Abstract
Patients with the severe form of coronavirus disease 2019 (COVID-19) have been frequently found to suffer from both arterial and venous thrombotic events due to the perpetuation of a hypercoagulable state. This phenomenon, termed COVID-19–associated coagulopathy, is now considered a major component of the pathophysiology of this novel infectious disease, leading to widespread thrombosis. While at first, the vascular insults may be limited to the pulmonary microvasculature, as the disease progresses, systemic involvement occurs, culminating in distant organ thrombosis and multiorgan dysfunction syndrome. In this review article, we discuss recent insights into the pathophysiologic mechanisms of COVID-19–associated coagulopathy and review the clinical, histopathologic, and laboratory evidence, which leads us to conclude that COVID-19 is both a pulmonary and vascular disorder.
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; ARDS, acute respiratory distress syndrome; CC, coronavirus 2019–associated coagulopathy; COVID-19, coronavirus disease 2019; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ICU, intensive care unit; IL, interleukin; LY30, lysis at 30 minutes; NO, nitric oxide; PAI-1, plasminogen activator inhibitor 1; PE, pulmonary embolism; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TF, tissue factor; TMA, thrombotic microangiopathy; tPA, tissue plasminogen activator; VTE, venous thromboembolism; vWF, von Willebrand factor
Article Highlights.
-
•
Arterial and venous thrombotic events are frequent in patients with severe coronavirus disease 2019 (COVID-19).
-
•
The underlying trigger of COVID-19–associated coagulopathy encompasses immuno-thrombo-inflammation.
-
•
The coagulopathy initiates with vascular insults to pulmonary microvasculature.
-
•
As the disease progresses, the prothrombotic state becomes systemic, culminating in multiple organ thrombosis.
Coronavirus disease 2019 (COVID-19) is the third severe outbreak of a member of the Coronaviridae family that has occurred during the past 20 years, following the severe acute respiratory syndrome (SARS) in 2002–2003 and the Middle-East respiratory syndrome in 2012.1 Unlike the two previous outbreaks, the infection disease sustained by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has dramatically spread around the world, affecting millions of people, causing hundreds of thousands of deaths, and being declared a pandemic disease by the WHO.2
Although COVID-19 was originally classified as a primary respiratory disease due to frequent lung involvement, presenting as a severe form of interstitial pneumonia and with a high risk of progression towards acute respiratory distress syndrome (ARDS), the evidence gradually accumulating over recent months has led to a clearer clinical picture. SARS-CoV-2 infection should be defined as a multisystem disease, characterized by high mortality in specific subsets of patients, especially older males, and those with important associated comorbidities, such as hypertension, diabetes, obesity, and cancer, as well as pulmonary, cardiovascular, liver, neurological, and renal disorders.3 Based on recent data, COVID-19 is characterized by dysregulation of multiple biological pathways, mirrored by an abnormal immune response and an exaggerated pro-inflammatory state, which finally converge to trigger the development of a profound hemostasis disturbance,4 in the form of localized and systemic coagulopathies and thrombotic events (Table ) whose presence is directly associated with poor outcomes. This dramatic evolution has been termed COVID-19–associated coagulopathy (CC). COVID-19–associated coagulopathy appears to correlate with severity of illness, with those in the intensive care unit (ICU) suffering the most significant derangements. This narrative review aims to provide some recent updates on the clinical and histopathological laboratory evidence showing the relationship between COVID-19 and hemostasis abnormalities, as well as explore the potential pathogenic mechanisms of CC.
Table.
Most Frequent Thrombotic Events/Complications Observed in COVID-19 Patients
| Venous thromboembolism |
| Deep vein thrombosis |
| Pulmonary embolism |
| In situ pulmonary thrombosis |
| Arterial thromboembolism |
| Myocardial infarction |
| Ischemic stroke |
| Other systemic thromboembolism |
| Disseminated intravascular coagulation |
| Systemic arterial events |
Search Strategy and Selection Criteria
Data for this review were identified by electronic searches of PubMed, Scopus, and Web of Science and references from relevant articles using the following search terms: coronavirus disease 2019, COVID-19, SARS-CoV-2, hemostasis, coagulation, fibrinolysis, and thrombosis. Only articles published in English between 2019 and October 5, 2020, were included. Because systematic selection criteria cannot be applied to include articles which explored the pathogenesis of coagulopathies in COVID-19, we arbitrarily included those articles which provided the most relevant contributions to describing the clinical, histopathological, laboratory, and pathogenetic evidence underlying this relationship. A narrative review was found to be better suited to discuss our results.
Clinical Evidence
Patients hospitalized with pneumonia often present with risk factors for venous thromboembolism (VTE), such as acute respiratory illness, active infection, pro-inflammatory state, diminished mobility, advanced age (>65 years), cancer, obesity, pregnancy, congestive heart failure, or history of prior VTE.5 In addition to these risk factors, COVID-19 itself is associated with hypercoagulability, which predisposes to a pro-thrombotic state.6 Biochemical characteristics of disseminated intravascular coagulation (DIC) and pulmonary embolism (PE), such as increased values of D-dimer and fibrin degradation products, are rather prevalent among severe COVID-19 patients.7 Therefore, these patients are at increased risk of suffering from both venous and arterial thrombotic events.6
Venous Thromboembolism and Pulmonary Embolism
In a recent report, in the absence of VTE prophylaxis, 25% (20 of 81) patients with severe COVID-19 admitted to ICU developed lower extremity deep vein thrombosis (DVT) diagnosed by Doppler ultrasound.8 Klok et al9 also reported that 31% (95% CI, 20% to 41%), of 184 severe COVID-19 patients admitted to ICU suffered thrombotic complications, 27% of whom had VTE confirmed by ultrasonography and 3.7% of whom suffered arterial thrombotic events (all ischemic strokes). Klok et al10 conducted an updated analysis of the same 184 ICU patients, of whom 41 died (22%) and 78 were discharged alive (43%), the cumulative incidence of thrombotic events adjusted for competing risk of death was 49% (95% CI, 41% to 57%). Pulmonary embolism was the most common thrombotic event (65 of 75 patients; 87%), whereas patients with thrombotic events were at higher risk of all-cause death (hazard ratio, 5.4; 95% CI, 2.4 to 12). Incardi et al11 also reported an incidence of 12% (12 of 99 patients) for VTE and 3% (3 of 99 patients) for arterial thromboembolism in 99 consecutive patients hospitalized for COVID-19 pneumonia.
Massive PE is also associated with COVID-19.12 Up to 5% to 10% of COVID-19 patients requiring mechanical ventilation may develop PE and/or DVT. In the study performed by Klok et al,9 PE was the most frequent thrombotic complication among 184 severe COVID-19 patients admitted to the ICU (25 of 31 patients; 81%). In a retrospective study performed between March 1 and April 16, 2020, in 135 COVID-19 patients with pneumonia (47% outpatients and 53% hospitalized for a median period of 5 days), a total of 32 (24%) cases of PE were identified with computed tomography pulmonary angiogram (CTPA), 50% (95% CI, 30% to 70%) in ICU patients and 18% (95% CI, 12% to 27%) in other patients, respectively.13 Fifteen of 32 PE cases were diagnosed in outpatients, whereas the remaining 17 were diagnosed during hospitalization.
