Abstract
Patient: Male, 40-year-old
Final Diagnosis: Central retinal vein occlusion
Symptoms: Blurring of vision • leg pain
Medication: —
Clinical Procedure: —
Specialty: General and Internal Medicine
Objective:
Rare co-existance of disease or pathology
Background:
COVID-19 is the disease caused by the novel virus, severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). The spectrum of disease seen in patients with COVID-19 infection ranges from asymptomatic or mild symptoms to severe pneumonia and even acute respiratory distress syndrome, which often requires invasive ventilation and intensive care. COVID-19-associated infection can be catastrophic, leading to both arterial and venous occlusion, microinfarcts, and multiorgan failure, although retinal vein occlusion has not yet been reported.
Case Report:
We present the case of a 40-year-old man who presented with a 3-day history of shortness of breath, cough, and fever. He also reported right calf pain and blurring of vision in both eyes. His medical history included hypertension and morbid obesity. The patient was found to have severe COVID-19 pneumonia on high-resolution computed tomography of the chest, right leg deep venous thrombosis on Doppler ultrasonography, and bilateral central retinal vein occlusion (RVO) on fundal examination. He was started on full-dose anticoagulation and discharged on rivaroxaban for 3 months. After 2 weeks of therapy, he had fully recovered from his COVID-19 symptoms and had near-normal vision.
Conclusions:
COVID-19 infection can cause RVO. Early full-dose anticoagulation should be considered in high-risk patients with severe COVID-19 infection. Ophthalmologists and other clinicians should have a high index of suspicion for RVO in patients with COVID-19 infection who presenting with blurred vision and severe pneumonia.
MeSH Keywords: COVID-19, Retinal Vein Occlusion
Background
Severe acute respiratory syndrome Coronavirus 2 (SARSCoV-2) is a novel, single-stranded RNA virus that causes SARS and Coronavirus disease 2019 (COVID-19). The pandemic that started in Wuhan, China, in December 2019 has since spread across the world, affecting more than 32 million people as of September 2020 [1].
There have been several reports of eye redness and irritation in patients with COVID-19, both anecdotal and published, suggesting that conjunctivitis may be a primary ocular manifestation in up to one-third of individuals with the infection [2]. Similarly, a recent report suggested that patients recovering from COVID-19 had subtle cotton wool spots and microhemorrhages on fundal examination [3].
We report the case of a 40-year-old man who presented with severe COVID-19 pneumonia, deep venous thrombosis (DVT) extending from the middle of the superficial right femoral vein down to the right distal peroneal vein, and bilateral central retinal vein occlusion (CRVO).
Case Report
A 40-year-old man presented with a 3-day history of shortness of breath, fever, cough, and pain in his right calf. He recently had been in contact with someone who tested positive for COVID-19. The patient’s medical history included controlled hypertension and morbid obesity. He was a nonsmoker with no family history of malignancy or thromboembolic disease. He had no history of recent travel or illness.
On examination, the patient’s heart rate was 106/min, blood pressure 132/77, respiratory rate 28/min, temperature 37°C, and oxygen saturation 97% on 4 L/min nasal cannula. His chest was clear. He had a swollen and tender right leg. He had elevated inflammatory markers, including ferritin (1518 µg/L), lactate dehydrogenase (402 IU/L), D-dimer (>20 µcg/mL), C-reactive protein (68 mg/L), and interlekin-6 (87.1 pg/mL) but his procalcitonin level, renal function, and complete blood count all were normal.
Infection with SARS-CoV-2 was confirmed based on testing of a nasopharyngeal swab with the U-TOP COVID-19 Detection Kit (Seasun Biomaterials Inc., Daejeon, Korea), which is a reverse transcriptase-polymerase chain reaction (RT-PCR) assay that has received Emergency Use Authorization from the US Food and Drug Administration. RT-PCR testing was performed using Clinical Laboratory Improvement Amendments diagnostic standards according to current testing guidelines [4].
