Abstract
Background
Although universal testing for mismatch repair deficiency (dMMR) has been recommended to all colorectal cancer (CRC) patients, related evidence for the Chinese population is lacking. Here, we investigated the prevalence and clinicopathological features of dMMR patients in a large Chinese CRC cohort.
Methods
We included 7,373 CRC patients treated at four Chinese medical centers between August 2010 and September 2016. Patients’ baseline characteristics and pathological features were recorded. The clinicopathological features were compared between patients with MLH1/PMS2 deficiency (dMLH1/PMS2) and MSH2/MSH6 deficiency (dMSH2/MSH6).
Results
Among the investigated patients, 654 (8.9%) were identified with dMMR CRCs and, of them, 401 (61.3%) were males, with a median age of 55 years (range, 22–87 years); 355 (54.3%) had stage II CRC based on American Joint Committee on Cancer 8th edition. The prevalence of the dMLH1/PMS2 group and the dMSH2/MSH6 group were 51.5% (337/654) and 25.1% (164/654), respectively. Compared with dMSH2/MSH6 patients, those with dMLH1/PMS2 were older (57 vs 52 years, P < 0.001), more likely to be female (45.7% vs 31.5%, P = 0.004), prone to having tumors located in the right-hand side of the colon (59.0% vs 47.6%, P = 0.015), and less likely to have a family history of tumors (29.7% vs 43.3%, P = 0.003).
Conclusions
The prevalence of dMMR in Chinese CRC patients was low, especially in the dMLH1/PMS2 group. The clinicopathological features were different between dMMR subgroups.
Keywords: prevalence, mismatch repair deficiency, colorectal cancer
Introduction
The DNA mismatch repair (MMR) system maintains genomic stability [1]. Mismatch repair deficiency (dMMR), usually caused by germline variants or epigenetic alterations in MMR genes, has been found to be associated with an elevated spontaneous mutation rate and cancer development [2]. Universal MMR testing is now recommended by the National Comprehensive Cancer Network guidelines [3] to all colorectal cancer (CRC) patients, as it was observed to be a prognostic factor for CRC [4], a predictive biomarker for immune checkpoint inhibitor [5] and chemotherapy with 5-fluorouracil alone [4], as well as an indicator for Lynch syndrome (LS) screening [6].
With the increasing burden of colorectal cancer both worldwide and in China [7], recent studies have found that ≤15% of CRC had dMMR worldwide [6, 8], but this incidence in Asian patients was comparatively much smaller (6%–11%) [9, 10], suggesting that ethnic diversity might play an important role in the prevalence of dMMR. Based on the findings of our previous research, we hypothesize that ethnicity might indeed be related to the observed differences in molecular features of LS between Chinese CRC patients and the Western populations [11]. Herein, we collected the MMR immunohistochemical (IHC) test results from four Chinese medical centers to investigate the prevalence of dMMR in a large cohort of CRC patients and to further evaluate their association with the patients’ clinicopathological features.
