Abstract
Background
The epithelial-to-mesenchymal transition (EMT) status is associated with programmed death-1 ligand 1 (PD-L1) expression in various cancers. However, the role and molecular mechanism of PD-L1 in the EMT of sorafenib-resistant hepatocellular carcinoma (HCC) cells remain elusive. In this study, we aimed to investigate the regulation of PD-L1 on the EMT in sorafenib-resistant HCC cells.
Methods
Initially, the sorafenib-resistant HCC cell lines HepG2 SR and Huh7 SR were established. Western-blot assays were used to detect the expression of PD-L1, E-cadherin, and N-cadherin. The intervention and overexpression of PD-L1 were used to explore the role of PD-L1 in the regulation of EMT in HepG2 SR and Huh7 SR cells. Cell migration and invasion were assessed by transwell assays. PD-L1 or Sterol regulatory element-binding protein 1 (SREBP-1) overexpression and knock-down were performed in order to study the mechanism of PD-L1 in sorafenib-resistant HCC cells.
Results
PD-L1 expression was upregulated, whereas E-cadherin levels were downregulated and N-cadherin expression was increased in HepG2 SR and Huh7 SR cells. The cell viabilities of HepG2 and Huh7 cells were lower than those of HepG2 SR and Huh7 SR cells. PD-L1 overexpression reduced E-cadherin expression and increased N-cadherin levels, whereas PD-L1 knock-down increased E-cadherin expression and decreased N-cadherin expression. PD-L1 expression promoted EMT and the migratory and invasive abilities of HepG2 SR and Huh7 SR cells. PD-L1 promoted the EMT of sorafenib-resistant HCC cells via the PI3K/Akt pathway by activating SREBP-1 expression in HepG2 SR and Huh7 SR cells.
Conclusions
The findings reveal that PD-L1 expression promotes EMT of sorafenib-resistant HCC cells.
Keywords: programmed death-1 ligand 1 (PD-L1), sorafenib-resistant hepatocellular carcinoma (HCC) cells, epithelial-to-mesenchymal transition (EMT)
Introduction
Hepatocellular carcinoma (HCC) has become the second leading cause of cancer-related mortality worldwide, notably in China [1]. Despite advances in liver resection and transplantation, long-term survival of HCC patients is still unsatisfactory due to recurrence of disease following surgical resection and adverse reactions to conventional chemotherapies including transcatheter arterial chemoembolization (TACE) or patient response to the targeted drug sorafenib [2, 3]. Sorafenib is a multikinase inhibitor, which can inhibit the proliferation and growth of tumors and reduce apoptosis and tumor angiogenesis of hepatocellular carcinoma cells [4]. Sorafenib can significantly improve the median survival time of patients only for 3–5 months due to drug resistance [5]. An in-depth understanding of the underlying molecular mechanisms of tumor resistance to sorafenib and of the ways to overcome this process is urgently required for the treatment of HCC.
Epithelial-mesenchymal transition (EMT) plays an important role in cancer progression. During EMT, epithelial cancer cells lose their epithelial properties, such as cell polarity and intercellular adhesion, and acquire interstitial properties, such as migration and invasion. Importantly, EMT is linked to drug resistance in multiple targeted therapies [6, 7]. EMT is a key step in the metastasis of HCC and is closely associated with patient survival. Previous studies have shown that sorafenib resistance may involve the EMT process [8]. The development of sorafenib resistance in Huh7 cells has been reported and the interaction of the PI3K/Akt-signaling pathway was studied. The PI3K/Akt-signaling pathway is activated in response to the sorafenib-targeted Raf/Ras/MAPK signaling pathway [9]. Recently, several interesting studies on HCC patient samples have shown that these cancer cells hijack part of the developmental EMT program, conferring resistance to sorafenib treatment [10, 11]. Recent studies have shown that sorafenib promotes EMT and enhances HCC metastasis by activating compensatory EMT-signaling pathways [12].
Recent studies further demonstrated that the EMT process could induce upregulation of programmed death-1 ligand 1 (PD-L1) expression and that PD-L1 signaling played an important role in maintaining the EMT status of renal cell carcinoma [13], glioblastoma [14], and breast cancer [15]. Patients with interstitial phenotype exhibited a considerably higher PD-L1-positive rate compared with those demonstrating an epithelial phenotype, notably in EGFR-mutated cancers [16]. As assessed by high vimentin and low E-cadherin expression, PD-L1 expression in head and neck squamous cell carcinoma (n = 50) was significantly associated with EMT status. In the TCGA cohort, the prognosis of the PD-L1+/EMT+ group was worse than that of the PD-L1+/EMT− patients [17]. Mesenchymal cell lines contain a higher percentage of PD-L1-positive cells and are characterized by enhanced activation of ZEB1 or Snail causing upregulation of PD-L1 expression in breast cancer [18]. Previous studies demonstrated that the molecular mechanism included bidirectional regulation between the EMT process and the PD-L1-signaling pathway in different cancers, which may help us to identify intrinsic or acquired resistance to inhibition of PD-L1 expression. In addition, the identification of novel biomarkers that can predict or monitor the anti-PD-L1 response may aid in the selection of patients and the adjustment of the therapeutic strategy [19].
