerged in the number of weeks participants in the intervention group (M = 8.64, SD = 3.52) and in the control group (M = 7.26, SD = 4.52) slept in the same room as their child at night (while the child was in his or her own cot), p = .77.
Perceived problems with infant crying, feeding, and sleeping
There was one significant difference with regard to perceived problems with infant crying, feeding, or sleeping: 16.2% of mothers in the intervention group reported problems with infant feeding during daytime at 6 weeks after birth, versus 6.9% in the control group; p = .05). No other differences between the intervention and the control groups emerged in the percentage of mothers reporting problems with the infant’s sleeping, crying, or feeding (assessed separately for day- vs. night-time) at 6 weeks after birth (T3) nor at 10 weeks after birth (T4; Table 6).
Table 6.
Percentage of mothers reporting problems with the infant’s sleep, crying or feeding
| Intervention T3 (6 weeks after birth) % reporting problem |
Control T3 (6 weeks after birth) % reporting problem |
p-value T3 |
Intervention T4 (10 weeks after birth) % reporting problem |
Control T4 (10 weeks after birth) % reporting problem |
p-value T4 |
|
|---|---|---|---|---|---|---|
| Infant sleep (night) | 29.3 | 32.8 | .69 | 14.5 | 15.0 | .95 |
| Infant sleep (day) | 50.0 | 36.8 | .13 | 34.5 | 35.0 | .96 |
| Infant crying (night) | 15.5 | 11.6 | .79 | 5.5 | 3.3 | .58 |
| Infant crying (day) | 31.0 | 34.5 | .69 | 27.3 | 21.7 | .48 |
| Infant feeding (night) | 13.8 | 5.2 | .11 | 3.6 | 6.7 | .47 |
| Infant feeding (day) | 16.2 | 6.9 | .05* | 16.4 | 10.0 | .31 |
Intervention uptake and satisfaction
Almost all mothers (98.5%) read the information booklet and watched the video before the birth of their child. For partners, these percentages were 85.7% (information booklet) and 92.9% (video). More than half of the mothers (55.6%) and about one third of the partners (32.1%) reported to have used the information after the birth of their child daily or several times a week. Moreover, participants reported the intervention to be useful. Both mothers and fathers found the information booklet the most useful part of the intervention (Table 7). The home visit and the video were rated as partly useful by both parents.
Table 7.
Means and standard deviations of intervention usefulness as reported by mothers and fathers (as indicated on a 1–5 Likert scale)
| Mothers | Partners | |
|---|---|---|
| Information booklet | 4.15 (.87) | 3.64 (.91) |
| Video | 3.08 (1.00) | 2.71 (1.05) |
| Home visit | 3.11 (.93) | 2.96 (.92) |
Discussion
The aim of this randomized controlled trial was to examine the effectiveness of a brief psychoeducational intervention to prevent postpartum parenting stress, to decrease symptoms of depression and anxiety, and to enhance parental well-being and the quality of caregiving behavior. The intervention was aimed at a universal population of parents (regardless of risk factors or (previous) symptoms), and the intervention was targeted at both parents. For both groups, there was a rise in distress scores between baseline and 6 weeks postpartum. No differences emerged in levels of parenting stress between the intervention and control group over time. This means that parents that received and digested the information and were visited at home during pregnancy did not report lower levels of parenting stress compared to parents that did not receive this support. Also, there was no effect of the intervention on symptoms of depression and anxiety, nor on the indices of parental well-being (satisfaction with the parenting role, self-efficacy, and sleep quality and quantity). With regard to quality of caregiving, no differences emerged in the quality of the parent-infant bond, nor in the duration infants received breastfeeding or slept in the parent’s room at night-time. Parents from the intervention group did not report less problems with their infant’s crying, feeding, or sleeping. In contrast, mothers from the intervention group reported more instead of less problems with infant feeding at 6 weeks postpartum than mothers from the control group.
While psycho-education has been indicated valuable in reducing symptoms of psychological distress [17], also when implemented during pregnancy [97], visiting upcoming parents at home and providing them with information about adapting to the parental role, and infant crying, feeding, and sleeping (arrangements) seemed not to be effective in preventing postpartum distress and enhancing caregiving quality. However, the intervention seemed to fill a gap in the information and tools that are currently available for parents-to-be. Parents rated the intervention as useful and of added value. The question arises how an intervention that is rated as useful, particularly by the mothers, did not result in any changes in our dependent variables. Several factors could have played a role here. First, it could be that the effect of the intervention becomes visible later on during the first year (i.e. after the initial 10 weeks). Second, it is possible that the intervention is effective on other measures that we did not take into account in this study (e.g. observed sensitive responsiveness; infant well-being). Third, the intervention might be more effective for specific groups of parents (i.e. our sample was relatively well-educated, reporting high SES). Also, we lack information on participants psychosocial history. Finally, it is possible that the intervention has no added value. We will review each of these possibilities below.