COVID-19 patients with PE are more frequently hospitalized in the ICU and/or under mechanical ventilation. In a retrospective study, which included 100 severe COVID-19 patients (66±13 years old; 70 men and 30 women) who underwent a contrast-enhanced computed tomography (CT) scan between March 15 to April 14, 2020, 23 patients had a PE (23%).14 Moreover, PEs were observed more frequently in the ICU and these patients required more frequent mechanical ventilation and had a longer delay from symptom onset to CT diagnosis. Helms et al15 also reported 64 thrombotic events among 150 COVID-19 patients (122 men; median age 63 years), 11 (16.7%) of which were PE, whereas Leonard-Lorant et al16 reported that 32 of 106 (30%) patients with COVID-19 infection had an acute PE diagnosed by CTPA. A case-series of COVID-19 patients with PE was recently reported by Poissy et al17 who observed 22 cases of PE among 107 consecutive confirmed COVID-19 patients (20.6%) studied between February 27 and March 31, 2020.
In another 1-month retrospective study, in which 328 COVID-19–positive patients underwent CTPA, 72 (22%) had a PE.18 The location of the PE was 37 (51%) segmental PE, 22 (31%) lobar PE, 9 (13%) central PE, and 4 (5.5%) subsegmental PE.18 No association was observed with age, sex, ethnicity, or history of cardiopulmonary disease (congestive heart failure or chronic obstructive pulmonary disease).18 Stoneham et al19 retrospectively analyzed the records of 274 inpatients with confirmed or possible COVID-19 infection from March 20, 2020, and April 9, 2020. A total of 21 (7.7%) patients were diagnosed with VTE, 16 (76.2%) with PE, and 5 (23.8%) with DVT.19 In 143 COVID-19 patients (aged 63±14 years; 51.7% men) hospitalized from January 29 to February 29, 2020, Zhang et al20 found that 66 patients developed lower extremity DVT (46.1%), 23 (34.8%) proximal DVT, and 43 (65.2%) distal DVT. Patients with DVT were older and had lower oxygenation index, a higher rate of cardiac injury, and worse prognosis compared with patients with no DVT.20 Nahum et al21 performed a venous ultrasonogram of the inferior limbs in 34 severe COVID-19 patients (age 62.2±8.6 years, 78% men) with pneumonia admitted to the ICU from mid-March to the beginning of April 2020. The investigators found DVT in 22 patients (65%) at admission and in 27 patients (79%) at 48 hours after ICU admission; 18 (53%) of these patients had bilateral thrombosis, whereas 9 (26%) had proximal thrombosis.21 Finally, Bilaloglu et al22 analyzed the incidence of venous and arterial thrombotic events in 3334 consecutive hospitalized COVID-19 patients at 4 hospitals in New York City. Any thrombotic events were identified in 533 (16.0%) patients, of which 207 (6.2%) were venous (3.2% PE and 3.9% DVT).22 These authors also observed that higher D-dimer levels at hospital presentation were associated with thrombotic events, as well as that all-cause mortality was higher in those COVID-19 patients with thrombotic events (43.2% vs 21.0%, P<.001).22
Stroke
Large-vessel stroke has also been reported in COVID-19. Five cases were described in COVID-19 patients younger than 50 years old hospitalized in New York City over a 2-week period from March 23 to April 7, 2020.23 Yaghi et al24 conducted a retrospective cohort study of consecutive COVID-19 patients hospitalized between March 15 and April 19, 2020, within a major health system in New York. During the study period, 32 of 3556 (0.9%) had imaging-proven ischemic stroke, whereas cryptogenic stroke was substantially more common among COVID-19 patients. Patients with COVID-19 also had higher admission National Institutes of Health Stroke Scale score, higher peak D-dimer levels, and higher mortality.24 Avula et al25 also reported four cases of ischemic stroke in COVID-19 patients confirmed by CT. Finally, Bilaloglu et al22 reported that 54 (1.6%) of all thrombotic events were ischemic stroke.
Disseminated Intravascular Coagulation
In the study from Tang et al26 on 183 COVID-19 patients, the authors reported that 15 of 21 (71%) nonsurviving COVID-19 patients fulfilled the International Society on Thrombosis and Haemostasis diagnostic criteria for DIC, whereas only 1 of 162 (0.6%) of survivors fulfilled DIC criteria. On a laboratory level, these patients presented with increased levels of D-dimer and fibrin degradation products, and prothrombin time prolongation. Although individuals may meet the diagnostic criteria for DIC, CC is a distinct entity from DIC, both in clinical presentation and laboratory findings. Whereas acute DIC can be generally characterized by profuse bleeding (although a thrombotic type can occur), CC is characterized primarily by thrombosis, with minimal bleeding complications described in the literature.
Acute Coronary Syndrome and Acute Myocardial Infarction
ST-segment elevation due to myocardial injury has been observed among COVID-19 patients at admission and/or during hospitalization. Myocardial interstitial edema has also been reported in these patients.27 Bangalore et al28 identified 18 COVID-19 patients (10 at admission and 8 during hospitalization) with ST-segment elevation on electrocardiography, which was potentially indicative of acute myocardial infarction. In these patients, there was a high prevalence of nonobstructive disease and poor prognosis, whereas all patients showed elevated D-dimer levels. On the other hand, only anecdotal cases of COVID-19 patients with acute coronary syndrome due to plaque-rupture have been described, although no cases have been published thus far.6
Histopathological Evidence
The evidence that COVID-19 is an intricate pathology with a strong thrombotic component has been shown in several histopathological investigations. Studies selected for further discussion in this article were chosen by presence of a sample size of more than two COVID-19 patients. The first case series of 12 autopsies on patients who died from COVID-19, published by Wichmann et al,29 found that massive PE originating from the deep veins of lower extremities was the cause of death in 4 of 12 patients (33%), whereas DVT could be diagnosed in 3 other patients (25%, 3 of 12). Altogether, VTE (ie, PE and/or DVT) could be identified in the majority of cases (58%). Along with a clear histopathological picture of ARDS, as attested by the presence of diffuse alveolar damage in all patients, microthrombi were regularly observed within the small lung arteries. In a second clinicopathologic case series published by Lax et al,30 thrombotic material, varying in extent from focal to extensively localized, could be detected in the pulmonary arteries of all autopsied patients (11 of 11; 100%), especially in the small and medium-size vessels, despite the administration of prophylactic anticoagulation. Infarction of lung tissue was also observed in all but one patient (91%). The organization of the thrombotic material, which filled the lumen of the vessels, was suggestive for in situ thrombosis rather than for embolization from peripheral vessels. With respect to other organs and tissues, intraventricular endocardial mural thrombi were found in one patient, and central liver vein thrombosis in another. In a further post-mortem investigation, Ackermann et al31 examined the lung tissue of seven patients who died from COVID-19 and compared their findings with those obtained in the lung tissue of seven other patients who died of ARDS caused by influenza A/H1N1 and 10 age-matched uninfected controls.31 Widespread thrombosis and microangiopathy could be found in pulmonary vessels of all COVID-19 patients. The thrombi had a diameter between 1 and 2 mm and did not fill the lumen of the pulmonary arteries involved. Alveolar capillary thrombosis was common and was found to be nine-fold more prevalent in COVID-19 patients than in those with influenza A/H1N1. In a case series of 10 autopsies of patients who died from COVID-19 published by Nunes et al,32 the authors found cytopathic effects attributable to SARS-CoV-2 in many organs and tissues, with clear signs of thromboembolic involvement frequently observed. Microthrombi could be found in the pulmonary arteries of 8 of 10 (80%) autopsied patients, accompanied by the evidence of fibrin thrombi in the vessels of testis (2 of 2; 100%), kidney (6 of 8; 75%), skin (3 of 10; 30%), heart (2 of 10; 20%), and spleen (1 of 5; 20%). Ischemic necrosis could also be seen in the liver of 3 of 10 (30%) patients. Fox et al33 performed autopsies on 10 African Americans (aged 44 to 78 years) who died as a consequence of SARS-CoV-2 infection in New Orleans. The authors found thrombosis and microangiopathy in the small vessels and capillaries of the lungs, with associated hemorrhage, as well as the typical characteristics of diffuse alveolar damage with presence of hyaline membranes.33 Finally, Nadkarni et al34 reported thromboembolic disease not clinically suspected in 11 of 26 autopsies (42%), whereas 3 (3 of 11; 27%) were from COVID-19 patients with early pre-mortem therapeutic anticoagulant therapy.