Taken together, these studies indicated that the expression levels of PD-L1 may be abnormal and thereby the regulation of the EMT and the migratory and invasive activities of sorafenib-resistant HCC cells is a significant strategy in the treatment of this disease. To assess this hypothesis, we initially established HepG2 and Huh7 cell lines resistant to sorafenib, denoted as HepG2 SR and Huh7 SR, and examined the expression levels of PD-L1 in the two cell lines. We further investigated the function and mechanism of PD-L1 in the regulation of EMT and its effect on the invasion of sorafenib-resistant HCC cells. Our findings indicated that upregulation of PD-L1 promoted EMT by activating the Sterol regulatory element-binding protein 1 (SREBP-1) via the PI3K/AKT-signaling pathway.
Methods
Cell culture and compounds
The human HCC cell lines HepG2 and Huh-7 were obtained from the Cell Bank of the Chinese Academy of Sciences and were grown in DMEM medium with high glucose (Gibco BRL, Grand Island, NY) in the presence of 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA). The cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Sorafenib was purchased from Selleck Chemicals (Houston, TX, USA) and dissolved in 100% dimethylsulfoxide (DMSO) (Saint Louis, MO, USA).
Sorafenib-resistant cells
Initially, we examined the 50% inhibiting concentration (IC50) of HepG2 and Huh-7 cells following sorafenib treatment. Subsequently, we seeded HepG2 and Huh-7 cells in six-well plates at a density of 1 × 104 cells per well and incubated the cells with sorafenib concentrations just below their respective IC50. During the following time periods, the concentration of sorafenib was slowly increased by 0.25 μmol/L per week. Following a 6-month treatment period, the two cell lines resistant to sorafenib were established and were named HepG2 SR and Huh7 SR. The cells were continuously cultured in the presence of sorafenib. Finally, we incubated HepG2, Huh7, HepG2-SR, and Huh7-SR cells with gradually increasing doses of sorafenib in 96-well plates and determined the cell viability by the MTT assay following 2 days of incubation.
Western-blot analysis
The proteins were extracted from the cells using RIPA buffer (Cell Signaling Technology) containing protease inhibitor (Sigma-Aldrich, St Louis, MO, USA). Protein detection was performed by Western blotting. The protein products were separated using 10% sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA). Subsequently, the membranes were blocked with 5% BSA in Tris Buffered saline Tween buffer prior to incubation with the following specific antibodies: mouse anti-E-cadherin and anti-N-cadherin (BD Biosciences, San Jose, CA, USA); rabbit anti-PD-L1, anti-AKT, and anti–p-AKT (Cell Signaling Technology, Houston, TX, USA); mouse anti-SREBP-1 (Santa Cruz, Dallas, TX, USA); mouse anti-β-actin; and anti-mouse, rabbit secondary antibodies (Santa Cruz Biotechnology). The proteins were visualized with ECL reagents (GEHealthcare Life Sciences, Piscataway, NJ, USA). The expression levels of the specific proteins were normalized to those of β-actin. We performed all the experiments in triplicate.
RNA extraction, cDNA synthesis, and quantitative real-time polymerase chain reactions
Total RNA was extracted from HepG2, HepG2 SR, Huh7, and Huh7 SR cells using an HP Total RNA Kit (Omega Biotech, Stamford, CT, USA) according to the manufacturer’s instructions. The concentration of the RNA samples was measured on a NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA). The synthesis of cDNA was carried out with the enzyme reverse transcriptase using an M-MLV First Strand kit (Life Technologies, Gaithersburg, MD, USA). Polymerase chain reaction (PCR) were performed using the Platinum®SYBR®Green qPCR supermix-UDG with ROX (Invitrogen) on an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). The cycling conditions were the following: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles, each consisting of 95°C for 15 s and 60°C for 1 min. The relative amounts of the Ct values of the gene mRNA- and microRNA-expression levels were normalized to those of β-actin mRNA and U6, respectively, which served as internal controls. The fold change was calculated using the 2–ΔΔCt method.
Construction of stable cell lines overexpressing PD-L1 and generation of PD-L1-silenced stable cell lines
HepG2 SR and Huh7 SR cells, which overexpressed PD-L1, were established according to the following procedure: the PD-L1 gene fragments (NM_014143.2; GenBank) were synthesized by Sangon Biotech (Shanghai) Co., Ltd (Shanghai, China) and subsequently amplified by PCR. The PCR fragments were subcloned into the EcoRI and BamHI sites in a pLVX-IRES-Neo vector for expression via a Lenti-X lentiviral expression system (Clontech, Mountain View, CA, USA). Subsequently, we co-transfected the PD-L1 expression construct with packaging plasmids into 293 T-cells using Lipofectamine 2000 (Invitrogen). The empty vector was used as a negative control. HEK293T cells were incubated in DMEM medium with 10% FBS in a humidified incubator with 5% CO2 at 37°C. Following the initial incubation for 48 h, the packaged lentiviruses were harvested and used to infect HepG2 SR and Huh7 SR cells. The infected cells were grown in DMEM medium for 2 days and stable transfectants were selected using 400 μg/mL G418 (Amresco, Solon, OH, USA). The selected cells were subsequently named LV-PD-L1-WT-HepG2 SR (HepG2 SR cells overexpressing wild-type PD-L1) and LV-PD-L1-WT-Huh7 SR (Huh7 SR cells overexpressing wild-type PD-L1). The LV-Vector-Ctrl (control vector transfection) cells were used as a control. The amplification of PD-L1 was conducted using the following primers: forward, 5′-TAGAATTCATGAGGATATTTGCTGTCTT-3′; reverse: 5′-TAGGATCCTTACGTCTCCTCCAAATGTG-3′.
