Abstract
Background
The presence of olfactory dysfunction or “loss of smell” has been reported as an atypical symptom in patients with coronavirus disease 2019 (COVID-19). We performed a systematic review and meta-analysis of the available literature to evaluate the prevalence of “loss of smell” in COVID-19 as well as its utility for prognosticating the disease severity.
Methods
An exhaustive search of the PubMed/Medline, Embase, Web of Science, Cochrane Library, LitCovid NIH, and WHO COVID-19 database was conducted through August 6th, 2020. All studies reporting the prevalence of “loss of smell” (anosmia and/or hyposmia/microsmia) in laboratory-confirmed COVID-19 patients were included. Pooled prevalence for cases (positive COVID-19 through reverse transcriptase (RT-PCR) and/or serology IgG/IgM) and controls (negative RT-PCR and/or serology) was compared, and the odds ratio (OR), 95% confidence interval (CI) and the p-value were calculated. A p-value of <0.05 was considered statistically significant.
Results
A total of 51 studies with 11074 confirmed COVID-19 patients were included. Of these, 21 studies used a control group with 3425 patients. The symptom of “loss of smell” (OR: 14.7, CI: 8.9–24.3) was significantly higher in the COVID-19 group when compared to the control group. Seven studies comparing severe COVID-19 patients with- and without “loss of smell” demonstrated favorable prognosis for patients with “loss of smell” (OR: 0.36, CI 0.27–0.48).
Conclusions
Olfactory dysfunction or “loss of smell” is a prevalent symptom in COVID-19 patients. Moreover, COVID-19 patients with “loss of smell” appear to have a milder course of the disease.
Keywords: COVID-19, Olfactory dysfunction, Loss of smell, SARS-CoV-2, Coronavirus
Introduction
The pandemic coronavirus disease-2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).1 The pandemic has resulted in significant economic and healthcare burden. Along with the pulmonary symptoms, the disease is also associated with neurological manifestations such as headache, impaired consciousness, altered gait/ataxia, seizures, diarrhea, nausea/vomiting, loss of smell, and altered taste/dysgeusia.2, 3, 4 The disease severity is associated with laboratory abnormalities such as low albumin, elevated interleukin 6, increased alanine/aspartate aminotransferase, increased total bilirubin, increased procalcitonin, increased C-reactive protein (CRP), etc.4, 5, 6, 7, 8
The “loss of smell” is an atypical symptom of COVID-19 and has been reported with varying prevalence in literature. Further, it has been observed that loss of smell is usually associated with milder form of disease compared to severe disease.2 We performed a systematic review and meta-analysis of available studies to evaluate the prevalence of “loss of smell” in COVID-19 and its utility as a prognostic indicator.
Methods
Search Strategy
A systematic search of the PubMed/Medline, Embase, Web of Science, Cochrane Library, LitCovid NIH, and WHO COVID-19 databases through August 6th, 2020, was conducted. The author (W.L.S.) created the initial search strategy using the vocabulary for “COVID-19” and “smell,” which was cross-checked by another reviewer (M.A.). We highlight an example search strategy using EMBASE in Supplementary table 1. Two independent reviewers (M.A. and H.H.) performed the initial screening and data extraction from the articles. Any discrepancy in article screening or data extraction was resolved through mutual discussion.
Inclusion and exclusion criteria
Only articles reporting the laboratory confirmed COVID-19 patients and “loss of smell” were included. Articles were excluded if they had <10 cases of interest. Articles with suspected cases of COVID-19 without a definitive laboratory diagnosis were also excluded. An adherence to “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines was observed.
Study Definition
Severe disease is defined as the presence of either respiratory distress (i.e., rate >30/min, PaO2/FiO2 <300, and/or SpO2 <93%), need for hospitalization, and/or death. Given the heterogeneity in defining the “loss of smell” across studies, we included the concepts of “anosmia (complete loss of smell)” and “hyposmia/microsmia (diminished or partial loss of smell)” collectively as “loss of smell”. Positive COVID-19 cases are defined as patients with laboratory confirmed COVID-19 (through reverse transcriptase polymerase chain reaction (RT-PCR) and/or serological evidence of COVID-19 through IgG/IgM). Controls are defined as patients with negative RT-PCR and/or serological testing.
Statistical measures and synthesis of results
The pooled prevalence of cases (COVID-19) and controls (non-COVID-19) were compared using the DerSimonian-Laird/Random-effect meta-analysis, and outcomes were reported using forest plots, proportions with 95% confidence interval (CI), odds ratio (OR) with 95% CI, p-value (<0.05 was considered statistically significant) and I2 heterogeneity (>50% considered substantial heterogeneity).9, 10, 11 Meta-analysis was conducted using comprehensive meta-analysis (BioStat, Englewood, New Jersey, USA) and Open Meta Analyst (CEBM, University of Oxford, Oxford, United Kingdom).
