Graphical abstract
Keywords: Processing method, Growth year, Post-harvest, Active ingredient, Industrial production, Scutellaria baicalensis
Highlights
-
•
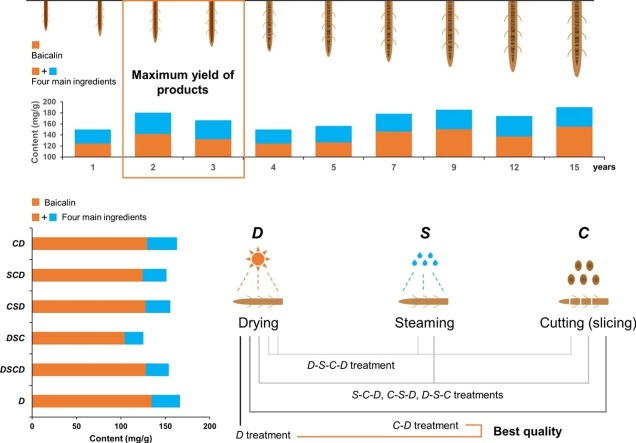
The best harvesting time for S. baicalensis was determined as 2–3 years.
-
•
The main medicinal active ingredients were higher in cortex than in stele.
-
•
The reduction of ingredients in fresh-cutting was lower than that in fresh-crushing.
-
•
The treatments of drying and cutting-drying were optimal for the yield of products.
-
•
Novel processing methods for industrial production of S. baicalensis was provided.
Abstract
Optimizing the processing technology is an effective way to improve the yield of active ingredients for the industrial production of medicinal crops. Baikal Skullcap (Scutellaria baicalensis Georgi) is a perennial herb in the Lamiaceae family and its dried root is used as a famous traditional Chinese medicine (TCM). Modern pharmacological studies have shown that the active ingredients of S. baicalensis have important pharmacological effects including anti-oxidation, anti-bacterial, anti-viral, anti-tumor, and anti-inflammation. Specifically, it is recently found that S. baicalensis has significant curative effects on the treatment of corona virus disease 2019 (COVID-19). In recent years, the market demand for the medicinal products of S. baicalensis is increasing because of its great medicinal values. However, the annual yield of active ingredients originated from the root of S. baicalensis is limited due to that little progress has been made on the traditional processing technology used in the extraction process. A pressing issue faced by both herbalists and scientists is how to improve the processing efficiency, thereby obtaining the maximum yield of products for S. baicalensis. In this study, a systematic analysis on the effects of growth years and post-harvest processing on the contents of medicinal active ingredients of S. baicalensis was conducted. The contents of eight active ingredients (baicalin, wogonoside, baicalein, wogonin, scutellarin, scutellarein, apigenin, and chrysin) in roots of S. baicalensis of different growth years (ranging from 1 year to 15 years) were estimated using high performance liquid chromatography (HPLC) and further analyzed to determine the optimal harvest period. In particular, the contents of six active ingredients in different parts (cortex and stele) of the root of S. baicalensis were estimated and compared. Meanwhile, the dynamic changes of the contents of active ingredients in fresh-crush and fresh-cut roots of S. baicalensis at room temperature were compared and analyzed to reveal the influence of post-harvest treatment on the contents of active ingredients. In addition, the effects of six different post-harvest treatments on the contents of active ingredients were systematically designed and compared to determine the best primary processing technology. The results showed that the best harvesting period for S. baicalensis should be determined as 2–3 years based on comprehensive evaluation of active ingredient content, annual yield increment, and land use efficiency. The contents of active ingredients including baicalin, wogonoside, baicalein, and wogonin in cortex were significantly higher than those in stele (P ≤ 0.05). The contents of baicalin, wogonoside, and scutellarin in fresh roots of S. baicalensis significantly reduced as the storage time increased, but the reduction of fresh-cutting was significantly lower than that of fresh-crushing. For the effects of different processing treatments, the contents of four main active ingredients (baicalin, wogonoside, baicalein, and wogonin) under drying (D) and cutting-drying (C–D) treatments were significantly higher than those of the other four treatments (P ≤ 0.05). Collectively, the above results will not only provide novel processing methods that will improve the yield of active ingredients for S. baicalensis, but also shed light on the optimization of processing technology for the industrial production of medicinal crops.
1. Introduction
Medicinal plants with effective pharmacological activities, low side effects as well as high economic values have long been used as raw materials and reservoirs of new drugs in pharmaceutical industry (Tschofen et al., 2016; Wurtzel and Kutchan, 2016; Tu, 2011; Balandrin et al., 1985). As an important part of the traditional Chinese medicine (TCM) system, medicinal plants have shown great therapeutic potentials in severe and acute diseases including malaria, diabetes, cancer, etc. (Chukwuma et al., 2019; Feachem et al., 2019; Manukumar et al., 2017; Miller and Su, 2011). Specifically, in the recent pandemic of corona virus disease 2019 (COVID-19), TCM preparation and drugs showed effective anti-viral and anti-inflammatory activities against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Chan et al., 2020; Li et al., 2020a; Liu et al., 2020; Luo et al., 2020; Ren et al., 2020; Su et al., 2020; Yang et al., 2020). Exploring the potential pharmacological functions and promoting the introduction and development of TCM will bring benefits to human health globally.
Currently, the primary aim of studies in TCM cultivation and production is how to improve the yield and quality of medicinal crops (specifically the active ingredients with high medicinal values). In recent years, the market demand of raw materials originated from medicinal crops is increasing with the development of pharmaceutical industry (Barata et al., 2016; Chen et al., 2016). Nevertheless, the yield of medicinal crops with high quality is decreasing due to growing human activities and habitat loss (Cao et al., 2020; He, 2018; Zhang et al., 2018; Chi et al., 2017). A pressing challenge faced by scientists is how to obtain the maximum yield of active ingredients in medicinal crops.
Improving the yield of active ingredients by optimizing the processing methods is a good way to achieve the above goals (Mishra et al., 2020; Nabet et al., 2019; Wang et al., 2018a). Typically, the active ingredients in medicinal plants are secondary metabolites that are closely related to cultivation methods, field management, harvest time, primary processing in the producing area, deep processing, storage, and transportation, etc. (Kessler and Kalske, 2018; Zhao et al., 2012; Xiao et al., 2009; Canter et al., 2005). Specifically, the processing methods including removing impurities, stacking, steaming, slicing, and drying have significant effects on the contents of active ingredients of Chinese medicinal materials (Nozad et al., 2016; Guo et al., 2015; Azizi, 2008). The primary processing in the producing area is the first key step for the quality formation of Chinese medicinal materials. Previous studies have demonstrated that primary processing in the producing area have a great influence on the yield, quality, and efficacy of the medicinal materials (Chen et al., 2015; Duan et al., 2009). In the traditional technology of primary processing, it is specified that post-harvest treatment and processing of medicinal materials in the producing area should follow the natural rules of the formation of active ingredients. Meanwhile, the specific characteristics of raw materials during the process of storage and transportation should be carefully considered to ensure the quality of medicinal materials (Wang et al., 2013; Zhao et al., 2012).
Nevertheless, most of the active ingredients (e.g., alkaloid salts, inorganic salts, etc.) of TCM have different affinity degrees with water (Zhang et al., 2017; Jin et al., 2016; Yang et al., 2016). Improper processing treatments (long-term exposure to water, water flushing, immersion, etc.) will cause considerable loss of active ingredients (Gao et al., 2011). In addition, the contents of active ingredients in medicinal materials are closely related to the enzyme activity of plant tissues (Gao et al., 2019; Kotwal et al., 2019; Yang et al., 2019; Liu et al., 2017). For medicinal crops that are rich in alkaloids and polysaccharides, the contents of active ingredients as well as the quality of medicinal materials will rapidly decline, due to the absence of high-temperature treatment that can inactivate enzyme activity in tissues and organs after harvest. For example, for the important medicinal crop tall gastrodia tuber (Gastrodia elata Bl.), high-temperature steam is firstly used to inactivate enzyme activity in the root after harvesting, and then the steamed roots are dried. As an alternative, it can also inactivate enzyme activity by boiling the root in water. However, the boiling process will significantly reduce the content of gastrodin, which will further reduce the quality of medicinal materials (Xiao, 2017). Accordingly, it is necessary to investigate the effects of processing methods on the quality formation of different active ingredients in medicinal crops, thereby providing optimum processing methods that will obtain the maximum yield of products for the industrial production of traditional Chinese medicinal materials.
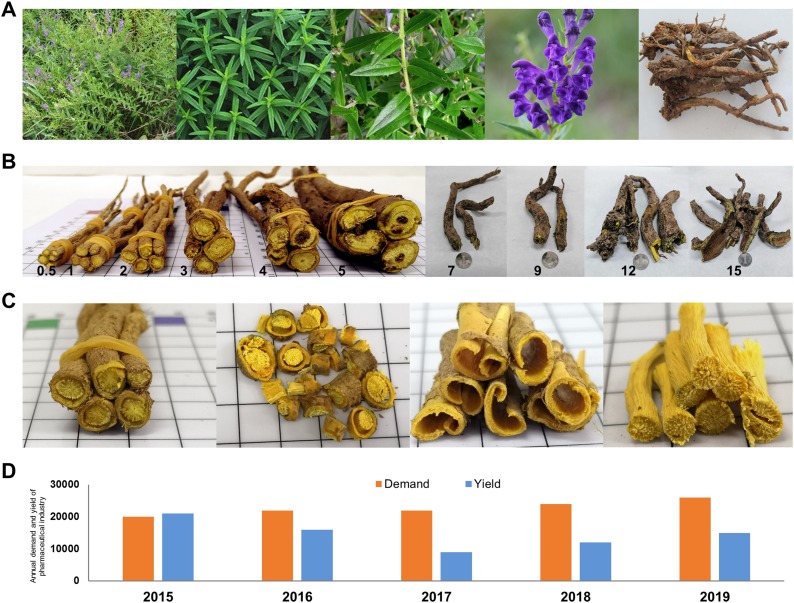
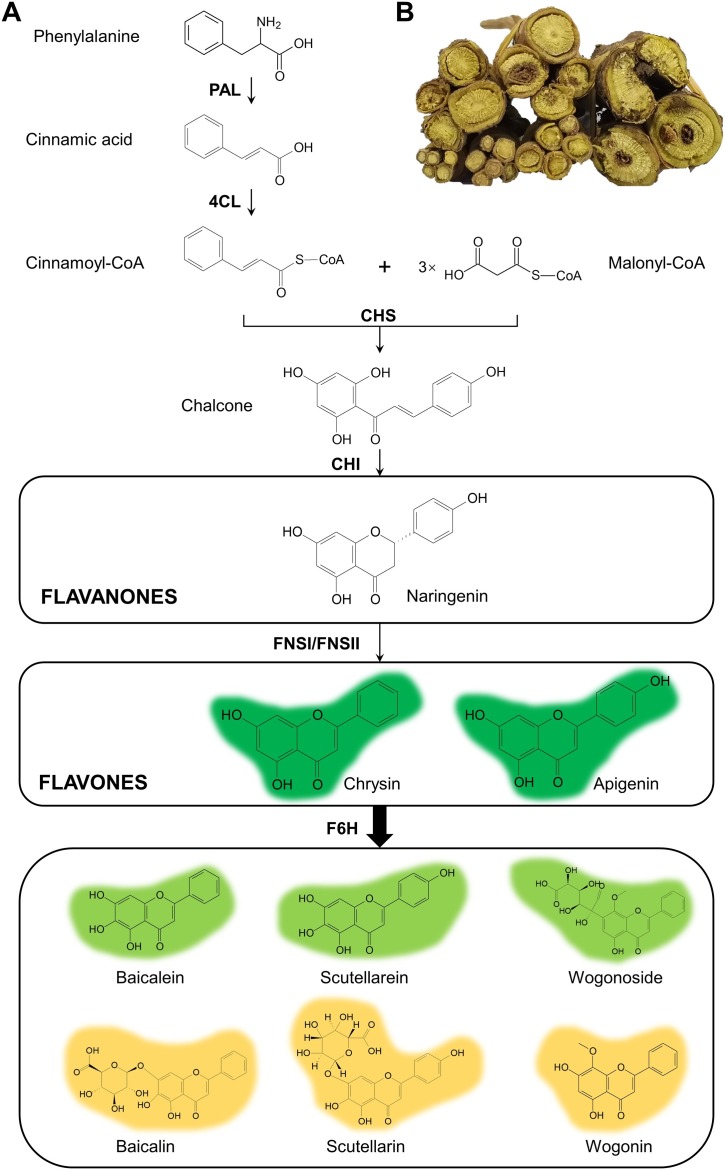
Baikal Skullcap (Scutellaria baicalensis Georgi) is a perennial herb in the Lamiaceae family and its dried root is used as a famous traditional Chinese medicine, which was first recorded in the Shen-nong-ben-cao-jing (The Classic of Herbal Medicine) for the treatment of diarrhea, dysentery, hypertension, hemorrhaging, insomnia, inflammation, and respiratory infection (Zhao et al., 2016a; Fig. 1 ). As one of the most commonly used Chinese medicinal materials in China, it has been used as medicinal materials for more than 2000 years since being recorded in the ancient medical books. Up to date, it has been included in over 90 % TCM formulas for treating cold (National Pharmacopoeia Committee, 2015). Modern pharmacological studies show that the active ingredients of S. baicalensis have important pharmacological effects such as anti-oxidation, anti-bacterial, anti-viral, anti-tumor, and anti-inflammation (Ma et al., 2018; Wu et al., 2018a, 2018b; Park et al., 2011; Zhao et al., 2007; Gao et al., 1999). Specifically, it is recently found that S. baicalensis has significant curative effects on the treatment of COVID-19 (Liu et al., 2020; Su et al., 2020). The main pharmaceutical active ingredients are flavonoids, of which the glycosides (e.g., wogonoside) and the aglycones (e.g., baicalein, wogonin) have the strongest medicinal activities (Fig. 2 ). Among them, the content of baicalin is determined as the main evaluation index and quality control of S. baicalensis by the Pharmacopoeia (National Pharmacopoeia Committee, 2015).
