Introduction
Chronic granulomatous disease (CGD) is a rare immunodeficiency affecting approximately 1 in 200,000 people [1]. Patients with CGD are unable to produce normal amounts of neutrophil superoxide and other phagocyte reactive oxygen species. As a result, phagocytes have decreased microbicidal activity and patients develop recurrent bacterial and fungal infections.
A portion of CGD patients develop inflammatory complications of the bowel that resemble inflammatory bowel disease (IBD). CGD-IBD consists of inflammatory, stricturing, and penetrating phenotypes with perianal involvement [2]. CGD-IBD is challenging to manage, as patients are inherently immunocompromised and at risk for more infections with further immunosuppression. The use of tumor necrosis factor inhibitors in this population has been associated with severe infections and mortality [3]. Other biologics, such as anakinra, have also had minimal efficacy [4]. The current mainstays of medical management are steroids, immunomodulators, antibiotics, and antifungal agents. Previous case reports have shown promising results for vedolizumab in CGD-IBD [5]. Vedolizumab is a humanized monoclonal antibody that targets the integrin α4β7. It prevents the adhesion of T-lymphocytes to mucosal addressin cell adhesion molecule 1 (MAdCAM-1), but not to vascular cell adhesion molecule-1 (VCAM-1), making its mechanism of action gut-specific.
Methods
Eleven patients with CGD-IBD were treated with vedolizumab while receiving care at the National Institutes of Health from 2015 to 2018 as a part of protocol 93-I-0119 or 05-I-0213. Clinical, laboratory, endoscopic, and pathologic data were extracted. Extent of bowel involvement was described using the Montreal classification. Mucosal healing was determined by the primary endoscopist and recorded as reported in the endoscopy report. All available endoscopies were reviewed after approximately 6 months of treatment and again at the end of follow-up. Descriptive statistics were performed using Excel (v.16.16.1).
Results
The 11 cases are summarized in Table 1. The cohort consists of eight males and three females, aged from 10 to 48 years old. There were eight Caucasian, two Asian, and one African American patient. Six patients had X-linked and five autosomal recessive CGD. Of note, one female patient was a highly Lyonized X-linked CGD carrier. Patients were diagnosed with CGD-IBD from infancy to 22 years of age. Two patients had a reported family history of colitis, three had luminal bowel surgery, and none of the patients used non-steroidal anti-inflammatory medications or smoked. The most frequently reported symptoms were increased stooling and hematochezia prior to treatment with vedolizumab. The mean erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) prior to the start of therapy was 31 mm/hr and 52 mg/L, respectively. Seven patients had colonic disease and four had ileocolonic disease. Three patients had an inflammatory phenotype, seven had a penetrating phenotype, one patient had a stricturing phenotype, and seven had perianal involvement. All patients were on steroids at the start of vedolizumab, eight patients were on aminosalicylates, nine were on immunomodulators, and six had previously tried other biologic agents including adalimumab, anakinra, ustekinumab, and infliximab.
Table 1.
