Abstract
Coronavirus disease 2019 (COVID-19) outbreak is proving to be an unprecedented disaster that lays its dark shadow on global health, economics and personal freedom. Severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) epidemics provide scientific data that is useful in better understanding and resolution of COVID-19. Similarities among SARS-CoV, MERS-CoV and SARS-CoV-2 have been investigated in the light of available data. SARS-CoV, MERS-CoV and SARS-CoV-2 evolved in bats and have positive-sense RNA genomes of 27.9 kb, 30.1 kb and 29.9 kb, respectively. Molecular and serological tools used for diagnosis of SARS and MERS patients resemble COVID-19 diagnostic tools. Stability and longevity data of SARS and MERS epidemics contribute in the current pandemic precaution policies. Trials to produce vaccines for SARS-CoV and MERS-CoV failed, therefore different strategies were employed for SARS-CoV2 vaccines production and during the past period antiviral agents, Convalescent plasma and monoclonal antibodies provide potential treatments for sever patients. The mortality rate caused by the SARS-CoV and MERS-CoV reached 15% and 37%, respectively. The first declarations about mortality rate of SARS-CoV-2 was around 2–4% but now this rate differed globally and reached more than 13% in some countries. A realistic COVID-19 outbreak scenario suggest that the pandemic might last for three years with fluctuation in the number of infected cases, unless vaccination process goes faster and/or antiviral drug is discovered.
Keywords: SARS-CoV, MERS-CoV, SARS-CoV-2, Future predictions
Coronoviridae is a family of viruses containing a large number of virus variants that are well known to infect various animals universally; however they have been scarcely recognized to infect humans. Coronaviruses (CoVs) as members of coronoviridae can infect humans resulting in a variety of symptoms including lung infection, pneumonia, fever, and breathing difficulties. CoVs have been recognized in various hosts of mammalians for instance Camelus sp., Paguma larvata, Mus sp., Canis lupus familiaris, Felis catus and bats; as well as in birds. Lately, data were accumulating regarding variety of COVID-19 symptoms that include neurological disorders which vary among headache, decrease in smell and taste functions, cerebrovascular disorders, brain dysfunction, epileptic seizure, and acute polyneuropathy [1]; coagulative alternations in the form of prolonged prothrombin time, thrombocytopenia, elevated D-dimer, and disseminated intravascular coagulation [2]; and hypercytokinemia that resembles sepsis in many ways [3].
Viruses of the family Coronaviridae as well as in many animal viruses enclose 26–32 kb positive ssRNA [4]. Based on genotype and serological tests, CoVs are classified in 4 subfamilies assigned as α, β, γ, and δ-CoVs. Subfamilies γ- and δ-CoVs have affinity towards birds as hosts while α- and β-CoVs are capable of colonizing mammalian cells. Subfamilies α- and β-CoVs are responsible for known Human CoVs related diseases [5,6]. Earlier, around 6 CoVs were confirmed as human pathogens with mild pathogenicity and mild symptoms that resemble common cold; examples include HCoV-229 E and HCoV-NL63 as members of α-CoVs; and HCoV-HKU1 and HCoV-OC43 as members of β-CoVs. Surprisingly members of coronoviridae were recognized in 1930s, however CoVs gained attentions in 2002 when severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) epidemic took place in China [7], in 2012 when middle east respiratory syndrome (MERS) coronavirus (MERS-CoV) was noticed in Saudi Arabia [8] and recently, when severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), previously called 2019-nCoV, the causative pathogen of Coronavirus Disease 2019 (COVID-19) appeared initially in China then became a pandemic [9]. Molecular analysis of SARS-CoV and MERS-CoV showed that they stand as members of β-CoVs [5,7]; as well as SARS-CoV-2 [10].
Studying the similarities among SARS-CoV-2, MERS-CoV and SARS-CoV is essential to limit the spread and to treat the patients with COVID-19. This review attempts to predict the scenario of SARS-CoV-2 in the future particularly with the absence of vaccines and antiviral drugs in MERS-CoV and SARS-CoV published data.
Discovery and similarities
In November 2002, southern China, SARS-CoV was noticed for the first time in Guangdong [11], over 8000 cases and around 774 deaths were recorded in 37 different countries between 2002 and 2003 [12]. MERS-CoV as an agent that cause severe human respiratory disease was first recognized in Saudi Arabia in 2012 [8]. MERS-CoV was identified for the first time in a sample collected from a 60 years old patient who suffered severe acute pneumonia and multiple organ failure in Saudia Arabia. The highest prevalence and outbreaks MERS-CoV outside Saudi Arabia were discovered later in South Korea in 2015 [13]. MERS-CoV was responsible for 2494 patients and 858 mortalities [14]. After seven years of MERS-CoV outbreaks, WHO data reflected that nearly 80% of MERS-CoV patients were reported in Saudi Arabia; with 1983 confirmed cases and mortality rate of 37.5% [15].