Laboratory Evidence
Laboratory findings, even early in the disease course, strongly suggest the presence of coagulopathy consistent with clinical outcomes. A recent meta-analysis of laboratory values measured at admission (or earliest time point in hospitalization), inclusive of 21 studies with 3377 patients, found lower platelet counts, mildly increased prothrombin time, and increased D-dimers in COVID-19 patients who progress to severe or fatal disease.35 These three findings can be considered the typical features of early CC.36
Interestingly, significant variability has been reported among studies concerning coagulation parameters in patients with COVID-19. This may reflect differing disease status/severity at the time of measurement, comorbidities, genetics, or environmental factors. Although variability has been reported with respect to platelet counts and D-dimers in patients with COVID-19, with many patients reported within the normal ranges, unsurprisingly, thrombocytopenia and high D-dimers are associated with poor outcomes.37 , 38
Although some characteristics of CC are similar to DIC, such as increased D-dimer and low platelet counts, distinctions between these pathologies should be made. First, the thrombocytopenia and increase in D-dimer does not reach the severity as observed in DIC, such as in patients with sepsis.36 Second, fibrinogen is increased in patients with CC, which contradicts the presence of a consumption coagulopathy like DIC.39 As noted earlier, increases in proteins such as fibrinogen and von Willebrand factor (vWF) are likely reflective of their status as acute phase reactants, driven by the high levels of interleukin (IL)–6 observed in patients with severe COVID-19.39
In addition to DIC, CC also shares some characteristics with thrombotic microangiopathy, including high lactate dehydrogenase and high ferritin serum values35 , 39 , 40; lactate dehydrogenase has been found to be associated with increased odds of progression to severe or fatal COVID-19.40 Moreover, complement-associated microvascular injury has been reported in patients with COVID-19, characterized by terminal complement components C5b-9 (membrane attack complex), C4d, and mannose-binding lectin–associated serine protease (MASP)2 deposition in the pulmonary microvasculature.41 Further studies measuring vWF antigen and activity, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) activity, and complement studies are needed to further elucidate the underlying mechanisms of CC and COVID-19–induced microvascular thrombosis. Significant elevations in vWF have been reported in patients with COVD-19.42 , 43 It may be suspected that high levels of vWF, because of its role as an acute phase reactant, may lead to a secondary ADAMTS13 deficiency.36 As ADAMTS13 cleaves ultra-large vWF multimers, the gradual depletion of this enzyme may lead to enhanced platelet-endothelial interaction propagating in a thrombotic microangiopathy (TMA)–like phenomenon.36 , 44 In a cohort of 88 patients, Bazzan et al45 observed low ADAMTS13 in patients with COVID-19, which was significantly lower in patients who died. The clinical picture observed by Bazzan et al45 is suggestive of a TMA-like phenomenon, as ADAMTS13 is not usually reduced in DIC. These findings were further confirmed by Martinelli et al46 who also reported a relative deficiency of ADAMTS13 in COVID-19 patients, as well as Tiscia et al47 who reported that reduced ADAMTS13 level predicts mortality in patients infected with SARS-CoV-2. Finally, in a small cohort of 12 patients, Huisman et al43 reported marked elevations in vWF:Ag:ADAMTS13 ratio (mean, 8.5 (SD: 6.7); reference range, 0.5 to 2.0).
Newer evidence has also emerged showing significant impairment of fibrinolysis in patients with severe COVID-19. Wright et al,48 using thromboelastography in a cohort of 44 critically ill COVID-19 patients, showed that 57% (25 of 44) of patients had complete lack of clot lysis at 30 minutes (LY30). Moreover, they observed that patients with a high D-dimer and low LY30 had a VTE rate of 50% versus 0% in patients with neither factor, and a need for renal replacement therapy rate of 80% versus 14%.48 Interestingly, the LY30 was accompanied by a high D-dimer, suggestive of some activation of endogenous fibrinolysis prior to inhibition. Henry et al49 reported significantly lower levels of plasminogen in patients developing critical COVID-19, suggestive of a consumptive phenomenon. Providing insight into a potential mechanism, Nougier et al50 demonstrated a significant imbalance between inhibitors (plasminogen activator inhibitor 1 [PAI-1]) and activators (tissue plasminogen activator, [tPA]) of fibrinolysis. They observed significantly elevated levels of PAI-1 and low levels of tPA, along with concomitantly high thrombin generation.50
In summary, the current laboratory evidence suggests that COVID-19 induces CC, which can be compared with a low-grade DIC, as well as microvascular immunothrombosis similar to TMA.36 , 44 Although this may be localized to the pulmonary microvasculature at first, as the infection progresses, systemic vasculature involvement occurs, complicated further by inhibition of fibrinolysis, and culminating in multiorgan dysfunction syndrome.
Pathogenetic Mechanisms
The previously described clinical, histopathological, and laboratory evidence shows that COVID-19 is a pathology often complicated by thrombotic events, localized and systemic, macro- and/or micro-vascular. A clear understanding of the underlying pathogenetic mechanisms contributing to trigger and/or amplify thrombosis in COVID-19 represents a crucial aspect in the managed care of this illness, which will pave the way to establishing specific therapeutic options, tailored to target the affected hemostasis pathways.
