Abstract
STUDY QUESTION
Do the long-term health outcomes following IVF differ depending upon the duration of embryo culture before transfer?
SUMMARY ANSWER
Using a mouse model, we demonstrate that in male but not female offspring, adverse cardiovascular (CV) health was more likely with prolonged culture to the blastocyst stage, but metabolic dysfunction was more likely if embryo transfer (ET) occurred at the early cleavage stage.
WHAT IS KNOWN ALREADY
ART associate with increased risk of adverse CV and metabolic health in offspring, and these findings have been confirmed in animal models in the absence of parental infertility issues. It is unclear which specific ART treatments may cause these risks. There is increasing use of blastocyst, versus cleavage-stage, transfer in clinical ART which does not appear to impair perinatal health of children born, but the longer-term health implications are unknown.
STUDY DESIGN, SIZE, DURATION
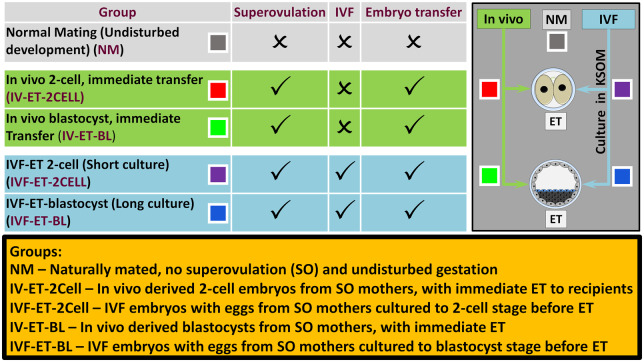
Five mouse groups were generated comprising: (i) natural mating (NM)—naturally mated, non-superovulated and undisturbed gestation; (ii) IV-ET-2Cell—in-vivo derived two-cell embryos collected from superovulated mothers, with immediate ET to recipients; (iii) IVF-ET-2Cell—IVF generated embryos, from oocytes from superovulated mothers, cultured to the two-cell stage before ET to recipients; (iv) IV-ET-BL—in-vivo derived blastocysts collected from superovulated mothers, with immediate ET to recipients; (v) IVF-ET-BL—IVF generated embryos, from oocytes from superovulated mothers, cultured to the blastocyst stage before ET to recipients. Both male and female offspring were analysed for growth, CV and metabolic markers of health. There were 8–13 litters generated for each group for analyses; postnatal data were analysed by multilevel random effects regression to take account of between-mother and within-mother variation and litter size.
PARTICIPANTS/MATERIALS, SETTINGS, METHODS
C57/BL6 female mice (3–4 weeks old) were used for oocyte production; CBA males for sperm with human tubal fluid medium were used for IVF. Embryos were transferred (ET) to MF1 pseudo-pregnant recipients at the two-cell stage or cultured in synthetic oviductal medium enriched with potassium medium to the blastocyst stage before ET. Control in-vivo embryos from C57BL6 × CBA matings were collected and immediately transferred at the two-cell or blastocyst stage. Postnatal assays included growth rate up to 27 weeks; systolic blood pressure (SBP) at 9, 15 and 21 weeks; lung and serum angiotensin-converting enzyme (ACE) activity at time of cull (27 weeks); glucose tolerance test (GTT; 27 weeks); basal glucose and insulin levels (27 weeks); and lipid accumulation in liver cryosections using Oil Red O imaging (27 weeks).
MAIN RESULTS AND THE ROLE OF CHANCE
Blastocysts formed by IVF developed at a slower rate and comprised fewer cells that in-vivo generated blastocysts without culture (P < 0.05). Postnatal growth rate was increased in all four experimental treatments compared with NM group (P < 0.05). SBP, serum and lung ACE and heart/body weight were higher in IVF-ET-BL versus IVF-ET-2Cell males (P < 0.05) and higher than in other treatment groups, with SBP and lung ACE positively correlated (P < 0.05). Glucose handling (GTT AUC) was poorer and basal insulin levels were higher in IVF-ET-2Cell males than in IVF-ET-BL (P < 0.05) with the glucose:insulin ratio more negatively correlated with body weight in IVF-ET-2Cell males than in other groups. Liver/body weight and liver lipid droplet diameter and density in IVF-ET-2Cell males were higher than in IVF-ET-BL males (P < 0.05). IVF groups had poorer health characteristics than their in-vivo control groups, indicating that outcomes were not caused specifically by background techniques (superovulation, ET). No consistent health effects from duration of culture were identified in female offspring.
LARGE SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
Results from experimental animal models cannot be extrapolated to humans. Nevertheless, they are valuable to develop conceptual models, in this case, in the absence of confounding parental infertility, in assessing the safety of ART manipulations.
WIDER IMPLICATIONS OF THE FINDINGS
The study indicates that longer duration of embryo culture after IVF up to blastocyst before ET leads to increased dysfunction of CV health in males compared with IVF and shorter cleavage-stage ET. However, the metabolic health of male offspring was poorer after shorter versus longer culture duration. This distinction indicates that the origin of CV and metabolic health phenotypes after ART may be different. The poorer metabolic health of males after cleavage-stage ET coincides with embryonic genome activation occurring at the time of ET.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported through the European Union FP7-CP-FP Epihealth programme (278418) and FP7-PEOPLE-2012-ITN EpiHealthNet programme (317146) to T.P.F., the Biotechnology and Biological Sciences Research Council (BBSRC) (BB/F007450/1) to T.P.F., and the Saudi government, University of Jeddah and King Abdulaziz University to A.A. The authors have no conflicts of interest to declare.
Keywords: ART, mouse IVF and embryo culture, embryo transfer, blastocyst, DOHaD, offspring long-term health, growth trajectory, CV health, metabolic health, liver phenotype
Introduction
Infertility is thought to affect an estimated 186 million people globally (Inhorn and Patrizio, 2015). The development of ART has provided a partial clinical resolution to infertility with over 8 million children born to date, representing some 2–6% births in developed countries (Berntsen et al., 2019; Crawford and Ledger, 2019). Although most IVF children appear healthy according to numerous systematic reviews, ART has been linked with a small increased risk of adverse obstetric and perinatal outcomes and birth defects compared with naturally conceived children (Pinborg et al., 2013; Qin et al., 2017; Berntsen et al., 2019). In addition, longer-term health concerns of ART offspring have been associated mainly with: altered birthweight and growth (Ceelen et al., 2009; Kleijkers et al., 2014, 2016); increased risk of cardiovascular (CV) dysfunction comprising CV remodelling during pregnancy with vascular impairment and raised blood pressure evident in children through to at least adolescence (Ceelen et al., 2008, 2009; Sakka et al., 2010; Scherrer et al., 2012; Valenzuela-Alcaraz et al., 2013; Zhou et al., 2014; von Arx et al., 2015; Guo et al., 2017; Meister et al., 2018); and susceptibility to metabolic dysfunction including poorer glucose handling, insulin resistance and increased triglycerides (Sakka et al., 2010; Chen et al., 2014; Gkourogianni et al., 2014; Pontesilli et al., 2015; Guo et al., 2017). In a minority of studies, impairment to neurological and cognitive health have also been reported (Sandin et al., 2013; Liu et al., 2017; Goldsmith et al., 2018).
These sustained health effects have been linked to the ‘Developmental Origins of Health and Disease’ (DOHaD) concept suggesting environmental factors during development, especially the peri-conceptional period, may alter subsequent growth and morphogenesis through epigenetic, cellular and physiological processes (Feuer and Rinaudo, 2016; Fleming et al., 2018). However, evaluation of ART children’s health is complex and confounded by the actual technologies and precise protocols applied in clinics, the gradual refinement in practice over time, and appropriateness of controls and comparator groups to distinguish between consequences mediated through parental infertility and ART practice (Berntsen et al., 2019).
With these considerations in mind, animal models have been invaluable to assess effects of ART-associated technologies on long-term offspring health, removing confounders such as parental infertility and treatment variability and including suitable controls. These indicate ART treatments do indeed affect long-term health. Thus, IVF and/or mouse embryo culture and transfer result in offspring with altered growth trajectory, CV abnormalities and glucose/insulin dysfunction (Watkins et al., 2007; Scott et al., 2010; Le et al., 2013; Rexhaj et al., 2013; Chen et al., 2014; Donjacour et al., 2014; Feuer et al., 2014; Ramirez-Perez et al., 2014; Schenewerk et al., 2014; Cerny et al., 2017; Wang et al., 2018).
In the last decade, there has been a gradual switch in ART practice from cleavage-stage embryo transfer (ET) to blastocyst stage ET to facilitate embryo selection and improve synchronicity with the uterine environment, despite the potential risk of increased embryo environmental perturbation. Whilst fresh blastocyst ET may marginally improve the live birth rate (Glujovsky et al., 2016) without significantly affecting birthweight (De Vos et al., 2018) or the risk of adverse perinatal outcomes (Shi et al., 2019), it is unknown whether extended culture negatively impacts on later health status. In the current study, we have used a mouse model to assess the effect of cleavage or blastocyst ET on offspring health across a range of growth, CV and metabolic criteria.
Materials and methods
Animals
Animal treatments were conducted in accordance with the UK Home Office Animal (Scientific procedure) Act 1986 and local ethics committee at the University of Southampton. CBA male and C57/BL6 female mice (source of embryos) and MF1 females (pseudo-pregnant recipients) were bred in-house (University of Southampton, Biomedical Research Facility) on a 07:00–19:00 light cycle, 24°C and fed ad libitum from weaning on a standard chow diet (Special Diet Service, Ltd, Witham, Essex, UK) and water.
Embryo production and treatment
Virgin female C57/BL6 mice (3–4 weeks old) were superovulated by i.p. injection of 5 IU pregnant mare’s serum gonadotropin (PMSG, Intervet, Cambridge, UK) and 46 h later, 5 IU hCG (Intervet, Cambridge, UK). For in-vivo produced embryos, females were housed overnight with CBA males. Plug positive females at embryonic day 0.5 (E0.5) (i.e. midday of plug detection day) were housed individually and, at E1.5 and E3.5, females were killed by cervical dislocation and two-cell embryos and blastocysts were flushed from dissected oviducts and uteri, respectively, into pre-warmed H6 medium supplemented with 4 mg/ml bovine serum albumin (BSA, A3311, Sigma, UK) (Nasr-Esfahani et al., 1990). Some females were also naturally mated without superovulation.
