Abstract
Cardiovascular disease is a competing cause of death in patients with cancer with early-stage disease. This elevated cardiovascular disease risk is thought to derive from both the direct effects of cancer therapies and the accumulation of risk factors such as hypertension, weight gain, cigarette smoking, and loss of cardiorespiratory fitness. Effective and viable strategies are needed to mitigate cardiovascular disease risk in this population; a multimodal model such as cardiac rehabilitation may be a potential solution. This statement from the American Heart Association provides an overview of the existing knowledge and rationale for the use of cardiac rehabilitation to provide structured exercise and ancillary services to cancer patients and survivors. This document introduces the concept of cardio-oncology rehabilitation, which includes identification of patients with cancer at high risk for cardiac dysfunction and a description of the cardiac rehabilitation infrastructure needed to address the unique exposures and complications related to cancer care. In this statement, we also discuss the need for future research to fully implement a multimodal model of cardiac rehabilitation for patients with cancer and to determine whether reimbursement of these services is clinically warranted.
Keywords: AHA Scientific Statements, cancer, cardiac rehabilitation, cardiovascular diseases
Advances in early detection and treatment have significantly improved the 5-year disease-specific survival rates for the 10 most common malignancies.1 As a result, there are >16.7 million cancer survivors in the United States today.1–4 Many of these individuals are at increased risk of morbidity and mortality from noncancer causes, predominantly cardiovascular disease (CVD). Specifically, cancer survivors living at least 5 years beyond diagnosis have a 1.3- to 3.6-fold increased risk of cardiovascular-specific mortality and a 1.7- to 18.5-fold increased incidence of CVD risk factors such as hypertension, diabetes mellitus, and dyslipidemia compared with age-matched counterparts with no cancer history.5,6 The elevated risk of CVD in cancer survivors is likely the result of normal age-related pathologies coupled with the direct (eg, radiation, chemotherapy, targeted therapy) and indirect (eg, deconditioning, weight gain)7 effects of cancer therapy that extend across multiple systems (ie, whole-organism cardiovascular toxicity).8 CVD is likely to become even more pervasive in the oncology setting as a result of continued improvements in cancer-specific mortality in conjunction with the rapidly aging population.9
Effective and viable strategies are needed to mitigate CVD risk in patients with cancer. The use of a delivery model similar to that used in cardiac rehabilitation (CR) programs may provide a potential solution for selected patients. CR has been defined as “the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk factor modification, and education, counseling, and behavioral interventions.”10 The objectives of contemporary CR are to increase functional capacity (cardiorespiratory fitness [CRF]), to decrease anginal symptoms, to facilitate cardiovascular risk reduction, to improve psychosocial well-being, and to reduce recurrent hospitalizations and the associated morbidity/mortality of CVD.10a Meta-analyses demonstrate that CR reduces CVD mortality and hospital admissions and improves health-related quality of life in patients with coronary heart disease.11 Referral to a comprehensive CR program is a Class I American Heart Association (AHA)/American College of Cardiology (ACC) Foundation guideline recommendation for patients with acute coronary syndromes12 and is a clinical performance measure.13
In this statement, we propose the development of a comprehensive model (ie, cardio-oncology rehabilitation [CORE]) to identify patients at high risk of CVD including cardiotoxicity related to cancer therapies14 and use the multimodality approach of CR (eg, exercise plus nutritional counseling and cardiovascular risk factor assessment) to prevent or mitigate cardiovascular events. Overall, this scientific statement is designed to present the rationale for multimodal CR in patients with cancer, to provide guidance for CR personnel on the specific CR needs of cancer survivors, and to highlight knowledge gaps and propose steps to facilitate the development and integration of CORE as an aspect of standard of care for cancer patients and survivors at high risk for CVD.
RATIONALE FOR IMPLEMENTATION OF A MULTIMODAL CR FOR PATIENTS WITH CANCER
Rationale for Structured Exercise Training in CR for Patients With Cancer
Traditionally, cardiovascular toxicity in cancer survivors has focused predominantly on the detection and management of cardiocentric dysfunction (eg, declines in left ventricular ejection fraction), which predisposes to the development of overt heart failure. The direct and indirect adverse consequences of anticancer therapeutics, however, extend beyond the heart to affect the entire cardiovascular-skeletal muscle axis. Indeed, CRF, an integrative assessment of global cardiovascular function, declines during exposure to various systemic combination regimens and may not recover after treatment cessation.15–17 For example, patients with breast cancer who are 40 to 50 years of age have a mean CRF level that is 30% to 32% lower than that of age-matched healthy, sedentary control subjects.15 Low CRF has also been demonstrated in other populations such as young adult cancer survivors18,19 and women with a history of gynecological cancers.16
Exercise training is the cornerstone of contemporary CR and is an established therapy to improve CRF, leading to reductions in cardiovascular morbidity and its attendant symptoms in those with existing CVD. Although less well established, a growing body of work evaluates the efficacy of structured exercise therapy on cardiovascular outcomes in patients with cancer.20 A comprehensive recent review of this literature is available20,21 and summarized in Table 1.
Table 1.
Studies of Exercise on Clinical and CVD Outcomes in Patients With Cancer in the Adjuvant and Postadjuvant Setting
| Setting | Clinical Outcomes | Cardiovascular Outcomes |
|---|---|---|
| Adjuvant | ||
| Breast | ↓ CVD events22 | ↑23 ↔24 ↓25 CRF |
| ↓ CAD mortality22 | ↓ LVEF26 | |
| Prostate | ↑ CRF27 | |
| Colorectal | ↑ CRF | |
| Mixed (meta-analysis) | ↑ CRF21 | |
| Postadjuvant | ||
| Breast | ↓ CVD events28 | ↔ ↑ CRF |
| ↓ All-cause mortality29 | ↑ Vascular function | |
| Prostate | ↑ CRF30 | |
| ↑ Vascular function30 | ||
| ↔ Lipid profile30 | ||
| ↔ Blood pressure30 | ||
| ASCC | ↓ CVD events31 | |
| ↓ All-cause mortality31 | ||
| Testicular | ↑ CRF32 | |
| ↑ Vascular function32 | ||
| ↑ Framingham Risk Score32 | ||
| Colorectal | ↓ All-cause mortality29 | ↑33 ↔34 CRF |
| Leukemia | ↑ CRF35 | |
| Lymphoma | ↑ CRF36 | |
| Mixed (meta-analysis) | ↑ CRF21 | |
Downward-pointing arrow (↓) indicates a decrease; upward-pointing arrow (↑) indicates an increase; and sideways-pointing arrow (↔) indicates no change. ASCC indicates adult survivors of childhood cancer; CAD, coronary artery disease; CRF, cardiorespiratory fitness; CVD, cardiovascular disease; and LVEF, left ventricle ejection fraction.
In brief, randomized trials have examined the efficacy of various exercise prescriptions on CRF in various cancer populations during and after primary adjuvant therapy. In general, current evidence indicates that exercise may attenuate the cancer treatment–induced declines in CRF, although confirmation in larger studies is needed. There is reasonable evidence to support the conclusion that exercise improves CRF after the completion of cancer therapy. For example, in a meta-analysis of 27 randomized clinical trials, exercise training after the completion of adjuvant therapy significantly increased CRF compared with usual care (weighted mean differences, 2.45 mL O2·kg−1·min−1 [95% CI, 1.71–3.19]).21 Overall, current investigation of exercise on CVD outcomes in cancer survivors is limited primarily to CRF, an end point of significant clinical importance because low CRF is associated with a higher incidence of short- and long-term treatment-related toxicities (eg, CVD), higher symptom burden (eg, fatigue), and increased risk of all-cause and cancer-specific mortality in patients with cancer.15,36,37
Feasibility of Exercise Training in CR for Patients With Cancer
A limited but growing number of studies have investigated the feasibility and utility of CR to deliver exercise interventions to cancer patients and survivors (Table 2). For example, Dolan et al40 reported that among 152 breast cancer survivors (177±167 weeks from surgery), aerobic and resistance training exercise performed once weekly in a CR supervised group setting resulted in significant improvements in CRF (P<0.001), quality of life (P<0.001), and fatigue (P<0.001). In another study, 280 cancer survivors (mixed diagnoses; 2.5±3.7 years from diagnosis) participating in 12 weeks of supervised aerobic training and 2 d/wk of resistance training exercises38 had significantly improved 6-minute walk duration (P=0.003), 1-repetition maximum leg press (P<0.01), and arm strength (P<0.01). Collectively, current investigations indicate that CR models are feasible and can improve CRF, muscular strength, and quality of life in cancer survivors. Given the multisystem consequences of cancer therapy resulting in increased risk of morbidity and mortality, there is a strong rationale both to identify survivors at greatest risk and to deliver individualized interventions.
