Abstract
Patient: Male, 3-day-old
Final Diagnosis: Neonatal leukemia
Symptoms: Skin lesions
Medication: —
Clinical Procedure: Biopsy • stem cell transplant
Specialty: Hematology • Pediatrics and Neonatology
Objective:
Rare disease
Background:
Neonatal acute leukemia is a rare condition. Little is known about its incidence and outcomes, and treatment options have not been standardized.
Case Report:
A 3-day old, apparently healthy male newborn was referred to the pediatric intensive care unit with multiple violaceous macules and a few papules on his face and upper trunk. After initial spontaneous regression, the lesions reappeared. Skin biopsy and bone marrow aspirate revealed a diagnosis of acute lymphoblastic leukemia (ALL). ALL induction therapy was initiated on day 24, resulting in morphological remission at the end of induction therapy. ALL chemotherapy was guided by sequential PCR-based monitoring of minimal residual disease (MRD). The patient received a transplant from an unrelated HLA high-resolution matched (10/10 loci) permissive donor. He was followed-up after transplant conducted by sequential PCR-based measurements of MRD in bone marrow.
Conclusions:
Neonatal leukemia often presents as congenital skin lesions known as blueberry muffin rash. ALL induction therapy was started at the end of the neonatal period. Treatment was well-tolerated and effective. Early donor search and PCR-MRD guided treatment surveillance can help to achieve and maintain molecular remission.
MeSH Keywords: Exanthema; Neoplasm, Residual; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Term Birth; Transplantation, Homologous; Unrelated Donors
Background
Neonatal leukemia, also called congenital leukemia, is a rare event, accounting for fewer than 1% of all childhood leukemias [1–3]. Neonatal leukemia is characterized clinically by cutaneous infiltrates, along with hepatomegaly and splenomegaly, whereas lymphadenopathy is rare [1]. This unusual clinical presentation results in diagnostic difficulty. Skin involvement typically presents with violaceous or blue-grey, nonblanchable papules and nodules, especially on the head, neck, and trunk, a cutaneous pattern called blueberry muffin baby [4,5]. This cutaneous phenotype, however, is non-specific and is also observed in neonates with congenital infections [6]. Moreover, this cutaneous pattern may be caused by dermal erythropoiesis in infants with hemolytic diseases of the newborn, or by neoplastic infiltrations, as in neonates with disseminated neuroblastoma, rhabdomyosarcoma or Langerhans cell histiocytosis [1, 5].
To add to the complexity of the diagnostic process, bone marrow biopsies may initially be non-diagnostic; furthermore, secondary progression or spontaneous regression may occur [7]. Indications for treatment are also unclear [8], especially as the phenotypes of neonatal leukemias vary. Although acute myeloid leukemia (AML) is most common, neonatal acute lymphoblastic leukemia (ALL) and mixed phenotype leukemia (MPAL) have also been observed [1]. Due to the high risk of disease relapse and treatment-related complications, the overall prognosis is guarded [9,10]. Recent analyses of children younger than 12 months with ALL found that the presence of a KMT2A/MLL rearrangement, initial white blood count at diagnosis, and response to prednisone treatment were the strongest predictors of patient outcome [11,12].
The current case report describes a patient with neonatal ALL and a t(9;11) translocation (KMT2A/MLL rearrangement), who was successfully treated with systemic multi-agent chemotherapy and allogeneic stem cell transplantation from an unrelated donor and was followed-up during the post-transplant period by PCR-based measurement of minimal residual disease (MRD) in sequential bone marrow samples. The diagnostic workup as well as the basic treatment strategies may be instructive in the diagnosis and treatment of future patients with neonatal ALL.