For IVF embryo production, sperm was retrieved from the cauda epididymis of CBA males (8 weeks old) and placed into 90 µl sperm pre-incubation medium TYH-MBCD (Takeo and Nakagata, 2011) made in-house and equilibrated for 1 h at 37°C in 5% CO2 in air. C57/BL6 females were superovulated as above and cumulus masses, collected from the oviduct ampulla 13 h post-hCG injection, were placed directly into 200 µl fertilisation drop containing human tubal fluid (HTF) medium made in-house with 1.0 mM reduced glutathione (GSH, Sigma: G4251). Sperm (3-5 µl from pre-equilibrated TYH-MBCD drop) were added to the fertilisation drop and incubated for 3–4 h to allow fertilisation to occur (Ishizuka et al., 2013). Presumptive zygotes were washed through four drops HTF medium without GSH and then cultured in the fourth drop under oil at 37°C and 5% CO2 in air to the next day (E1.5) before calculating the fertilisation rate. IVF embryos (two-cell stage) were then divided into two groups, the first was washed in pre-warmed M2 medium (Sigma; Cat No. M7167) before transfer to E0.5 MF1 pseudo-pregnant mothers. The second group was cultured in potassium simplex optimised medium with amino acids and BSA (synthetic oviductal medium enriched with potassium; Sigma-Aldrich) (Biggers et al., 2005) at 37°C in 5% CO2 in air to the blastocyst stage before washing in M2 medium and transfer to E2.5 MF1 pseudo-pregnant mothers.
In-vivo and IVF-generated blastocyst trophectoderm (TE) and inner cell mass (ICM) cell numbers were determined by differential nuclear staining as described (Handyside and Hunter, 1984) with modifications (Velazquez et al., 2018).
Embryo transfer
ET was performed by flank laparotomy in pseudo-pregnant MF1 recipients (7–8.5 weeks) obtained by mating with vasectomised MF1 males. Two-cell embryos and blastocysts were washed three times in M2 medium prior to ET into the oviduct and uteri, respectively, in minimal medium, as previously described (Velazquez et al., 2018). Recipients were anaesthetised by a single intraperitoneal injection of Ketamine (50 mg/kg, Ketaset, Pfizer, UK) and Xylazine (10 mg/kg, Rompun, Bayer, UK). Embryos were transferred (19.7 ± 6.05 per recipient) in equal numbers into both maternal tracts with separate recipients used for different treatments, as below. After transfer, exposed tracts were placed back into the abdominal cavity, the peritoneum was sutured, and the skin was closed with wound clips. Recipients were then kept individually in a clean cage in a warm room (28–30°C) to recover from anaesthesia. Females were then housed in a quiet room for the rest of their pregnancy and lactation. Litter size was adjusted to up to 8 per dam at birth with similar numbers of males and females.
Animal treatment groups
Eight to 13 litters were generated from each of five treatments with groups termed as follows: (i) natural mating (NM)—naturally mated, non-superovulated and undisturbed gestation; (ii) IV-ET-2Cell—in-vivo derived two-cell embryos collected from superovulated mothers, with immediate ET to recipients; (iii) IVF-ET-2Cell—IVF generated embryos with oocytes from superovulated mothers cultured to the two-cell stage before ET to recipients; (iv) IV-ET-BL—in vivo derived blastocysts collected from superovulated mothers, with immediate ET to recipients; (v) IVF-ET-BL—IVF generated embryos with oocytes from superovulated mothers cultured to the blastocyst stage before ET to recipients. These treatment groups are shown in Fig. 1.
Figure 1.
Experimental design showing the five treatment groups used.
Offspring analysis
All offspring from the five treatment groups were weaned at 3 weeks and males and females were caged separately per litter. Offspring body weight was recorded weekly for 27 weeks. Systolic blood pressure (SBP) was measured at postnatal weeks 9, 15 and 21 by tail-cuff plethysmography with Non-Invasive Blood Pressure Monitor (NIBP-8, Columbus Instruments, Columbus, OH, USA) in a pre-warmed room (28–30°C) to which mice were acclimatised for 90 min, as described previously (Velazquez et al., 2018). Five SBP readings with good waveforms and good overall quality were taken per mouse, and the mean value of the three middle readings was calculated and recorded. Heart rate was monitored as an indicator of stress, and if reaching >500 beats per minute, SPB readings were delayed until heart rate reduced. Glucose tolerance tests (GTT) were conducted at postnatal week 27 in unrestrained conscious mice after 15 h overnight fast, with access to water. A standard protocol for GTT using a blood glucose meter (Accu-Chek Aviva, Roche Diagnostics GmbH, Germany) to measure blood glucose in small drops collected by tail tipping was employed. Topical anaesthetic cream (Lidocaine 5%, Teva, UK) was applied to the tail 20 min before starting the GTT. After recording of fasting glucose level (0 min), a glucose (G8270, Sigma) solution (20%, in sterile distilled water) was i.p. injected at a dose of 2 g/kg. Blood glucose levels were measured at 15, 30, 60 and 120 min after glucose administration. Area under the curve (AUC) values were calculated by the trapezoidal rule (Matthews et al., 1990). Organ Allometry was determined 2 days after GTT: mice were sacrificed by cervical dislocation, blood was collected by heart puncture and organs (i.e. liver, heart, left and right kidneys, lung and spleen) were weighed, snap frozen in liquid nitrogen and stored at −80°C. Blood samples were centrifuged at 4°C for serum collection and stored at −80°C.
Angiotensin-converting enzyme activity
The method was used as previously (Watkins et al., 2006, 2007) to measure serum and lung angiotensin-converting enzyme (ACE) activities, the classical enzyme regulator of the renin-angiotensin system converting Angiotensin I to the vasopressor Angiotensin II (Li et al., 2017). The assay is based on the colourimetric determination of hippurate with cyanuric chloride/dioxan reagent. Briefly, for serum ACE activity, samples were incubated in hippuryl-l-histidyl-l-leucine (HHL; Sigma) solution in H3PO3 buffer at 37°C, the reaction was terminated with HCl (Sigma) followed by addition of cyanuric chloride (Sigma) in 1,4-dioxan (Sigma) for yellow colouration to develop. Four replicates per sample were analysed using a plate reader (Varioskan Flash Multimode Reader; Thermo Scientific) at 380 nm. Negative controls comprised addition of HCl before HHL. A Hippurate standard curve (20–100 µM) was prepared from 112 mg Hippuric acid (Sigma) solution in 250 ml 20 mmol/l NaOH, treated as samples except the addition of HHL. Each of the four replicates per sample were analysed in duplicate, and the average of these eight readings taken. For lung ACE activity, lung samples of 50 ± 1 mg were homogenised in 300 μl ice-cold boric buffer (H3BO3, 2M NaCl, pH 8.3; Sigma) with a PowerGen homogeniser, centrifuged at 16 400 rpm for 10 min at 4°C and the supernatant was removed and stored at −80°C. Pellets were homogenised in 300 μl buffer and centrifuged, and the supernatant was removed and stored. Duplicate analysis of four replicate supernatants per sample was analysed as described for serum ACE activity. Total protein content of samples was measured using a BioRad kit. Serum ACE activity was expressed as amount (in µM) of hippurate formed per millilitre of serum per minute; lung ACE activity was expressed as amount (in nanomolar) of hippurate formed per milligram of protein per minute. Serum and lung samples were selected at 27 weeks from the same offspring at the middle weight across litters from the five treatment groups (one male and one female from each of 7–9 mothers per treatment) and stored frozen. These same offspring were used for serum glucose and insulin assays and for the liver lipid metabolism assay.
Serum glucose and insulin analysis
Glucose concentration in offspring serum was measured using the glucometer as described in the GTT procedure. Serum insulin concentration was determined using an ELISA kit (Mercodia, Sweden, Mouse: 10-1247-01) based on the manufacturer’s instructions. Briefly, 10 µl of each calibrator 0, 1, 2, 3, 4 and 5 and serum samples were incubated in coated microplate wells with 100 µl enzyme conjugate solution on a plate shaker at room temperature at 750 rpm for 2 h, before washing in 350 µl of wash buffer repeated five times, before addition of 200 µl TMB substrate and incubation for 15 min, before addition of 50 µl stop solution. Absorbance was measured at 450 nm using a Varioskan Flash Multimode Reader (Thermo Scientific). Standard deviation and coefficient of variance were calculated for each sample run in duplicate in three plates and mean insulin values were calculated. The glucose/insulin ratio (G:I) ratio was calculated to assess insulin resistance (McAuley et al., 2001). A total of 6–8 samples from each treatment, both male and female and each from a separate mother, were used for combined glucose and insulin analyses.
Liver morphometrics and metabolism
Frozen-stored adult offspring median lobe liver samples were embedded in OCT-compound and cryosections at 7 µm were generated and stained with Oil Red O to visualise lipid accumulation and Mayer’s Haematoxylin as counterstain before mounting in aqueous medium and applying coverslips. Images of sections were analysed and photographed using an Olympus dotSlide Virtual Microscopy System with an Olympus BX61 Microscope Frame at 10× magnification. Images (3 per liver sample) were quantified using Fiji software for red-stained lipid accumulation with the Watershed tool applied to separate grouped lipid droplets. A total of 6–9 offspring from each treatment, both male and female and each from a separate mother, were used for liver analyses.
Statistics
Statistical analyses were performed with the IBM SPSS Statistics software, version 21 (IBM Corporation) and significance was taken as P ≤ 0.05. If a P-value of between 0.1 and 0.05 was observed, a trend was assumed to exist. Blastocyst cell number, rates of blastocyst development and ET outcome (i.e. pregnancy rate, ET efficiency and litter size) were analysed using a one-way ANOVA followed by a pairwise t-test with Bonferroni correction analysis. Percentage data were arcsine transformed before ANOVA analysis. Postnatal data, comprising offspring weights, SBP, GTT, organ weights and ratios, post-culling serum glucose and insulin, serum and lung ACE activities and liver lipid accumulation data, were analysed using multilevel random effects regression models to compare treatment groups (Kwong et al., 2004) and to analyse relationships between different readouts (i.e. correlations) within each treatment group (Velazquez et al., 2018). All postnatal data were converted to Z-scores before being analysed with the regression models which took into account between-mother and within-mother variation and litter size (Kwong et al., 2004; Watkins et al., 2008).