First, we followed parents until 10 weeks after birth. We expected parental distress levels to be highest within these first 10 weeks, because infant crying rises until 6 weeks and gradually decreases thereafter [96]. Indeed, in both groups, distress scores over time showed a peak at 6 weeks, and returned to baseline at 10 weeks after birth. It could be that the tools we provided parents with have a buffering effect on other peaks of parental distress during the first year. This reasoning is in line with Hiscock et al. [67] who found an effect of their psychoeducational intervention implemented shortly after birth on primary caregiver’s (in 99.6% of cases the mother) depressive symptoms at 6, but not at 4 months postpartum. Moreover, effects of the intervention on breastfeeding and room-sharing might also become visible later in the first year of life. While not leading to group differences during the first weeks postpartum, when many parents are breastfeeding and room-sharing, differences in total breastfeeding and room-sharing duration might become visible after this period. Future studies into universal prevention during pregnancy should consider to extend the study period to examine whether effects of interventions emerge later in the first year of the infant’s life.
Second, it is possible that the intervention was not effective in decreasing parental distress or increasing caregiving quality measures but impacted other measures such as observed parental caregiving quality, parental stress physiology, or infant behavior. Given that mothers from the intervention group reported more problems with infant feeding, it is possible that the intervention increased maternal awareness for potential problems, making them more prone to report these. However, no differences emerged with regard to crying and sleeping. Measuring parent-infant interaction at various moments during the first year would shed more light on whether the intervention supports parents in developing these skills. Also, our study relied heavily on self-report measures, which are known for their problems with social desirability [98]. Parental physiological stress measures, such as cortisol measurements in hair, could give insight in parental stress physiology. Also, direct measures of infant behavior should be added, such as a registration of infant crying patterns, to monitor potential effects of the intervention on infant behavior [99].
Third, our sample mainly consisted of relatively highly educated first-time parents. These parents are more likely to seek and find information about pregnancy and childrearing themselves. It is possible that less educated parents have less resources to find the necessary information and would benefit more when this information would be consequently provided by midwives and nurses, for example through a clear and brief information booklet as provided in the current study. However, as Henshaw et al. [1] showed among a sample of parents varying in income, education, and ethnicity; having access to much information does not necessarily reduce stress. There is especially a need for reliable and evidence-based information [2]. Therefore, we aimed to provide parents with up to date and scientifically validated information about the first months postpartum, and intervention effects were expected also among highly educated parents.
Related to the previous point is that we have no information about the participants psychosocial history, while it is possible that the intervention is (only) effective for parents with a history of psychosocial problems. In future research, psychosocial history should be added as a moderator of intervention effectiveness. Other risk factors that have been shown to moderate postpartum distress symptomatology could also determine intervention effectiveness, for example delivery complications [11], and relational problems [100]. In sum, it could be that a more mixed sample of parents, including less highly educated parents, as well as a sample of parents varying in risk factors, would generate different results. On a related note, while there were no between-group differences, gender differences emerged with regard to depression, anxiety, and sleep at 6 weeks postpartum. Mothers reported higher levels of symptomatology than fathers. This is in line with earlier research [59, 101]. Previous research into depression suggested that the rise in symptomatology fathers experience after birth takes place later in the first year and develops more slowly than is the case for mothers [59]. Future studies following parents longitudinally could shed more light on whether mothers and fathers need different types of support, and/or at different times during the perinatal period.
Finally, while absence of evidence is not evidence of absence, we should also acknowledge the possibility that a brief psychoeducational intervention aimed at providing information is not sufficient to influence parents’ levels of distress and their caregiving quality. It might be that interventions incorporating more sessions, as well as state-of-the-art therapeutic elements, are needed to prevent parents from developing symptomatology. For example, Haga, Drozd, Lisoy, Wentzel-Larsen, and Slining [102] reported an effect on depressive symptoms of an online intervention among a universal population of pregnant women at 6 weeks postpartum. This extensive intervention consisted of 44 online 10-min sessions and incorporated a mix of elements of cognitive-behavioural therapy, mindfulness, psychoeducation, meta-cognitive therapy, acceptance and commitment therapy, and positive psychology. Thus, this intervention differed from ours with regard to the content (psychoeducation combined with elements of a range of therapeutic techniques); the relatively high frequency of (short) sessions, and the fact that all sessions could be followed online. These factors could explain why the intervention was successful in reducing depressive symptoms, at least during the first weeks after birth.
Strengths and limitations
This randomized controlled trial has several strengths. First, while most studies focusing on the prevention of psychopathology after birth included mothers who already displayed symptomatology (indicated prevention [47, 48]; or on mothers belonging to risk groups for developing postpartum psychopathology (selected prevention [49–51], in this study a universally applicable intervention was tested. In contrast to indicated or selected prevention, much less is known about the effectiveness of universal prevention during pregnancy [56, 58]. Second, while the majority of previous intervention focused on mothers only, we were able to include mothers as well as a substantial part of fathers. Third, while most prevention studies focused on depression as an outcome measure [56, 58], we included a variety of distress outcomes, including stress related to the parenting role and symptoms of anxiety.