Lately, SARS-CoV-2 that is responsible for COVID-19 emerged in December 2019 initially in Wuhan, China. Then at 31 January 2020 around 11,791 cases with 213 deaths were confirmed in 19 different countries [9]. This epidemic had caused outbreak of serious infectious pneumonia in most countries around the world with more than 30, 055, 710 cases including 943,433 death cases by September, 18, 2020 [16].
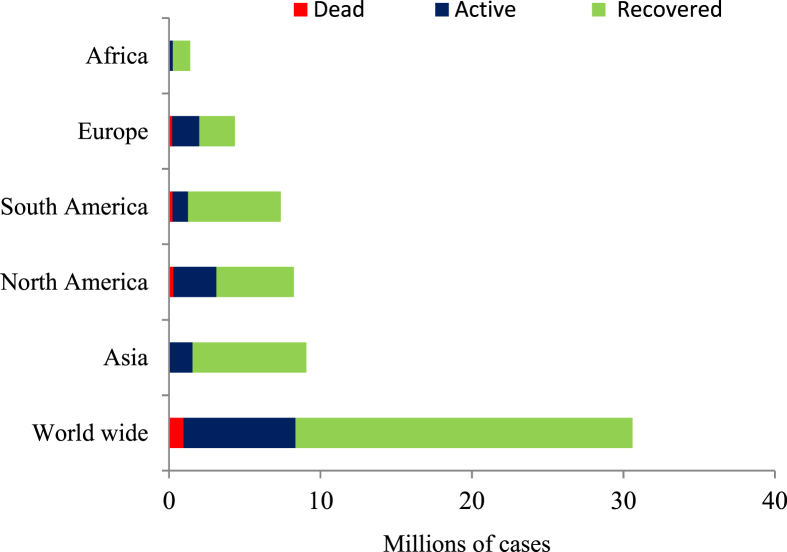
SARSCoV-2 isolate, collected from an employee in Wuhan seafood market, had a genome size of 29.9 kb [17]. Prior study exhibited that genomes of SARS-CoV and MERS-CoV had sizes of 27.9 kb and 30.1 kb, respectively [18]. Genetic distance between SARS-CoV-2 and SARS-CoV is less than the genetic distance between SARS-CoV-2 and MERS-CoV [19]. Whole genome sequences comparison between SARS-CoV and SARS-CoV-2 revealed that 79.5% of sequences are similar [17,20]. Both SARS-CoV-2 and SARS-CoV spike (S) proteins were found to be able to bind to angiotensin converting enzyme 2 receptor, hence penetrate and colonize epithelial cells of the alveoli [20]. The current review could be able to predict a futuristic scenario of SARS-CoV-2 based on similarities among SARS-CoV, MERS-CoV, and SARS-CoV-2; taking into consideration the experiences gained during SARS-CoV and MERS-CoV epidemics that may contribute in SARS-CoV-2 infection control. Several scientists suggested that SARS-CoV-2 outbreak decreases in summer season because of temperature rising but unfortunately, recent reports confirmed the continuous of SARS-CoV infections in summer. A study was carried out during 2017 by Altamimi et al. in Saudi Arabia mentioned that most MERS-CoV- infected cases were recorded during summer [21]. Also, earlier study observed that the maximum number of MERS-CoV occurrence and mortalities were not recorded in the winter, but in the spring between the end of April and the beginning of May [22]. Aly et al. recorded the presence of MERS-CoV confirmed cases in relatively high numbers in summer season in Saudi Arabia [23]. The current review expects SARS-CoV-2 new cases will be recorded for a minimum three years in most countries but not in the same rate of outbreaks in 2020 unless a drug or vaccines is discovered to inhibit the outbreak. Several studies on MERS-CoV support this expectation, while MERS-CoV was discovered in 2012, cases were still reported until 2017. Altamimi et al. reported 119 confirmed cases during 2017 in the capital and central region of Saudi Arabia, in 2016 the number of cases was 48.7% higher than 2017 and 25.4% lower than 2015 reported cases these findings were supported in other researches [21,24]. Although SARS-CoV-2 is more transmissive when compared to SARS-CoV and MERS-CoV it still has lower severity and mortality rates, according to available data and such findings were confirmed in the review by Yi et al. [25]. According to WHO recent situation report, global death rate among SARS-CoV-2 infected patients is around 3.14% [Fig. 1], however the rate varies among countries to range between 0.20 and 28.90% [16,26,27].
Fig. 1.
