Abstract
Objective
Systemic loss of estradiol (E2) during menopause is associated with increased adiposity which can be prevented with E2 replacement. Rodent studies suggest that E2, or lack of, is a key mediator in menopause-related metabolic changes. We have previously demonstrated that E2 treatment produces a rapid, dose-dependent activation of AMP-activated protein kinase (AMPK) in murine skeletal muscle. Activation of AMPK is implicated in the therapeutic benefits of many insulin sensitizing agents including metformin and thiazolidinediones. Here, we expand our observations and provide novel data which demonstrate that in addition to E2, its metabolite 2-hydroxyestradiol (2-HE2), activate AMPK in C2C12 myotubes.
Methods and Procedures
C2C12 myotubes were used to examine the effects on E2 and the by-products of its metabolism on AMPK activation.
Results
Low concentrations of E2 (10 and 100 nmol/l) were found to increase AMPK phosphorylation by ~1.6-fold, while a higher concentration (10 µmol/l) resulted in a ~3.0-fold increase. In comparison to E2 treatment alone, incubation of myotubes with E2 and 1-aminobenzotriazole (ABT) (a CYP450 inhibitor that blocks metabolism of E2) caused AMPK activation to be enhanced at low E2 concentrations, but attenuated at higher concentrations. The effects of ABT suggested that one or more E2 metabolites contribute to the maximal activation of AMPK at high E2 concentrations. Indeed, the estrogen metabolite 2-HE2, but not 2-methoxyestradiol (2-ME2), directly activated AMPK in C2C12 myotubes.
Discussion
We propose a model where E2, acting through its metabolite 2-HE2 and the estrogen receptors (ERs), activates AMPK in myotubes. Finally, activation is abolished when all E2 is metabolized to 2-ME2.
INTRODUCTION
Systemic loss of estrogen during menopause is associated with increased adiposity which can be prevented with estrogen replacement (alone or in combination with progesterone) (1–6). Recent data from the Women’s Health Initiative demonstrated that when compared to placebo, women randomized to hormone replacement therapy were more lean and insulin sensitive, as well as less likely to develop type 2 diabetes (7). Using an ovariectomized mouse model of menopause, we have identified a number of novel mechanisms by which estradiol (E2) acts to reduce adiposity and improves metabolic health (8). In addition to E2-mediated changes in gene expression, we demonstrated that in vivo and in vitro E2 treatment rapidly activates AMP-activated protein kinase (AMPK) in skeletal muscle, potentially acting to reduce body fat and promote greater insulin sensitivity.
AMPK is an intracellular signaling molecule that is activated at times of low-fuel availability (i.e., low ATP levels). It has a multitude of physiological effects including increasing fat oxidation, preventing fat synthesis, and inducing glucose uptake (9). The AMPK pathway is activated by many known “insulin sensitizers” such as metformin, thiazolidinediones, and exercise (9–11) as well as by the adipokines adiponectin and leptin (12–14). Therefore, activation of AMPK is considered a target for the treatment of metabolic diseases (9,15).
We recently demonstrated that E2 activates AMPK in a dose and time-dependent manner (8). In a published review of our previous findings (16), the authors astutely pointed out that maximal activation of AMPK was not achieved until E2 concentration reached 10 µmol/l, though the estrogen receptors (ERs) are saturated at a much lower concentration. Therefore, the aim of this study was to further explore this observation and enhance our understanding of how E2 activates AMPK in skeletal muscle. We now demonstrate that the product of cytochrome P450 1A1 (CYP 1A1)-mediated metabolism of E2, 2-HE2, activates AMPK in C2C12 myotubes. As 2-HE2 is not a ligand for ER (17), this suggests dual mechanisms of activation of AMPK by E2 (via ER) and 2-HE2 (via an unknown mechanism). These findings provide new insight into mechanisms of systemic metabolism and body weight regulation by estrogens.
Methods And Procedures
C2C12 culture
Mouse C2C12 myoblasts (American Type Culture Collection, Rockville, MD) were cultured and differentiated into myotubes as previously described (18). On day 5, cells were serum-depleted in Dulbecco’s modified Eagle’s medium for 16 h before experiments. 17-β E2 and 1-aminobenzotriazole (ABT) were purchased from Sigma (St Louis, MO). 2-HE2 and 2-ME2 were purchased from Steraloids (Newport, RI) and were prepared with 0.1% ascorbic acid to minimize oxidation. Incubations with control cells had equivalent concentrations of ascorbic acid. Incubations were terminated by addition of ice-cold phosphate-buffered saline containing 10 nmol/l NaF and 1 mmol/l Na3VO4.