Results
IVF and embryo culture delay blastocyst development and reduce cell proliferation
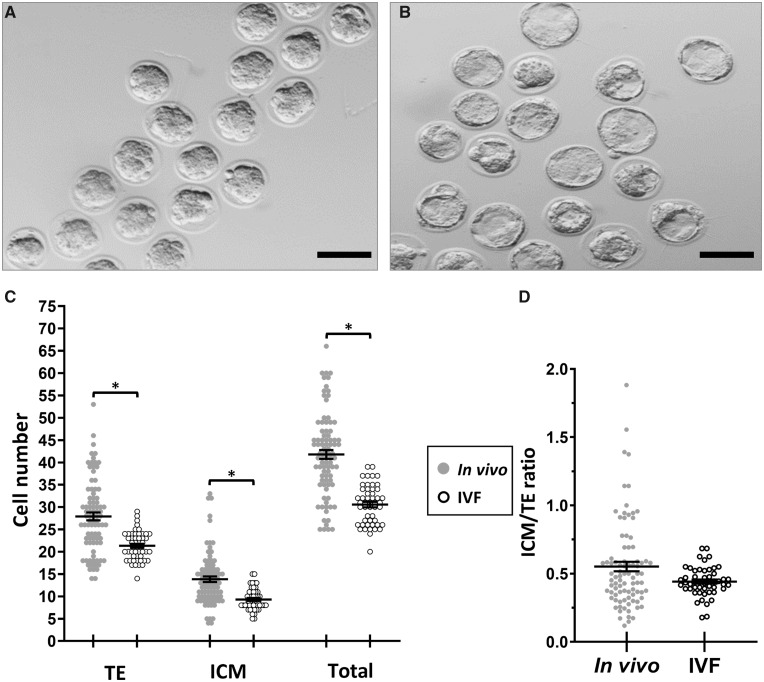
Routine analysis of IVF embryo development was conducted throughout the study, with oocytes (n = 1720) collected from 40 superovulated dams, used in 14 separate IVF experiments, leading to an overall mean success rate of two-cell embryo formation of 92%, and from those allocated to culture, 81% formed morulae and 72% developed to blastocysts. The developmental rate of IVF embryos was compared with in-vivo embryos (superovulated; naturally mated; develop in vivo; collected at E3.5). IVF embryos developed more slowly and only reached the morula stage at E3.5 whilst in-vivo embryos had become expanding blastocysts by then (Table I). IVF embryos became expanding blastocysts by E4.5 (Fig. 2A and B; Table II). Some IVF and in-vivo mid-expanded blastocysts at E4.5 and E3.5 days, respectively, were subjected to differential cell staining which showed increased TE, ICM and total cell numbers in in-vivo versus IVF embryos (P < 0.05) although the ICM:TE ratio did not differ between the two groups (Fig. 2C and D). IVF and prolonged culture therefore delayed blastocyst formation and reduced associated proliferation of both cell lineages compared with in-vivo development.
Table I.
Developmental rate of in vivo and IVF embryos at E3.5 and E4.5.
| Group | Number (%) | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD ± SEM) from each dam | |||||||
| Dam number | Embryo number | Morula | Early blastocyst | Mid blastocyst | Late blastocyst | Arrested (early cleavage) | |
| in vivo | 22 | 469 | 15 (3.2) | 40 (8.5) | 114 (24.3) | 295 (62.9) | 5 (1.1) |
| E3.5 | 0.71 (0.72 ± 0.15) | 1.9 (0.89 ± 0.19) | 5.43 (1.29 ± 0.27) | 14.05 (3.31 ± 0.7) | 0.24 (0.44 ± 0.09) | ||
|
| |||||||
| IVF | 40 | 10761 | 876 (81.4) | 0 | 0 | 0 | 200 (18.6) |
| E3.5 | 62.57 (38.14 ± 6.03) | 14.28 (5.69 ± 0.9) | |||||
| IVF | 109 (12.4)2 | 51 (5.8)2 | 83 (9.5)2 | 633 (72.3)2 | 0 | ||
| E4.5 | 7.78 (5.1 ± 0.81) | 3.64 (1.98 ± 0.31) | 5.92 (3.19 ± 0.5) | 45.21 (28.12 ± 4.45) | |||
Early blastocyst has a blastocoel volume less than half of the total embryo volume.
Mid blastocyst has a blastocoel volume equal to or larger than the total embryo volume.
Late blastocyst blastocoel fully expanded within the embryo whilst the zona pellucida (ZP) is thinning.
Number of 2-cell embryos cultured after IVF.
Per cent of morulae at E3.5.
Figure 2.
Effect of IVF and prolonged embryo culture on blastocyst development cell number. IVF embryos at E3.5 comprise morulae (A) and at E4.5 comprise blastocysts (B); bar = 100 µm. (C) IVF embryos (n = 50) have fewer cells than in-vivo embryos (n = 87) at the blastocyst stage. Mean (±SEM) blastocyst cell number for IVF compared with in-vivo embryos (P < 0.05). (D) Mean (±SEM) ICM/TE ratio of blastocysts. * P < 0.05.
Table II.
Offspring production criteria for the five treatment groups as shown in Fig. 1.
| Treatment Group | ET pregnancy rate 1 % (dam numbers) | ET efficiency 2 % (pups/ embryos transferred) | Birth litter size 3 Mean (SD ± SEM) [litter number] | Offspring number | No. male/ female pups | Ratio Male:Female |
|---|---|---|---|---|---|---|
| NM | N/A | N/A | 8 (1.33 ± 0.42)a [10] | 80 | 40/40 | 1 |
| IV-ET-2Cell | 88.9 (8/9) | 31.7 (57/180)a | 7.12 (4.36 ± 1.54)b [8] | 57 | 32/25 | 1.3 |
| IVF-ET-2Cell | 73.3 (11/15) | 16.7 (75/450)a | 8.33 (3.74 ± 1.25)a1 [9] | 75 | 42/33 | 1.2 |
| IV-ET-BL | 81.8 (9/11)a | 30.5 (47/154)a | 5.88 (1.73 ± 0.61)b [8] | 47 | 22/25 | 0.9 |
| IVF-ET-BL | 48.3 (14/29)b | 9.1 (42/464)b | 3.23 (1.79 ± 0.5)b,b1 [13] | 42 | 26/16 | 1.6 |
Data were analysed using ANOVA (mean± SEM).
Dams that gave birth/total number of ETs performed.
Total number of pups at birth (before litter size correction)/total embryos transferred (7–15 per side).
Calculated on dams with live pups at birth (before litter size correction).
Within a column, values with different letters are significantly different (P < 0.05).
Postnatal offspring from ART treatments display increased body weight
To study the effect of ART and embryo culture duration on postnatal development, we generated the five treatment groups as shown in Fig. 1 with the offspring production criteria shown in Table II. The ET pregnancy rate (% dams giving birth) was significantly higher in the IV-ET-BL group compared with IVF-ET-BL, otherwise no differences were found between groups (Table II). ET efficiency (pups generated per numbers of embryos transferred) was lower in IVF-ET-BL than other groups. Litter size in the ET groups IV-ET-2Cell, IV-ET-BL and IVF-ET-BL was lower than the NM group. The IVF-ET-BL litter size was also lower than the IVF-ET-2Cell group. Male:female ratio was not different between any of the treatment groups (Table II).
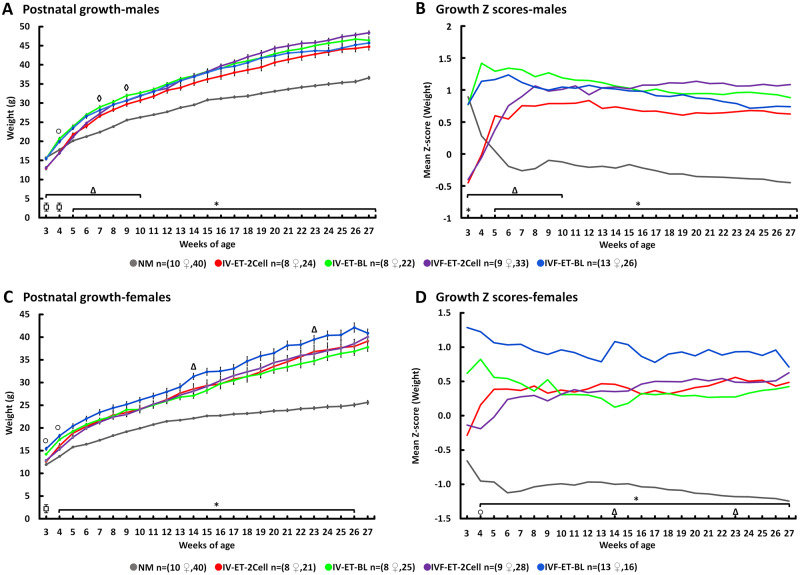
Male and female offspring body weight differences between groups were analysed from weaning through to Week 27, taking into consideration litter size and individual maternal origin. All four ET groups were significantly heavier compared with the NM control group, evident from Week 5 (males) and 4 (females) through to Week 27 (Fig. 3A and C). Z-score plots confirmed increased body weight for all ET groups compared with the NM group up to Week 27 (Fig. 3B and D). Generally, weight differences between different ET groups were minimal and are itemised in the Fig. 3 legend. Notably, IVF-ET-BL female mean weight was heavier than other ET groups throughout the 27-week period (Fig. 3C and D). Thus, the combined techniques of ART (superovulation, IVF, culture, transfer, recipient gestation) in our model, or just some of them (minimal superovulation, transfer, recipient gestation), resulted in sustained increase in postnatal weight in both sexes compared with natural, unstimulated reproduction.
Figure 3.
Effect of ART techniques on growth of offspring. Body weight and Z-score analysis in male (A and B) and female (C and D) offspring. Multilevel random effects regression analysis. *Indicates a significant difference (P < 0.05) between NM and other treatments; ⧮ denotes NM vs. (IV-ET-2Cell, IVF-ET-2Cell and IVF-ET-BL) at Week 3 and NM vs. IV-ET-BL at Week 4 (P ≤ 0.05), Δ indicates IV-ET-2-Cell vs. IV-ET-BL and ○ indicates IVF-ET-BL vs. IVF-ET-2Cell (P ≤ 0.05). Mean (±SEM) body weight from 3 to 27 weeks (from 8 to 13 litters); n of mothers or foster mothers ♀, n of offspring.
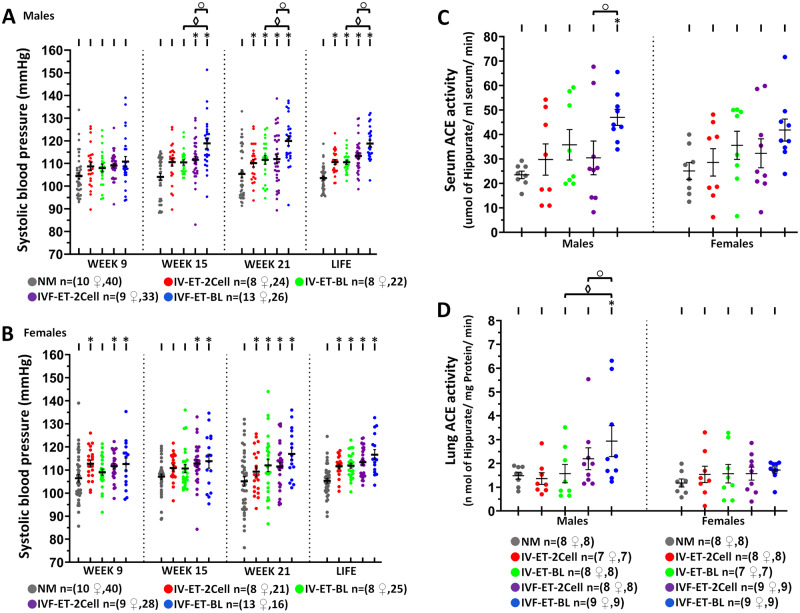
Male offspring from IVF and prolonged culture before ET develop CV dysfunction
SBP was determined at 9, 15 and 21 weeks and the mean of these also recorded as LIFE (Fig. 4). In males, mean SBP for all time points was consistently highest in IVF-ET-BL, reduced in IVF-ET-2Cell, the two IV-ET control groups and lowest in the NM group (Fig. 4A). IVF-ET-BL male SBP was increased at Weeks 15, 21 and LIFE compared with IVF-ET-2Cell (P = 0.032, 0.034 and 0.017, respectively) and with IV-ET-BL (P = 0.003, 0.014 and 0.001, respectively) (Fig. 4A). In females, although a similar SBP pattern existed across treatment groups, the differences were not significant between ET groups (Fig. 4B). However, NM females showed significant lower SBP than females in IV-ET and IVF-ET groups at Weeks 15, 21 and LIFE (P < 0.05).
