Abstract
Background
Endoscopic treatment is recommended for the management of esophageal varices. However, variceal recurrence or rebleeding is common after endoscopic variceal eradication. Our study aimed to systematically evaluate the prevalence of esophageal collateral veins (ECVs) and the association of ECVs with recurrence of esophageal varices or rebleeding from esophageal varices after endoscopic treatment.
Methods
We searched the relevant literature through the PubMed, EMBASE, and Cochrane Library databases. Prevalence of paraesophageal veins (para-EVs), periesophageal veins (peri-EVs), and perforating veins (PVs) were pooled. Risk ratio (RR) and odds ratio (OR) with 95% confidence intervals (CIs) were calculated for cohort studies and case–control studies, respectively. A random-effects model was employed. Heterogeneity among studies was calculated.
Results
Among the 532 retrieved papers, 28 were included. The pooled prevalence of para-EVs, peri-EVs, and PVs in patients with esophageal varices was 73%, 88%, and 54%, respectively. The pooled prevalence of para-EVs and PVs in patients with recurrence of esophageal varices was 87% and 62%, respectively. The risk for recurrence of esophageal varices was significantly increased in patients with PVs (OR = 9.79, 95% CI: 1.95–49.22, P = 0.006 for eight case–control studies), but not in those with para-EVs (OR = 4.26, 95% CI: 0.38–38.35, P = 0.24 for four case–control studies; RR = 1.81, 95% CI: 0.83–3.97, P = 0.14 for three cohort studies). Patients with para-EVs had a significantly higher incidence of rebleeding from esophageal varices (RR = 13.00, 95% CI: 2.43–69.56, P = 0.003 for two cohort studies). Statistically significant heterogeneity was notable across the meta-analyses.
Conclusions
ECVs are common in patients with esophageal varices. Identification of ECVs could be helpful for predicting the recurrence of esophageal varices or rebleeding from esophageal varices after endoscopic treatment.
Keywords: esophageal collateral veins, esophageal varices, prevalence, rebleeding
Introduction
Portal hypertension is a major consequence of advanced liver disease, which leads to the development of collateral veins between the portal and systemic circulations [1], thereby causing gastroesophageal variceal bleeding, splenorenal shunt, and ascites, etc. [2]. Esophageal varices are the most important collateral veins secondary to portal hypertension with a prevalence of 30%–40% in compensated patients and 80% in decompensated patients [3]. Gastrointestinal bleeding secondary to rupture of esophageal varices results in a 6-week mortality of 15%–20% [2, 3]. Endoscopic treatment, such as endoscopic variceal ligation (EVL) or endoscopic injection sclerotherapy (EIS), remains the standard treatment for high-risk varices and bleeding varices [4, 5], but does not influence the pathophysiology of portal hypertension. Recurrence of esophageal varices and rebleeding from esophageal varices after endoscopic therapy are common [3, 6].
Esophageal collateral veins (ECVs), which are characterized as hypoechoic shadow on endoscopic ultrasound (EUS) or serpentine enhanced shadow on computed tomography (CT) [7, 8], are associated with esophageal mucosal varices in patients with portal hypertension [9, 10]. They are classified into paraesophageal veins (para-EVs), which are large vessels distal to the muscularis externa of the esophagus; periesophageal veins (peri-EVs), which are small vessels adjacent to the muscularis externa of the esophagus; and perforating veins (PVs), which are vessels connecting the para-EVs to the submucosal varices [7, 11]. The presence of ECVs for assessing the severity and outcomes of portal hypertensive bleeding remains inconclusive among the major practice guidelines and consensus.
We performed a systematic review and meta-analysis of available evidence to explore the prevalence of ECVs in patients with portal hypertension and to evaluate the association of ECVs with recurrence of esophageal varices or rebleeding from esophageal varices after endoscopic treatment.
Methods
Search strategy
This study was registered on the PROSPERO database (registration number: CRD42019129555).
We searched all relevant papers via the PubMed, EMBASE, and Cochrane library databases. The search items were (paraesophageal or para-esophageal or periesophageal or peri-esophageal or perforating) AND (endoscopic or endoscopy) AND (variceal or varices). The last search was performed on 22 March 2019.
Study selection
Inclusion criteria were as follows: (i) studies reporting on the prevalence of ECVs in patients with esophageal varices and/or recurrence of esophageal varices; and (ii) reporting on the association of recurrence of esophageal varices and/or rebleeding from esophageal varices with ECVs. There was no language limitation.
Exclusion criteria were as follows: (i) duplicates; (ii) reviews and/or meta-analyses; (iii) case reports; (iv) comments, letters, and/or editorials; (v) studies that did not explore the prevalence of ECVs or association of ECVs with recurrence of esophageal varices or rebleeding from esophageal varices; and (vi) studies with incomplete data.