Western blotting
Phosphorylation of AMPK on threonine 172 was assessed in lysates of C2C12 myotubes following sonication in SDS lysis buffer containing phosphatase and protease inhibitors. Lysates were electrophoresed, transferred to nitrocellulose, and probed with antibodies against total AMPK and phosphorylated AMPK (Cell Signaling, Beverly, MA). Chemiluminescence (Super Signal, Pierce, Rockford, IL) was quantified by laser densitometry within the linear range of detection. For detection of CYP 1A1 and catechol O-methyl transferase (COMT) protein expression, whole quadriceps, liver and C2C12 myotubes (day 5) were processed as described earlier and probed with an antibodies specific for the proteins (Santa Cruz Biotechnology, Santa Cruz, CA). Equal amounts of total protein were loaded for each tissue.
Real-time PCR
Liver and muscle (quadriceps) were dissected from C57/B6J mice and total RNA was extracted from ~100 mg of frozen tissue using commercially available kits (Qiagen, Valencia, CA). RNA was also isolated from C2C12 myotubes on day 5 for evaluation of CYP1A1 and COMT mRNA expression. RNA was quantified by RiboGreen Quantitation Assay (Molecular Probes, Eugene, OR) and complementary DNA was synthesized from 1 µg of total RNA using a reverse transcription system (Promega, Madison, WI). Real-time PCR was performed in triplicate on each sample with an ABI PRISM 7700 Sequence Detection System in 20 µl total volume using SYBR Green PCR Master MIX (Applied Biosystems, Foster City, CA). Primers sequences were as follows: CYP 1A1, F-5′TTAACTCTTCCCTGGATGCC3′ and R-5′GGTTGGTTA CCAGGTACATG3′; COMT, F-5′ATGTGAGGGGGAGCAGTAGC3′ and R-5′GGGGGTCAGAGTGAGTGTGT3′. Data were analyzed using a comparative critical threshold (Ct) method (19), with the amount of target gene normalized to the average of two endogenous control genes (18S ribosomal RNA and cyclophilin B). Percent difference was calculated by 2−ΔΔCt (20).
RESULTS
Dose-dependent activation of AMPK by E2
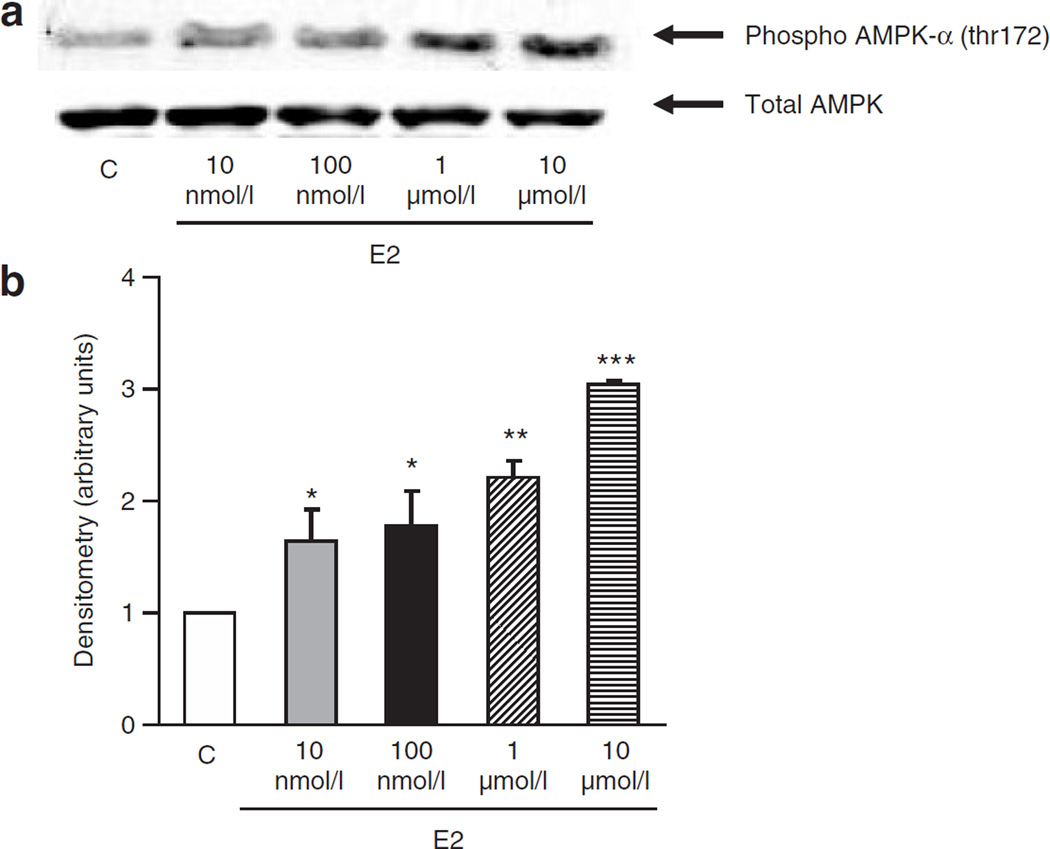
