Abstract
Immunocompromised patients, including HSCT recipients, may have a poor prognosis after contracting COVID-19 due to the absence of a pathogen-specific adaptive immune response. One of the possible options for severe COVID-19 treatment may be the transfusion of hyperimmune SARS-CoV-2 convalescent plasma.
A 9-month-old girl with juvenile myelomonocytic leukemia received an HSCT from a haploidentical donor. On day +99, during routine virologic monitoring, SARS-CoV-2 was detected without any clinical symptoms. On day +144, the child developed a polysegmental bilateral viral pneumonia with 60 % damage to the lung tissue and confirm a positive SARS-Cov-2 results in throat swab. The patient was treated with tocilizumab and three doses of fresh frozen plasma obtained from a SARS-CoV-2 convalescent patient.
Therapy with tocilizumab and three doses of fresh frozen plasma was well tolerated. In spite of full resolution of the lung lesions, complete elimination of SARS-CoV-2 has not been achieved 4 months after the first detection, which is due to persistence of secondary immunodeficiency after HSCT and the lack of reconstitution of the adaptive immune response.
This case represents a demonstration of an atypical course of COVID-19 and the delayed development of lung lesions, which was most likely associated with the features of the patient's immune status after HSCT. SARS-CoV-2 convalescent plasma in combination with other therapeutic approaches is one of the possible curative options for this clinical situation.
Keywords: COVID-19, Stem cell transplantation, Convalescent plasma, Immunocompromised patients, Pediatric
1. Introduction
It is known that patients with impaired immunity, including patients with cancer and recipients of hematopoietic stem cells, may have higher risks and are more likely to have a poor prognosis when infected with SARS-CoV-2. Data from China thus far have shown that cancer patients infected with COVID-19 have a 3.5 times higher risk of requiring mechanical ventilation or ICU admission than the general population [1]. The reason for the poor prognosis among these groups of patients is secondary immunodeficiency and, as a result, a decrease or absence of a pathogen-specific adaptive immune response to viral infection, which is necessary for the elimination of SARS-CoV-2 [2]. Thus, providing care to immunocompromised patients and those suffering from cancer amidst this pandemic has been extremely challenging. Aggressive combinations of immunosuppressive therapy are an additional factor that may worsen patient prognosis in a severe course of COVID-19 [3,4].
In children, severe COVID-19 is less common than it is in adults, which is due to a number of factors. First, children have a lower expression of the ACE2 receptor – a target for SARS-CoV-2. s, a high number of B- and T-regulatory cells reduces the likelihood of developing severe cytokine inflammatory reactions [5,6]. Nevertheless, a reduced immune response, as well as the absence of specific SARS-CoV-2-specific drugs, can worsen the prognosis of immunocompromised patients with severe COVID-19.
The most challenging category of immunocompromised patients is recipients of allogeneic hematopoietic stem cell transplantation (HSCT). Restoration of the immune response is usually observed no earlier than 4–6 months after HSCT. In addition, problems such as GVHD and prolonged immunosuppressive therapy may further delay the process of immune reconstitution. Therefore, patients have severe secondary immunodeficiency and an extremely low likelihood of the formation of a pathogen-specific immune response in the first months after HSCT. This case presents our experience of treating a child with a severe course of COVID-19 soon after allogeneic HSCT.
One of the possible options for pathogen-specific immunotherapy for severe COVID-19 may be transfusion of convalescent plasma originating from patients who have previously recovered from the viral infection and are now able to donate their anti-SARS-CoV-2 immunoglobulin-containing blood. Recent experience and data from China showed that human convalescent plasma is a potential therapeutic option to lessen the severity and/or shorten the length of illness caused by COVID-19 [7]. The true clinical effect of this intervention is being verified through several ongoing research clinical trials in adults [8,9]. Recently was published first report on the convalescent plasma treatment in pediatric cohort [10]. But to the best of our knowledge, this is the first experience of using convalescence plasma in a pediatric immunocompromised patient after HSCT.
2. Clinical case
In January 2020, a 9-month-old girl with juvenile myelomonocytic leukemia received a stem cell transplantation from a haploidentical related donor. The conditioning regimen consisted of treosulfan (42 g/m2 total dose; days -5, -4, and - 3), fludarabine (150 mg/m2 total dose; days -6, -5, -4, -3, and -2), melphalan (140 mg/m2; day -2), rabbit ATG (5 mg/kg; days -5 and -4) and rituximab (100 mg/m2; day -1). Immunomagnetic TCRαβ+/CD19+ graft depletion (Miltenyi Biotec, Bergisch Gladbach, Germany) was the basic technology for GVHD prophylaxis, and the patient did not receive immunosuppressive therapy after transplantation. Neutrophil engraftment occurred on day +13. Unfortunately, acute grade III GVHD with skin and intestinal involvement developed on day +10, but the patient had a good response to steroids, etanercept and tocilizumab. Later, due to mild symptoms of intestinal GVHD, the patient continued treatment with budesonide.
