Abstract
Objectives
The aim of this study was to assess the clinical course and outcomes of all heart transplant recipients affected by coronavirus disease-2019 (COVID-19) who were followed at the leading heart transplant centers of Northern Italy.
Background
The worldwide severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) pandemic has created unprecedented challenges for public health, demanding exceptional efforts for the successful management and treatment of affected patients. Heart transplant patients represent a unique cohort of chronically immunosuppressed subjects in which SARS-CoV-2 may stimulate an unpredictable clinical course of infection.
Methods
Since February 2020, we enrolled all 47 cases (79% male) in a first cohort of patients, with a mean age of 61.8 ± 14.5 years, who tested positive for SARS-CoV-2, out of 2,676 heart transplant recipients alive before the onset of the COVID-19 pandemic at 7 heart transplant centers in Northern Italy.
Results
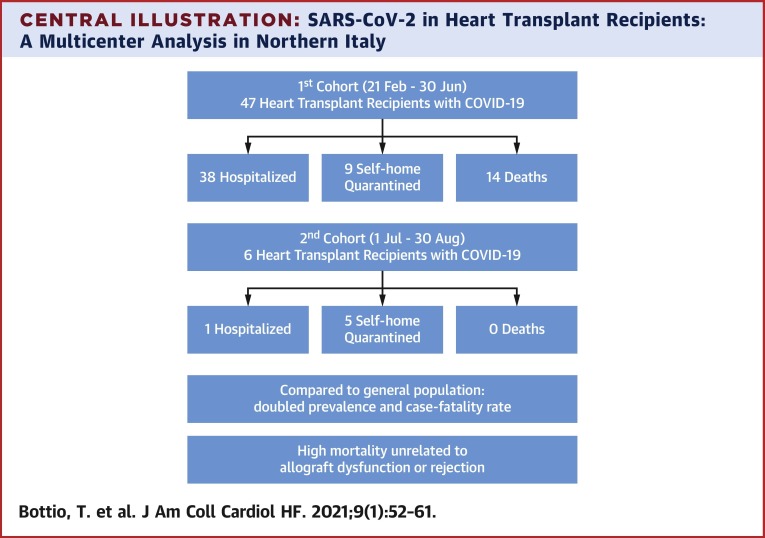
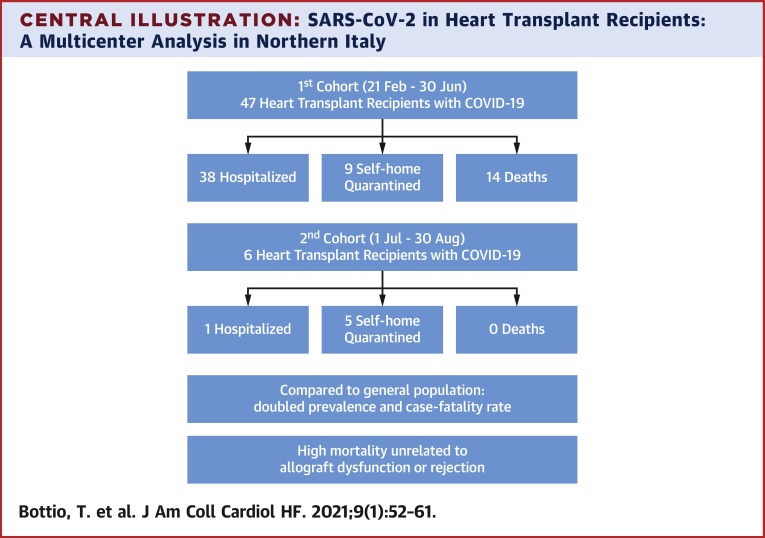
To date, 38 patients required hospitalization while 9 remained self-home quarantined and 14 died. Compared to the general population, prevalence (18 vs. 7 cases per 1,000) and related case fatality rate (29.7% vs. 15.4%) in heart transplant recipients were doubled. Univariable analysis showed older age (p = 0.002), diabetes mellitus (p = 0.040), extracardiac arteriopathy (p = 0.040), previous PCI (p = 0.040), CAV score (p = 0.039), lower GFR (p = 0.004), and higher NYHA functional classes (p = 0.023) were all significantly associated with in-hospital mortality. During the follow-up two patients died and a third patient has prolonged viral-shedding alternating positive and negative swabs. Since July 1st, 2020, we had 6 new patients who tested positive for SARS-CoV-2, 5 patients asymptomatic were self-quarantined, while 1 is still hospitalized for pneumonia. A standard therapy was maintained for all, except for the hospitalized patient.
Conclusions
The prevalence and mortality of SARS-CoV-2 should spur clinicians to immediately refer heart transplant recipients suspected as having SARS-CoV2 infection to centers specializing in the care of this vulnerable population.
Key Words: COVID-19 and heart transplant recipients, heart transplantation, immunosuppressive therapy, SARS-CoV-2
Abbreviations and Acronyms: CMV, cytomegalovirus; COVID-19, coronavirus disease-2019; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Central Illustration
The worldwide severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) pandemic has created unprecedented challenges for public health, demanding exceptional efforts for the successful management and treatment of affected patients. Northern Italy was the first region in Western Europe to experience a large outbreak of coronavirus disease 2019 (COVID-19), which has since rapidly spread throughout the world. As of the end of June 2020, more than 70% of the total number of positive patients nationally and almost 80% of deaths occurred in this area (1).
In this context, information on the incidence and clinical characteristics of COVID-19 in heart transplant recipients is still limited (2,3). Challenges remain in fully characterizing SARS-CoV-2 infection in this population given the scant published reports available on the prevalence, clinical presentation, outcomes, and relationship between immunosuppressive therapy and severe forms of the disease.