Activation of AMPK is believed to be a target for the prevention of insulin resistance and the subsequent development of type 2 diabetes (9,15). We have previously shown (8) that AMPK is activated (phosphorylated) with µmol/l concentrations of E2 with questionable phosphorylation at lower (nmol/l) E2 concentrations. Using longer exposures of the western blots, (Figure 1a) and careful quantification by densitometry (Figure 1b), we found that E2 rapidly activates AMPK in a dose-dependent manner (10 nmol/l to 10 µmol/l). Although there is moderate activation at low E2 concentrations (1.5 and 1.7-fold with 10 and 100 nmol/l, respectively), further AMPK activation was observed at higher E2 concentrations (two- and threefold with 1–10 µmol/l, respectively) (Figure 1b). This concentration–response relationship is surprising in light of the fact that ERs are saturated with nmol/l concentrations of E2, and suggests the metabolism of E2 may be involved. For example, high concentrations of E2 may be required, above normal saturating concentrations, if the metabolism of E2 is rapid and AMPK activation is completely ER dependent (16). Alternatively, it is possible that there is a non-ER mediated signal, potentially elicited by a product of E2 metabolism, which acts either alone or in concert with an ER-dependent mechanism to further activate AMPK. Therefore, we decided to investigate whether a product of E2 metabolism mediates some or all of E2 actions on AMPK activation.
Figure 1.
Dose-dependent activation of AMP-activated protein kinase (AMPK) with estradiol (E2) treatment. (a) Western blots of phosphorylated (threonine 172) AMPK (upper blot) and total AMPK (lower blot). C2C12 myotubes were treated for 5 min with 10 nmol/l to 10 µmol/l E2. AMPK is activated by E2 in a dose-dependent manner. Representative blot of four separate experiments. (b) Densitometry of western blots. Experiments were repeated four times and average values are reported. (*P < 0.05, **P < 0.01, ***P < 0.001, relative to control).