Data collection
We collected the data as follows: first author, publication year, country, target population, enrollment period, follow-up period, endoscopic treatment approaches (EIS, EVL, or EIS combined EVL), type of ECVs (para-EVs, peri-EVs, and PVs), diagnostic approaches for ECVs (including CT, EUS, endoscopic color Doppler ultrasonography [ECDUS], magnetic resonance angiography [MRA], and percutaneous transhepatic portography [PTP]), prevalence of ECVs, and incidence of recurrence of esophageal varices or rebleeding from esophageal varices.
Study quality assessment
The quality of included studies was evaluated by using the Newcastle-Ottawa Scale (NOS). The maximum score was 9 points. High quality was considered as a NOS score of ≥6 points.
Statistical analysis
All meta-analyses were performed by using the Stata software version 12.0 (Stata Corp, College Station, Texas, USA) and Review Manager software version 5.3 (Cochrane collaboration, the Nordic Cochrane Centre, Copenhagen). First, we pooled the prevalence of ECVs in patients with esophageal varices or recurrence of esophageal varices along with the respective 95% confidence interval (CI). Thereafter, we explored the association between ECVs and recurrence of esophageal varices or rebleeding from esophageal varices. The risk ratio (RR) or odds ratio (OR) with 95% CI was calculated. Only a random-effects model was employed. I2 and P-values were calculated to assess the heterogeneity, and I2 > 50% and/or P < 0.1 were considered to have statistically significant heterogeneity. Because every study we included would be biased toward reporting some non-standard results, the publication bias was not evaluated. Subgroup analyses were performed according to the approaches used for diagnosing ECVs (EUS, ECDUS, CT, MRA, or PTP), choices of endoscopic treatment (EIS, EVL, or EIS combined with EVL), and regions (Asia or Europe).
Results
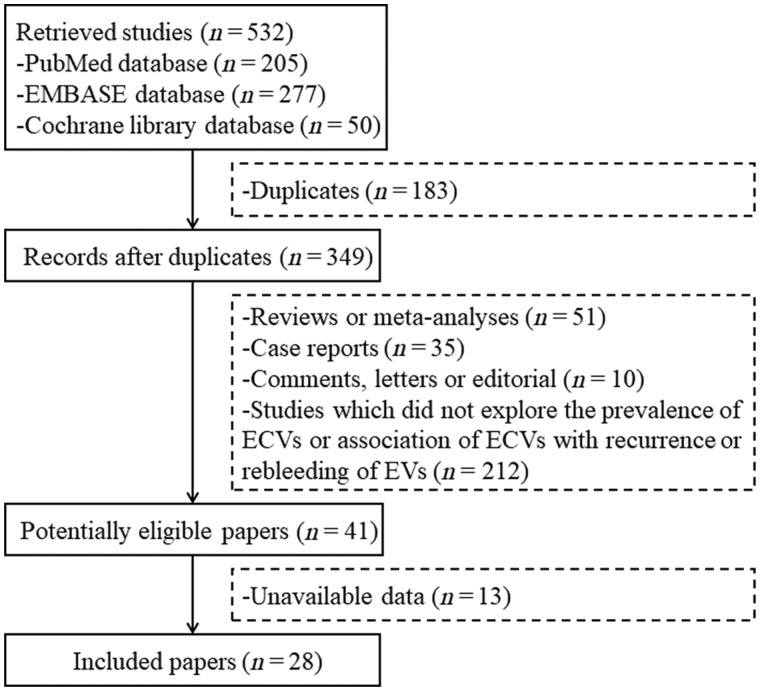
A total of 532 studies were identified through the PubMed, EMBASE, and Cochrane library databases and, finally, 28 studies were included [12–39] (Figure 1). Twenty-one studies were of high quality while seven were of low quality (Supplementary Table 1).
Figure 1.
Flowchart of study selection. ECVs, esophageal collateral veins; EVs, esophageal varices.
Prevalence of ECVs
Fifteen studies explored the prevalence of ECVs in patients with esophageal varices. The sample size ranged from 13 to 251, with a total of 994 patients (Supplementary Table 2). EUS, ECDUS, CT, and MRA were employed in eight, five, one, and one study, respectively. The prevalence of para-EVs, peri-EVs, and PVs were explored in 7, 3, and 12 studies, respectively. Meta-analyses demonstrated that the overall prevalence of para-EVs, peri-EVs, and PVs were 73% (95% CI: 60%–85%), 88% (95% CI: 75%–101%), and 54% (95% CI: 45%–63%), respectively. Heterogeneity was statistically significant in all of the three meta-analyses (Table 1).
Table 1.