Of course, the study has limitations as well. First, while we aimed to include a sample of expecting parents varying in demographic characteristics and backgrounds, our sample was relatively well-educated, and reported a relatively high income. This decreases the generalizability of this study. Notably, to be able to digest the information booklet and the video, parents needed to be able to read Dutch and have access to the internet. Since 98% of Dutch households have access to the internet at home [103], we assume that this criterion has not lead to the exclusion of lower educated parents. Instead, it seems more likely that higher SES parents actively search for and make use of this type of interventions. Indeed, a study investigating predictors of eHealth usage found that people with a lower socio-economic status were less likely to engage in a number of eHealth activities compared to their counterparts with higher socio-economic status [104]. It is important that future studies assess the reasons for these differences in the implementation of eHealth and online interventions, because these differences can contribute to persistent disparities in health across social groups [104]. Second, we were only able to measure until 10 weeks after birth. While we captured the first stressful weeks after birth including the infant crying peak, it could be that potential effects on parental distress levels or our measures of caregiving quality, including breastfeeding and room-sharing, become visible later on during the first year. Third, we did not measure parental use of external support services, including the use and quality of external breastfeeding courses and support services. It might be that, as a result of the intervention, parents used these support services more often, or that differences in our outcome variables only become visible among groups of parents who have limited access to (high-quality) support services. Fourth, as stated earlier, it could also be that the intervention did not impact on parental distress, but on other measures we could not take into account in this study. Observations and physiological measurements of both parental and infant behavior could provide more fine-grained information about the potential effect of the intervention.
Conclusion
The current study offered no evidence that a universal prevention program implemented during pregnancy, and aimed at both mothers and fathers, is effective in preventing symptoms of parenting stress, depression, or anxiety during the first 10 weeks after birth. Also, we found no evidence that the intervention enhanced the quality of parental caregiving. However, parents reported the intervention, especially the information booklet, to be of added value. Future research should detect whether this type of brief psychoeducation might be effective on other measures, samples or periods in time. While absence of evidence is not evidence of absence, it is also possible that a brief psychoeducational intervention aimed at providing information is not sufficient to improve parental well-being and caregiving quality, and other, more intensive, types of interventions are needed. Since parental distress symptomatology and parental caregiving quality after birth can affect infant development [16, 21, 65], detecting effective ways of intervening in an early stage -thus already during pregnancy- is of vital importance for both parent’s as well as children’s health and development.
Acknowledgements
Not applicable.
Abbreviations
- AAP
American Academy of Pediatrics
- ECR
Experiences in Close Relationships Questionnaire
- EPDS
Edinburgh Postnatal Depression Scale
- HADS
Hospital Anxiety and Depression Scale
- IMS
The Investment Model Scale
- MLM
Multilevel Lineair Modeling
- MPAS
Maternal Postnatal Attachment Scale
- PSI
Parenting Stress Index
- PSQI
Pittsburgh Sleep Quality Index
- SES
Socioeconomic status
- SIDS
Sudden Infant Death Syndrome
- T
Time
- WHO
World Health Organization
Authors’ contributions
MM participated in the study design, participant recruitment, trial coordination, data analysis, and drafted the manuscript. RB participated in the study design and data analysis, and supervised writing the manuscript. JD is initiator of the study, obtained funding and provided feedback on the draft of this paper. TD participated in the study design and supervised writing the manuscript. AvS is the principle investigator, participated in the study design and supervised writing the manuscript. All authors have read and approved the final manuscript.
Funding
This research was funded by the Netherlands Organization for Scientific Research (NWO: 406.14.106). The funding body had no role in the design of the study and in the collection, analysis, and interpretation of data nor in the writing of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was registered at the Netherlands Trial Register (NTR6065). The study was approved by the Medical Ethical Committee Brabant (NL58528.028.16/P1620). All participants were informed about the study’s aims and procedures, and provided written consent for participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
At t2, one questionnaire measuring delivery characteristics was sent to both parents. One of the parents could fill in this questionnaire. For all participating fathers, a questionnaire was returned at t2 (but not necessarily filled in by the father).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marjolein Missler, Email: m.a.missler@vu.nl.
Annemieke van Straten, Email: a.van.straten@vu.nl.
Jaap Denissen, Email: j.j.a.denissen@uu.nl.
Tara Donker, Email: t.donker@vu.nl.
Roseriet Beijers, Email: r.beijers@psych.ru.nl.