Risk of bias
Publication bias was assessed using a funnel plot and Egger's regression analysis. If significant publication bias was suspected, we utilized the “trim-and-fill” method and Fail-Safe N test. The presence of bias in the individual study was assessed using the Quality in Prognostic Studies (QUIPS) tool.12
Results
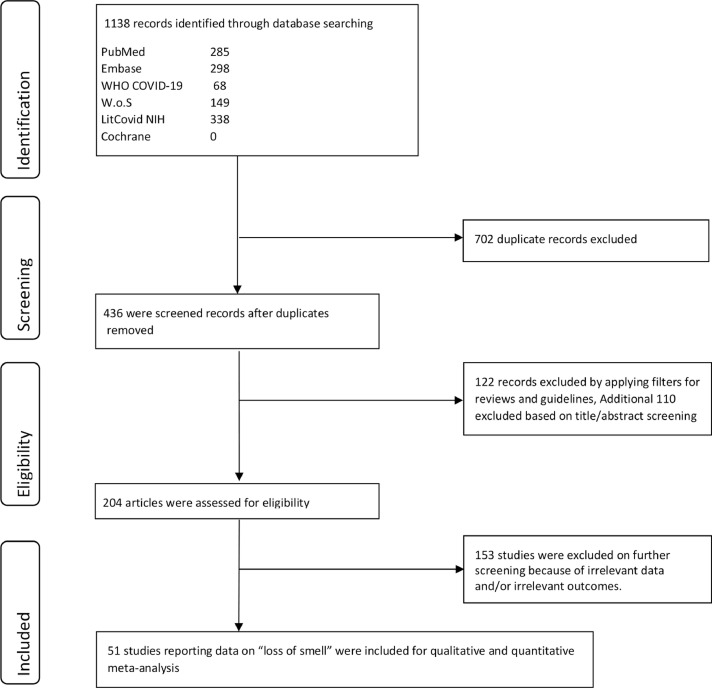
A total of 51 studies were included based on the search strategy mentioned previously (Fig. 1 ). Publication bias based on prevalence for “loss of smell” was noted based on visual assessment of the funnel plot and Egger's regression analysis (p = 0.01). We then used the “trim-and-fill” method to create adjusted funnel plot that did not significantly differ from the original funnel plot (Supplementary Fig. 1). The Fail-Safe N test was 504 with an alpha of 0.05. This signifies that 504 studies with effect size zero will be needed to nullify the effect noted for the current analysis. Using the QUIPS tool, only seven studies were considered low risk. The other remaining studies either did not account for confounders in their statistical analyses or outcome/prognostic factors were not adequately assessed (Table 1 ).
Figure 1.
PRISMA flow diagram.
Table 1.
The Quality in Prognostic Studies (QUIPS) table for risk of bias
| Study, year | Participation (The study sample represents population of interest on key characteristics?) | Attrition (The proportion of study sample providing outcome data is adequate?) | Prognostic factor measurement (Prognostic factor is adequately measured in study subjects?) | Outcome measurement (The outcome of interest is adequately measured in study subjects?) | Study confounders (Potential confounders are accounted for?) | Statistical analysis? (Statistical analysis appropriately designed for the study?) |
|---|---|---|---|---|---|---|
| Abalo-Lojo | Yes | Yes | No | Partly | No | No |
| Aggrawal | Yes | Partly | No | Partly | No | Yes |
| Altin | Yes | Yes | Yes | Yes | No | Yes |
| Beltran-Corbellini | Yes | Yes | Yes | Yes | Yes | Yes |
| Brandstetter | Yes | Yes | Yes | Yes | No | Yes |
| Carigan | Yes | Yes | Yes | Yes | Yes | Yes |
| Chiesa-Estomba | Yes | Yes | No | Yes | No | No |
| Chiesa-Estomba 2 | Yes | No | No | Yes | No | Yes |
| D'Ascanio | Yes | Yes | Yes | Partly | Partly | Yes |
| Dawson | Yes | Yes | Yes | Yes | No | Yes |
| Dell'Era | Yes | Yes | No | Yes | Yes | Yes |
| Giacomelli | Yes | Yes | No | Yes | No | No |
| Gorzkowski | Yes | Yes | No | Partly | No | Yes |
| Guner | Yes | Yes | No | Partly | No | Yes |
| Haehner | Yes | Partly | Yes | Yes | No | No |
| Hintschich | Yes | Partly | Yes | Yes | No | Yes |
| Hornus | Yes | Yes | Yes | Partly | No | No |
| Izquierdo-Domínguez | Yes | Yes | Yes | Yes | Yes | Yes |
| Jalessi | Yes | Yes | No | Yes | Yes | Yes |
| Kai Chua | Yes | Yes | Yes | Yes | No | No |
| Kempker | Yes | Partly | Yes | Yes | No | No |
| Kim | Yes | Partly | No | Partly | No | No |
| Klopfenstein | Yes | Yes | No | Partly | No | Partly |
| Lechien (1) | Yes | Yes | No | Partly | Yes | Partly |
| Lechien (2) | Yes | Yes | Partly | Partly | Yes | Yes |