Prevalence of esophageal collateral veins in patients with esophageal varices: results of meta-analyses
| Group | No. of studies | No. of patients | Pooled prevalence using random-effects model (95% CI) | Heterogeneity |
|
|---|---|---|---|---|---|
| I 2 | P-value | ||||
| Paraesophageal veins | |||||
| Overall (all from Asia) | 7 | 358 | 73% (60%–85%) | 97.4% | <0.001 |
| Using EUS | 3 | 106 | 61% (29%–92%) | 95.5% | <0.001 |
| Using ECDUS | 2 | 149 | 100% (99%–101%) | 0.0% | 0.850 |
| Using CT | 1 | 59 | 29% (17%–40%) | − | − |
| Using MRA | 1 | 44 | 45% (31%–60%) | − | − |
| Periesophageal veins | |||||
| Overall | 3 | 97 | 88% (75%–101%) | 75.7% | 0.016 |
| Using EUS | 3 | 97 | 88% (75–101%) | 75.7% | 0.016 |
| Asia | 2 | 57 | 91% (77%–106%) | 67.8% | 0.078 |
| Europe | 1 | 40 | 80% (68%–92%) | − | − |
| Perforating veins | |||||
| Overall | 12 | 851 | 54% (45%–63%) | 85.9% | <0.001 |
| Using EUS | 7 | 259 | 55% (43%–66%) | 72.5% | 0.001 |
| Using ECDUS | 5 | 592 | 54% (39%–68%) | 92.0% | <0.001 |
| Asia | 11 | 800 | 56% (46%–65%) | 86.1% | <0.001 |
| Europe | 1 | 51 | 39% (26%–53%) | − | − |
CI, confidence interval; EUS, endoscopic ultrasound; ECDUS, endoscopic color Doppler ultrasonography; CT, computed tomography; MRA, magnetic resonance angiography.
Fourteen studies explored the prevalence of ECVs in patients with recurrence of esophageal varices. The sample size ranged from 3 to 294, with a total of 684 patients (Supplementary Table 3). Among them, EUS, ECDUS, CT, and PTP were employed in nine, three, one, and one study, respectively; EIS alone, EVL alone, and EIS or EVL were performed in seven, three, and four studies, respectively. The prevalence of para-EVs, peri-EVs, and PVs were explored in 9, 1, and 10 studies, respectively. Meta-analyses demonstrated that the overall prevalence of para-EVs, peri-EVs, and PVs were 87% (95% CI: 79%–94%), 76% (95% CI: 56%–97%), and 62% (95% CI: 35%–90%), respectively. Heterogeneity was statistically significant in the two meta-analyses regarding para-EVs and PVs (Table 2).
Table 2.
Prevalence of esophageal collateral veins in patients with esophageal variceal recurrence after endoscopic treatment: results of meta-analyses
| Group | No. of studies | No. of patients | Pooled prevalence using random-effects model (95% CI) | Heterogeneity |
|
|---|---|---|---|---|---|
| I 2 | P-value | ||||
| Paraesophageal veins | |||||
| Overall (all from Asia) | 9 | 488 | 87% (79%–94%) | 91.2% | <0.001 |
| Using EUS | 4 | 84 | 96% (92%–100%) | 0.0% | 0.592 |
| Using ECDUS | 3 | 353 | 95% (89%–101%) | 83.6% | 0.002 |
| Using CT | 1 | 14 | 57% (31%–88%) | − | − |
| Using PTP | 1 | 37 | 32% (17%–48%) | − | − |
| EIS | 6 | 380 | 75% (55%–94%) | 92.1% | <0.001 |
| EVL | 2 | 49 | 98% (93%–102%) | 0.0% | 0.555 |
| Periesophageal veins | |||||
| Overall (from Asia) | 1 | 17 | 76% (56%–97%) | − | − |
| Using EUS | 1 | 17 | 76% (56%–97%) | − | − |
| EIS | 1 | 17 | 76% (56%–97%) | − | − |
| Perforating veins | |||||
| Overall | 10 | 584 | 62% (35%–90%) | 98.4% | <0.001 |
| Using EUS | 7 | 231 | 70% (49%–90%) | 92.0% | <0.001 |
| Using ECDUS | 3 | 353 | 46% (−6%–98%) | 98.3% | <0.001 |
| EIS | 5 | 385 | 54% (13%–95%) | 98.1% | <0.001 |
| EVL | 4 | 131 | 65% (30%–99%) | 95.3% | <0.001 |
| Asia | 9 | 565 | 63% (34%–93%) | 98.5% | <0.001 |
| Europe | 1 | 19 | 53% (30%–75%) | − | − |
CI, confidence interval; EUS, endoscopic ultrasound; ECDUS, endoscopic color Doppler ultrasonography; CT, computed tomography; PTP, percutaneous transhepatic portography; EIS, endoscopic injection sclerotherapy; EVL, endoscopic variceal ligation.