Material and methods
Case inclusion and stratification
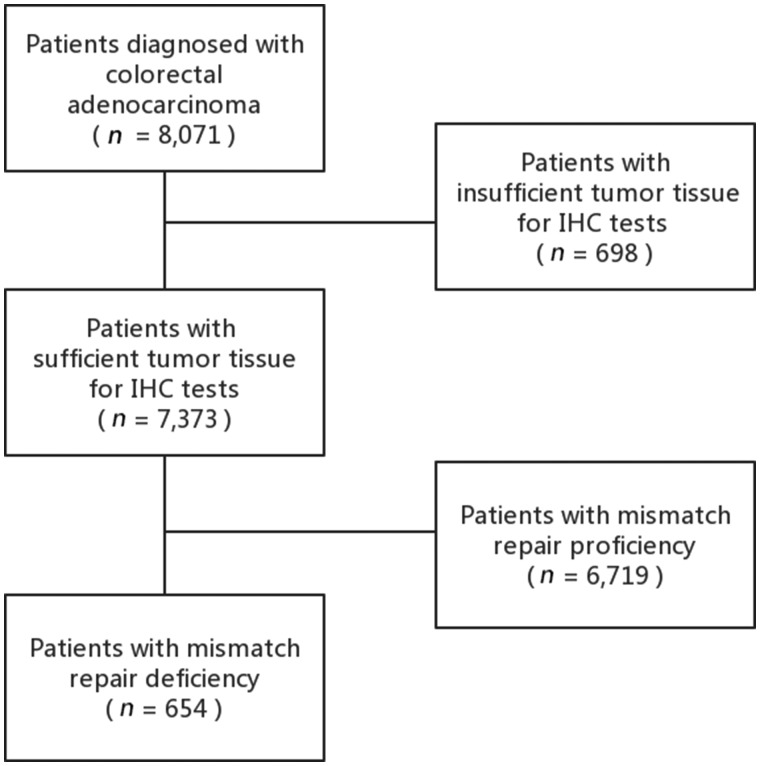
Data for this retrospective study were collected from four Chinese medical centers, namely the Sun Yat-sen University Cancer Center (Guangzhou, China), Zhejiang Cancer Hospital (Hangzhou, China), the First Affiliated Hospital of Guangzhou Medical University (Guangzhou, China), and the First Affiliated Hospital of Kunming Medical University (Kunming, China). The inclusion criteria comprised: (i) patients who had surgical resection for newly diagnosed colorectal adenocarcinoma between August 2010 and September 2016 and (ii) dMMR status detected using IHC tests in tumor tissues. Patients with insufficient tumor tissues for IHC tests were excluded. The screening strategy is detailed in Figure 1. All data in our study have been recorded at Sun Yat-sen University Cancer Center for future reference (number RDDA2020001385).
Figure 1.
Flow diagram of the screening strategy and main results of the study. CRC, colorectal cancer; dMMR, mismatch repair deficiency; pMMR, mismatch repair proficiency; IHC, immunohistochemistry.
Immunohistochemistry test
IHC tests for the MLH1, MSH2, MSH6, and PMS2 proteins were performed on tumors and adjacent normal tissues. All specimens were prepared as 4-μm fixed formalin paraffin-embedded sections. Protein deficiency was defined as negative nuclear staining in tumor cells and the presence of nuclear staining in normal tissues, whereas positive expression was defined as nuclear staining in tumor cells consistent with labeling in control cells. Patients with MLH1 deficiency alone or together with PMS2 deficiency were stratified into a dMLH1/PMS2 group and those with MSH2 deficiency alone or together with MSH6 deficiency were stratified into a dMSH2/MSH6 group.
Statistical analysis
Descriptive statistical methods were used to analyse demographic data. Data for continuous and discrete variables are presented as their corresponding mean and median, respectively. The comparison between group expression patterns was carried out using the t-test, chi-squared test, or Kruskal–Wallis test. All P-values of <0.05 were considered statistically significant. Statistical analyses were performed using the SPSS software, version 19.0 (Chicago, IL, USA).
Results
Baseline characteristics
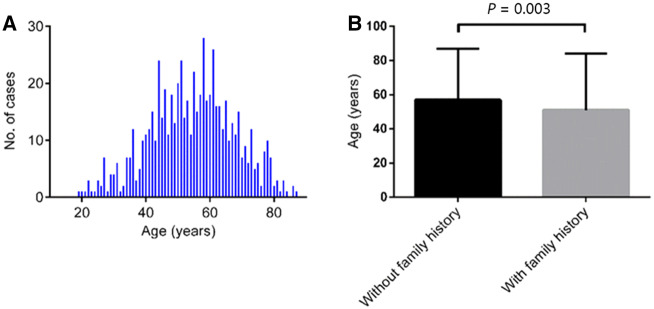
A total of 7,373 CRC patients from four medical centers were enrolled in our study, among whom 654 (8.9%) was identified with dMMR CRCs. The prevalence of dMLH1/PMS2 and dMSH2/MSH6 was 51.5% (337/654) and 25.1% (164/654), respectively. The baseline characteristics of the 654 dMMR patients are presented in Table 1. Of them, 401 (61.3%) were males, with a median age of 55 years (range, 22–87 years) (Figure 2A); 229 (35.0%) patients were younger than 50 years old, whereas 85 (13.0%) were >70 years old. There were 223 (34.1%) patients who had a family history of LS-related cancers, with a younger median age than that of patients without tumor family history (51 vs 57 years, P = 0.003) (Figure 2B). For dMMR patients >70 years old, 76.5% (65/85) had no tumor family history. The right-hand side of the colon was the most common location (n = 340; 52.0%) for dMMR CRCs, followed by the left-hand side of the colon (n = 146; 22.3%) and the rectum (n = 144; 22.0%).