C2C12 myotubes express the enzymes involved in E2 metabolism
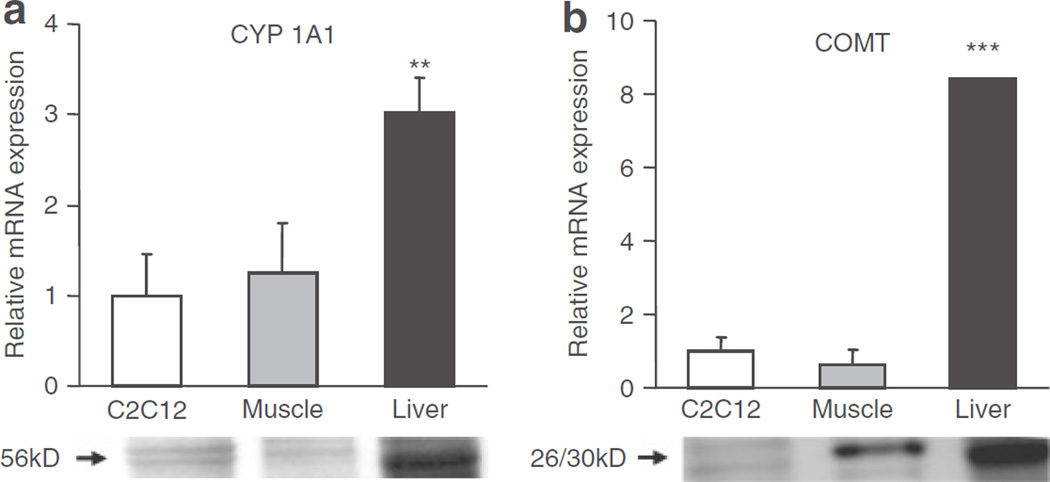
In vivo, E2 is believed to be metabolized primarily in the liver, where CYP450 enzymes are highly expressed. Previous studies have suggested that only the vascular cells in muscle express CYP450 enzymes (21), rendering it highly unlikely that cultured C2C12 myotubes are capable of this conversion. Therefore, we wanted to determine whether the in vitro model being used (C2C12 myotubes) expressed the enzymes needed for E2 metabolism. We compared the mRNA (top panel) and protein (bottom panel) expression of CYP 1A1, the enzyme responsible for conversion of E2 to 2-HE2, in C2C12 myotubes, mouse muscle (which would also contain vascular cells), and liver (Figure 2a). We demonstrated the presence of CYP 1A1 in C2C12 msyotubes.
Figure 2.
Estradiol-metabolizing enzymes, cytochrome P450 1A1 (CYP 1A1) and catechol O-methyl transferase (COMT), are expressed in C2C12 myotubes. (a) Relative mRNA (top panel) and protein (bottom panel) expression of CYP 1A1 and (b) COMT. (n = 3–5 samples per tissue). (**P < 0.01, ***P < 0.001, relative to C2C12).
Subsequently, 2-HE2 is further metabolized to 2-ME2 by the enzyme COMT. We demonstrate a similar expression pattern for COMT at both the mRNA (top panel) and protein (bottom panel) level suggesting our cell system is capable of complete E2 breakdown in this pathway (Figure 2b).
2-HE2 directly activates AMPK
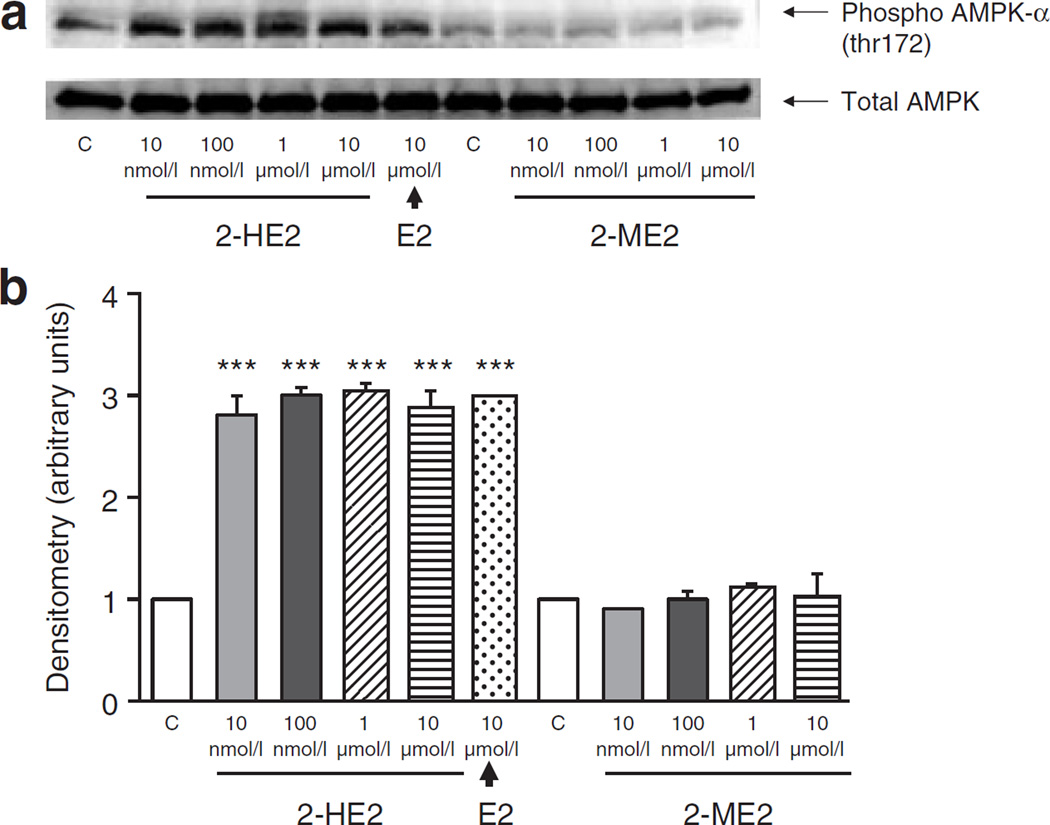
To determine whether E2 metabolites contribute to E2-mediated activation of AMPK, C2C12 myotubes were incubated with varying concentrations of 2-HE2 or 2-ME2. We demonstrate that 2-HE2 activates AMPK in myotubes (Figure 3). Unlike our observations with E2, this activation was maximal at 10 nmol/l. The by-product of 2-HE2 metabolism, 2-ME2, did not activate AMPK (Figure 3). In summary, the catechol E2 2-HE2, but not the methoxyestradiol 2-ME2, increases AMPK phosphorylation in C2C12 myotubes.