Table 2.
Feasibility and Outcomes of Exercise Training in CR Model for Patients With Cancer
| Author | Sample Size, n | Cancer Type | Stage | Timing | Supervision | Exercise Prescription | Length | Physiological Outcomes | PROs | Adherence, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Dittus et al38 | 280 | 68% Breast, 32% other | NA | 2.4±3.7 y from diagnosis | Supervised by exercise specialist; group setting in CR | 2 times/wk supervised Aerobic: 20 mins 3–4 times/wk 70%–85% PHR progressing to 40 mins 4–5 times/wk Resistance: 60%–70% 1-RM U/L extremity |
12 wk | ↑ 6-min walk ↑ U/L extremity strength |
↑ | 74 |
| Young-McCaughan et al39 | 62 | 22% Breast, 19% prostate, 59% other | Stage 0 or 1=30% Stage 2=39% Stage 3 or 4=29% |
During treatment: 24% Completed therapy: <6 mo, 37%; >6 mo, 39% |
Supervised by exercise specialist; group setting in CR | 2 times/wk supervised exercise Aerobic: walking 3–5 times/wk Resistance: none |
12 wk | ↑ METs | ↑ | 40–85, varied by stage and treatment |
| Dolan et al40 | 152 | Breast cancer survivors | Early stage | 177±167 wk from surgery | Supervised by exercise specialist; group setting in CR | 1 time/wk supervised Aerobic: 4 times/wk walking 1–3 miles 60–80% VO2 reserve Resistance: 2 times/wk |
22 wk | ↑ VO2peak | ↑ | 67 |
| Hubbard et al41 | 41 | Colorectal cancer survivors | NA | Recovery period after surgery | Supervised by exercise specialist; group setting in CR | 1–2 times/wk supervised Aerobic: 50 min based on Borg RPE Resistance: 25 min/bout |
6–12 wk | Feasibility | Feasibility | 62 |
| Hsieh et al42 | 96 | Breast cancer survivors | NA | Immediately after surgery alone (n=22), after surgery+chemotherapy (n=30), after surgery+radiation (n=17), after surgery+chemotherapy+radiation (n=27) | Individually supervised by exercise specialist in oncology rehabilitation setting based on CR | 2–3 times/wk supervised Aerobic: 40 mins 40%–70% PHR Resistance and stretching: 20 min |
6 mo | ↑ VO2max and time on treadmill (P<0.05) for all groups | ↑ For all groups | 90 |
| De Jesus et al43 | 20 | Breast cancer scoring high on fatigue scale | 1–3 | Within 4 mo of adjuvant therapy | Supervised by exercise specialist in group setting, CR administered at YMCA (community based) | 3 times/wk supervised Aerobic: 50%–70% VO2max; 15 min progressing 45 min/bout Resistance: none |
16 wk | Feasibility | Feasibility | 30 |
CR indicates cardiac rehabilitation; MET, metabolic equivalent; NA, not available; PHR, peak heart rate; PRO, patient-reported outcomes; RM, resistance maximum; RPE, rating of perceived exertion; and U/L, upper/lower.
Rationale for Other Components of CR
In addition to the therapy-related decline in CRF, preexisting and treatment-related modifiers may increase CVD risk. Risk factors such as smoking, hypertension, diabetes mellitus, dyslipidemia, and obesity may be higher in cancer survivors than in the general population, in part as a result of the shared pathways to CVD and cancer.45 Preexisting CVD risk factors are strong predictors of anthracycline-,46 trastuzumab-,47 and radiation-related48 CVD. Furthermore, the development of de novo risk factors after therapy also increases morbidity and mortality.49,50 Indeed, cancer survivors are more likely than control subjects51 to have hypertension (65.9% versus 59.5%, respectively; P <0.01) and diabetes mellitus (23.4% versus 21.5%, respectively; P<0.01); for hypertension, this risk extends to >10 years after diagnosis.52 Nichols et al53 estimated that each 5-kg weight gain after a breast cancer diagnosis was associated with a 19% increase in CVD mortality (P=0.04). Among 36 232 two-year survivors of adult-onset cancer, survivors with ≥2 CVD risk factors had a higher risk of CVD (incidence rate ratio, 1.83–2.59) compared with control subjects without cancer, whereas there was a 3.78-fold increased risk of all-cause mortality in cancer survivors who developed CVD compared with those without CVD (incidence rate ratio, 3.78 [95% CI, 3.55–4.01]; P=0.01).51 Collectively, the global nature and magnitude of impairment create a strong rationale for multimodal approaches to be centered on treatment strategies with the capacity to favorably modify the cardiovascular system.
Consideration of a CR model is appealing in cancer care for the following reasons: First, CR delivers exercise as its core program with the long-term goal of improving CVD outcomes. Second, CR offers an opportunity to measure and subsequently reduce CVD risk factors in patients with cancer. Third, CR provides an individualized approach to exercise and medical therapy that allows critical adjustments and tailored therapy for patients with cancer. Fourth, CR provides surveillance to communicate with providers about changes in patient status (eg, vital signs, symptoms, exercise intolerance). Finally, CR presents an opportunity to simultaneously deliver several bundled interventions for patients. Historically, the significant clinical benefits of CR services in patients with a recent, qualifying CVD event55–61 stem from the systematic application of exercise and medical therapies under close supervision and guidance from a well-organized, multidisciplinary team of healthcare professionals,65,66 thus providing a rationale for applying this approach to patients with cancer as well.
IDENTIFYING PATIENTS WITH CANCER FOR CR
Here, we present a targeted approach to identifying patients with cancer who may be expected to derive the greatest benefit from multimodal CR. To streamline terminology in the implementation of CR in patients with cancer, we refer to rehabilitation in this setting as CORE. These patients include those exposed to higher doses of cardiotoxic chemotherapeutic or radiation treatment regimens with untreated CVD risk factors as detailed below.67,68
We include the recent American Society of Clinical Oncology clinical practice guideline for the prevention and monitoring of cardiac dysfunction in survivors of adult cancers as part of risk stratification for CORE referral.14 No organization has reached consensus recommendations on all cancer therapies implicated in overall CVD risk (eg, stroke, thromboembolism, cardiomyopathy in patients with cancer (eg, vascular endothelial growth factor inhibitors, immunotherapy, androgen-deprivation therapy). However, the American Society of Clinical Oncology guideline provides a solid evidence base for selected cancer therapies and exposures predisposing patients with cancer to cardiac dysfunction (a subset of overall CVD). The guideline recommends that patients with cancer who meet the following criteria should be considered at increased risk: (1) treatment with high-dose anthracycline (eg, doxorubicin ≥250 mg/m2, epirubicin ≥600 mg/m2) or high-dose radiotherapy ≥30 Gy when the heart is in the treatment field or lower-dose anthracycline in combination with lower-dose radiotherapy; (2) treatment with lower-dose anthracycline or trastuzumab alone plus the presence of ≥2 risk factors (smoking, hypertension, diabetes mellitus, obesity, dyslipidemia), older age (≥60 years) at cancer treatment, or compromised cardiac function (history of myocardial infarction, borderline or low left ventricular ejection fraction, moderate valvular disease); or (3) treatment with lower-dose anthracycline followed by trastuzumab. Although the referenced guideline serves as a starting point for referral, it does not replace the expertise of the cardiologist or oncologist in terms of a patient’s underlying risk for treatment-related CVD, which will vary on the basis of age, diagnosis, medical history, and prior treatment exposures. Moreover, in addition to survivors of adult cancers, pediatric cancer survivors should be considered for CORE on the basis of prior high-risk exposures.69,70
Algorithm for Referral to CORE
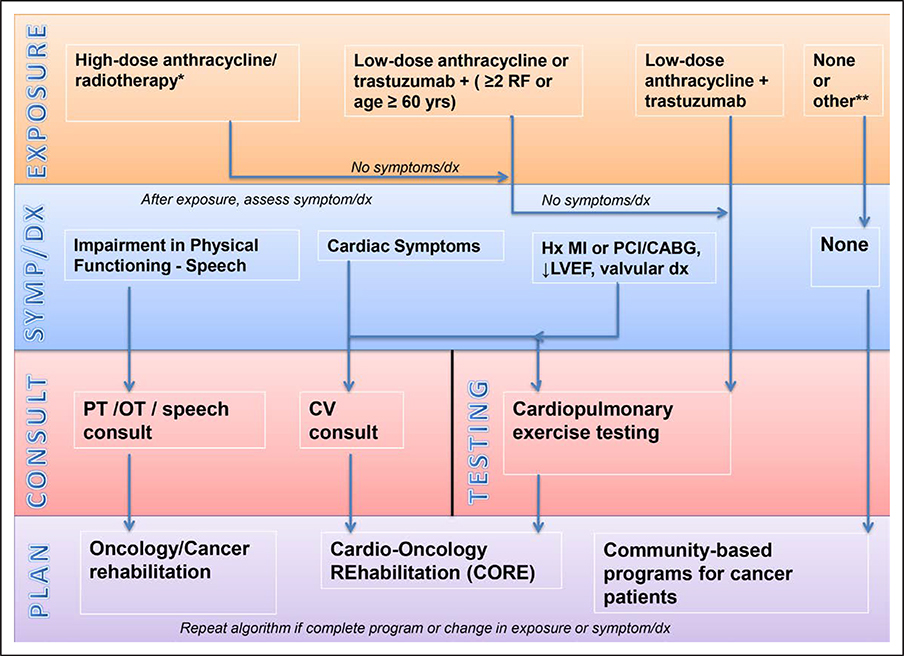
The algorithm shown in Figure 1 represents a proposed framework for testing and consultations before CORE is started. The CORE algorithm is not driven by a specific point in the cancer continuum but rather by a patient’s underlying risk of cardiac dysfunction (based on the American Society of Clinical Oncology clinical practice guideline), cardiac symptoms, or CVD history. Referrals to CORE flow from the treating provider (oncologist, internist, cardiologist) at the time of active treatment, in the survivorship setting when prior exposures are reviewed, or at any time after a cancer diagnosis in patients with existing CVD or in patients with cancer who develop cardiac symptoms. In patients who are eligible for CORE, cardiopulmonary safety should be assessed with cardiopulmonary exercise testing before CORE is started (Table 3, normal testing requirements).73
Figure 1. Cardio-oncology rehabilitation (CORE) algorithm for patients with cancer.