A high-resolution computed tomography scan of the patient’s chest showed features of severe COVID-19 pneumonia (Figure 1). Doppler ultrasonography of his right leg showed DVT extending from the middle of the superficial right femoral vein down to the right distal peroneal vein. Echocardiography showed severe dilation of the right ventricle with raised right ventricle pressure and volume overload, consistent with features of right heart strain, with a possible diagnosis of pulmonary embolism in the context of DVT and COVID-19. As a result, the patient was started on a therapeutic dose of low-molecular-weight heparin (LMWH). On Day 2 of admission, the patient complained of painless blurred vision in both eyes, which was worse in the left eye. He had no focal neurological signs. A magnetic resonance image of the brain/orbit ruled out any central ischemic vascular event secondary to COVID-19 infection or orbital pathology.
Figure 1.
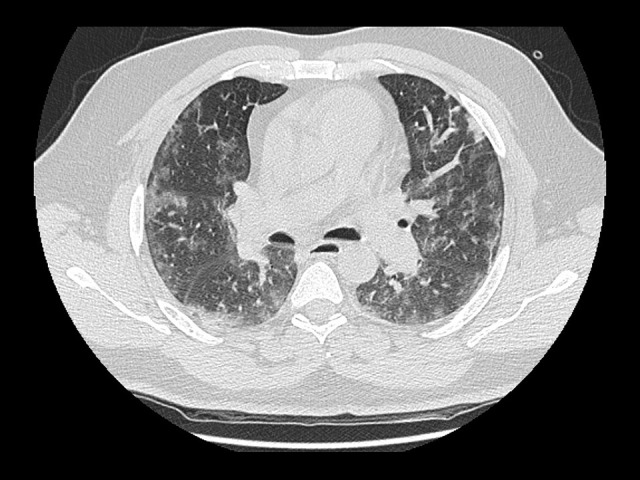
High-resolution computed tomography scan of the chest showing bilateral ground-glass opacities associated with severe COVID-19 pneumonia.
An evaluation by the Ophthalmology team at bedside revealed the patient’s visual acuity to be 6/9 in the right eye and 6/18 in the left eye. No fundal imaging was available at this stage, due to COVID-19 restrictions, but indirect fundal examination at the bedside showed bilateral dilated and tortuous veins, widespread cotton wool spots, dot and blot intraretinal hemorrhages, and optic disk edema. These findings were more pronounced in the left fundus. Based on the indirect ophthalmos-copy, a diagnosis of bilateral CRVO was made.
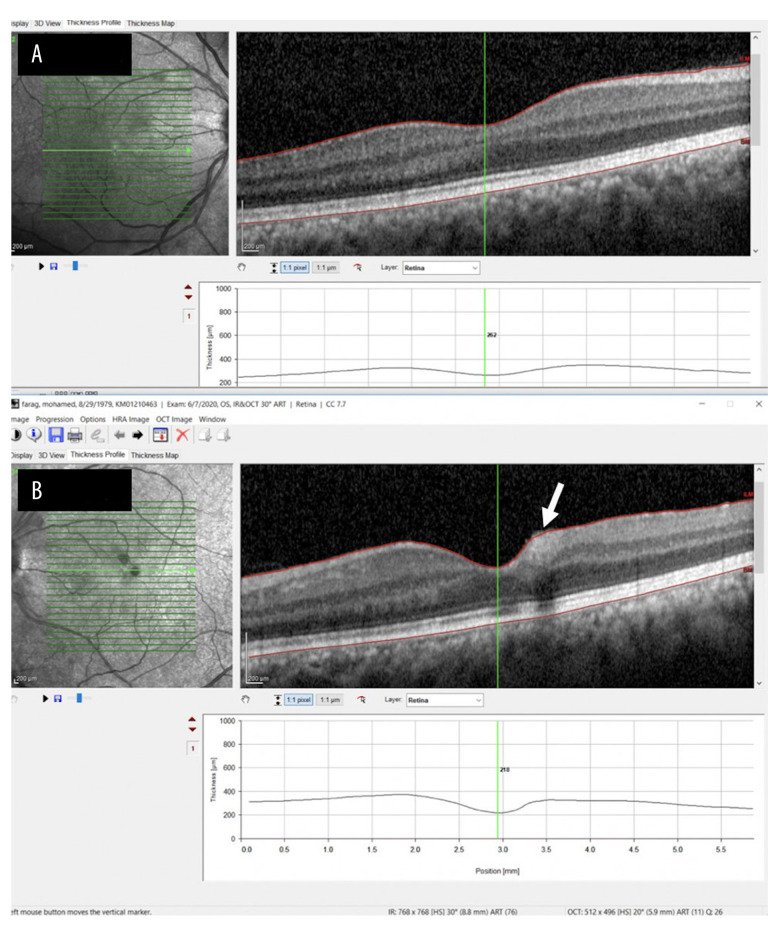
By the following week, as the patient’s general condition improved and he was off oxygen, slit-lamp examination revealed a normal anterior segment. Fundal retinal examination of both eyes revealed improvement in retinal findings compared with the initial bedside findings (Figure 2). Furthermore, there were 2 parafoveal hemorrhages in the left eye on optical coherence tomography (OCT) (Figure 3), which explained why the patient’s vision was worse in that eye. OCT examination of the fundus did not reveal clinically significant macular edema. Because there was also no improvement in the patient’s visual acuity (right eye 6/6, left eye 6/12), it was decided to observe him. Fluorescein angiography is required to assess ischemic or nonischemic areas in retinal vein occlusion, but it was not performed in our patient. Our clinical diagnosis – based on clinical fundus examination, OCT, symptoms and course of the disease – was bilateral CRVO.