References
- 1.Henshaw EJ, Cooper MA, Jaramillo M, Lamp MN, Jones AL, Wood TL. “Trying to figure out if you are doing things right, and where to get the info”: parents recall information and support needed during the first 6 weeks postpartum. Matern Child Health J. 2018;22:1668–1675. doi: 10.1007/s10995-018-2565-3. [DOI] [PubMed] [Google Scholar]
- 2.Young C, Roberts R, Ward L. Hindering resilience in the transition to parenthood: a thematic analysis of parents’ perspectives. J Rep Inf Psych. 2020. 10.1080/02646838.2020.1757630. [DOI] [PubMed]
- 3.Hong Law K, Dimmock J, Guelfi KJ, Nguyen T, Gucciardi D, Jackson B. Stress, depressive symptoms, and maternal self-efficacy in first-time mothers: modelling and predicting change across the first six months of motherhood. Appl Psych Health Well Being. 2019;11:126–147. doi: 10.1111/aphw.12147. [DOI] [PubMed] [Google Scholar]
- 4.Van Scheppingen MA, Denissen JJA, Chung JM, Tambs K, Bleidorn W. Self-esteem and relationship satisfaction during the transition to motherhood. J Pers Soc Psychol. 2018;114:973–991. doi: 10.1037/pspp0000156. [DOI] [PubMed] [Google Scholar]
- 5.Leigh B, Milgrom J. Risk factors for antenatal depression, postnatal depression, and parenting stress. BMC Psych. 2008;8:24. doi: 10.1186/1471-244X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heron J, O’Connor TG, Evans J, Golding J, Glover V. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Aff Dis. 2004;80:65–73. doi: 10.1016/j.jad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Yelland J, Sutherland G. Brown SJ. BMC Public Health. 2010;10:771. doi: 10.1186/1471-2458-10-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCoy SJ, Beal JM, Shipman SB, Payton ME, Watson GH. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Ost Ass. 2006;106:193–198. [PubMed] [Google Scholar]
- 9.Morris-Rush JK, Freda M, Bernstein PS. Screening for postpartum depression in an inner-city population. Am J Obst & Gyn. 2003;188:1217–1219. doi: 10.1067/mob.2003.279. [DOI] [PubMed] [Google Scholar]
- 10.Glasheen C, Richardson GA, Fabio A. A systematic review of the effects of postnatal maternal anxiety on children. Arch Womens Ment Health. 2010;13:61–74. doi: 10.1007/s00737-009-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Field T. Postnatal anxiety prevalence, predictors, and effects on development: a narrative review. Inf Beh Dev. 2018;51:24–32. doi: 10.1016/j.infbeh.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Ped. 2006;118:659–668. doi: 10.1542/peds.2005-2948. [DOI] [PubMed] [Google Scholar]
- 13.Matthey S, Barnett B, Howie P, Kavanagh DJ. Diagnosing postpartum depression in mothers and fathers: whatever happened to anxiety? J Aff Dis. 2003;74:139–147. doi: 10.1016/S0165-0327(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 14.Brennan P, Hammen C, Andersen MJ, Bor W, Najman JM, Williams GM. Chronicity, severity, and timing of maternal depressive symptoms: relationships with child outcomes at age 5. Dev Psych. 2000;36:759–766. doi: 10.1037/0012-1649.36.6.759. [DOI] [PubMed] [Google Scholar]
- 15.Meaney MJ. Perinatal maternal depressive symptoms as an issue for population health. Am J of Psych. 2018;175:11. doi: 10.1176/appi.ajp.2018.17091031. [DOI] [PubMed] [Google Scholar]
- 16.Murray L, Fearon P, Cooper P. Postnatal depression, mother-infant interactions, and child development - prospects for screening and treatment. In: Milgrom J, Gemmill A, editors. Identifying perinatal depression and anxiety: evidence-based practice in screening, psychosocial assessment and management. Oxford: Wiley Blackwell; 2015. pp. 139–164. [Google Scholar]
- 17.Donker T, Griffiths KM, Cuijpers P, Christensen H. Psychoeducation for anxiety, depression, and psychological distress: a meta-analysis. BMC Med. 2009;7:79. doi: 10.1186/1741-7015-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abidin RR. The determinants of parenting behavior. J Clin Child Psych. 1992;21:407–412. doi: 10.1207/s15374424jccp2104_12. [DOI] [Google Scholar]
- 19.Crnic KA, Gaze C, Hoffman C. Cumulative parenting stress across the preschool period: relations to maternal parenting and child behaviour at age 5. Inf Child Dev. 2005;14:117–132. doi: 10.1002/icd.384. [DOI] [Google Scholar]
- 20.Koss KJ, Gunnar MR. Annual research review: early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. J Child Psychol Psych. 2018;59:327–346. doi: 10.1111/jcpp.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384:1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- 22.Bakermans-Kranenburg MJ, Van IJzendoorn MH, Juffer F. Less is more: meta-analyses of sensitivity and attachment interventions in early childhood. Psychol Bull. 2003;129:195–215. doi: 10.1037/0033-2909.129.2.195. [DOI] [PubMed] [Google Scholar]