Fig. 1.
The plant and main medicinal material of S. baicalensis used in pharmaceutical industry.
(A) The plant of S. baicalensis. From left to right: whole plant, leaf, stem, flower, and root; (B) The roots of S. baicalensis with different growth years. From left to right: 0.5, 1, 2, 3, 4, 5, 7, 9, 12, 15; (C) The different parts of roots from 2-year-old S. baicalensis. From left to right: whole root, root slice, cortex, stele; (D) The annual demand and yield of medicinal products of S. baicalensis used in pharmaceutical industry in China. Data were collected and analyzed by our group. Unit: tons.
Fig. 2.
The flavonoid biosynthesis in roots of S. baicalensis.
(A) The flavonoid biosynthesis in S. baicalensis. PAL: phenylalanine ammonia lyase; 4CL: 4-coumarate CoA ligase; CHS: chalcone synthase; CHI: chalcone isomerase; FNSI: flavone synthase I; FNSII: flavone synthase II; F6H: flavone 6-hydroxylase. (B) The roots of S. baicalensis.
Due to its great medicinal values, there is a huge market demand for S. baicalensis every year and it is becoming one of the top ten sales of Chinese medicinal materials in China (Zhao et al., 2016a). Before the 1990s, the products of S. baicalensis are mainly originated from wild resources. Since the 21 st century, the decreasing wild resources of S. baicalensis were far from meeting the rapidly increasing market demand of pharmaceutical use. Accordingly, the market share of cultivated varieties has been increasing after extensive cultivation of S. baicalensis (Su et al., 2008).
For the industrial production of S. baicalensis, the harvesting time and processing in producing areas are considered as the key factors for the quality formation of medicinal materials (Hu et al., 2019). Since S. baicalensis is a perennial herb, its stele of the root gradually decays from the base after growing for about 4 years (known as “hollow roots”) (Fig. 1). Although it has been recorded in the classical prescriptions and the medical classics that the hollow roots of S. baicalensis has the best curative effect in TCM, the theoretical basis of this statement remains unclear. Meanwhile, the factor of harvesting time is very important for the quality and yield of medicinal materials as well as the income of farmers during the cultivation process. At present, the farmers and herbalists believe that harvesting time at two and a half years or three years can ensure the best quality, the highest yield, and the highest land use efficiency for S. baicalensis. However, there is no clear evidence to show whether the contents of medicinal active ingredients of S. baicalensis harvested at such time have the highest levels. In addition, the primary processing in producing areas (removing impurities, drying, slicing, etc.) is considered as another key factor that affects the contents of medicinal active ingredients. Previous studies have shown that the contents of medicinal active ingredients in S. baicalensis after harvesting are closely related to enzyme activities in roots (Wang et al., 2018b; Zhao et al., 2016b). The active ingredients such as baicalin and wogonoside in fresh roots are easily reduced in enzyme solution under humid environment or washing and soaking in water (Liu, 2015). Nevertheless, there is a lack of systematic research on the effects of post-harvest treatment and processing technology on the contents of medicinal active ingredients to reveal such mechanisms. Taken together, it is of great theoretical significance and practical prospect to reveal the effects of different processing technology on the medicinal active ingredients under the background of increasing market demand for S. baicalensis.
On the basis of our previous research in S. baicalensis over the past 15 years (Li et al., 2019; Bai et al., 2018; Guo et al., 2016; Zhang et al., 2016; Bai et al., 2013), in this study we conducted a comprehensive study from the following aspects by taking eight medicinal active ingredients from the root of S. baicalensis as indicators: (1) the contents of active ingredients in roots of S. baicalensis from growing 1–15 years were compared and analyzed to determine the optimal harvest time; (2) six medicinal active ingredients in the parts of stele and cortex in roots of 2-year and 15-year old S. baicalensis were compared and analyzed to reveal the differences of active ingredients in different growth years; (3) the dynamic changes of the contents of active ingredients in fresh-crush and fresh-cut roots of S. baicalensis at room temperature were compared and analyzed to reveal the influence of post-harvest treatment on the contents of active ingredients; (4) the effects of six different post-harvest treatment on the contents of active ingredients were systematically designed and compared to determine the best primary processing technology. Collectively, the above results will provide a theoretical basis for the scientific harvest and the primary processing in the producing area of S. baicalensis, and provide a reference for the selection of optimal processing technology of medicinal materials for the industrial production of medicinal crops.
2. Materials and methods
2.1. Plant materials
In this study, the germplasms of S. baicalensis from singe genetic source (originated from the main producing area in Shaanxi province) were planted from 2004 to present in the Germplasm Resources Garden for Medicinal Plants in Shaanxi Normal University (N34°09′, E108°54′), Xi’an, China. To determine the age of S. baicalensis for further experiments, the germplasms with different growth years were planted separately in the garden. For experiments regarding different ages, the root samples were collected from three plants of S. baicalensis with similar phenotypic and physiological characters of each age group (planted in 2004, 2007, 2010, 2012, 2014, 2015, 2016, 2017, and 2018) (Table S1). For experiments regarding different storage time and different processing methods, the root samples were collected from three plants of S. baicalensis with similar phenotypic and physiological characters planted in 2017. For experiments regarding different parts, the samples of cortex and stele were collected from three plants of S. baicalensis with similar phenotypic and physiological characters planted in 2017. All the plant samples of S. baicalensis were harvested in September 2019. After removing sediment and impurities, the samples were prepared for further experiments.
2.2. Processing methods of samples
To obtain the maximum yield of active ingredients, six different post-harvest processing methods were designed based on traditional technology in this study. The six treatments for processing the roots of S. baicalensis were listed as following (D for drying, S for steaming, and C for slicing, respectively):
D treatment: Fresh whole roots were dried at 80 °C in oven (101-2A, Taisite, Tianjin, China) to constant weight, and then crushed and filtered through a 60-mesh sieve;
D-S- C-D treatment: Fresh whole roots were dried at 80 °C in oven to constant weight, softened by steam for 20 min, then sliced and dried, and finally crushed and filtered through a 60-mesh sieve;
D-S-C treatment: Fresh whole roots were dried at 80 °C in oven to constant weight, softened by steam for 20 min, then crushed directly after slicing, and finally filtered through a 60-mesh sieve;
C-S-D treatment: Fresh whole roots were cut into slices, softened by steam for 20 min after slicing, dried to constant weight at 80 °C in oven, and finally crushed and filtered through a 60-mesh sieve;
S-C–D treatment: Fresh whole roots were softened by steam for 20 min, cut into slices, dried to constant weight at 80 °C in oven, and finally crushed and filtered through a 60-mesh sieve;
C–D treatment: Fresh whole roots were cut into slices, dried to constant weight at 80 °C in oven, and finally crushed and filtered through a 60-mesh sieve.
2.3. Slicing and crushing of fresh roots of S. baicalensis
After collecting the fresh roots of S. baicalensis, the soil was removed and the surface moisture of roots were dried. The dried roots were then sliced quickly and mixed thoroughly. The fresh slices were divided into two parts. One was crushed directly and another was left untreated. The two groups of samples were placed in a petri dish, sealed with plastic wrap to retain water, and then stored at room temperature for 0 h, 0.5 h, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 4.5 h, 5 h, 6 h, 8 h, 11 h, 22 h, 23 h, 24 h, and 25 h, respectively. Finally, these samples were immediately placed in a 10 mL centrifuge tube, quick-frozen with liquid nitrogen, and stored at -80 °C. After completely frozen, these samples were freeze-dried at -80 °C for 6 h using freeze dryer (GOLD-SIM, Newark, USA), and then crushed and filtered through a 60-mesh sieve.
2.4. Estimation of the contents of medicinal active ingredients
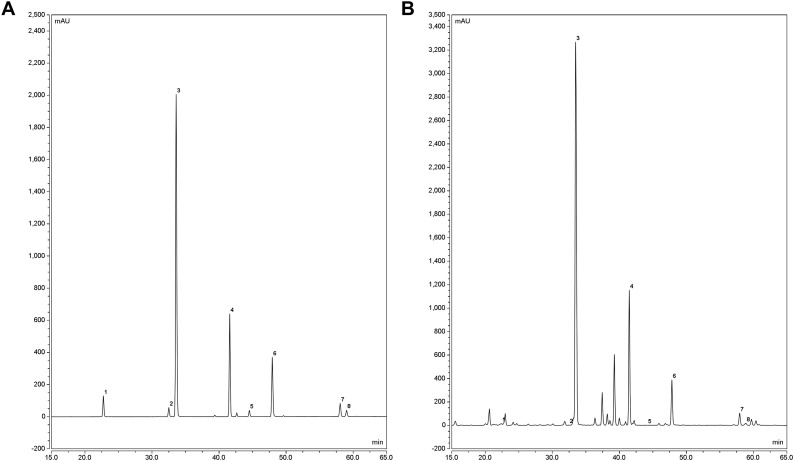
The extraction and measurement of eight active ingredients (baicalin, scutellarin, chrysin, scutellarein, wogonoside, apigenin, wogonin, and baicalein) were conducted using our previous protocol (Guo et al., 2016). First, 0.5 g dried sample was accurately weighed and placed in a centrifuge tube. Then the sample was added with 20 mL 70 % ethanol. After 30 min of ultrasonic treatment (500 W, 80 kHz), the sample was cooled to room temperature, and then filtered and the filtrate was collected. Next, 20 mL 70 % ethanol was added to the residue followed by sonicating for 30 min under the same condition. After filtration, the two filtrates were combined into a 50 mL volumetric flask and 70 % ethanol was added to the scale line. Then the sample was filtered through a 0.22 μm filter after thoroughly mixing to obtain the sample solution. The eight medicinal active ingredients of samples were extracted using high-performance liquid chromatograph (HPLC) (Thermo Fisher Scientific, New York, USA) (Fig. 3 ). The determination condition was as below. Mobile phase: acetonitrile to 0.1 % formic acid. Gradient elution: 0 ∼ 10 min, 10 % ∼ 15 % acetonitrile; 10 ∼ 20 min, 15 % ∼ 20 % acetonitrile; 20 ∼ 30 min, 20 % ∼ 25 % acetonitrile; 30 ∼ 60 min, 25 % ∼ 45 % acetonitrile; 60 ∼ 65 min, 45 % ∼ 10 % acetonitrile. Flow rate: 1.0 mL/min. Detection wavelength: 274 nm. Column temperature: 30 °C. Sample size: 10 μL.