Chronically immunosuppressed patients may have a greater viral burden, resulting in heightened infectivity and eventual worse prognosis. On the other hand, the interaction between immunomodulators and severe infection pathogenesis is still not fully understood. An abnormal immune response to the infection consisting of elevated cytokine production and perpetuation of a systemic inflammatory state may play a crucial role in the course of COVID-19 infection (4). In addition, the lung damage observed in the severe forms of the disease may be related to hyperactivation of the immune response rather than the viral infection itself. Heart transplant patients represent a unique cohort of chronically immunosuppressed subjects in which SARS-CoV-2 may stimulate an unpredictable clinical course of infection. Scientific published reports is still lacking organized and multi-institutional reports on this especially vulnerable population (2,3,5).
To date, this study provides the most comprehensive data on SARS-CoV-2 infection prevalence and clinical characteristics in heart transplant recipients. Here, we sought to identify clinical variables and therapeutic strategies associated with a worse outcome, focusing on immunosuppressive therapy modulation and its possible interactions with anti-inflammatory medication and antiviral drugs.
Methods
Study oversight
It is declared that every reasonable effort was made to obtain informed consent to participate in this study. Notably, the use of data for scientific and research purposes is already included in the informed consent agreements used at the participating centers. The local ethics committees approved of the study design, consent process, and review and analysis of the data.
We also guarantee the respect of anonymity and professional secrecy and use the collected data and the statistical analysis solely for the scientific purposes granted in accordance with the law in force (GDPR).
Study population
We designed a retrospective, observational, multicenter study across 7 leading heart transplant centers of Northern Italy: Milan, Padua, Bergamo, Bologna, Turin, Verona, Udine. All heart transplant recipients with positive nasopharyngeal reverse transcriptase-polymerase chain reaction tests for SARS-CoV-2 at these centers were included in this study.
Data sources
We obtained medical records and compiled data for hospitalized patients and outpatients with laboratory-proven COVID-19 infection. We extracted data on recent exposure history, clinical symptoms or signs, and laboratory findings on admission from electronic medical records. All laboratory testing and radiological assessments were performed according to standard of care. Pertinent information was compiled into a unique dataset and forwarded to the data-processing coordination center at the University of Padua. A team of experienced heart transplant clinicians abstracted and reviewed all data. If the core data were missing, requests for clarification were sent back to each group’s principal investigator for completion.
Study outcomes
Primary outcomes were COVID-19 infection prevalence and case fatality rate in heart transplant patients. Secondary outcomes were rate of hospitalization, intensive care unit (ICU) admissions, and hospital length of stay.
Statistical analysis
Continuous variables were presented as mean ± SD. The Student’s t-test for unpaired data or the Mann-Whitney U test were used to compare parametric and nonparametric continuous variables respectively (normal distribution was assessed by the Kolmogorov-Smirnov test). Categorical variables were presented as relative and absolute frequency; chi-square test analysis or the Fisher exact test were used to compare categorical variables where appropriate. A 2-sided p value of <0.05 was considered significant.
All statistical analyses were performed using SPSS software version 20 (IBM Corp., Armonk, New York). Data are updated to July 1, 2020.
Results
Of 2,676 heart transplant recipients alive before the onset of the COVID-19 pandemic at the end of February 2020, 47 patients were included in this analysis as part of a first cohort of patients. Among them, 2 patients had received combined heart-kidney transplants and 1 patient had undergone retransplantation. This preliminary series reports baseline patient characteristics, features of COVID-19 onset and hospital course, laboratory results, and outcomes (Table 1 ).
Table 1.
Baseline Characteristics of Heart Transplant Population With COVID-19
| All (N = 47) | Survivors (n = 33) | Nonsurvivors (n = 14) | P Value | Home Self-Quarantined Patients (n = 9) | Hospitalized Patients (n = 38) | p Value | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age (yrs) | 61.84 ± 14.51 | 57.67 ± 14.87 | 71.67 ± 7.24 | 0.002 | 48.64 ± 17.34 | 64.97 ± 12.02 | 0.002 |