To establish stable transfection clones of HepG2 SR and Huh7 SR cell lines in which PD-L1 was silenced, a small hairpin RNA (shRNA) fragment against the human PD-L1 gene (shRNA: 5′-GACCTATATGTGGTAGAGTAT-3′) was subcloned into the lentiviral vector pGLV2-U6-Puro (GenePharma, Shanghai, China). The PD-L1-silenced construct or negative control mock lentivirus was prepared and co-transfected with packaging plasmids into 293T cells using Lipofectamine 2000 (Invitrogen). Following 48 h of incubation, the packaged lentiviruses were collected and the HepG2 SR and Huh7 SR cells were infected with the packaged lentiviruses and cultured for 2 days. Finally, stable cell lines were selected using 1 μg/mL puromycin (Sigma-Aldrich, St Louis, MO, USA). The selected cells, including infected HepG2 SR and Huh7 SR cells as well as negative control cells, were named LV-PD-L1-shRNA-HepG2 SR, LV-PD-L1-shRNA-Huh7 SR, and LV-NC, respectively.
SREBP-1 siRNA transfection
The short interfering RNA (siRNA) sequences against SREBP-1 were directly synthesized by GenePharma (Shanghai, China). Scrambled siRNA served as a negative control. Huh7 SR cells were transiently transfected with 150 pmol of siRNA (SREBP-1siRNA or control siRNA) sequences using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Following 48 h of incubation, the cells were harvested and used for further experiments.
Transwell assay
Transwell migration and invasion assays were performed using transwell plates (BD Biosciences, Franklin Lakes, NJ, USA). The incubations were performed in the 24-well transwell chambers containing polycarbonate filters with 8-mm pores coated with (invasion) or without (migration) matrigel. According to the manufacturer's instructions, 5 × 104 cells were seeded in DMEM medium supplemented with 1% FBS and were added to the top chamber. DMEM medium with 10% FBS was put into the bottom chamber and used as a chemoattractant. Following 48 h of incubation at 37°C, the DMEM medium was discarded and the cells adhering to the upper surface of the membrane were gently removed with a cotton swab. The cells that had migrated to the lower surface of the membrane were subsequently stained with 1% crystal violet for 30 min at room temperature. The images of the migrated cells were captured by a light microscope (magnification, ×100; Olympus Corporation, Tokyo, Japan). The cells were stained and counted in at least three microscopic fields (magnification, ×100). The experiments were independently repeated three times.
Statistical analysis
Significant differences were analysed using the unpaired t-test (two-tailed). All data are presented as mean ± standard error of the mean and statistical differences were considered significant at P < 0.05. All statistical analyses were performed using GraphPad Prism 5.02 (GraphPad, San Diego, CA, USA).
Results
Establishment of HepG2 SR and Huh7 SR cell lines with acquired EMT characteristics is accompanied by elevated PD-L1 expression in HepG2 SR and Huh7 SR cells
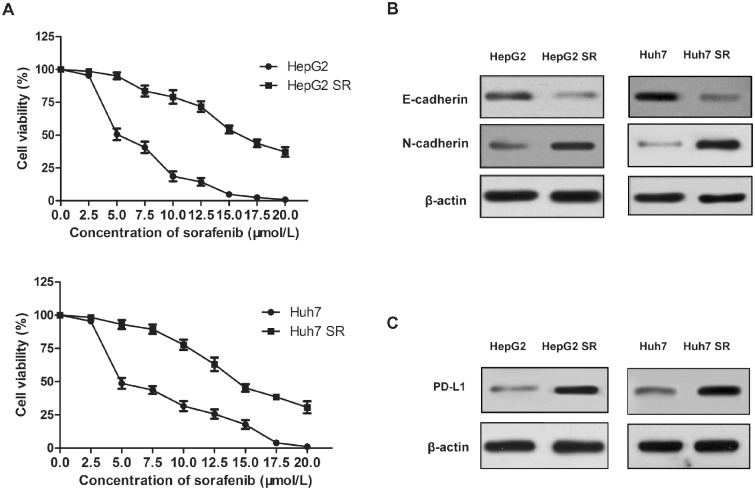
To explore the mechanism of acquired sorafenib resistance in HCC cells, the sorafenib-resistant cell lines HepG2 and Huh7 were established. Resistance was generated by increasing sorafenib concentrations step by step in DMEM medium. Following 6 months of culture, two resistant HCC cell lines, namely HepG2 SR and Huh7 SR, were developed. After 48 h of incubation with 10 μmol/L sorafenib, the viability of HepG2 and Huh7 cells (18.6% and 31.6%, respectively, P < 0.01) was significantly lower than that of HepG2 SR and Huh7 SR cells (79.1% and 77.8%, respectively, P < 0.01) (Figure 1A). Moreover, when the viability of HepG2 and Huh7 cells was ∼0, the viability of HepG2SR and Huh7SR cells was estimated to be 37.2% and 30.7%, respectively (Figure 1A).