Fig. 3.
The HPLC chromatograms of eight active ingredients in roots of S. baicalensis.
(A) The chromatograms of standards of eight active ingredients. 1: scutellarin; 2: scutellarein; 3: baicalin; 4: wogonoside; 5: apigenin; 6: baicalein; 7: wogonin; 8: chrysin. (B) The representative chromatograms of eight active ingredients of root sample from 2-year-old S. baicalensis. 1: scutellarin; 2: scutellarein; 3: baicalin; 4: wogonoside; 5: apigenin; 6: baicalein; 7: wogonin; 8: chrysin.
2.5. Quantity evaluation of medicinal active ingredients
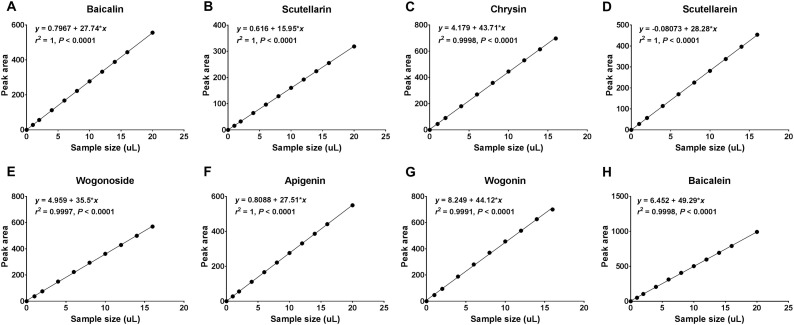
For the generation of standard curves, the standards of the eight active ingredients were prepared with a concentration of 5 × 10−4 mg/μL. The eight standards were then injected with volumes of 1, 2, 4, 6, 8, 10, 12, 14, 16, and 20 μL, respectively, and were detected at wavelength of 274 nm. The peak area values of each standard were recorded. The linear regression equations were then calculated using the sample volume of standards as abscissa (X) and the peak areas as ordinate (Y) (Fig. 4 ). For the quantity evaluation of estimated samples, the peak values of each active ingredient were substituted to the linear regression equations to obtain the sample sizes. Then the content of each active ingredient of 10 μL was calculated by multiplying by the concentration of standards (5 × 10−4 mg/μL). Finally, the contents of the eight active ingredients of samples (mg/g) were calculated by multiplying by the dilution ratio.
Fig. 4.
The HPLC standard curves of eight active ingredients in roots of S. baicalensis.
The HPLC standard curves of eight active ingredients in roots of S. baicalensis were calculated using linear regression. (A) baicalin; (B) scutellarin; (C) chrysin; (D) scutellarein; (E) wogonoside; (F) apigenin; (G) wogonin; (H) baicalein.
2.6. Data analysis
The data of this study were analyzed using IBM SPSS Statistics (Version 22.0). For the analysis of the contents of eight active ingredients of different growth years, the analysis of variance (ANOVA) was conducted. The growth years were chosen as dependent list, and the content of each active ingredients was chosen as factor. The LSD pattern was used as equal variances assumed. The options of “Descriptive” and “Homogeneity of variance test” were selected. For the comparisons of the contents of active ingredients among different part, different storage time, and different processing methods, the nonparametric tests for each group of data was first conducted. The differences of data were then analyzed using Student's t-test. P ≤ 0.05 indicates statistically significance.
3. Results
3.1. Effects of growth years on the contents of eight active ingredients in roots of S. baicalensis
To investigate the effects of growth years on the contents of active ingredients in S. baicalensis, the roots with different growth years (ranging from 1–15) were collected and then dried at 80 °C in oven after removing sediment and impurities. The contents of eight medicinal active ingredients were further estimated (Table 1 ). The results showed that the contents of baicalin in roots of S. baicalensis in different growth years were all higher than 12 % (corresponding to 120 mg/g), which met the minimum requirement of the Pharmacopoeia (2015 version; 9%, corresponding to 90 mg/g) and could be used as medicinal materials (National Pharmacopoeia Committee, 2015). Further ANOVA analysis showed that the content of baicalin exhibited a "V" trend changing with growth years (Table 1). It is relatively high at both ends (2 years old and older than 7 years old) but low at the middle period (3–5 years old). In general, the total amount of the four main active ingredients (baicalin, wogonoside, baicalein, and wogonin) (13.5 %, corresponding to 135 mg/g; National Pharmacopoeia Committee, 2020) in roots of S. baicalensis showed the following trend: 15 years > 9 years > 2 years > 7 years > 12 years > 3 years > 5 years > 4 years >=1 year. Among them, the total content of the four main active ingredients in 15-year-old roots was as high as 190 mg/g, followed by that of 9-year-old roots (185.57 mg/g). The content of 2-year-old roots reached 180 mg/g, which was significantly higher than that of 1-year-old roots (149.73 mg/g) (P ≤ 0.05). For the other four active ingredients (scutellarin, scutellarein, chrysin, and apigenin) with lower contents, they also showed significant differences among different growth years (Table 1). The contents of scutellarein and scutellarin were significantly lower than those of all the four main active ingredients (P < 0.001). For the former, its content continued to increase in the first three years and remained basically stable after the fourth year. While for the latter, its content continued to decrease with growth years, and reached the lowest level (0.03 %) in 15-year-old roots. Conversely, for chrysin which is the precursor of baicalin, wogonoside, baicalein, and wogonin in the flavonoid synthesis pathway, its content reached the highest level (about 0.08 %) in 15-year-old roots.
Table 1.
The contents of eight medicinal active ingredients in roots of S. baicalensis with different growth years (mg/g).
| Growth years | The contents of medicinal active ingredients (Dry weight, mg/g) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baicalin | Wogonoside | Baicalein | Wogonin | Scutellarin | Scutellarein | Apigenin | Chrysin | |
| 1-year-old | 124.371 ± 0.135 h | 22.037 ± 0.035 g | 2.691 ± 0.004 g | 0.642 ± 0.001 h | 1.073 ± 0.003 a | 0.017 ± 0.000 g | 0.082 ± 0.001 b | 0.223 ± 0.000 i |
| 2-year-old | 141.807 ± 0.415 d | 28.408 ± 0.064 b | 7.423 ± 0.020 c | 2.584 ± 0.006 c | 0.897 ± 0.006 b | 0.088 ± 0.001 f | 0.068 ± 0.000 c | 0.624 ± 0.003 d |
| 3-year-old | 132.303 ± 0.342 f | 27.795 ± 0.075 c | 4.490 ± 0.012 f | 1.904 ± 0.003 e | 0.564 ± 0.005 d | 0.174 ± 0.001 a | 0.091 ± 0.001 a | 0.397 ± 0.001 e |
| 4-year-old | 124.186 ± 0.218 i | 21.261 ± 0.021 h | 3.229 ± 0.005 h | 1.101 ± 0.003 f | 0.583 ± 0.005 c | 0.140 ± 0.000 c | 0.048 ± 0.001 d | 0.312 ± 0.000 g |
| 5-year-old | 126.376 ± 0.303 g | 20.800 ± 0.003 i | 6.169 ± 0.004 d | 3.107 ± 0.007 a | 0.364 ± 0.006 g | 0.131 ± 0.002 d | 0.031 ± 0.001 e | 0.765 ± 0.003 c |
| 7-year-old | 146.080 ± 0.152 c | 26.989 ± 0.073 d | 4.413 ± 0.011 h | 1.029 ± 0.004 g | 0.356 ± 0.011 g | 0.134 ± 0.006 d | 0.082 ± 0.001 b | 0.269 ± 0.004 h |
| 9-year-old | 150.286 ± 0.480 b | 24.020 ± 0.035 f | 8.532 ± 0.009 a | 2.734 ± 0.007 b | 0.378 ± 0.005 f | 0.131 ± 0.003 de | 0.068 ± 0.000 c | 0.806 ± 0.006 b |
| 12-year-old | 137.354 ± 0.170 e | 29.263 ± 0.008 a | 5.214 ± 0.004 e | 2.521 ± 0.003 d | 0.412 ± 0.001 e | 0.129 ± 0.001 e | 0.091 ± 0.001 a | 0.335 ± 0.003 f |
| 15-year-old | 155.148 ± 0.301 a | 24.272 ± 0.003 e | 8.438 ± 0.003 b | 2.522 ± 0.002 d | 0.321 ± 0.002 h | 0.143 ± 0.001 b | 0.048 ± 0.001 d | 0.832 ± 0.004 a |
Note: Different letters indicate significant differences of the content of each medicinal active ingredient in the same column (P ≤ 0.05).
3.2. Effects of selection for different parts on the contents of six active ingredients in roots of S. baicalensis
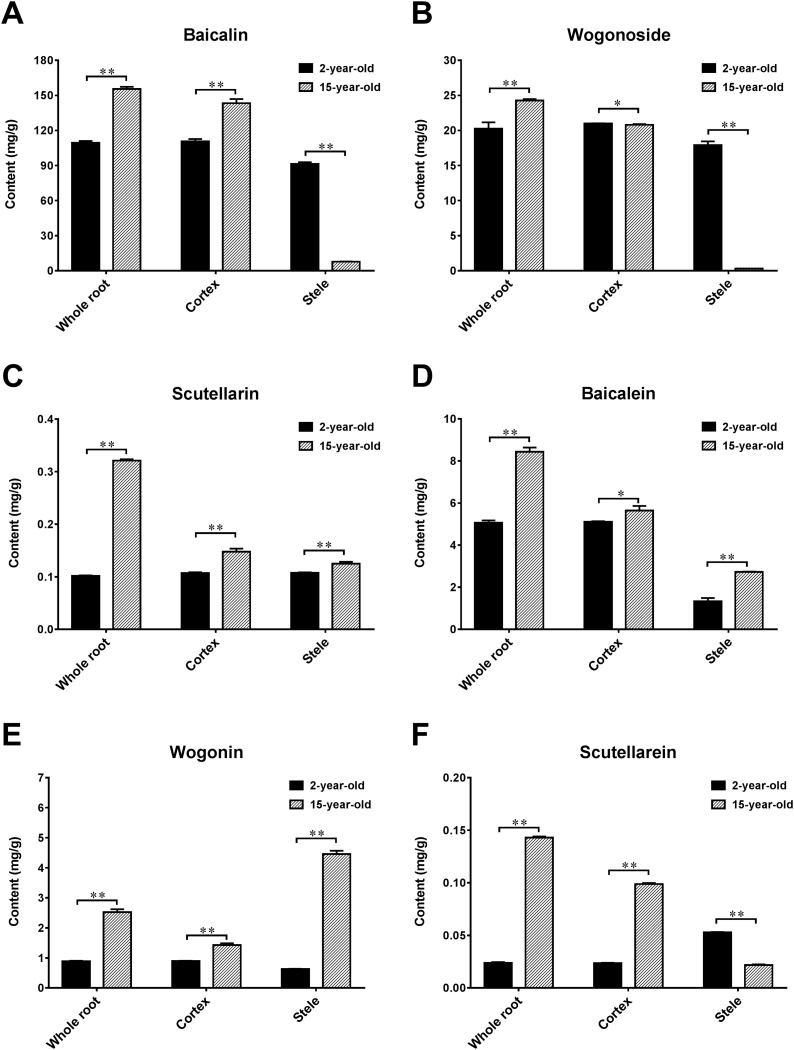
To further reveal the mechanism regarding the "V" trend of the contents of the four main active ingredients, we next investigated the disparity of active ingredients in different parts (cortex and stele). For S. baicalensis, as the growth period increases, the part of stele in the whole root gradually decays, and the proportion of cortex increases rapidly. Our results showed that the contents of active ingredients including baicalin, wogonoside, baicalein, and wogonin in cortex were significantly higher than those in stele (P ≤ 0.05, Fig. 5 ). Thus, the contents of older roots (rotten stele, older than 4 years) were significantly higher than those of younger roots (non-rotten-stele, not older than 4 years). The proportion of stele and cortex in the root of 2-years-old S. baicalensis was balanced, so the content of four active components retained high levels. However, for 3- to 5-year-old S. baicalensis, the enlargement of roots was mainly due to the rapid growth of stele. Therefore, the contents of active ingredients were relatively low. When S. baicalensis grows for more than three years, the part of stele in the whole roots begin to rot and the proportion of cortex increases year by year. Meanwhile, the contents of six main active ingredients in 15-year-old roots of S. baicalensis were significantly higher than those in 2-year-old roots (Fig. 5). Further comparative analysis of the contents of six active ingredients in cortex and stele revealed that the contents of five medicinal active ingredients (baicalin, wogonoside, scutellarin, baicalein, wogonin) in the cortex of 2-year-old and 15-year-old S. baicalensis were all significantly higher than those of the stele (P ≤ 0.05) except for scutellarein. However, the contents of baicalin, wogonoside, scutellarein in the stele of the roots of 2-year-old S. baicalensis were all significantly higher than those of the cortex. The contents of baicalein and wogoninin in the stele of 15-year-old S. baicalensis were all significantly higher than those of 2-year-old S. baicalensis. The above results indicated that the contents of active ingredients in S. baicalensis tended to decrease with the increase of growth years (more than 3 years). Since the stele began to rot after several years, the proportion of cortex in roots increased year by year. Due to the great difference of the contents of active ingredients in cortex and stele, the contents of six medicinal active ingredients in the whole root of 2-year-old S. baicalensis was lower than that of 15-year-old S. baicalensis, but it was higher than that of 3- to 5-year-old S. baicalensis. Collectively, these results will give reasonable interpretations why S. baicalensis with rotten stele is taken as top-quality products in TCM, thereby providing theoretical basis for determining the best harvesting time as 2 or 2.5 years.