Risk factors (RFs) include hypertension, dyslipidemia, diabetes mellitus, smoking, and obesity. CABG indicates coronary artery bypass graft; CV, cardiovascular; dx, diagnosis; Hx, history; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OT, occupational therapy; PCI, percutaneous coronary intervention; and PT, physical therapy. *High-dose anthracycline (eg, doxorubicin ≥250 mg/m2); high-dose radiotherapy (RT; ≥30 Gy) where the heart is in the treatment field; or lower-dose anthracycline + lower-dose RT (<30 Gy). ** Other therapies should be reviewed by treating healthcare provider to determine appropriateness for community-based program vs need for consultation or other testing.
Table 3.
Safety Check for Exercise Training in CORE
| Normal testing |
| CPET71 |
| Resting BP ≤160/90 mm Hg* |
| Normal BP response to exercise |
| No inducible ischemia |
| No atrial or ventricular arrhythmias |
| Maintain normal O2 saturations |
| No symptoms† |
| 6-min walk test72 |
| Resting blood pressure ≤160/90 mm Hg* |
| Maintain normal O2 saturations |
| Laboratory studies |
| Absence of severe anemia (<8.0 g/dL) |
| Absolute neutrophil count >500 mm3 |
| Platelet count >50 000/μL |
| No baseline symptoms |
| Acute nausea during exercise |
| Vomiting within 24 h |
| Disorientation |
| Blurred vision |
| Ongoing cancer complications |
| Acute infection |
| Acute metabolic disease‡ |
| New-onset lymphedema |
| Mental or physical impairment to exercise |
| Initial wound healing after surgery |
| Bone or brain metastasis§ |
| Displays exercise knowledge |
| Understands functions of aerobic and resistance equipment |
| Demonstrates correct form on equipment |
| Understands perceived exertion and heart rate goals; performs exercise accordingly |
BP indicates blood pressure; CORE, cardio-oncology rehabilitation; and CPET, cardiopulmonary exercise testing.
If elevated, recheck after 5 minutes. If still elevated, then reschedule CPET after patient is seen by provider to adjust BP medications.
Symptoms such as dyspnea, chest pain, or dizziness or other cardiac symptoms during exercise deemed abnormal by supervising physician.
Examples include abnormal thyroid function, uncontrolled diabetes mellitus, and electrolyte abnormalities.
For patients with bone or brain metastases, a plan in CORE needs to include a consultation with oncology rehabilitation to establish a patient-specific safe exercise plan.
The protocol used (ie, Bruce et al74, Balke and Ware,75 Naughton et al76) should consider age, habitual physical activity (PA), and the anticipated functional capacity of the patient. A modified Balke protocol has been used previously in a breast cancer population. In this protocol, treadmill speed is initially set at 3.3 mph. In the first minute, the grade is set at 0%, followed by a 2% increase in the second minute and a 1% increase every minute thereafter. After 25 minutes, the grade remained unchanged but the speed was increased 0.3 mph (5.4 m/min) for each additional minute until test termination.77 This test can be performed during the initial patient assessment visit in CORE. A 6-minute walk test is an alternative field assessment that can be used to evaluate the functional capacity of patients with cancer before they begin an exercise routine.78
Given that exercise may be limited or unsafe as a result of treatment-related frailty, musculoskeletal, neurological, or cognitive issues, bone loss, and ongoing treatment, cancer rehabilitation (eg, physical therapy, occupational therapy) should be initiated before CORE in this setting to address these impairments (Figure 1, left side of the CORE algorithm). Furthermore, if patients with cancer demonstrate functional impairments during or after active treatment, a cancer rehabilitation consultation should be done before or in concert with CORE. Multidisciplinary centers that could partner with CORE in managing the complex needs of patients with cancer include cancer rehabilitation, supportive care, and integrative medicine/integrative oncology.
Education of CR Staff
Patients with cancer experience unique challenges that may differ considerably from the challenges faced by their CR patient counterparts. Accordingly, CR staff (physician or exercise physiologist) should have the expertise and training to evaluate and administer an individually tailored exercise plan that is based on each patient’s health status, treatments received, and specific risks associated with the cancer type. Moreover, CR physicians and advanced practice providers should consider partnering with oncologists and oncology providers to provide key components of the medical assessment. We propose that patients with cancer should meet an initial set of exercise performance metrics within a supervised setting (defined in Table 3) to assess overall safety. To assist CR staff with the unique needs of patients with cancer, we combine the traditional components of CR79 with the following cancer-specific considerations for CORE (Table 4).
Table 4.
Core Components of CORE
| CR | CORE |
|---|---|
| Patient assessment | Review cancer therapies and potential side effects |
| Assess health conditions impairing exercise | |
| Assess for lymphedema, ostomy, and infection risks | |
| Review for metastatic disease, presence/stage, and readiness for exercise vs cancer rehabilitation if bony metastasis | |
| Review complete blood cell count | |
| Screen for depression, fatigue, and quality of life | |
| Perform cardiopulmonary assessment | |
| Nutrition counseling | Cancer-specific nutritional recommendations (eg, National Comprehensive Cancer Network) |
| Involve dietitians who specialize in cancer | |
| Weight management | Assess weight management issues—weight loss, loss of lean muscle mass, and gain in fat mass—that are cancer specific |
| Tailor aerobic and resistance training accordingly | |
| BP management | Review chemotherapeutic agents and molecularly targeted drugs causing hypertension such as VEGF signaling pathway inhibitors |
| Appropriately screen and reassess for those on active therapy | |
| Lipid/lipoprotein management | Primary CVD prevention setting: ACC and AHA cholesterol guidelines for lipid management, which recommend statin therapy for CVD risk score ≥7.5% over 10-y period |
| Recognize setting when CVD risk score not valid | |
| Diabetes mellitus management | Recognize chemotherapeutic agents that worsen glucose control |
| Tobacco cessation | Provide referral to smoking cessation program within cancer center |
| Psychosocial management | Develop referral network of social work and mental health professionals who support the care and treatment of patients with cancer |
| PA counseling | Emphasize the health risks of prolonged periods of sitting; goal is an increase in habitual lifestyle PA and a decrease in sedentary time |
| Exercise training | Aerobic and resistance exercise training prescription based on ACSM guidelines specific to patients with cancer |
| Supervised exercise training in the CORE setting | |
| Incorporation of behavioral change strategies demonstrated effective for cancer patients and survivors |
ACC indicates American College of Cardiology; ACSM, American College of Sports Medicine; AHA, American Heart Association; BP, blood pressure; CORE, cardio-oncology rehabilitation; CR, cardiac rehabilitation; CVD, cardiovascular disease; PA, physical activity; and VEGF, vascular endothelial growth factor.