| Male | 37 (79) | 25 (76) | 12 (86) | 0.700 | 6 (67) | 31 (82) | 0.377 |
| Time from Htx (yrs) | 10.46 ± 8.70 | 9.46 ± 8.61 | 12.75 ± 8.75 | 0.242 | 6.55 ± 9.81 | 11.29 ± 8.35 | 0.164 |
| Risk factors | |||||||
| BMI (kg/m2) | 25.27 ± 4.80 | 25.32 ± 4.21 | 25.15 ± 6.13 | 0.915 | 25.82 ± 4.10 | 25.13 ± 4.99 | 0.703 |
| Obesity | 8 (17) | 5 (15) | 3 (21) | 0.679 | 1 (11) | 7 (18) | 0.600 |
| Arterial hypertension | 30 (64) | 22 (67) | 8 (57) | 0.741 | 5 (56) | 25 (66) | 0.704 |
| Dyslipidemia | 22 (47) | 14 (42) | 8 (57) | 0.524 | 4 (44) | 18 (47) | 0.874 |
| Diabetes mellitus | 8 (17) | 3 (9) | 5 (36) | 0.040 | 1 (11) | 7 (18) | 0.600 |
| Former smoker | 11 (23) | 8 (24) | 3 (21) | 0.835 | 3 (33) | 8 (21) | 0.419 |
| Peripheral vascular disease | 8 (17) | 3 (9) | 5 (36) | 0.040 | 0 (0) | 8 (21) | 0.323 |
| COPD | 3 (6) | 2 (6) | 1 (7) | 0.890 | 0 (0) | 3 (8) | 0.384 |
| Stroke | 1 (2) | 0 (0) | 1 (7) | 0.298 | 0 (0) | 1 (2) | 0.623 |
| Malignancy | 5 (11) | 3 (9) | 2 (14) | 0.627 | 2 (22) | 3 (8) | 0.240 |
| Dialysis | 6 (13) | 4 (12) | 2 (14) | 0.839 | 0 (0) | 6 (16) | 0.579 |
| GFR (ml/min) | 48.17 ± 32.14 | 55.00 ± 33.93 | 30.82 ± 18.72 | 0.004 | 82.90 ± 35.30 | 39.72 ± 25.29 | <0.001 |
| Previous PCI | 11 (23) | 5 (15) | 6 (36) | 0.040 | 0 (0) | 11 (29) | 0.092 |
| CMR | 7 (15) | 4 (12) | 3 (21) | 0.404 | 1 (11) | 6 (16) | 0.771 |
| AMR | 1 (2) | 0 (0) | 1 (7) | 0.295 | 0 (0) | 1 (3) | 0.633 |
| CAV Score | 0.41 ± 0.89 | 0.17 ± 0.59 | 1.00 ± 1.21 | 0.039 | 0.14 ± 0.38 | 0.46 ± 0.95 | 0.398 |
| NYHA functional class | 0.023 | 0.218 | |||||
| I | 35 (74) | 28 (85) | 7 (50) | 8 (100) | 27 (71) | ||
| II | 8 (17) | 3 (9) | 5 (36) | 0 (0) | 8 (21) | ||
| III | 3 (6) | 1 (3) | 2 (14) | 0 (0) | 3 (8) | ||
| IV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| LVEF (%) | 54.48 ± 10.38 | 56.72 ± 9.04 | 48.71 ± 12.05 | 0.083 | 56.40 ± 9.48 | 54.00 ± 10.77 | 0.654 |
| Immunosuppressive therapy | |||||||
| Cyclosporine | 34 (81) | 21 (64) | 13 (93) | 0.073 | 6 (67) | 28 (74) | 0.939 |
| Tacrolimus | 12 (26) | 11 (33) | 1 (7) | 0.073 | 2 (22) | 10 (26) | 0.939 |
| Prednisone | 19 (40) | 14 (42) | 5 (36) | 0.749 | 6 (67) | 13 (34) | 0.033 |
| Mycophenolate | 26 (55) | 18 (55) | 8 (57) | 0.955 | 5 (56) | 22 (58) | 0.707 |
| Everolimus | 12 (26) | 8 (24) | 4 (29) | 0.800 | 2 (22) | 10 (26) | 0.939 |
| Azathioprine | 3 (6) | 1 (3) | 2 (14) | 0.216 | 0 (0) | 3 (8) | 0.411 |
| Anticoagulant therapy | 4 (9) | 3 (9) | 1 (7) | 0.782 | 0 (0) | 4 (11) | 0.330 |
| Laboratory results at last FU | |||||||
| WBC count (cells per 109/l) | 6.91 ± 2.75 | 6.67 ± 2.82 | 7.59 ± 2.63 | 0.461 | 5.56 ± 2.54 | 7.30 ± 2.74 | 0.176 |
| Lymphocyte (cells per 109/l) | 1.71 ± 1.05 | 1.57 ± 0.97 | 2.13 ± 1.22 | 0.234 | 1.24 ± 0.69 | 1.85 ± 1.10 | 0.217 |
Values are mean ± SD or n (%).
Abbreviations: AMR = antibody-mediated rejection; BMI = body mass index; CAV = cardiac allograft vasculopathy; CMR = cardiac magnetic resonance; COPD chronic obstructive pulmonary disease; COVID-19 = coronavirus disease 2019; GFR = glomerular filtration rate; Htx = heart transplant; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; NYHA = New York Heart Association; FU = follow up; LVEF = left ventricular ejection fraction; WBC = white blood cells.
Cohort 1 analysis
Baseline and risk factors
All collected data are presented in Table 1. Mean age was 61.8 ± 14.5 years and mean time from heart transplantation was 10.5 ± 8.7 years; 79% of patients were men. The most frequent risk factors were arterial hypertension (64%) and dyslipidemia (47%). Mean body mass index was 25.27 ± 5.80 kg/m2 with 17% meeting the body mass index criteria for obesity. Impaired renal function was present in most subjects, with a mean estimated glomerular filtration rate (eGFR) of 48.17 ± 32.14 ml/min (calculated with Chronic Kidney Disease Epidemiology Collaboration equation) and 13% of patients had end-stage kidney disease requiring hemodialysis; 91% of cases were in New York Heart Association (NYHA) functional class I to II. The most common immunosuppressive regimen consisted of cyclosporine combined with mycophenolate mofetil. Almost 50% of patients received corticosteroids. Heart allograft function, assessed by last available echocardiogram, was preserved in all subjects.
COVID-19 onset
All heart transplant recipients enrolled had a positive reverse transcriptase-polymerase chain reaction nasopharyngeal swab test for SARS-CoV-2, with 93% of being symptomatic. The most frequent symptom was fever (87%), followed by cough (70%) and shortness of breath (70%). Mean duration of symptoms was 9.80 ± 7.31 days. In febrile patients, the mean fever peak was 38.2 ± 0.6°C and fever lasted for a mean of 7.5 ± 3.8 days. In 72% of cases, radiographic signs of pneumonia were present. These data are summarized in Table 2 .
Table 2.