Figure 1.
Programmed death-1 ligand 1 (PD-L1) expression is elevated in HepG2-SR and Huh7-SR cells, which acquire epithelial-mesenchymal transition (EMT) characteristics. (A) HepG2, Huh7 cells, and the sorafenib-resistant hepatocellular carcinoma (HCC) cells denoted as HepG2-SR and Huh7-SR are incubated with increasing doses of sorafenib for 2 days. Cell viability (%) was compared to that of the corresponding untreated cells. (B) and (C) Western-blot analysis of E-cadherin, N-cadherin, and PD-L1 protein expression in HepG2-SR, Huh7-SR cells, and their corresponding parental lines. Densitometry values for each protein are normalized to those of β-actin and shown below as the corresponding bands.
Furthermore, the levels of the epithelial marker E-cadherin were decreased, whereas the expression levels of the mesenchymal marker N-cadherin were increased in HepG2 SR and Huh7 SR cells (Figure 1B). In addition, PD-L1 expression levels were higher in HepG2 SR and Huh7 SR cells than those noted in the corresponding parental cells (Figure 1C). The aforementioned results indicated that PD-L1 may play a role in the acquisition of EMT in sorafenib-resistant HCC cells.
PD-L1 expression promotes EMT and invasion of HepG2 SR and Huh7 SR cells
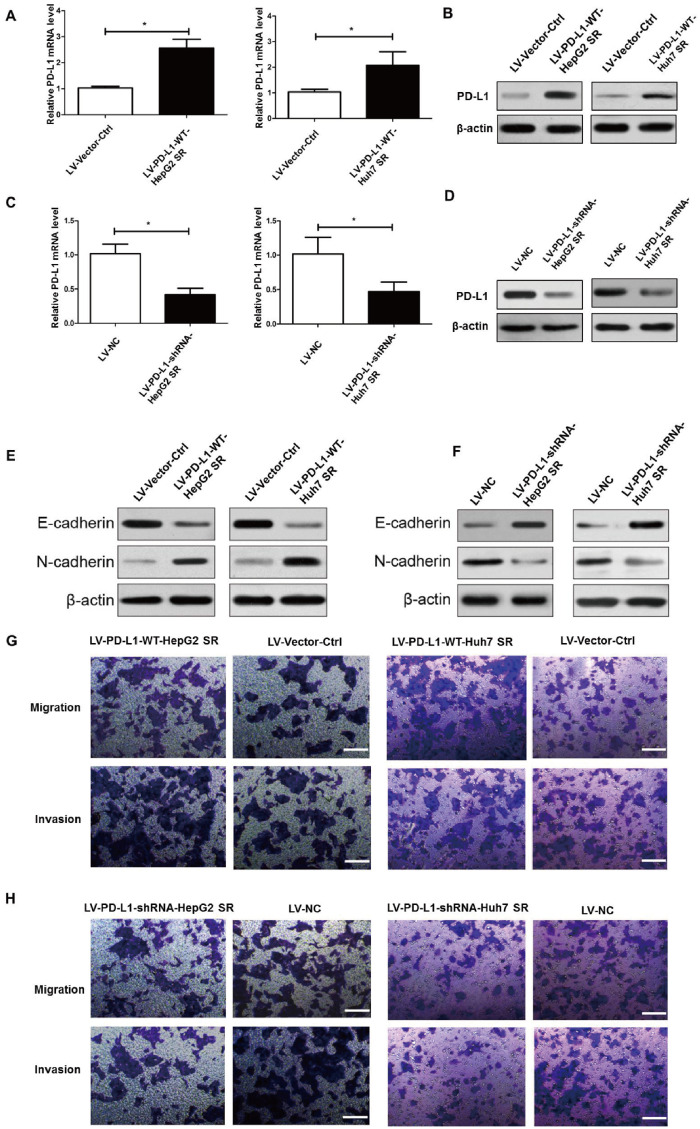
To further explore the role of PD-L1 in the regulation of the biological features of sorafenib-resistant HCC cells, the LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR cells were established with stable overexpression of PD-L1, whereas the LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells were generated with stable knock-down of PD-L1. PD-L1 levels were confirmed by quantitative real-time PCR (qRT–PCR) and Western-blot assay. As shown in Figure 2A and C, expression levels of PD-L1 mRNA were significantly higher in LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR cells than in LV-Vector-Ctrl cells, while expression levels of PD-L1 mRNA were significantly lower in LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells than in LV-NC cells. For PD-L1 protein levels, the similar results were obtained in the above cells (Figure 2B and D).
Figure 2.