Patient Assessment
General
CR staff should obtain medical and surgical histories; review current medications (including oncological medications) and patient compliance; perform a physical examination; develop an individual patient treatment plan; verify that the patient is taking appropriate cardioprotective medications; identify modifiable cardiovascular risk factors; establish goals for risk factor control and a plan for assessing their attainment; develop a discharge plan summarizing long-term goals; and identify the responsible healthcare provider for follow-up of long-term goals.
Cancer Specific
CR staff should review current and prior cancer therapies, potential side effects, and risk of late toxicities80; assess musculoskeletal impairments/peripheral neuropathy and provide referral to oncology rehabilitation or oncology provider if the above impairment is found; determine the presence of lymphedema and alter the exercise prescription on the basis of these findings81; assess the presence of an ostomy and other infection risks; review complete blood cell count results; screen for depression, fatigue, and quality of life; and perform cardiopulmonary assessment (eg, cardiopulmonary exercise testing).
Nutritional Counseling
General
CR staff should assess current dietary practices; determine target areas for intervention; prescribe specific dietary modifications; educate/counsel the patient and family; incorporate behavior change models and compliance strategies; develop a plan to address unhealthy eating behaviors; and plan periodic assessment of patient adherence to recommended dietary changes.
Cancer Specific
CR staff should adopt nutritional recommendations that are cancer specific (eg, National Comprehensive Cancer Network) and involve dietitians who specialize in cancer care.
Weight Management
General
CR staff should measure height, weight, and waist circumference; calculate body mass index; consider objective measurement of body composition to determine fat and lean body mass; establish short- and long-term goals; develop a permanent lifestyle plan for prudent eating habits, exercise training, and PA to favorably modify body composition; and periodically assess progress toward goals.
Cancer Specific
CR staff should recognize the spectrum of weight management issues, including weight loss, loss of lean muscle mass, and gain in fat mass, which are cancer specific, and tailor aerobic and resistance training accordingly.82
Blood Pressure Management
General
CR staff should measure blood pressure (BP) ≥2 times in the seated posture; rule out orthostatic hypotension by measuring BP lying, seated, and standing at program entry and after increases in antihypertensive medication dosing; assess the patient’s treatment plan for BP control and compliance; determine the appropriate BP goal; and periodically assess progress toward the goal.
Cancer Specific
CR staff should check BP in both arms (unless contraindicated by lymphedema or other impairments) given that unilateral subclavian steal can be seen in patients treated with mediastinal or neck irradiation; review chemotherapeutic agents and molecularly targeted drugs that cause hypertension, such as vascular endothelial growth factor signaling pathway inhibitors, and review the recommended antihypertensive medications in this setting83; and apply appropriate screening and reassessment for those on active therapy.84–86
Aggressive BP targets endorsed in the recent AHA/ACC BP guidelines should be considered in cancer survivors considered CORE eligible, especially those with compromised left ventricular function or patients on vascular endothelial growth factor signaling pathway inhibitors.87 Reassessment of BP is recommended before each training session for patients with cancer on active cancer therapies known to cause hypertension/hypotension.
Lipid Management
General
CR staff should determine the current levels of fasting total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol (or non–high-density lipoprotein cholesterol if triglycerides are >400 mg/dL), and triglycerides from the patient’s medical record or obtain levels if not available (wait 6 weeks after hospitalization before blood testing) in all patients with CVD; assess current medical treatment plan and patient compliance; educate patient and family about a therapeutic lifestyle, including diet, exercise training, lifestyle PA, and body fat loss; and set a goal low-density lipoprotein cholesterol of <70 mg/dL or non–high-density lipoprotein cholesterol of <100 mg/d for those with existing CVD.
Cancer Specific
In the primary CVD prevention setting, CR staff should use the ACC and AHA cholesterol guidelines88 for lipid management, which recommend statin therapy for CVD risk score ≥7.5% over 10-year period. The CVD risk score developed by the ACC and AHA incorporates age, sex, total cholesterol, low-density lipoprotein cholesterol, systolic BP, history of diabetes mellitus, and smoking history into a validated CVD risk algorithm.88 Pursnani et al89 demonstrated that patients with cancer identified as having elevated CVD risk on the basis of the AHA/ACC CVD risk algorithm (≥7.5% 10-year absolute CVD risk) are also at heightened risk for cancer-related mortality in view of shared risk factors between CVD and cancer. However, the CVD risk score does not take into account cancer therapies such as mediastinal irradiation, which are associated with an increased risk of myocardial infarction and cardiac-specific death independently from standard CVD risk factors.90–92 Therefore, the CVD risk algorithm may be helpful as a starting point for a discussion of the use of lipid-lowering medication for cancer survivors as long as their therapy did not include an exposure that would render the calculator invalid. CVD risk algorithms are published for patients with certain site-specific cancers, including childhood cancer survivors93,94 and patients with breast cancer who received adjuvant trastuzumab.95 If statin therapy is recommended, a rereview of existing medications is needed to avoid potential drug-drug interactions in patients with cancer.
Diabetes Mellitus Management
General
CR staff should confirm the presence or absence of diabetes mellitus in all patients by reviewing the medical record; if the patient has diabetes mellitus, determine the presence of complications such as autonomic or peripheral neuropathy, retinopathy, orthostatic hypotension, chronic kidney disease, or peripheral artery disease; determine whether the patient experiences symptoms related to diabetic complications or if episodes of hypoglycemia or hyperglycemia occur; identify the healthcare provider who manages the patient’s diabetes mellitus; test blood glucose levels before and after the initial supervised exercise sessions to establish the patient’s glycemic response to exercise and to determine whether further routine testing is required; have policies in place for treating both hypoglycemia and hyperglycemia; educate the patient about the potential interaction between exercise training and glycemic control; reinforce the patient’s self-monitoring skills; consider referral to a certified diabetes mellitus educator; consider referral to a dietitian for medical nutrition therapy; set a hemoglobin A1c goal of ≤7%; and minimize the likelihood of exercise training complications.
Cancer Specific
CR staff should recognize chemotherapeutic agents that worsen glucose control (eg, phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin inhibitors, androgen-deprivation therapy) and therapeutic exposures that increase the risk of diabetes mellitus (eg, total body or abdominal irradiation),84,96,97 and refer patients with cancer with diabetes mellitus to a certified diabetes mellitus educator and registered dietitian.
Tobacco (Nicotine) Cessation
General
CR staff should ask the patient about current and past use of tobacco and nicotine products, including e-cigarettes; document tobacco/nicotine use status as never, former, or current; specify the amount of exposure; if the patient is currently using these products, assess readiness to change; discuss treatment strategies and resources; consider referral to a nicotine cessation clinic or program; teach relapse prevention skills; and set a goal of complete cessation of use and no exposure to environmental tobacco smoke.
Cancer Specific
CR staff should provide a referral to a smoking cessation program within a cancer center or designated program within the institution.
Psychosocial Management
General
CR staff should identify psychosocial distress, especially depression, but also anxiety, anger or hostility, social isolation, marital/family distress, sexual dysfunction, and substance abuse; identify use of psychotropic medications; identify the healthcare provider who provides care for the patient’s psychosocial distress; develop a supportive environment in CR; offer individual or group counseling/education sessions; refer appropriate patients to mental health professionals; and periodically reassess psychosocial health.
Cancer Specific
CR staff should develop a referral network of social work and mental health professionals who support the care and treatment of patients with cancer, and work with the primary oncology team to support psychosocial concerns.
PA Counseling
General
CR staff should assess current lifestyle PA level via a validated questionnaire or step count with a pedometer or other technology; provide education/counseling on the importance of increasing PA in the normal routine of daily life, emphasizing the health risks of prolonged periods of sitting; and set a goal of an increase in habitual lifestyle PA and a decrease in sedentary time.
Exercise Training Counseling
General
CR staff should develop an individualized exercise prescription for each patient that includes aerobic and resistance exercise, warm-up and cool-down activities, and flexibility stretching exercises; specify frequency, intensity, types, or modes of exercise, as well as duration and volume of training and the rate of progression of the exercise dose98,99; prescribe both supervised and independent exercise sessions while the patient is enrolled in CR; counsel the patient about cardiac warning signs and symptoms that may occur during exercise and the proper responses to the warnings; and discuss goals that include lifelong exercise training with the benefits of reduced cardiac symptoms and improved CRF, muscle strength and endurance, and joint flexibility.
Cancer Specific
CR staff should develop an exercise prescription that is based on the underlying structure of guidelines by the American College of Sports Medicine and delivered by American College of Sports Medicine/American Cancer Society–certified cancer exercise trainers. The CORE algorithm provides guidance on which patients may benefit most from supervised exercise training in the CORE setting; this recommendation is determined by prior exposures and symptoms. Figure 2 illustrates the potential benefits that exercise training may confer to patients with cancer at heightened risk for CVD.