Fig. 5.
The contents of six active ingredients in whole root, cortex and stele of S. baicalensis.
The contents of six active ingredients (from A to E: baicalin, wogonoside, scutellarin, baicalein, wogonin, and scutellarein) in whole root, cortex and stele of 2-year-old and 15-year-old S. baicalensis were estimated and compared. Data were presented as Mean ± SD. *P ≤ 0.05, **P ≤ 0.01 (Student’s t test).
3.3. Effects of storage time on the contents of active ingredients in fresh-crushed roots
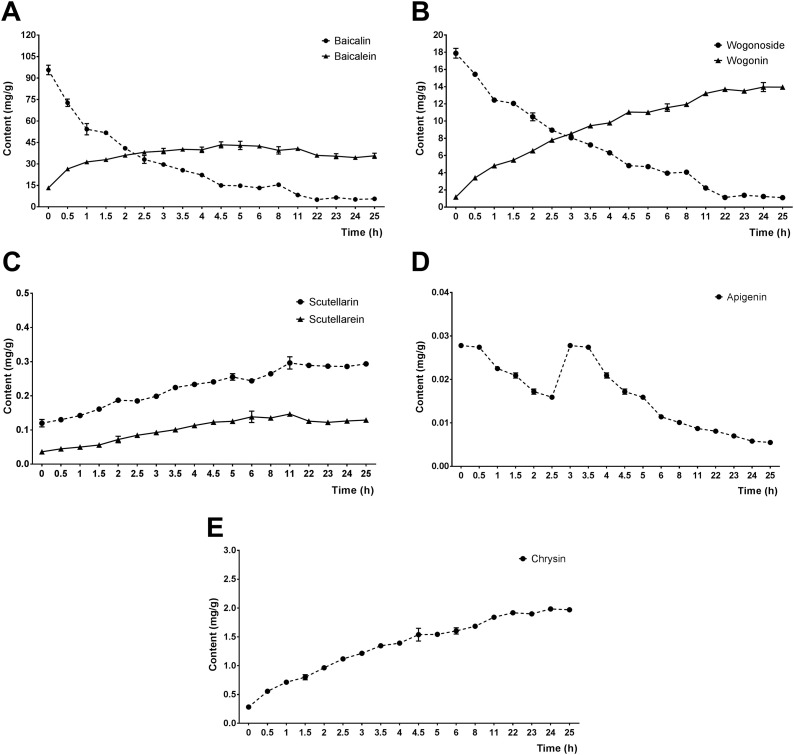
In general, the plant of S. baicalensis needs to be dried after harvesting in the primary processing process. During the subsequent extracting process, it will be softened with water or steam, and then sliced, and finally dried for extraction. Thus, it is of great importance to investigate how the storage time before extraction affects the contents of active ingredients in the industrial production of S. baicalensis. In this study, the fresh roots of S. baicalensis were directly crushed after removing sediments. The samples were then stored for different times at room temperature to reveal the trend of the contents of eight active ingredients changing with storage time. The results showed that the contents of chrysin, baicalein, wogonin and scutellarein in the fresh-crushed roots of S. baicalensis increased from 0 h to 25 h at room temperature (Fig. 6 ). Among them, the contents of chrysin and wogonin showed similar trend, and then they reached the highest level at 25 h (Dry weight: 1.97 mg/g and 13.94 mg/g, respectively), which were 6.0 % and 10.9 % higher than those in the beginning time (0 h). The content of baicalein increased slowly, reaching a stable level at about 38.84 mg/g after 2 h. On the contrary, the contents of baicalin and wogonoside continued to decline with the extension of storage time, and tended to be stable at around 22 h. The two active ingredients decreased from 95.67 mg/g and 17.89 mg/g (0 h storage) to 5.04 mg/g and 1.13 mg/g (22 h storage), with decreases of 94.7 % and 93.7 %, respectively. In particular, the contents of baicalin and wogonoside decreased significantly from 0 h to 1 h. The content of apigenin showed a generally downward trend with the extension of storage time, and it rebounded at 3 h–4 h with a peak of 0.03 mg/g, and then continued to decline after 4 h. Collectively, the above results showed that the storage time at room temperature had a significant effect on the eight active ingredients in fresh-crushed roots of S. baicalensis. According to the trends of active ingredients to be extracted, specific extraction process should be developed for the industrial production of S. baicalensis.
Fig. 6.
The contents of eight active ingredients in fresh-crushed roots of S. baicalensis with different storage time.
The contents of eight active ingredients in fresh-crushed roots of S. baicalensis with different storage time (0 h, 0.5 h, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 4.5 h, 5 h, 6 h, 8 h, 11 h, 22 h, 23 h, 24 h, and 25 h) were estimated and compared. (A) baicalin and baicalein; (B) wogonoside and wogonin; (C) scutellarin and scutellarein; (D) apigenin; (E) chrysin. Data were presented as Mean ± SD.
3.4. Effects of storage time on the contents of active ingredients in fresh-cut roots
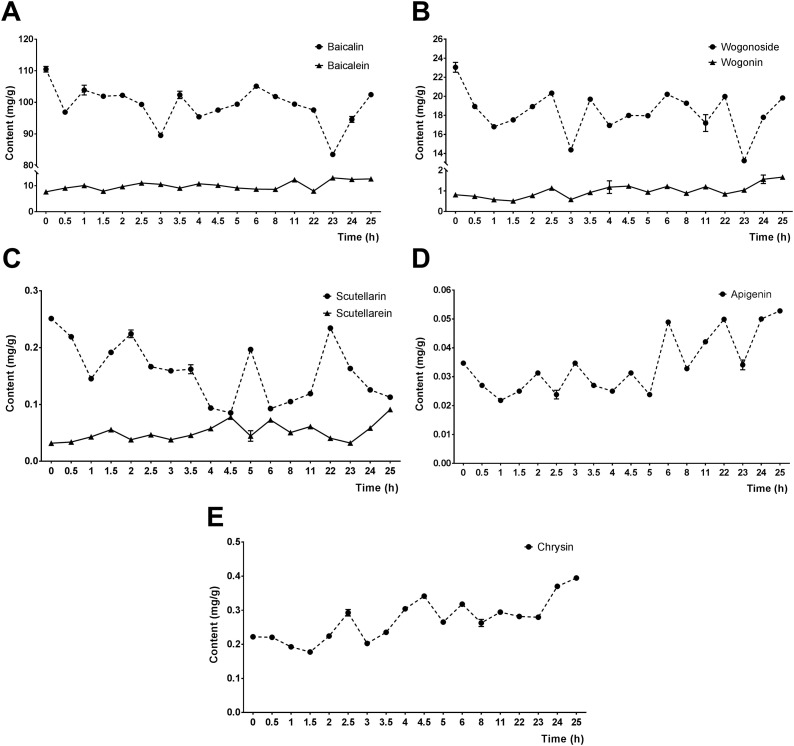
In traditional processing methods, the roots of S. baicalensis are dried after harvesting, and then softened by steam or boiling water, which is convenient for further cutting. However, this process takes a long time and consumes energy. Moreover, the contents of effective active ingredients in the slices will decrease once the dried roots are softened with steam or boiling water. It is not clear how the active ingredients change after the fresh roots of S. baicalensis were cut to slices and then placed at room temperature for different times. In this study, the fresh slices of roots of S. baicalensis were stored for different times at room temperature, and then the contents of active ingredients were determined after drying and comminuting. The results showed that the contents of baicalein (Fig. 7 A), wogonin (Fig. 7B), and chrysin (Fig. 7E) gradually increased with the extension of storage time at room temperature, reaching the maximum of 0.39 mg/g, 12.67 mg/g and 1.67 mg/g, respectively at about 25 h. Compared with those at 0 h, the contents of baicalin (Fig. 7A), wogonoside (Fig. 7B), and scutellarin (Fig. 7C) all decreased significantly with the extension of storage time, but were observed with a repeated fluctuation at different storage time. Comparatively, the two active ingredients apigenin (Fig. 7D) and scutellarein (Fig. 7C) had relatively low contents with a gradually increasing trend, reaching a maximum of 0.053 mg/g and 0.091 mg/g, respectively, at storage time of 25 h. The above results indicated that the contents of baicalin, wogonoside, and scutellarin in the slices of fresh roots of S. baicalensis will significantly reduce as the storage time increases, but the reduction of fresh-cutting was significantly lower than that of fresh-crushing.
Fig. 7.
The contents of eight active ingredients in fresh-cut roots of S. baicalensis with different storage time.
The contents of eight active ingredients in fresh-cut roots of S. baicalensis with different storage time (0 h, 0.5 h, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 4.5 h, 5 h, 6 h, 8 h, 11 h, 22 h, 23 h, 24 h, and 25 h) were estimated and compared. (A) baicalin and baicalein; (B) wogonoside and wogonin; (C) scutellarin and scutellarein; (D) apigenin; (E) chrysin. Data were presented as Mean ± SD.