Table 1.
Baseline characteristics of 654 colorectal cancer patients with mismatch repair deficiency
| Characteristic | No. of cases (%) |
|---|---|
| Sex | |
| Female | 253 (38.7) |
| Male | 401 (61.3) |
| Age (years) | |
| <50 | 229 (35.0) |
| 50∼ | 186 (28.4) |
| 60∼ | 154 (23.5) |
| 70∼ | 85 (13.0) |
| Tumor site | |
| Right colon | 340 (52.0) |
| Left colon | 146 (22.3) |
| Rectum | 144 (22.0) |
| Multiple locations | 24 (3.7) |
| Family history | |
| Yes | 223 (34.1) |
| No | 431 (65.9) |
| Tumor differentiation | |
| Well or moderate | 433 (66.2) |
| Poor | 185 (28.3) |
| N.A. | 36 (5.5) |
| Histology of primary tumor | |
| Adenocarcinoma | 400 (61.2) |
| Mucinous adenocarcinoma | 250 (38.2) |
| Others | 4 (0.6) |
| Lymphovascular invasion | |
| Yes | 128 (19.6) |
| No | 522 (79.8) |
| N.A. | 4 (0.6) |
| Perineural invasion | |
| Yes | 94 (14.4) |
| No | 555 (84.9) |
| N.A. | 5 (0.7) |
| T category | |
| Tis | 2 (0.3) |
| 1 | 27 (4.1) |
| 2 | 90 (13.8) |
| 3 | 287 (43.9) |
| 4 | 244 (37.3) |
| Tx | 4 (0.6) |
| N category | |
| 0 | 480 (73.4) |
| 1 | 123 (18.8) |
| 2 | 46 (7.0) |
| Nx | 5 (0.8) |
| M category | |
| 0 | 576 (88.1) |
| 1 | 75 (11.5) |
| Mx | 3 (0.4) |
| Stage | |
| 0 | 1 (0.2) |
| I | 104 (15.9) |
| II | 355 (54.3) |
| III | 115 (17.6) |
| IV | 75 (11.5) |
| N.A. | 4 (0.6) |
N.A., not available.
Figure 2.
Age of onset for patients with mismatch repair deficiency. (A) Fitting curve showing the distribution of the ages of onset for all patients with mismatch repair deficiency. (B) Bar graph showing the median diagnosed age for patients with and without family history.
Pathological features and staging
Lymphovascular and perineural invasion occurred in 19.6% (128/654) and 14.4% (94/654) of dMMR patients, respectively. Histologically, 38.2% (250/654) of the patients were diagnosed with mucinous carcinoma and 28.3% (180/654) of the cases were classified as poorly differentiated. Among the 654 patients, 531 (81.2%) had advanced T category (T3/4) disease, 169 (25.8%) had lymph-node metastasis, 75 (11.5%) had distant metastasis, and 355 (54.3%) had stage II CRC based on American Joint Committee on Cancer 8th edition.