PD-L1 expression promoted EMT, migration, and invasion of HepG2 SR and Huh7 SR cells. (A) and (B) Quantitative real-time PCR (qRT–PCR) analysis and Western-blot assay of PD-L1-expression levels in HepG2 SR and Huh7 SR cells following stable overexpression of PD-L1 (LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR cells). LV-Vector-Ctrl represents control vector transfection. (C) and (D) qRT–PCR analysis and Western-blot assay of PD-L1 expression levels in HepG2 SR and Huh7 SR cells following stable knock-down of PD-L1 with shRNAs (LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells). LV-NC represents mock lentiviral infection. (E) and (F) Western-blot assay of the epithelial marker proteins E-cadherin and N-cadherin demonstrating their expression in HepG2 SR and Huh7 SR cells following stable overexpression or stable knock-down of PD-L1. β-actin was used as an endogenous control. (G) Cell migratory and invasive abilities of PD-L1-overexpressing cells and those of the corresponding control cells were examined by the transwell assay. Scale bar, 200 μm. (H) Cell migratory and invasive activities of PD-L1 ablation cells and of the corresponding control cells were examined by transwell assays. Scale bar, 200 μm. Cell transwell assays were performed in 24-well transwell chambers containing polycarbonate filters with (invasion) or without (migration) 8-mm pores coated with matrigel. Migrated and invaded cells were stained and counted in at least three microscopic fields. *P < 0.05.
Subsequently, the role of PD-L1 on the EMT of HepG2 SR and Huh7 SR cells was investigated by examining E-cadherin and N-cadherin protein levels. LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR cells overexpressing PD-L1 showed reduced expression of E-cadherin and increased expression of N-cadherin as compared with LV-Vector-Ctrl cells transfected with empty vector (Figure 2C). LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells with stable knock-down of PD-L1 showed increased expression of E-cadherin and decreased expression of N-cadherin as compared with LV-NC cells transfected with scrambled shRNA (Figure 2D). Moreover, we performed transwell assays to analyse the effects of PD-L1 in regulating the migratory and invasive abilities of HepG2 SR and Huh7 SR cells. As illustrated in Figure 2E, transwell assays indicated that PD-L1-overexpressing (LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR) cells exhibited higher migratory and invasive abilities compared to those of the LV-Vector-Ctrl groups. However, PD-L1 ablation (LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells) significantly decreased the number of migrated and invasive cells compared with that of the cells transfected with scrambled shRNA (LV-NC cells) (Figure 2F). Taken together, the above results demonstrated that overexpression of PD-L1 could enhance EMT as well as migration and invasion of HepG2 SR and Huh7 SR cells.
PD-L1 facilitates EMT via the PI3K/AKT-signaling pathway
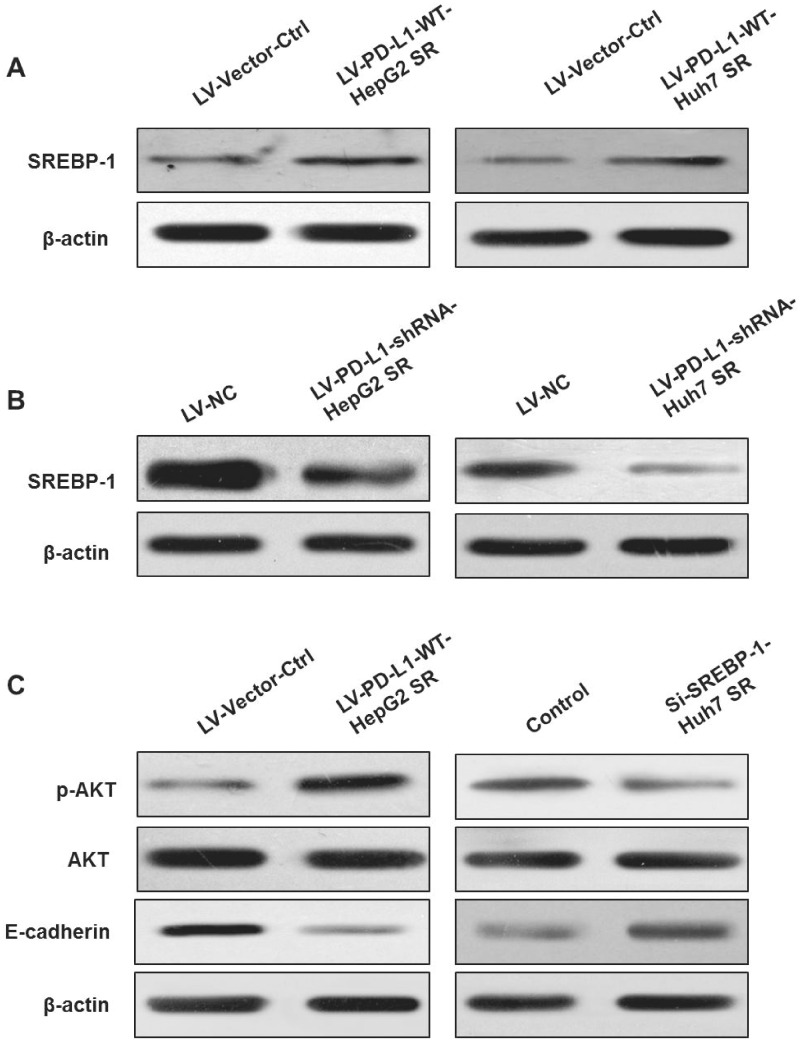
The aforementioned results obtained in Figure 2A–D confirmed that, compared with the control cells, PD-L1-expression levels were higher in LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR cells and lower in LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells. To test whether PD-L1 played a biological role by modulating SREBP-1 in sorafenib-resistant HCC cells, we initially detected SREBP-1 expression in LV-PD-L1-WT-HepG2 SR cells. Western-blot analysis indicated that overexpression of PD-L1 resulted in upregulation of SREBP-1 levels. The expression levels of SREBP-1 protein were increased in LV-PD-L1-WT-Huh7 SR cells compared with those in LV-Vector-Ctrl cells (Figure 3A). Subsequently, the levels of SREBP-1 were assessed in LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells. The results demonstrated that knock-down of PD-L1 reduced SREBP-1 expression (Figure 3B).