Association between ECVs and recurrence of esophageal varices
Fourteen studies explored the association between ECVs and recurrence of esophageal varices. The sample size ranged from 18 to 306, with a total of 1,021 patients (Supplementary Tables 4 and 5). Among them, 4 were cohort studies and 10 were case–control studies; EUS, ECDUS, CT, and PTP were employed in 10, 2, 1, and 1 study, respectively; EIS alone, EVL alone, and EIS or EVL were performed in 7, 4, and 3 studies, respectively.
Three cohort studies including 206 patients explored the association between para-EVs and recurrence of esophageal varices. Meta-analysis demonstrated that there was no significant association between para-EVs and recurrence of esophageal varices (RR = 1.81, 95% CI: 0.83–3.97, P = 0.14). Heterogeneity was statistically significant in the meta-analysis (Table 3). Only one cohort study including 22 patients explored the association between PVs and recurrence of esophageal varices and no significant association was demonstrated (RR = 1.88, 95% CI: 0.83–4.22, P = 0.13). No cohort study explored the association between peri-EVs and recurrence of esophageal varices.
Table 3.
Association between esophageal collateral veins and esophageal variceal recurrence in cohort studies: results of meta-analyses
| Group | No. of studies | No. of patients | Pooled-effect sizes using random-effects model |
Heterogeneity |
||
|---|---|---|---|---|---|---|
| Risk ratio (95% CI) | P-value | I 2 | P-value | |||
| Paraesophageal veins | ||||||
| Overall (all from Asia) | 3 | 206 | 1.81 (0.83–3.97) | 0.14 | 79% | 0.008 |
| Using EUS | 1 | 40 | 2.19 (1.37–3.52) | 0.001 | − | − |
| Using CT | 1 | 51 | 3.52 (1.49–8.34) | 0.004 | − | − |
| Using PTP | 1 | 115 | 0.82 (0.49–1.54) | 0.62 | − | − |
| EIS | 2 | 166 | 1.68 (0.43–6.65) | 0.46 | 86% | 0.008 |
| EVL | 1 | 40 | 2.19 (1.37–3.52) | 0.001 | − | − |
| Perforating veins | ||||||
| Overall (from Asia) | 1 | 22 | 1.88 (0.83–4.22) | 0.13 | − | − |
| Using EUS | 1 | 22 | 1.88 (0.83–4.22) | 0.13 | − | − |
| EVL | 1 | 22 | 1.88 (0.83–4.22) | 0.13 | − | − |
CI, confidence interval; EUS, endoscopic ultrasound; CT, computed tomography; PTP, percutaneous transhepatic portography; EIS, endoscopic injection sclerotherapy; EVL, endoscopic variceal ligation.
Four case–control studies including 447 patients explored the association between para-EVs and recurrence of esophageal varices. Meta-analysis demonstrated that there was no significant association between para-EVs and recurrence of esophageal varices (OR = 4.26, 95% CI: 0.38–38.35, P = 0.24). Heterogeneity was statistically significant in the meta-analysis (Table 4). Eight case–control studies including 696 patients explored the association between PVs and recurrence of esophageal varices. Meta-analyses demonstrated that PVs significantly increased the risk of recurrence of esophageal varices (OR = 9.79, 95% CI: 1.95–49.22, P = 0.006). Heterogeneity was statistically significant in the meta-analysis (Table 4). Only one case–control study including 44 patients explored the association between peri-EVs and recurrence of esophageal varices and no significant association was demonstrated (OR = 1.37, 95% CI: 0.34–5.51, P = 0.66).
Table 4.
Association between esophageal collateral veins and esophageal variceal recurrence in case–control studies: results of meta-analyses
| Group | No. of studies | No. of patients | Pooled-effect quantities using random-effects model |
Heterogeneity |
||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | I 2 | P-value | |||
| Paraesophageal veins | ||||||
| Overall (all from Asia) | 4 | 447 | 4.26 (0.38–48.35) | 0.24 | 77% | 0.005 |
| Using EUS | 3 | 141 | 7.50 (0.35–161.19) | 0.20 | 75% | 0.020 |
| Using ECDUS | 1 | 306 | 0.90 (0.11–7.23) | 0.92 | − | − |
| EIS | 4 | 403 | 3.97 (0.37–43.25) | 0.26 | 72% | 0.010 |
| EVL | 1 | 44 | 18.75 (1.91–184.20) | 0.01 | − | − |
| Periesophageal veins | ||||||
| Overall (from Asia) | 1 | 44 | 1.37 (0.34–5.51) | 0.66 | − | − |
| Using EUS | 1 | 44 | 1.37 (0.34–5.51) | 0.66 | − | − |
| EIS | 1 | 44 | 1.37 (0.34–5.51) | 0.66 | − | − |
| Perforating veins | ||||||
| Overall | 8 | 696 | 9.79 (1.95–49.22) | 0.006 | 80% | <0.001 |
| Using EUS | 6 | 335 | 11.27 (3.24–39.28) | <0.001 | 54% | 0.060 |
| Using ECDUS | 2 | 361 | 5.12 (0.00–11,618.16) | 0.68 | 95% | <0.001 |
| EIS | 4 | 418 | 5.83 (0.37–92.96) | 0.21 | 88% | <0.001 |
| EVL | 2 | 70 | 8.21 (2.33–28.93) | 0.001 | 0% | 0.630 |
| Asia | 7 | 656 | 11.10 (1.54–79.82) | 0.02 | 83% | <0.001 |
| Europe | 1 | 40 | 6.67 (1.46–30.43) | 0.01 | − | − |
CI, confidence interval; EUS, endoscopic ultrasound; ECDUS, endoscopic color Doppler ultrasonography; EIS, endoscopic injection sclerotherapy; EVL, endoscopic variceal ligation.