| Lechien (3) | Yes | Yes | No | Partly | Yes | Yes |
| Lechien (4) | Yes | Yes | No | Partly | Partly | Partly |
| Lee | Yes | No | Yes | Yes | Yes | Yes |
| Liang | Yes | Yes | No | Partly | No | Yes |
| Magnavita | Yes | Yes | Yes | Yes | No | Yes |
| Mao | Yes | Yes | No | Partly | Partly | Partly |
| Martin-Sanz | Yes | Yes | Yes | Yes | No | Yes |
| Mishra | Yes | Yes | No | Partly | No | No |
| Moein | Yes | Yes | Yes | Yes | Yes | Partly |
| Noh | Yes | Yes | No | Partly | No | Yes |
| Paderno (1) | Yes | No | No | Partly | Yes | Yes |
| Paderno (2) | Yes | Yes | No | Partly | Yes | Yes |
| Parente-Arias | Yes | Yes | No | Partly | No | Yes |
| Patel | Yes | No | No | Partly | Partly | No |
| Petrocelli | Yes | No | No | Partly | No | Yes |
| Qiu | Yes | Yes | No | Partly | Partly | Yes |
| Romero-Sanchez | Yes | Yes | No | Partly | Yes | No |
| Sakalli | Yes | Yes | No | Partly | Yes | Yes |
| Sayin | Yes | Partly | Yes | Yes | Yes | Yes |
| Tostmann | Yes | Yes | Yes | Yes | Partly | Partly |
| Tsivgoulis | Yes | Partly | Yes | Yes | Yes | No |
| Vaira (1) | Yes | Yes | No | Partly | No | Partly |
| Vaira (2) | Yes | Yes | No | Partly | No | Yes |
| Yan (1) | Yes | Yes | Yes | Yes | Yes | Yes |
| Yan (2) | Yes | Yes | No | Partly | Yes | Yes |
| Zayet | Yes | Yes | Yes | Yes | Yes | Yes |
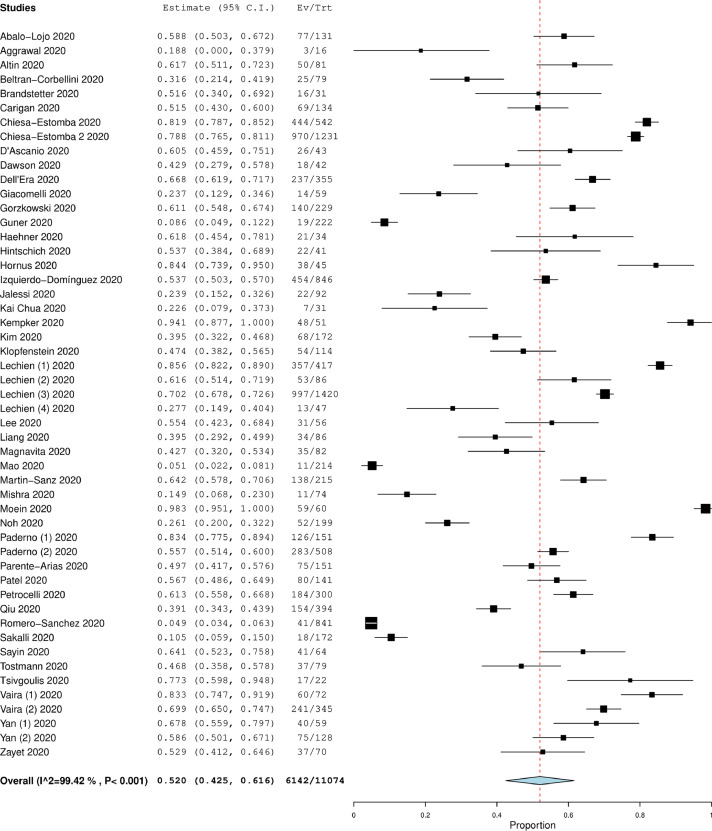
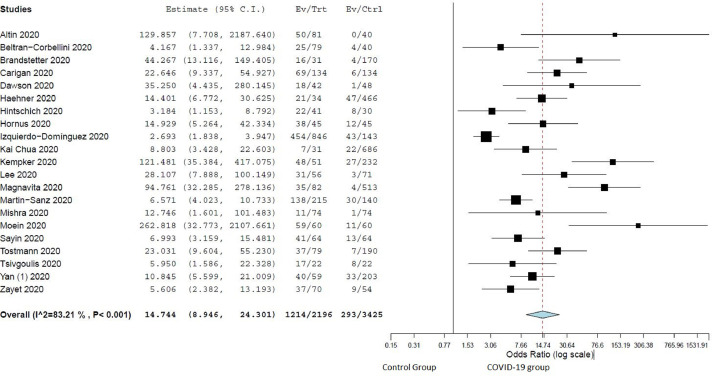
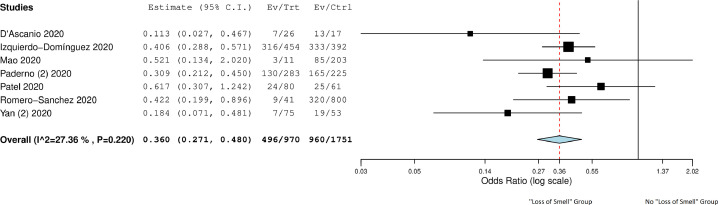
A total of 11074 COVID-19 patients (mean age 46.7 ± 10.4 years and males 46.9%) were included in the final analysis (Table 2).2 , 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 The overall prevalence of “loss of smell” in COVID-19 patients was 52.0% (CI: 42.5%-61.6%, I2 = 99.4%) (Fig. 2 ). A total of 21 studies compared these symptoms in COVID-19 patients (n = 2196) and controls (n = 3425). 13 , 14 , 18 , 19 , 21 , 25 , 27 , 28 , 34, 35, 36, 37 , 39 , 40 , 45 , 47, 48, 49 , 59 , 60 , 62 “Loss of smell” was associated significantly more in the COVID-19 group compared to non-COVID-19 group (OR: 14.7, CI: 8.9–24.3, p < 0.001, I2 = 83.2%) (Fig. 3 ). Among COVID-19 patients, the odds of patients with severe disease and “loss of smell” were significantly lower when compared to patients with severe disease and without “loss of smell” (OR: 0.36, CI 0.27–0.48, p < 0.01, I2 = 27.4% (Fig. 4 ).2 , 21 , 32 , 37 , 52 , 54 , 57
Table 2.
Study characteristics, baseline demographics and prevalence of “loss of smell” in COVID and control group (N: No. of patients)
| Study, year | Country | Center (single, dual, multi) | Study Period | Type of study | Total Patients, non COVID group, N | Total Patients, COVID group, N | Mean age, COVID group (years) | Male gender, COVID group (%) | “Loss of smell” in COVID group, N (%) | “Loss of smell” in non COVID group, N (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abalo-Lojo, 2020 | Spain | Single | – | Cohort | – | 131 | 50.4 | 56 (42.6%) | 77 (58.8%) | – |
| Aggrawal, 2020 | USA | Single | Mar 1-Apr 4 | Cohort | – | 16 | 65.5 | 12 (75.0%) | 3 (18.8%) | – |