On day +99, during routine virologic monitoring, SARS-CoV-2 was detected (PCR, throat swab), without any clinical symptoms of the disease or radiological findings on CT. Budesonide therapy was discontinued, but after the reactivation of GVHD (grade III; skin, intestinal) on Day +113, the patient started methylprednisolone treatment at 2 mg/kg with good response and a tapered dose reduction down to 0,3 mg/kg. After two negative results (day+ 117 and +122), a positive SARS-Cov-2 results was again obtained on day +125.
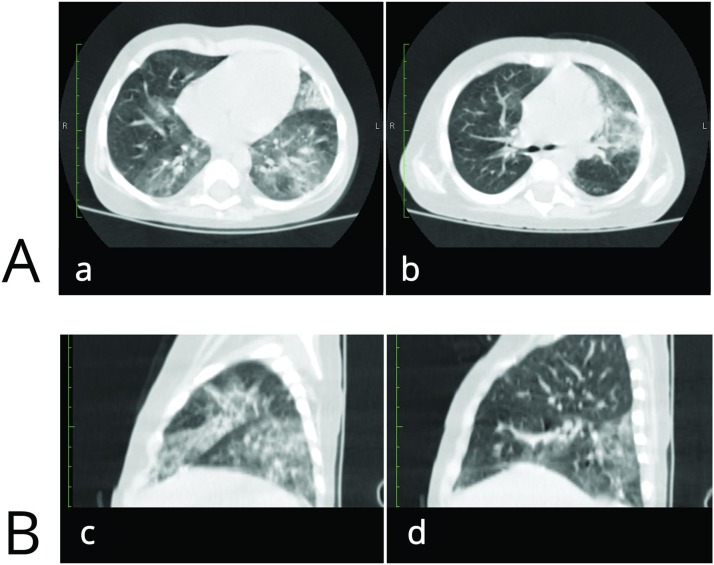
On day +144, the child developed a dry cough, shortness of breath, and a decrease in SaO2 to 94 %. A CT scan revealed signs of polysegmental bilateral viral pneumonia with 60 % damage and multiple areas of ground glass opacity of perebronchial and subpleural areas (Fig. 1 ). SARS-CoV-2 was positive again in throat swab. On day +145, tocilizumab was administered at a dose of 10 mg/kg, and the dose of methylprednisolone was increased up to 1 mg/kg. Signs of severe secondary immune deficiency after HSCT, namely, the absence of T and B cell compartments and, accordingly, agammaglobulinemia, were considered serious problems. In addition, the situation was exacerbated by the need for immunosuppressive therapy to control GVHD. Thus, the possibility for a specific intrinsic immune response to SARS-CoV-2 remained extremely poor. To slow the progression of respiratory failure, a dose of fresh frozen plasma (10 mL/kg) obtained from a SARS-CoV-2 convalescent patient (apheresis, pathogen inactivated with riboflavin + UV; titer of specific SARS-Cov-2Ab - 1:160) was administered on day+146. Marked improvement in the lung lesions was revealed on CT after 14 days, and these findings were also accompanied by a normalization of the patient's clinical condition. Later, another 2 transfusions of FFP obtained from different SARS-CoV-2 convalescent donors were performed (10 mL/kg; pathogen inactivated with riboflavin + UV; titers of specific Cov-2Ab - 1:160 and 1:80), and full resolution of the lung lesions on CT was achieved (Fig. 2 ). An assessment of IgG antibodies specific to SARS-CoV-2 was performed 5 days after the last convalescent donor FFP transfusion with chemiluminescent microparticle immunoassay (ARCHITECT, Abbott, USA), and a positive result with 5,47 S/C was detected (the cutoff is 1.40 index S/C).
Fig. 1.
Initial signs of polysegmental bilateral viral pneumonia (+144 day after HSCT).
A –Axial CT scan; multiple areas of ground glass opacity of perebronchial and subpleural areas (a - a slice at the level of the upper lobes of both lungs; b - a slice at the level of the lower lobes of both lungs).
B – Coronal CT scan; multiple areas of ground glass opacity mainly in the left lung (c - left lung; d - right lung).
Fig. 2.
Positive changes after 1 month due to the involution of some areas of frosted glass opacity.
A –Axial CT scan (a - a slice at the level of the upper lobes of both lungs; b - a slice at the level of the lower lobes of both lungs).
B – Coronal CT scan (c - left lung; d - right lung).
According to blood flow cytometry data, 488 CD3+ cells/μL and 6 naïve (CD3+ CDRA45+ CD197+) cells/μL were detected with the complete absence of the B-cell compartment and lack of endogenous immunoglobulin synthesis. Additionally, we did not detect SARS-CoV-2-specific T cells by an IFN-γ ELISPOT assay 90 days after initial virus detection (Elispot reader, ImmunoSpot Series 5 UV Analyzer; C.T.L, Cleveland, OH). Despite the absence of clinical manifestations of COVID-19, the child retained positive detections of SARS-CoV-2 (PCR, throat swab) 4 months after the initial detection.