Association between ECVs and rebleeding from esophageal varices
Three studies explored the association between ECVs and rebleeding from esophageal varices. The sample size ranged from 40 to 79, with a total of 170 patients (Supplementary Tables 4 and 5). Among them, two were cohort studies and one was a case–control study; EUS and CT were employed in two and one study, respectively; EIS alone, EVL alone, and EIS or EVL were performed in one study each, respectively.
Two cohort studies including 91 patients explored the association between para-EVs and rebleeding from esophageal varices. Meta-analysis demonstrated that para-EVs significantly increased the incidence of rebleeding from esophageal varices (RR = 13.00, 95% CI: 2.43–69.56, P = 0.003). There was no significant heterogeneity in the meta-analysis (Table 5).
Table 5.
Association between esophageal collateral veins and esophageal variceal rebleeding in cohort studies: results of meta-analyses
| Group | No. of studies | No. of patients | Pooled-effect sizes using random-effects model |
Heterogeneity |
||
|---|---|---|---|---|---|---|
| Risk ratio (95% CI) | P-value | I 2 | P-value | |||
| Paraesophageal veins | ||||||
| Overall (all from Asia) | 2 | 91 | 13.00 (2.43–69.56) | 0.003 | 0% | 0.980 |
| Using EUS | 1 | 40 | 12.60 (0.70–227.89) | 0.09 | − | − |
| Using CT | 1 | 51 | 13.21 (1.69–103.38) | 0.01 | − | − |
| EIS | 1 | 51 | 13.21 (1.69–103.38) | 0.01 | − | − |
| EVL | 1 | 40 | 12.60 (0.70–227.89) | 0.09 | − | − |
CI, confidence interval; EUS, endoscopic ultrasound; CT, computed tomography; EIS, endoscopic injection sclerotherapy; EVL, endoscopic variceal ligation.
Only one case–control study including 79 patients explored the association between para-EVs and rebleeding from esophageal varices and demonstrated that para-EVs significantly increased the incidence of rebleeding from esophageal varices (OR = 19.27, 95% CI: 1.09–341.82, P = 0.044).
Discussion
Our systematic review and meta-analysis aimed at exploring the prevalence of ECVs in patients with esophageal varices and assessing the association of ECVs with recurrence of esophageal varices or rebleeding from esophageal varices. Based on the 28 included studies, we found that the prevalence of para-EVs, peri-EVs, and PVs was 73%, 88%, and 54%, respectively, in patients with esophageal varices; and 87%, 76%, and 62%, respectively, in patients with recurrence of esophageal varices. PVs increased the risk of recurrence of esophageal varices by nearly 10-fold and para-EVs increased the risk of rebleeding from esophageal varices after endoscopic treatment by more than 13-fold.
Recently, a systematic review by Masalaite et al. [40] also explored the association of ECVs with recurrence of esophageal varices and demonstrated that ECVs might be related to a higher risk of recurrence of esophageal varices. Compared to Masalaite’s study, our findings might be more reliable, since we included a larger number of studies. The prevalence of ECVs was one of the major interests in our study, but not in Masalaite’s study. The association of ECVs with rebleeding from esophageal varices was explored in our study, but not in Masalaite’s study. Routine endoscopy cannot detect ECVs and there are multiple diagnostic approaches for identification of ECVs [41]. The approach for detecting ECVs was not limited in our study, in contrast to Masalaite’s study in which only EUS was employed. We also pooled the effect size according to the study design (i.e. RR and OR for cohort and case–control studies, respectively) compared to the study by Masalaite et al. in which only the RR was calculated.