Figure 4.
Effect of ART techniques on cardiovascular function in offspring. Postnatal systolic blood pressure (SBP) at indicated weeks of age and mean of these for individual offspring (LIFE), in male (A) and female (B) offspring; mean (±SEM) from 8 to 13 litters. Serum ACE activity (C) and lung ACE activity (D) in male and female offspring; mean (±SEM) from 7 to 9 litters per treatment. Multilevel random effects regression analysis. *Indicates a significant difference (P < 0.05) between NM and selected groups; ◊ indicates IV-ET-BL vs. IVF-ET-BL, and ○ indicates IVF-ET-BL vs. IVF-ET-2Cell differences (P < 0.05). n of mothers or foster mothers ♀, n of offspring. ACE, angiotensin-converting enzyme.
Serum and lung ACE activity, known to associate with increased SBP (Li et al., 2017), were further measured in offspring. Male IVF-ET-BL offspring recorded the highest serum and lung ACE activity, both being higher (P < 0.05) than in the IVF-ET-2Cell males (Fig. 4C and D). IVF-ET-BL lung ACE in males was also higher than the control IV-ET-BL males (P < 0.05) (Fig. 4D). However, ACE activities were not different across groups in female offspring (Fig. 4C and D). Correlation analysis of SBP and ACE activity revealed a significant positive correlation between both SBP 21 weeks and SBP LIFE with Lung ACE activity in male IVF-ET-BL offspring but not in females nor in any other treatment group (Table III).
Table III.
Phenotypic correlations between different offspring outcomes across treatments.
| Natural mating |
IV-ET-2cell |
IV-ET-BL |
IVF-ET-2cell |
IVF-ET-BL |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 8) | Female (n = 8) | Male (n = 6–8) | Female (n = 7–8) | Male (n = 6–8) | Female (n = 6–7) | Male (n = 8–9) | Female (n = 7–9) | Male (n = 7–9) | Female (n = 7–9) | |
| Cardiovascular phenotype | ||||||||||
| SBP wk21 − Lung ACE | −0.190 | 0.415 | 0.556 | 0.370 | 0.184 | 0.130 | −0.026 | 0.223 | 0.902* | −0.119 |
| SBP LIFE − Lung ACE | 0.118 | 0.107 | 0.415 | 0.353 | 0.693$ | 0.328 | 0.101 | 0.195 | 0.714* | −0.177 |
| G:I and body weight | ||||||||||
| G:I ratio − BW3 | −0.305 | −0.351 | −0.211 | −0.566 | −0.403 | 0.080 | −0.224 | 0.160 | −0.676$ | −0.268 |
| G:I ratio − BW9 | 0.111 | 0.169 | −0.621 | −0.649 | 0.936* | 0.789$ | −0.806* | −0.073 | −0.664 | −0.013 |
| G:I ratio − BW15 | 0.128 | −0.303 | −0.761$ | −0.735$ | −0.904* | 0.749$ | −0.807* | −0.309 | −0.700$ | −0.053 |
| G:I ratio − BW21 | 0.287 | −0.184 | −0.848* | −0.710$ | −0.871* | 0.664 | −0.812* | 0.008 | −0.748$ | 0.122 |
| G:I ratio − BW27 | 0.001 | −0.448 | −0.832* | −0.883* | −0.768$ | 0.910* | −0.935* | −0.026 | −0.806* | −0.741$ |
| G:I ratio − FG | −0.523 | −0.183 | −0.040 | −0.431 | −0.651 | 0.471 | −0.640$ | −0.090 | 0.437 | 0.198 |
| G:I ratio − GTT 15 min | −0.010 | −0.021 | −0.082 | −0.282 | −0.383 | 0.679 | −0.806* | 0.137 | −0.767* | 0.234 |
| G:I ratio − GTT 120 min | 0.298 | −0.350 | −0.753$ | −0.547 | −0.432 | 0.927* | −0.648$ | 0.043 | −0.707$ | −0.201 |
| Insulin – AUC | 0.023 | −0.111 | −0.013 | 0.208 | 0.252 | −0.912* | 0.860* | −0.315 | 0.659 | 0.420 |
| G:I ratio − AUC | −0.195 | −0.107 | −0.263 | −0.371 | −0.540 | 0.960* | −0.862* | 0.116 | −0.819* | −0.530 |
SBP LIFE, average (SBP9, SBP15 and SBP21); ACE, angiotensin-converting enzyme; G:I G:I ratio, Serum glucose: Serum insulin ratio; BW, body weight as specific week; AUC, area under the curve for GTT test; Insulin, Serum insulin; FG, fasting glucose.
P < 0.1;
P < 0.05.
The combined techniques of ART (superovulation, IVF, culture, ET, recipient gestation) therefore contribute to adverse postnatal CV health compared with natural unstimulated reproduction but with prolonged versus short embryo culture exacerbating these effects in male offspring.
Male offspring from IVF and short culture before ET develop impaired glucose and insulin metabolism
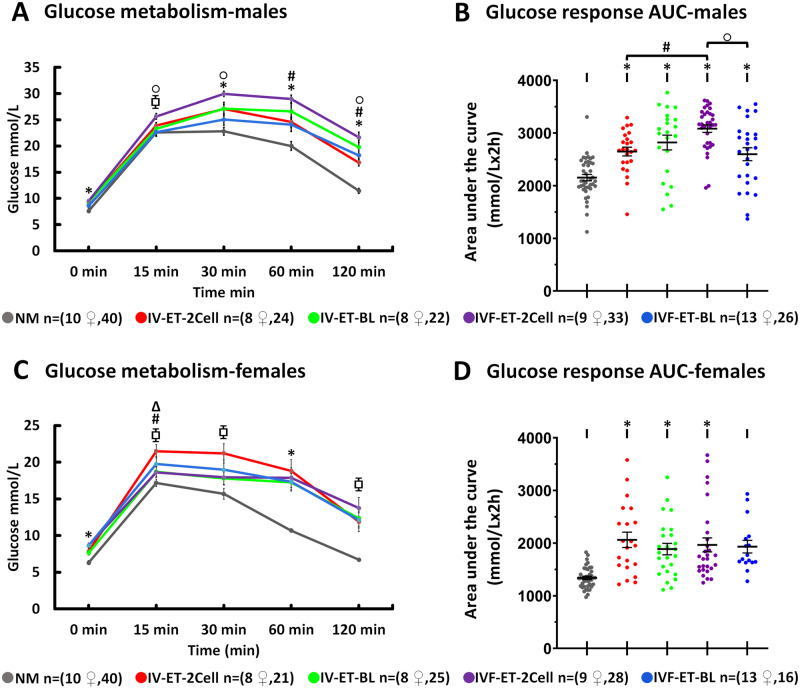
Glucose metabolism of offspring was assessed by glucose tolerance test (GTT) at postnatal Week 27. Male offspring fasting glucose level (i.e. 0 min) and after 15, 30 min, 1 and 2 hr of i.p glucose injection showed all treatment groups to have significantly slower recoveries and larger AUC than the NM control group (Fig. 5A and B). Glucose recovery and AUC for IVF-ET-2Cell was poorer compared with both IV-ET-2Cell (P = 0.05–0.004) and IVF-ET-BL males (P = 0.03–0.003). In female offspring, fasting glucose level, glucose recovery and AUC also appeared to be poorer in treatment groups compared with the NM control although the differences were not always significant. No significant differences were detected between the four treatment groups in females (Fig. 5C and D).
Figure 5.
Effect of ART techniques on glucose metabolism in offspring. Intraperitoneal GTT at 0, 15, 30, 60 and 120 min and AUC in male (A and B) and female (C and D) offspring; mean (±SEM) in 8–13 litters per treatment. Multilevel random effects regression analysis. *Indicates a significant difference (P < 0.05) between NM and selected groups; Δ indicates IV-ET-2-Cell vs. IV-ET-BL, # indicates IV-ET-2Cell vs. IVF-ET-2Cell, and ○ indicates IVF-ET-BL vs. IVF-ET-2Cell differences (P ≤ 0.05). n of mothers or foster mothers ♀, n of offspring.
Serum samples collected at 27 weeks during animal culling were used to measure insulin and glucose levels and the glucose:insulin ratio (G:I), a measure of insulin effectiveness in glucose homeostasis. In male offspring, glucose levels were similar across treatments with IV-ET-BL higher than IV-ET-2Cell and NM (P < 0.05; Fig. 6A). In contrast, insulin levels differed substantially across treatments with IVF-ET-2Cell males being significantly higher than all other groups (P < 0.05; Fig. 6B). The lowest insulin level was in NM males which led to the highest G:I ratio in NM males and this was significantly higher than in IV-ET-2Cell, IVF-ET-2Cell and IV-ET-BL groups (P = 0.005, P = 0.001 and P = 0.038, respectively; Fig. 6C). Female serum glucose was unchanged across treatments (Fig. 6A) while insulin was lowest in the NM group and significantly raised in IVF-ET-2Cell females (P < 0.05; Fig. 6B), resulting in G:I ratio highest in NM females, as in males, and significantly higher than in IV-ET-BL and IVF-ET-2Cell females (P < 0.05; Fig. 6C).
Figure 6.
