Abstract
Behavioral lifestyle factors are associated with cardiometabolic disease and obesity, which are risk factors for coronavirus disease 2019 (COVID-19). We aimed to investigate whether physical activity, and the timing and balance of physical activity and sleep/rest, were associated with SARS-CoV-2 positivity and COVID-19 severity. Data from 91,248 UK Biobank participants with accelerometer data and complete covariate and linked COVID-19 data to July 19, 2020, were included. The risk of SARS-CoV-2 positivity and COVID-19 severity—in relation to overall physical activity, moderate-to-vigorous physical activity (MVPA), balance between activity and sleep/rest, and variability in timing of sleep/rest—was assessed with adjusted logistic regression. Of 207 individuals with a positive test result, 124 were classified as having a severe infection. Overall physical activity and MVPA were not associated with severe COVID-19, whereas a poor balance between activity and sleep/rest was (odds ratio [OR] per standard deviation: 0.71; 95% confidence interval [CI], 0.62 to 0.81]). This finding was related to higher daytime activity being associated with lower risk (OR, 0.75; 95% CI, 0.61 to 0.93) but higher movement during sleep/rest being associated with higher risk (OR, 1.26; 95% CI, 1.12 to 1.42) of severe infection. Greater variability in timing of sleep/rest was also associated with increased risk (OR, 1.21; 95% CI, 1.08 to 1.35). Results for testing positive were broadly consistent. In conclusion, these results highlight the importance of not just physical activity, but also quality sleep/rest and regular sleep/rest patterns, on risk of COVID-19. Our findings indicate the risk of COVID-19 was consistently approximately 1.2-fold greater per approximately 40-minute increase in variability in timing of proxy measures of sleep, indicative of irregular sleeping patterns.
Abbreviations and Acronyms: BMI, body mass index; COVID-19, coronavirus disease 2019; MVPA, moderate-to-vigorous physical activity; OR, odds ratio; SD, standard deviation
There is evidence that the risk of coronavirus disease 2019 (COVID-19) is higher in people with cardiometabolic diseases.1 Recent research also suggests that the risk is associated with lifestyle-related factors, including obesity2 and self-reported slow walking pace,3 a marker of physical fitness.
There has been limited attention to the risk of COVID-19 and behavioral lifestyle factors. Low levels of physical activity are known to be associated with increased risk of cardiometabolic disease, obesity, and lower fitness.4 Furthermore, evidence suggests that irregular sleep timing and increased variability in sleep duration are detrimentally associated with cardiovascular disease risk and markers of cardiometabolic health.5 This evidence also suggests that a balance between active behaviors and quality sleep/rest across the 24-hour day is important for cardiometabolic health. We hypothesize that these behavioral factors could similarly influence the risk of COVID-19.
Our aim was to investigate whether device-measured physical activity—the balance between activity during waking hours and the main sleep/rest period during the day—and the timing of the main sleep/rest and activity periods are associated with the risk of testing positive for SARS-CoV-2 and developing severe COVID-19.
Materials and Methods
We used data from UK Biobank (application 36371), a prospective cohort of >500,000 adults age 40-69 years.6 Assessments were conducted between March 2006 and July 2010 with data on 24-hour movement patterns from Axivity AX3 wrist-worn accelerometers (Axivity, Newcastle, UK) in >100,000 adults gathered between June 2013 and December 2015.7 UK Biobank data are linked to national SARS-CoV-2 laboratory test data through Public Health England’s Second Generation Surveillance System; data were available from March 16, 2020, to July 19, 2020, and included specimen origin (hospital inpatient vs other): a positive test result for SARS-CoV-2 with hospitalization was considered as evidence of a severe infection, in line with guidance for this dataset.8 Two SARS-CoV-2 outcomes were used: (1) severe infection with SARS-CoV-2 and (2) positive test result for SARS-CoV-2.