- 23.De Wolff MS, Van IJzendoorn MH. Sensitivity and attachment: a meta-analysis on parental antecedents of infant attachment. Soc Res Child Dev. 1997;68:571–591. doi: 10.1111/j.1467-8624.1997.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 24.Fearon RP, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Lapsley A, Roisman GI. The significance of insecure attachment and disorganization in the development of children’s externalizing behavior: a meta-analytic study. Child Dev. 2010;81:435–456. doi: 10.1111/j.1467-8624.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- 25.Groh AM, Roisman GI, Van IJzendoorn MH, Bakermans-Kranenburg MJ, Fearon RP. The significance of insecure and disorganized attachment for children’s internalizing symptoms: a meta-analytic study. Child Dev. 2012;83:591–610. doi: 10.1111/j.1467-8624.2011.01711.x. [DOI] [PubMed] [Google Scholar]
- 26.Isabella RA, Belsky J, Von Eye A. Origins of infant-mother attachment: an examination of interactional synchrony during the infant’s first year. Dev Psych. 1989;25:12–21. doi: 10.1037/0012-1649.25.1.12. [DOI] [Google Scholar]
- 27.Smeekens S, Riksen-Walraven JM, Van Bakel HJA. Multiple determinants of externalizing behavior in 5-year-olds: a longitudinal model. J Abn Child Psychol. 2007;35:347–361. doi: 10.1007/s10802-006-9095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sroufe AL. Attachment and development: a prospective, longitudinal study from birth to adulthood. Attach & Hum Dev. 2005;7:349–367. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- 29.De Cock ESA, Henrichs J, Vreeswijk CMJM, Maas AJBM, Rijk CHAM, Van Bakel HJA. Continuous feelings of love? The parental bond from pregnancy to toddlerhood. J Fam Psych. 2016;30:125–134. doi: 10.1037/fam0000138. [DOI] [PubMed] [Google Scholar]
- 30.Jansen J, De Weerth C, Riksen-Walraven M. Breastfeeding and the mother–infant relationship—A review. Dev Rev. 2008; 28, 503–521. 10.1016/j.dr.2008.07.001.
- 31.Osher D, Cantor P, Berg J, Steyer L, Rose T. Drivers of human development: how relationships and context shape learning and development. Appl Dev Sci. 2020;24:6–36. doi: 10.1080/10888691.2017.1398650. [DOI] [Google Scholar]
- 32.Mason ZS, Briggs RD, Silver EJ. Maternal attachment feelings mediate between maternal reports of depression, infant social–emotional development, and parenting stress. J Rep and Inf Psych. 2011;29:382–394. doi: 10.1080/02646838.2011.629994. [DOI] [Google Scholar]
- 33.De Cock ES, Henrichs J, Klimstra TA, Maas AJBM, Vreeswijk CMJM, Meeus WHJ, Van Bakel HJA. Longitudinal associations between parental bonding, parenting stress, and executive functioning in toddlerhood. J Child Fam Stud. 2017;26:1723–1733. doi: 10.1007/s10826-017-0679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro Dias C, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Aff Dis. 2014;171:142–154. doi: 10.1016/j.jad.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 35.Hoff CE, Movva N, Rosen Vollmar AK, Pérez-Escamilla R. Impact of Maternal Anxiety on Breastfeeding Outcomes: A Systematic Review. Adv Nutri. 10:816–26. 10.1093/advances/nmy132. [DOI] [PMC free article] [PubMed]
- 36.World Health Organization . 10 facts on breastfeeding. 2017. [Google Scholar]
- 37.Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Act Paed. 2015;104:14–19. doi: 10.1111/apa.13139. [DOI] [PubMed] [Google Scholar]
- 38.Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, Murch S, Jeeva Sankar M, Walker N, Rollins NC. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 39.Moon R. AAP Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: evidence base for updated recommendations for a safe infant sleeping environment. Ped. 2016;138. 10.1542/peds.2016-2940. [DOI] [PubMed]
- 40.Rollins J. Sharing a room: updated recommendations for a safe infant sleeping environment. Ped Nurs. 2017;43:7. [PubMed] [Google Scholar]
- 41.Tappin D, Ecob R, Brooke H. Bedsharing, roomsharing, and sudden infant death syndrome in Scotland: a case-control study. J of Ped. 2005;147:32–27. doi: 10.1016/j.jpeds.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 42.Beijers RJ, Riksen-Walraven M, De Weerth C. Cortisol regulation in 12-month-old human infants: associations with the infant’s early history of breastfeeding and co-sleeping. Stress. 2013;16:267–277. doi: 10.3109/10253890.2012.742057. [DOI] [PubMed] [Google Scholar]
- 43.Tollenaar MS, Beijers R, Jansen J, Riksen-Walraven M, De Weerth C. Solitary sleeping in young infants is associated with heightened cortisol reactivity to a bathing session but not to a vaccination. Psychoneuro. 2012;37:167–177. doi: 10.1016/j.psyneuen.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Ball HL. Breastfeeding, bed-sharing, and infant sleep. Birth. 2003;30:181–188. doi: 10.1046/j.1523-536X.2003.00243.x. [DOI] [PubMed] [Google Scholar]