Total numbers of infected cases divided into recovered, active, and dead cases until 18th, September, 2020 [16,26,27].
Sex desegregation
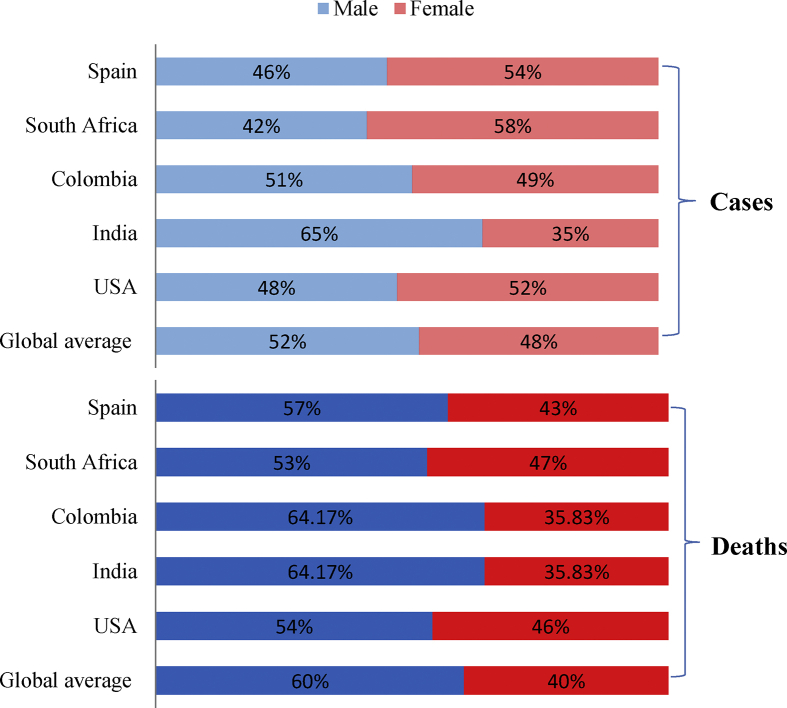
Numerous literatures showed that the rate of male infection was more than female infection by MERS-CoV. In Saudi Arabia, Alghamdi et al. focused on fatality rate as a result of MERS-CoV infection, and found that the fatality rate among women (23%) was less than that among men which reached to 52% [22]. Several explanations have been written regarding male to female infection, although some are not convincing, for example Park et al. stated that women care more for a healthy and hygienic attitude [28]. Some opinions went to attribute women relatively low infections to decreasing exposure to MERS-CoV infection because of face veils, but even if this explanation could be right regarding Saudi Arabia, it still could not clarify the same pattern in South Korea in which women do not wear face veils. Also Arabi et al. showed that MERS-CoV infects female less than males [29]. Although Choi et al. mentioned that more evidence is required to confirm the relationship between infection and smoking [30], Jansen et al. stated that the male predominance for infection by MERS-CoV may be associated with the habit of smoking that is more common among men [31]. The same scenario was also observed in infections by SARS-CoV-2, where Porcheddu et al. stated that some sex disaggregation data revealed greater mortality rates in men as compared with women as men have up to three times higher infection probabilities than women [32]. The higher incidence of SARS-CoV-2 among men may be due to the men spend more time outdoors in work and also may use the means of transportation such as trains and buses; thus it has a higher risk of contact to a source of infection. It is necessary to search for the real causes of this phenomenon. There could be a relationship between infection ratio and physiology of human that may be associated with sex hormones. Although males infection percentage in some countries such as Spain, South Africa and USA were lower than females at certain times, the rate of fatality caused by SARS-CoV-2 was higher in males [Fig. 2] [33].
Fig. 2.
Covid 19 sex-disaggregated data of confirmed cases and deaths until 21st, September, 2020 [33].
Comorbidity
The severity of COVID-19, SARS and MERS disease is associated with increased age and comorbidity, although severe disease is not limited to these risk groups. Patients with defective immune system for example, elderly or individuals with a history of medical cases including hypertension, obesity, diabetes or cancer, might quickly develop a severe or critical status due to SARS-CoV and MERS-CoV infection [34]. Yang et al. compared SARS-CoV-2 patients and concluded that coexisting diseases increase risk factors for patients [35].
Patient's age plays a critical role for infection severity of CoVs, numerous reports revealed that highest prevalence of SARS-CoV-2 infections occur within relatively over age groups. Altamimi et al. found that in 2017, 41.2% of MERS-CoV-infected patients were between 41 and 60 years old [21]. According to available data the same is true for SARS-CoV-2, where individuals with mean age of 60 years and older showed increased susceptibility [36]. Age (above 50 years) has emerged as one independent risk factor for severe disease [37].