Analyses were restricted to English centers, individuals with known sleep disorders (identified with ICD-10 code G47 in UK Biobank), and those who died before March 16, 2020, were excluded. Participant characteristics, including body mass index (BMI), sex, ethnicity, and self-reported sleep duration were collected at the baseline assessment.
For each participant, accelerometer data (5-second epoch time series) were extracted from UK Biobank7 and converted to R-format for processing and analysis with GGIR (version 1.11-0; http://cran.r-project.org).9 Participants were excluded if they failed calibration (including those not calibrated on their own data), had fewer than 3 days of valid wear (defined as >16 hours per day), or wear data were not present for each 15-minute period of the 24-hours cycle.
Accelerometer outcomes (Table 1 ) were averaged across valid days and divided into three categories: standard physical activity outcomes, the balance between activity level and sleep/rest, and the variability in timing of activity and sleep/rest.
Table 1.
Accelerometer Outcome Variables for Physical Activity and Sleep/Resta
| Outcome | Unit | Abbreviation | Interpretation | |
|---|---|---|---|---|
| Physical activity | ||||
| 1 | Average acceleration over the 24-hour day | mg | Overall physical activity | Proxy for total physical activity |
| 2 | Moderate-to-vigorous physical activity in 1-min boutsb | min | MVPA | Purposeful activity (e.g. walking) accumulated in 1-min bouts |
| Activity and sleep/rest | ||||
| 3 | Average acceleration over most active continuous 16 h | mg | Activity during waking hours | Overall intensity of movement during the most active 16 h of the day as a proxy for waking hours. Greater values present a higher level of physical activity within this window. |
| 4 | Average acceleration over least active continuous 8 hc | mg | Movement during sleep/rest | Overall intensity of movement during the least active 8 h of the day sleep/rest as a proxy for the sleep window (main rest period). Lower values represent a more restful window of recovery. |
| 5 | The intensity of the most active 16 h expressed as % of average acceleration over the 24-h day | % | Balance between activity and sleep/rest | A proxy for the balance between activity and rest/sleep in a 24-h day. A value of 100% would mean no distinction between activity and sleep/rest (ie, no drop in movement levels during the main sleep/rest period). As the value gets closer to 150% it indicates an increasingly distinct activity/rest cycle with two thirds of the day active and one third resting. |
| Variability in timing of activity and sleep/rest | ||||
| 6 | Variability (SD) in the start time of the most active continuous 16 h | min | Variability in timing of activity | Proxy for variability in time of sleep offset (wake) |
| 7 | Variability (SD) in the start time of the least active continuous 8 hb | min | Variability in timing of sleep/rest | Proxy for variability in time of sleep onset |
| 8 | Variability (SD) in the midpoint of the time difference between the start of the most active continuous 16 h and least active continuous 8 hc | min | Variability in sleep/rest midpoint | Proxy for variability in mid-sleep time |
MVPA = moderate-to-vigorous physical activity.
Accelerometer cut-point for classification of MVPA = 100 mg.10
To allow for differences in the duration of sleep, we conducted sensitivity analyses assessing the effects of the average acceleration and timing of the least active 6 h rather than 8 h.
Statistical Analysis
Logistic regression was used to assess associations of a severe infection with SARS-CoV-2 (N = 124) with no test or a negative test result (whole cohort, N = 91,041) as comparator (model 1) and a positive test result (N = 207) with a negative result (N = 2009) as comparator (model 2).
For model 1, participants who tested positive for COVID-19 but were not classified as severe (ie, they tested positive in the community; N = 83) were excluded because it is possible that these individuals went on to develop severe COVID-19 but were not retested on hospital admission. Model 1 can be interpreted as the overall population level risk of being admitted to hospital with COVID-19 during the linkage period within UK Biobank. This population level method of assessing risk is commonly reported within COVID-19 risk factor research, and it is of value here as it enables comparison to the literature in terms of how the risk factors assessed compare with other commonly reported risk factors (eg, obesity).3 , 11
Model 2 relates specifically to the tested population, and it can be interpreted as the risk of a positive test in anyone within UK Biobank who has been tested for COVID-19.