COVID-19 Onset Characteristics Among Heart Transplant Population
| All (N = 47) | Survivors (n = 33) | Nonsurvivors (n = 14) | P Value | |
|---|---|---|---|---|
| COVID-19 onset | ||||
| Presenting symptoms | 44 (93) | 30 (91) | 14 (100) | 0.544 |
| Fever | 41 (87) | 27 (82) | 14 (100) | 0.159 |
| Cough | 33 (70) | 21 (64) | 12 (86) | 0.175 |
| Shortness of breath | 33 (70) | 19 (58) | 14 (100) | 0.004 |
| Myalgia | 21 (45) | 15 (45) | 6 (43) | 0.870 |
| Headache | 9 (19) | 5 (15) | 4 (29) | 0.419 |
| Anosmia | 7 (15) | 7 (21) | 0 (0) | 0.166 |
| Sinusitis | 3 (6) | 3 (9) | 0 (0) | 0.548 |
| Gastrointestinal | 10 (21) | 9 (27) | 1 (7) | 0.242 |
| Nasopharyngeal swab test positive | 47 (100) | 33 (100) | 14 (100) | 1.000 |
| X-ray pneumonia | 34 (72) | 20 (61) | 14 (100) | 0.005 |
| Fever peak (°C) | 38.16 ± 0.56 | 38.02 ± 0.55 | 38.40 ± 0.53 | 0.041 |
| Duration of fever (days) | 7.46 ± 3.79 | 6.58 ± 2.90 | 9.08 ± 4.75 | 0.141 |
| Duration of symptoms (days) | 9.80 ± 7.31 | 10.05 ± 7.82 | 9.00 ± 5.93 | 0.766 |
| Hospitalization | 38 (81) | 24 (73) | 14 (100) | 0.030 |
| SpO2 at admission (%) | 91.90 ± 5.92 | 93.93 ± 5.04 | 86.55 ± 4.68 | <0.001 |
| Worst SpO2 during hospitalization (%) | 87.81 ± 8.95 | 91.30 ± 7.49 | 79.27 ± 6.13 | <0.001 |
| Acute respiratory distress syndrome | 13 (28) | 2 (6) | 11 (79) | <0.001 |
| Laboratory results at admission | ||||
| WBC count (cells per 109/l) | 5.57 ± 3.03 | 5.06 ± 2.70 | 7.47 ± 3.63 | 0.061 |
| Hb (g/dl) | 11.00 ± 2.27 | 11.17 ± 2.42 | 10.57 ± 1.85 | 0.489 |
| Platelets (cells per 109/l) | 180.82 ± 69.79 | 184.71 ± 72.52 | 155.50 ± 60.10 | 0.600 |
| Lymphocyte (cells per 109/l) | 1.61 ± 2.17 | 1.73 ± 2.28 | 0.70 ± 0.53 | 0.544 |
| Eosinophilis (cells per 109/l) | 0.15 ± 0.35 | 0.16 ± 0.36 | 0.18 ± 0.41 | 0.933 |
| CRP (mg/dl) | 31.58 ± 42.45 | 26.22 ± 32.53 | 50.70 ± 67.33 | 0.383 |
| PCT (ng/ml) | 1.14 ± 2.53 | 0.51 ± 1.12 | 4.56 ± 6.07 | 0.519 |
| Serum creatinine (mg/dl) | 3.28 ± 3.98 | 3.26 ± 4.54 | 3.34 ± 2.26 | 0.149 |
| Serum creatinine in hospital peak (mg/dl) | 3.12 ± 2.36 | 2.70 ± 2.31 | 4.14 ± 2.27 | 0.034 |
| AST (U/l) | 25.69 ± 15.85 | 21.01 ± 11.19 | 46.00 ± 19.29 | 0.008 |
| ALT (U/l) | 20.88 ± 15.57 | 17.08 ± 9.21 | 32.25 ± 25.91 | 0.092 |
| TnI in hospital peak | 41.85 ± 88.18 | 13.07 ± 6.67 | 99.40 ± 144.58 | 0.253 |
Values are n (%) or mean ± SD.
Abbreviations: ALT = alanine transaminase; AST = aspartate aminotransferase; COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; Hb = hemoglobin; PCT = procalcitonin; SpO2 = oxygen saturation; TnI = troponin I; WBC = white blood cells.
In-hospital stay
Pertinent data are summarized in Tables 2 and 3 . Of 47 subjects, 38 (81%) required hospitalization, with a mean length of stay of 17.79 ± 10.70 days; 4 (9%) patients required intensive care unit stay. Mean O2 saturation at admission was 92% ± 6%. Thirteen (28%) patients developed acute respiratory distress syndrome. Noninvasive and invasive ventilation were required in 15 (32%) and 2 (4%) patients, respectively. Only 1 patient required a tracheostomy for failure of weaning from ventilatory support, and 1 patient was treated with nitric oxide. Pronation therapy was performed for 3 patients. Vasopressor support with noradrenaline was required in 3 patients due to septic shock; extracorporeal life support was never used. Major adverse events observed were superimposed bacterial infection requiring targeted antibiotic treatment (11%) and sepsis (9%). During hospitalization, allograft function was assessed by laboratory and echocardiographic monitoring, which did not show evidence of myocardial dysfunction or injury (Table 2). For this analysis, we report the lowest echocardiographic left ventricular ejection fraction (LVEF) and the peak troponin I levels. Mean LVEF during hospitalization was 59.1% ± 6.7% and comparable to that measured in the last pre-COVID-19 echocardiogram (p = 0.201). No clinically significant allograft rejections were observed.
Table 3.