In a study on MERS- CoV patients diabetes and hypertension were prevalent in 50% of the severe patients; furthermore 15% of studied patients were diabetic, 16% were obese, 50% had hypertension, and 30% suffered cardiovascular diseases [38]. SARS-CoV patients who died were mostly old and had other health problems such as diabetes, and hyperglycemia [39]. Patients with SARS-CoV-2 and hypertension or diabetes are more likely to develop severe disease [37].
Cancer is one of the clinical risk factors that determine the mortality rate among MERS-CoV-infected patients [40,41]. Although SARS-CoV-2-infected patients that pre-diagnosed with cancer exhibited severe symptoms, patients with lung cancer did not exhibit severe symptoms when compared to those with other types of cancers [42]. Recent reports suggested that some anti-cancer regimes did not affect the risk of SARS-CoV-2 infection or worsens of the symptoms [43].
Origin, host and transmission
MERS-CoV and SARS-CoV were identified for the first time in 2002 and 2012, respectively, even though their original hosts were not confirmed in any virological study. According to Zumla et al. the exact origin of MERS-CoV remains unidentified but some reports propose that dromedary camels are the main reservoir host [44]. MERS-CoV outbreaks in Saudi Arabia were probably as a result of interaction with camels and other animals. Nevertheless more than 50% of confirmed cases did not occur near by zoonotic habitats as well as camels. Another study of MERS-CoV infection among camel handlers observed no positive cases [45]. Ithete et al. did not determine the animal host of MERS-CoV but stated that the MERS-CoV transfers among humans as well as from animals to humans [46]. MERS-CoV was isolated from dromedary camels not only in Saudi Arabia but also in other countries such as Egypt, Oman and Qatar [47]. Serological studies suggest that around 90% of mature camels in Africa and Middle-East were serologically positive for MERS-CoV [48]. According to many literatures, antibodies of MERS-CoV were detected in many camels [[49], [50], [51]], this evidence supports the suggestion that camels might play a role as an intermediate host. Woo et al. stated that infection by MERS-CoV is due to contact to mammalians mostly dromedary camels and bats [52].
Although the outbreak of SARS-CoV and MERS-CoV occurred from 2003 to 2012, the research is still being done to establish the true reservoir of these viruses. Recently epidemiologic investigations suggested that bat is the probable origin of SARS-CoV and MERS-CoV that were spread to human through an intermediate host represented by palm civet or raccoon dog for SARS-CoV, while dromedary camel for MERS-CoV [53,54].
There are limited studies on CoV infections particularly MERS-CoV, therefore major discrepancies about the routs responsible for transmitting the virus from the infected animal are still fogy. Zumla et al. mentioned that milk and uncooked meat as well as nasal mucous, saliva, and sputum may be sources of spread [44]. Hence, droplets and direct contact are considered one of the most important sources of infection transmission. Surprisingly Ge et al. suggested that the original host of MERS-CoV might be bats [55]. Goats, cows, sheep and/or poultry might play essential role in the spread of virus [56].
The origin of SARS-CoV-2 is still unclear to scientific society; therefore, Chinese health authorities investigate the origin of SARS-CoV-2 [57]. Initial epidemiological investigations suggested that the origin of the outbreak of SARS-CoV-2 was Huanan Seafood Market; however, the first confirmed case had no clear connection to the Huanan Seafood Market which disputes this suggestion [58]. Numerous studies endorsed that bats might be the original host of most recently identified CoVs including SARS-CoV, MERS-CoV and SARS-CoV-2, but their transmission to human might take place through intermediate host. Virus genome sequencing analyses suggest that SARS-CoV-2 might be transmitted from bats to humans through unidentified intermediate animal host(s) [Fig. 3]. Direct contact with intermediate host considered the key way of SARS-CoV-2 transmission. The presence of intermediate host is supported by the fact that human communities are relatively far from bat habitats. Intermediate hosts represent appropriate media for CoVs mutations. Nosocomial workers were reported as main contributors for SARS-CoV and MERS-CoV transmission [59,60], infection by SARS-CoV among healthcare workers was 33–42% while transmission among patients in MERS-CoV cases was 62–79%; so it is highly recommended to monitor SARS-CoV-2 transmission rates among health care workers and to raise protection standards. Published data mentioned several ways of SARS-CoV-2 transmission; most of them agreed that respiratory drops represent the main path for infection [61].
Fig. 3.
Human coronaviruses evolution, intermediate hosts, and approximate numbers of infected cases.