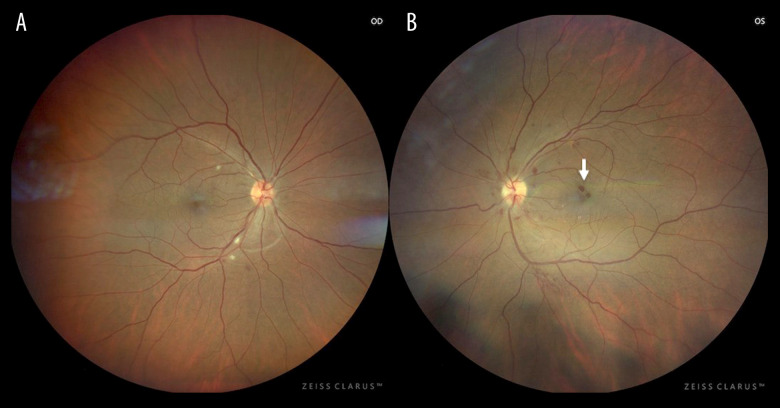
Figure 2.
Fundal images. (A) In the right eye, there are multiple cotton wool spots surround the peripapillary area and minimal intraretinal hemorrhages in the inferior arcade. (B) In the left eye, there are multiple intraretinal hemorrhages affecting the peripapillary area and macula and a minimal increase in tortuosity of the vessels.
Figure 3.
Optical coherence tomography scans. (A) In the right eye, the anatomy is normal. (B) In the left eye, a parafoveal intraretinal hemorrhage is visible but there is no clinically significant macular edema.
The patient’s condition improved during admission and the LMWH was switched to rivaroxaban, 15 mg twice daily for 21 days, then 20 mg once daily for 3 months on discharge. His thromophilia workup was normal, including testing for anti-thrombin III, protein C, protein S, lupus anticoagulant, cardiolipin antibodies, HIV, hepatitis B and C, syphilis, and B2 glycoprotein. Ten days after hospital admission, the patient had 2 consecutive negative COVID-19 nasopharyngeal PCR swab results. He was discharged home, fully recovered from his COVID-19 symptoms and had near-normal vision, and was followed up as an outpatient by the Internal Medicine and Ophthalmology teams. However, because the patient did not come to our follow-up clinic, we were unable to do the necessary follow-up tests.
Our final diagnosis was bilateral CRVO associated with DVT, right heart strain on echocardiography, hypertension, and severe COVID-19 infection.
Discussion
RVO is defined as a retinal vascular disorder characterized clinically by dilatation, engorgement, and tortuosity of the retinal blood vessels. If it is not relieved, the result is intra-retinal bleeding, macular edema, development of ischemic retinal areas, optic disc swelling, and reduced visual acuity [5]. Hypertension, diabetes, vasculitis, and high cholesterol levels are the factors most commonly associated with vascular diseases such as retinal occlusion. A number of studies have reported that increased blood viscosity, factor V Leiden mutation, thrombophilia, and protein C or S deficiency may play an important role in the etiology of CRVO in younger patients [6,7]. Our patient was at risk because of his hypertension, obesity and right ventricular dilatation, but his thrombophilia screening was negative.