| Altin, 2020 | Turkey | Dual | Mar 25-Apr 20 | Cohort | 40 | 81 | 54.2 | – | 50 (61.7%) | 0 (0%) |
| Beltran-Corbellini, 2020 | Spain | Dual | Mar 23-Mar 25 | Case-control | 40 | 79 | – | 48 (60.8%) | 25 (31.6%) | 4 (10.0%) |
| Brandstetter, 2020 | Germany | Single | – | Cohort | 170 | 31 | – | 30 (14.9%) | 16 (51.6%) | 4 (2.4%) |
| Carigan, 2020 | Canada | Single | Mar 10- Mar 23 | Case-control | 134 | 134 | 57.1 | – | 69 (51.5%) | 6 (4.5%) |
| Chiesa-Estomba (1), 2020 | South America (multiple countries) | Multi | – | Cross-sectional | – | 542 | 34 | 218 (40.2%) | 444 (819%) | – |
| Chiesa-Estomba (2), 2020 | Europe (multiple countries) | Multi | – | Cohort | – | 1231 | 41 | – | 970 (78.8%) | – |
| D'Ascanio, 2020 | Italy | Single | Febr 1-Apr 24 | Case-control | 25 | 43 | 58.1 | – | 26 (60.5%) | – |
| Dawson, 2020 | USA | Single | Mar-Apr | Cohort | 48 | 42 | – | 48 (53.3%) | 18 (42.9%) | 1 (2.1%) |
| Dell'Era, 2020 | Italy | Single | Mar 10- Mar 30 | Cross-sectional | – | 355 | 50 | 192 (54.1%) | 237 (66.8%) | – |
| Giacomelli, 2020 | Italy | Single | – | Cross-sectional | – | 59 | 60 | 40 (67.8%) | 14 (23.7%) | – |
| Gorzkowski, 2020 | France | Single | Mar 1- Mar 31 | Cross-sectional | – | 229 | 39.7 | 82 (35.8%) | 140 (61.1%) | – |
| Guner, 2020 | Turkey | Single | Mar 10-Apr 10 | Cohort | – | 222 | 50.6 | 132 (59.5%) | 19 (8.6%) | – |
| Haehner, 2020 | Germany | Single | – | Cross-sectional | 466 | 34 | – | 15 (44.1%) | 21 (61.7%) | 47 (10.1%) |
| Hintschich, 2020 | Germany | Single | – | Cohort | 30 | 41 | 37 | 13 (31.7%) | 22 (53.7%) | 8 (26.7%) |
| Hornus, 2020 | Germany | Single | – | Cross-sectional | 45 | 45 | 56 | – | 38 (84.4%) | 12 (26.7%) |
| Izquierdo-Domínguez, 2020 | Spain | Multi | Mar 21-Apr 18 | Cross-sectional | 143 | 846 | 56.8 | – | 454 (53.6%) | 43 (30.1%) |
| Jalessi, 2020 | Iran | Single | Feb-Mar | Cohort | – | 92 | 52.9 | 62 (67.4%) | 22 (23.9%) | – |
| Kai Chua, 2020 | Singapore | Single | Mar 23-Apr 4 | Cohort | 686 | 31 | – | – | 7 (22.6%) | 22 (3.2%) |
| Kempker, 2020 | USA | Single | – | Cohort | 232 | 51 | – | 10 (19.6%) | 48 (94.1%) | 27 (11.6%) |
| Kim, 2020 | Korea | Single | Mar 12-16 | Cross-sectional | – | 172 | 26 | 66 (38.4%) | 68 (39.5%) | – |
| Klopfenstein, 2020 | France | Single | March 1-Mar 17 | Cohort | – | 114 | – | – | 54 (47.4%) | – |
| Lechien (1), 2020 | 18 European hospitals | Multi | – | Cross-sectional | – | 417 | – | – | 357 (85.6%) | – |
| Lechien (2), 2020 | Belgium | Single | – | Cross-sectional | – | 86 | 41.7 | 30 (34.9) | 53 (61.6%) | – |
| Lechien (3), 2020 | 12 European hospitals | Multi | Mar 22-Apr 10 | Cross-sectional | – | 1420 | 39.2 | – | 997 (70.2%) | – |
| Lechien (4), 2020 | Belgium | Single | Mar 20-Apr 16 | Cross-sectional | – | 47 | 58.8 | 22 (46.8%) | 13 (27.6%) | – |
| Lee, 2020 | Canada | Single | Mar 16-Apr 15 | Cross-sectional | 71 | 56 | 38 | 23 (41.1%) | 31 (55.4%) | 3 (4.2%) |
| Liang, 2020 | China | Single | Mar 16-Apr 12 | Cohort | – | 86 | 25.5 | 44 (51.2%) | 34 (39.5%) | – |
| Magnavita, 2020 | Italy | Multi | Mar 27-Apr 30 | Cross-sectional | 513 | 82 | – | – | 35 (42.7%) | 4 (0.8%) |