Clinical appearances
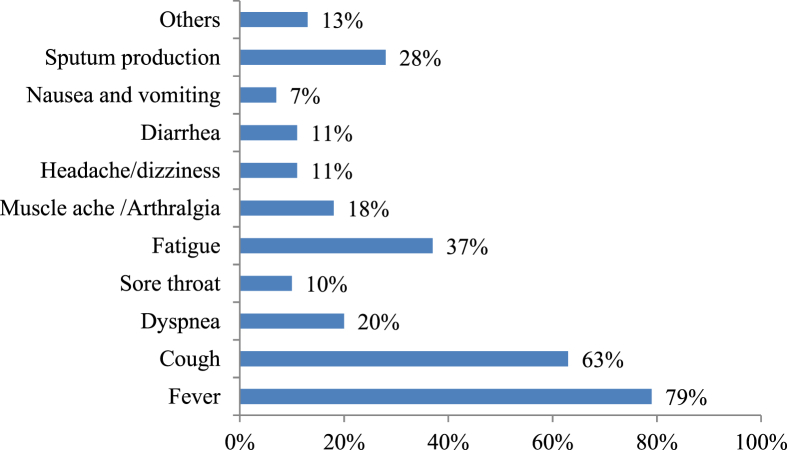
The medical pictures of CoVs patients ranged in symptoms from no symptoms to respiratory syndrome and death [61]. Although the complete clinical manifestation of COVID-19 is unclear yet [62]; reported symptoms include fever, cough, fatigue and gastrointestinal infection [63]. Fever, fatigue and dry cough are the main symptoms of SARS-CoV-2 infection that represent 99%, 70% and 59% in reported symptoms, respectively beside another symptoms [Fig. 4] [58,64]. The highest risk groups among patients are elderly and those suffer underlying diseases. Approximately similar findings with minor differences were reported in MERS and SARS patients where fever, dry cough and dyspnea were main symptoms [58,65]. The resemblances in symptoms in CoVs patients suggest that all CoVs may have the same target and/or mechanisms.
Fig. 4.
Common symptoms of SARS-CoV-2 infections and their percentage among patients [58,64].
The period of recovery from COVID-19 may differ in relation to health conditions of patients and symptoms severity. Patients with minor symptoms have been reported to cure within a week while patients with severe symptoms have been reported to experience advanced respiratory failure as a result of alveolar damage caused by the virus, which could result in death [10]. Mortality has been reported principally among elderly and middle aged patients especially with medical situations that include tumor surgery, cirrhosis, hypertension, coronary heart disease, diabetes, and Parkinson's disease [1,43,66,67].
Mortality rate caused by the SARS-CoV was around 10%–15% [68], while MERS-CoV mortality rates could reach 37% [53]. However, death rate among patients of COVID-19 ranged between 2 and 4% and exceeded 13% in some countries which suggest that the mortality rate of COVID-19 might be less than mortality rates of SARS and MERS taking into consideration improvement in medical services and facilities through years.
Diagnosis, treatment and prevention
Rapid and accurate laboratory diagnostic tools are essential for discovery and treatment of CoVs infections. A variety of molecular and serological diagnostic tools were developed for CoVs [69]. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) is the most acceptable used method tool for detection of CoVs because of its specificity and simplicity as a qualitative assay, however samples may be misdiagnosed as false negative or false positive [70]. Immunoassays are not commonly conducted for diagnosis. Although antibody seroconversion provides reliable proof of infection, serological tests lags behind the molecular testing because it is not suitable for early diagnosis [71]. Chest computed tomography (CCT) provided a potential imaging technique to identify lung involvement caused by SARS-CoV-2 (COVID-19), even in asymptomatic patients [72]. The use of CCT along with RT-qPCR provides high chance of accurate diagnosis [70,73].
Common SARS, MERS and COVID-19 treatment options are mostly supportive and symptomatic [74]. Although vaccination may be the optimal choice, no COVs vaccines were successfully developed. Trials for putative vaccines production are running in full swing, however it is expected to take more time to be applied [75]. Currently treatment is carried out mainly using broad spectrum antibiotics, corticosteroids [76]. Several drugs that include antiviral, immunosuppressant, steroids are also used as supportive and symptomatic treatments.
The use of plasma from recovered patients has been also anticipated as a treatment [58,76]. Convalescent plasma, a classic adaptive immunotherapy, has been used for the treatment of many infectious diseases that include SARS and MERS [77,78]. Although convalescent plasma is a potential effective treatment against COVID-19 that requires further investigations [79,80], it is still a temporary solution until new antiviral agents and vaccines are developed. Lately the use of Tocilizumab, a monoclonal antibody against interleukin-6, was investigated as an alternative treatment for COVID-19 patients with severe respiratory symptoms [81]. Di Giambenedetto et al. highlighted the efficacy of tocilizumab in the treatment of COVID-19 without detected adverse events and suggested that tocilizumab may be an effective and safe treatment of COVID-19 patients with severe pneumonia [82].