The physical activity and rest variables listed in Table 1 were used as independent variables. These variables were standardized before entry into the models and the odds ratios (ORs) per standard deviation (SD) were reported for ease of comparison across variables.
In model 2, regressions were adjusted for the following potential confounders selected on current clinical knowledge: age on March 16, 2020; sex; ethnicity; Townsend Deprivation Index; number of people in household; fruit, vegetable, and red meat consumption; smoking status; alcohol intake; number of self-reported cancers and non-cancer illnesses; and number of treatments or medications taken. The model for severity of infection (model 1) was adjusted for key demographic variables only (age, sex, and ethnicity) because of the smaller number of outcome events. The activity during waking hours (3, Table 1) and amount of movement during sleep/rest (4, Table 1) were also mutually adjusted for one another and for sleep duration. When assessing the balance between activity and sleep/rest (5, Table 1), and the variability in timing of activity and sleep/rest (6-8, Table 1), sleep duration was added to the models. Finally, when assessing the variability in timing of activity and sleep/rest (6-8, Table 1) overall activity was also added to the models.
Sensitivity Analyses
-
1.
To allow for differences in sleep duration, we conducted analyses with the average acceleration and timing of the least active 6 hours rather than 8 hours.
-
2.
Assuming individuals testing positive in the community to be non-severe, we added them to the comparator group for severe infection with SARS-CoV-2 (severe infection N = 124; comparator group N = 91,124).
-
3.
We did not adjust for BMI in our main analyses as it is potentially on the causal pathway from physical activity to COVID-19 risk. However, we performed sensitivity analyses for: (1) model 1 further adjusted for BMI and number of cancer and non-cancer illnesses (underlying health conditions) and (2) model 2 further adjusted for BMI (initial model already adjusted for underlying health conditions).
-
4.
Although we controlled for deprivation and household size, it is difficult to determine risk due to level of exposure. The United Kingdom was under lockdown during the period of the study, with people requested to stay at home. We ran sensitivity analyses excluding the group likely to have had the greatest exposure to the virus: health care workers as they continued working throughout lockdown (UK Biobank codes 2211001-2216012, N = 1665, of whom 62 were tested [11 positive, 5 severe infection]). All analyses were performed using Stata version 16.0 (StataCorp, College Station, TX). Statistical significance was set at the alpha level of 0.05.
Results