| Mao, 2020 | China | Multi | Jan 16 -Feb 19 | Cohort | – | 214 | – | – | 11 (5.1%) | – |
| Martin-Sanz, 2020 | Spain | Single | Mar 1-Apr7 | Case-control | 140 | 215 | – | 44 (20.5%) | 138 (64.1%) | 30 (24.8%) |
| Mishra, 2020 | India | Single | – | Cross-sectional | 74 | 74 | – | 43 (58.1%) | 11 (14.8%) | 1 (1.4%) |
| Moein, 2020 | Iran | Single | March 21 - Apr 5 | Case-control | 60 | 60 | 46.6 | 40 (66.7%) | 59 (98.3%) | 11 (18.3%) |
| Noh, 2020 | Korea | Single | NR | Cohort | – | 199 | 38 | 69 (34.7%) | 52 (26.1%) | – |
| Paderno (1), 2020 | Italy | Single | Mar 27-Apr 1 | Cohort | – | 151 | 45 | 56 (37.1%) | 126 (83.4%) | – |
| Paderno (2), 2020 | Italy | Single | Mar 27-Apr 1 | Cross-sectional | – | 508 | 55 | 284 (55.9%) | 283 (55.7%) | – |
| Parente-Arias, 2020 | Spain | Single | Mar 3-Mar 24 | Cohort | – | 151 | – | 53 (35.1%) | 75 (49.7%) | – |
| Patel, 2020 | UK | Single | Mar 1-Apr 1 | Cross-sectional | – | 141 | 45.6 | 83 (58.8%) | 80 (56.7%) | – |
| Petrocelli, 2020 | Italy | Single | Apr 16-May 2 | Cohort | – | 300 | 43.6 | 75 (25.0%) | 184 (61.3%) | – |
| Qiu, 2020 | China, France, Germany | Multi | Mar 15-Apr 5 | Cohort | – | 394 | – | – | 154 (40.9%) | – |
| Romero-Sanchez, 2020 | Spain | Dual | Mar 1-Apr 1 | Cohort | – | 841 | 66.4 | 473 (56.2%) | 41 (64.1%) | – |
| Sakalli, 2020 | Turkey | Single | – | Cross-sectional | – | 172 | 37.8 | 84 (48.8%) | 18 (10.4%) | – |
| Sayin, 2020 | Turkey | Single | – | Cross-sectional | 64 | 64 | 37.8 | 25 (39.1%) | 41 (64.1%) | 13 (20.3%) |
| Tostmann, 2020 | Netherlands | Single | Mar 10 -Mar 29 | Cross-sectional | 190 | 79 | – | – | 37 (46.8%) | 7 (3.7%) |
| Tsivgoulis, 2020 | Greece | Single | Mar 19- Apr 8 | Case-control | 22 | 22 | 55 | 6 (54.5%) | 17 (77.3%) | 8 (36.4%) |
| Vaira (1), 2020 | Italy | Single | Mar 31 - Apr 6 | Cross-sectional | – | 72 | – | – | 60 (83.3%) | – |
| Vaira (2), 2020 | Italy | Mutli | – | Cohort | – | 345 | 48.5 | 146 (42.3%) | 241 (69.9%) | – |
| Yan (1), 2020 | USA | Single | Mar 3 -Mar 29 | Cross-sectional | 203 | 59 | – | 29 (49.2%) | 40 (67.8%) | 33 (16.3%) |
| Yan (2), 2020 | USA | Single | Mar 3 - Apr 8 | Cohort | – | 128 | – | – | 75 (59.6%) | – |
| Zayet, 2020 | France | Single | Feb 26-Mar 14 | Cohort | 54 | 70 | 50.4 | 29 (41.4%) | 37 (54.2%) | 9 (16.7%) |
Figure 2.
Forest plot demonstrating overall prevalence of “loss of smell” in COVID-19 patients.
Figure 3.
Forest plot comparing prevalence in COVID-19 vs control group for “loss of smell”.
Figure 4.
Forest plot comparing severe cases in COVID-19 group presenting with “loss of smell” to patients without “loss of smell”.
Discussion
We summarized the overall prevalence of “loss of smell” for COVID-19 patients and compared with control patients i.e. those without laboratory confirmation of COVID-19 from the same study period. The overall prevalence of “loss of smell” was significantly higher for the COVID-19 group compared to control group. In addition, “loss of smell” had a lower association with severe COVID-19 compared to COVID-19 patients without “loss of smell”.