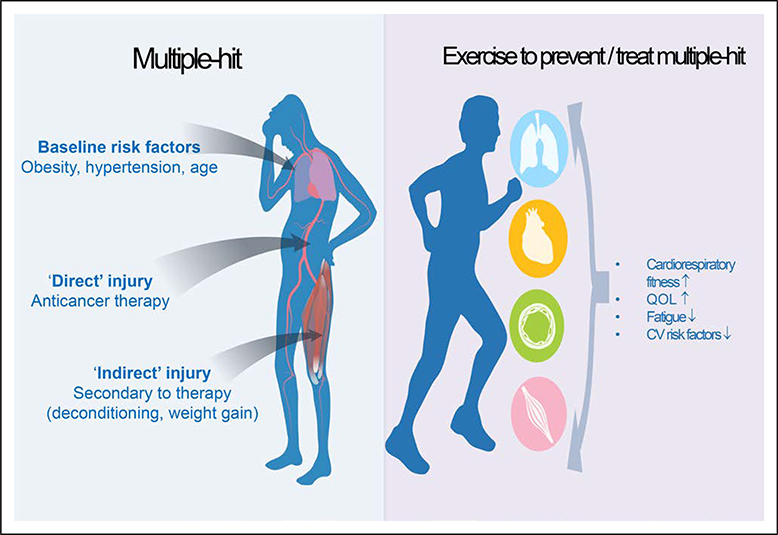
Figure 2.
Potential benefits that exercise training may confer to patients with cancer at heightened risk for cardiovascular (CV) disease.
QOL indicates quality of life.
At CORE entry, the medical assessment performed by physician or midlevel provider ascertains additional comorbidities that may affect an individualized exercise prescription such as balance or musculoskeletal complaints (see the Patient Assessment section). Resistance exercises should be based on both 1-repetition maximum (60%–70% 1-repetition maximum leg and chest press) in concert with appropriate load to maintain optimal form for 8 to 10 repetitions until muscle fatigue for the major muscle groups. Moderate aerobic exercise is recommended for patients with cancer100,101 on the basis of the best available evidence, although determining moderate intensity can be challenging in a CORE population given the use of β-blockers, the prevalence of autonomic dysfunction,102 and the use of current therapies that affect resting heart rate. Therefore, formalized testing with cardiopulmonary exercise testing allows objective guidance to determine the appropriate intensity of aerobic exercise. A determination of moderate intensity based on perceived exertion (rating of perceived exertion of 4–6 on the Borg scale [range, 0–10]) and peak heart rate (50%–70% peak heart rate) achieved during cardiopulmonary exercise testing can guide moderate-intensity recommendations. Volume recommendations (frequency and duration of exercise bout) should take into account prior exercise exposure/CRF, current cancer therapies, and musculoskeletal/fracture risk. The initial exercise dose (intensity, volume as described above) ultimately prescribed should be made in concert with a working knowledge of the most up-to-date American College of Sports Medicine guideline recommendations for patients with cancer. Behavioral change strategies to progress and develop consistent exercise routine should be incorporated.103
Timing and Referral Considerations for CORE
The mode of delivery, schedule, and frequency of cancer therapies are unique to a particular cancer and may differ considerably from patient to patient. Consequently, the type and duration of treatment are highly individualized, as is the optimal time to begin a patient-specific rehabilitation program. Uptake of self-reported exercise levels after a diagnosis of breast cancer is enhanced by an oncologist recommendation that is integrated into clinic visits at the time of adjuvant chemotherapy.104 Incorporating exercise rehabilitation during active treatment is in line with the UK model that advocates for the initiation of exercise referral at the time of diagnosis and treatment.105 However, barriers such as fatigue, physical deconditioning, and depression during regular treatments, some of which may be debilitating, along with the associated side effects that the patient is coping with and the regular follow-up appointments the patient has, raise the question of the most appropriate time for patients with cancer to initiate exercise formally in a rehabilitation setting. Thus, according to the presented CORE algorithm, referral is driven by exposures and symptoms, not timing after a cancer diagnosis.
CENTER- VERSUS HOME-BASED CR AND OPTIONS FOR CORE
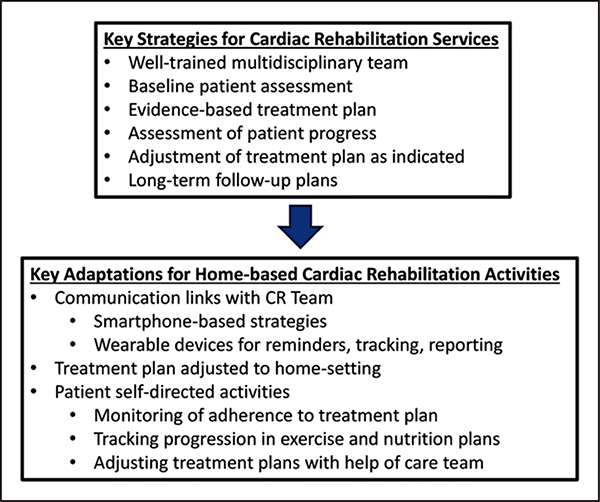
New models of CR delivery are being explored, such as home-based and community center–based CR, to help improve participation by expanding its capacity and flexibility.107,108 Home-based CR involves components similar to those of center-based CR, but such services are applied in locations that are more convenient for the patient (eg, in the patient’s home or local exercise center) rather than the typical CR center (Figure 3). For patients with cancer, both center- and home-based exercise programs are possible options, although consideration of patient preference, safety, and efficacy is required. Patient preference for CORE may differ, depending on the timing of exercise related to the phase of diagnosis, intervention, and recovery. Center-based programs may be more realistic for patients with cancer who are preparing for or have completed their most intensive surgical, chemotherapy, and radiation therapy interventions and thus have fewer structured medical appointments. Center-based programs may be advantageous for pursuing reimbursement by third-party payers because there currently is no reimbursement for non–center-based rehabilitation for CVD or cancer. However, home-based programs may have an advantage for providing lower-cost, more convenient options for CORE, especially for those who are deemed safe to exercise (Table 4). Whether a center- or home-based program is chosen, measuring the efficacy of CORE, with a particular focus on the individual’s response to the dose and type of exercise, is important.
Figure 3.
Key strategies and activities of home-based cardiac rehabilitation (CR) services.
REIMBURSEMENT FOR CR AND CORE
Reimbursement is well established for center-based CR, is based on a large body of supporting evidence, and requires physician referral and medical supervision.109,110 Reimbursement for centered-based CR was first established in 1982 for patients who experienced a recent myocardial infarction, coronary artery bypass graft surgery, or stable angina.79 Unfortunately, no reimbursement strategy is currently available to provide access to a multimodality CR program for patients with cancer. This scientific statement is a first step to pave the way for reimbursement for patients with cancer within the CR model. Further work is needed to establish the science base for CR in the cancer population and to generate guidelines and accompanying policy metrics to shape referrals and reimbursement. In the future, possible options for reimbursement include patient self-pay, direct contracting with employers/private insurance companies, or coverage by governmental payers. An additional pathway, growing in use across the United States, is a global or bundled payment for care rendered to a patient with a diagnosis or condition over a specific period of time. Institutions and payers establish the financial and health outcomes of the bundle, the components of care to be included, and the risk-sharing aspects.
RESEARCH GAPS FOR CORE
Research needed to move toward referral and reimbursement for CORE among patients with cancer includes (1) developing and conveying the evidence base for CORE to patients and families, clinicians, health systems, payers, and employers; (2) demonstrating which patients are most likely to benefit and, when possible, showing improved economic outcomes (eg, downstream healthcare use, ability to return work); (3) identifying the most effective, efficient, and patient-centric delivery practices in varied settings to quickly adopt what program components work; (4) testing the impact of CORE on cardiac-specific outcomes in patients with cancer (often, these efforts to implement best practices highlight significant gaps in evidence, providing a great opportunity to engage health services researchers, particularly experts in the implementation of science and patient-reported outcomes); (5) creating automatic or opt-out referral systems and stratifying participation data by cancer type, stage, and cardiac risk level to help ensure participation by all who can benefit; and (6) defining and testing the effects of embedding a small set of metrics in quality reporting and performance programs, ideally in both fee-for-service models and value-based arrangements.