Case Report
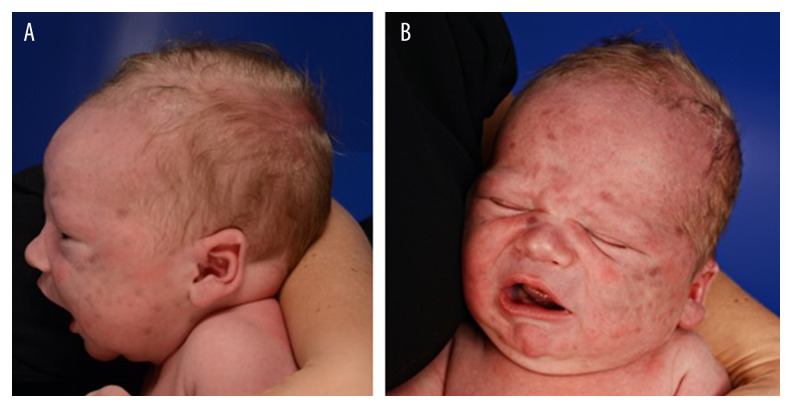
A 3-day old male newborn, spontaneously delivered after 41 weeks of gestation at a birth weight of 4390 g and with Apgar scores of 9/10/10, was referred to the neonatal intensive care unit (NICU) for evaluation of multiple cutaneous skin lesions on his face and right shoulder. The pregnancy had been uneventful, and the patient’s family history was unremarkable for both dermatological and hematological diseases. A clinical examination upon admission to the NICU showed multiple, purple-toviolaceous, nonblanchable macules several millimeters in size on his face, upper back and right shoulder; only a few lesions were slightly elevated (Figure 1A, 1B). A complete blood count revealed 13 980 leucocytes/µL, hemoglobin 16.0 g/dL, hematocrit 54%, and platelets 256 000/µL. A peripheral blood smear showed 30% neutrophils, 57% lymphocytes, 9% monocytes, and few pro- and metamyelocytes, but no leukemic blasts. His reticulocyte count was 38‰, corresponding to 185 000/µL. He was negative for neonatal sepsis. Transcranial and abdominal ultra-sound yielded unremarkable results, and his liver and spleen were normal in morphology and size. Ophthalmological examination including fundoscopy showed neither chorioretinitis nor hematopoietic foci. Parvovirus-B19-PCR (blood) and CMV-PCR (urine) were negative, as were the results of a Coombs test and red blood cell membrane and enzyme assays.
Figure 1.
(A, B) Skin lesions on post-partum day 3, showing multiple purple-to-violaceous, nonblanchable macules several millimeters in size on the patient’s face, with only a few lesions being slightly elevated.
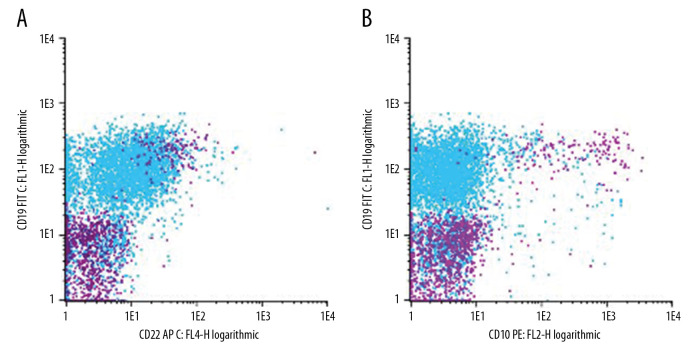
A skin biopsy performed on day 7 of life showed the presence of leukemic blasts (Figure 2A–2C), indicating a diagnosis of neonatal ALL. A bone marrow examination on day 7 did not provide conclusive results, but a peripheral blood smear on day 14 and a bone marrow aspirate on day 16 confirmed the diagnosis of neonatal ALL. Flow cytometry analysis of a peripheral blood sample showed a monomorphic lymphatic blast population with a rare phenotype of CD19 +, CD45low, CD22low, CD79α+, CD10–, CD20–, CD34–, and TdT– (Figure 3). Molecular analysis showed absence of the GATA1 mutation, but confirmed the MLL-AF9 (KMT2A-MLLT3) fusion transcript [t (9;11)] [9]. The patient’s cerebrospinal fluid contained <5 leucocytes/µL, although cytospin revealed leukemic blasts, resulting in a central nervous system status of CNS2a. Strikingly, his skin lesions spontaneously regressed over the next several days, but reappeared on day 13, with dissemination over the entire integument. His leucocyte counts gradually increased, peaking at 125 700/µL on post-partum day 24.
Figure 2.
