Sir,
At the end of 2019, a novel human coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged and rapidly spread throughout the world. Although close exposure to respiratory droplets from an infected patient is the main transmission route of SARS-CoV-2, touching contaminated surfaces and objects might also contribute to transmission of this virus. In addition, the evidence for gastrointestinal infection of SARS-CoV-2 and the presence of SARS-CoV-2 RNA in faecal specimens raised the question of a faecal–oral transmission route [1,2]. Here, we a report our study on the stability of SARS-CoV-2 on various environmental surfaces and in human excreta (faeces and urine). These data have great importance for understanding the transmission of this virus.
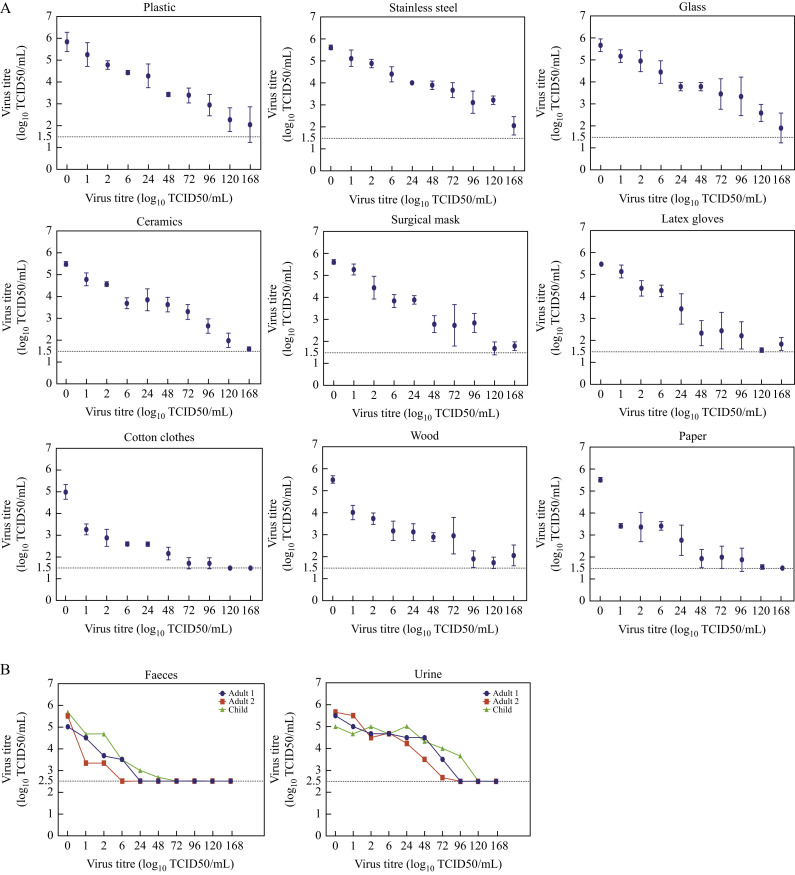
Nine different objects representing a variety of household and hospital situations were collected and cut into small pieces of about 1×1 cm. SARS-CoV-2 BetaCoV/Beijing/AMMS01/2020 was the strain used. Fifty microlitres of virus stock with an infectious titre of 106 50% tissue culture infectious dose (TCID50) per millilitre was deposited on each surface and left at room temperature. At predefined time-points, the viruses were recovered by adding 500 μL of viral transport medium. The infectivity of residual virus was titrated in quadruplicate on 96-well plates containing 100 μL of Vero cells (2×105 cells/mL). The plates were incubated in 5% CO2 at 37°C. On the fifth day, the cytopathic effect was observed under a microscope, and the TCID50 for each sample at a different time was calculated with the Reed–Muench method. All experiments were repeated three times. SARS-CoV-2 was stable on plastic, stainless steel, glass, ceramics, wood, latex gloves, and surgical mask, and remained viable for seven days on these surfaces. The virus titre declined slowly on these seven surfaces (Figure 1 A). However, no infectious virus was recovered from cotton clothes after four days and from paper after five days.
Figure 1.
Stability of SARS-CoV-2 on environmental surfaces and in human excreta. (A) Survival time of SARS-CoV-2 on nine surfaces. The limit of detection (LOD) for the assays was 101.5 tissue culture infectious dose (TCID50)/mL. (B) Survival time of SARS-CoV-2 in three faecal and urine specimens. The LOD for the assays was 102.5 TCID50/mL due to cytotoxicity caused by faecal and urine specimens.
The specimens of faeces and urine were collected from three healthy donors, including two adults and one seven-year-old child. A 10% suspension of each faecal specimen was prepared in phosphate-buffered saline (pH, 7.4) as described previously [3]. A total of 2.7 mL of each filtered faecal suspension and urine sample was inoculated with 0.3 mL of virus stock and left at room temperature for seven days. At desired time-points, 50 μL of each sample was taken and virus titre was determined with the same method described above. Figure 1B shows the duration of SARS-CoV-2 survival in three faeces. In the first adult faecal specimen, no viable SARS-CoV-2 was measured after 6 h, and in the second adult faecal specimen, no virus remained viable after 2 h. However, the virus survived for two days in the child faeces. SARS-CoV-2 was more stable in urine than in faeces, and infectious virus was detected up to three days in two adult urine samples and four days in one child urine sample.
Prior to our study, two research teams had reported on the stability of SARS-CoV-2 on different material surfaces [4,5]. In comparison with the above two studies, our data displayed a prolonged survival time of this virus on environmental surfaces. In general, the stability of virus in environments was derived from simulated experiments, which were influenced by many factors. The titre of virus stock and the volume of virus inoculation were related with the final results. Compared with van Doremalen's experiments, we used the same volume of inoculation, but the titre of virus stock was one logarithmic unit higher. In Chin's study, 5 μL of virus stock with an infectious titre of 106.8 TCID50/mL was deposited on the surface. Therefore, we highly recommended that a technical specification should be drafted to guide further research into the survival of newly emerging virus.
Fomites such as human excreta are a matter of considerable public concern for the transmission of SARS-CoV-2. Several studies reported the presence of viral RNA in faeces of patients in which respiratory samples had switched negative [2,6]. In this study, we showed that this virus remained viable for several hours in faeces and for three to four days in urine. With respect to virus isolation, it is not easy to isolate SARS-CoV-2 from faecal samples, in spite of high virus RNA concentration. Until recently, three SARS-CoV-2 strains were successfully isolated from faecal specimens [7]. The short duration of SARS-CoV-2 survival in faeces has practical implications for virus isolation from faecal samples. A reasonable suggestion is that we should shorten the time from sample collection to virus isolation. There was scarce evidence for the presence of viral RNA of SARS-CoV-2 in urine, and no infectious virus has yet been isolated from a urine sample. However, isolation of SARS-CoV, closely related to SARS-CoV-2, has indeed been reported. Taken together, the transmission of SARS-CoV-2 by human excreta is plausible. Effective hand hygiene and adequate disinfection in toilets is recommended to limit the transmission of this virus.
Conflict of interest statement
None declared.
Funding source
This work is financially supported by National Key Research and Development Project of China (2020YFC0846200).
References
- 1.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai M.Y.Y., Cheng P.K.C., Lim W.W.L. Survival of severe acute respiratory syndrome coronavirus. Clin Infect Dis. 2005;41:e67–e71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang A., Tong Z.-D., Wang H.-L., Dai Y.-X., Li K.-F., Liu J.-N. Detection of novel coronavirus by RT–PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26:1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao H., Lu X., Chen Q., Xu K., Chen Y., Cheng L. Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv. 2020 2020.04.14.20060160. [Google Scholar]