3.5. Correlation analysis of medicinal active ingredients in fresh-crushed roots of S. baicalensis
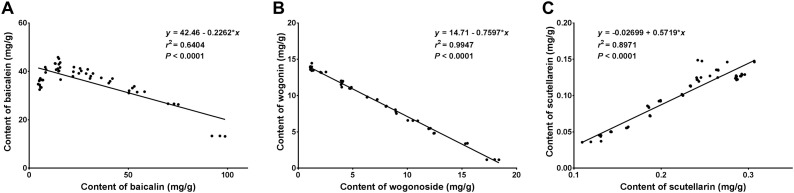
The above results demonstrated that the eight medicinal active ingredients exhibited increasing or decreasing trends changing with storage time after crushing the fresh roots of S. baicalensis at room temperature. To investigate whether there exist correlations among the changes of active ingredients, we further conducted correlation analysis on the contents of different active ingredients. The results showed that there was significant negative correlations between the contents of baicalin and baicalein (correlation coefficient: -0.815, P < 0.0001, Fig. 8 A), as well as the contents of wogonoside and wogonin (correlation coefficient: -0.998, P < 0.0001, Fig. 8B), indicating that there exists mutual conversion between baicalin and baicalein, and between wogonoside and wogonin. The contents of baicalein and wogonin increase significantly with the extension of storage time, while the contents of baicalin and wogonoside decrease significantly. On the contrary, the contents of scutellarin and scutellarein (Fig. 8C) have a very significant positive correlation with correlation coefficient as high as 0.95 (P < 0.0001). The contents of several other ingredients were relatively low, and the correlations among them were not significant (P > 0.05). These results suggested that the secondary metabolism in fresh roots of S. baicalensis had not been stopped after crushing, and there were still mutual transformations among different components.
Fig. 8.
The correlation analysis of medicinal active ingredients in fresh-crushed roots of S. baicalensis.
The correlations among eight active ingredients in fresh-crushed roots of S. baicalensis were analyzed. (A) The correlation of the contents between baicalin and baicalein; (B) The correlation of the contents between wogonoside and wogonin; (C) The correlation of the contents between scutellarin and scutellarein. Data were presented as Mean ± SD.
3.6. Effects of different processing treatments on the contents of eight medicinal active ingredients in S. baicalensis
In recent years, the market demand for the raw medicinal materials of S. baicalensis is growing, with the development of pharmacological studies (Yuan et al., 2010). Specifically, pharmaceutical enterprises mainly focus on how to obtain the maximum contents of active ingredients in medicinal materials. In this study, six different post-harvest treatments were designed and used to systematically reveal the effects of different processing treatments on the contents of eight medicinal active ingredients (Table 2 ). The results showed that the contents of four main active ingredients (baicalin, wogonoside, baicalein, and wogonin) under D and C-D treatments were significantly higher than those of the other four treatments (P ≤ 0.05), indicating that the active ingredients in roots of S. baicalensis lost considerably after softening treatment by steam. The contents of the other four medicinal active ingredients were all lower than 0.06 % (corresponding to 0.6 mg/g), which were significantly lower than those of the four main active components in roots (P ≤ 0.05). Among the six different treatments, the contents of baicalin were all higher than 10 %, which was in accordance with the standard of the Pharmacopoeia. The highest content was observed in D treatment (134.54 mg/g), followed by C-D treatment (129.48 mg/g). The contents of baicalin and wogonoside of D-S-C treatment were the lowest (104.09 mg/g and 18.27 mg/g, respectively). Collectively, the above results suggested that pharmaceutical companies should adopt appropriate processing treatments according to the characteristics of medicinal materials, since the active ingredients were significantly influenced by processing methods. For instance, the crushing process after direct drying (C-D treatment) is very suitable for improving the yield of active ingredients. In addition to the D-S-C treatment (cutting after drying and softening), the C-S-D treatment (killing enzyme by steam after cutting, and then drying the slices) is a potential processing method for the industrial production of S. baicalensis.
Table 2.
The contents of eight medicinal active ingredients of S. baicalensis under different treatments (mg/g).
| Treatments | The contents of medicinal active ingredients (Dry weight, mg/g) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baicalin | Wogonoside | Baicalein | Wogonin | Scutellarin | Scutellarein | Apigenin | Chrysin | |
| D | 134.538 ± 0.190 a | 26.582 ± 0.021 b | 3.962 ± 0.008 b | 1.387 ± 0.007 b | 0.463 ± 0.003 c | 0.099 ± 0.001 a | 0.057 ± 0.000 a | 0.313 ± 0.001 b |
| D-S- C-D | 128.146 ± 0.295 bc | 22.314 ± 0.087 e | 2.426 ± 0.014 c | 0.998 ± 0.006 c | 0.591 ± 0.007 a | 0.042 ± 0.001 c | 0.055 ± 0.000 b | 0.345 ± 0.002 a |
| D-S-C | 104.085 ± 0.194 d | 18.274 ± 0.048 f | 2.085 ± 0.011 d | 0.890 ± 0.002 d | 0.574 ± 0.004 b | 0.033 ± 0.000 e | 0.036 ± 0.001 c | 0.298 ± 0.001 c |
| C-S-D | 128.001 ± 0.155 bc | 26.106 ± 0.077 c | 0.900 ± 0.004 e | 0.630 ± 0.003 f | 0.365 ± 0.004 e | 0.036 ± 0.001 d | 0.028 ± 0.001 d | 0.106 ± 0.000 f |
| S- C-D | 124.634 ± 0.084 c | 25.059 ± 0.124 d | 0.860 ± 0.003 f | 0.733 ± 0.003 e | 0.392 ± 0.002 d | 0.062 ± 0.001 b | 0.055 ± 0.000 a | 0.137 ± 0.001 e |
| C-D | 129.478 ± 0.185 b | 26.946 ± 0.052 a | 5.200 ± 0.012 a | 1.522 ± 0.004 a | 0.191 ± 0.005 f | 0.026 ± 0.001 f | 0.026 ± 0.000 e | 0.192 ± 0.002 d |
Note: Different letters indicate significant differences of the content of each medicinal active ingredient in the same column (P ≤ 0.05).
3.7. Effects of different processing treatments on the total content of four main medicinal active ingredients in S. baicalensis
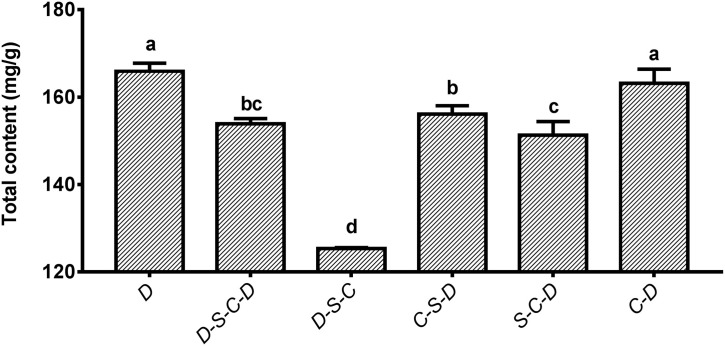
Recent studies have revealed that there exists conversion among different active ingredients in plants, and only one active ingredient is difficult to reflect the quality and stability of medicinal materials (Li et al., 2020b). Accordingly, in the latest release of Chinese Pharmacopoeia, it is stipulated that the total content of baicalin, wogonoside, baicalein, and wogonin should not less than 13.5 % for S. baicalensis (National Pharmacopoeia Committee, 2020). In this study, the total content of four main active ingredients in the root of S. baicalensis treated with six different processing treatments was further analyzed (Fig. 9 ). The results showed that the total content of the four main active ingredients of S. baicalensis roots under D treatment was the highest (166.47 mg/g), followed by that of C-D treatment (163.15 mg/g), which was much higher than the standard of 13.5 % (corresponding to 135 mg/g) stipulated in the Chinese Pharmacopoeia (2020) (National Pharmacopoeia Committee, 2020). For S. baicalensis roots under D-S-C treatment, the total content of the four main active ingredients decreased to 125.33 mg/g, which was below the minimum requirement of 13.5 % by the Pharmacopoeia. The total contents of the four main active ingredients under D-S- C-D, C-S-D, and S- C-D treatments were 153.88 mg/g, 155.64 mg/g, and 151.29 mg/g, respectively, which were all lower than those of D treatment (16.6 %) and C-D treatment (16.32 %), but were higher than the minimum requirement of 13.5 %. Collectively, the above results indicated that different post-harvest processing treatments had significant effects on the total contents of medicinal active ingredients of S. baicalensis. Specifically, the D-S- C-D treatment can be adopted for the medicinal materials used in traditional formulations of Chinese medicine, while the D and C-D treatments can be used in industrial production since neither of the two treatments significantly reduce the total content of four main active ingredients in medicinal materials. Compared with D and C-D treatments, the total content of the four main active ingredients under C-S-D treatment significantly decreased. Nevertheless, the traditional processing treatment (D-S- C-D) requires four steps including drying, softening, cutting, and drying treatment, which is considered to high consumption of energy and time. In contrast, the C-S-D treatment with energy conservation and high efficiency is a potential processing technology to be further studied in the future.
Fig. 9.
The total content of four main active ingredients in roots of S. baicalensis under different treatments.
The total content of four main active ingredients (baicalin, wogonoside, baicalein, and wogonin) in roots of S. baicalensis under different treatments were estimated and analyzed. D: drying; S: steaming; C: slicing. Different letters indicate significant differences of the total content of four main active ingredients among different treatments (P ≤ 0.05). Data were presented as Mean ± SD.
4. Discussion
The contradictions between harvesting years and contents of active ingredients have long been the primary issue for the industrial production of medicinal crops (Saha and Basak, 2020; Wang et al., 2017). At present, the medicinal materials of S. baicalensis used in pharmaceutical industry are mostly collected from cultivation resources. According to our field investigation as of May 2020, the cultivated area of S. baicalensis is more than 26,000 ha in China, with an annual production of about 60,000 tons for raw materials. However, an emerging challenge faced by herbalists and pharmaceutical enterprises is how to obtain the maximum yield of active ingredients from limited medicinal materials. For S. baicalensis, its root gradually grows thicker, and the production of medicinal materials increases with cultivation years. However, the yield of active ingredients showed different regular patterns changing with growth years (Xu et al., 2018). In this study, the contents of four main medicinal active ingredients (baicalin, wogonoside, baicalein, and wogonin) showed a changing trend of "V" type from 1-year-old to 15-year-old S. baicalensis, indicating that the contents of 2 or 3 growth years and 15 growth years were higher than those of 4 or 5 growth years. The main reason is mostly due to the age of plant tissues. As the growth year increases, the phenotypic, biochemical, and physiological characters (e.g. leaf area, chlorophyll content) of plants change gradually. Previous studies have found that leaf area as well as chlorophyll content have critical effects on the contents of primary and secondary metabolites of crops (Munz et al., 2018; Senger et al., 2014; Hudina and Štampar, 2002). For S. baicalensis, its capacity of sugar biosynthesis increases with growing leaf area and chlorophyll content, thereby increasing the contents of secondary metabolites (e.g. the four main active ingredients). However, the increasing leaf area will also lead to more consumptions of organic matters through cellular respiration. Thus, there exists a trade-off between accumulation of secondary metabolites and consumption of organic matters. This is rational to explain the “V” type trend of active ingredients of 1-year-old to 15-year-old S. baicalensis. In addition, another reason is probably the significant differences of the contents of active ingredients between cortex and stele in roots of S. baicalensis. In 2-year and 15-year old S. baicalensis, the contents of baicalin, wogonoside, scutellarin, scutellarein, baicalein, and wogonin in the part of cortex were significantly higher than those of stele (P ≤ 0.01, Fig. 5). Previous studies have shown that baicalein and wogonin are mainly distributed in cortex, while baicalin and wogonoside are evenly distributed in both cortex and stele (Wei et al., 2015). Meanwhile, it is found that the contents of baicalin, wogonoside, baicalein, and wogonin in roots of 2-years-old S. baicalensis is higher than that of 1.5-year and 3-year-old S. baicalensis (Chen and Hu, 2006; Li et al., 2008). After growing for more than 3 years, the part of stele in the whole root will gradually decay, and the proportion of cortex will increase year after year. Therefore, the contents of five medicinal active ingredients in roots of S. baicalensis older than 4 growth years are significantly higher than those of 2 growth years. Considering only land use efficiency and product yield, the contents of active ingredients, and the yield of medicinal materials in perennial S. baicalensis (more than 3 years) were both higher than those of 2 years. However, the increment of yield per hectare and per year significantly reduced in S. baicalensis growing over three years (Table 1). Moreover, the increment of economic benefit per unit area and per year significantly reduced with growing years of land utilization. In addition, for S. baicalensis growing more than 3 years, the part of stele in the whole root will gradually rot and the proportion of cortex will increase. The contents of baicalin, wogonoside, scutellarin, baicalein, and wogonin in cortex were significantly higher than those in stele (P < 0.001) (Fig. 5). Thus, the contents of active ingredients in roots (with decayed stele) growing more than 3 years is significantly higher than that of roots (without decayed stele) growing less than 3 years. Taken together, the best harvesting year of S. baicalensis is determined as cultivated 2–3 years based on comprehensive evaluation of the content of medicinal active ingredients, the increment of annual yield, and the efficiency of land utilization. Collectively, the above results not only gave interpretations for the differences of medicinal active ingredients in different growing years and different parts of roots, but also provided valuable references that old S. baicalensis (rotten or hollow stele) was best quality of Chinese medicinal materials in ancient pharmacopoeia.