SARS-CoV and MERS-CoV can survive up to 5 days in a dry environment (40%–50% humidity) at 20 °C [83]. Transmission of MERS-CoV is enhanced by relative low humidity and high temperature [22]. SARS-CoV-2 may have the same characteristics. Kampf et al. mentioned that SARS-CoV-2 like other viruses could be inactivated with 70% concentration of isopropanol and ethanol apart from 0.1% sodium hypochlorite within 1 min exposure [83]. Protection protocols recommended to avoid SARS-CoV and MERS-CoV infection included hands disinfection, wearing protective face masks and surfaces sterilization as well as avoiding intimate personal contact with infected patients [84], the same protocols are applying for protection from SARS-CoV-2 that include airborne precautions and other protective measurements that may reduce the risk of infection [85].
Conclusions
SARS-CoV-2, SARS-CoV and MERS-CoV have considerable degree of similarities that could be basic data in predicting SARS-CoV-2 behavior. Although SARS-CoV-2 has a higher transmission rate than SARS-CoV and MERS-CoV, its mortality rate is obviously lower. Several reports investigated vaccination, epidemiology, symptoms, mode of action, control and origin of SARS-CoV-2, however confirmed data are relatively limited and require further investigations not only for SARS-CoV-2, but also SARS-CoV and MERS-CoV. No specific treatment or vaccination was developed against CoVs. Therefore, SARS-CoV-2 infection control measures are essential for the prevention of its spread in healthcare facilities, as well as in the community. COVID-19 pandemic increased studies manipulating SARS-CoV-2 in comparison with SARS-CoV and MERS-CoV. The current review recommends that the scholarly community conduct further research to provide valid and reliable ways to manage this kind of public health emergency in both the short term and long-term.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Pennisi M., Lanza G., Falzone L., Fisicaro F., Ferri R., Bella R. SARS-CoV-2 and the nervous system: from clinical features to molecular mechanisms. Int J Mol Sci. 2020;21:5475. doi: 10.3390/ijms21155475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellinvia S., Edwards C.J., Schisano M., Banfi P., Fallico M., Murabito P. The unleashing of the immune system in COVID-19 and sepsis: the calm before the storm? Inflamm Res. 2020;69:757–763. doi: 10.1007/s00011-020-01366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv Virus res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Ahdal M.N., Al-Qahtani A.A., Rubino S. Coronavirus respiratory illness in Saudi Arabia. J Infect Dev Ctries. 2012;6:692–694. doi: 10.3855/jidc.3084. [DOI] [PubMed] [Google Scholar]
- 8.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 9.Adhikari S.P., Meng S., Wu Y.J., Mao Y.P., Ye R.X., Wang Q.Z. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Taisheng, Cao Wei, Fan Hongwei, Shi Juhong. Beijing Union Medical College Hospital on “pneumonia of novel coronavirus infection” diagnosis and treatment proposal (V2.0) Med J Beijing Union Med Coll Hosp. 2020 [Google Scholar]
- 11.Peiris J.S.M., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan-Yeung M., Xu R.H. SARS: epidemiology. Respirology. 2003;8:S9–S14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X., Chughtai A.A., Dyda A., Macintyre C.R. Comparative epidemiology of Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia and South Korea. Emerg Microb Infect. 2017;6:e51. doi: 10.1038/emi.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J.Y., Kim Y.J., Chung E.H., Kim D.W., Jeong I., Kim Y. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC Infect Dis. 2017;17:498. doi: 10.1186/s12879-017-2576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . 2020. WHO EMRO | MERS situation update. MERS-CoV | Epidemic and pandemic diseases.http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-december-2019.html [Google Scholar]
- 16.World Health Organization . 2020. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ [Google Scholar]
- 17.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altamimi A., Abu-Saris R., El-Metwally A., Alaifan T., Alamri A. Demographic variations of MERS-CoV infection among suspected and confirmed cases: an epidemiological analysis of laboratory-based data from riyadh regional laboratory. BioMed Res Int. 2020;2020:9629747. doi: 10.1155/2020/9629747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alghamdi I.G., Hussain, Almalki S.S., Alghamdi M.S., Alghamdi M.M., El-Sheemy M.A. The pattern of Middle east respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aly M., Elrobh M., Alzayer M., Aljuhani S., Balkhy H. Occurrence of the Middle East respiratory syndrome coronavirus (MERS-CoV) across the gulf corporation council countries: four years update. PloS One. 2017;12 doi: 10.1371/journal.pone.0183850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Da’Ar O.B., Ahmed A.E. Underlying trend, seasonality, prediction, forecasting and the contribution of risk factors: an analysis of globally reported cases of Middle East Respiratory Syndrome Coronavirus. Epidemiol Infect. 2018;146:1343–1349. doi: 10.1017/S0950268818001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johns Hopkins Coronavirus Resource Center . 2020. Mortality analyses.https://coronavirus.jhu.edu/data/mortality/ [Google Scholar]