Olfactory and gustatory changes are one of the most underreported symptoms in COVID-19 and can sometimes be only presenting symptoms in these patients.3 As demonstrated in our study, “loss of smell” was associated with somewhat favorable prognosis of the disease and hence careful screening should be undertaken to identify potential patients with COVID-19. These patients should undergo testing to rule out COVID-19. This will help in preventing the spread of the virus
We noted significant variations in the reporting of symptoms (i.e., dysosmia/anosmia/hyposmia/microsmia) in the studies. Mao et al. noted “loss of smell” in 5.1% of their cohort, while Moein et al. noted that roughly 98% of patients had “loss of smell”.2 , 18 Earlier studies such as by Mao et al. relied on the retrospective data collection and questionnaire based survey. As the olfactory symptoms became well-recognized, the newer studies might have assessed these patients specifically for these symptoms, resulting in a higher prevalence of olfactory symptoms. Further, only few studies objectively evaluated the “loss of smell” using validated tools.18 , 20 , 25 , 35 , 42 , 44 , 60 , 61 The objective methods used in literature to assess “loss of smell” included: “Sniffin Sticks test”, “The University of Pennsylvania Smell Identification Test (UPSIT)”, “Quick Smell Identification Test (Q-SIT)”, and “Connecticut Chemosensory Clinical Research Center Test (CCCRC test)”. We feel that the actual prevalence of olfactory symptoms could be much higher than what is reported as we have combined data from relatively older studies as well. Our results should be interpreted as such keeping in mind this important limitation.
Only 7 studies compared the disease severity in patients with “loss of smell” versus those without “loss of smell”. Although our results are limited due to the very small sample size, “loss of smell” was characterized by the less severe disease compared to those without this symptom. This finding is noteworthy and needs to be further explored in more extensive studies. The limitation of our analysis is the observational nature of the studies with significant variations in the reporting of symptoms and follow-up. A temporospatial association of the disease severity and the symptom was not possible. However, our study is novel as we performed a pooled analysis combining the statistical power and further compared and demonstrated the prevalence in the control group.
In conclusion, we demonstrate here that alteration in smell is prevalent in COVID-19 and should be included as one of the essential symptoms to screen the population. Further larger studies are urgently needed to evaluate the utility of olfactory dysfunction in patients with COVID-19, as demonstrated in our study. Therefore, alteration in the sense of smell should be added as a screening question to identify not only the symptomatic disease but also possible healthy (or presumed asymptomatic) carriers of the disease.
Author contributions
Conception and design: Muhammad Aziz, Hemant Goyal, Literature search: Wade M. Lee-Smith, First draft: Muhammad Aziz, Critical revision and editing: All authors, Final approval: All authors.