3. Discussion
The effect of primary and secondary immunodeficiency in the course of SARS-CoV-2 remains controversial. It is commonly believed that viral agents are associated with an increased risk of a more severe disease course and respiratory complications in immunocompromised patients [3,12]. According to data published by Minotti C et al., the prognosis of adults and children receiving immunosuppressive therapy with new COVID-19 infections is more favorable than that of patients with other comorbid conditions [4]. Indeed, the severe respiratory complications caused by coronaviruses are thought to be driven by aberrant inflammatory and cytokine responses perpetuated by the host immune system [2]. This may be a possible explanation for the milder course of COVID-19 in patients with impaired immune responses and the virtual absence of a cytokine storm. However, according to a limited meta-analysis conducted by Gao Y et al., immunosuppressive therapy and immunodeficiency were associated with a more severe course of COVID-19 [11]. This is likely a consequence of severe progressive uncontrolled damage of the lung parenchyma caused primarily by a viral (SARS-CoV-2) agent, not by cytokines.
Moreover, hematopoietic stem cell recipients represent a very special population of patients. An adaptive specific immune response to infection is formed 4–6 months after allogeneic HSCT and may even be delayed in the case of GVHD and immunosuppressive therapy. Thus, the lack of an immune reconstitution and persistence of secondary immunodeficiency can lead to the severe course and poor prognosis of SARS-CoV-2 as well as other viral infections after HSCT. Additionally, it should be noted that the timing of the clinical manifestations of lung insufficiency in patients with severe immune deficiencies may significantly differ from the course of COVID-19 in immunocompetent patients, whose own inflammatory response in the early stages is most likely to be the pathophysiology of lung damage. In our case, the development of respiratory failure was diagnosed only 43 days after the detection of SARS-CoV-2, and this may be indirect evidence that lesions in the pulmonary interstitium caused by a replicating virus may play a major role in developing a severe course of COVID-19. A low probability of the patient forming her own immune response became the argument for starting therapy with hyperimmune SARS-CoV-2 convalescent plasma in combination with other therapeutic options, despite the lack of published data on similar experiences in the pediatric cohort. A complete clinical response to therapy was reported in a few days, and the patient had no clinical manifestations of the infection 4 months after the first detection of SARS-CoV-2. Unfortunately, complete elimination of SARS-CoV-2 has not been achieved, which is due to the lack of reconstitution of the adaptive immune response. This was confirmed by the absence of B-cells according to flow cytometry, as well as lack of SARS-CoV-2-specific T-cells according to IFNγ ELISPOT test result. Consider this, we cannot exclude the risk of reactivation of the disease after prolonged persistence of the virus in this patient due to long-term immunodeficiency.
This case represents a demonstration of an atypical course of COVID-19 and the delayed development of lung lesions, which was most likely associated with the features of the patient's immune status after HSCT. Convalescent plasma with high titers of pathogen-specific antibodies can play a crucial role that cannot be ignored while treating patients with no prospects for the formation of an adaptive response to the pathogen. In our opinion, this kind of therapy should be discussed as a curative option for this group of high-risk patients.
4. Conclusion
This clinical case demonstrates that COVID-19 may lead to different symptoms in patients with immune insufficiency. The time to pulmonary damage may be delayed, and the lung tissue injury itself is associated with specific damage caused by a viral agent rather than an immuno-inflammatory process. The inability to develop an adaptive immune response in patients with severe immune deficiency can be a predictor of a poor prognosis for COVID-19. Convalescent plasma therapy is one of the possible and effective options in such a clinical situation in pediatric practice.
Disclaimers
The authors have nothing to disclose in relation to the submitted manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
We express our gratitude to physicians as well as the nursing for dedicated care of patient.
References
- 1.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y., Wang Y., Shao C., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 4.Minotti C., Tirelli F., Barbieri E., Giaquinto C., Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. 2020;81(1):e61–e66. doi: 10.1016/j.jinf.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodin P. Why is COVID-19 so mild in children? Acta Paediatr. 2020;109(6):1082–1083. doi: 10.1111/apa.15271. [DOI] [PubMed] [Google Scholar]
- 6.Abdulamir A.S., Hafidh R.R. The Possible Immunological Pathways for the Variable Immunopathogenesis of COVID—19 Infections among Healthy Adults, Elderly and Children. Electron J Gen Med. 2020;17(4):1–4. doi: 10.29333/ejgm/7850. em202. [DOI] [Google Scholar]
- 7.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyner M.J., Wright R.S., Fairweather D., et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diorio D., Anderson E.M., McNerney K.O., et al. Convalescent plasma for pediatric patients with SARS‐CoV‐2‐associated acute respiratory distress syndrome. Pediatr Blood Cancer. 2020;67:e28693. doi: 10.1002/pbc.28693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y., Chen Y., Liu M., Shi S., Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):e93–e95. doi: 10.1016/j.jinf.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogoyavlenskaya A.A., Laberko A.L., Shelikhova L.N., et al. Herpesvirus infection following allogenic hematopoietic stem cell transplantation with TCRαβ and CD19 depletion: risk factors and outcome. Pediatr Hematol/Oncol Immunopathol. 2017;16(1):10–21. doi: 10.24287/1726-1708-2017-16-1-10-21. [DOI] [Google Scholar]