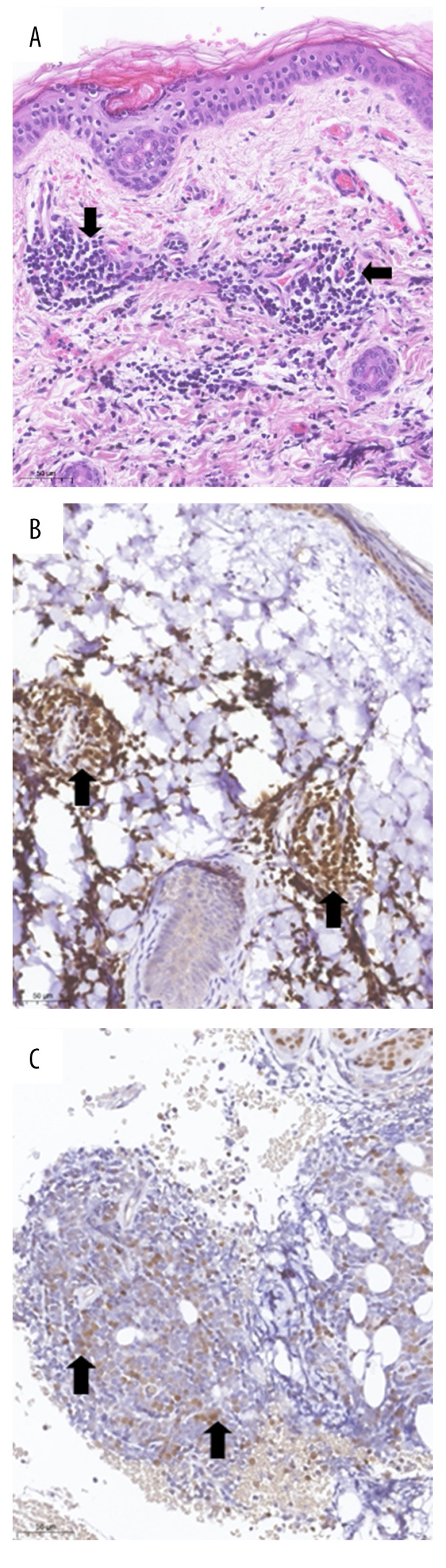
Skin biopsy: (A) Dermal lymphoblastic infiltrate (arrow), H&E stain; (B, C) Immunohistochemistry, showing (B) positive staining of lymphoblasts for the B cell marker PAX5; and (C) weak nuclear expression of TdT by lymphoblasts.
Figure 3.
Flow cytometry of bone marrow: Phenotype overlay of blast cells at the time of diagnosis characterized as CD19+, CD22low, CD10– (A, B; blast population in light blue
 ) and regenerating normal B cell compartment (A, B; normal B cell population in purple
) and regenerating normal B cell compartment (A, B; normal B cell population in purple
 ) just prior to allogeneic stem cell transplantation. Lymphoid cells were gated in a SSC/FSC plot. The phenotype of the blast population was characterized by additional flow cytometric markers as CD45low, CD79α+, CD20–, CD34–, TdT– (data not shown). Results of phenotyping were confirmed by the national ALL reference laboratory.
) just prior to allogeneic stem cell transplantation. Lymphoid cells were gated in a SSC/FSC plot. The phenotype of the blast population was characterized by additional flow cytometric markers as CD45low, CD79α+, CD20–, CD34–, TdT– (data not shown). Results of phenotyping were confirmed by the national ALL reference laboratory.
Based on ALL-BFM protocols and attempting to avoid toxicity during the neonatal period, the patient was started on the prednisone pre-phase of ALL induction therapy on post-partum day 24. Examination on day 8 showed that this patient responded poorly to prednisone, whereas examination of a bone marrow sample on day 15 showed only a moderate response to induction therapy (>10% blasts). Morphological remission was achieved on day 33 at the end of induction therapy, although minimal residual disease persisted. Subsequent ALL therapy was guided by sequential PCR-based MRD monitoring with the aim of achieving a state of <10 (–4) MRD prior to the planned stem cell transplantation from an unrelated HLA high-resolution matched (10/10 loci) permissive donor. The choice of the stem cell donor was based on high-resolution best match (10/10) and HLA-DP permissiveness. Because the patient was very young, the conditioning regimen consisted of fludarabine, thiotepa, melphalan, and ATG. Post-transplant follow-up care was also guided by sequential PCR-based measurements of MRD in bone marrow at 6–8-week intervals. The patient is now 2 years old and is in excellent clinical condition. He has remained MRD-negative for 15 months after transplantation.