The duration of storage time is also an important factor that effects the yield of active ingredients. In traditional processing of S. baicalensis products, the farmers usually dried the roots immediately in the sun after harvesting, and then softened the dried roots with steam and cut the soft roots into slices. Finally, the dried slices were sold to pharmaceutical companies or hospitals for further processing. Typically, this process will take considerable time and lead to loss of active ingredients. Thus, new processing approaches are needed to solve this problem. Reducing the effect of storage time is a potential way to achieve such gorals. For the industrial production of S. baicalensis, it will greatly improve efficiency and reduce cost if the fresh roots are directly used for the extraction of medicinal active ingredients. However, the medicinal active ingredients of S. baicalensis are mainly flavonoids, which are secondary metabolites synthesized by a variety of key enzymes (Zhao et al., 2016b). It is necessary to systematically reveal how the contents of active ingredients changes with the prolongation of storage time at room temperature after crushing or cutting, thereby determining whether the processing methods are effective and reliable. Previous studies have demonstrated that the four main medicinal active ingredients (baicalin, wogonoside, baicalein, and wogonin) in roots of S. baicalensis are located in the downstream of flavonoid synthesis pathway (Zhao et al., 2016b). Glycosides and aglycones can be mutually transformed by the enzymes of UDP-glucuronate: baicalein 7-O-glucuronosyltransferase (UBGAT) (Nagashima et al., 2000) and glucuronidase (GUS) (Sakurama et al., 2013). When the plant of S. baicalensis is affected by external stress environment, the cells will produce a large amount of H2O2 (Fu et al., 2018). Baicalin, a glycoside component in roots of S. baicalensis, is immediately hydrolyzed to baicalein (an aglycone component) under the action of GUS (Dikaya et al., 2018; Matsuda et al., 2000). In this study, we revealed the dynamic change of the effects of storage time on the contents of eight active ingredients in fresh-crushed and fresh-cut roots at room temperature. The results showed that the contents of glycosides (baicalin and wogonoside) decreased linearly with the extension of storage time after the fresh roots were crushed or cut, while those of aglycones (baicalein and scutellarin) increased linearly (P ≤ 0.01). These results indicated that the secondary metabolism in tissues of fresh roots did not stop immediately after crushing or cutting. With the extension of storage time at room temperature, the UBGAT enzyme hydrolyzed the glycosides to produce glucuronic acid and aglycones in suitable conditions. Based on these observations, the best processing methods for the extraction of active ingredients should be determined according to characteristics of specific products. For instance, for the extraction of chrysin, baicalein, and wogonin, the fresh slices should be stored at room temperature (22°C) for more than 25 h after roots were fresh-crushed or fresh-cut. On the contrary, for the extraction of baicalin, wogonoside, and apigenin, the fresh slices should be extracted as soon as possible after roots were fresh-crushed or fresh-cut. Compared with fresh-crushing treatment, the content of glycosides under fresh-cutting treatment in roots has a little decrease. Collectively, the processing technology for fresh-crushed or fresh-cut roots of S. baicalensis should be further studied in the future.
Among the factors (excellent germplasm, cultivation area, water and fertilizer management, pest control, growth years, harvesting time, etc.) that affect the quality of Chinese medicinal materials, the post-harvest processing has a great impact on the content of medicinal active ingredients, which would further affect the clinical efficacy and stability of TCM (Wu et al., 2018a, 2018b; Xiao et al., 2016; Zhao et al., 2010). For S. baicalensis, the traditional post-harvest processing technology is to remove impurities after harvesting and to dry naturally in sun. Generally, it will not directly kill the enzymes in fresh roots of S. baicalensis using steam. Instead, the dried roots will be softened by steam, and then cut to slices, and finally to be dried again. Previous studies have confirmed that steam treatment is better than water boiling treatment to soften the dried roots of S. baicalensis (Liao, 2006). The former has high temperature, strong penetration and good enzyme-killing effect, while the latter has the dried roots expose to water with slower softening speed so that some glycoside components will be dissolved in water (Qin et al., 2017; Liao et al., 2007). Therefore, the steam treatment is used to soften dried roots and to kill enzyme activity before cutting to slices, thereby preventing the content of glycosides from decreasing in traditional methods. In recent years, a series of new drugs including extracts, drop pills, formula particles and injection originated from S. baicalensis have been developed, leading to increasing demands for the raw medicinal materials of S. baicalensis. Thus, it is bringing urgent needs that novel processing approaches should be proposed accordingly based on the traditional methods. To develop effective and reliable processing methods, a primary issue is that it remains unclear how the different treatments and processing technology affect the contents of medicinal active ingredients. In this study, we systematically designed six kinds of processing and technology according to several traditional treatments of post-harvest processing of S. baicalensis. The total contents of the four main medicinal active ingredients in roots of S. baicalensis using different processing treatments after harvest were shown as (from high to low): D > C-D > C-S-D > D-S- C-D > S- C-D > D-S-C (Table 2). Among them, the total contents of four main active ingredients of C-D treatment declined markedly compared with those of D treatment, which may be related to the fact that baicalin and wogonoside can be transformed into baicalein and wogonin by the key enzyme UBGAT during the cutting process of fresh roots of S. baicalensis (Nagashima et al., 2000). Compared with other treatments of softening and slicing, the contents of active ingredients under D treatment (drying and cutting directly) have the highest levels, indicating that this treatment could effectively reduce the loss of active ingredients during the processing process. For traditional methods, it is very difficult to cut the dried roots to slices following the D treatment. Meanwhile, the cutting process of dried root is easy to form debris and irregular particles, which will not pass the quality control for use in medicine. However, for pharmaceutical enterprises, it is easy to cut the dried roots directly and then extract effective active ingredients including baicalin, wogonoside, and other ingredients. As a traditional processing method, the D-S- C-D treatment typically softened the dried roots first and then cut to slices. The contents of active ingredients under such treatment is significantly higher than those of D-S-C treatment (drying-softening-cutting) (P ≤ 0.05). This result is related to the fact that moisture is often absorbed into dried roots during the process of steaming and wet roots that are not dried in time. Therefore, the baicalin in roots is susceptible to be hydrolyzed into other components in moisture conditions by steaming. To prevent the reduction and conversion of glycosides, the most effective treatment should be killing the enzyme activity by steaming. In the industrial production, the steam treatment and the drying treatment with high temperature can kill the enzyme in roots of S. baicalensis to prevent the reduction or conversion of baicalin. For the efficiency of killing enzyme activity in fresh root slices of S. baicalensis, the result showed that the contents of medicinal active ingredients with drying and cutting treatment (C-D) are higher than those of C-S-D treatment (cutting-softening-drying). The most possible reason should be that the enzyme activity quickly lost under high temperature during the drying process, while the moisture could transform glycosides into aglycones in the process of steaming treatment. Meanwhile, the other active components are reduced and lost with the condensation and dissolution of water vapor. The contents of active ingredients of C-S-D treatment are higher than those of S- C-D treatment. This result may be related to that the enzyme inactivation is faster and more complete in C-S-D treatment. Consequently, the dried slices can retain more active ingredients in roots of S. baicalensis.
In traditional processing methods, the roots of S. baicalensis need to be dried twice (one for killing enzyme activity by steam, while another for cutting to slices), which is energy-consuming and time-consuming. Therefore, it is necessary to optimize the traditional processing technology of S. baicalensis to minimize energy consumption during the drying process and the softening treatment by steam. A prospective strategy is that the fresh medicinal materials being cut into slices and processed after harvest in the original cultivated areas, which will not only save time and energy during the processing process, but also maintain the quality of medicinal materials (Zhao et al., 2017). In recent years, researchers have paid more attention to the processing technology of Chinese medicinal materials using fresh slicing on the premise of ensuring the quality (Tang et al., 2010). Specifically, it is clearly proposed that further studies should focus on the deep processing technology of fresh-cutting for medicinal materials in the Protection and Development Plan of Chinese Medicinal Materials (2015–2020). Up to date, a series of studies aimed at reducing the effects of softening in dried medicinal materials and exploring the fresh-cutting methods have been reported in Chinese medicinal materials including common peony (Paeonia lactiflora Pall.) (Jin et al., 2011), dan shen (Salvia miltiorrhiza Bge.) (Zhao et al., 2017), and cassia bark tree (Cinnamomum cassia Presl) (Luo et al., 2017). Inevitably, the traditional processing technology (D-S- C-D) for S. baicalensis have the issues of mutual conversion and hydrolysis of active ingredients. Our results first demonstrated that the C-S-D treatment of S. baicalensis would be a candidate of optimal processing approaches that produce the maximum yield of active ingredients. The comparative analysis results of six processing technology in this study showed that the total contents of eight active ingredients and four main active ingredients in roots of S. baicalensis were less affected under C-S-D treatment than that of the traditional processing technology (D-S-C–D). Collectively, the processing technology of fresh-cutting for S. baicalensis will become the mainstream processing technology with cost-saving, environment-friendly and energy-saving for the extraction of active ingredients in pharmaceutical enterprises in the future, which is of epochal significance for accelerating the modernization of traditional Chinese medicinal materials.
5. Conclusion
In this study, we first revealed the effects of growth years and post-harvest processing on the contents of medicinal active ingredients of S. baicalensis. By estimating the contents of eight active ingredients (baicalin, wogonoside, baicalein, wogonin, scutellarin, scutellarein, apigenin, and chrysin) in roots of S. baicalensis of different growth years (ranging from 1 year to 15 years) using HPLC, we conducted a systematic analysis to determine the optimal harvest period of this important medicinal crop. Our results showed that the best harvest period for S. baicalensis should be determined as 2–3 years based on comprehensive evaluation of active ingredient content, annual yield increment, and land use efficiency. Meanwhile, we compared the contents of six active ingredients in different parts (cortex and stele) of roots of S. baicalensis, and monitored the dynamic changes of the contents of active ingredients in fresh-crush and fresh-cut roots of S. baicalensis at room temperature. In addition, we observed the effects of six different post-harvest treatments on the contents of active ingredients to determine the best primary processing technology. We concluded that the contents of baicalin, wogonoside, baicalein, and wogonin in cortex were significantly higher than those in stele (P ≤ 0.05). The contents of four main active ingredients (baicalin, wogonoside, baicalein, and wogonin) under drying (D) and cutting-drying (C-D) treatments were significantly higher than those of the other four treatments (P ≤ 0.05). Collectively, these results will provide valuable references for determining the optimal harvesting and processing technology that will obtain the maximum products of active ingredients for S. baicalensis. Furthermore, the processing methods of softening, cutting, and drying proposed in this study will help improving the product yield for pharmaceutical industry of other medicinal crops.
Data and materials availability
All data is available in the main text and the supplementary materials.