Data were available for 91,248 individuals, of whom 2009 had been tested for COVID-19, 207 had a positive test result, and 124 were classified as having a severe infection. Participant characteristics for both models are reported in Table 2 .
Table 2.
Characteristics of UK Biobank Participants by COVID-19 Positive and Severitya
| Variable | Severity of infection (in UK Biobank), model 1 |
Test result, model 2 |
||||
|---|---|---|---|---|---|---|
| Negative/not tested | Severe | Total | Negative | Positive | Total | |
| Participants (N) | 91,041 | 124 | 91,165 | 1802 | 207 | 2009 |
| Age at COVID-19 diagnosis | 68.1 (61.0-73.2) | 69.6 (58.7-75.5) | 68.1 (61.0-73.2) | 70.5 (62.6-75.3) | 64.9 (56.2-73.4) | 70.1 (61.6-75.2) |
| Sex (female) | 51,908 (57.0%) | 52 (41.9%) | 51,960 (57.0%) | 949 (52.7%) | 103 (49.8%) | 1052 (52.4%) |
| Ethnicity | ||||||
| White European | 87,951 (98.4%) | 115 (95.8%) | 88,066 (98.4%) | 1725 (98.0%) | 191 (95.0%) | 1916 (97.7%) |
| South Asian | 684 (0.8%) | 2 (1.7%) | 686 (0.8%) | 22 (1.3%) | 3 (1.5%) | 25 (1.3%) |
| Black/Afro-Caribbean | 760 (0.9%) | 3 (2.5%) | 763 (0.9%) | 13 (0.7%) | 7 (3.5%) | 20 (1.0%) |
| Townsend deprivation index | –2.5 (–3.8 to –0.2) | –2.5 (–3.6 to 0.6) | –2.5 (–3.8 to –0.2) | –2.3 (–3.7 to 0.3) | –2.3 (–3.6 to 0.5) | –2.3 (–3.7 to 0.3) |
| Number in household | 2.0 (2.0-3.0) | 2.0 (2.0-3.0) | 2.0 (2.0-3.0) | 2.0 (2.0-3.0) | 2.0 (2.0-4.0) | 2.0 (2.0-3.0) |
| Fruit and vegetable scoreb | 4.3 (3.0-6.0) | 4.3 (2.7-6.0) | 4.3 (3.0-6.0) | 4.3 (3.0-6.0) | 4.0 (2.7-6.0) | 4.3 (3.0-6.0) |
| Red meat scorec | 1.5 (1.5-2.5) | 2.0 (1.5-3.0) | 1.5 (1.5-2.5) | 1.5 (1.5-2.5) | 2.0 (1.5-2.5) | 1.5 (1.5-2.5) |
| Smoking status | ||||||
| Never | 52,357 (57.7%) | 56 (45.2%) | 52,413 (57.6%) | 889 (49.4%) | 107 (51.2%) | 996 (49.7%) |
| Previous | 32,328 (35.6%) | 54 (43.5%) | 32,382 (35.6%) | 754 (41.9%) | 79 (38.2%) | 833 (41.5%) |
| Current | 6119 (6.7%) | 14 (11.3%) | 6133 (6.7%) | 155 (8.6%) | 21 (10.1%) | 176 (8.8%) |
| Alcohol intake frequency | 1.5 (0.5-3.5) | 1.5 (0.5-3.5) | 1.5 (0.5-3.5) | 1.5 (0.5-3.5) | 1.5 (0.5-3.5) | 1.5 (0.5-3.5) |
| Number of self-reported cancers | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) |
| Number of self-reported non-cancer illnesses | 1.0 (0.0-2.0) | 2.0 (1.0-3.0) | 1.0 (0.0-2.0) | 2.0 (1.0-3.0) | 1.0 (0.0-3.0) | 2.0 (1.0-3.0) |
| Number of treatments/medications taken | 1.0 (0.0-3.0) | 2.0 (1.0-5.0) | 1.0 (0.0-3.0) | 2.0 (1.0-4.0) | 2.0 (0.0-4.0) | 2.0 (1.0-4.0) |
| Body mass index (kg/m2) | 26.0 (23.6-28.9) | 27.3 (24.1-31.9) | 26.0 (23.6-28.9) | 26.8 (24.2-30.2) | 27.3 (24.1-31.1) | 26.9 (24.2-30.3) |
| Self-reported sleep duration (h) | 7.0 (7.0-8.0) | 7.0 (7.0-8.0) | 7.0 (7.0-8.0) | 7.0 (6.0-8.0) | 7.0 (6.0-8.0) | 7.0 (6.0-8.0) |
| Physical activity | ||||||
| Overall physical activity (mg) | 27.4 (22.7-33.0) | 26.9 (21.3-32.8) | 27.4 (22.7-33.0) | 25.8 (21.1-31.0) | 26.8 (21.9-32.1) | 25.9 (21.1-31.1) |