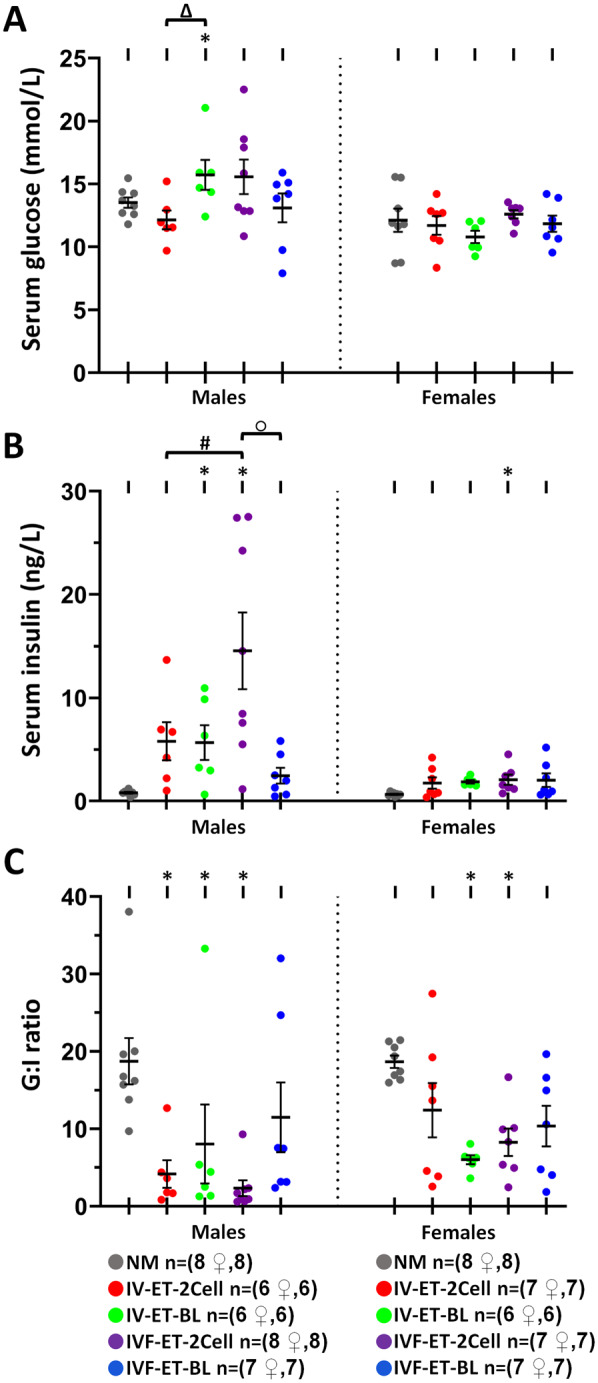
Effect of ART techniques on glucose and insulin levels in offspring. Serum glucose (A), serum insulin (B) and G:I ratio (C) in male and female offspring; mean (±SEM) from 6 to 8 litters per treatment. Multilevel random effects regression analysis. *Indicates a significant difference (P < 0.05) between NM and selected groups; Δ indicates IV-ET-2-Cell vs. IV-ET-BL, # indicates IV-ET-2Cell vs. IVF-ET-2Cell, and ○ indicates IVF-ET-BL vs. IVF-ET-2Cell differences (P ≤ 0.05). n of mothers or foster mothers ♀, n of offspring.
Metabolic outcomes were analysed for possible associations with other phenotypes; the G:I ratio in particular was found to be significantly negatively correlated both with body weight throughout postnatal life and with AUC from the GTT in the IVF-ET-2Cell male but not female offspring (Table III). Other groups with ET treatment also showed weaker associations between these parameters but there were no associations on the NM group (Table III).
The combined techniques of ART (superovulation, IVF, culture, ET, recipient gestation) therefore contribute to adverse postnatal metabolic health as measured by glucose homeostasis compared with natural unstimulated reproduction. Here, evidence of insulin resistance was most pronounced after short embryo culture particularly in male offspring.
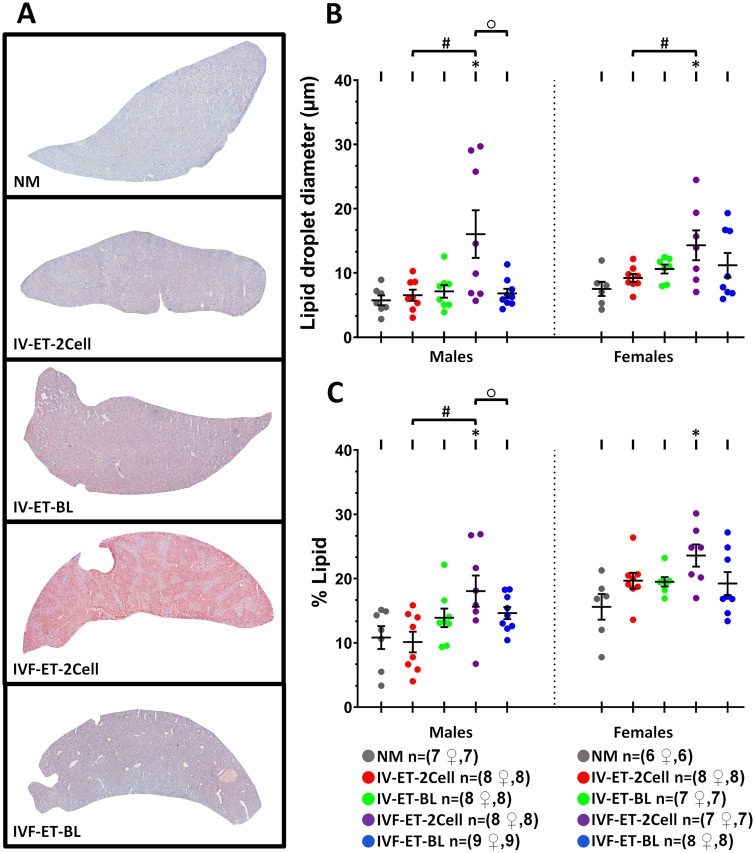
Offspring from IVF and short culture before ET develop increased lipid accumulation in liver
Metabolic health of offspring was also assessed by analysis of lipid accumulation in liver cryosections stained with Oil Red O using organs stored at 27 weeks at culling. Representative images of lipid accumulation in male liver sections are shown in Fig. 7A. Lipid droplet size was increased in IVF-ET-2Cell offspring relative to other groups and especially in males. IVF-ET-2Cell lipid size was increased compared with IVF-ET-BL (P = 0.015) and with IV-ET-2Cell (P = 0.015) in males (Fig. 7B). Moreover, the relative percentage area of lipid accumulation was increased in IVF-ET-2Cell versus IVF-ET-BL at trend level (t = 0.065) and versus control IV-ET-2Cell (P = 0.003) in males (Fig. 7C). Thus, IVF and transfer after short rather than long culture contribute to adverse liver lipid accumulation as well as impaired glucose-insulin metabolism, especially in males.
Figure 7.
Effect of ART techniques on lipid accumulation in offspring liver. Representative images of liver cryosections from male offspring stained with Oil Red O from each treatment group (A). Average lipid droplet diameter (B) and percentage lipid-stained area (C) in male and female offspring; mean (±SEM) from 6 to 9 litters per treatment. *Indicates a significant difference (P < 0.05) between NM and selected groups; ○ indicates IVF-ET-BL vs. IVF-ET-2Cell (B, P = 0.015; C, t = 0.065), and # indicates IV-ET-2Cell vs. IVF-ET-2Cell differences (P < 0.05). n of mothers or foster mothers ♀, n of offspring.
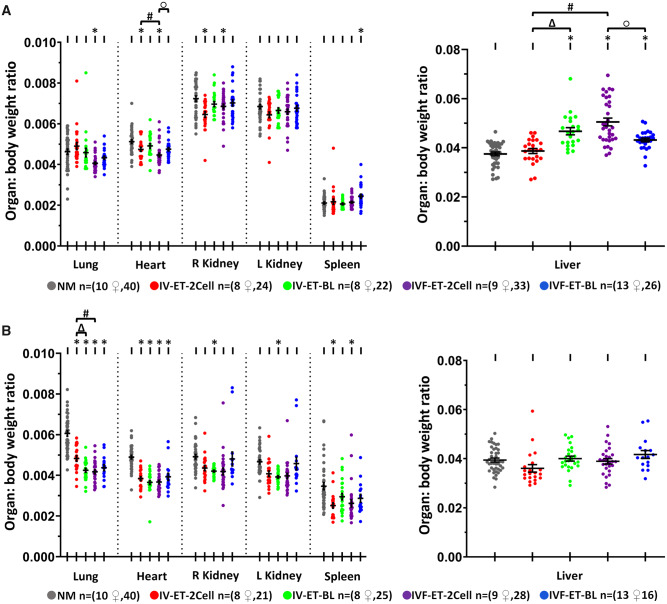
Postnatal offspring from ART treatments display altered organ allometry
Offspring were sacrificed at postnatal Week 27 and organ/body weight ratios were determined before organ freeze storage. Male offspring organ weight was generally proportional to body weight but with exceptions (see Fig. 8 for details). Notably, IVF-ET-2Cell males had relatively smaller lungs, hearts and right kidneys and larger livers compared with NM males, whilst IVF-ET-BL males also had larger livers and spleens compared with NM males (Fig. 8A). IVF-ET-BL males had larger hearts and smaller livers than IVF-ET-2Cell males. The IV-ET-2Cell and IV-ET-BL control groups had few organ size differences from NM males. In contrast, female offspring from ART treatments generally had smaller proportioned organ sizes, especially lungs and hearts, compared with NM females but differences between the two IVF groups were not apparent (Fig. 8B). The combined techniques of ART (superovulation, IVF, culture, transfer) therefore contribute to altered organ allometry in both male and female offspring compared with natural unstimulated reproduction.
Figure 8.
Effect of ART techniques on organ/body weight in adult offspring. Organ allometry variables in male (A) and female (B); mean (±SEM) organ: body weight ratio from 8 to 13 litters per treatment. Multilevel random effects regression analysis. *Indicates a significant difference (P < 0.05) between NM and selected groups, Δ indicates IV-ET-2-Cell vs. IV-ET-BL, # indicates IV-ET-2Cell vs. IVF-ET-2Cell, and ○ indicates IVF-ET-BL vs. IVF-ET-2Cell differences (P < 0.05). n mothers or foster mothers ♀, n offspring.