- 45.McKenna JJ, Ball HL, Gettler LT. Mother-infant co-sleeping, breastfeeding, and sudden infant death syndrome: what biological anthropology has discovered about normal infant sleep and pediatric sleep medicine. Am J of Phys Anthro. 2007;134:133–161. doi: 10.1002/ajpa.20736. [DOI] [PubMed] [Google Scholar]
- 46.Baddock A, Purnell MT, Blair PS, Pease AS, Elder DE, Galland BC. The influence of bed-sharing on infant physiology, breastfeeding, and behaviour: a systematic review. Sleep Med Rev. 2019;43:106–117. doi: 10.1016/j.smrv.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Bittner A, Peukert J, Zimmerman C, Junge-Hoffmeister J, Parker LS, Stöbel-Richter Y, Weidner K. Early intervention in pregnant women with elevated anxiety and depressive symptoms. Efficacy of a cognitive-behavioural program. J Peri & Neo Nurs. 2014;28:185–195. doi: 10.1097/JPN.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 48.Austin MP, Frilingos M, Lumley J, Hadzi-Pavlovic D, Roncolato W, Acland S, Saint K, Segal N, Parker G. Brief antenatal cognitive behaviour therapy group intervention for the prevention of postnatal depression and anxiety: a randomised controlled trial. J Aff Dis. 2008;105:35–44. doi: 10.1016/j.jad.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Bayrampour H, Vinturache A, Hetherington E, Lorenzetti DL, Tough S. Risk factors for antenatal anxiety: a systematic review of the literature. J Rep & Inf Psych. 2018;36:476–503. doi: 10.1080/02646838.2018.1492097. [DOI] [PubMed] [Google Scholar]
- 50.Dennis C, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Brit J Psych. 2017;210:315–323. doi: 10.1192/bjp.bp.116.187179. [DOI] [PubMed] [Google Scholar]
- 51.Doyle O, Delaney L, O’Farrelly C, Fitzpatrick N, Daly M. Can early intervention improve maternal well-being? Evidence from a randomized controlled trial. PLoS One. 2017;12. 10.1371/journal.pone.0169829. [DOI] [PMC free article] [PubMed]
- 52.Wajid A, Kingston D, Bright KS, Mughal MK, Charrois EM, Giallo R. (2020). Psychosocial factors associated with trajectories of maternal psychological distress over a 10-year period from the first year postpartum: an Australian population-based study. J Aff Dis. 2020;263:31–38. doi: 10.1016/j.jad.2019.11.138. [DOI] [PubMed] [Google Scholar]
- 53.Clatworthy J. The effectiveness of antenatal interventions to prevent postnatal depression in high-risk women. J Aff Dis. 2012;137:25–34. doi: 10.1016/j.jad.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 54.Sockol LE, Epperson CN, Barber JP. Preventing postpartum depression: a meta-analytic review. Clin Psych Rev. 2013;33:1205–1217. doi: 10.1016/j.cpr.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Aff Dis. 2015;177:7–21. doi: 10.1016/j.jad.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 56.Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Aff Dis. 2018;232:316–328. doi: 10.1016/j.jad.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 57.US Preventive Services Task Force Interventions to prevent perinatal depression: US Preventive Task Force recommendation statement. JAMA. 2019;321:580–587. doi: 10.1001/jama.2019.0007. [DOI] [PubMed] [Google Scholar]
- 58.Evans K, Morrell CJ, Spiby H. Systematic review and meta-analysis of non-pharmalogical interventions to reduce the symptoms of mild to moderate anxiety in pregnant women. J Adv Nurs. 2018;74:289–309. doi: 10.1111/jan.13456. [DOI] [PubMed] [Google Scholar]
- 59.Kiviruusu O, Pietikäinen JT, Kylliäinen A, Pölkki P, Saarenpää-Heikkilä O, Marttunen M, Paunio T, Paavonen JE. Trajectories of mothers’ and fathers’ depressive symptoms from pregnancy to 24 months postpartum. J Aff Dis. 2020;260:629–637. doi: 10.1016/j.jad.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 60.Hughes C, Devine RT, Foley S, Ribner AD, Mesman J, Blair C. Couples becoming parents: trajectories for psychological distress and buffering effects of social support. J Aff Dis. 2020;265:372–380. doi: 10.1016/j.jad.2020.01.133. [DOI] [PubMed] [Google Scholar]
- 61.Kvalevaag AL, Ramchandani PG, Hove O, Assmus J, Eberhard-Gran M, Biringer E. Paternal mental health and socioemotional and behavioural development in their children. Ped. 2013;131:1–7. doi: 10.1542/peds.2012-0804. [DOI] [PubMed] [Google Scholar]
- 62.Sweeney S, MacBeth A. The effects of paternal depression on child and adolescent outcomes: a systematic review. J Aff Dis. 2016;205:44–59. doi: 10.1016/j.jad.2016.05.073. [DOI] [PubMed] [Google Scholar]