Figure 3.
2-Hydroxyestradiol (2-HE2) but not 2-methoxyestradiol (2-ME2) activates AMP-activated protein kinase (AMPK). (a) Western blots of phosphorylated (threonine 172) AMPK (upper blot) and total AMPK (lower blot). C2C12 myotubes were treated with estradiol (E2)-metabolites as described for 5 min. AMPK is activated by 2-HE2 but not by 2-ME2. (b) Densitometry of western blots. Experiments were repeated three times and average values are reported. (***P < 0.001, relative to control).
E2 activates AMPK in the absence of 2-HE2 conversion
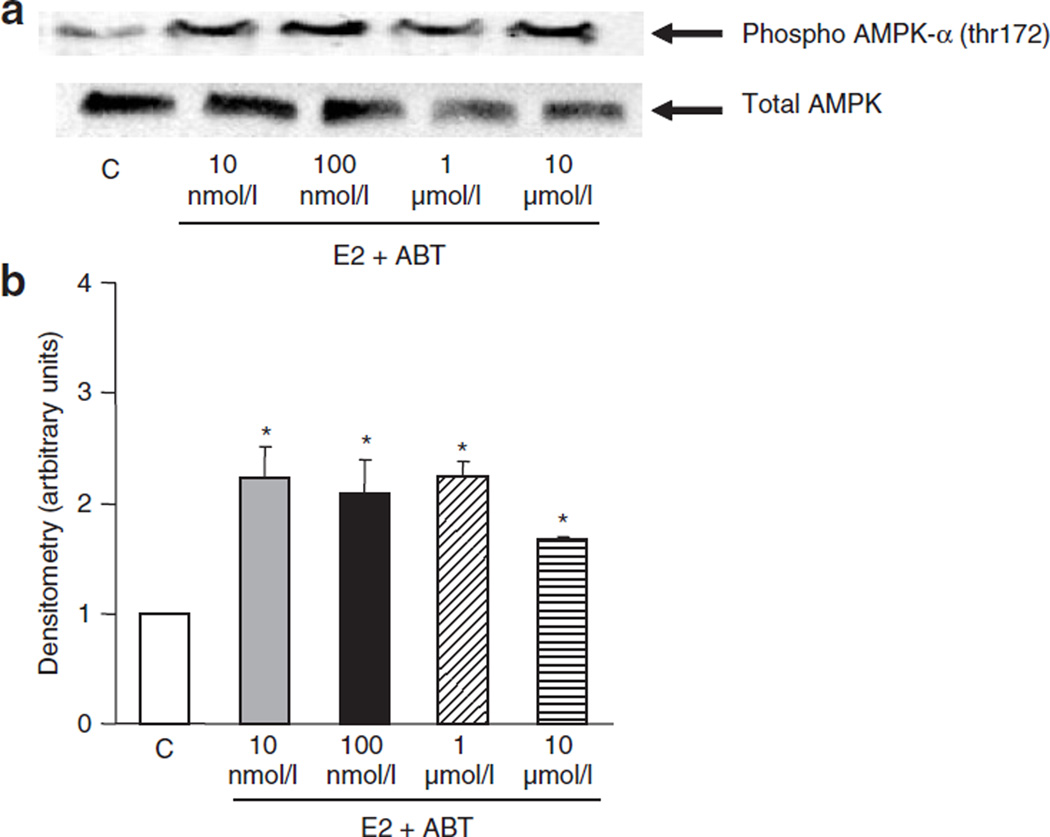
To determine whether E2-induced activation of AMPK is entirely due to 2-HE2, we used a CYP450 inhibitor, ABT (Figure 4), which prevents E2 breakdown and the subsequent production of catechol estrogens (22). Addition of ABT shifted the concentration–response curve to the left, such that 10 nmol/l E2 enhanced AMPK activity twofold with no further activation observed at higher E2 concentrations (Figure 4). However, ABT also prevented the maximal stimulation we observed with 10 µmol/l E2 (maximal activation was twofold compared to threefold with E2 alone (Figure 1)). This suggests that both ER-dependent and independent pathways are involved in the maximal activation of AMPK.