Characteristics of this cohort of patients with chronic granulomatous disease-related inflammatory bowel disease (CGD-IBD)
| Patient | Age at CGD-IBD diagnosis | Sex/ race | Genotype | Previous biologic treatment | Duration of Ved (months) | No. of bowel movements |
Hematochezia |
C-reaction protein (mg/L) |
Steroid dose (mg) |
Adverse drug reaction | Drug discontinued | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre- Ved | Post- Ved | Pre- Ved | Post- Ved | Pre- Ved | Post- Ved | Pre- Ved | Post- Ved | ||||||||
| 1 | 17 | F/A | gp91 | No | 8 | 4 | 6 | Yes | No | 8.3 | 6.8 | 15 | 20 | Yes | Yes |
| 2 | 22 | M/C | gp91 | Yes | 6 | 2 | 2 | No | NA | 33.5 | 48.5 | 5 | 5 | No | No |
| 3 | 8 | M/AA | gp91 | Yes | 11 | 7 | 10 | Yes | Yes | 36.7 | 10.2 | 40 | 10 | No | Yes |
| 4 | 20 | M/C | p47 | Yes | 1 | 18 | 6 | Yes | NA | 73.8 | 35.5 | 40 | 30 | No | No |
| 5 | 17 | F/C | p47 | No | 19 | 3 | 3 | No | No | 62.3 | 42.2 | 20 | 20 | Yes | No |
| 6 | 1 | M/C | gp91 | Yes | 36 | NA | 3 | No | No | 88.1 | 6.8 | 5 | 1.5* | No | No |
| 7 | 19 | M/C | gp91 | No | 12 | 6 | 3 | Yes | NA | 72 | 156 | 10 | 10 | No | Yes |
| 8 | 15 | M/C | p47 | No | 9 | 7 | 9 | Yes | Yes | 0.8 | 0.6 | 20 | 17.5 | No | Yes |
| 9 | 5 | F/A | p67 | Yes | 16 | 3 | 2 | No | No | 112.4 | 1.7 | 3 | 2 | Yes | No |
| 10 | 8 | M/C | gp91 | No | 6 | 5 | 5 | NA | NA | 10.4 | 6.2 | 8.75 | 8.75 | Yes | No |
| 11 | 15 | M/C | p47 | Yes | 12 | 7 | 2 | No | No | 69.9 | 71.8 | 30 | 15 | No | No |
Budesonide. F, female; M, male; A, Asian; C, Caucasian; AA, African American; Ved, vedolizumab; NA, not available.
Response to therapy was assessed by clinical, laboratory, and endoscopic data after approximately 6 months and again at the end of follow-up, which was 12 months on average. Seven patients (64%) reported subjective improvement on vedolizumab. Four patients (36%) reported improvement in the number of bowel movements but only one of seven patients (14%) reported improvement in hematochezia at the end of follow-up. The average ESR and CRP improved to 26 mm/h and 35 mg/L, respectively. Four of seven patients (57%) had endoscopic improvement on an interim endoscopy at approximately 6 months (Figure 1). None of the patients had endoscopic improvement beyond 6 months of follow-up. Three patients (27%) had to decrease the interval of dosing from every 8 weeks to every 4 weeks due to inadequate response. Therapy was discontinued in four patients (36%) due to lack of meaningful response. None of the patients discontinued steroids but six patients (55%) decreased their dose, on average by 10 mg, by the end of follow-up. Four (36%) patients had adverse events while being treated with vedolizumab, two patients had infectious complications including pneumonia and the formation of abscesses, and two patients had symptoms consistent with infusion reactions.
Figure 1.
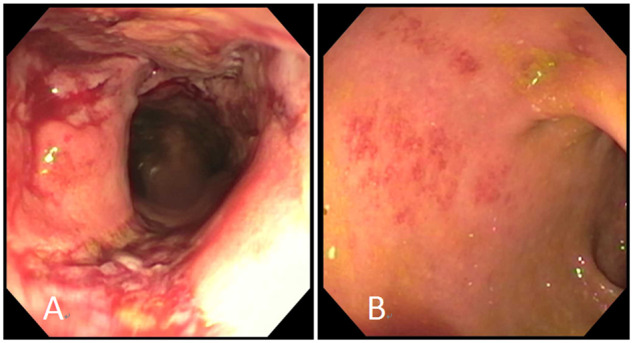
Endoscopic improvement after vedolizumab therapy in a patient with chronic granulomatous disease-related inflammatory bowel disease. (A) Erythema, friability, and ulceration prior to vedolizumab therapy. (B) Mucosal erythema and granularity after vedolizumab therapy.
Discussion
Vedolizumab for CGD-IBD resulted in limited success. While more than half of patients reported subjective clinical improvement, it did not lead to sustained mucosal healing nor did it have a steroid-sparing effect. Interestingly, more than half of patients with interim endoscopy had mucosal improvement approximately 6 months after starting therapy, which was not sustained until the end of therapy. It is not clear why the response to therapy was short-lived. Antibody levels were not checked in our cohort, but vedolizumab antibody production rates were <5% in the GEMINI trials [6–8]. However, a systematic review and meta-analysis found that loss of response to vedolizumab in Crohn’s disease (CD) and ulcerative colitis (UC) of 47.9 and 39.8 per 100 person-years of follow-up, respectively [9]. Lastly, while none of our patients were able to discontinue steroids, 55% were able to reduce their steroid doses by 10 mg on average while on vedolizumab.