- 27.Worldometer Coronavirus cases. Worldometer. 2020:1–22. [Google Scholar]
- 28.Park J.H., Cheong H.K., Son D.Y., Kim S.U., Ha C.M. Perceptions and behaviors related to hand hygiene for the prevention of H1N1 influenza transmission among Korean university students during the peak pandemic period. BMC Infect Dis. 2010;10:222. doi: 10.1186/1471-2334-10-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arabi Y.M., Arifi A.A., Balkhy H.H., Najm H., Aldawood A.S., Ghabashi A. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 30.Choi S.M., Jeong Y.J., Park J.S., Kang H.J., Lee Y.J., Park S.S. The impact of lifestyle behaviors on the acquisition of pandemic (H1N1) influenza infection: a case-control study. Yonsei Med J. 2014;55:422–427. doi: 10.3349/ymj.2014.55.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen A., Chiew M., Konings F., Lee C.K., Ailan L. Sex matters - a preliminary analysis of Middle East respiratory syndrome in the Republic of Korea, 2015. West Pacific Surveill Response. J WPSAR. 2015;6:68–71. doi: 10.5365/WPSAR.2015.6.3.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porcheddu R., Serra C., Kelvin D., Kelvin N., Rubino S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. The Journal of Infection in Developing Countries. 2020;14(02):125–128. doi: 10.3855/jidc.12600. [DOI] [PubMed] [Google Scholar]
- 33.Global Health 50/50. 2020. https://globalhealth5050.org/ [Google Scholar]
- 34.Renu K., Prasanna P.L., Valsala Gopalakrishnan A. Coronaviruses pathogenesis, comorbidities and multi-organ damage – a review. Life Sci. 2020;255:117839. doi: 10.1016/j.lfs.2020.117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen K., Yang Y., Wang T., Zhao D., Jiang Y., Jin R. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J Pediatr. 2020;16:223–231. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhihong L., Bin Y., Kaipan G., Wanping B., Guoliang H., Jianping W. Clinical feature in patients of sever acute respiratory syndrome with type 2 diabetes and secondary hyperglycemia. Chinese J Endocrinol Metab. 2004;20:14–15. [Google Scholar]
- 40.Park J.E., Jung S., Kim A. MERS transmission and risk factors: a systematic review. BMC Publ Health. 2018;18:574. doi: 10.1186/s12889-018-5484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuyama R., Nishiura H., Kutsuna S., Hayakawa K., Ohmagari N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Publ Health. 2016;16:1203. doi: 10.1186/s12889-016-3881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vivarelli S., Falzone L., Grillo C.M., Scandurra G., Torino F., Libra M. Cancer management during covid-19 pandemic: is immune checkpoint inhibitors-based immunotherapy harmful or beneficial? Cancers. 2020;12:1–22. doi: 10.3390/cancers12082237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reusken C.B.E.M., Farag E.A.B.A., Haagmans B.L., Mohran K.A., Godeke G.J., Raj V.S. Occupational exposure to dromedaries and risk for MERS-CoV infection, Qatar, 2013–2014. Emerg Infect Dis. 2015;21:1422–1425. doi: 10.3201/eid2108.150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ithete N.L., Stoffberg S., Corman V.M., Cottontail V.M., Richards L.R., Schoeman M.C. Close relative of human middle east respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19:1697–1699. doi: 10.3201/eid1910.130946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan R.W.Y., Hemida M.G., Kayali G., Chu D.K.W., Poon L.L.M., Alnaeem A. Tropism and replication of Middle East respiratory syndrome coronavirus from dromedary camels in the human respiratory tract: an in-vitro and ex-vivo study. Lancet Respir Med. 2014;2:813–822. doi: 10.1016/S2213-2600(14)70158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kayali G., Peiris M. A more detailed picture of the epidemiology of Middle East respiratory syndrome coronavirus. Lancet Infect Dis. 2015;15:495–497. doi: 10.1016/S1473-3099(15)70128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corman V.M., Kallies R., Philipps H., Gopner G., Muller M.A., Eckerle I. Characterization of a novel betacoronavirus related to Middle East respiratory syndrome coronavirus in European hedgehogs. J Virol. 2014;88:717–724. doi: 10.1128/JVI.01600-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milne-Price S., Miazgowicz K.L., Munster V.J. The emergence of the Middle East Respiratory Syndrome coronavirus. Pathog Dis. 2014;71:121–136. doi: 10.1111/2049-632X.