- 63.Maycock B, Binns CW, Dhaliwal S, Tohotoa J, Hauck Y, Burns S, Howat P. Education and support for fathers improves breastfeeding rates: a randomized controlled trial. J Hum Lac. 2013;29:484–490. doi: 10.1177/0890334413484387. [DOI] [PubMed] [Google Scholar]
- 64.Pisacane A, Continisio GI, Aldinucci M, D'Amora S, Continisio P. A controlled trial of the father's role in breastfeeding promotion. Ped. 2005;116:494–498. doi: 10.1542/peds.2005-0479. [DOI] [PubMed] [Google Scholar]
- 65.Goodman SH, Rouse MH, Connell AM, Robbins Broth M, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- 66.Missler MA, Beijers R, Denissen JJA, Van Straten A. Effectiveness of a psycho-educational intervention to prevent postpartum parental distress and enhance infant well-being: study protocol of a randomized controlled trial. Trials. 2018;19:4. doi: 10.1186/s13063-017-2348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hiscock HA, Cook F, Bayer J, Le H, Mensah F, Cann W, Symon B, St James-Roberts I. Preventing early infant sleep and crying problems and postnatal depression: a randomized trial. Pediatrics. 2014;133:346–354. doi: 10.1542/peds.2013-1886. [DOI] [PubMed] [Google Scholar]
- 68.Feldman R, Eidelman AI, Rotenberg N. Parenting stress, infant emotion regulation, maternal sensitivity, and the cognitive development of triplets: a model for parent and child influences in a unique ecology. Child Dev. 2004;75:1774–1791. doi: 10.1111/j.1467-8624.2004.00816.x. [DOI] [PubMed] [Google Scholar]
- 69.Hechler C, Beijers R, Riksen-Walraven M, De Weerth C. Prenatal predictors of postnatal quality of caregiving behavior in mothers and fathers. Parenting. 2019;19:101–119. doi: 10.1080/15295192.2019.1556010. [DOI] [Google Scholar]
- 70.Panter-Brick C, Burgess A, Eggerman M, McAllister F, Pruett K, Leckman JF. Practitioner review: engaging fathers – recommendations for a game change in parenting interventions based on a systematic review of the global evidence. J Child Psychol Psychiatr. 2014;55:1187–1212. doi: 10.1111/jcpp.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abidin R. Parenting stress index: manual. Charlottesville: Pediatric Psychology Press; 1983. [Google Scholar]
- 72.De Brock AJLL, Vermulst AA, Gerris JRM, Abidin RR. Nijmeegse ouderlijke stress index: meetinstrument voor de vaststelling van stress bij opvoeders. Lisse: Swets & Zeitlinger; 1992. [Google Scholar]
- 73.Missler MA, Van der Laan G, Stroebe MS. The work-home interface: The role of home-based predictors of burnout among mothers. Fam Sci. 4:148–60. 10.1080/19424620.2013.871740.
- 74.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Brit J Psych. 1987;150:782–790. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 75.Pop VJ, Komproe IH, Van Son MJ. Characteristics of the Edinburgh postnatal depression scale in the Netherlands. J Aff Dis. 1992;26:105–110. doi: 10.1016/0165-0327(92)90041-4. [DOI] [PubMed] [Google Scholar]
- 76.Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen H, Bunevicius R, Van Baar A, Pop V. Validation of the Edinburgh Depression Scale during pregnancy. J Psych Res. 2011;70:385–389. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 77.Matthey S, Henshaw C, Elliott S, Barnett B. Variability in use of cut-off scores and formats on the Edinburgh postnatal depression scale – implications for clinical and research practice. Arch Womens Ment Health. 2006;9:309–315. doi: 10.1007/s00737-006-0152-x. [DOI] [PubMed] [Google Scholar]
- 78.Leung BMY, Letourneau NL, Giesbrecht GF, Ntanda H, Heart M. Predictors of postpartum depression in partnered mothers and fathers from a longitudinal cohort. Community Ment Health J. 2017;53:420–431. doi: 10.1007/s10597-016-0060-0. [DOI] [PubMed] [Google Scholar]
- 79.Olssøn I, Mykletun A, Dahl AA. The hospital anxiety and depression rating scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psych. 2005;5. 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed]
- 80.Spinhoven PH, Ormel J, Sloekers PPA, Kempen GIJM, Speckens AEM, Van Hemert AM. A validation study of the hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psych Med. 1997;27:363–370. doi: 10.1017/S0033291796004382. [DOI] [PubMed] [Google Scholar]
- 81.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale: an updated literature review. J of Psych Res. 2002;52:69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 82.Loyd BH, Abidin RR. Revision of the parenting stress index. J Ped Psych. 1985;10:169–177. doi: 10.1093/jpepsy/10.2.169. [DOI] [PubMed] [Google Scholar]
- 83.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatric Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 84.Cook F, Bayer J, Le HND, Mensah F, Cann W, Hiscock H. Baby business: a randomized controlled trial of a universal parenting program that aims to prevent early infant sleep and cry problems and associated parental depression. BMC Ped. 2012;13. 10.1186/1471-2431-12-13. [DOI] [PMC free article] [PubMed]