Hemostasis can be basically divided into three major stages. Primary hemostasis, which involves blood vessels and platelets, aims to generate a temporary and somewhat unstable blood clot, which attempts to stop bleeding after an endothelial injury has occurred. Secondary hemostasis, which develops immediately afterwards, encompasses the sequential activation of many clotting factors, leading to the generation of sufficient fibrin to stabilize the initial platelet plug. Fibrinolysis involves a series of events with the purpose to dissolve the blood clot and restore normal flow within the blood vessel. Notably, all these three essential hemostasis phases seem variably deranged in COVID-19, all characterized by onset of many significant prothrombotic abnormalities that will be summarized below, and which can be referred to as “immunothrombosis” or “thromboinflammation.”
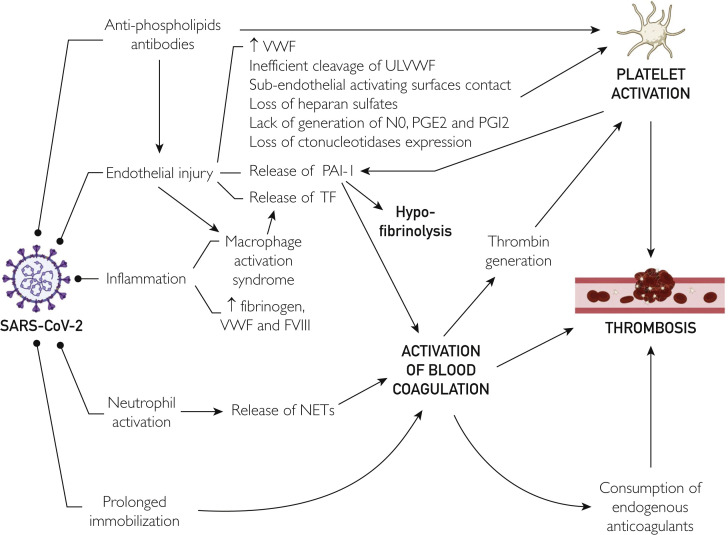
The triggering factors of COVID-19–associated immuno-thrombo-inflammation foster platelet hyperreactivity, hypercoagulability, and hypofibrinolysis, which seem to coexist in SARS-CoV-2 infection, thus contributing to define the portrait of a perfect storm (Figure ).51 Notably, most of these causal factors of SARS-CoV-2–induced immuno-thrombo-inflammation are also hallmarks of ARDS.52 Thus, it is not surprising that they would also be deeply involved in the pathogenesis of the pulmonary intravascular coagulopathy seen in COVID-19.53 Understandably, as the inflammation amplifies and propagates outside the lung tissue as a consequence of SARS-CoV-2 colonization of other organs where angiotensin-converting enzyme 2 (ACE2) is strongly expressed, such as in the heart, kidneys, intestine, liver, testis, adipose tissue, and central nervous system,54 the coagulopathy also progresses systematically, with development of distant organ thrombosis, up to the development of DIC, which may occur in some patients with late-stage COVID-19.26
Figure.
Pathogenesis of thrombosis in coronavirus disease 2019. FVIII, factor VIII; NET, neutrophil extracellular trap; NO, nitric oxide; PAI-1, plasminogen activator inhibitor 1; PGE2, prostaglandin E2; PGI2, prostaglandin I2; SARS-CoV-2, severe acute respiratory syndrome coronavirus; TF, tissue factor; ULVWF, ultra-large von Willebrand factor; VWF, von Willebrand factor.
Derangement of Primary Hemostasis
Endothelial injury, along with the ensuing disruption of blood vessel integrity, is the main trigger of primary hemostasis, encompassing a series of sequential events characterized by platelet activation, aggregation, and adhesion, culminating in the generation of a primary platelet plug, as previously underlined. Several lines of evidence now concur to confirm that endothelial injury and dysfunction are commonplace in patients with COVID-19. First, the SARS-CoV-2 receptor ACE2 is physiologically expressed at the surface of arterial and venous endothelial cells and arterial smooth muscle cells,55 and a cytopathic effect consequent to direct viral infection of these cells is likely. This has been recently confirmed in an interesting study from Varga et al56 which shows the presence of viral particles within endothelial cells, followed by onset of endotheliitis, cellular degeneration, and necrosis. This process has been shown to occur in the lungs as the primary site of viral infection, but may later spread and involve the blood vessels of many other organs and tissues.
The endothelial injury is then rapidly followed by a series of events leading to platelet activation, adhesion to the sub-endothelial matrix and aggregation, with the final generation of a platelet plug.57 These events include production and acute release of vWF, inefficient cleavage of ultra-large vWF catalyzed by ADAMTS13, direct contact with activating surfaces in the sub-endothelial matrix, loss of heparan sulfates at the surface of injured blood vessels, lack of generation of nitric oxide (NO), prostaglandin E2, and prostacyclin (also known as prostaglandin I2), and loss of surface expression of ectonucleotidases.58 , 59 Platelet activation may also occur as a consequence of the generation of a considerable amount of thrombin after activation of blood coagulation, as will be discussed in detail in the following section. Indirect evidence that platelet hyperactivation may play a substantial role in the pathogenesis of SARS-CoV-2 coagulopathy emerges from the study of Viecca et al,60 who showed that the administration of acetylsalicylic acid (250 mg infusion, followed by 75 mg daily for 1 month) and oral clopidogrel (300 mg initially, followed by 75 mg daily for 1 month) was effective in improving the ventilation/perfusion ratio in COVID-19 patients with severe respiratory failure. However, this investigation was performed as a retrospective case-control study, which may suffer from considerable bias; thus, the results must be interpreted with caution.