As endoscopic treatment eradicates esophageal varices locally but does not decrease the portal pressure, esophageal varices usually recur after variceal eradication. Routine endoscopic surveillance and early secondary prophylaxis should be strictly performed after variceal eradication [3]. The frequency of endoscopic surveillance should be dependent upon the risk of recurrence and rebleeding. As for patients who are at a high risk of variceal recurrence and rebleeding, the interval of endoscopic surveillance could be made shorter. Defining the risk factors for recurrence or rebleeding after endoscopic variceal treatment within current guidelines and consensus remains an unmet need. Conventionally, the major risk factors for recurrence and rebleeding may include the severity of liver disease, treatment approaches, and hepatic venous pressure gradient (HVPG) response to treatment [1]. Response was defined as a reduction in HVPG to ≤12 mmHg or a decrement of ≥20% from baseline. HVPG responders had a lower risk of rebleeding [42–45]. However, HVPG response is not completely equal to the recurrence of esophageal varices. Some HVPG responders remain at risk of rebleeding and some HVPG non-responders do not rebleed [46]. Besides, HVPG measurement is invasive, expensive, and requires technical skill. In the absence of the availability of HVPG measurement, evaluating the presence of ECVs may be of value in predicting the recurrence of esophageal varices or rebleeding from esophageal varices.
EVL and EIS are the most common endoscopic approaches for esophageal varices. Only two studies demonstrated that the prevalence of PVs was higher in patients undergoing EVL than in those undergoing EIS [20, 39]. Our meta-analysis further confirmed that the presence of PVs significantly increased the risk of recurrence of esophageal varices in patients undergoing EVL, but not in those undergoing EIS. These might be explained by the heterogeneity in the effects of types of endoscopic treatment on esophageal varices. Despite the fact that EVL achieved superficial variceal eradication through mechanical constriction, it did not have any effect on PVs. By comparison, EIS acted on submucosal tissues through chemical reaction and led to obliteration of PVs.
There were several limitations in our study. First, the heterogeneity was significant in most of our meta-analyses. In spite of subgroup and meta-regression analysis, the source of heterogeneity remained unclear. Second, the follow-up duration for evaluating the recurrence of esophageal varices or rebleeding from esophageal varices was variable. Third, two studies included were published in the abstract form, so some detailed data could not be extracted. Fourth, 21% of studies included were of low quality.
In conclusion, ECVs are common in patients with esophageal varices. The risk of recurrence of esophageal varices was 10-fold higher in patients with PVs and the risk of rebleeding from esophageal varices was more than 13-fold higher in patients with para-EVs. In clinical practice, contrast-enhanced cross-sectional imaging or advanced endoscopic modalities in assessing ECVs associated with esophageal varices could help in identifying the patients at risk for variceal recurrence or rebleeding. Patients with PVs should be considered to shorten the interval of endoscopic surveillance and actively employ the strategy for portal pressure reduction, especially in patients with history of EVL.
Supplementary data
Supplementary data is available at Gastroenterology Report online.
Authors’ contributions
Q.Q.L. performed literature search and selection, data extraction, quality assessment, and drafted manuscript. Z.H.B. performed statistical analysis and gave critical comments. H.Y.L., C.A.P., and X.Z.G. gave critical comments and revised the manuscript. X.S.Q. conceived the work, reviewed the literature, gave critical comments, and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was partially supported by the Natural Science Foundation of Liaoning Province [20180530057].
Supplementary Material
Acknowledgements
None.
Conflicts of interest
None declared.
Contributor Information
Qian-Qian Li, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, Liaoning, P. R. China; Postgraduate College, Dalian Medical University, Dalian, Liaoning, P. R. China.
Hong-Yu Li, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, Liaoning, P. R. China.
Zhao-Hui Bai, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, Liaoning, P. R. China; Postgraduate College, Shenyang Pharmaceutical University, Shenyang, Liaoning, China.
Cyriac Abby Philips, The Liver Unit and Monarch Liver Lab, Cochin Gastroenterology Group, Ernakulam Medical Center, Kochi, India.
Xiao-Zhong Guo, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, Liaoning, P. R. China.
Xing-Shun Qi, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, Liaoning, P. R. China.