| Moderate to vigorous PA accumulated in 1-min bouts (min) | 42.1 (24.7-65.2) | 36.0 (19.4-55.4) | 42.0 (24.7-65.2) | 34.6 (18.5-55.5) | 36.9 (22.2-57.3) | 35.0 (18.8-55.9) |
| Activity and sleep/rest | ||||||
| Activity during waking hours (16 h) (mg) | 39.0 (32.2-47.1) | 38.2 (29.8-45.9) | 39.0 (32.2-47.1) | 36.5 (29.8-44.5) | 38.1 (30.5-45.5) | 36.7 (29.9-44.6) |
| Amount of movement during sleep (8 h) (mg) | 3.8 (3.1-4.9) | 4.2 (3.3-5.8) | 3.8 (3.1-4.9) | 3.9 (3.2-4.9) | 4.2 (3.3-5.7) | 3.9 (3.2-5.0) |
| Amount of movement during sleep (6 h) (mg)d | 2.8 (2.4-3.2) | 3.0 (2.6-3.7) | 2.8 (2.4-3.2) | 2.8 (2.5-3.3) | 3.0 (2.5-3.6) | 2.8 (2.5-3.4) |
| Balance between activity and sleep/rest (%) | 142.5 (140.4-144.0) | 141.1 (138.0-143.4) | 142.5 (140.4-144.0) | 141.9 (139.4-143.6) | 141.4 (138.6-143.2) | 141.8 (139.4-143.6) |
| Timing of activity and sleep/rest | ||||||
| Start time of most active 16h per day (hh:mm) | 7:05 (6:34-7:37) | 7:04 (6:34-7:47) | 7:05 (6:34-7:37) | 7:07 (6:34-7:38) | 7:02 (6:28-7:43) | 7:07 (6:34-7:38) |
| Variability in timing of activity (SD, min) | 37.6 (25.0-55.1) | 46.4 (31.5-68.4) | 37.6 (25.0-55.1) | 38.2 (26.4-56.5) | 46.7 (31.4-70.8) | 38.8 (26.6-57.6) |
| Start time of least active 8h per day (hh:mm) | 23:04 (22:34-23.35) | 23:02 (22:28-23:41) | 23:04 (22:34-23:35) | 23:07 (22:34-23.37) | 23:02 (22:28-23:43) | 23:05 (22:33-23:37) |
| Variability in timing of sleep/rest (8 h, SD, min) | 37.7 (25.0-55.4) | 46.7 (30.4-67.1) | 37.6 (25.0-55.1) | 38.3 (26.5-56.5) | 47.1 (31.0-71.2) | 39.1 (26.7-57.7) |
| Start time of least active 6h per day (hh:mm)d | 00:08 (23:34-00:43) | 00:08 (23:34-00:58) | 00:08 (23:34-00:43) | 00:10 (23:34-00:48) | 00:07 (23:34-00:53) | 00:08 (23:34-00:48) |
| Variability in timing of sleep/rest (6h, SD, min)d | 53.1 (38.0-70.6) | 56.6 (40.3-80.8) | 53.1 (38.0-70.6) | 53.2 (38.9-72.0) | 57.2 (40.7-81.8) | 53.5 (39.3-72.6) |
| Mid-point of difference between start of least active 8 h and most active 16 h (hh:mm) | 3:04 (2:34-3:36) | 3:02 (2:29-3:41) | 3:04 (2:34-3:36) | 3:07 (2:34-3:38) | 3:02 (2:28-3:43) | 3:06 (2:34-3:38) |
| Variability in sleep/rest mid-point (8 h, SD, min) | 37.6 (25.0-55.1) | 46.7 (30.6-68.1) | 37.6 (25.0-55.1) | 38.3 (26.4-56.5) | 47.1 (30.6-71.8) | 38.8 (26.6-57.6) |
| Midpoint of difference between start of least active 6 h and most active 16 h (hh:mm)d | 3:36 (3:05-4:09) | 3:38 (3:04-4:22) | 3:36 (3.05-4:09) | 3:38 (3:04-4:13) | 3:36 (3:04-4:10) | 3:38 (3:04-4:13) |
| Variability in sleep/rest midpoint (6 h, SD, min)d | 39.3 (28.0-54.9) | 43.1 (30.1-70.1) | 39.3 (28.0-54.9) | 40.5 (28.8-55.5) | 46.1 (29.8-69.8) | 40.9 (28.9-56.5) |
Continuous variables are reported as median (interquartile range), categorical variables as number (percentage)
COVID-19 = coronavirus disease 2019.