Discussion
We have used an animal model to address the safety for long-term offspring health of specific ART techniques in common practice in clinics and in the absence of confounding parental infertility. Given the past record of adverse offspring health risk mediated through embryo culture (Cagnone and Sirard, 2016; Sunde et al., 2016; Fleming et al., 2018), the model was designed to distinguish specifically between short and long culture duration either up to cleavage-stage (two-cell) or blastocyst transfer, respectively. Both groups (IVF-ET-2Cell; IVF-ET-BL) were supported by direct in-vivo controls for transfer at these two stages (IV-ET-2Cell; IV-ET-BL) which included the background ART techniques (superovulation; ET) but in the absence of the tested techniques (IVF; short or long culture). These four groups were also compared with a NM group where no ART techniques were applied. Thus, the model is suitable for direct comparison of the health consequences for offspring arising from IVF and culture duration independent of other techniques, but also permits evaluation of the background techniques and the collective of all ART techniques. However, our design required the use of atmospheric oxygen rather than 5% for culture, although the former is reported to still be practised in some 40% of IVF cycles worldwide (van Montfoort et al., 2020). This choice was necessary to maintain consistency between the two IVF groups and their two IV controls where embryo incubation was kept to an absolute minimum, essentially the time to complete ET in the surgery room, and could not be accomplished at 5% O2 for practicalities. Finally, the statistical approach of random effects regression analysis on the dataset permits outcomes to be evaluated in the entire offspring generated by each treatment rather than just on litter means, thereby integrating variability both within- and between-mothers and independent of the effect of litter size (Kwong et al., 2004), as used in our previous periconceptional DOHaD models (Watkins et al., 2008; Velazquez et al., 2016, 2018).
One enduring feature of the dataset was the distinction between offspring phenotype arising from all four manipulated groups with that of the NM group. Thus, compared with the NM group, offspring from manipulated groups exhibited increased postnatal growth and poorer CV and metabolic health across the spectrum of assays undertaken, commonly in both male and female offspring at significant levels. This broad and unequivocal phenotypic consequence at one level demonstrates the collective effect of the ART techniques applied over the lifespan but is likely to be exaggerated because of the use of MF1 recipients for gestation and lactation. For example, it is established that the maternal uterine genotype of mouse recipients can influence offspring phenotype such as postnatal growth rate (Cowley et al., 1989). Whilst we used inbred C57BL6/CBA embryos for genomic stability and capacity to overcome the ‘2-cell block’ in culture, outbred MF1 recipients were necessary to enhance pregnancy efficiency, a combination we have used successfully previously for DOHaD-related mouse studies (Velazquez et al., 2016). Thus, the growth rate of offspring from the manipulated groups here broadly matched that previously reported (Velazquez et al., 2016) and is similar to MF1 offspring from natural pregnancies (Watkins et al., 2008) or slightly below that following MF1 embryo manipulations and transfer to MF1 recipients (Velazquez et al., 2018).
In the critical group comparison of culture duration after IVF with all other ART techniques normalised, we found a curious dichotomy between IVF-ET-2Cell and IVF-ET-BL offspring, and particular males, in that CV outcomes (SBP; ACE activity; larger heart/body mass) were poorer in IVF-ET-BL treatments but conversely, metabolic outcomes (glucose response; raised basal insulin; increased liver/body mass; increased liver lipid accumulation) were poorer in the IVF-ET-2Cell group. Both CV phenotype in IVF-ET-BL and metabolic phenotype in IVF-ET-2Cell males were poorer than their respective controls (IV-ET-BL; IV-ET-2Cell) indicating outcomes were predominantly derived from IVF and culture duration, perhaps in combination with the timing of ET (discussed later), rather than by in-vitro manipulations and ET per se. To assess the basis for this dichotomy in health outcomes in IVF-ET-BL and IVF-ET-2Cell offspring, we first need to consider the direct effects of in-vitro culture on the early embryo.
Our study showed that in-vitro culture, although permissive for blastocyst formation, was suboptimal, slowing development and reducing proliferation of TE and ICM cells, as previously reported in other mouse ART models (Watkins et al., 2007; Chen et al., 2019). Culture conditions can interfere with two critical aspects of preimplantation development, namely embryo metabolism and the epigenetic regulation of the new embryonic genome. Embryo metabolism matures progressively from a low rate during fertilisation and early cleavage, dependent upon mitochondrial oxidative phosphorylation for energy production, and increases substantially at the blastocyst stage (Houghton et al., 1996; Leese, 2012). This progression is accompanied by upregulated glycolysis in late cleavage stages, further enhancing energy availability for blastocyst morphogenesis, especially epithelial transport activity and increased protein synthesis for growth (Houghton et al., 1996; Leese, 2012). Mitochondrial morphology also matures during cleavage with normal transverse cristae formation coinciding with the increased efficiency of ATP production at morula and blastocyst stages (Harvey, 2019). The unnatural metabolite milieu experienced in embryo culture can induce oxidative stress through increased production of reactive oxygen species alongside ATP in the mitochondrial electron transport chain (Takahashi, 2012; Cagnone and Sirard, 2016). Whilst natural protective mechanisms exist through antioxidant enzymes to maintain the redox balance, culture conditions can perturb this balance leading to impaired development affecting growth, gene expression and survival (Leese, 2012; Takahashi, 2012; Cagnone and Sirard, 2016). Indeed, direct manipulation of energy substrates, mitochondrial activity and redox potential in mouse zygotes leads to altered postnatal growth rates (Banrezes et al., 2011). Furthermore, a range of environmental factors including maternal over-nutrition and obesity have also been shown to disturb mitochondrial functioning, localisation and mtDNA copy number in oocytes and early cleavage embryos with enduring effects on foetal and postnatal growth and metabolism (Igosheva et al., 2010; Grindler and Moley, 2013; Wu et al., 2015).
The second consequence of adverse culture environment is to interfere with the epigenetic reprogramming of the new embryonic genome (Chason et al., 2011; Cagnone and Sirard, 2016; Sunde et al., 2016). Global demethylation of the genome during cleavage is followed by a gradual, lineage-specific pattern of de-novo methylation initiated in the blastocyst to coordinate development (Seisenberger et al., 2013). Thus, culture environment may alter the expression and methylation level of imprinted genes within the embryo persisting into later developmental stages (Doherty et al., 2000; de Waal et al., 2014). Non-imprinted genes are also vulnerable to culture conditions with the global pattern of gene expression (Feuer et al., 2017) and DNA methylation distinct from that of in-vivo embryos (Wright et al., 2011; Salilew-Wondim et al., 2015; Canovas et al., 2017). Epigenetic disturbance may at least partially derive from mitochondrial dysfunction since mitochondria supply intermediates in DNA methylation and histone acetylation through the 1-carbon metabolism pathway (Xu and Sinclair, 2015; Cagnone and Sirard, 2016; Ducker and Rabinowitz, 2017).
The poorer CV outcomes identified in IVF-ET-BL males after long culture versus both control IV-ET-BL and short culture IVF-ET-2Cell groups likely reflects the progressive negative effects of in-vitro culture on embryo metabolism and epigenetic stability. Indeed, we show a progressive increase in SBP in male offspring based upon the duration of culture from IV controls through to IVF-ET-BL offspring. Cardiac and associated vasculature form very early during development, from E8.5 in mouse, and is a complex morphogenetic process essential for embryo survival with recent research identifying significant epigenetic regulation (Kathiriya et al., 2015). Adult CV dysfunction occurs in response to a wide range of peri-conceptional environments, indicating its sensitivity (Fleming et al., 2018). Moreover, an epigenetic basis for adverse CV health including arterial hypertension has been reported in a mouse ART model, mediated through altered DNA methylation of the endothelial eNOS gene in the aorta, leading to reduced eNOS expression and disturbed NO signalling (Rexhaj et al., 2013). Notably, the CV phenotype and associated epigenetic alteration in the eNOS gene can be prevented by inclusion of the epigenetic regulator, melatonin, in embryo culture medium (Rexhaj et al., 2015). Indeed, the significant positive correlation identified between SBP and lung ACE level in the IVF-ET-BL males, but not other groups, suggests that ACE expression, known to be epigenetically regulated (Mudersbach et al., 2019) and sensitive to the peri-conceptional environment (Watkins et al., 2006, 2007), may contribute an epigenetic pathway to affect later CV health.
In contrast to the clear link between extended culture and offspring CV dysfunction, it was the IVF-ET-2Cell group with shorter culture duration that lead to the poorer metabolic phenotype in offspring. Collectively, male offspring from this treatment demonstrated poorer glucose handling which correlated negatively with body mass, increased basal insulin levels, increased relative liver sizing and liver lipid accumulation, compared with either the direct control group (IV-ET-2Cell) or the IVF-ET-BL group. Increased birth weight and poorer glucose and insulin regulation were previously reported in mouse IVF offspring following ET at the two-cell stage but predominantly in females (Scott et al., 2010). Similar poorer glucose handling mainly in female offspring following IVF was found after mouse blastocyst ET and coincided with metabolic dysfunction across several tissues including liver, evidenced by microarray analysis (Feuer et al., 2014). Furthermore, liver metabolic dysfunction including accumulation of monounsaturated fatty acids has been reported following mouse IVF and ET at the two-cell stage (Wang et al., 2013). Mouse IVF also leads to increased phospholipid accumulation in foetal liver (Li et al., 2016), indicating prenatal origin of ART-mediated metabolic impairment. Given the increased accumulation of lipid in the male IVF-ET-2Cell liver, it would be interesting in future studies to determine serum lipid levels and adipose tissue composition for a broader understanding of lipid dysregulation in this group. The co-occurrence of markers of metabolic disease risk in several studies, as well as in our current study, confirm the link between ART and adult metabolic health.
This distinction in outcomes between IVF-ET-2Cell and IVF-ET-BL groups suggests different mechanisms and biological pathways may be at work for metabolic and CV outcomes. Apart from the shorter culture duration, the IVF-ET-2Cell group experienced ET during the two-cell stage when the mouse embryonic genome is predominantly activated (EGA) (Flach et al., 1982). The period of EGA at the transition from maternal to embryonic control of development is recognised as one of particular sensitivity to culture conditions across mammalian species, affecting embryo potential (Lonergan et al., 2003; Zander et al., 2006) and is discussed in detail elsewhere (Brison et al., 2014). A convincing argument suggests that stressful manipulations during the EGA (such as ET here) may be accentuated by the absence of gap junction communication between blastomeres to coordinate homogeneity and protection in intercellular maturation (Brison et al., 2014). EGA in the human occurs slightly later in cleavage, at the 4- to 8-cell transition (Braude et al., 1988; Vassena et al., 2011), but cleavage ET in human ART normally coincides with this cellular stage.
A further characteristic of our study has been the clear disparity in outcomes based upon offspring sex with males being far more sensitive that females. Sexual dimorphism has been commonly found in periconceptional DOHaD programming studies in response to diverse challenges including ART-based models and evident in small and large mammals and humans (Hansen et al., 2016; Fleming et al., 2018). In mouse studies of embryo culture effects on offspring cardiometabolic health, males commonly show increased sensitivity, as here (Donjacour et al., 2014; Velazquez et al., 2018), but female vulnerability has been shown elsewhere (Feuer et al., 2014), indicating that strain differences may be contributory. This also likely reflects different susceptibilities to CV disease based on sex, which arise in utero (Schalekamp-Timmermans et al., 2016). Environmental conditions such as nutrient and metabolite levels both in vivo and in vitro can differentially influence embryo response in terms of signalling activity, gene expression and morphogenesis in a sex-specific manner that can persist through gestation and postnatal life (Hansen et al., 2016).