Treatment and Outcome of Heart Transplant Population With COVID-19
| All (N = 47) | Survivors (n = 33) | Nonsurvivors (n = 14) | p Value | |
|---|---|---|---|---|
| Treatment and outcome | ||||
| Hydroxychloroquine | 38 (81) | 24 (73) | 14 (100) | 0.155 |
| Lopinavir/Ritonavir | 21 (45) | 13 (39) | 8 (57) | 0.526 |
| Tocilizumab | 1 (2) | 1 (3) | 0 (0) | 0.482 |
| Iperimmune plasma | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Corticosteroid bolus therapy | 10 (21) | 8 (24) | 2 (14) | 0.361 |
| Management of immunosuppressive therapy∗ | ||||
| Reduction cyclosporine dose (n = 34) | 6 (18) | 3 (14) | 3 (30) | 0.653 |
| Reduction tacrolimus dose (n = 12) | 5 (42) | 5 (45) | 0 (0) | 0.377 |
| Reduction mycophenolate dose (n = 30) | 17 (57) | 13 (62) | 4 (44) | 0.623 |
| Reduction everolimus dose (n = 12) | 3 (25) | 2 (25) | 1 (25) | 1.000 |
| Reduction azathioprine dose (n = 3) | 3 (100) | 1 (100) | 2 (100) | 1.000 |
| Antibiotics prophylaxis | 39 (83) | 26 (79) | 13 (93) | 0.735 |
| Anticoagulant therapy | 13 (28) | 11 (33) | 2 (14) | 0.282 |
| ICU stay | 4 (9) | 2 (6) | 2 (14) | 0.616 |
| NIV | 15 (32) | 4 (12) | 11 (79) | <0.001 |
| Invasive ventilation | 2 (4) | 0 (0) | 2 (14) | 0.129 |
| Pronation | 3 (6) | 2 (6) | 1 (7) | 0.896 |
| CVVH | 0 (0) | 0 (0) | 1 (7) | 0.368 |
| ECMO support | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Inotropic support | 3 (6) | 0 (0) | 3 (21) | 0.043 |
| Nitric oxide | 1 (2) | 0 (0) | 1 (7) | 0.310 |
| Complications | ||||
| Neurological | 1 (2) | 0 (0) | 1 (7) | 0.333 |
| Gastrointestinal | 1 (2) | 1 (3) | 0 (0) | 0.464 |
| Infective | ||||
| Bacterial coinfection | 5 (11) | 2 (6) | 3 (21) | 0.307 |
| Fungal coinfection | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Viral coinfection | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Sepsis | 4 (9) | 2 (6) | 2 (14) | 0.307 |
| In hospital length of stay (days) | 17.79 ± 10.70 | 23.21 ± 8.91 | 8.50 ± 6.17 | <0.001 |
Values are n (%) or mean ± SD
Abbreviations: COVID-19 = coronavirus disease 2019; CVVH = continuous venovenous hemofiltration; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; NIV = noninvasive ventilation.
Percentage of patients with a reduction in immunosuppressant dosage.
Of the 38 hospitalized patients, 14 (37%) died and 24 (63%) have been discharged home. Cause of death for the deceased patients was respiratory failure in all cases, with the exception of one who died due to multiorgan failure.
Pharmacological treatment
More than 80% of patients received hydroxychloroquine, whereas antiretroviral therapy with ritonavir/lopinavir was used 50% of the subjects. Prophylaxis with broad-spectrum antibiotics was administered in 83% of cases, mainly in the form of beta-lactamases and macrolides (Table 1). One patient received a monoclonal antibody against interleukin-6 (tocilizumab). A corticosteroid administration as bolus medication was performed in 10 patients (21%).
Doses of immunosuppressive drugs were reduced in all 38 (100%) hospitalized patients. Mycophenolate mofetil dose was decreased in 57% of patients, everolimus in 25%, cyclosporine in 18%, tacrolimus in 5%, and azathioprine in all patients receiving this drug. Thirteen (28%) patients received anticoagulation, with low molecular weight heparin. Laboratory findings during hospitalization are shown in Table 3.
Non-hospitalized SARS-CoV-2–positive heart transplant recipients
Nine patients in our cohort were either asymptomatic or had mild symptoms. These patients did not require hospitalization, but were instructed to self-quarantine at home according to a protocol that included frequent telephone monitoring by the corresponding heart transplant team. Compared with remainder of the population, the nonhospitalized patients exhibited significant differences in mean age (p = 0.002), eGFR (p < 0.001), and prednisone use (p = 0.033) (Table 1). Cyclosporine and mycophenolate mofetil were the most common immunosuppression regimen, and 67% of patients in this group also received corticosteroids. At COVID-19 onset, 5 (71%) recipients were symptomatic, presenting mostly with fever (78%) and cough (67%); median oxygen saturation at onset was 98 ± 1%. Hydroxychloroquine was administered to 3 (33%) patients, ritonavir/lopinavir to 2 (22%), and antibiotic prophylaxis to 4 (44%). Unlike hospitalized patients, 4 (44%) home self-quarantined patients continued their immunosuppressive regimen without adjustment. In the remainder of the nonhospitalized subgroup, mycophenolate mofetil was reduced in 50% of the patients, everolimus in 50%, and the only patient receiving tacrolimus had his dosage halved. Cyclosporine and prednisone doses were unchanged. All patients had a rapid resolution of symptoms and did not require further treatment.
Univariable survival analysis
Univariable analysis (Table 1) of baseline characteristics showed older age (p = 0,002), diabetes mellitus (p = 0.040), peripheral vascular disease (p = 0.040), previous percutaneous coronary intervention (p = 0.040), cardiac allograft vasculopathy score (p = 0.039), lower eGFR (p = 0.004), and higher NYHA functional class (p = 0.023) were all significantly associated with in-hospital mortality. Chronic immunosuppressive therapy was similar in survivors and nonsurvivors (Central Illustration ). Symptoms at COVID-19 onset were similar, with the exception of shortness of breath (p = 0.004). In the subgroup of deceased patients, radiographic signs of pneumonia (p = 0.005) and acute respiratory distress syndrome (p < 0.001) were more frequent with a lower O2 saturation at admission (p < 0.001) and lowest value during hospitalization (p < 0.001). During hospitalization, nonsurvivors required noninvasive ventilation at higher rates (p < 0.001). Immunosuppressive therapy adjustment and use of anti-inflammatory and antiretroviral drugs were similar between survivors and patients who succumbed to COVID-19. We observed a significant difference in length of hospital stay (23.2 ± 8.9 vs. 8.5 ± 6.2 days; p < 0.001) between survivors and nonsurvivors.
Central Illustration.
SARS-CoV-2 in Heart Transplant Recipients: A Multicenter Analysis in Northern Italy
COVID-19 = coronavirus disease-2019; SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2.