Number of portions reported per day.
Number of portions reported per week.
To allow for differences in the duration of sleep, we conducted sensitivity analyses assessing the effects of the average acceleration and timing of the least active 6 h rather than 8 h.
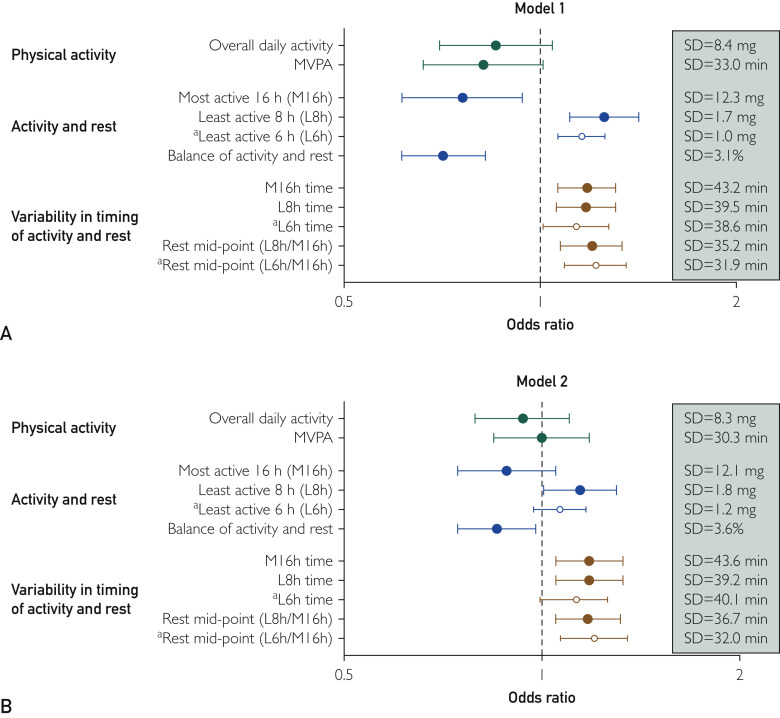
The results of the regression models are shown in Figure and Supplemental Table 1 (available online at http://www.mayoclinicproceedings.org). Results for severe infection (Figure A) and positive test results (Figure B) were broadly consistent. Overall physical activity level and moderate-to-vigorous physical activity (MVPA) were not significantly associated with the risk of testing positive for SARS-CoV-2 or developing severe COVID-19. A higher amount of movement during the main sleep/rest period (least active 8 hours; OR, 1.14-1.26; P < .05) and lower activity during waking hours (model 1 only, OR, 0.75 [95% CI, 0.61-0.93]; P = .01) were associated with increased odds independent of each other (ie, both were significant in the same model). Consequently, a worse (lower) balance between activity and sleep/rest (ie, a smaller drop in movement during the main sleep/rest period) was also predictive (OR, 0.71-0.86; P < .05). Irregular sleeping patterns (greater variability in the start times of activity during waking hours, amount of movement during the main sleep/rest period , and midpoint of the difference between these times) were consistently associated with significantly greater odds (OR, 1.17-1.21; P < .01) across both models. Results of unadjusted models were consistent with the adjusted models (Supplemental Table 1 available online at http://www.mayoclinicproceedings.org).
Figure.