The important role played by the platelets in the pathogenesis of COVID-19 coagulopathies has hence been shown by several other independent studies. Roncati et al61 showed with a postmortem biopsy report that naked megakaryocyte nuclei within lungs and bone marrow of COVID-19 patients with severe illness are increased by more than 10-fold. This phenomenon has been attributed to excess IL-6 stimulation of megakaryocytopoiesis and platelet production, which would then contribute to generate a hypercoagulability state, especially within the lung tissue, thus increasing the likelihood of developing immunothrombosis. Convincing evidence of a direct interaction between platelets and SARS-CoV-2 has been provided by Zhang et al,62 who showed that human platelets express both ACE2 and transmembrane serine protease 2 on their surface, so that the virus, through its spike protein, can directly stimulate platelets, triggering the release of clotting factors, inflammatory mediators, and generation of leukocyte-platelet aggregates. Further evidence that severity of COVID-19 depends on platelet activation has been provided in the study of Hottz et al.63 Briefly, the authors found a higher degree of platelet hyper-activation in COVID-19 patients with severe illness compared with those with milder symptoms. Not only were platelets hyperactivated in severe COVID-19, but platelet-monocyte aggregates were also found to be considerably increased in COVID-19 patients compared with healthy controls, and were even higher in patients with severe illness compared with those with milder disease. Finally, monocyte expression of tissue factor, which is the initiator of the blood coagulation cascade, was found to be hyperexpressed in COVID-19 patients with severe illness. Evidence that platelets are hyperactivated in COVID-19 patients and show a considerable remarkable predisposition to generate leukocyte aggregates has been confirmed by Manne et al.64 In this original investigation, both P-selectin expression and the number of platelet-neutrophil and platelet-monocyte aggregates were found to be significantly enhanced in patients with SARS-CoV-2 infection. Moreover, platelet aggregation in response to adenosine diphosphate, thrombin, and collagen was found to be substantially higher in COVID-19 patients compared with controls. Overlapping evidence has been reported by Zaid et al65 showing that SARS-CoV-2 was able to bind to platelet surface, that the platelet content of platelet factor 4 and serotonin were significantly reduced in COVID-19 patients, especially in those with severe illness, and that the relative concentration of these two molecules was consequently higher in these patients’ plasma. Importantly, platelet aggregation and adhesion were also enhanced in patients with COVID-19, especially in those with severe illness, thus confirming that platelets are much more predisposed to clotting in this condition. It has been finally shown that agonist-stimulated expression of active fibrinogen receptor on platelet surface was reduced by more than 50% in patients with SARS-CoV-2 infection, while a vast array of cytokines, chemokines, growth factors and even procoagulant factors (especially fibrinogen and vWF) are released in large amounts after stimulating platelets collected from COVID-19 patients.66
Platelet activation with ensuing generation and release into the bloodstream of a vast array of cytokines and inflammatory mediators would hence further contribute to worsening the endothelial injury, both directly (eg, further decreasing NO availability and releasing reactive oxygen species) and/or indirectly (eg, enhancing leukocyte-endothelial interaction, promoting the migration of inflammatory cells).67
Derangement of Secondary Hemostasis
The activation of blood coagulation is a second essential aspect for effective prevention of bleeding following vessel injury. Unlike older theories, it has now been clearly elucidated that physiological hemostasis originates mainly with the exposure of tissue factor (TF) due to and resulting in vascular disintegration (eg, the so-called extrinsic pathway), followed by downstream activation of the coagulation cascade, which involves a series of sequential catalytic reactions finalized to generate a sufficient amount of fibrin for strengthening and stabilizing platelet plugs.68 , 69 Notably, the role of the so-called intrinsic pathway in activation of secondary hemostasis has been considerably resized during the past decades, whereby the presence of factor (F) XII appears unnecessary for physiologic activation of blood coagulation. However, its capacity to activate FXI is retained in some prothrombotic conditions, such as atherosclerosis and severe infections.70
Endothelial injury and/or dysfunction appear to be the main driver in the COVID-19–dependent activation of blood coagulation. The widespread damage of endothelia, as previously described, is likely associated with consistent release of TF, both in the pulmonary circuit, as well as in the blood vessels of other organs and tissues, which would hence contribute to activating secondary hemostasis. Substantial exposure and release of TF can also occur from cells of the macrophage/monocyte lineage and in microvesicles directly shed by these cells,71 which may be highly activated in COVID-19, as noted by the occurrence of macrophage activation syndrome that is frequently observed in patients with severe or critical forms of COVID-19,72 as well as in other life-threatening viral diseases such as Ebola.73 Macrophage activation can occur because of direct interaction with SARS-CoV-2. Viral particles have been detected within these cells, either penetrating the cell directly or being opsonized through the Fc receptor, where they likely exert both an activating and cytotoxic effect.74 On the other hand, extensive macrophage/monocyte activation can also occur as a consequence of an exaggerated pro-inflammatory reaction (also known as “cytokine storm”), which is common in the severe/critical forms of COVID-19 and is characterized by extremely high values of IL-6, IL-8, IL-12, transforming growth factor-β, interferon-γ, CCL2, and C-X-C motif chemokine 9 and 10.75
Neutrophil activation is another essential mechanism underlying the common observation of a prothrombotic state in patients with COVID-19. Neutrophils can be colonized by SARS-CoV-2 by internalization through the Fc receptor or can be activated by endothelial cells, platelets, and monocytes/macrophages, and are then capable of producing neutrophil extracellular traps (NETs), which can directly activate FXII and thereby the intrinsic pathway of blood coagulation.76 In addition, complement may also interact with the platelet/NET/thrombin axis.77 In effect, increased plasma levels of NETs, TF activity, and sC5b-9 has been detected in COVID-19 patients, while thrombin or NETosis inhibition or C5aR1 blockade attenuated thrombogenicity.77
Importantly, the severe pro-inflammatory condition is then associated with a remarkable associated increase in the circulating levels of many acute-phase proteins, including fibrinogen, vWF, and FVIII,78 , 79 and which may hence contribute to amplify the thrombotic process. Evidence has also been provided that the ongoing thrombotic process would contribute to sustain or even amplify the prothrombotic state, as mirrored by a progressive decline in the activity of the major endogenous anticoagulants such as antithrombin, tissue factor pathway inhibitor, and anticoagulation proteins C and S.75 In fact, Lippi et al80 recently found in a meta-analysis that low antithrombin levels were significantly associated with COVID-19 severity.
Prolonged immobilization and venous stasis, as consequences of the long stay in subintensive units and ICUs of COVID-19 patients with respiratory failure and/or multiple organ dysfunctions, are likely additional contributing factors of thrombosis in COVID-19.81
Derangement of Fibrinolysis
As discussed earlier, significant evidence has emerged as of late to support a major derangement of fibrinolysis in COVID-19. Plasminogen activator inhibitor 1, the major inhibitor of the fibrinolytic pathway, is largely contained in endothelial cells, megakaryocytes, and circulating platelets.82 It is thus reasonable to suspect that the endothelial injury and dysfunction that develops in the advanced stages of COVID-19 would be associated with enhanced endothelial release of PAI-1, as well as release from platelets following activation.83 This concept is supported by evidence of increased PAI-1 activity that is common in patients with ARDS, and which cumulatively contributes to a declining clinical status by inhibiting fibrinolysis, thus worsening the thrombotic burden.84 , 85 As noted above, PAI-1 levels have been reported to be elevated in patients with severe COVID-19.50 On the contrary, elevated levels of bradykinin likely occur due to the inflammatory response to the virus, which may in turn induce the release of tPA from endothelium.44 However, this release appears insignificant in comparison to the elevation of PAI-1 and consumption by endogenous fibrinolysis, as noted by the significantly lower levels of tPA that were observed in a patient developing severe COVID-19.50
Based on the data reported to date, we proffer that elevated D-dimer early in the COVID-19 disease course is reflective of pulmonary inflammation with local activation of platelets and blood coagulation.44 Initially, there is a sufficient balance of tPA/PAI-1 allowing for adequate activation of fibrinolysis. However, as the disease progresses, there is consumption of plasminogen, as observed by the low plasma values in severe COVID-19 as reported by Henry et al,49 along with inflammation driven elevations of PAI-1 and depletion of tPA, which leads to a state of hypofibrinolysis (as observed by thromboelastography), allowing perpetuation of pulmonary and systemic thrombi. This hypofibrinolysis is accompanied by a marked decrease in D-dimers over 24 hours in patients with low LY30 as opposed to mild increase in patients with normal LY30 noted by Wright et al.48
Development of Antiphospholipid Antibodies
As in other infections, the emergence of antiphospholipid antibodies has been reported in patients with COVID-19,86, 87, 88 which may then contribute to trigger and/or amplify the coagulopathy. However, a persistent presence of these antibodies has not been observed, and many severe infections are noted to be associated with a temporary appearance and disappearance of such antibodies.44
Although the exact pathogenetic mechanisms remain uncertain, it has been shown that these antibodies may activate endothelial cells, monocytes, and platelets, as well as directly interfere with some proteins of the coagulation pathways.89 More specifically, antiphospholipid antibodies may directly trigger endothelial cell activation90 and the further development of a pro-inflammatory and procoagulant endothelial phenotype,91 as well as upregulation and enhanced expression of TF in monocytes.92 These specific antibodies bind to the platelets and contribute to triggering platelet hyperreactivity.93 The effects of antiphospholipid antibodies on blood coagulation include inhibition of natural inhibitors such as antithrombin and activated protein C94, and hyperactivation of some clotting factors such as thrombin and FXa.95 , 96 Elevated C-reactive protein values may interfere in lupus anticoagulant (LAC) determination, causing transient LAC positivity.97 , 98 Therefore, LAC results should be carefully interpreted in patients with high C-reactive protein levels.