Figure 3.
PD-L1 promotes EMT by activating PI3K/AKT signaling. (A) Western-blot assay of Sterol regulatory element-binding protein 1 (SREBP-1) in LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR cells following overexpression of PD-L1. (B) Western-blot assay of SREBP-1 in LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells following knock-down of PD-L1. (C) p-AKT and E-cadherin levels in LV-PD-L1-WT-HepG2 SR cells overexpressing PD-L1 and Huh7 SR cells following knock-down of SREBP-1. β-actin was used as an endogenous control.
The PI3K/Akt pathway has been shown to play an important role in promoting EMT and drug resistance in various types of cancer [7]. Therefore, we examined whether PD-L1 promoted the EMT process of sorafenib-resistant HCC cells via the PI3K/Akt pathway. As expected, p-AKT expression was elevated in LV-PD-L1-WT-HepG2 SR cells overexpressing PD-L1. It is interesting to note that knock-down of SREBP-1 by siRNA decreased p-AKT levels in Huh7 SR cells (Figure 3C). Moreover, E-cadherin levels were reduced in LV-PD-L1-WT-HepG2 SR cells, while they were increased following downregulation of SREBP-1 in Huh7 SR cells (Figure 3C). These findings suggested that the PD-L1/SREBP-1 axis promoted the EMT process by activating the PI3K/AKT-signaling pathway in sorafenib-resistant HCC cells.
Discussion
To date, the exact mechanism by which PD-L1 contributes to EMT and invasion of sorafenib-resistant HCC cells remains unclear. In the present study, we demonstrated that PD-L1 levels were elevated in HepG2 SR and Huh7 SR cells, and that they could promote EMT, migration, and invasion of these cells. PD-L1 activated SREBP-1 to promote EMT of HepG2 SR and Huh7 SR cells via the PI3K/AKT-signaling pathway. The results also revealed that the viability of HepG2 and Huh7 cells was apparently lower than that of HepG2 SR and Huh7 SR cells following incubation of the cells with increasing concentration of sorafenib. These findings indicated that targeting PD-L1 could have considerable therapeutic effects for HCC patients with resistance to sorafenib treatment.
Sorafenib is one of several targeted drugs used by oncologists and has been approved by the FDA for the treatment of various human cancers including lung, prostate, and kidney cancers, as well as melanoma and HCC. However, the response rate is not satisfactory due to drug resistance [20, 21]. Accumulating evidence from recent studies has stressed the idea that EMT is an important mechanism for sorafenib resistance in advanced HCC [12, 22]. In the present study, we found that the enhanced migratory ability of sorafenib-resistant HCC cells was significantly associated with changes in the EMT phenotype, such as reduced expression of the epithelial marker E-cadherin and increased levels of the mesenchymal marker N-cadherin. Furthermore, we found that the viability of HepG2 and Huh7 cells was significantly lower than that of HepG2 SR and Huh7 SR cells following incubation with increasing concentration of sorafenib. PD-L1 expression is elevated in a wide range of human cancers and is often associated with poor patient prognosis and prediction of Abs response to PD-1/PD-L1. The emergence of promising clinical results by the clinical application of the programmed death-1 (PD-1) and PD-L1 inhibitors has suggested that the understanding of the biological regulatory mechanism of PD-L1 can aid in the identification of biomarkers and the development of combinatorial strategies for clinical use [23, 24]. Recently, PD-L1 overexpression has also been reported in drug-resistant cells. PD1- and PD-L1-expression levels were higher in SCLC cells resistant to cisplatin (H69R, H82R) compared with those noted in the corresponding parental counterparts [25]. A study by Zhang et al. [26] revealed that cisplatin treatment upregulated PD-L1 expression in NSCLC cell lines. In the present study, the levels of PD-L1 were detected in the two sorafenib-resistant HCC cell lines, HepG2 SR and Huh7 SR. The findings indicated that PD-L1 expression was elevated in HepG2 SR and Huh7 SR cells compared with that noted in the corresponding parental cells.