Clinicopathological features between the two dMMR subgroups
Compared with the dMSH2/MSH6 group, patients in the dMLH1/PMS2 group were older (57 vs 52 years, P < 0.001), more likely to be female (45.7% vs 31.5%, P = 0.004), prone to having tumors located in the right-hand side of the colon (59.0% vs 47.6%, P = 0.015), and less likely to have a family history of LS-related cancers (29.7% vs 43.3%, P = 0.003). On the other hand, differences in tumor differentiation, histology of the primary tumor, lymphovascular invasion, perineural invasion, and staging between the dMLH1/PMS2 and dMSH2/MSH6 groups were not statistically significant between the two groups in Table 2.
Table 2.
Differences in clinicopathological features between the two mismatch repair deficiency subgroups
| Characteristic | dMLH1 alone or with dPMS2 (n = 337) | dMSH2 alone or with dMSH6 (n = 164) | P |
|---|---|---|---|
| Median age (years) | 57 | 52 | <0.001 |
| Sex | 0.004 | ||
| Male | 182 (54.3) | 111 (68.5) | |
| Female | 155 (45.7) | 53 (31.5) | |
| Tumor site | 0.015 | ||
| Right colon | 199 (59.0) | 78 (47.6) | |
| Left colon | 76 (22.6) | 36(21.9) | |
| Rectum | 53 (15.7) | 40 (24.4) | |
| Multiple | 9 (2.7) | 10 (6.1) | |
| Family history | 0.003 | ||
| Yes | 100 (29.7) | 71 (43.3) | |
| No | 237 (70.3) | 93 (56.7) | |
| Tumor differentiation | 0.599 | ||
| Well or moderate | 223 (66.2) | 106 (64.6) | |
| Poor | 96 (28.5) | 51 (31.1) | |
| N.A. | 18 (5.3) | 7 (4.3) | |
| Histology of primary tumor | 0.075 | ||
| Adenocarcinoma | 216 (64.1) | 91 (55.5) | |
| Mucinous adenocarcinoma | 121 (35.9) | 73 (44.5) | |
| Lymphovascular invasion | 0.052 | ||
| Yes | 76 (22.6) | 23 (14.0) | |
| No | 258 (76.5) | 140 (85.4) | |
| N.A. | 3 (0.9) | 1 (0.6) | |
| Perineural invasion | 0.696 | ||
| Yes | 45 (13.4) | 25 (15.2) | |
| No | 289 (85.7) | 137 (83.5) | |
| N.A. | 3 (0.9) | 2 (1.3) | |
| Stage | 0.627 | ||
| 0 | 1 (0.3) | 0 (0.0) | |
| I | 49 (14.5) | 24 (14.6) | |
| II | 187 (55.5) | 91 (55.5) | |
| III | 57 (16.9) | 32 (19.5) | |
| IV | 42 (12.5) | 15 (9.1) | |
| N.A. | 1 (0.3) | 2 (1.2) |
All values (except age) are presented as number of patients followed by percentage in the parentheses. N.A., not available.
Discussion
Our study investigated the largest dMMR cohort by far in Chinese CRC patients, in which a low prevalence (8.9%, 654/7,373) of dMMR was confirmed. Further evaluation demonstrated that subgroups of dMMR had different clinicopathological features; compared with the dMSH2/MSH6 group, patients with dMLH1/PMS2 were more likely to be female, to have late disease onset, a right-sided tumor, and no family history of cancer.
Since the MMR IHC testing has been gradually adopted worldwide, the difference of dMMR prevalence among nations has raised special attention. CRC patients from the USA were found to have a prevalence of dMMR as high as 14%–16% [6, 8], while, in contrast to a Malaysian study, only 6.2% of patients consisting of Chinese migrants had CRCs with dMMR [9]. Our previous single-center study showed that abnormal IHC results were detected in 10.2% (330/3,250) of CRC patients, and most of them were from Southern China [11]. In this current study, the patients from the four medical centers were from different parts of China. So, the epidemiological data were more convincing and a great supplementation to the ethnic diversity of dMMR CRCs. Interestingly, the spectrum of dMMR was different from that in Western patients as well. An Australian population-based study found that dMLH1/PMS2 patients accounted for 83.7% of all dMMR CRCs [12], while only 51.5% of the dMMR patients in our cohort were dMLH1/PMS2. One possible explanation for this dissimilarity might be the difference in frequency of MLH1 promoter methylation, which is responsible for a large number of MMR somatic mutations and contributes to nearly 80% of MLH1 deficiency [13]. Hence, it is supposed that Chinese CRC patients were less likely to have MLH1 promoter hypermethylation and this may have resulted in fewer patients with dMMR tumors.