Derangement of the Renin-Angiotensin-Aldosterone System
The renin-angiotensin-aldosterone system has been a focus of pathophysiologic interest since ACE2 has been identified as the host receptor for SARS-CoV-2. Angiotensin-converting enzyme 2 metabolizes angiotensin II (Ang II) into angiotensin 1,7 (Ang 1,7), which opposes the vasoconstrictive and pro-inflammatory properties of Ang II.99 It has been hypothesized that the binding of the virus to ACE2 attenuates the activity of the enzyme, resulting in a state of high Ang II and low Ang 1,7.99 Such a derangement, in theory, would lead to alterations fostering a hypercoagulable state. High Ang II would lead to increased PAI-1 and TF expression, further promoting hypercoagulability and impairing fibrinolysis, as well as further inflammation and vasoconstriction, thus exacerbating an underlying endothelial dysfunction.44 Moreover, Ang II receptors present on platelets potentiates platelet aggregation and activation.100 However, variability has been reported with respect to Ang II levels in patients with COVID-19. Whereas Liu et al101 reported drastically elevated Ang II levels in a small cohort of 12 COVID-19 patients from China, Henry et al,102 in a cohort of 30 patients not taking a direct renin-angiotensin-aldosterone system–modifying drug, reported normal physiologic levels of Ang II and aldosterone in patients with COVID-19, that did not increase with disease severity, nor was different from levels measured in healthy controls.102 On the contrary, Henry et al103 observed significantly low levels of Ang 1,7 as compared with healthy controls. Moreover, Ang 1,7 was found to be significantly lower in those who progressed to severe disease.103
Physiologically, Ang 1,7 possesses multiple properties that may be important to the maintenance of normal hemostasis.44 , 104 Within microcirculation, Ang 1,7 exerts a vasoprotective effect through via NO-mediated vasodilation by endothelial cells and antithrombotic effects via NO-mediated release from platelets, which inhibits platelet aggregation and activation.104, 105, 106 Thus, low Ang 1,7 in patients with COVID-19 may likely contribute to trigger a coagulopathy. Multiple clinical trials with angiotensin II receptor blockers in COVID-19 are ongoing, and trials using Ang 1,7 peptide have recently begun.
Finally, obese patients have worse outcomes with COVID-19, including respiratory failure, need for mechanical ventilation, and higher mortality.107, 108, 109 Obesity and overweight are associated with an increased risk of developing VTE.110, 111, 112 Hypercoagulability has been reported in overweight patients, increasing with the severity of obesity,113 mainly due to mechanisms such as action of adipocytokines, coagulation factors hyperactivity, hypofunctional fibrinolysis, increased inflammation, Ang II/Ang 1,7 imbalance, increased oxidative stress and endothelial dysfunction, lipid and glucose tolerance disorders together with metabolic syndrome, and venous stasis and impaired venous return.114, 115, 116 Thus, obesity may have additive effects in the hypercoagulability status and thrombosis observed in certain COVID-19 patients.
Residual Questions
Vast questions remain on COVID-19–associated coagulopathy. First and foremost, we must investigate what mechanisms are driving this prothrombotic phenomenon. Importantly, we must evaluate whether the coagulopathy is driving the underlying pathophysiology of SARS-CoV-2 or if this coagulopathy is a result of secondary factors during the infection. Second, we must identify targets for pharmacologic therapy, determine appropriate anticoagulation, antiplatelet, and antifibrinolytic regimens, and discern the ideal timing for initiation of such therapies. Finally, we must work to risk stratify patients at initial presentation for individual risk for development of severe COVID-19 and thromboembolism, to enable early intervention and careful monitoring. To likely achieve improved outcomes in COVID-19, a personalized therapeutic approach is likely needed for each individual patient based on one’s personal risk of progressing towards severe illness and their current biological and metabolic derangements. Pooling data obtained from studies that used different methods and measurement units is an objective challenge. Better harmonization of both analytical and postanalytical (eg, measurement units) variables shall be considered a research priority.
Conclusion
It is now clear that the outcome of COVID-19 depends on the severity of both pulmonary and circulatory involvement, thus encompassing alveolar damage and local (ie, lung) and systemic thrombosis. The current evidence supports the development of a thrombotic process in COVID-19, which can be defined as immuno-thrombo-inflammation. This is likely the consequence of derangement of multiple biological pathways, including endothelial injury, macrophage/monocyte and neutrophil activation, exacerbated by prolonged immobilization, and development of antiphospholipid antibodies.
Footnotes
Potential Competing Interests: The authors report no potential competing interests.