There are several limitations to our observational study. The lack of a validated clinical index to objectively assess clinical remission or clinical response in CGD-IBD limits direct comparison of response rates in CGD-IBD, CD, and UC. It is possible that vedolizumab could be more successful with implementation of therapeutic drug monitoring for dose optimization. The data from CD and UC studies suggest that >50% of patients who lose response to vedolizumab can recapture it by dose intensification [9]. Furthermore, there is evidence to suggest that vedolizumab levels >18 μg/mL 6 weeks after starting therapy are associated with mucosal healing in both CD and UC [10]. Drug monitoring may provide further insight into the short-lived response to vedolizumab in CGD-IBD. Early and aggressive optimization may improve and prolong the early positive signals in this challenging population and should be further investigated.
Author’s contributions
Study concept and design: N.K. Acquisition of data: N.K., B.M., B.C., A.S., A.B., H.L.M., S.M.H., C.Z, and T.H. Data analysis and interpretation: N.K. and B.C. Study supervision: T.H. Drafting of manuscript: N.K. Critical revision of the manuscript for important intellectual content: B.M., B.C., A.S., S.S.D., A.B., C.K., H.L.M., S.M.H., C.Z., and T.H. All authors have read and approved the manuscript.
Funding
This work was supported by intramural program at the National Institutes of Health.
Acknowledgements
None.
Conflicts of interest
Dr Athos Bousvaros has several disclosures as follows. Research support (subinvestigator on protocols): Promehteus, Jannssen, Abbvie, Takeda, and Buhlmann. Consulting: Shire, Takeda, Best Doctors, Alivio, and Eli Lilly. Honoraria and royalties: up to date—royalties; Boston University—honorarium; Nutricia—honorarium—2017 September. All the other co-authors have no disclosures to report.
Contributor Information
Natasha Kamal, Digestive Disease Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Beatriz Marciano, Laboratory of Host Defenses, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Bryan Curtin, Digestive Disease Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Anna Strongin, Digestive Disease Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Suk See DeRavin, Laboratory of Host Defenses, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Athos Bousvaros, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, MA, USA.
Christopher Koh, Liver Disease Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Harry L Malech, Laboratory of Host Defenses, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Steven M Holland, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Christa Zerbe, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Theo Heller, Liver Disease Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
References
- 1. Kuhns DB, Alvord WG, Heller T et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med 2010;363:2600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khangura SK, Kamal N, Ho N et al. Gastrointestinal features of chronic granulomatous disease found during endoscopy. Clin Gastroenterol Hepatol 2016;14:395–402.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uzel G, Orange JS, Poliak N et al. Complications of tumor necrosis factor-alpha blockade in chronic granulomatous disease-related colitis. Clin Infect Dis 2010;51:1429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hahn KJ, Ho N, Yockey L et al. Treatment with anakinra, a recombinant IL-1 receptor antagonist, unlikely to induce lasting remission in patients with CGD colitis. Am J Gastroenterol 2015;110:938–9. [DOI] [PubMed] [Google Scholar]
- 5. Campbell N, Chapdelaine H. Treatment of chronic granulomatous disease-associated fistulising colitis with vedolizumab. J Allergy Clin Immunol Pract 2017;5:1748–9. [DOI] [PubMed] [Google Scholar]
- 6. Sands BE, Feagan BG, Rutgeerts P et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–27.e3. [DOI] [PubMed] [Google Scholar]
- 7. Sandborn WJ, Feagan BG, Rutgeerts P et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 8. Feagan BG, Rutgeerts P, Sands BE et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 9. Peyrin-Biroulet L, Danese S, Argollo M et al. Loss of response to vedolizumab and ability of dose intensification to restore response in patients with Crohn's disease or ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019;17:838–46, e2. [DOI] [PubMed] [Google Scholar]
- 10. Yacoub W, Williet N, Pouillon L et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018;47:906–12. [DOI] [PubMed] [Google Scholar]