Conclusion
We have shown that IVF and embryo culture in a mouse model specifically associate with adverse CV and metabolic outcomes particularly in male offspring independent of background superovulation and ET techniques. Our study shows a clear effect of culture duration after IVF with long culture to the blastocyst stage before ET leading to a poorer CV phenotype and shorter culture to the two-cell stage before transfer resulting in a poorer metabolic health phenotype. We consider this distinction in outcome likely reflects different pathways leading to these health conditions initiated from preimplantation environment and the interaction between culture duration and the timing of ET in relation to EGA. These findings further pinpoint the risks of preimplantation manipulations in the programming of long-term health outcomes. From a clinical perspective, whilst our data do not identify a safer strategy for IVF and culture duration, they do show the biological and health implications that derive from the ART culture protocol.
Acknowledgements
We are grateful for the technical support of the Biomedical Research Facility at the University of Southampton.
Authors’ roles
A.A. performed experiments, analysed data and wrote and edited the manuscript. R.K.R.I.A, B.S. and K.W. performed experiments. M.A.V. provided technical support, analysed data and edited the paper. A.J.W. and J.J.E. analysed data and edited the paper. C.O. provided statistical expertise. N.R.S. performed experiments, analysed data and edited the paper. T.P.F. conceived and designed the study, and wrote and edited the manuscript.
Funding
This work was supported through the European Union FP7-CP-FP Epihealth programme (278418) and FP7-PEOPLE-2012-ITN EpiHealthNet programme (317146) to T.P.F., the BBSRC (BB/F007450/1) to T.P.F. and the Saudi government, University of Jeddah and King Abdulaziz University to A.A.
Conflict of interest
The authors have no conflicts of interest to declare.
Contributor Information
Anan Aljahdali, School of Biological Sciences, University of Southampton, Southampton General Hospital, Southampton SO16 6YD, UK; University of Jeddah, Jeddah, Saudi Arabia.
R K Raja Ili Airina, School of Biological Sciences, University of Southampton, Southampton General Hospital, Southampton SO16 6YD, UK.
Miguel A Velazquez, School of Natural and Environmental Sciences, Newcastle University, Newcastle Upon Tyne NE1 7RU, UK.
Bhavwanti Sheth, School of Biological Sciences, University of Southampton, Southampton General Hospital, Southampton SO16 6YD, UK.
Katrina Wallen, School of Biological Sciences, University of Southampton, Southampton General Hospital, Southampton SO16 6YD, UK.
Clive Osmond, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton SO16 6YD, UK.
Adam J Watkins, Division of Child Health, Obstetrics and Gynaecology, Faculty of Medicine, University of Nottingham, Nottingham NG7 2UH, UK.
Judith J Eckert, Human Development and Health, Faculty of Medicine, University of Southampton, Southampton General Hospital, Southampton SO16 6YD, UK.
Neil R Smyth, School of Biological Sciences, University of Southampton, Southampton General Hospital, Southampton SO16 6YD, UK.
Tom P Fleming, School of Biological Sciences, University of Southampton, Southampton General Hospital, Southampton SO16 6YD, UK.
References
- Banrezes B, Sainte-Beuve T, Canon E, Schultz RM, Cancela J, Ozil JP. Adult body weight is programmed by a redox-regulated and energy-dependent process during the pronuclear stage in mouse. PLoS One 2011;6:e29388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen S, Soderstrom-Anttila V, Wennerholm UB, Laivuori H, Loft A, Oldereid NB, Romundstad LB, Bergh C, Pinborg A. The health of children conceived by ART: ‘the chicken or the egg?’. Hum Reprod Update 2019;25:137–158. [DOI] [PubMed] [Google Scholar]
- Biggers JD, McGinnis LK, Lawitts JA. One-step versus two-step culture of mouse preimplantation embryos: is there a difference? Hum Reprod 2005;20:3376–3384. [DOI] [PubMed] [Google Scholar]
- Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 1988;332:459–461. [DOI] [PubMed] [Google Scholar]
- Brison DR, Sturmey RG, Leese HJ. Metabolic heterogeneity during preimplantation development: the missing link? Hum Reprod Update 2014;20:632–640. [DOI] [PubMed] [Google Scholar]
- Cagnone G, Sirard MA. The embryonic stress response to in vitro culture: insight from genomic analysis. Reproduction 2016;152:R247–R261. [DOI] [PubMed] [Google Scholar]
- Canovas S, Ivanova E, Romar R, Garcia-Martinez S, Soriano-Ubeda C, Garcia-Vazquez FA, Saadeh H, Andrews S, Kelsey G, Coy P. DNA methylation and gene expression changes derived from assisted reproductive technologies can be decreased by reproductive fluids. Elife 2017;6:e23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Prein J, Smit JJ, Vermeiden JP, Spreeuwenberg M, van Leeuwen FE, Delemarre-van de Waal HA. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8-18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod 2009;24:2788–2795. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab 2008;93:1682–1688. [DOI] [PubMed] [Google Scholar]
- Cerny D, Sartori C, Rimoldi SF, Meister T, Soria R, Bouillet E, Scherrer U, Rexhaj E. Assisted reproductive technologies predispose to insulin resistance and obesity in male mice challenged with a high-fat diet. Endocrinology 2017;158:1152–1159. [DOI] [PubMed] [Google Scholar]
- Chason RJ, Csokmay J, Segars JH, DeCherney AH, Armant DR. Environmental and epigenetic effects upon preimplantation embryo metabolism and development. Trends Endocrinol Metab 2011;22:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wong SL, Wu LL, Gordon YE, Heilbronn LK, Robker RL. Differential impacts of gonadotrophins, IVF and embryo culture on mouse blastocyst development. Reprod Biomed Online 2019;39:372–382. [DOI] [PubMed] [Google Scholar]
- Chen M, Wu L, Zhao J, Wu F, Davies MJ, Wittert GA, Norman RJ, Robker RL, Heilbronn LK. Altered glucose metabolism in mouse and humans conceived by IVF. Diabetes 2014;63:3189–3198. [DOI] [PubMed] [Google Scholar]
- Cowley DE, Pomp D, Atchley WR, Eisen EJ, Hawkins-Brown D. The impact of maternal uterine genotype on postnatal growth and adult body size in mice. Genetics 1989;122:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford GE, Ledger WL. In vitro fertilisation/intracytoplasmic sperm injection beyond 2020. BJOG 2019;126:237–243. [DOI] [PubMed] [Google Scholar]
- De Vos A, Santos-Ribeiro S, Van Landuyt L, Van de Velde H, Tournaye H, Verheyen G. Birthweight of singletons born after cleavage-stage or blastocyst transfer in fresh and warming cycles. Hum Reprod 2018;33:196–201. [DOI] [PubMed] [Google Scholar]
- de Waal E, Mak W, Calhoun S, Stein P, Ord T, Krapp C, Coutifaris C, Schultz RM, Bartolomei MS. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol Reprod 2014;90:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod 2000;62:1526–1535. [DOI] [PubMed] [Google Scholar]
- Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod 2014;90:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab 2017;25:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuer S, Liu X, Donjacour A, Simbulan R, Maltepe E, Rinaudo P. Transcriptional signatures throughout development: the effects of mouse embryo manipulation in vitro. Reproduction 2017;153:107–122. [Google Scholar]
- Feuer S, Rinaudo P. From embryos to adults: a DOHaD perspective on in vitro fertilization and other assisted reproductive technologies. Healthcare (Basel) 2016;4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuer SK, Liu X, Donjacour A, Lin W, Simbulan RK, Giritharan G, Piane LD, Kolahi K, Ameri K, Maltepe E et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology 2014;155:1956–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach G, Johnson MH, Braude PR, Taylor RA, Bolton VN. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J 1982;1:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, Barker M, Saffery R, Yajnik CS, Eckert JJ et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet 2018;391:1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkourogianni A, Kosteria I, Telonis AG, Margeli A, Mantzou E, Konsta M, Loutradis D, Mastorakos G, Papassotiriou I, Klapa MI et al. Plasma metabolomic profiling suggests early indications for predisposition to latent insulin resistance in children conceived by ICSI. PLoS One 2014;9:e94001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 2016;6:CD002118. [DOI] [PubMed] [Google Scholar]
- Goldsmith S, McIntyre S, Badawi N, Hansen M. Cerebral palsy after assisted reproductive technology: a cohort study. Dev Med Child Neurol 2018;60:73–80. [DOI] [PubMed] [Google Scholar]
- Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod 2013;19:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XY, Liu XM, Jin L, Wang TT, Ullah K, Sheng JZ, Huang HF. Cardiovascular and metabolic profiles of offspring conceived by assisted reproductive technologies: a systematic review and meta-analysis. Fertil Steril 2017;107:622–631.e5. [DOI] [PubMed] [Google Scholar]
- Handyside AH, Hunter S. A rapid procedure for visualising the inner cell mass and trophectoderm nuclei of mouse blastocysts in situ using polynucleotide-specific fluorochromes. J Exp Zool 1984;231:429–434. [DOI] [PubMed] [Google Scholar]
- Hansen PJ, Dobbs KB, Denicol AC, Siqueira LG. Sex and the preimplantation embryo: implications of sexual dimorphism in the preimplantation period for maternal programming of embryonic development. Cell Tissue Res 2016;363:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AJ. Mitochondria in early development: linking the microenvironment, metabolism and the epigenome. Reproduction 2019;157:R159–R179. [DOI] [PubMed] [Google Scholar]
- Houghton FD, Thompson JG, Kennedy CJ, Leese HJ. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev 1996;44:476–485. [DOI] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One 2010;5:e10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–426. [DOI] [PubMed] [Google Scholar]
- Ishizuka Y, Nishimura M, Matsumoto K, Miyashita M, Takeo T, Nakagata N, Hosoi Y, Anzai M. The influence of reduced glutathione in fertilization medium on the fertility of in vitro-matured C57BL/6 mouse oocytes. Theriogenology 2013;80:421–426. [DOI] [PubMed] [Google Scholar]
- Kathiriya IS, Nora EP, Bruneau BG. Investigating the transcriptional control of cardiovascular development. Circ Res 2015;116:700–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijkers SH, Mantikou E, Slappendel E, Consten D, van Echten-Arends J, Wetzels AM, van Wely M, Smits LJ, van Montfoort AP, Repping S et al. Influence of embryo culture medium (G5 and HTF) on pregnancy and perinatal outcome after IVF: a multicenter RCT. Hum Reprod 2016;31:2219–2230. [DOI] [PubMed] [Google Scholar]