Association of physical activity, the balance between activity and sleep/rest, and variability in the timing of sleep/rest and activity. (A) Model 1, severe COVID-19 (UK Biobank cohort as comparator). (B) Model 2, positive test for COVID-19 (negative test result as comparator). Odds ratios expressed per SD of each variable. Model 2 adjusted for age, sex, ethnicity, Townsend Deprivation Index, number of people in household, fruit/vegetable consumption, red meat consumption, smoking status, alcohol intake, number of self-reported cancers and non-cancer illnesses, and number of treatments/medications. Model 1 adjusted for age, sex, and ethnicity owing to the smaller number of outcome events. Activity during waking hours and movement during sleep/rest mutually adjusted. “Activity & sleep/rest” and “Variability in timing of activity and sleep/rest” variables additionally adjusted for sleep duration. “Variability in timing of activity and sleep/rest” additionally adjusted for overall physical activity. COVID-19 = coronavirus disease 2019; MVPA = moderate-to-vigorous physical activity; OR = odds ratio; SD = standard deviation. aSensitivity analyses using the least active 6 hours rather than 8 hours (open circles).
Sensitivity analyses broadly confirmed the associations:
-
1.
If the least active 6 hours was as the main sleep/rest period instead of the least active 8 hours
-
2.
If individuals testing positive in the community were assumed to be nonsevere and added to the comparator group for severe infection with SARS-CoV-2 (Supplemental Figure 1, available online at http://www.mayoclinicproceedings.org)
-
3.
With further adjustment for BMI (models 1 and 2) and cancer and non–cancer illnesses (model 1; Supplemental Figure 2, available online at http://www.mayoclinicproceedings.org)
-
4.
When excluding health care workers (Supplemental Figure 3, available online at http://www.mayoclinicproceedings.org).
Discussion
The balance and variability in timing of activity and rest were more strongly associated with the risk of testing positive for SARS-CoV-2 or incidence of severe COVID-19 than “standard” measures of activity (ie, MVPA). This highlights the importance of not just physical activity alone, but also adequate quality sleep/rest. A distinct activity cycle (better balance between activity and rest), reflecting a clear drop in movement during the main sleep/rest period (one third of the day), was associated with a lower risk, independent of self-reported sleep duration. The importance of quality sleep/rest was further evident in the positive association between the level of movement during sleep/rest (the least active continuous 8 hours) and risk, independent of activity level during waking hours, in all models.
A better balance between activity and the dominant sleep/rest period (by 1 SD of the sample mean) was associated with approximately 30% lower risk of severe COVID-19. This more distinct activity cycle could reflect greater movement during waking hours (16 hours), less movement during the main sleep/rest period (8 hours), or both. For example, a 1 SD higher balance between activity and sleep/rest could reflect the equivalent of 90 minutes of extra-brisk walking12; or lower movement during the main sleep/rest period by 1 SD (ie, approximately 2 mg) of the sample mean; or approximately 45 minutes of brisk walking and lower movement during the main sleep/rest period by approximately 0.5 SD (ie, approximately 1 mg) of the sample mean.
Sleep disruption and physical inactivity can contribute to chronic inflammation13 and to cardiometabolic disease, which in turn is associated with an increased risk of COVID-19.1 Furthermore, as COVID-19 is an acute inflammatory disease, it might exacerbate existing chronic inflammation associated with poor activity and rest behaviors or existing cardiometabolic disease. Alongside other risk factors (eg, psychological stress and genetic predisposition), the virus might be associated with a “cytokine storm”13 contributing to the observed increased risk of severe COVID-19.
The consistently 1.2-fold higher risk of COVID-19 per approximately 40-minute increase in variability in timing of sleep/rest, indicative of irregular sleeping patterns, further supports the finding that risk factors for cardiovascular and cardiometabolic disease4 , 5 are also risk factors for COVID-19.2 , 3
Limitations
Characteristics of participants, including accelerometer data, were measured before the current pandemic. Although the analyses were controlled for deprivation and household size, it was not possible to determine level of exposure to infection; however, results were robust when excluding the group likely to have had the highest level of exposure to the virus, health care workers. The definition of severe COVID-19 was a positive test from a hospital inpatient, consistent with the definition proposed by the researchers who developed the linkage method8; however, actual disease severity cannot be confirmed from the linkage data available at the time of analysis. Furthermore, participants in UK Biobank might not be representative of the wider population and testing in the UK has not been universal, making analyses vulnerable to bias. However, participants might not need to be representative when estimating relative risk factor associations, as empirically demonstrated for UK Biobank.14 As such, our results point to the potential importance of rest and physical activity as predictive of later risk of COVID-19 infection and should be confirmed with current databases from other populations.