It is well known that CRC patients with dMMR are usually diagnosed at an early age, located in the right-hand side of the colon, and have good prognoses [14, 15]. In this present study, 35.0% of the CRC patients with dMMR were diagnosed before 50 years old and most of them had stage II disease. However, 13.0% of the dMMR patients were diagnosed after their 70s. The dMMR patients with a family history of LS-related cancers had a significantly earlier age of CRC onset as compared to those without a tumor family history. This phenomenon may be attributed to the heterogeneity between germline and sporadic dMMR in CRCs. As a result, genetic tests may help to further stratify dMMR CRCs and provide better guidance for clinical management.
Another notable finding from our present study was the clinicopathological differences between the dMLH1/PMS2 and dMSH2/MSH6 groups. The reason for such differences was probably related to certain gene variations corresponding to the loss of MMR-protein expression. As previously reported, the estimated cumulative risks of CRC by age 70 years were 41% for MLH1 and 48% for MSH2 mutation carriers, respectively [16]. Similarly, patients with dMLH1/PMS2 in our cohort were more likely to have later onset than the dMSH2/MSH6 group. Although the MMR status could be evaluated either by IHC or a microsatellite instability test, one advantage of the IHC test is the indication of potential mutant genes. When genetic sequencing is not available, the dMMR subgroup could be used as an alternative biomarker for individual treatment.
Certain limitations exist in our study. First, insufficient information regarding the methylation test and genetic analysis made it impossible to clarify the etiology of each dMMR tumor. Second, ethnic information was not available from the obtained database for evaluation. Considering that China is a multi-ethnic nation, the genetic background between Han Chinese and other ethnic minorities might possibly explain, to a certain extent, the different prevalence of dMMR among four hospitals.
In conclusion, our study showed that the prevalence of dMMR in Chinese CRC patients was 8.9%, which is relatively low compared to Western populations, especially for patients with dMLH1/PMS2. There was heterogeneity among dMMR patients and it caused clinicopathological differences between dMMR subgroups.
Authors’ contributions
W. J., Q.Q.S., H.X.J., and P.R.D. conceived of the study and participated in its design and coordination. Z. Z., W.L.L, C.F.K., and J.L. performed data curation. Y.H.L., H.Z.Z., and M.Y.C. assisted in pathological interpretation. L.E.L. and L.L.M performed the statistical analyses and interpretation. W.J. and Q.Q.S. drafted the manuscript. D.S.W., Z.Z.P., H.X.J., and P.R.D. reviewed and edited. All authors read and approved the final manuscript.
Funding
This study was funded by the National Key R&D Program of China [grant number 2017YFC0908200], the National Natural Science Foundation of China [grant number 81871971] and Science and Technology Program of Guangzhou [grant number 201803010117], and the Science and Technology Program of Guangzhou [grant number 201802020030].
Acknowledgements
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
None declared.
Contributor Information
Wu Jiang, Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, P. R. China; State Key Laboratory of Oncology in South China, Guangzhou, Guangdong, P. R. China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P. R. China.
Qiao-Qi Sui, Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, P. R. China; State Key Laboratory of Oncology in South China, Guangzhou, Guangdong, P. R. China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P. R. China.
Wen-Liang Li, Department of Surgical Oncology, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, P. R. China.
Chuan-Feng Ke, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, P. R. China.
Yi-Hong Ling, State Key Laboratory of Oncology in South China, Guangzhou, Guangdong, P. R. China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P. R. China; Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, P. R. China.