Figure 4.
Inhibition of E2 breakdown (and 2-hydroxyestradiol synthesis) enhances AMP-activated protein kinase (AMPK) activation at lower E2 concentration and prevents maximal stimulation at higher E2 concentration. C2C12 myotubes were treated with E2 in the presence of CYP450 inhibitor, ABT (10 µmol/l), for 5 min. Preventing E2 breakdown and the subsequent synthesis of 2-HE2 increased AMPK activation at low E2 concentrations (10–100 nmol/l), but prevented maximal stimulation at the higher concentration (10 µmol/l). (a) Western blots of phosphorylated (threonine 172) AMPK (upper blot) and total AMPK (lower blot) (b) Densitometry of western blots. (*P < 0.05, relative to control).
DISCUSSION
Estrogen is a steroid hormone which elicits its effects through both genomic and nongenomic mechanisms (23–26). It circulates in the human body primarily in the form of 17-β E2, but there is a growing body of literature suggesting that some biological effects may be mediated by its metabolites (22). One of the major pathways of E2 metabolism involves the hydroxylation of E2 to form a catechol E2, 2-HE2, and the subsequent methylation of this catechol E2 to form 2-ME2.
Previously, we demonstrated the ability of E2 to activate AMPK in a dose and time-dependant manner. However, maximal activation of AMPK was not achieved until the E2 concentration used (10 µmol/l) was well above the saturation level of the ERs. There are at least two possible scenarios that explain why concentrations of E2 that exceed ER binding capacity can enhance AMPK activation. One hypothesis, provided by Simpson and McInnes (16), is that if E2 activates AMPK solely through activation of ER, there might be “extremely rapid metabolism of E2” by the C2C12 myotubes. If this were true, high concentrations of E2 may constantly replenish the rapidly metabolized supply of E2 for ER binding, thus prolonging and enhancing the activation of AMPK. Alternatively, it is possible that there is a non-ER mediated signal, potentially elicited by a product of E2 metabolism, which acts either alone or in concert with an ER-dependent mechanism to further activate AMPK.
Although, we previously demonstrated that the ER antagonist, ICI 182,780 completely inhibits E2-mediated activation of AMPK at 10 µmol/l, suggesting an ER-dependent mechanism (8), recent studies have demonstrated that ICI 182, 780 (10 µmol/l) in addition to blocking the actions of ERs, can also prevent the conversion of E2 to its metabolites (27). Therefore, this study used new approaches to test the ability of estrogen and its metabolites to activate AMPK. Incubation of myotubes with 2-HE2 or 2-ME2 identified 2-HE2 as a potent activator of AMPK (Figure 3). In order to assess the relative contributions of E2 and 2-HE2 to AMPK activation, an inhibitor of E2 metabolism (ABT) was employed. Preventing the breakdown of E2 increased activation of AMPK at low E2 concentrations possibly by increasing its half-life and the amount of E2 available for binding to ERs. In contrast, maximal activation at high E2 concentrations was blunted by ABT, suggesting that an E2 metabolite, in addition to E2 acting via ERs, may be responsible for maximal activation of AMPK.