CONCLUSIONS AND FUTURE DIRECTIONS
Given the available evidence on the risk of CVD in patients with cancer and the benefits of exercise to reduce CVD risk in the general population, there is a great need to develop and test programs specific to the care of patients with cancer. Previous success in incorporating exercise into the CR setting is reviewed here, along with an algorithm to stratify patients with cancer who may benefit most from supervised exercise using a CORE model. Nevertheless, challenges remain. CR staff are not currently educated on the complex issues that affect patients with cancer, as detailed in this document. The responsibility of identifying and referring patients with cancer at risk for cardiac dysfunction remains in the hands of oncology and primary care providers. Although cardiologists have traditionally worked with oncology teams after cardiac toxicities have already occurred, a proactive stance is now needed between cardiologists and oncologists to develop CORE. CR programs across the country need to develop an infrastructure to provide services aligned with the unique exposures and needs of patients with cancer as described in this statement. The effects of cancer stage, treatment types, and modulating variables need to be clarified when identifying the periods of greatest physical decline and potential recovery. CR participation is already established to reduce CVD events in those with established myocardial infarction; patients undergoing coronary artery bypass graft surgery or percutaneous coronary intervention; and symptomatic patients with angina pectoris, chronic stable systolic heart failure, and heart valve repair or replacement. Research is needed to demonstrate a reduction in cardiac dysfunction among patients with cancer enrolled in CORE, thereby enhancing referrals and the likelihood of insurance coverage for patients with cancer. If realized, CORE has the potential to grow exponentially within an existing CR infrastructure, thus providing a widely accessible multimodality program to patients with cancer across the United States that is not presently available.
Writing Group Disclosures
| Writing Group Member | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Susan C. Gilchrist | University of Texas MD Anderson Cancer Center | None | None | None | None | None | None | None |
| Ana Barac | Medstar Heart and Vascular Institute, Medstar Washington Hospital Center | Genentech (immediate family members: cardiology PI on investigator-initiated study; no salary or direct support)* | None | None | None | None | None | None |
| Philip A. Ades | University of Vermont | Dr Ades was supported in part by NIH Center of Biomedical Research Excellence Award P20GM103644 from the National Institute of General Medical Sciences† | None | None | None | None | None | None |
| Catherine M. Alfano | American Cancer Society | None | None | None | None | None | None | None |
| Barry A. Franklin | William Beaumont Hospital | None | None | None | None | None | None | None |
| Lee W. Jones | Memorial Sloan Kettering Cancer Center | None | None | None | None | Stock ownership† | None | None |
| Andre La Gerche | Baker Heart and Diabetes Institute | None | None | None | None | None | None | None |
| Jennifer A. Ligibel | Dana-Farber Cancer Institute and Harvard Medical School | None | None | None | None | None | None | None |
| Gabriel Lopez | MD Anderson Palliative, Rehabilitation and Integrative Medicine, UT MD Anderson Cancer Center | None | None | None | None | None | None | None |
| Kushal Madan | Sir Ganga Ram Hospital (New Delhi, India) | None | None | None | None | None | None | None |
| Kevin C. Oeffinger | Duke University/Duke Cancer Institute | None | None | None | None | None | None | None |
| Jeannine Salamone | Georgetown Breast Cancer Advocates, Georgetown University, Lombardi Cancer Center | None | None | None | None | None | None | None |
| Jessica M. Scott | Memorial Sloan Kettering Cancer Center | AKT IV Against Cancer* | None | None | None | None | None | None |
| Ray W. Squires | Mayo Clinic | None | None | None | None | None | None | None |
| Randal J. Thomas | Mayo Clinic | None | None | None | None | None | None | None |
| Diane J. Treat-Jacobson | University of Minnesota School of Nursing | NIH (LITE study site PI for RCT comparing low- and high-intensity exercise training in patients with PAD)†; Margaret A. Cargill Foundation (grant to promote community awareness and improved detection and treatment for patients with PAD in rural Minnesota; closer to a quality improvement project than a research study)†; PCORI (site PI on Dr McDermott’s HONOR study evaluating efficacy of home-based exercise in patients with PAD)† | None | None | None | None | None | None |
| Janet S. Wright | CDC/CMS Million Hearts | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Kim Dittus | University of Vermont | None | None | None | None | None | None | None |
| Dennis J. Kerrigan | Henry Ford Hospital | Henry Ford Hospital Game on Cancer (PI for an internally funded [$28 600] pilot study to investigate the effects of exercise training on individuals with preclinical cardiotoxicity)† | None | None | None | None | None | None |
| Ronald J. Krone | Washington University in St. Louis | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Significant.
Footnotes
ARTICLE INFORMATION
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on January 7, 2019, and the American Heart Association Executive Committee on February 19, 2019. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 843-216-2533 or kelle.ramsay@wolterskluwer.com.
The American Heart Association requests that this document be cited as follows: Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, La Gerche A, Ligibel JA, Gabriel Lopez G, Madan K, Oeffinger KC, Salamone J, Scott JM, Squires RW, Thomas RJ, Treat-Jacobson DJ, Wright JS; on behalf of the American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Peripheral Vascular Disease. Cardiooncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997-e1012. doi: 10.1161/CIR.0000000000000679.
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/about-us/statements-and-policies/copyright-request-form).
Endorsed by the American Cancer Society
REFERENCES
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 2.Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25:1683–1690. doi: 10.1200/JCO.2006.09.2106 [DOI] [PubMed] [Google Scholar]
- 3.Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, Sorror ML, Horowitz MM, Bolwell B, Rizzo JD, Socié G. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, Taylor CW, van Leeuwen FE. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064 [DOI] [PubMed] [Google Scholar]
- 6.Chow EJ, Mueller BA, Baker KS, Cushing-Haugen KL, Flowers ME, Martin PJ, Friedman DL, Lee SJ. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004 [DOI] [PubMed] [Google Scholar]
- 7.Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, Sklar C, Forman S, Weisdorf D, Gurney JG, Bhatia S. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplantation Survivor Study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037 [DOI] [PubMed] [Google Scholar]
- 9.Koelwyn GJ, Khouri M, Mackey JR, Douglas PS, Jones LW. Running on empty: cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J Clin Oncol. 2012;30:4458–4461. doi: 10.1200/JCO.2012.44.0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenger NK, Froelicher ES, Smith LK, Ades PA, Berra K, Blumenthal JA, Certo CM, Dattilo AM, Davis D, DeBusk RF. Cardiac rehabilitation as secondary prevention: Agency for Health Care Policy and Research and National Heart, Lung, and Blood Institute. Clin Pract Guidel Quick Ref Guide Clin. 1995;52:1–23. [PubMed] [Google Scholar]
- 10a.Franklin BA, Brinks J. Cardiac rehabilitation: underrecognized/underutilized. Curr Treat Options Cardio Med. 2015;17:62. [DOI] [PubMed] [Google Scholar]
- 11.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 12.Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation [published correction appears in Circulation. 2015;131:e408]. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d [DOI] [PubMed] [Google Scholar]
- 13.Thomas RJ, King M, Lui K, Oldridge N, Pina IL, Spertus J. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation). Circulation. 2010;122:1342–1350. doi: 10.1161/CIR.0b013e3181f5185b [DOI] [PubMed] [Google Scholar]
- 14.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 15.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE 2nd, Douglas PS, Haykowsky M. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peel AB, Barlow CE, Leonard D, DeFina LF, Jones LW, Lakoski SG. Cardiorespiratory fitness in survivors of cervical, endometrial, and ovarian cancers: the Cooper Center Longitudinal Study. Gynecol Oncol. 2015;138:394–397. doi: 10.1016/j.ygyno.2015.05.027 [DOI] [PubMed] [Google Scholar]
- 17.Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc. 2014;3:e000432. doi: 10.1161/JAHA.113.000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller AM, Lopez-Mitnik G, Somarriba G, Lipsitz SR, Hinkle AS, Constine LS, Lipshultz SE, Miller TL. Exercise capacity in long-term survivors of pediatric cancer: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study . Pediatr Blood Cancer. 2013;60:663–668. doi: 10.1002/pbc.24410 [DOI] [PubMed] [Google Scholar]