Early-midterm follow-up analysis
During the follow-up, 2 patients died and a third patient has prolonged viral-shedding alternating positive and negative swabs in absence of symptoms and not requiring hospitalization. An asymptomatic patient previously self-home quarantined with no therapy modifications and negativized swab test, died due to cytomegalovirus (CMV) pneumonia. The second patient, who was treated by reducing the immunosuppressive regimen and discharged home with standard therapy, 2 months later was rehospitalized due to cardiogenic shock for suspected allograft rejection. No endomyocardial biopsy was performed. He was treated with thymoglobulin infusion but he was complicated with pneumonia and cholecystitis and died due to septic shock. He had negative nasopharyngeal swab test for SARS-CoV-2. All the other patients are doing well and are strictly monitored.
Cohort 2 analysis
From July 1, 2020, we had 6 new patients with a positive test for SARS-CoV-2. Five patients are self-quarantined, 1 is still hospitalized for pneumonia. Patients at home maintained immunosuppressive therapy unchanged and antibiotic therapy was not administered if they were afebrile. The hospitalized patient had fever and bilateral interstitial pneumonia for which immunosuppressive therapy was reduced and prophylactic antibiotic therapy was added along with supplemental oxygen therapy. Neither antiviral drugs nor hydroxychloroquine were administered (Table 4 ).
Table 4.
Characteristics of Cohort 2 SARS-CoV-2–Positive Heart Transplant Population (N = 6)
| Demographics | |
| Age (yrs) | 59.0 (48.3–73.5) |
| Male | 4 (67) |
| Time from Htx (yrs) | 7.8 (3.7–18.3) |
| Risk factors | |
| BMI (kg/m2) | 26.9 (22.7–29.9) |
| Obesity | 2 (33) |
| Arterial hypertension | 4 (67) |
| Dyslipidemia | 2 (33) |
| Diabetes mellitus | 2 (33) |
| Former smoker | 0 (0) |
| Peripheral vascular disease | 0 (0) |
| COPD | 1 (17) |
| Stroke | 0 (0) |
| Malignancy | 2 (33) |
| Dialysis | 0 (0) |
| GFR (ml/min) | 43.5 (39.0–73.5) |
| Previous PCI | 1 (17) |
| CMR | 1 (17) |
| AMR | 0 (0) |
| CAV Score | 0 (0–3) |
| NYHA functional class | |
| I | 4 (66) |
| II | 2 (33) |
| III | 0 |
| IV | 0 |
| LVEF (%) | 58.5 (55.8–62.8) |
| Immunosuppressive therapy | |
| Cyclosporine | 3 (50) |
| Tacrolimus | 1 (17) |
| Prednisone | 3 (50) |
| Mycophenolate | 4 (67) |
| Everolimus | 1 (17) |
| Azathioprine | 0 (0) |
| Anticoagulant therapy | 0 (0) |
| Laboratory results at last follow-up | |
| WBC count (cells per 109/l) | 5.48 (5.11–6.66) |
| Lymphocyte (cells per 109/l) | 1.22 (0.92–1.77) |
| COVID-19 onset | |
| Presenting symptoms | 4 (66) |
| Fever | 4 (66) |
| Cough | 2 (33) |
| Shortness of breath | 2 (33) |
| Myalgia | 2 (33) |
| Headache | 1 (17) |
| Anosmia | 0 (0) |
| Sinusitis | 0 (0) |
| Gastrointestinal | 0 (0) |
| Nasopharyngeal swab test positive | 6 (100) |
| X-ray pneumonia | 1 (17) |
| Fever peak (°C) | 38.1 (37.7–38.4) |
| Duration of fever (days) | 4.8 (4.5–5.0) |
| Duration of symptoms (days) | 6.0 (4.8–7.0) |
| Hospitalization | 1 (17) |
| Modification of immunosuppressive therapy | 1 (17) |
| Antibiotics prophylaxis | 4 (67) |
| Death | 0 (0) |
Values are mean (range) or n (%).
AMR = antibody-mediated rejection; BMI = body mass index; CAV = cardiac allograft vasculopathy; CMR = cardiac magnetic resonance; COPD = chronic obstructive pulmonary disease; GFR = glomerular filtration rate; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cells.
Discussion
We present the largest retrospective and first Italian multicenter case series of heart transplant recipients with COVID-19. We enrolled a total of 53 patients with documented SARS-CoV-2 infection among 2,676 heart transplant recipients alive at the onset of the COVID-19 pandemic from 7 leading heart transplant centers in Northern Italy. The close collaboration between the participating centers enabled the collection of data from a unique cohort of heart transplant recipients experiencing the infection early in the SARS-CoV-2 pandemic. The timing of infection permits analysis of unique aspects of the clinical course and outcomes of this disease. Furthermore, by assessing the clinical conditions at admission, chronic immunosuppressive therapy and its modulation, supportive measures during hospitalization, and specific COVID-19 treatments, we were able to identify baseline characteristics and medical interventions that could impact patients’ prognosis.
To date, in the Italian regions considered (Lombardy, Veneto, Emilia-Romagna, Piedmont, and Friuli Venezia Giulia), the prevalence of the disease in the overall population accounts for 7 cases per 1,000 people (1). The median age of all Italian positive cases with COVID-19 is 61 years and 54% of those affected are female (1). Among deaths, the median age was 82 (0–109) years and 57% were male. Arterial hypertension (66%), diabetes mellitus (30%), and ischemic heart disease (28%) were the most common pre-existing conditions. More than 60% of the deaths had 3 or more diseases at the time of infection. Fever (75%), along with dyspnea (73%) and cough (38%), was the most common presenting symptom (1).