- Kleijkers SH, van Montfoort AP, Smits LJ, Viechtbauer W, Roseboom TJ, Nelissen EC, Coonen E, Derhaag JG, Bastings L, Schreurs IE et al. IVF culture medium affects post-natal weight in humans during the first 2 years of life. Hum Reprod 2014;29:661–669. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Osmond C, Fleming TP. Support for Barker hypothesis upheld in rat model of maternal undernutrition during the preimplantation period: application of integrated ‘random effects’ statistical model. Reprod BioMed Online 2004;8:574–576. [DOI] [PubMed] [Google Scholar]
- Le F, Wang LY, Wang N, Li L, Li le J, Zheng YM, Lou HY, Liu XZ, Xu XR, Sheng JZ et al. In vitro fertilization alters growth and expression of Igf2/H19 and their epigenetic mechanisms in the liver and skeletal muscle of newborn and elder mice. Biol Reprod 2013;88:75. [DOI] [PubMed] [Google Scholar]
- Leese HJ. Metabolism of the preimplantation embryo: 40 years on. Reproduction 2012;143:417–427. [DOI] [PubMed] [Google Scholar]
- Li B, Xiao X, Chen S, Huang J, Ma Y, Tang N, Sun H, Wang X. Changes of phospholipids in fetal liver of mice conceived by in vitro fertilization. Biol Reprod 2016;94:105. [DOI] [PubMed] [Google Scholar]
- Li XC, Zhang J, Zhuo JL. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res 2017;125:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Gao J, He X, Cai Y, Wang L, Fan X. Association between assisted reproductive technology and the risk of autism spectrum disorders in the offspring: a meta-analysis. Sci Rep 2017;7:46207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan P, Rizos D, Kanka J, Nemcova L, Mbaye AM, Kingston M, Wade M, Duffy P, Boland MP. Temporal sensitivity of bovine embryos to culture environment after fertilization and the implications for blastocyst quality. Reproduction 2003;126:337–346. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Diabetes Care 2001;24:460–464. [DOI] [PubMed] [Google Scholar]
- Meister TA, Rimoldi SF, Soria R, von Arx R, Messerli FH, Sartori C, Scherrer U, Rexhaj E. Association of assisted reproductive technologies with arterial hypertension during adolescence. J Am Coll Cardiol 2018;72:1267–1274. [DOI] [PubMed] [Google Scholar]
- Mudersbach T, Siuda D, Kohlstedt K, Fleming I. Epigenetic control of the angiotensin-converting enzyme in endothelial cells during inflammation. PLOS One 2019;14:e0216218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr-Esfahani M, Johnson MH, Aitken RJ. The effect of iron and iron chelators on the in-vitro block to development of the mouse preimplantation embryo: BAT6 a new medium for improved culture of mouse embryos in vitro. Hum Reprod 1990;5:997–1003. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Anttila V, Nygren KG, Hazekamp J, Bergh C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- Pontesilli M, Painter RC, Grooten IJ, van der Post JA, Mol BW, Vrijkotte TG, Repping S, Roseboom TJ. Subfertility and assisted reproduction techniques are associated with poorer cardiometabolic profiles in childhood. Reprod Biomed Online 2015;30:258–267. [DOI] [PubMed] [Google Scholar]
- Qin JB, Sheng XQ, Wu D, Gao SY, You YP, Yang TB, Wang H. Worldwide prevalence of adverse pregnancy outcomes among singleton pregnancies after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Arch Gynecol Obstet 2017;295:285–301. [DOI] [PubMed] [Google Scholar]
- Ramirez-Perez FI, Schenewerk AL, Coffman KL, Foote C, Ji T, Rivera RM, Martinez-Lemus LA. Effects of the use of assisted reproductive technologies and an obesogenic environment on resistance artery function and diabetes biomarkers in mice offspring. PLoS One 2014;9:e112651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, Fuster DG, Anderegg M, Somm E, Bouillet E, Allemann Y, Sartori C, Scherrer U. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest 2013;123:5052–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexhaj E, Pireva A, Paoloni-Giacobino A, Allemann Y, Cerny D, Dessen P, Sartori C, Scherrer U, Rimoldi SF. Prevention of vascular dysfunction and arterial hypertension in mice generated by assisted reproductive technologies by addition of melatonin to culture media. Am J Physiol Heart Circ Physiol 2015;309:H1151–H1156. [DOI] [PubMed] [Google Scholar]
- Sakka SD, Loutradis D, Kanaka-Gantenbein C, Margeli A, Papastamataki M, Papassotiriou I, Chrousos GP. Absence of insulin resistance and low-grade inflammation despite early metabolic syndrome manifestations in children born after in vitro fertilization. Fertil Steril 2010;94:1693–1699. [DOI] [PubMed] [Google Scholar]
- Salilew-Wondim D, Fournier E, Hoelker M, Saeed-Zidane M, Tholen E, Looft C, Neuhoff C, Besenfelder U, Havlicek V, Rings F et al. Genome-wide DNA methylation patterns of bovine blastocysts developed in vivo from embryos completed different stages of development in vitro. PLoS One 2015;10:e0140467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Nygren KG, Iliadou A, Hultman CM, Reichenberg A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA 2013;310:75–84. [DOI] [PubMed] [Google Scholar]
- Schalekamp-Timmermans S, Cornette J, Hofman A, Helbing WA, Jaddoe VW, Steegers EA, Verburg BO. In utero origin of sex-related differences in future cardiovascular disease. Biol Sex Differ 2016;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenewerk AL, Ramirez FI, Foote C, Ji T, Martinez-Lemus LA, Rivera RM. Effects of the use of assisted reproduction and high-caloric diet consumption on body weight and cardiovascular health of juvenile mouse offspring. Reproduction 2014;147:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer U, Rimoldi SF, Rexhaj E, Stuber T, Duplain H, Garcin S, de Marchi SF, Nicod P, Germond M, Allemann Y et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation 2012;125:1890–1896. [DOI] [PubMed] [Google Scholar]
- Scott KA, Yamazaki Y, Yamamoto M, Lin Y, Melhorn SJ, Krause EG, Woods SC, Yanagimachi R, Sakai RR, Tamashiro KL. Glucose parameters are altered in mouse offspring produced by assisted reproductive technologies and somatic cell nuclear transfer. Biol Reprod 2010;83:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc Lond B Biol Sci 2013;368:20110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Zhang W, Li N, Xue X, Liu C, Qu P, Shi J, Huang C. Comparison of perinatal outcomes following blastocyst and cleavage-stage embryo transfer: analysis of 10 years' data from a single centre. Reprod Biomed Online 2019;38:967–978. [DOI] [PubMed] [Google Scholar]
- Sunde A, Brison D, Dumoulin J, Harper J, Lundin K, Magli MC, Van den Abbeel E, Veiga A. Time to take human embryo culture seriously. Hum Reprod 2016;31:2174–2182. [DOI] [PubMed] [Google Scholar]
- Takahashi M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev 2012;58:1–9. [DOI] [PubMed] [Google Scholar]
- Takeo T, Nakagata N. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin. Biol Reprod 2011;85:1066–1072. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Alcaraz B, Crispi F, Bijnens B, Cruz-Lemini M, Creus M, Sitges M, Bartrons J, Civico S, Balasch J, Gratacos E. Assisted reproductive technologies are associated with cardiovascular remodeling in utero that persists postnatally. Circulation 2013;128:1442–1450. [DOI] [PubMed] [Google Scholar]
- van Montfoort AP, Arts EGJM, Wijnandts L, Sluijmer A, Pelinck M-J, Land JA, van Echten-Arends J. Reduced oxygen concentration during human IVF culture improves embryo utilization and cumulative pregnancy rates per cycle. Hum Reprod Open 2020. doi:10.1093/hropen/hoz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassena R, Boue S, Gonzalez-Roca E, Aran B, Auer H, Veiga A, Izpisua Belmonte JC. Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development. Development 2011;138:3699–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez MA, Sheth B, Smith SJ, Eckert JJ, Osmond C, Fleming TP. Insulin and branched-chain amino acid depletion during mouse preimplantation embryo culture programmes body weight gain and raised blood pressure during early postnatal life. Biochim Biophys Acta Mol Basis Dis 2018;1864:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez MA, Smith CG, Smyth NR, Osmond C, Fleming TP. Advanced maternal age causes adverse programming of mouse blastocysts leading to altered growth and impaired cardiometabolic health in post-natal life. Hum Reprod 2016;31:1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arx R, Allemann Y, Sartori C, Rexhaj E, Cerny D, de Marchi SF, Soria R, Germond M, Scherrer U, Rimoldi SF. Right ventricular dysfunction in children and adolescents conceived by assisted reproductive technologies. J Appl Physiol (1985) 2015;118:1200–1206. [DOI] [PubMed] [Google Scholar]
- Wang LY, Le F, Wang N, Li L, Liu XZ, Zheng YM, Lou HY, Xu XR, Chen YL, Zhu XM et al. Alteration of fatty acid metabolism in the liver, adipose tissue, and testis of male mice conceived through assisted reproductive technologies: fatty acid metabolism in ART mice. Lipids Health Dis 2013;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Le F, Wang N, Zhang F, Luo Y, Lou Y, Hu M, Wang L, Thurston LM et al. Alteration in the expression of the renin-angiotensin system in the myocardium of mice conceived by in vitro fertilization. Biol Reprod 2018;99:1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins A, Wilkins A, Osmond C, Warner CM, Comiskey M, Hanson M, Fleming TP. The influence of mouse Ped gene expression on postnatal development. J Physiol 2006;571:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Platt D, Papenbrock T, Wilkins A, Eckert JJ, Kwong WY, Osmond C, Hanson M, Fleming TP. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci U S A 2007;104:5449–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, Wilkins A, Perry VH, Sheth B, Kwong WY et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod 2008;78:299–306. [DOI] [PubMed] [Google Scholar]
- Wright K, Brown L, Brown G, Casson P, Brown S. Microarray assessment of methylation in individual mouse blastocyst stage embryos shows that in vitro culture may have widespread genomic effects. Hum Reprod 2011;26:2576–2585. [DOI] [PubMed] [Google Scholar]
- Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, Norman RJ, Febbraio MA, Carroll J, Robker RL. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015;142:681–691. [DOI] [PubMed] [Google Scholar]
- Xu J, Sinclair KD. One-carbon metabolism and epigenetic regulation of embryo development. Reprod Fertil Dev 2015;27:667–676. [DOI] [PubMed] [Google Scholar]
- Zander DL, Thompson JG, Lane M. Perturbations in mouse embryo development and viability caused by ammonium are more severe after exposure at the cleavage stages. Biol Reprod 2006;74:288–294. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liu H, Gu HT, Cui YG, Zhao NN, Chen J, Gao L, Zhang Y, Liu JY. Association of cardiac development with assisted reproductive technology in childhood: a prospective single-blind pilot study. Cell Physiol Biochem 2014;34:988–1000. [DOI] [PubMed] [Google Scholar]