Supplemental Online Material
References
- 1.Lippi G., Plebani M. The novel coronavirus (2019-nCoV) outbreak: think the unthinkable and be prepared to face the challenge. Diagnosis (Berl) 2020;7(2):79–81. doi: 10.1515/dx-2020-0015. [DOI] [PubMed] [Google Scholar]
- 2.Mahase E. COVID-19: WHO declares pandemic because of "alarming levels" of spread, severity, and inaction. BMJ. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 3.Lauretani F., Ravazzoni G., Roberti M.F. Assessment and treatment of older individuals with COVID 19 multi-system disease: clinical and ethical implications. Acta Biomed. 2020;91(2):150–168. doi: 10.23750/abm.v91i2.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes G.D., Burnett A., Allen A. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzik T.J., Mohiddin S.A., Dimarco A. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klok F.A., Kruip M., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inciardi R.M., Adamo M., Lupi L. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bompard F., Monnier H., Saab I. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56(1):2001365. doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296(3):E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard-Lorant I., Delabranche X., Severac F. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to d-Dimer levels. Radiology. 2020;296(3):E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poissy J., Goutay J., Caplan M. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 18.Poyiadji N., Cormier P., Patel P.Y. Acute pulmonary embolism and COVID-19. Radiology. 2020;297(3):E335–E338. doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoneham S.M., Milne K.M., Nuttal E. Thrombotic risk in COVID-19: a case series and case-control study. Clin Med (Lond) 2020;20(4):e76–e81. doi: 10.7861/clinmed.2020-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., Feng X., Zhang D. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 21.Nahum J., Morichau-Beauchant T., Daviaud F. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3(5):e2010478. doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oxley T.J., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaghi S., Ishida K., Torres J. SARS2-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avula A., Nalleballe K., Narula N. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with COVID-19 — a case series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wichmann D., Sperhake J.P., Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lax S.F., Skok K., Zechner P. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunes Duarte-Neto A., de Almeida Monteiro R.A., da Silva L.F.F. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77(2):186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadkarni G.N., Lala A., Bagiella E. Anticoagulation, bleeding, mortality, and pathology among patients with COVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 36.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120(5):876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in intensive care unit. a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 38(9):1722-1726. [DOI] [PMC free article] [PubMed]
- 41.Magro C., Mulvey J.J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ladikou E.E., Sivaloganathan H., Milne K.M. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin Med (Lond) 2020;20(5):e178–e182. doi: 10.7861/clinmed.2020-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huisman A., Beun R., Sikma M., Westerink J., Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42(5):e211–e212. doi: 10.1111/ijlh.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henry B.M., Vikse J., Benoit S., Favaloro E.J., Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bazzan M., Montaruli B., Sciascia S., Cosseddu D., Norbiato C., Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern Emerg Med. 2020;15(5):861–863. doi: 10.1007/s11739-020-02394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinelli N., Montagnana M., Pizzolo F. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb Res. 2020;193:170–172. doi: 10.1016/j.thromres.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiscia G.L., Favuzzi G., De Laurenzo A. Reduction of ADAMTS13 levels predicts mortality in SARS-CoV-2 patients. TH Open. 2020;4(3):e203–e206. doi: 10.1055/s-0040-1716379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright F.L., Vogler T.O., Moore E.E. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231(2):193–203.e1. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry B.M., Benoit S., Hoehn J., Lippi G., Favaloro E.J., Benoit J.L. Circulating plasminogen concentration at admission in patients with coronavirus disease 2019 (COVID-19) Semin Thromb Hemost. 2020;46(7):859–862. doi: 10.1055/s-0040-1715454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nougier C., Benoit R., Simon M. Hypofibrinolytic state and high thrombin generation may play a major role in sars-cov2 associated thrombosis. J Thromb Haemost. 2020;18(9):2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lippi G., Sanchis-Gomar F., Henry B.M. Coronavirus disease 2019 (COVID-19): the portrait of a perfect storm. Ann Transl Med. 2020;8(7):497. doi: 10.21037/atm.2020.03.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGuire W.W., Spragg R.G., Cohen A.B., Cochrane C.G. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest. 1982;69(3):543–553. doi: 10.1172/JCI110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gheblawi M., Wang K., Viveiros A. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lippi G., Franchini M., Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011;8(9):502–512. doi: 10.1038/nrcardio.2011.91. [DOI] [PubMed] [Google Scholar]
- 58.van Hinsbergh V.W. Endothelium—role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34(1):93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yau J.W., Teoh H., Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viecca M., Radovanovic D., Forleo G.B., Santus P. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe COVID-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res. 2020;158:104950. doi: 10.1016/j.phrs.2020.104950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roncati L., Ligabue G., Nasillo V. A proof of evidence supporting abnormal immunothrombosis in severe COVID-19: naked megakaryocyte nuclei increase in the bone marrow and lungs of critically ill patients. Platelets. 2020;31(8):1085–1089. doi: 10.1080/09537104.2020.1810224. [DOI] [PubMed] [Google Scholar]
- 62.Zhang S., Liu Y., Wang X. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13(1):120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manne B.K., Denorme F., Middleton E.A. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaid Y., Puhm F., Allaeys I. Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ Res. 2020;127(11):1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taus F., Salvagno G., Canè S. Platelets promote thrombo-inflammation in SARS-CoV-2 pneumonia. Arterioscler Thromb Vasc Biol. 2020;40(12):2975–2989. doi: 10.1161/ATVBAHA.120.315175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixon J.T., Gozal E., Roberts A.M. Platelet-mediated vascular dysfunction during acute lung injury. Arch Physiol Biochem. 2012;118(2):72–82. doi: 10.3109/13813455.2012.665463. [DOI] [PubMed] [Google Scholar]
- 68.Lippi G., Favaloro E.J. Laboratory hemostasis: from biology to the bench. Clin Chem Lab Med. 2018;56(7):1035–1045. doi: 10.1515/cclm-2017-1205. [DOI] [PubMed] [Google Scholar]
- 69.Lippi G., Adcock D., Favaloro E.J. Understanding the "philosophy" of laboratory hemostasis. Diagnosis (Berl) 2019;6(3):223–226. doi: 10.1515/dx-2018-0099. [DOI] [PubMed] [Google Scholar]
- 70.Danese E., Montagnana M., Lippi G. Factor XII in hemostasis and thrombosis: active player or (innocent) bystander? Semin Thromb Hemost. 2016;42(6):682–688. doi: 10.1055/s-0036-1571338. [DOI] [PubMed] [Google Scholar]
- 71.Grover S.P., Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(4):709–725. doi: 10.1161/ATVBAHA.117.309846. [DOI] [PubMed] [Google Scholar]
- 72.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogers K.J., Maury W. The role of mononuclear phagocytes in Ebola virus infection. J Leukoc Biol. 2018;104(4):717–727. doi: 10.1002/JLB.4RI0518-183R. [DOI] [PubMed] [Google Scholar]