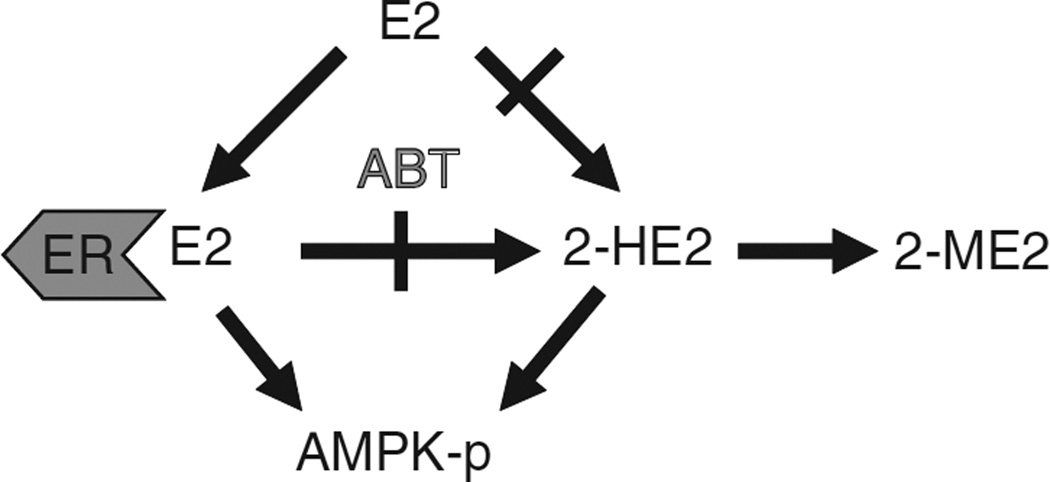
Overall, our findings demonstrate that both E2 and its metabolite, 2-HE2, activate AMPK in muscle. We propose a model where E2 acts both directly and through its metabolite 2-HE2 to increase AMPK activation (Figure 5). Within this model, AMPK activation is ceased when E2 is completely metabolized to 2-ME2. Consistent with this hypothesis, E2-mediated phosphorylation of AMPK is diminished after 15 min (data not shown).
Figure 5.
Model of estradiol’s (E2’s) effects on AMP-activated protein kinase (AMPK) activation in muscle. E2, acting through ERs, activates AMPK. In addition, the E2 metabolite 2-HE2 activates AMPK in an ER-independent manner, and thus increases AMPK activation at high E2 concentrations.
AMPK is an intracellular signaling molecule with a growing list of functions related to muscle metabolism (9). Its activation acutely increases fat oxidation, inhibits fat synthesis, and increases glucose uptake (9). In addition, chronic AMPK activation has been shown to prevent obesity and insulin resistance, at least in part through increasing mitochondrial biogenesis (15,28). Our studies suggest that activation of AMPK in muscle may render 2-HE2 protective against the development of metabolic disease. In support of this, continuous 2-HE2 treatment in rats reduced adiposity and the development of diabetes (29) suggesting this may be related to 2-HE2-activation of AMPK. Further support for our model (Figure 5) comes from a recent paper by Zhang et al. (30) which demonstrates that unlike 2-HE2, 2-ME2 is not protective against obesity and diabetes. In addition, 2-HE2 has been shown to activate AMPK in endothelial cells suggesting beneficial effects on multiple tissues (31). Interestingly, in endothelial cells E2 alone did not have any effect on AMPK activation. Although the reason for this difference is not immediately evident, it may be related to tissue-specific differences in upstream kinases, E2 receptor expression (i.e., ER-α vs. ER-β density) and/or methodological differences.
In summary, we demonstrate that 2-HE2 rapidly activates AMPK in myotubes suggesting that some of the reported metabolic benefits of E2 may be related to 2-HE2 actions. Although the rapid degradation of 2-HE2 does not make it a good pharmacological agent, identification of its receptor and the mechanism by which it activates AMPK, may have implications for the prevention of adiposity and metabolic disease.