COVID-19 infection can cause a hyperinflammatory state and has been reported to increase the incidence of venous thromboembolism (VTE). Although the pathogenesis is not entirely understood, there may be many elements that contribute to the acute inflammatory response in severe COVID-19 infection. To our knowledge, this is the first case of PCR-confirmed COVID-19 infection presenting with RVO. As per our hospital protocol, all of our patients who test positive for COVID-19 are started on anticoagulation prophylaxis or treatment at a dose dependent upon D-dimers, weight, and thromboembolic risk. We assume that the early initiation of anticoagulation halted and reversed the progression of bilateral CRVO in the patient described here. This was evident in the improvement in his visual acuity and the resolution of vascular changes in both retinas.
Similarly, Wu et al. reported that ocular involvement in COVID-19 is more likely to be associated with severe pneumonia, compared with the presentation in patients with milder symptoms and normal test results [2]. However, Marinho et al. reported retinal and OCT findings in 12 adults, the majority of whom were healthcare workers; only 2 were admitted to the hospital but they did not require intensive care. All of the patients showed hyperreflective lesions at the level of the ganglion cell and inner plexiform layers, more prominently in the papillomacular junction. The results of OCT-angiography studies appeared normal, and 4 of the patients presented with subtle cotton wool spots and microhemorrhages along the retinal arcade [3]. The clinical picture in these patients was not similar to that in the present case, but the report does show that COVID-19 can predispose patients to retinal vascular signs.
Furthermore, a report by Loon et al. suggests that SAR-CoV RNA is present in tears [8]. However, neither the pathogenesis nor the existence of SARS-CoV in ocular tissue has been confirmed. Seah et al. reported that ocular manifestations in animal models are linked to ACE2 receptors, which are present in the aqueous humor and may lead to ocular manifestations of the virus: conjunctivitis, uveitis, choroiditis with retinal detachment, and retinal vasculitis [9].
The cytokine cascade seen in COVID-19 is a consequence of the inflammatory response to severe infection and also due to the direct effect of SARS-CoV-2 on the endothelium. The inflammatory response and the virus’s direct effect contribute to the severity of illness, complications such as VTE, and mortality associated with COVID-19.
The severe COVID-19 infection in our patient, as evidenced by the raised inflammatory markers, would have triggered a hyperinflammatory response and cytokine cascade, which would explain the occurrence of bilateral CRVO. He also had other predisposing risk factors, including hypertension, morbid obesity, and right ventricular dilatation, but no evidence of hypercoagulopathy.
Conclusions
COVID-19 has varied presentations with hyperinflammatory response. It can cause both arterial and venous occlusion, including in unusual sites, such as the retinal veins. Early full-dose anticoagulation should be considered in high-risk patients with severe COVID-19 infection. Ophthalmologists and other clinicians should have a high index of suspicion for RVO in patients with COVID-19 who present with blurred vision and severe pneumonia. Our patient had hypertension as a risk factor, although it is uncommon in young patients, and elevation in inflammatory markers. We concluded that in him, COVID-19 pneumonia combined with hypertension and morbid obesity led to an inflammatory state that resulted in bilateral CRVO.
Footnotes
Conflicts of interest
None
References:
- 1.World Health Organization Coronavirus Disease Dashboard (COVID-19) https://covid19.who.int/
- 2.Wu P, Duan F, Luo C. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei province, China. JAMA Ophthalmol. 2020;138(5):575–78. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinho P, Marcos A, Romano A, et al. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organisation (WHO) Use of laboratory methods for SARS diagnosis. 2020. https://who.int/csr/sars/labmethods/en/
- 5.Coscas G, Loewenstein A, Augustin A, et al. Management of retinal vein occlusion – consensus document. Ophthalmologica. 2011;226:4–28. doi: 10.1159/000327391. [DOI] [PubMed] [Google Scholar]
- 6.Fisus A, Pop D, Rusu M, et al. Nonishcemic central retinal vein occlusion associated with hereditary thrombophilia. Rom J Ophthalmol. 2015;59(3):172–76. [PMC free article] [PubMed] [Google Scholar]
- 7.Risk factors for central retinal vein occlusion. Arch Ophthalmol. 1996;114(5):545–54. [PubMed] [Google Scholar]
- 8.Loon S, Teoh S, Oon L, et al. The severe acute respiratory syndrome coronavirus in tears. Br J Ophthalmol. 2004;88(7):861–63. doi: 10.1136/bjo.2003.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391–95. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]