- 19.De Caro E, Smeraldi A, Trocchio G, Calevo M, Hanau G, Pongiglione G. Subclinical cardiac dysfunction and exercise performance in childhood cancer survivors. Pediatr Blood Cancer. 2011;56:122–126. doi: 10.1002/pbc.22606 [DOI] [PubMed] [Google Scholar]
- 20.Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;137:1176–1191. doi: 10.1161/CIRCULATIONAHA.117.024671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, Moskowitz CS, Matsoukas K, Iyengar NM, Dang CT, Jones LW. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36:2297–2305. doi: 10.1200/JCO.2017.77.5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palomo A, Ray RM, Johnson L, Paskett E, Caan B, Jones L, Okwuosa T. Associations between exercise prior to and around the time of cancer diagnosis and subsequent cardiovascular events in women with breast cancer: a Women’s Health Initiative (WHI) analysis. J Am Coll Cardiol. 2017;69(suppl):1774. doi: 10.1016/S0735-1097(17)35163-X28385306 [DOI] [Google Scholar]
- 23.MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res. 1989;38:348–351. [PubMed] [Google Scholar]
- 24.Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, Woodard S, Wells G, Reid R. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657 [DOI] [PubMed] [Google Scholar]
- 25.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, de Maaker-Berkhof M, Boven E, Schrama J, Geenen MM, Meerum Terwogt JM, van Bochove A, Lustig V, van den Heiligenberg SM, Smorenburg CH, Hellendoorn-van Vreeswijk JA, Sonke GS, Aaronson NK. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081 [DOI] [PubMed] [Google Scholar]
- 26.Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009;15:4963–4967. doi: 10.1158/1078-0432.CCR-09-0628 [DOI] [PubMed] [Google Scholar]
- 27.Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, Malone SC, Wells GA, Scott CG, Slovinec D’Angelo ME. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–351. doi: 10.1200/JCO.2007.15.4963 [DOI] [PubMed] [Google Scholar]
- 28.Jones LW, Habel LA, Weltzien E, Castillo A, Gupta D, Kroenke CH, Kwan ML, Quesenberry CP Jr, Scott J, Sternfeld B, Yu A, Kushi LH, Caan BJ. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34:2743–2749. doi: 10.1200/JCO.2015.65.6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012 [DOI] [PubMed] [Google Scholar]
- 30.Jones LW, Hornsby WE, Freedland SJ, Lane A, West MJ, Moul JW, Ferrandino MN, Allen JD, Kenjale AA, Thomas SM, Herndon JE 2nd, Koontz BF, Chan JM, Khouri MG, Douglas PS, Eves ND. Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014;65:852–855. doi: 10.1016/j.eururo.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott JM, Li N, Liu Q, Yasui Y, Leisenring W, Nathan PC, Gibson T, Armenian SH, Nilsen TS, Oeffinger KC, Ness KK, Adams SC, Robison LL, Armstrong GT, Jones LW. Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol. 2018;4:1352–1358. doi: 10.1001/jamaoncol.2018.2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams SC, DeLorey DS, Davenport MH, Stickland MK, Fairey AS, North S, Szczotka A, Courneya KS. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: a phase 2 randomized controlled trial. Cancer. 2017;123:4057–4065. doi: 10.1002/cncr.30859 [DOI] [PubMed] [Google Scholar]
- 33.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22:54–64. doi: 10.1002/pon.2047 [DOI] [PubMed] [Google Scholar]
- 34.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl). 2003;12:347–357. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Zhu J, Gu Z, Yin X. Efficacy of exercise interventions in patients with acute leukemia: a meta-analysis. PLoS One. 2016;11:e0159966. doi: 10.1371/journal.pone.0159966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, Tankel K, Basi S, Chua N, Mazurek A, Reiman T. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634 [DOI] [PubMed] [Google Scholar]
- 37.Lakoski SG, Willis BL, Barlow CE, Leonard D, Gao A, Radford NB, Farrell SW, Douglas PS, Berry JD, DeFina LF, Jones LW. Midlife cardiorespiratory fitness, incident cancer, and survival after cancer in men: the Cooper Center Longitudinal Study. JAMA Oncol. 2015;1:231–237. doi: 10.1001/jamaoncol.2015.0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dittus KL, Lakoski SG, Savage PD, Kokinda N, Toth M, Stevens D, Woods K, OʼBrien P, Ades PA. Exercise-based oncology rehabilitation: leveraging the cardiac rehabilitation model. J Cardiopulm Rehabil Prev. 2015;35:130–139. doi: 10.1097/HCR.0000000000000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young-McCaughan S, Mays MZ, Arzola SM, Yoder LH, Dramiga SA, Leclerc KM, Caton JR, Sheffler RL, Nowlin MU. Research and commentary: change in exercise tolerance, activity and sleep patterns, and quality of life in patients with cancer participating in a structured exercise program. Oncol Nurs Forum. 2003;30:441–54. doi: 10.1188/03.ONF.441-454 [DOI] [PubMed] [Google Scholar]
- 40.Dolan LB, Barry D, Petrella T, Davey L, Minnes A, Yantzi A, Marzolini S, Oh P. The cardiac rehabilitation model improves fitness, quality of life, and depression in breast cancer survivors. J Cardiopulm Rehabil Prev. 2018;38:246–252. doi: 10.1097/HCR.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 41.Hubbard G, Adams R, Campbell A, Kidd L, Leslie SJ, Munro J, Watson A. Is referral of postsurgical colorectal cancer survivors to cardiac rehabilitation feasible and acceptable? A pragmatic pilot randomised controlled trial with embedded qualitative study. BMJ Open. 2016;6:e009284. doi: 10.1136/bmjopen-2015-009284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsieh CC, Sprod LK, Hydock DS, Carter SD, Hayward R, Schneider CM. Effects of a supervised exercise intervention on recovery from treatment regimens in breast cancer survivors. Oncol Nurs Forum. 2008;35:909–915. doi: 10.1188/08.ONF.909-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Jesus S, Fitzgeorge L, Unsworth K, Massel D, Suskin N, Prapavessis H, Sanatani M. Feasibility of an exercise intervention for fatigued breast cancer patients at a community-based cardiac rehabilitation program. Cancer Manag Res. 2017;9:29–39. doi: 10.2147/CMAR.S117703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deleted in press. [Google Scholar]
- 45.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in car-diovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005;23:8597–8605. doi: 10.1200/JCO.2005.02.5841 [DOI] [PubMed] [Google Scholar]
- 47.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle JN, Winer EP, Gelmon KA, Gersh BJ, Jaffe AS, Rodeheffer RJ. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 49.Jones LW, Haykowsky M, Peddle CJ, Joy AA, Pituskin EN, Tkachuk LM, Courneya KS, Slamon DJ, Mackey JR. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev. 2007;16:1026–1031. doi: 10.1158/1055-9965.EPI-06-0870 [DOI] [PubMed] [Google Scholar]
- 50.Jones LW, Haykowsky M, Pituskin EN, Jendzjowsky NG, Tomczak CR, Haennel RG, Mackey JR. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor–positive operable breast cancer. Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156 [DOI] [PubMed] [Google Scholar]
- 51.Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK, Douglas PS, Bhatia S, Chao C. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. 2016;34:1122–1130. doi: 10.1200/JCO.2015.64.0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leach CR, Weaver KE, Aziz NM, Alfano CM, Bellizzi KM, Kent EE, Forsythe LP, Rowland JH. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv. 2015;9:239–251. doi: 10.1007/s11764-014-0403-1 [DOI] [PubMed] [Google Scholar]
- 53.Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ, Holick CN, Hampton JM, Stampfer MJ, Willett WC, Newcomb PA. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009:18:1403–1409. doi: 10.1158/1055-9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deleted in press.
- 55.Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med. 2014;127:538–546. doi: 10.1016/j.amjmed.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. doi: 10.1161/CIRCULATIONAHA.110.983536 [DOI] [PubMed] [Google Scholar]
- 57.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. doi: 10.1161/CIRCULATIONAHA.109.876383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jolliffe J, Rees K, Taylor R, Thompson D, Oldridge N, Ebrahim S. Exercisebased rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001:CD001800. [DOI] [PubMed] [Google Scholar]
- 59.O’Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS Jr, Hennekens CH. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–244. [DOI] [PubMed] [Google Scholar]
- 60.Pack QR, Goel K, Lahr BD, Greason KL, Squires RW, Lopez-Jimenez F, Zhang Z, Thomas RJ. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community-based study. Circulation. 2013;128:590–597. doi: 10.1161/CIRCULATIONAHA.112.001365 [DOI] [PubMed] [Google Scholar]
- 61.Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac re-habilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. doi: 10.1016/j.jacc.2009.01.078 [DOI] [PubMed] [Google Scholar]
- 62. Deleted in press.
- 63. Deleted in press.
- 64. Deleted in press.