Conclusion
This report provides evidence of an association between markers of sleep/rest and physical activity and the risk or severity of COVID-19 infection. Public health studies could incorporate such measures to better identify and protect individuals at high risk of COVID-19 or cardiometabolic disease.
Acknowledgments
Data were analyzed using UK Biobank application number 36371.
Footnotes
Grant Support: This research was supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre, the NIHR Applied Research Collaborations – East Midlands, and a grant from the UKRI-DHSC COVID-19 Rapid Response Rolling Call (MR/V020536/1). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Potential Competing Interests: Dr Khunti is a member of the independent SAGE group. The other authors report no competing interests.
Availability of data and materials: This research has been conducted using the UK Biobank Resource under Application 36371 (http://www.ukbiobank.ac.uk/).
Code availability: Accelerometer data were processed using the open-source R-package GGIR (version 1.11-0, http://cran.r-project.org).
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yates T., Razieh C., Zaccardi F., Davies M.J., Khunti K. Obesity and risk of COVID-19: Analysis of UK Biobank. Prim Care Diabetes. 2020;14(5):566–567. doi: 10.1016/j.pcd.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates T., Razieh C., Zaccardi F. Obesity, walking pace and risk of severe COVID-19: Analysis of UK Biobank. medRxiv. 2020 doi: 10.1101/2020.07.10.20150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburton D.E., Nicol C.W., Bredin S.S. Health benefits of physical activity: The evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang T., Redline S. Cross-sectional and prospective associations of actigraphy-assessed sleep regularity with metabolic abnormalities: The multi-ethnic study of atherosclerosis. Diabetes Care. 2019;42(8):1422–1429. doi: 10.2337/dc19-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudlow C., Gallacher J., Allen N. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty A., Jackson D., Hammerla N. Large scale population assessment of physical activity using wrist worn accelerometers: The UK Biobank Study. PLoS One. 2017;12(2):e0169649. doi: 10.1371/journal.pone.0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong J., Rudkin J.K., Allen N. Dynamic linkage of COVID-19 test results between Public Health England’s Second Generation Surveillance System and UK Biobank. Microb Genom. 2020;6(7) doi: 10.1099/mgen.0.000397. mgen000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migueles J.H., Rowlands A.V., Huber F. GGIR: A research community–driven open-source r package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Measure Phys Behav. 2019;2(3):188–196. [Google Scholar]
- 10.Hildebrand M., van Hees V.T., Hansen B.H., Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46(9):1816–1824. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 11.Yates T., Zaccardi F., Razieh C., Gillies C. 2020. Framework for analysis and interpretation of ongoing COVID-19 research. Wellcome Open Research website.https://wellcomeopenresearch.org/articles/5-208 Accessed October 26, 2020. [Google Scholar]
- 12.Chudasama Y.V., Khunti K., Zaccardi F. Physical activity, multimorbidity, and life expectancy: A UK Biobank longitudinal study. BMC Med. 2019;17(1):108. doi: 10.1186/s12916-019-1339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vepa A., Bae J.P., Ahmed F., Pareek M., Khunti K. COVID-19 and ethnicity: A novel pathophysiological role for inflammation. Diabetes Metab Syn Clin Res Rev. 2020;14(5):1043–1051. doi: 10.1016/j.dsx.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batty G.D., Gale C.R., Kivimäki M. Comparison of risk factor associations in UK Biobank against representative, general population-based studies with conventional response rates: Prospective cohort study and individual participant meta-analysis. Brit Med J. 2020;368:m131. doi: 10.1136/bmj.m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.