References
- 1. Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823–32. [DOI] [PubMed] [Google Scholar]
- 2. Sanyal AJ, Bosch J, Blei A et al. Portal hypertension and its complications. Gastroenterology 2008;134:1715–28. [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Tsao G, Abraldes JG, Berzigotti A et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017;65:310–35. [DOI] [PubMed] [Google Scholar]
- 4. Haq I, Tripathi D. Recent advances in the management of variceal bleeding. Gastroenterol Rep (Oxf) 2017;5:113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia-Tsao G, Sanyal AJ, Grace ND et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterology 2007;102:2086–102. [DOI] [PubMed] [Google Scholar]
- 6. Kravetz D. Prevention of recurrent esophageal variceal hemorrhage: review and current recommendations. J Clin Gastroenterol 2007;41:318–22. [DOI] [PubMed] [Google Scholar]
- 7. Tajiri T, Yoshida H, Obara K et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc 2010;22:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Bandali MF, Mirakhur A, Lee EW et al. Portal hypertension: imaging of portosystemic collateral pathways and associated image-guided therapy. World J Gastroenterol 2017;23:1735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCormack T, Rose J, Smith P et al. Perforating veins and blood flow in oesophageal varices. Lancet 1983;2:1442–4. [DOI] [PubMed] [Google Scholar]
- 10. Hashizume M, Kitano S, Sugimachi K et al. Three-dimensional view of the vascular structure of the lower esophagus in clinical portal hypertension. Hepatology 1988;8:1482–7. [DOI] [PubMed] [Google Scholar]
- 11. Irisawa A, Shibukawa G, Obara K et al. Collateral vessels around the esophageal wall in patients with portal hypertension: comparison of EUS imaging and microscopic findings at autopsy. Gastrointest Endosc 2002;56:249–53. [DOI] [PubMed] [Google Scholar]
- 12. Caletti G, Brocchi E, Baraldini M et al. Assessment of portal hypertension by endoscopic ultrasonography. Gastrointest Endosc 1990;36:S21–7. [DOI] [PubMed] [Google Scholar]
- 13. Lin CY, Lin PW, Tsai HM et al. Influence of paraesophageal venous collaterals on efficacy of endoscopic sclerotherapy for esophageal varices. Hepatology 1994;19:602–8. [DOI] [PubMed] [Google Scholar]
- 14. Burtin P, Cales P, Oberti F et al. Endoscopic ultrasonographic signs of portal hypertension in cirrhosis. Gastrointest Endosc 1996;44:257–61. [DOI] [PubMed] [Google Scholar]
- 15. Choudhuri G, Dhiman RK, Agarwal DK. Endosonographic evaluation of the venous anatomy around the gastro-esophageal junction in patients with portal hypertension. Hepatogastroenterology 1996;43:1250–5. [PubMed] [Google Scholar]
- 16. Dhiman RK, Choudhuri G, Saraswat VA et al. Role of paraoesophageal collaterals and perforating veins on outcome of endoscopic sclerotherapy for oesophageal varices: an endosonographic study. Gut 1996;38:759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leung VK, Sung JJ, Ahuja AT et al. Large paraesophageal varices on endosonography predict recurrence of esophageal varices and rebleeding. Gastroenterology 1997;112:1811–6. [DOI] [PubMed] [Google Scholar]
- 18. Sakai T, Iwao T, Oho K et al. Influence of extravariceal collateral channel pattern on recurrence of esophageal varices after sclerotherapy. J Gastroenterol 1997;32:715–9. [DOI] [PubMed] [Google Scholar]
- 19. Irisawa A, Obara K, Sato Y et al. EUS analysis of collateral veins inside and outside the esophageal wall in portal hypertension. Gastrointest Endosc 1999;50:374–80. [DOI] [PubMed] [Google Scholar]
- 20. Lo GH, Lai KH, Cheng JS et al. Prevalence of paraesophageal varices and gastric varices in patients achieving variceal obliteration by banding ligation and by injection sclerotherapy. Gastrointest Endosc 1999;49:428–36. [DOI] [PubMed] [Google Scholar]
- 21. Sato T, Yamazaki K, Toyota J et al. Perforating veins in recurrent esophageal varices after endoscopic therapy visualized by endoscopic color Doppler ultrasonography. Dig Endosc 1999;11:236–40. [Google Scholar]
- 22. Irisawa A, Saito A, Obara K et al. Endoscopic recurrence of esophageal varices is associated with the specific EUS abnormalities: severe peri-esophageal collateral veins and large perforating veins. Gastrointest Endosc 2001;53:77–84. [DOI] [PubMed] [Google Scholar]
- 23. Hino S, Kakutani H, Ikeda K et al. Hemodynamic assessment of the left gastric vein in patients with esophageal varices with color Doppler EUS: factors affecting development of esophageal varices. Gastrointest Endosc 2002;55:512–7. [DOI] [PubMed] [Google Scholar]
- 24. Konishi Y, Nakamura T, Kida H et al. Catheter US probe EUS evaluation of gastric cardia and perigastric vascular structures to predict esophageal variceal recurrence. Gastrointest Endosc 2002;55:197–203. [DOI] [PubMed] [Google Scholar]