In our cohort of heart transplant recipients, we observed a 2-fold prevalence of infection, approximately 17 cases per 1,000 individuals. Although solid organ transplant recipients are typically more aware of infection prevention and use of personal protective equipment, the prevalence of COVID-19 in this population was higher than in the general population. This higher prevalence is likely the result of the higher susceptibility of heart transplant recipients to infections due to their chronic immunosuppressed state. In Northern Italy, the case-fatality rate of the overall population is 15.4%, whereas that of our heart transplant patients was 29.7%. The Italian population older than 70 years accounts for 85% of all COVID-19–related deaths with a case-fatality rate >26% (1). The case-fatality rate of heart transplant recipients with COVID-19 is similar to the 25% mortality reported by Latif et al. (3) in their cohort. A recent meta-analysis of COVID-19 cases showed a greater risk of developing severe and lethal disease in male patients, especially if they are older than 65 years and active smokers. Comorbidities such as hypertension, diabetes, and cardiovascular and respiratory disease also can influence prognosis (6). Our findings confirm these observations. According to our univariable analysis, age, higher NYHA functional class, diabetes, chronic kidney disease, peripheral vascular disease, cardiac allograft vasculopathy, and lower oxygen saturation at admission (related to severe pulmonary involvement and delay in hospital referral), may account for the observed higher case-fatality rate. In the nonsurviving population, diabetes-related cardiovascular effects are exemplified by the higher rate of extracardiac arteriopathy and previous percutaneous coronary intervention, as well as a lower mean eGFR. As reported in a meta-analysis by Li et al. (7), diabetes mellitus was 2-fold more frequent in ICU/severe cases than in nondiabetic individuals. Diabetes mellitus itself also can be with an abnormal immune response characterized by increased cytokine production and inhibition of macrophages and T-cell activation, which leads to higher susceptibility to infection (8). Impaired glycemic control also is associated with an increased risk of bacterial coinfection and pneumonia (9).
Differences in age, eGFR, and prednisone use were observed between hospitalized and nonhospitalized patients, but the relevance of these findings remains unknown because of the small sample size of this study.
Most (81%) patients in this case series were admitted to the hospital because of fever and hypoxemic respiratory failure. Of the individuals with severe primary pulmonary involvement, 32% needed only noninvasive ventilation, whereas only 4% required mechanical ventilation. Nevertheless, mortality rate was much higher (30%) for patients with primarily pulmonary presentation and, in fact, respiratory failure was their leading cause of death. These poor outcomes may be related to the rapidity with which the pandemic spread in Northern Italy, leading to a critical shortage of ICU beds over a period of only a few days. In these circumstances, heroic efforts were made to manage very ill patients in non–acute-care settings. It is plausible that lack of health care resources in the midst of an overwhelming emergency may have contributed to the high mortality rate of our patients’ cohort.
Elimination of immunosuppressive therapy has been reported in a few cases of solid organ transplant recipients infected with the SARS-CoV-2 virus (10); however, we hypothesized that suspension could lead to allograft failure due to rebound activation of immunologic memory, potentially exacerbated by the hyperinflammatory state. Instead, the center participating in this prospective observational study elected to reduce immunosuppressive therapy in markedly lymphopenic and febrile patients so that the components of acquired immune system could be recruited against the viral infection. Although we are aware of the ongoing controversies surrounding the use of hydroxychloroquine, this drug was used in most patients based on its anti-inflammatory activity (11). Despite the lack of agreement in the scientific community and awareness of drug interactions with calcineurin and mammalian target of rapamycin inhibitors (12), antiretroviral therapy with ritonavir/lopinavir was used in nearly 50% of the patients. In only 1 case we used tocilizumab, a monoclonal antibody against interleukin-6. Tocilizumab is currently used in some forms of cytokine-release syndromes and is currently under investigation for treatment of COVID-19, although clear benefits have not yet been demonstrated (13).
We observed no COVID-19–related myocarditis. Although endomyocardial biopsies have not been performed due to the enormously strained health care resources, persistently normal LVEF, only slight changes in myocardial biomarkers, and lack of requirement for mechanical circulatory support, are consistent with the belief that COVID-19 myocarditis did not occur in our heart transplant recipients.
Broad-spectrum prophylactic antibiotics were used to avoid superimposed bacterial infection, the risk of which was heightened by lymphocytopenia and protracted hospitalization. Beta-lactamases and macrolides were predominantly used and a second line of targeted antibiotic therapy was used when a specific pathogen was identified. The fact that of 5 patients with confirmed superimposed bacterial infections 4 developed sepsis and 2 died, confirms the wisdom of our approach, which may have averted more deaths related to superimposed bacterial infections.
In patients with severe disease, we used therapeutic doses of low molecular weight heparin to prevent venous thromboembolic risk. Coagulopathy with microcirculation thrombophilia in severe forms is related to hyperinflammatory state due to the infection itself and correlates with the poorest outcomes (14). Tests of aggregometry and thromboelastography were not performed due to the urgency of the situation and the scarcity of resources.
During the follow-up, unfortunately, we observed the occurrence of 2 new deaths and a second wave of infections involving 6 new patients.
Regarding the 2 deaths, the first patient was self-quarantined and his immunosuppressive regimen was left unchanged. He died due to CMV-related pulmonary infection. It can be hypothesized that an alteration in the immunosuppressive state has favored the CMV replication. The second patient, in whom immunosuppressive therapy was previously reduced, was discharged home with standard therapy. Probably, this was enough to trigger a rejection that proved itself later, with cardiogenic shock. The death then occurred from complications following hospitalization. Immunosuppressant plasma levels should be strictly monitored after therapy modifications during the disease and follow-up.
As for the new infections we registered, in 5 cases, being asymptomatic, as in the previous cases described, we did not modify the immunosuppressive therapy, we did not add antiviral drugs, and we treated the patients with careful home monitoring. In 1 patient, in whom pulmonary involvement was more important, the patient was hospitalized and treated as discussed previously by reducing immunosuppressive therapy and adding antibiotics to avoid opportunistic infections. The clinical characteristics of the patients self-quarantined were comparable regardless the cohort considered.