CRediT authorship contribution statement
Chengke Bai: Conceptualization, Writing - original draft, Supervision. Jingjing Yang: Investigation, Resources, Writing - review & editing. Bo Cao: Investigation, Methodology, Writing - review & editing. Ying Xue: Resources, Writing - review & editing. Pufan Gao: Investigation, Resources. Hui Liang: Investigation, Resources. Guishuang Li: Methodology, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
We thank Drs. Junru Yu and Lei Zhang for their helpful revision on this manuscript. This work was supported by the Key Research and Development Projects of Industry Innovation of Shaanxi Province [2020ZDLSF05-11 to C.K.B.], the National Key Research and Development Program of China [2019YFC1712600 to C.K.B.], the Innovation Team Project of Breeding and Standardized Production of New Varieties of Traditional Chinese Medicine in Fundamental Research Funds for the Central Universities [GK201801008 to C.K.B.], and the Youth Program of the Second Affiliated Hospital, School of Medicine, Xi’an Jiaotong University [YJ(QN)201714 to B.C.].
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.indcrop.2020.112985.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Azizi M. Change in content and chemical composition of Hypericum perforatum L. oil at three harvest time. J. Herb. Spice. Med. Plant. 2008;13:79–85. doi: 10.1300/J044v13n02_07. [DOI] [Google Scholar]
- Bai C., Wen M., Zhang L., Li G.S. Genetic diversity and sampling strategy of Scutellaria baicalensis germplasm resources based on ISSR. Genet. Resour. Crop Ev. 2013;60:1673–1685. doi: 10.1007/s10722-012-9949-9. [DOI] [Google Scholar]
- Bai C., Xu J., Cao B., Li X., Li G.S. Transcriptomic analysis and dynamic expression of genes reveal flavonoid synthesis in Scutellaria viscidula. Acta Physiol. Plant. 2018;40:161. doi: 10.1007/s11738-018-2733-5. [DOI] [Google Scholar]
- Balandrin M.F., Klocke J.A., Wurtele E.S., Bollinger W.H. Natural plant chemicals: sources of industrial and medicinal materials. Science. 1985;228:1154–1160. doi: 10.1126/science.3890182. [DOI] [PubMed] [Google Scholar]
- Barata A.M., Rocha F., Lopes V., Carvalho A.M. Conservation and sustainable uses of medicinal and aromatic plants genetic resources on the worldwide for human welfare. Ind. Crop. Prod. 2016;88:8–11. doi: 10.1016/j.indcrop.2016.02.035. [DOI] [Google Scholar]
- Canter P.H., Thomas H., Ernst E. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 2005;23:180–185. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Cao B., Bai C., Wu K., Xue Y., Yang J., Gao P., Liang H., Li G. Concentrated conservation and utilization: four medicinal crops for diabetes treatment showed similar habitat distribution patterns in China. Ind. Crop. Prod. 2020;152:112478. doi: 10.1016/j.indcrop.2020.112478. [DOI] [Google Scholar]
- Chan K.W., Wong V.T., Tang S.C.W. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western medicine for the management of 2019 novel coronavirus disease. Am. J. Chin. Med. 2020;48:737–762. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Hu J.H. The origin and growth period of cultivated Scutellaria baicalensis Georgi. Chin. Tradit. Herb. Drug. 2006;37:614–615. [Google Scholar]
- Chen L.W., Qin K.M., Zhu Y.H., Cai H., Li W.D., Cai B.C. Research status and prospect of primary processing of traditional Chinese medicinal materials. China J. Chin. Mater. Med. 2015;40:602–606. [PubMed] [Google Scholar]
- Chen S.L., Yu H., Luo H.M., Wu Q., Li C.F., Steinmetz A. Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin. Med. 2016;11:37. doi: 10.1186/s13020-016-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Zhang Z., Xu X., Zhang X., Zhao Z., Liu Y., Wang Q., Wang H., Li Y., Yang G., Guo L., Tang Z., Huang L. Threatened medicinal plants in China: distributions and conservation priorities. Biol. Conserv. 2017;210:89–95. doi: 10.1016/j.biocon.2017.04.015. [DOI] [Google Scholar]
- Chukwuma C.I., Matsabisa M.G., Ibrahim M.A., Erukainure O.L., Chabalala M.H., Islam M.S. Medicinal plants with concomitant anti-diabetic and anti-hypertensive effects as potential sources of dual acting therapies against diabetes and hypertension: a review. J. Ethnopharmacol. 2019;235:329–360. doi: 10.1016/j.jep.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Dikaya V.S., Solovyeva A.I., Sidorov R.A., Solovyev P.A., Stepanova A.Y. The relationship between endogenous β-glucuronidase activity and biologically active flavones-aglycone contents in hairy roots of baikal skullcap. Chem. Biodivers. 2018;15:e1700409. doi: 10.1002/cbdv.201700409. [DOI] [PubMed] [Google Scholar]
- Duan J., Su S., Lv J., Yan H., Ding A. Traditional experiences and modern cognition on primary processing of traditional Chinese medicinal materials. China J. Chin. Mater. Med. 2009;34:3151–3157. [PubMed] [Google Scholar]
- Feachem R.G.A., Chen I., Akbari O., Bertozzi-Villa A., Bhatt S., Binka F., Boni M.F., Buckee C., Dieleman J., Dondorp A., Eapen A., Sekhri Feachem N., Filler S., Gething P., Gosling R., Haakenstad A., Harvard K., Hatefi A., Jamison D., Jones K.E., Karema C., Kamwi R.N., Lal A., Larson E., Lees M., Lobo N.F., Micah A.E., Moonen B., Newby G., Ning X., Pate M., Quiñones M., Roh M., Rolfe B., Shanks D., Singh B., Staley K., Tulloch J., Wegbreit J., Woo H.J., Mpanju-Shumbusho W. Malaria eradication within a generation: ambitious, achievable, and necessary. Lancet. 2019;394:1056–1112. doi: 10.1016/S0140-6736(19)31139-0. [DOI] [PubMed] [Google Scholar]
- Fu X.Y., Guo H.M., Cong W., Meng X.C. Exogenous H2O2 regulated secondary metabolism of Scutellaria baicalensis and enhanced drug quality. China J. Chin. Mater. Med. 2018;43:271–287. doi: 10.19540/j.cnki.cjcmm.20171030.009. [DOI] [PubMed] [Google Scholar]
- Gao Z., Huang K., Yang X., Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta. 1999;1472:643–650. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- Gao H., Wang Z., Li Y., Qian Z. Overview of the quality standard research of traditional Chinese medicine. Front. Med. 2011;5:195–202. doi: 10.1007/s11684-011-0134-x. [DOI] [PubMed] [Google Scholar]
- Gao H., Li F., Xu Z., Huang C., Xiong C., Jiang C., Xie N., Leng L., Zhang Y., Yousaf Z., Liu X., Sun W. Genome-wide analysis of methyl jasmonate-regulated isoform expression in the medicinal plant Andrographis paniculata. Ind. Crop. Prod. 2019;135:39–48. doi: 10.1016/j.indcrop.2019.04.023. [DOI] [Google Scholar]
- Guo P., Brand E., Zhao Z. Chinese medicinal processing: a characteristic aspect of the ethnopharmacology of traditional Chinese medicine. In: Heinrich M., Jäger A.K., editors. Ethnopharmacology. John Wiley & Sons Ltd.; Chichester, UK: 2015. [DOI] [Google Scholar]
- Guo L., Lei C., Yang F., Duan C., Bai C. Similarity and diversity evaluation of bioactive ingredients in S. baicalensis and S. viscidula by HPLC. Northwest Pharm. J. 2016;31:9–12. [Google Scholar]
- He J. Harvest and trade of caterpillar mushroom (Ophiocordyceps sinensis) and the implications for sustainable use in the Tibet Region of Southwest China. J. Ethnopharmacol. 2018;221:86–90. doi: 10.1016/j.jep.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Hu L.Q., Xiong Y., Wang Y.Q., Cao L.J., Yang Y.Z., Jiao J.J., Yang M. Preliminary study on standardization of production and processing of Scutellaria baicalensis pieces. China J. Chin. Mater. Med. 2019;44:3281–3286. doi: 10.19540/j.cnki.cjcmm.20190522.301. [DOI] [PubMed] [Google Scholar]
- Hudina M., Štampar F. Influence of leaf area on the sugar and organic acids content in pear (Pyrus communis) fruits cultivar Williamss. Acta Hortic. 2002;596:749–752. doi: 10.17660/ActaHortic.2002.596.128. [DOI] [Google Scholar]
- Jin C.S., Li S.L., Wu D.L., Zhang W. Study on industrialized production technology of fresh-cut for Paeonia Radix Alba. China J. Chin. Mater. Med. 2011;36:3444–3448. [PubMed] [Google Scholar]
- Jin H., Liu Y., Guo Z., Wang J., Zhang X., Wang C., Liang X. Recent development in liquid chromatography stationary phases for separation of Traditional Chinese Medicine components. J. Pharm. Biomed. Anal. 2016;130:336–346. doi: 10.1016/j.jpba.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Kessler A., Kalske A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. S. 2018;49:115–138. doi: 10.1146/annurev-ecolsys-110617-062406. [DOI] [Google Scholar]
- Kotwal S., Kaul S., Dhar M.K. Comparative expression analysis of flavonoid biosynthesis genes in vegetative and reproductive parts of medicinally important plant, Plantago ovata Forssk. Ind. Crop. Prod. 2019;128:248–255. doi: 10.1016/j.indcrop.2018.11.016. [DOI] [Google Scholar]
- Li H., Huang L.Q., Yang B., Feng X.F., Li W., Tang N. Changes of active components in Scutellaria baicalensis Georgi at different growth stages. Chin. Tradit. Herb. Drug. 2008;39:604–607. [Google Scholar]
- Li X., Hu D., Bai C., Zhao N., Liang H. Cloning and bioinformatic analysis of SVFNSII-2 gene in Scutellaria viscidula. J. Chin. Med. Mater. 2019;42:37–44. [Google Scholar]
- Li R., Hou Y., Huang J., Pan W., Ma Q., Shi Y., Li C., Zhao J., Jia Z., Jiang H., Zheng K., Huang S., Dai J., Li X., Hou X., Wang L., Zhong N., Yang Z. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kong D., Fu Y., Sussman M.R., Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020;148:80–89. doi: 10.1016/j.plaphy.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Liao J.P. Hunan University of Chinese Medicine; Changsha, China: 2006. Study on the Standardization of Softening and Cutting Technology of Scutellaria baicalensis Georgi. [Google Scholar]
- Liao J.P., Ren W.Q., Yan M. Comparative study on different softening methods of Scutellaria baicalensis Georgi. J. Chinese Pharm. Sci. 2007;16:59–61. [Google Scholar]
- Liu S.Y. Zhejiang University; Hangzhou, China: 2015. Studies on Quality Control Methods for the Purification Process of Scutellaria baicalensis Water Extract. [Google Scholar]
- Liu X., Liu Y., Huang P., Ma Y., Qing Z., Tang Q., Cao H., Cheng P., Zheng Y., Yuan Z., Zhou Y., Liu J., Tang Z., Zhuo Y., Zhang Y., Yu L., Huang J., Yang P., Peng Q., Zhang J., Jiang W., Zhang Z., Lin K., Ro D.K., Chen X., Xiong X., Shang Y., Huang S., Zeng J. The genome of medicinal plant Macleaya cordata provides new insights into benzylisoquinoline alkaloids metabolism. Mol. Plant. 2017;10:975–989. doi: 10.1016/j.molp.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Liu H., Ye F., Sun Q., Liang H., Li C., Lu R., Huang B., Tan W., Lai L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. bioRxiv. 2020 doi: 10.1101/2020.04.10.035824. 2020.04.10.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S.X., Wang Y.Z., Li W.H., Zhang Y., Sun Z.Y., Nie R.J. Study on the feasibility of fresh cutting of Cinnamomum cassia Presl. J. Chin. Med. Mater. 2017;40:2836–2838. [Google Scholar]
- Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., Liu J.P. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020;26:243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Yu Q., Xing X., Liu S., Shi C., Luo J. San Wu Huangqin decoction, a Chinese herbal formula, inhibits influenza a/PR/8/34 (H1N1) virus infection in vitro and in vivo. Viruses. 2018;10:117. doi: 10.3390/v10030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manukumar H.M., Shiva Kumar J., Chandrasekhar B., Raghava S., Umesha S. Evidences for diabetes and insulin mimetic activity of medicinal plants: present status and future prospects. Crit. Rev. Food Sci. Nutr. 2017;57:2712–2729. doi: 10.1080/10408398.2016.1143446. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Hatano K., Harioka T., Taura F., Tanaka H., Tateishi N., Shan S., Morimoto S., Shoyama Y. Histochemical investigation of β-glucuronidase in culture cells and regenerated plants of Scutellaria baicalensis Georgi. Plant Cell Rep. 2000;19:390–394. doi: 10.1007/s002990050745. [DOI] [PubMed] [Google Scholar]
- Miller L.H., Su X. Artemisinin: discovery from the Chinese herbal garden. Cell. 2011;146:855–858. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M.K., Pandey S., Misra P., Niranjan A., Srivastava A. An efficient protocol for clonal regeneration and excised root culture with enhanced alkaloid content in Thalictrum foliolosum DC. - an endemic and important medicinal plant of temperate Himalayan region. Ind. Crop. Prod. 2020;152:112504. doi: 10.1016/j.indcrop.2020.112504. [DOI] [Google Scholar]
- Munz S., Präger A., Merkt N., Claupein W., Graeff-Hönninger S. Leaf area index, light interception, growth and steviol glycoside formation of Stevia rebaudiana Bertoni under field conditions in southwestern Germany. Ind. Crop. Prod. 2018;111:520–528. doi: 10.1016/j.indcrop.2017.11.021. [DOI] [Google Scholar]
- Nabet N., Gilbert-López B., Madani K., Herrero M., Ibáñez E., Mendiola J.A. Optimization of microwave-assisted extraction recovery of bioactive compounds from Origanum glandulosum and Thymus fontanesii. Ind. Crop. Prod. 2019;129:395–404. doi: 10.1016/j.indcrop.2018.12.032. [DOI] [Google Scholar]
- Nagashima S., Hirotani M., Yoshikawa T. Purification and characterization of UDP-glucuronate: baicalein 7-O-glucuronosyltransferase from Scutellaria baicalensis Georgi. cell suspension cultures. Phytochemistry. 2000;53:533–538. doi: 10.1016/s0031-9422(99)00593-2. [DOI] [PubMed] [Google Scholar]
- National Pharmacopoeia Committee . China Medical Science and Technology Press; Beijing: 2015. Pharmacopoeia of the People’s Republic of China. [Google Scholar]
- National Pharmacopoeia Committee . China Medical Science and Technology Press; Beijing: 2020. Pharmacopoeia of the People’s Republic of China. [Google Scholar]
- Nozad M., Khojastehpour M., Tabasizadeh M., Azizi M., Miraei Ashtiani S.H., Salarikia A. Characterization of hot-air drying and infrared drying of spearmint (Mentha spicata L.) leaves. J. Food Meas. Charact. 2016;10:466–473. doi: 10.1007/s11694-016-9325-0. [DOI] [Google Scholar]
- Park J., Kim R., Park E. Antioxidant and α-glucosidase inhibitory activities of different solvent extracts of skullcap (Scutellaria baicalensis) Food Sci. Biotechnol. 2011;20:1107. doi: 10.1007/s10068-011-0150-2. [DOI] [Google Scholar]
- Qin S.Z., Li F., Zhang Y., Li J., Zhao X., Zhao C.G. Effects of different softening methods on the baicalin content of baicalin decoction pieces. Strait Pharm. J. 2017;29:30–31. [Google Scholar]
- Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A., Basak B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crop. Prod. 2020;145 doi: 10.1016/j.indcrop.2019.111979. [DOI] [Google Scholar]
- Sakurama H., Kishino S., Uchibori Y., Yonejima Y., Ashida H., Kita K., Takahashi S., Ogawa J. β-Glucuronidase from Lactobacillus brevis useful for baicalin hydrolysis belongs to glycoside hydrolase family 30. Appl. Microbiol. Biotechnol. 2013;98:4021–4032. doi: 10.1007/s00253-013-5325-8. [DOI] [PubMed] [Google Scholar]
- Senger E., Peyrat A., Martin M., Montes J.M. Genetic variation in leaf chlorophyll content of Jatropha curcas L. Ind. Crop. Prod. 2014;58:204–211. doi: 10.1016/j.indcrop.2014.04.003. [DOI] [Google Scholar]
- Su S., He C.M., Li L.C., Chen J.K., Zhou T.S. Genetic characterization and phytochemical analysis of wild and cultivated populations of Scutellaria baicalensis. Chem. Biodivers. 2008;5:1353–1363. doi: 10.1002/cbdv.200890123. [DOI] [PubMed] [Google Scholar]
- Su H., Yao S., Zhao W., Li M., Liu J., Shang W.J., Xie H., Ke C., Gao M., Yu K., Liu H., Shen J., Tang W., Zhang L., Zuo J., Jiang H., Bai F., Wu Y., Ye Y., Xu Y. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv. 2020 doi: 10.1101/2020.04.13.038687. 2020.04.13.038687. [DOI] [Google Scholar]
- Tang L.Y., Wang Z.J., Song B.S., He Z.Q., Wu H.W., Huang L.Q. Study on feasibility of cutting process of fresh Angelica sinensis Radix. China J. Chin. Mater. Med. 2010;35:3147–3150. [PubMed] [Google Scholar]
- Tschofen M., Knopp D., Hood E., Stöger E. Plant molecular farming: much more than medicines. Annu. Rev. Anal. Chem. 2016;9:271–294. doi: 10.1146/annurev-anchem-071015-041706. [DOI] [PubMed] [Google Scholar]
- Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011;17:1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- Wang D., Fan S., Li A. Progress of studies on Chinese herbal medicine storage method. Mod. Chin. Med. 2013;15:416–419. [Google Scholar]
- Wang J., Li J.L., Li J., Li J.X., Liu S.J., Huang L.Q., Gao W.Y. Production of active compounds in medicinal plants: from plant tissue culture to biosynthesis. Chin. Herb. Med. 2017;9:115–125. doi: 10.1016/S1674-6384(17)60085-6. [DOI] [Google Scholar]
- Wang Y., Zhang G., Chi X., Chen S. Green and efficient extraction of podophyllotoxin from Sinopodophyllum hexandrum by optimized subcritical water extraction combined with macroporous resin enrichment. Ind. Crop. Prod. 2018;121:267–276. doi: 10.1016/j.indcrop.2018.05.024. [DOI] [Google Scholar]
- Wang Z.L., Wang S., Kuang Y., Hu Z.M., Qiao X., Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018;56:465–484. doi: 10.1080/13880209.2018.1492620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.Y., Wang X., Zhang L., Zhou J., Geng Y.L., Liu W. Study on the distribution and microstructure of active components in the root of Scutellaria baicalensis Georgi. J. Chin. Med. Mater. 2015;38:911–914. [Google Scholar]
- Wu R., Murali R., Kabe Y., French S.W., Chiang Y.M., Liu S., Sher L., Wang C.C., Louie S., Tsukamoto H. Baicalein targets GTPase-mediated autophagy to eliminate liver tumor-initiating stem cell-like cells resistant to mTORC1 inhibition. Hepatology. 2018;68:1726–1740. doi: 10.1002/hep.30071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Wang S., Lu J., Jing Y., Li M., Cao J., Bian B., Hu C. Seeing the unseen of Chinese herbal medicine processing (Paozhi): advances in new perspectives. Chin. Med. 2018;13:4. doi: 10.1186/s13020-018-0163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzel E.T., Kutchan T.M. Plant metabolism, the diverse chemistry set of the future. Science. 2016;353:1232–1236. doi: 10.1126/science.aad2062. [DOI] [PubMed] [Google Scholar]
- Xiao J.J. Chengdu University of Traditional Chinese Medicine; Chengdu, China: 2017. Study on Quality Assessment and Processing of Tianma Decoction Pieces. [Google Scholar]
- Xiao X., Chen S., Huang L., Xiao P. Survey of investigations on Daodi Chinese medicinal materials in China since 1980s. China J. Chin. Mater. Med. 2009;34:519–523. [PubMed] [Google Scholar]
- Xiao Y.Q., Li L., Liu Y., Ma Y.L., Yu D.R. Development and innovation of traditional Chinese medicine processing discipline and Chinese herbal pieces industry. China J. Chin. Mater. Med. 2016;41:24–27. doi: 10.4268/cjcmm20160105. [DOI] [PubMed] [Google Scholar]
- Xu J., Yu Y., Shi R., Xie G., Zhu Y., Wu G., Qin M. Organ-specific metabolic shifts of flavonoids in Scutellaria baicalensis at different growth and development stages. Molecules. 2018;23:428. doi: 10.3390/molecules23020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yang C., Li C., Zhao Q., Liu L., Fang X., Chen X.Y. Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Sci. Bull. (Beijing). 2016;61:3–17. doi: 10.1007/s11434-015-0929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Cao B., Xue Y., Liang H., Wu Y., Zhao N., Hu D., Gao P., Li G., Bai C. The medicinal active ingredients and their associated key enzyme genes are differentially regulated at different growth stages in Cornus officinalis and Cornus controversa. Ind. Crop. Prod. 2019;142 doi: 10.1016/j.indcrop.2019.111858. [DOI] [Google Scholar]
- Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q.J., Zhang Z.Y., Hu J., Guo L.P., Shao A.J., Huang L.Q. Impacts of recent cultivation on genetic diversity pattern of a medicinal plant, Scutellaria baicalensis (Lamiaceae) BMC Genet. 2010;11:29. doi: 10.1186/1471-2156-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Cao B., Bai C., Li G.S., Bai C. Predicting suitable cultivation regions of medicinal plants with Maxent modeling and fuzzy logics: a case study of Scutellaria baicalensis in China. Environ. Earth Sci. 2016;75:361. doi: 10.1007/s12665-015-5133-9. [DOI] [Google Scholar]
- Zhang Q., Yang F.Q., Ge L., Hu Y.J., Xia Z.N. Recent applications of hydrophilic interaction liquid chromatography in pharmaceutical analysis. J. Sep. Sci. 2017;40:49–80. doi: 10.1002/jssc.201600843. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhuang H., Zhang Y., Wang L., Zhang Y., Geng Y., Gou Y., Pei S., Wang Y. Plants for health: An ethnobotanical 25-year repeat survey of traditional medicine sold in a major marketplace in North-west Yunnan, China. J. Ethnopharmacol. 2018;224:119–125. doi: 10.1016/j.jep.2018.05.029. [DOI] [PubMed] [Google Scholar]
- Zhao T.H., Deng S.H., Yang H.S., Chen S.P. Study of antibacterial activity of active fraction from stems and leaves of Scutellaria baicalensis Georgi. Chin. Pharmacol. Bull. 2007;23:882–886. [Google Scholar]
- Zhao Z., Liang Z., Chan K., Lu G., Lee E.L., Chen H., Li L. A unique issue in the standardization of Chinese Materia Medica: processing. Planta Med. 2010;76:1975–1986. doi: 10.1055/s-0030-1250522. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Guo P., Brand E. The formation of daodi medicinal materials. J. Ethnopharmacol. 2012;140:476–481. doi: 10.1016/j.jep.2012.01.048. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Chen X.Y., Martin C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. (Beijing) 2016;61:1391–1398. doi: 10.1007/s11434-016-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Zhang Y., Wang G., Hill L., Weng J.K., Chen X.Y., Xue H., Martin C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2016;2:e1501780. doi: 10.1126/sciadv.1501780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.G., Gao S.R., Yan B.B., Xie J., Zhang X.M., Li J., Hou J.L., Wang W.Q. Study on feasibility of cutting fresh Radix et Rhizoma Salviae Miltiorrhizae in planting area. China J. Tradit. Chin. Med. Pharm. 2017;32:797–800. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.