Discussion
The first or initiating events in childhood leukemia occur prenatally during fetal development. This evidence comes from 2 sources – identical twin infants or children with concordant ALL [13,14] and retrospective analysis of neonatal blood spots or Guthrie cards [15,16]. According to the two-hit hypothesis, a second postnatal hit is required for ALL to develop later in childhood. Thus, neonatal acute leukemia is an extremely rare event [2].
The clinical features of this patient were somewhat unusual. The cutaneous lesions in this newborn were concentrated on his face and were mainly of a macular nature, in contrast to the papular and nodular morphology of lesions described in many previous patients with neonatal leukemia. Moreover, these lesions regressed spontaneously before recurring and spreading to other parts of the skin, as previously described [1]. Skin involvement preceded the appearance of other clinical signs, such as hepatosplenomegaly, anemia, and petechiae. The differential diagnosis of blueberry muffin lesions includes congenital infections, neonatal hemolytic diseases, and neoplastic-infiltrative processes other than leukemia, possibilities that can be excluded by PCR, red blood cell assays, and histological examination. Diagnosis was confirmed by skin biopsy, peripheral blood smear, and bone marrow aspiration. Workup for neonatal sepsis was negative.
In addition, the leukemic blasts in this patient displayed an aberrant phenotype, consisting of a monomorphic population positive for CD19 and CD79α, determining the lineage commitment, but negative for CD10, CD34, and TdT, as determined by flow cytometry. Molecular analysis excluded transient myeloproliferation and confirmed the presence of the MLL-AF9 (KMT2A-MLLT3) fusion transcript, a finding typical of early infant ALL. Interestingly, although flow cytometry showed that bone marrow samples, assessed in 2 different laboratories, were negative for TdT (data not shown), leukemic blasts in skin biopsy samples were positive for TdT. The reason for this discrepancy is unknown.
Indications of whether and when to treat patients with neonatal ALL remain undetermined [1,2,8]. Based on the clinical course of his symptoms and in an attempt to reduce undue toxicity, the interdisciplinary physician team of neonatologists and pediatric oncologists decided to start the prednisone pre-phase of the ALL-BFM protocol in this patient on day 24 of the neonatal period.
Based on its rarity, the prognosis of patients with neonatal ALL is guarded [9,10]. Treatment of this patient was guided by PCR-based measurements of MRD with the aim to achieve a state of <10 (–4) MRD [17,18] prior to the carefully planned allogeneic stem cell transplantation from an unrelated donor matched at 10/10 loci.
A similar strategy was pursued during the post-transplant period with PCR-based measurement of MRD in sequential bone marrow specimens obtained every 6-8 weeks. Regular monitoring allowed early intervention with bispecific antibodies [19] or conjugated antibodies [20,21] should MRD increase during serial follow-up. The patient has remained MRD-negative and is alive and well at age 2 years.
Conclusions
Neonatal leukemia can be diagnosed early if the warning signs of blueberry muffin lesions are correctly interpreted. Treatment of neonatal ALL in our patient was well-tolerated and effective. Early identification of a donor and PCR-MRD-guided treatment surveillance helped achieve and maintain molecular remission. Treatment of neonatal ALL may be life-saving.
Footnotes
Conflicts of interest
None.