Acknowledgments
This work was supported in part by NIH R01 DK50647 and US Department of Agriculture, Agricultural Research Service, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture. T.M.D. was supported by the Canadian Institute for Health Research Doctoral Fellowship and the Woodrow-Wilson Johnson & Johnson Dissertation Fellowship for Research in Women’s Health. We thank Dr Perfield and Dr Curtis for critical review of the article.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 2.Park Y-W, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gambacciani M, Ciaponi M, Cappagli B, et al. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas. 2001;39:125–132. doi: 10.1016/s0378-5122(01)00194-3. [DOI] [PubMed] [Google Scholar]
- 4.Jensen LB, Vestergaard P, Hermann AP, et al. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2003;18:333–342. doi: 10.1359/jbmr.2003.18.2.333. [DOI] [PubMed] [Google Scholar]
- 5.Kritz-Silverstein D, Barrett-Connor E. Long-term postmenopausal hormone use, obesity, and fat distribution in older women. JAMA. 1996;275:46–49. [PubMed] [Google Scholar]
- 6.Sumino H, Ichikawa S, Yoshida A, et al. Effects of hormone replacement therapy on weight, abdominal fat distribution, and lipid levels in Japanese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27:1044–1051. doi: 10.1038/sj.ijo.0802371. [DOI] [PubMed] [Google Scholar]
- 7.Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 8.D’Eon TM, Souza SC, Aronovitz M, et al. Estrogen regulation of adiposity and fuel partitioning: Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–39991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 9.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeBrasseur NK, Kelly M, Tsao T-S, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase (AMPK) in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–E181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 12.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee RR, Rangwala SM, Shapiro JS, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 15.Pold R, Jensen LS, Jessen N, et al. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes. 2005;54:928–934. doi: 10.2337/diabetes.54.4.928. [DOI] [PubMed] [Google Scholar]
- 16.Simpson ER, McInnes KJ. Sex and fat—can one factor handle both? Cell Metab. 2005;2:346–347. doi: 10.1016/j.cmet.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Philips BJ, Ansell PJ, Newton LG, et al. Estrogen receptor-independent catechol estrogen binding activity: protein binding studies in wild-type, estrogen receptor-α KO, and aromatase KO mice tissues. Biochemistry. 2004;43:6698–6708. doi: 10.1021/bi036154j. [DOI] [PubMed] [Google Scholar]
- 18.Shih HH, Tevosian SG, Yee AS. Regulation of differentiation by HBP1, a target of the retinoblastoma protein. Mol Cell Biol. 1998;18:4732–4743. doi: 10.1128/mcb.18.8.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Mikhailova ON, Gulyaeva LF, Filipenko ML. Gene expression of drug metabolizing enzymes in adult and aged mouse liver: a modulation by immobilization stress. Toxicology. 2005;210:189–196. doi: 10.1016/j.tox.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Smith C, Stamm SC, Riggs JE, et al. Ethanol-mediated CYP1A1/2 induction in rat skeletal muscle tissue. Exp Mol Pathol. 2000;69:223–232. doi: 10.1006/exmp.2000.2328. [DOI] [PubMed] [Google Scholar]
- 22.Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther. 2004;308:403–409. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- 23.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 24.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 25.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 26.Evinger AJ, III, Levin ER. Requirements for estrogen receptor α membrane localization and function. Steroids. 2005;70:361–363. doi: 10.1016/j.steroids.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Dubey RK, Jackson EK, Gillespie DG, et al. Cytochromes 1A1/1B1- and catechol-O-methyltransferase-derived metabolites mediate estradiol-induced antimitogenesis in human cardiac fibroblast. J Clin Endocrinol Metab. 2005;90:247–255. doi: 10.1210/jc.2003-032154. [DOI] [PubMed] [Google Scholar]
- 28.Bergeron R, Ren JM, Cadman KS, et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 29.Tofovic SP, Dubey RK, Jackson EK. 2-Hydroxyestradiol attenuates the development of obesity, the metabolic syndrome, and vascular and renal dysfunction in obese ZSF1 rats. J Pharmacol Exp Ther. 2001;299:973–977. [PubMed] [Google Scholar]
- 30.Zhang X, Jia Y, Jackson EK, Tofovic SP. 2-Methoxyestradiol and 2-ethoxyestradiol retard the progression of renal disease in aged, obese, diabetic ZSF1 rats. J Cardiovasc Pharmacol. 2007;49:56–63. doi: 10.1097/FJC.0b013e31802cb88e. [DOI] [PubMed] [Google Scholar]
- 31.Schulz E, Anter E, Zou M-H, Keaney JF., Jr Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation. 2005;111:3473–3480. doi: 10.1161/CIRCULATIONAHA.105.546812. [DOI] [PubMed] [Google Scholar]