- 74.Park M.D. Macrophages: a Trojan horse in COVID-19? Nat Rev Immunol. 2020;20(6):351. doi: 10.1038/s41577-020-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gould T.J., Vu T.T., Swystun L.L. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34(9):1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 77.Skendros P., Mitsios A., Chrysanthopoulou A. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130(11):6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lippi G., Franchini M., Targher G., Poli G., Guidi G.C. The significance of evaluating conventional inflammatory markers in von Willebrand factor measurement. Clin Chim Acta. 2007;381(2):167–170. doi: 10.1016/j.cca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 79.Fish R.J., Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108(3):419–426. doi: 10.1160/TH12-04-0273. [DOI] [PubMed] [Google Scholar]
- 80.Lippi G., Henry B.M., Sanchis-Gomar F. Plasma Antithrombin Values Are Significantly Decreased in Coronavirus Disease 2019 (COVID-19) Patients with Severe Illness. Semin Thromb Hemost. 2020 doi: 10.1055/s-0040-1716873. [DOI] [PubMed] [Google Scholar]
- 81.Kotfis K., Williams Roberson S., Wilson J.E., Dabrowski W., Pun B.T., Ely E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24(1):176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cesari M., Pahor M., Incalzi R.A. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28(5):e72–91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morrow G.B., Whyte C.S., Mutch N.J. Functional plasminogen activator inhibitor 1 is retained on the activated platelet membrane following platelet activation. Haematologica. 2019 doi: 10.3324/haematol.2019.230367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ozolina A., Sarkele M., Sabelnikovs O. Activation of coagulation and fibrinolysis in acute respiratory distress syndrome: a prospective pilot study. Front Med (Lausanne) 2016;3:64. doi: 10.3389/fmed.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spadaro S., Park M., Turrini C. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm (Lond) 2019;16:1. doi: 10.1186/s12950-018-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harzallah I., Debliquis A., Drenou B. Lupus anticoagulant is frequent in patients with COVID-19. J Thromb Haemost. 2020;18(8):2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beyrouti R., Adams M.E., Benjamin L. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Negrini S., Pappalardo F., Murdaca G., Indiveri F., Puppo F. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17(3):257–267. doi: 10.1007/s10238-016-0430-5. [DOI] [PubMed] [Google Scholar]
- 90.Haviv Y.S. Association of anticardiolipin antibodies with vascular injury: possible mechanisms. Postgrad Med J. 2000;76(900):625–628. doi: 10.1136/pmj.76.900.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierangeli S.S., Chen P.P., Raschi E. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost. 2008;34(3):236–250. doi: 10.1055/s-0028-1082267. [DOI] [PubMed] [Google Scholar]
- 92.Amengual O., Atsumi T., Khamashta M.A., Hughes G.R. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thromb Haemost. 1998;79(2):276–281. [PubMed] [Google Scholar]
- 93.Vega-Ostertag M., Harris E.N., Pierangeli S.S. Intracellular events in platelet activation induced by antiphospholipid antibodies in the presence of low doses of thrombin. Arthritis Rheum. 2004;50(9):2911–2919. doi: 10.1002/art.20434. [DOI] [PubMed] [Google Scholar]
- 94.Willis R., Pierangeli S.S. Pathophysiology of the antiphospholipid antibody syndrome. Auto Immun Highlights. 2011;2(2):35–52. doi: 10.1007/s13317-011-0017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Forastiero R.R., Martinuzzo M.E., Lu L., Broze G.J. Autoimmune antiphospholipid antibodies impair the inhibition of activated factor X by protein Z/protein Z-dependent protease inhibitor. J Thromb Haemost. 2003;1(8):1764–1770. doi: 10.1046/j.1538-7836.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 96.Yang Y.H., Chang C.J., Chuang Y.H., Hsu H.Y., Chen P.P., Chiang B.L. Identification of anti-prothrombin antibodies in the anti-phospholipid syndrome that display the prothrombinase activity. Rheumatology (Oxford) 2010;49(1):34–42. doi: 10.1093/rheumatology/kep328. [DOI] [PubMed] [Google Scholar]
- 97.Schouwers S.M., Delanghe J.R., Devreese K.M. Lupus Anticoagulant (LAC) testing in patients with inflammatory status: does C-reactive protein interfere with LAC test results? Thromb Res. 2010;125(1):102–104. doi: 10.1016/j.thromres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 98.Devreese K.M., Verfaillie C.J., De Bisschop F., Delanghe J.R. Interference of C-reactive protein with clotting times. Clin Chem Lab Med. 2015;53(5):e141–e145. doi: 10.1515/cclm-2014-0906. [DOI] [PubMed] [Google Scholar]
- 99.Henry B.M., Vikse J. Clinical Characteristics of COVID-19 in China. N Engl J Med. 2020;382(19):1860–1861. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 100.Kalinowski L., Matys T., Chabielska E., Buczko W., Malinski T. Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertension. 2002;40(4):521–527. doi: 10.1161/01.hyp.0000034745.98129.ec. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henry B.M., Benoit S., Lippi G., Benoit J. Letter to the Editor — Circulating plasma levels of angiotensin II and aldosterone in patients with coronavirus disease 2019 (COVID-19): a preliminary report. Prog Cardiovasc Dis. 2020;63(5):702–703. doi: 10.1016/j.pcad.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henry B.M., Benoit J.L., Berger B.A. Coronavirus disease 2019 is associated with low circulating plasma levels of angiotensin 1 and angiotensin 1,7. J Med Virol. 2020 doi: 10.1002/jmv.26479. [DOI] [PubMed] [Google Scholar]
- 104.Durand M.J., Zinkevich N.S., Riedel M. Vascular actions of angiotensin 1-7 in the human microcirculation: novel role for telomerase. Arterioscler Thromb Vasc Biol. 2016;36(6):1254–1262. doi: 10.1161/ATVBAHA.116.307518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fraga-Silva R.A., Pinheiro S.V., Goncalves A.C., Alenina N., Bader M., Santos R.A. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med. 2008;14(1-2):28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fang C., Stavrou E., Schmaier A.A. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121(15):3023–3032. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanchis-Gomar F., Lavie C.J., Mehra M.R., Henry B.M., Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95(7):1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharma A., Garg A., Rout A., Lavie C.J. Association of obesity with more critical illness in COVID-19. Mayo Clin Proc. 2020;95(9):2040–2042. doi: 10.1016/j.mayocp.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lavie C.J., Sanchis-Gomar F., Henry B.M., Lippi G. COVID-19 and obesity: links and risks. Expert Rev Endocrinol Metab. 2020;15(4):215–216. doi: 10.1080/17446651.2020.1767589. [DOI] [PubMed] [Google Scholar]
- 110.Darvall K.A., Sam R.C., Silverman S.H., Bradbury A.W., Adam D.J. Obesity and thrombosis. Eur J Vasc Endovasc Surg. 2007;33(2):223–233. doi: 10.1016/j.ejvs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Lorenzet R., Napoleone E., Cutrone A., Donati M.B. Thrombosis and obesity: cellular bases. Thromb Res. 2012;129(3):285–289. doi: 10.1016/j.thromres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 112.Rosito G.A., D'Agostino R.B., Massaro J. Association between obesity and a prothrombotic state: the Framingham Offspring Study. Thromb Haemost. 2004;91(4):683–689. doi: 10.1160/th03-01-0014. [DOI] [PubMed] [Google Scholar]
- 113.Campello E., Zabeo E., Radu C.M. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thromb Haemost. 2015;113(1):85–96. doi: 10.1160/TH14-02-0156. [DOI] [PubMed] [Google Scholar]
- 114.Reaven G.M., Scott E.M., Grant P.J. Hemostatic abnormalities associated with obesity and the metabolic syndrome. J Thromb Haemost. 2005;3(5):1074–1085. doi: 10.1111/j.1538-7836.2005.01277.x. [DOI] [PubMed] [Google Scholar]
- 115.Mertens I., Van Gaal L.F. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3(2):85–101. doi: 10.1046/j.1467-789x.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 116.Samad F., Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122(20):3415–3422. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.