Footnotes
Conflict of Interest and Ethical statement: The authors have no commercial associations or sources of support that might pose a conflict of interest.
Source of funding: None.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjms.2020.09.017.
Appendix. SUPPLEMENTARY MATERIALS
Supplementary Figure 1: Funnel plot signifying visible asymmetry in studies evaluating “loss of smell” as outcome.
References
- 1.“WHO director-general's opening remarks at the media briefing on COVID-19 - 11 March 2020.” World Health Organization, World Health Organization, www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020. (Accessed on May 15, 2020).
- 2.Mao L, Jin H, Wang M, Hu Y. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz M, Perisetti A, Lee-Smith WM, Gajendran M. Taste Changes (Dysgeusia) in COVID-19: a systematic review and metaanalysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.003. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aziz M, Haghbin H, Lee-Smith W, Goyal H. Gastrointestinal predictors of severe COVID-19: systematic review and meta-analysis. Ann Gastroenterol. 2020 doi: 10.20524/aog.2020.0527. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58:1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 6.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aziz M, Fatima R, Assaly R. Elevated interleukin‐6 and severe COVID‐19: a meta‐analysis. J Med Virol. 2020 doi: 10.1002/jmv.25948. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24:255. doi: 10.1186/s13054-020-02995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 13.Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez-Jorge F. Acute-onset smell and taste disorders in the context of Covid-19: a pilot multicenter PCR-based case-control study. Eur J Neurol. 2020 doi: 10.1111/ene.14273. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson P, Rabold EM, Laws RL. Loss of taste and smell as distinguishing symptoms of COVID-19. Clin Infect Dis. 2020:ciaa799. doi: 10.1093/cid/ciaa799. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacomelli A, Pezzati L, Conti F, Bernacchia D. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY. Features of anosmia in COVID-19. Med Mal Infect. 2020;50:436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moein ST, Hashemian SMR, Mansourafshar B, Khorram-Tousi A. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tostmann A, Bradley J, Bousema T, Yiek W. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaira LA, Deiana G, Fois AG, Pirina P. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck. 2020;42:1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan CH, Faraji F, Prajapati DP, Boone CE. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10:806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan CH, Faraji F, Prajapati DP, Ostrander BT. Self-reported olfactory loss associates with outpatient clinical course in Covid-19. Int Forum Allergy Rhinol. 2020;10:821–831. doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abalo-Lojo JM, Pouso-Diz JM, Gonzalez F. Taste and smell dysfunction in COVID-19 patients. Ann Otol Rhinol Laryngol. 2020;129:1041–1042. doi: 10.1177/0003489420932617. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis (Berl) 2020;7:91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 25.Altin F, Cingi C, Uzun T, Bal C. Olfactory and gustatory abnormalities in COVID-19 cases. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06155-9. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dell'Era V, Farri F, Garzaro G, Gatto M. Smell and taste disorders during COVID-19 outbreak: cross-sectional study on 355 patients. Head Neck. 2020;42:1591–1596. doi: 10.1002/hed.26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandstetter S, Roth S, Harner S, Buntrock-Dopke H. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatr Allergy Immunol. 2020 doi: 10.1111/pai.13278. [article in press] [DOI] [PubMed] [Google Scholar]
- 28.Carignan A, Valiquette L, Grenier C, Musonera JB. Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. CMAJ. 2020;192:E702–E707. doi: 10.1503/cmaj.200869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiesa-Estomba CM, Lechien JR, Portillo-Mazal P, Martinez F. Olfactory and gustatory dysfunctions in COVID-19. first reports of Latin-American ethnic patients. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiesa-Estomba CM, Lechien JR, Radulesco T, Michel J. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur J Neurol. 2020 doi: 10.1111/ene.14440. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorzkowski V, Bevilacqua S, Charmillon A, Jankowski R. Evolution of olfactory disorders in COVID-19 patients. Laryngoscope. 2020 doi: 10.1002/lary.28957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Ascanio L, Pandolfini M, Cingolani C. Olfactory dysfunction in COVID-19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820943530. [article in press] [DOI] [PubMed] [Google Scholar]
- 33.Güner R, Hasanoğlu İ, Kayaaslan B, Aypak A. COVID-19 experience of the major pandemic response center in the capital: results of the pandemic's first month in Turkey. Turk J Med Sci. 2020 doi: 10.3906/sag-2006-164. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haehner A, Draf J, Dräger S, de With K. Predictive value of sudden olfactory loss in the diagnosis of COVID-19. ORL J Otorhinolaryngol Relat Spec. 2020;82:175–180. doi: 10.1159/000509143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hintschich CA, Wenzel JJ, Hummel T, Hankir MK. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22655. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornuss D, Lange B, Schröter N, Rieg S. Anosmia in COVID-19 patients. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.05.017. S1198-743X(20)30294-9. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izquierdo-Domínguez A, Rojas-Lechuga MJ, Chiesa-Estomba C, Calvo-Henriquez C. Smell and taste dysfunctions in COVID-19 are associated with younger age in ambulatory settings - a multicenter cross-sectional study. J Investig Allergol Clin Immunol. 2020 doi: 10.18176/jiaci.0595. [article in press] [DOI] [PubMed] [Google Scholar]