Several studies have demonstrated a strong correlation between EMT status and PD-L1 expression in multiple solid cancers [27]. It is interesting to note that some reports have also shown that PD-L1 signaling plays an important role in maintaining the EMT status of renal-cell carcinoma and breast cancer [13, 15]. In the present study, the role of PD-L1 was explored in the EMT in HepG2 SR and Huh7 SR cells by examining E-cadherin and N-cadherin protein levels. Overexpression of PD-L1 cells (LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR) revealed reduced expression levels of E-cadherin and elevated expression levels of N-cadherin compared with those noted in the control cells (LV-Vector-Ctrl) that were transfected with empty vector. Moreover, the cells with stable knock-down of PD-L1 expression (LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR) presented markedly increased E-cadherin levels and decreased expression of N-cadherin than the LV-NC cells transfected with scrambled shRNA. It has been proposed that EMT plays an important role in a large number of cellular processes, including cell migration and invasion [28, 29]. In the present study, transwell assays revealed that LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR cells exhibited higher migratory and invasive abilities compared with those of LV-Vector-Ctrl groups. Furthermore, LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells revealed the opposite effects. This finding was consistent with the study demonstrating that PD-L1 promoted the migratory ability of renal-cell-carcinoma cells [13].
SREBP-1 is a transcription factor that has been found to be associated with malignant characteristics in several types of human cancers [30–33]. SREBP-1 exhibited higher expression in ovarian cancer compared with that noted in benign and borderline ovarian tumors. Furthermore, the cell growth and migratory and invasive activities of an ovarian-cancer cell line were suppressed following knock-down of SREBP-1 [30]. Du et al. [31] revealed that SREBP-1 facilitated tumor growth in bladder cancer by controlling the expression levels of key lipogenic enzymes. A previous study demonstrated that SREBP-1 promoted invasion and metastasis of HCC cells [34]. In the present study, we found that SREBP-1 levels were upregulated in LV-PD-L1-WT-HepG2 SR and LV-PD-L1-WT-Huh7 SR cells. Moreover, the expression levels of SREBP-1 were downregulated in LV-PD-L1-shRNA-HepG2 SR and LV-PD-L1-shRNA-Huh7 SR cells. It is well known that PI3K/Akt is involved in various cellular processes, including cell growth, survival, and proliferation, and therefore may contribute to malignant phenotypes. The PI3K/AKT-signaling pathway has been shown to be closely associated with chemoresistance [35–37]. Chen and colleagues indicated that the PI3K/Akt-signaling pathway exhibited an effect on sorafenib resistance in hepatocellular carcinoma cells in vitro [9]. In the present study, it was shown that p-AKT expression was elevated in LV-PD-L1-WT-HepG2 SR cells. In addition, knock-down of SREBP-1 by siRNA decreased p-AKT levels in Huh7 SR cells, whereas E-cadherin expression was reduced in LV-PD-L1-WT-HepG2 SR cells and it was increased by knock-down of SREBP-1 in Huh7 SR cells.
In conclusion, the findings demonstrated that sorafenib led to an EMT phenotype with reduced expression of E-cadherin and increased levels of N-cadherin, while PD-L1-expression levels were elevated during that process. It was further shown that PD-L1 promoted EMT and the migratory and invasive activities of the sorafenib-resistant HCC cell lines by activating SREBP-1 via the PI3K/AKT-signaling pathway. Therefore, targeting PD-L1 may have considerable therapeutic effects to overcome sorafenib resistance in hepatocellular carcinoma. However, the present study has not fully investigated a certain number of patient samples. Therefore, further studies are required to validate our results in a number of patient tissues.
Authors’ contributions
Study concept and design: X.L.Z., G.L.X., and C.F.N. Performed the experiments: G.L.X., H.S.L., Y.H.X., W.S.W., J.S., and M.M.L. Data collection: All authors. Statistical analysis: X.L.Z., G.L.X., and C.F.N. Drafted the manuscript: X.L.Z., G.L.X., and C.F.N. All authors read and approved the final manuscript.
Funding
This study was supported by Natural Science Foundation of China [No. 81771945].
Acknowledgements
We would like to thank all the participants in this study. We would like to thank Jiangsu Institute of Clinical Immunology of The First Affiliated Hospital of Soochow University for technical assistance.
Conflict of interest
None declared.
Contributor Information
Gui-Li Xu, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, P. R. China.
Cai-Fang Ni, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, P. R. China.
Han-Si Liang, Jiangsu Institute of Clinical Immunology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, P. R. China.
Yun-Hua Xu, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, P. R. China.
Wan-Sheng Wang, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, P. R. China.
Jian Shen, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, P. R. China.
Ming-Ming Li, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, P. R. China.