Le-En Liao, Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, P. R. China; State Key Laboratory of Oncology in South China, Guangzhou, Guangdong, P. R. China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P. R. China.
Zhu Zhu, Department of Surgical Oncology, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, P. R. China.
Mu-Yan Cai, State Key Laboratory of Oncology in South China, Guangzhou, Guangdong, P. R. China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P. R. China; Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, P. R. China.
Jun Luo, Department of Colorectal Surgery, Zhejiang Cancer Hospital, Hangzhou, Zhejiang, P. R. China.
Lin-Lin Mao, Department of Clinical Genome Center, Guangzhou KingMed Diagnostics Group Co., Ltd, Guangzhou, Guangdong, P. R. China.
Hui-Zhong Zhang, State Key Laboratory of Oncology in South China, Guangzhou, Guangdong, P. R. China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P. R. China; Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, P. R. China.
De-Sen Wan, Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, P. R. China; State Key Laboratory of Oncology in South China, Guangzhou, Guangdong, P. R. China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P. R. China.
Zhi-Zhong Pan, Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, P. R. China; State Key Laboratory of Oncology in South China, Guangzhou, Guangdong, P. R. China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P. R. China.
Hai-Xing Ju, Department of Colorectal Surgery, Zhejiang Cancer Hospital, Hangzhou, Zhejiang, P. R. China.
Pei-Rong Ding, Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, P. R. China; State Key Laboratory of Oncology in South China, Guangzhou, Guangdong, P. R. China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P. R. China.
References
- 1. Jascur T, Boland CR. Structure and function of the components of the human DNA mismatch repair system. Int J Cancer 2006;119:2030–5. [DOI] [PubMed] [Google Scholar]
- 2. Boland PM, Yurgelun MB, Boland CR. Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. CA Cancer J Clin 2018;68:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Care Network: Genetic/Familial High-Risk Assessment: Colorectal Version 2.2019. https://wwwnccnorg/professionals/physician_gls/pdf/genetics_colonpdf [DOI] [PubMed]
- 4. Sargent DJ, Marsoni S, Monges G et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le DT, Uram JN, Wang H et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hampel H, Frankel WL, Martin E et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 2008;26:5783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng RM, Zong YN, Cao SM et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heald B, Plesec T, Liu X et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. J Clin Oncol 2013;31:1336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheah PL, Looi LM, Teoh KH et al. Colorectal carcinoma in Malaysians: DNA mismatch repair pattern in a multiethnic population. Asian Pac J Cancer Prev 2014;15:3287–91. [DOI] [PubMed] [Google Scholar]
- 10. Li P, Xiao ZT, Braciak TA et al. Impact of age and mismatch repair status on survival in colorectal cancer. Cancer Med 2017;6:975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang W, Cai MY, Li SY et al. written on behalf of AME Colorectal Cancer Cooperative Group. Universal screening for Lynch syndrome in a large consecutive cohort of Chinese colorectal cancer patients: high prevalence and unique molecular features. Int J Cancer 2019;144:2161–8. [DOI] [PubMed] [Google Scholar]
- 12. Ward RL, Hicks S, Hawkins NJ. Population-based molecular screening for Lynch syndrome: implications for personalized medicine. J Clin Oncol 2013;31:2554–62. [DOI] [PubMed] [Google Scholar]
- 13. Haraldsdottir S, Hampel H, Tomsic J et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology 2014;147:1308–16.e1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chou CL, Lin JK, , Wang HS et al. Microsatellite instability screening should be done for right-sided colon cancer patients less than 60 years of age. Int J Colorectal Dis 2010;25:47–52. [DOI] [PubMed] [Google Scholar]
- 15. Lin CC, , Lai YL, Lin TC et al. Clinicopathologic features and prognostic analysis of MSI-high colon cancer. Int J Colorectal Dis 2012;27:277–86. [DOI] [PubMed] [Google Scholar]
- 16. Møller P, Seppälä T, Bernstein I et al. Mallorca Group (http://mallorca-group.eu). Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 2017;66:464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]