The data presented here highlight that heart transplant recipients are extremely vulnerable to the unfavorable consequences of the COVID-19 infection. The fact that some patients have been successfully treated during self-quarantine at home does not warrant lowering the attention of care, and indeed underlines the importance of early referral of infected heart transplant recipients to their respective programs. In fact, there is a risk, even if the cases observed are few in our experience, that on the one hand we go toward a state of excessive immunosuppression with the evidence of opportunistic infections, and on the other toward a state of underimmunosuppression with the possibility of rejection.
The high mortality we observed seems to be unrelated to allograft dysfunction or rejection. The reduction of immunosuppression in severe forms of disease is crucial for 2 fundamental aspects: first, promoting the immune response against viral infection, and second, reducing the risk of superinfections related to hospitalization. For future hospitalized heart transplant recipients with COVID-19, our strategy will consist of reduction of immunosuppression and antibiotic prophylaxis, along with the best supportive care available. We believe that in the asymptomatic forms, immunosuppressive therapy may remain unchanged until the first symptoms emerge. One of the main findings, in light of this experience, is that there is always a careful monitoring of the patient, because at any moment the patient can take different paths. Furthermore, availability of targeted SARS-CoV-2 antiviral drugs seems to be closer; hopefully they will soon be available for the physician.
Study limitations
First, in the absence of a control group, despite enrolling the largest series to date of heart transplant recipients with COVID-19, the study is observational in nature. The cohort presented may not represent the heart transplant population at large, because of its geographic restriction to Northern Italy and their diagnosis early in the course of the pandemic. The prevalence of SARS-CoV-2 infection may have been underestimated because asymptomatic heart transplant recipients were not tested. As mounting evidence is emerging about the absence of benefits of using anti-inflammatory or antiviral therapies (11), some of the treatments proposed for our patients may seem outdated. These limitations should be viewed in the context of a rapidly spreading infection that overwhelmed the Northern Italian Health System, despite the heroic efforts of its health care providers.
Conclusions
Heart transplant recipients are especially vulnerable to infection with SARS-CoV-2 infection and experienced a 2-fold higher mortality compared with the general population. Specific risk factors have been identified for severe disease and higher mortality. The wisdom of reducing the intensity of immunosuppression and of using anti-inflammatory and antibiotic prophylaxis requires study in larger populations. The severity of COVID-19 disease in heart transplant recipients underscores the critical importance of early and timely referral to specialized heart transplant centers. Despite the acknowledged limitations, this study evaluated the largest population of heart transplant recipients infected with SARS-CoV-2 to date.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Data on heart transplant recipients in the COVID-19 era are still very limited. This is a retrospective, observational, and multicentric study that has included the leading heart transplant centers of Northern Italy, where the SARS-CoV-2 pandemic has been more devasting. We included all heart transplant recipients positive for SARS-CoV-2 at these centers. Thus, this paper is the study with largest comprehensive data, among those already published, on the SARS-CoV-2 infection prevalence and clinical presentation. The risk of SARS-CoV-2 infection in patients treated by immunosuppressive agents is double in comparison with the general population. Even the mortality that resulted was doubled in the heart transplant population. The reduction of immunosuppressive therapy, associated with hydroxychloroquine and broad-spectrum antibiotics administration, has been the common strategy, and it accounted for a mortality of 30%.
TRANSLATIONAL OUTLOOK: The dataset created for the pandemic emergency is prospective, and we are collecting data relating to new cases, as well as the clinical evolution of those already recognized as infected. Future studies will concern the effects of the reduction of immunosuppressive therapy, and the systemic effects of the Covid-19 virus by controlling the immune response elicited by the virus itself in each patient.
Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Giuseppe Toscano, MD, and Angela Fraiese, MD (Ospedale di Padova, Padova), Francesco Serafini, MD (Ospedale dell’Angelo, Mestre Venezia), Angela Ribola, MD (Ospedale di Cremona, Cremona), and Attilio Lanzini, MD (Ospedale di Manerbio, Manerbio) for support in data collection.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Istituto Superiore di Sanità Sorveglianza Integrata COVID-19 in Italia, aggiornamento del 30 Giugno 2020. https://www.epicentro.iss.it/coronavirus/sars-cov-2-dashboard Available at:
- 2.Li F., Cai J., Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;39:496–497. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latif F., Farr M.A., Clerkin K.J., et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020 May 13 doi: 10.1001/jamacardio.2020.2159. (E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira M.R., Mohan S., Cohen D.J., et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Z., Peng F., Xu B., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B., Yang J., Zhao F., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Critchley J.A., Carey I.M., Harris T., DeWilde S., Hosking F.J., Cook D.G. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. 2018;41:2127–2135. doi: 10.2337/dc18-0287. [DOI] [PubMed] [Google Scholar]
- 9.Ferlita S., Yegiazaryan A., Noori N., et al. Type 2 diabetes mellitus and altered immune system leading to susceptibility to pathogens, especially Mycobacterium tuberculosis. J Clin Med. 2019;8:2219. doi: 10.3390/jcm8122219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu L., Xu X., Ma K., et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20:1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA letter revoking of emergency use authorization for emergency use of chloroquine phosphate and hydroxychloroquine sulfate, June 15, 2020. US Food and Drug Administration. https://www.fda.gov/media/138945/download Available at:
- 12.Elens L., Langman L.J., Hesselink D.A., et al. Pharmacologic treatment of transplant recipients infected with SARS-CoV-2: considerations regarding therapeutic drug monitoring and drug-drug interactions. Ther Drug Monit. 2020;42:360–368. doi: 10.1097/FTD.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alzghari S.K., Acuña V.S. Supportive treatment with tocilizumab for COVID-19: a systematic review. J Clin Virol. 2020;127:104380. doi: 10.1016/j.jcv.2020.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiezia L., Boscolo A., Poletto F., et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]