- 38.Jalessi M, Barati M, Rohani M, Amini E. Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19. Neurol Sci. 2020;41:2331–2338. doi: 10.1007/s10072-020-04590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kai Chua AJ, Yun Chan EC, Loh J, Charn TC. Acute olfactory loss is specific for Covid-19 at the Emergency Department. Ann Emerg Med. 2020 doi: 10.1016/j.annemergmed.2020.05.015. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kempker RR, Kempker JA, Peters M, Rebolledo PA. Loss of smell and taste among healthcare personnel screened for coronavirus 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa877. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim GU, Kim MJ, Ra SH, Lee J. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;26:948.e1–948.e3. doi: 10.1016/j.cmi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020;42:1583–1590. doi: 10.1002/hed.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lechien JR, Chiesa-Estomba CM, Place S, Laethem YV. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lechien JR, Ducarme M, Place S, Chiesa-Estomba C. Objective Olfactory Findings in Hospitalized Severe COVID-19 Patients. Pathogens. 2020;9:E627. doi: 10.3390/pathogens9080627. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DJ, Lockwood J, Das P, Wang R. Self-reported anosmia and dysgeusia as key symptoms of coronavirus disease 2019. CJEM. 2020 doi: 10.1017/cem.2020.420. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang Y, Xu J, Chu M, Mai J. Neurosensory dysfunction: a diagnostic marker of early COVID-19. Int J Infect Dis. 2020;98:347–352. doi: 10.1016/j.ijid.2020.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnavita N, Tripepi G, Di Prinzio RR. Symptoms in health care workers during the COVID-19 epidemic. a cross-sectional survey. Int J Environ Res Public Health. 2020;17:5218. doi: 10.3390/ijerph17145218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin-Sanz E, Riestra J, Yebra L, Larran A. Prospective study in 355 patients with suspected COVID-19 infection. value of cough, subjective hyposmia, and hypogeusia. Laryngoscope. 2020 doi: 10.1002/lary.28999. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mishra P, Gowda V, Dixit S, Kaushik M. Prevalence of new onset anosmia in COVID-19 patients: is the trend different between european and indian population? Indian J Otolaryngol Head Neck Surg. 2020 doi: 10.1007/s12070-020-01986-8. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noh JY, Yoon JG, Seong H. Asymptomatic infection and atypical manifestations of COVID-19: Comparison of viral shedding duration. J Infect. 2020 doi: 10.1016/j.jinf.2020.05.035. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paderno A, Mattavelli D, Rampinelli V, Grammatica A. Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820939538. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paderno A, Schreiber A, Grammatica A, Raffetti E. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 2020;10:955–962. doi: 10.1002/alr.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parente-Arias P, Barreira-Fernandez P, Quintana-Sanjuas A, Patiño-Castiñeira B. Recovery rate and factors associated with smell and taste disruption in patients with coronavirus disease 2019. Am J Otolaryngol. 2020 doi: 10.1016/j.amjoto.2020.102648. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel A, Charani E, Ariyanayagam D, Abdulaal A. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26:1236–1241. doi: 10.1016/j.cmi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrocelli M, Ruggiero F, Baietti AM, Pandolfi P. Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: the Bologna experience of 300 cases. J Laryngol Otol. 2020 doi: 10.1017/S0022215120001358. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu C, Cui C, Hautefort C, Haehner A. Olfactory and gustatory dysfunction as an early identifier of COVID-19 in adults and children: an international multicenter study. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820934376. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romero-Sanchez CM, Diaz-Maroto I, Fernandez-Diaz E, Sanchez-Larsen A. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020 doi: 10.1212/wnl.0000000000009937. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakalli E, Temirbekov D, Bayri E, Alis EE. Ear nose throat-related symptoms with a focus on loss of smell and/or taste in COVID-19 patients. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sayin İ, Yaşar KK, Yazici ZM. Taste and smell impairment in COVID-19: an AAO-HNS anosmia reporting tool-based comparative study. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820931820. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsivgoulis G, Fragkou PC, Delides A, Karofylakis E. Quantitative evaluation of olfactory dysfunction in hospitalized patients with Coronavirus (COVID-19) J Neurol. 2020;267:2193–2195. doi: 10.1007/s00415-020-09935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaira LA, Hopkins C, Salzano G, Petrocelli M. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42:1560–1569. doi: 10.1002/hed.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zayet S, Kadiane-Oussou NJ, Lepiller Q, Zahra H. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes Infect. 2020 doi: 10.1016/j.micinf.2020.05.016. [article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Funnel plot signifying visible asymmetry in studies evaluating “loss of smell” as outcome.