- 65.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115:2675–2682. doi: 10.1161/CIRCULATIONAHA.106.180945 [DOI] [PubMed] [Google Scholar]
- 66.Hamm LF, Sanderson BK, Ades PA, Berra K, Kaminsky LA, Roitman JL, Williams MA. Core competencies for cardiac rehabilitation/secondary prevention professionals: 2010 update: position statement of the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2011;31:2–10. doi: 10.1097/HCR.0b013e318203999d [DOI] [PubMed] [Google Scholar]
- 67.Lenneman CG, Sawyer DB. Cardio-oncology: an update on cardiotoxic-ity of cancer-related treatment. Circ Res. 2016;118:1008–1020. doi: 10.1161/CIRCRESAHA.115.303633 [DOI] [PubMed] [Google Scholar]
- 68.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407 [DOI] [PubMed] [Google Scholar]
- 69.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, Stovall M, Chow EJ, Sklar CA, Mulrooney DA, Mertens AC, Border W, Durand JB, Robison LL, Meacham LR. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berkman AM, Lakoski SG. Treatment, behavioral, and psychosocial components of cardiovascular disease risk among survivors of childhood and young adult cancer. J Am Heart Assoc. 2015;4:e001891. doi: 10.1161/JAHA.115.001891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Piña IL, Rodney R, Simons-Morton DA, Williams MA, Bazzarre T. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. [DOI] [PubMed] [Google Scholar]
- 72.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 73.Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespira-tory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5 [DOI] [PubMed] [Google Scholar]
- 74.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. [DOI] [PubMed] [Google Scholar]
- 75.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 76.Naughton J, Balke B, Nagle F. Refinements in Method of Evaluation and Physical Conditioning Before and After Myocardial Infarction. Am J Cardiol. 1964;14:837–843. [DOI] [PubMed] [Google Scholar]
- 77.Lakoski SG, Barlow CE, Koelwyn GJ, Hornsby WE, Hernandez J, Defina LF, Radford NB, Thomas SM, Herndon JE 2nd, Peppercorn J, Douglas PS, Jones LW. The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: the Cooper Center Longitudinal Study. Breast Cancer Res Treat. 2013;138:909–916. doi: 10.1007/s10549-013-2478-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631–636. doi: 10.1055/s-0032-1323746 [DOI] [PubMed] [Google Scholar]
- 79.Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84:373–383. doi: 10.1016/S0025-6196(11)60548-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3:e000665. doi: 10.1161/JAHA.113.000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, Bryan CJ, Williams-Smith CT, Greene QP. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664–673. doi: 10.1056/NEJMoa0810118 [DOI] [PubMed] [Google Scholar]
- 82.Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32:335–346. doi: 10.1200/JCO.2013.49.5523 [DOI] [PubMed] [Google Scholar]
- 83.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, Remick SC, Steingart R, Tang WH; Cardiovascular Toxicities Panel, Convened by the Angiogenesis Task Force of the National Cancer Institute Investigational Drug Steering Committee . Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–1467. doi: 10.1056/NEJMra1100265 [DOI] [PubMed] [Google Scholar]
- 85.Nazer B, Humphreys BD, Moslehi J. Effects of novel angiogenesis in-hibitors for the treatment of cancer on the cardiovascular system: focus on hypertension. Circulation. 2011;124:1687–1691. doi: 10.1161/CIRCULATIONAHA.110.992230 [DOI] [PubMed] [Google Scholar]
- 86.Abi Aad S, Pierce M, Barmaimon G, Farhat FS, Benjo A, Mouhayar E. Hypertension induced by chemotherapeutic and immunosuppresive agents: a new challenge. Crit Rev Oncol Hematol. 2015;93:28–35. doi: 10.1016/j.critrevonc.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 87.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Hypertension. 2018;71:e140–e144]. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 88.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(suppl 2):S74–S75]. Circulation. 2014;129(suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 89.Pursnani A, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Guideline-based statin eligibility, cancer events, and noncardiovascular mortality in the Framingham Heart Study. J Clin Oncol 2017;35:2927–2933. doi: 10.1200/JCO.2016.71.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Nimwegen FA, Ntentas G, Darby SC, Schaapveld M, Hauptmann M, Lugtenburg PJ, Janus CPM, Daniels L, van Leeuwen FE, Cutter DJ, Aleman BMP. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood. 2017;129:2257–2265. doi: 10.1182/blood-2016-09-740332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Petersen EJ, Raemaekers JM, Kok WE, Aleman BM, van Leeuwen FE. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180 [DOI] [PubMed] [Google Scholar]
- 92.Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, Hoskin PJ, Lister A, Radford JA, Rohatiner AZ, Linch DC. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029 [DOI] [PubMed] [Google Scholar]
- 93.Chow EJ, Chen Y, Hudson MM, Feijen EAM, Kremer LC, Border WL, Green DM, Meacham LR, Mulrooney DA, Ness KK, Oeffinger KC, Ronckers CM, Sklar CA, Stovall M, van der Pal HJ, van Dijk IWEM, van Leeuwen FE, Weathers RE, Robison LL, Armstrong GT, Yasui Y. Prediction of ischemic heart disease and stroke in survivors of childhood cancer. J Clin Oncol. 2018;36:44–52. doi: 10.1200/JCO.2017.74.8673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chow EJ, Chen Y, Kremer LC, Breslow NE, Hudson MM, Armstrong GT, Border WL, Feijen EA, Green DM, Meacham LR, Meeske KA, Mulrooney DA, Ness KK, Oeffinger KC, Sklar CA, Stovall M, van der Pal HJ, Weathers RE, Robison LL, Yasui Y. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33:394–402. doi: 10.1200/JCO.2014.56.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009;181:1998–2006. doi: 10.1016/j.juro.2009.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meacham LR, Sklar CA, Li S, Liu Q, Gimpel N, Yasui Y, Whitton JA, Stovall M, Robison LL, Oeffinger KC. Diabetes mellitus in long-term survivors of childhood cancer: increased risk associated with radiation therapy: a report for the Childhood Cancer Survivor Study. Arch Intern Med. 2009;169:1381–1388. doi: 10.1001/archinternmed.2009.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.American College of Sports Medicine. ACSM’s Guidelines for Graded Exercise Testing and Prescription. 10th ed. Philadelphia: Wolters Kluwer; 2018:226–267. [Google Scholar]
- 99.Squires RW, Kaminsky LA, Porcari JP, Ruff JE, Savage PD, Williams MA. Progression of exercise training in early outpatient cardiac rehabilitation: An Official Statement From The American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2018;38:139–146. doi: 10.1097/HCR.0000000000000337 [DOI] [PubMed] [Google Scholar]
- 100.Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T; Exercise for People With Cancer Guideline Development Group. Exercise for people with cancer: a clinical practice guideline. Curr Oncol. 2017;24:40–46. doi: 10.3747/co.24.3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brown JC, Troxel AB, Ky B, Damjanov N, Zemel BS, Rickels MR, Rhim AD, Rustgi AK, Courneya KS, Schmitz KH. Dose-response effects of aerobic exercise among colon cancer survivors: a randomized phase II trial. Clin Colorectal Cancer. 2018;17:32–40. doi: 10.1016/j.clcc.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lakoski SG, Jones LW, Krone RJ, Stein PK, Scott JM. Autonomic dysfunction in early breast cancer: Incidence, clinical importance, and underlying mechanisms. Am Heart J. 2015;170:231–241. doi: 10.1016/j.ahj.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berkman AM, Gilchrist SC. Behavioral change strategies to improve phys-ical activity after cancer treatment. Rehabil Oncol. 2018;36:152–160. doi: 10.1097/01.REO.0000000000000112 [DOI] [Google Scholar]
- 104.Jones LW, Courneya KS, Fairey AS, Mackey JR. Effects of an on-cologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: a single-blind, randomized controlled trial. Ann Behav Med. 2004;28:105–113. doi: 10.1207/s15324796abm2802_5 [DOI] [PubMed] [Google Scholar]
- 105.Jefford M, Rowland J, Grunfeld E, Richards M, Maher J, Glaser A. Implementing improved post-treatment care for cancer survivors in England, with reflections from Australia, Canada and the USA. Br J Cancer. 2013;108:14–20. doi: 10.1038/bjc.2012.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Deleted in press.
- 107.DeBusk RF, Miller NH, Superko HR, Dennis CA, Thomas RJ, Lew HT, Berger WE 3rd, Heller RS, Rompf J, Gee D, Kraemer HC, Bandura A, Ghandour G, Clark M, Shah RV, Fisher L, Taylor CB. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120:721–729. [DOI] [PubMed] [Google Scholar]
- 108.Ma J, Berra K, Haskell WL, Klieman L, Hyde S, Smith MW, Xiao L, Stafford RS. Case management to reduce risk of cardiovascular disease in a county health care system. Arch Intern Med. 2009;169:1988–1995. doi: 10.1001/archinternmed.2009.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011:Cd001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124–132. doi: 10.1016/j.ypmed.2017.07.015 [DOI] [PubMed] [Google Scholar]