References:
- 1.Roberts I, Fordham NJ, Rao A, Bain BJ. Neonatal leukaemia. Br J Haematol. 2018;182(2):170–84. doi: 10.1111/bjh.15246. [DOI] [PubMed] [Google Scholar]
- 2.Ishii E, Oda M, Kinugawa N, et al. Features and outcome of neonatal leukemia in Japan: experience of the Japan Infant Leukemia Study Group. Pediatr Blood Cancer. 2006;47(3):268–72. doi: 10.1002/pbc.20599. [DOI] [PubMed] [Google Scholar]
- 3.Bader JL, Miller RW. US cancer incidence and mortality in the first year of life. Am J Dis Child. 1979;133(2):157–59. doi: 10.1001/archpedi.1979.02130020047010. [DOI] [PubMed] [Google Scholar]
- 4.Bowden JB, Hebert AA, Rapini RP. Dermal hematopoiesis in neonates: report of five cases. J Am Acad Dermatol. 1989;20(6):1104–10. doi: 10.1016/s0190-9622(89)70141-9. [DOI] [PubMed] [Google Scholar]
- 5.Shown TE, Durfee MF. Blueberry muffin baby: Neonatal neuroblastoma with subcutaneous metastases. J Urol. 1970;104(1):193–95. doi: 10.1016/s0022-5347(17)61698-7. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Latif ME, Sugo E. Images in clinical medicine. Congenital cytomegalovirus infection. N Engl J Med. 2010;362(9):833. doi: 10.1056/NEJMicm0804100. [DOI] [PubMed] [Google Scholar]
- 7.Coenen EA, Zwaan CM, Reinhardt D, et al. Pediatric acute myeloid leukemia with t(8;16)(p11;p13), a distinct clinical and biological entity: A collaborative study by the International-Berlin-Frankfurt-Münster AML-study group. Blood. 2013;122(15):2704–13. doi: 10.1182/blood-2013-02-485524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliver R, Juergens AL, 2nd, Hatch D, et al. Neonatal acute lymphoblastic leukemia. Pediatr Emerg Care. 2020;36(2):e102–3. doi: 10.1097/PEC.0000000000001956. [DOI] [PubMed] [Google Scholar]
- 9.Van der Linden MH, Valsecchi MG, De Lorenzo P, et al. Outcome of congenital acute lymphoblastic leukemia treated on the Interfant-99 protocol. Blood. 2009;114(18):3764–68. doi: 10.1182/blood-2009-02-204214. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Ren Z, Yang J, Yin A. Analysis of 59 cases of congenital leukemia reported between 2001 and 2016. J Int Med Res. 2019;47(10):4625–35. doi: 10.1177/0300060519872899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown P, Pieters R, Biondi A. How I treat infant leukemia. Blood. 2019;133(3):205–14. doi: 10.1182/blood-2018-04-785980. [DOI] [PubMed] [Google Scholar]
- 12.Pieters R, De Lorenzo P, Ancliffe P, et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol; Results from an international phase III randomized study. J Clin Oncol. 2019;37(25):2246–56. doi: 10.1200/JCO.19.00261. [DOI] [PubMed] [Google Scholar]
- 13.Ford AM, Ridge SA, Cabrera ME, et al. In utero rearrangements in the trithorax-related oncogene in infant leukaemias. Nature. 1993;363(6427):358–60. doi: 10.1038/363358a0. [DOI] [PubMed] [Google Scholar]
- 14.Wiemels JL, Ford AM, Van Wering ER, et al. Protracted and variable latency of acute lymphoblastic leukemia after TEL AML1gene fusion in utero. Blood. 1999;94(3):1057–62. [PubMed] [Google Scholar]
- 15.Gale KB, Ford AM, Repp R, et al. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci USA. 1997;94(25):13950–54. doi: 10.1073/pnas.94.25.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiemels JL, Cazzaniga G, Daniotti M, et al. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354(9189):1499–503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 17.Bader P, Kreyenberg H, Henze GHR, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: The ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–84. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 18.Bader P, Salzmann-Manrique E, Balduzzi A, et al. More precisely defining risk peri-HCT in pediatric ALL: Pre- vs. post-MRD measures, serial positivity, and risk modeling. Blood Adv. 2019;3(21):3393–405. doi: 10.1182/bloodadvances.2019000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–89. doi: 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 20.Bhojwani D, Sposto R, Shah NN, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia. 2019;33(4):884–92. doi: 10.1038/s41375-018-0265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafei H, Kantarjian HM, Jabbour EJ. Targeted therapy paves the way for the cure of acute lymphoblastic leukaemia. Br J Haematol. 2020;188(02):207–23. doi: 10.1111/bjh.16207. [DOI] [PubMed] [Google Scholar]