- 25. Irisawa A, Obara K, Bhutani MS et al. Role of para-esophageal collateral veins in patients with portal hypertension based on the results of endoscopic ultrasonography and liver scintigraphy analysis. J Gastroenterol Hepatol 2003;18:309–14. [DOI] [PubMed] [Google Scholar]
- 26. Oikawa K, Ohara S, Sugiyama K et al. Analysis of portal hemodynamics in long-term recurrence-free patients after endoscopic variceal ligation (EVL): evaluation with endoscopic ultrasonography. Gastroenterol Endosc 2003;45:3–11. [Google Scholar]
- 27. Sato T, Yamazaki K, Toyota J et al. Experience with electronic radial endoscopic color Doppler ultrasonography in esophageal variceal patients. Dig Endosc 2003;15:275–9. [Google Scholar]
- 28. Sato T, Yamazaki K, Toyota J et al. Perforating veins in recurrent esophageal varices evaluated by endoscopic color Doppler ultrasonography with a galactose-based contrast agent. J Gastroenterol 2004;39:422–8. [DOI] [PubMed] [Google Scholar]
- 29. Sato T, Yamazaki K, Toyota J et al. Evaluation of the alternate blood flow in esophageal variceal patients by endoscopic color Doppler ultrasonography. Dig Endosc 2004;16:208–12. [Google Scholar]
- 30. Ishii S, Kakemura T, Fujinuma S et al. Endoscopic ultrasonography findings and clinical backgrounds in cases with early recurrence of esophageal varices after endoscopic injection sclerotherapy. Dig Endosc 2005;17:310–7. [Google Scholar]
- 31. Nakano S, Hachiya A. Effect of endoscopic variceal ligation for esophageal varices: vascular structure images by miniature ultrasound probe. Dig Endosc 2005;17:203–9. [Google Scholar]
- 32. Sato T, Yamazaki K, Toyota J et al. Usefulness of electronic radial endoscopic color Doppler ultrasonography in esophageal varices: comparison with convex type. J Gastroenterol 2006;41:28–33. [DOI] [PubMed] [Google Scholar]
- 33. Sato T, Yamazaki K, Toyota J et al. Endoscopic ultrasonographic evaluation of hemodynamics related to variceal relapse in esophageal variceal patients. Hepatol Res 2009;39:126–33. [DOI] [PubMed] [Google Scholar]
- 34. Erden A, Idilman R, Erden I et al. MR angiography of esophageal mural veins in portal hypertension: a correlation with endoscopic grades of esophageal varices. Turk J Gastroenterol 2010;21:275–9. [DOI] [PubMed] [Google Scholar]
- 35. Shim J, Hwangbo Y, Young Jang J et al. Predicting perforating veins in patients with esophageal varices using conventional endoscopy. Hepatology 2010;52:1075A. [Google Scholar]
- 36. Kume K, Yamasaki M, Watanabe T et al. Mild collateral varices and a fundic plexus without perforating veins on EUS predict endoscopic non-recurrence of esophageal varices after EVL. Hepatogastroenterology 2011;58:798–801. [PubMed] [Google Scholar]
- 37. Shim JJ, Kim JW, Lee CK et al. Mass-like esophageal varices (F3) are different from tortuous varices (F2) in clinical outcome and eus findings. J Hepatol 2013;58:S254. [Google Scholar]
- 38. Masalaite L, Valantinas J, Stanaitis J. Endoscopic ultrasound findings predict the recurrence of esophageal varices after endoscopic band ligation: a prospective cohort study. Scand J Gastroenterol 2015;50:1322–30. [DOI] [PubMed] [Google Scholar]
- 39. Zheng J, Zhang Y, Li P et al. The endoscopic ultrasound probe findings in prediction of esophageal variceal recurrence after endoscopic variceal eradication therapies in cirrhotic patients: a cohort prospective study. BMC Gastroenterol 2019;19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Masalaite L, Valantinas J, Stanaitis J. The role of collateral veins detected by endosonography in predicting the recurrence of esophageal varices after endoscopic treatment: a systematic review. Hepatol Int 2014;8:339–51. [DOI] [PubMed] [Google Scholar]
- 41. Caletti GC, Brocchi E, Ferrari A et al. Value of endoscopic ultrasonography in the management of portal hypertension. Endoscopy 1992;24:342–6. [DOI] [PubMed] [Google Scholar]
- 42. D’Amico G, Garcia-Pagan JC, Luca A et al. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology 2006;131:1611–24. [DOI] [PubMed] [Google Scholar]
- 43. Villanueva C, Lopez-Balaguer JM, Aracil C et al. Maintenance of hemodynamic response to treatment for portal hypertension and influence on complications of cirrhosis. J Hepatol 2004;40:757–65. [DOI] [PubMed] [Google Scholar]
- 44. Abraldes JG, Tarantino I, Turnes J et al. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology 2003;37:902–8. [DOI] [PubMed] [Google Scholar]
- 45. Bureau C, Peron JM, Alric L et al. ‘A La Carte’ treatment of portal hypertension: adapting medical therapy to hemodynamic response for the prevention of bleeding. Hepatology 2002;36:1361–6. [DOI] [PubMed] [Google Scholar]
- 46. Augustin S, Gonzalez A, Badia L et al. Long-term follow-up of hemodynamic responders to pharmacological therapy after variceal bleeding. Hepatology 2012;56:706–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.