- 85.Condon JT, Corkindale CJ. The assessment of parent-to-infant attachment: development of a self-report questionnaire instrument. J Rep Inf Psych. 1998;16:57–76. doi: 10.1080/02646839808404558. [DOI] [Google Scholar]
- 86.Van Bussel JCH, Spitz B, Demyttenaere K. Three self-report questionnaires of the early mother-to-infant bond: reliability and validity of the Dutch version of the MPAS, PBQ and MIBS. Arch Womens Ment Health. 2010;13:373–384. doi: 10.1007/s00737-009-0140-z. [DOI] [PubMed] [Google Scholar]
- 87.Edelstein RS, Weede AK, Shaver PR, Schaaf JM, Quas JA, Lovas GS, Goodman GS. Adult attachment style and parental responsiveness during a stressful event. Attach & Hum Dev. 2004;6:31–52. doi: 10.1080/146167303100001659584. [DOI] [PubMed] [Google Scholar]
- 88.Jones JD, Cassidy J, Shaver PR. Parents’ self-reported attachment styles: a review of links with parenting behaviors, emotions, and cognitions. Pers Soc Psych Rev. 2015;19:44–76. doi: 10.1177/1088868314541858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lafontaine MF, Brassard A, Lussier Y, Valois P, Shaver PR, Johnson SM. Selecting the best items for a short-form of the experiences in close relationships questionnaire. Eur J Psychol Assess. 2006;32:140–154. doi: 10.1027/1015-5759/a000243. [DOI] [Google Scholar]
- 90.Conradi HJ, Gerlsma C, Van Duijn M, De Jonge P. Internal and external validity of the experiences in close relationships questionnaire in an American and two Dutch samples. Eur J Psych. 2006;20:258–269. [Google Scholar]
- 91.Brennan KA, Clark C, Shaver PR. Self-report measurement of adult attachment: an integrative overview. In: Simpson JA, Rholes WS, editors. Attachment and close relationships. New York: Guilford Press; 1998. pp. 46–76. [Google Scholar]
- 92.Bartholomew K, Horowitz LM. Attachment styles among young adults: a test of a four category model. J Pers Soc Psych. 1991;61:226–244. doi: 10.1037/0022-3514.61.2.226. [DOI] [PubMed] [Google Scholar]
- 93.Rusbult CE, Martz JM, Agnew CR. The investment model scale: measuring commitment level, satisfaction level, quality of alternatives, and investment size. Pers Rel. 1998;5:357–391. doi: 10.1111/j.1475-6811.1998.tb00177.x. [DOI] [Google Scholar]
- 94.Montgomery AJ, Peeters MCW, Schaufeli WB, Panagopoulou EP. Cross-over and work-home interference. Irish J Psych. 2008;29:61–76. doi: 10.1080/03033910.2008.10446274. [DOI] [Google Scholar]
- 95.Tabachnik BG, Fidell LS. Multilevel linear modeling. In: Hartman S, editor. Using multivariate statistics. Boston, MA: Pearson Education Inc; 2007. pp. 781–857. [Google Scholar]
- 96.Barr RG. The normal crying curve: what do we really know? Dev Med Child Neuro. 1990;32:356–362. doi: 10.1111/j.1469-8749.1990.tb16949.x. [DOI] [PubMed] [Google Scholar]
- 97.Missler MA, Donker T, Beijers R, Ciharova M, Moyse C, De Vries R, Denissen J, Van Straten A. (Submitted). Preventing postpartum maternal distress: a systematic review and meta-analysis of psychological interventions. [DOI] [PMC free article] [PubMed]
- 98.Morsbach SK, Prinz RJ. Understanding and improving the validity of self-report of parenting. Clin Child Fam Psych Rev. 2006;9:1–21. doi: 10.1007/s10567-006-0001-5. [DOI] [PubMed] [Google Scholar]
- 99.Barr RG, Kramer MS, Boisjoly C, McVey-White L, Pless IB. Parental diary of infant cry and fuss behavior. Arch Dis Child. 1988;63:380–387. doi: 10.1136/adc.63.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tissera H, Auger E, Séguin L, Kramer MS, Lydon JE. Happy prenatal relationships, healthy postpartum mothers: a prospective study of relationship satisfaction, postpartum stress, and health. Psych Health. 2020. 10.1080/08870446.2020.1766040. [DOI] [PubMed]
- 101.Gay CL, Lee KA, Lee S. Sleep patterns and fatigue in new mothers and fathers. Bio Res Nurs. 2004;5:311–318. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haga SM, Drozd F, Lisoy C, Wentzel-Larsen T, Slining K. Mamma Mia – a randomized controlled trial of an internet-based intervention for perinatal depression. Psych Med. 2019;49:1850–1858. doi: 10.1017/S0033291718002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Statistics Netherlands . The Netherlands leads Europe in internet access. 2018. [Google Scholar]
- 104.Kontos E, Blake KD, Chou WYS, Prestin A. Predictors of eHealth usage: Insights on the digital divide from the health information National Trends Survey 2012. J Med Internet Res. 2014;16. 10.2196/jmir.3117. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.