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yusof M.F., Eltahir Y.M., Serhan W.S., Hashem F.M., Elsayed E.A., Marzoug B.A. Prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Abu Dhabi Emirate, United Arab Emirates. Virus Gene. 2015;50:509–513. doi: 10.1007/s11262-015-1174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woo P.C.Y., Lau S.K.P., Li K.S.M., Tsang A.K.L., Yuen K.Y. Genetic relatedness of the novel human group C betacoronavirus to Tylonycteris bat coronavirus HKU4 and Pipistrellus bat coronavirus HKU5. Emerg Microb Infect. 2012;1:e35. doi: 10.1038/emi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge X.Y., Li J.L., Lou Yang X., Chmura A.A., Zhu G., Epstein J.H. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raj V.S., Smits S.L., Provacia L.B., van den Brand J.M.A., Wiersma L., Ouwendijk W.J.D. Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4-mediated entry of the Middle East respiratory syndrome coronavirus. J Virol. 2014;88:1834–1838. doi: 10.1128/JVI.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W. Virology, epidemiology, pathogenesis, and control of covid-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chowell G., Abdirizak F., Lee S., Lee J., Jung E., Nishiura H. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang C.K., Song K.H., Choe P.G., Park W.B., Bang J.H., Kim E.S. Clinical and epidemiologic characteristics of spreaders of middle east respiratory syndrome coronavirus during the 2015 outbreak in Korea. J Kor Med Sci. 2017;32:744–749. doi: 10.3346/jkms.2017.32.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lupia T., Scabini S., Mornese Pinna S., Di Perri G., De Rosa F.G., Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–27. doi: 10.1016/j.jgar.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cdc Centers for Disease Control Symptoms of coronavirus | CDC. Centers Dis Control Prev. 2019 [Google Scholar]
- 63.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- A n update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheleme T., Bekele F., Ayela T. Clinical presentation of patients infected with coronavirus disease 19: a systematic review. Infect Dis Res Treat. 2020;13:1–8. doi: 10.1177/1178633720952076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espinosa O.A., Zanetti A.D.S., Antunes E.F., Longhi F.G., de Matos T.A., Battaglini P.F. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2020;62:1–13. doi: 10.1590/S1678-9946202062043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L.M. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan Y., Chang L., Wang L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): current status, challenges, and countermeasures. Rev Med Virol. 2020;30:e2106. doi: 10.1002/rmv.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20:453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Q., Xia S., Sun Z., Wang Q., Du L., Lu L. Testing of middle east respiratory syndrome coronavirus replication inhibitors for the ability to block viral entry. Antimicrob Agents Chemother. 2015;59:742–744. doi: 10.1128/AAC.03977-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sánchez-Oro R., Torres Nuez J., Martínez-Sanz G. Radiological findings for diagnosis of SARS-CoV-2 pneumonia (COVID-19) Med Clínica. 2020;155:36. doi: 10.1016/j.medcle.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Şendur H.N. Debate of chest CT and RT-PCR test for the diagnosis of COVID-19. Radiology. 2020;297:3. doi: 10.1148/radiol.2020203627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Center for disease control and prevention About Middle East respiratory syndrome (MERS) CDC. Centers Dis Control Prev. 2020:1. [Google Scholar]
- 75.Mallapaty S., Ledford H. COVID-vaccine results are on the way — and scientists' concerns are growing. Nature. 2020;586:16–17. doi: 10.1038/d41586-020-02706-6. [DOI] [PubMed] [Google Scholar]
- 76.Center for disease control and prevention . 2020. COVID-19 Treatment Guidelines.https://www.covid19treatmentguidelines.nih.gov/ [Google Scholar]
- 77.Cheng Y., Wong R., Soo Y.O.Y., Wong W.S., Lee C.K., Ng M.H.L. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ko J.H., Seok H., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 79.Liu S.T.H., Lin H.M., Baine I., Wajnberg A., Gumprecht J.P., Rahman F. Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study. Nat Med. 2020;26:1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 80.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di Giambenedetto S., Ciccullo A., Borghetti A., Gambassi G., Landi F., Visconti E. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J Med Virol. 2020;92:1787–1788. doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geller C., Varbanov M., Duval R.E. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qattan I., Aljohani A., Alfarsi M., Aljohani E., Alsubhi M., MERS-CoV An epidemic whirlwind. Biol Med. 2016;8:1–15. [Google Scholar]
- 85.Centers for Disease Control and Prevention . 2020. How to protect yourself & others.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention-H.pdf [Google Scholar]