Xiao-Li Zhu, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, P. R. China.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Xiang D, Cheng Z, Liu H et al. Shp2 promotes liver cancer stem cell expansion by augmenting beta-catenin signaling and predicts chemotherapeutic response of patients. Hepatology 2017;65:1566–80. [DOI] [PubMed] [Google Scholar]
- 3. Cidon EU. Systemic treatment of hepatocellular carcinoma: past, present and future. World J Hepatol 2017;9:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan W, Luo X, Li W et al. TNF-alpha is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine 2019;40:446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu YJ, Zheng B, Wang HY et al. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin 2017;38:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009;9:265–73. [DOI] [PubMed] [Google Scholar]
- 7. Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29:4741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Malenstein H, Dekervel J, Verslype C et al. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett 2013;329:74–83. [DOI] [PubMed] [Google Scholar]
- 9. Chen KF, Chen HL, Tai WT et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther 2011;337:155–61. [DOI] [PubMed] [Google Scholar]
- 10. Chen J, Jin R, Zhao J et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett 2015;367:1–11. [DOI] [PubMed] [Google Scholar]
- 11. Zhang PF, Li KS, Shen YH et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis 2016;7:e2201–e2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang XY, Ke AW, Shi GM et al. alphaB-crystallin complexes with 14-3-3zeta to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology 2013;57:2235–47. [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Wang H, Zhao Q et al. PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med Oncol 2015;32:212. [DOI] [PubMed] [Google Scholar]
- 14. Qiu XY, Hu DX, Chen WQ et al. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim Biophys Acta Mol Basis Dis 2018;1864:1754–69. [DOI] [PubMed] [Google Scholar]
- 15. Alsuliman A, Colak D, Al-Harazi O et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer 2015;14:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S, Koh J, Kim MY et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol 2016;58:7–14. [DOI] [PubMed] [Google Scholar]
- 17. Yang WF, Wong MCM, Thomson PJ et al. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol 2018;86:81–90. [DOI] [PubMed] [Google Scholar]
- 18. Dongre A, Rashidian M, Reinhardt F et al. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res 2017;77:3982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang Y, Zhan H. Communication between EMT and PD-L1 signaling: new insights into tumor immune evasion. Cancer Lett 2020;468:72–81. [DOI] [PubMed] [Google Scholar]
- 20. Kabir TD, Ganda C, Brown RM et al. A microRNA-7/growth arrest specific 6/TYRO3 axis regulates the growth and invasiveness of sorafenib-resistant cells in human hepatocellular carcinoma. Hepatology 2018;67:216–31. [DOI] [PubMed] [Google Scholar]
- 21. Dong XF, Liu TQ, Zhi XT et al. COX-2/PGE2 axis regulates HIF2alpha activity to promote hepatocellular carcinoma hypoxic response and reduce the sensitivity of sorafenib treatment. Clin Cancer Res 2018;24:3204–16. [DOI] [PubMed] [Google Scholar]
- 22. Chen HA, Kuo TC, Tseng CF et al. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatology 2016;64:1637–51. [DOI] [PubMed] [Google Scholar]
- 23. Herbst RS, Soria JC, Kowanetz M et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao Q, Wang XY, Qiu SJ et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971–9. [DOI] [PubMed] [Google Scholar]
- 25. Yan F, Pang J, Peng Y et al. Elevated cellular PD1/PD-L1 expression confers acquired resistance to cisplatin in small cell lung cancer cells. PLoS One 2016;11:e0162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang P, Ma Y, Lv C et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci 2016;107:1563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Q, Zhang Y, Chen Y et al. A Novel mTORC1/2 Inhibitor (MTI-31) inhibits tumor growth, epithelial-mesenchymal transition, metastases, and improves antitumor immunity in preclinical models of lung cancer. Clin Cancer Res 2019;25:3630–42. [DOI] [PubMed] [Google Scholar]
- 28. Zheng X, Carstens JL, Kim J et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015;527:525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 2017;14:611–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nie LY, Lu QT, Li WH et al. Sterol regulatory element-binding protein 1 is required for ovarian tumor growth. Oncol Rep 2013;30:1346–54. [DOI] [PubMed] [Google Scholar]
- 31. Du X, Wang QR, Chan E et al. FGFR3 stimulates stearoyl CoA desaturase 1 activity to promote bladder tumor growth. Cancer Res 2012;72:5843–55. [DOI] [PubMed] [Google Scholar]
- 32. Huang WC, Li X, Liu J et al. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res 2012;10:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li W, Tai Y, Zhou J et al. Repression of endometrial tumor growth by targeting SREBP1 and lipogenesis. Cell Cycle 2012;11:2348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li C, Yang W, Zhang J et al. SREBP-1 has a prognostic role and contributes to invasion and metastasis in human hepatocellular carcinoma. IJMS 2014;15:7124–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guerreiro AS, Fattet S, Fischer B et al. Targeting the PI3K p110alpha isoform inhibits medulloblastoma proliferation, chemoresistance, and migration. Clin Cancer Res 2008;14:6761–9. [DOI] [PubMed] [Google Scholar]
- 36. Zhu Y, Yu J, Wang S et al. Overexpression of CD133 enhances chemoresistance to 5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric cancer cells. Oncol Rep 2014;32:2437–44. [DOI] [PubMed] [Google Scholar]
- 37. Yuge K, Kikuchi E, Hagiwara M et al. Nicotine induces tumor growth and chemoresistance through activation of the PI3K/Akt/mTOR pathway in bladder cancer. Mol Cancer Ther 2015;14:2112–20. [DOI] [PubMed] [Google Scholar]